Background
Whether lipoprotein(a) [Lp(a)] is associated with recurrent cardiovascular events (RCVEs) still remains controversial. The present study aimed to investigate the prognostic value of Lp(a) for long-term RCVEs and each component of it in people with acute coronary syndrome (ACS).
Methods
This multicenter, observational and retrospective study enrolled 765 ACS patients at 11 hospitals in Chengdu from January 2014 to June 2019. Patients were assigned to low-Lp(a) group [Lp(a) < 30 mg/dl] and high-Lp(a) group [Lp(a) ≥ 30 mg/dl]. The primary and secondary endpoints were defined as RCVEs and their elements, including all-cause death, nonfatal myocardial infarction (MI), nonfatal stroke and unplanned revascularization.
Results
Over a median 17-month follow-up, 113 (14.8%) patients presented with RCVEs were reported, among which we observed 57 (7.5%) all-cause deaths, 22 (2.9%) cases of nonfatal stroke, 13 (1.7%) cases of nonfatal MI and 33 (4.3%) cases of unplanned revascularization. The incidences of RCVEs and revascularization in the high-Lp(a) group were significantly higher than those in the low-Lp(a) group (P < 0.05), whereas rates of all-cause death, nonfatal stroke and nonfatal MI were not statistically different (P > 0.05). Kaplan–Meier analysis also revealed the same trend. Multivariate Cox proportional hazards analysis showed that 1-SD increase of Lp(a) was independently associated with both the primary endpoint event [hazard ratio (HR), 1.285 per 1-SD; 95% confidence interval (CI), 1.112–1.484; P < 0.001] and revascularization (HR, 1.588 per 1-SD; 95% CI, 1.305–1.932; P < 0.001), but not with the other secondary events.
Conclusion
Increased Lp(a) is an independent predictor of RCVEs and unplanned revascularization in patients with ACS.
Keywords: acute coronary syndrome, lipoprotein(a), recurrent cardiovascular events, revascularization
Introduction
Acute coronary syndrome (ACS), including acute myocardial infarction (MI) and unstable angina, is a type of clinical critical syndrome and is a major cause of hospitality and death of coronary heart disease (CHD). More seriously, despite significant advances in the optimal secondary prevention treatment, such as statin therapy, there still remain substantial residual risks in patients who had cardiovascular events (CVEs) before [1,2]. Therefore, besides the existing indicators, it is urgent to find a new one to help predict the recurrent CVEs (RCVEs) improve the prognosis.
Recently, lipoprotein(a) [Lp(a)] has been recognized as a novel independent risk factor for the incidence of CHD [3–7], which is consisted of a low-density lipoprotein-like particle and apolipoprotein B100, with apolipoprotein(a) [apo(a)], the characteristic protein of Lp(a), covalently binding to it via a disulfide binding [8]. Compositionally, Lp(a) shows higher pathogenicity compared with LDL-cholesterol (LDL-C) in CHD due to the presence of apo(a), which is regarded as the major causative factor of atherosclerosis, thrombosis and inflammation [9].
It has been demonstrated the predictive value of Lp(a) on cardiovascular diseases (CVD) in the general people around the world and current guideline suggests Lp(a) should be one-off measured to stratify people who have a substantial lifetime risk of CVD [10]. However, controversy still exists in people who had previous CVEs, especially ACS [11–13]. Aside from the negative results, among studies that reported poor prognosis, there is also evidence to suggest differential predictive values of Lp(a) in individual components of RCVEs [7,12,14–17]. Therefore, we conducted this study to further discuss the effects of Lp(a) on predicting RCVEs and components of it in patients with ACS.
Methods
Study design and population
This retrospective, multicenter and observational cohort study (registration number for clinical trials: ChiCTR1900025138) consecutively recruited Chinese patients with ACS [defined as ST-segment elevation MI (STEMI), non-ST-segment elevation MI and unstable angina pectoris, and definitions were determined by current guidelines [18]] from 11 tertiary general hospitals located in Chengdu between January 2014 and June 2019 (http://www.medresman.org). Patients were excluded according to the criteria as follows: (a) younger than 18 years of age; (b) severe liver and kidney diseases, severe infectious diseases, decompensated heart failure and malignant tumors; (c) incomplete plasma Lp(a) records on admission; (d) died in the hospital at baseline and (e) lost to follow-up. Finally, 765 patients were included in the analysis. After admission, all enrolled patients received optimal secondary prevention therapy. Demographic and clinical data, medical history and medicine at discharge were collected from hospital records at baseline. Since plasma Lp(a) concentrations ≥30 mg/dl have been reported to have positive relation to increased risk of CVD [6,19], the study population was assigned to high-Lp(a) group [Lp(a) ≥ 30 mg/dl] (n = 203) and low-Lp(a) group [Lp(a) < 30 mg/dl] (n = 562). The study was approved by local ethics review board.
Follow-up and clinical endpoints
Patients were followed up routinely at the data of discharge, 1, 6 and 12 months, and then annually after that. The information about RCVEs was obtained through telephone contacts with patients and their family members, or records from outpatient service and readmission. The baseline and follow-up data were collected by trained cardiovascular professionals. The primary observational endpoint of the present study was RCVEs and was the composite of all-cause death, nonfatal MI, nonfatal stroke and unplanned revascularization. The secondary observational endpoints were each component of the primary composite endpoint. Nonfatal MI was defined as the value of at least one of myocardial biomarkers greater than the upper limit of reference and with at least one of the following: (a) symptoms of myocardial ischemia and (b) electrocardiogram changes: new ST-T segment changes or left bundle branch block or emergence of pathological Q waves. Stroke was defined as acute or focal brain dysfunction with imaging changes due to a variety of vascular (ischemic or hemorrhagic) etiologies. Unplanned revascularization was defined as revascularization of any ischemic vessel by percutaneous coronary intervention (PCI) or coronary artery bypass grafting (CABG), but scheduled revascularization such as second-stage operation was not included.
Statistical analysis
Data were statistically analyzed with the SPSS version 26.0 software. Continuous data of normal or nonnormal distribution were presented as mean ± SD or median (25th–75th percentiles: Q1–Q3), and the differences of two groups were analyzed by using unpaired t-test or Mann–Whitney U test, respectively. Categorical variables were expressed as number (percentage) and examined by Chi-square test or Fisher’s exact test, as appropriate. The cumulative incidences of primary endpoint and secondary endpoints were assessed by Kaplan–Meier survival analysis, and we used the log-rank test to compare between the two groups. In order to estimate whether elevated Lp(a) was a predictor of poor prognosis, based on the univariate regression analysis for Lp(a), three models were established using Cox proportional hazards regression analysis: (a) model 1: sex, age and types of ACS were adjusted; (b) model 2: variables in model 1 and smoking, diabetes and hypertension were adjusted and (c) model 3: adjusted for variables that were included in model 2, LDL-C, serum creatinine and PCI. The association of Lp(a) with each component of RCVEs was aslo evaluated by model 3. Lp(a) was analyzed in two ways: (a) as a categorical variable and (b) as a continuous variable. A two-tailed P value <0.05 was considered to indicate statistical significance.
Results
Baseline characteristics of the population
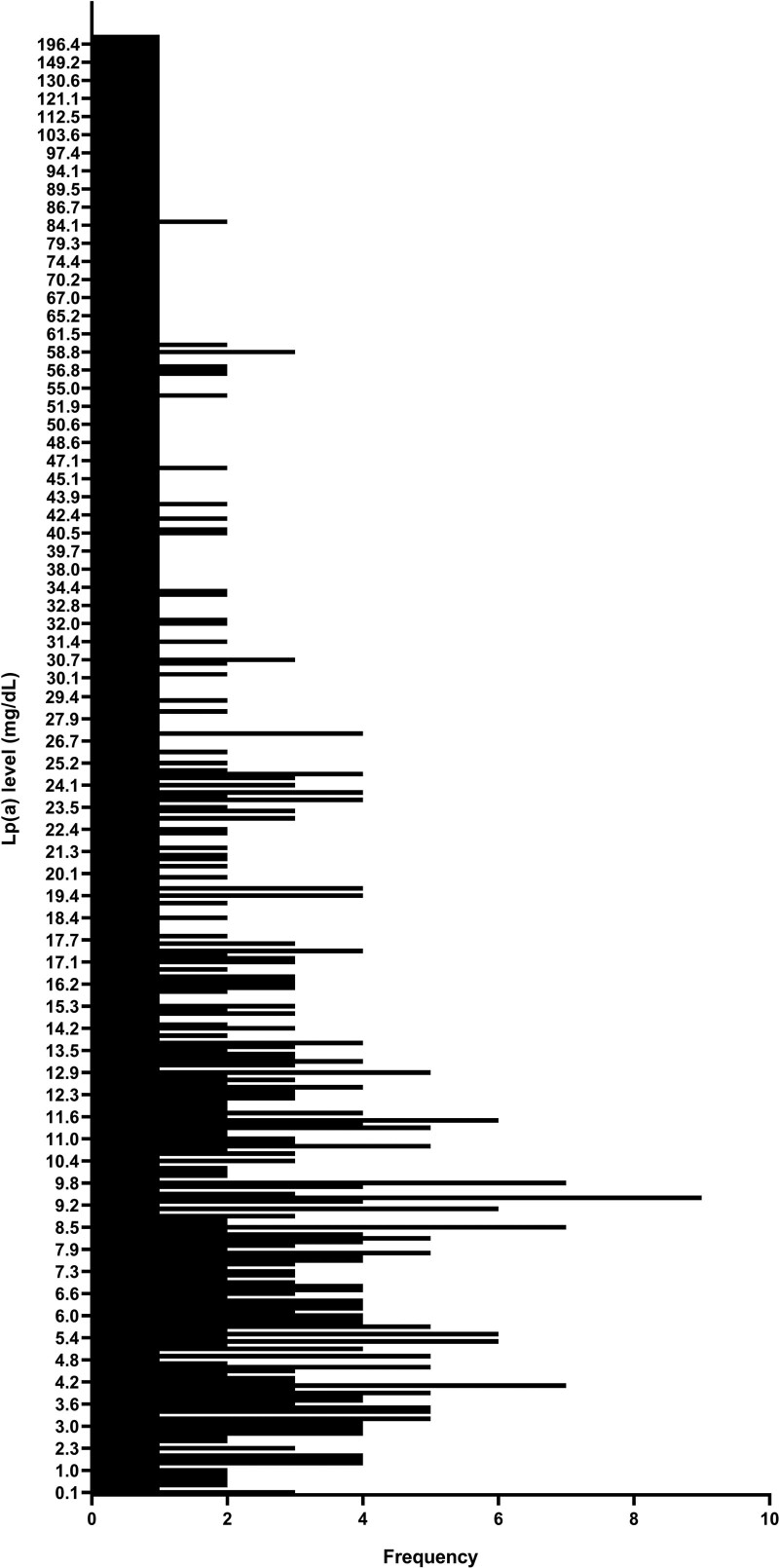
A total of 765 patients were enrolled in this study, including 574 males (75%) and 191 females (25%). The average age was 65.67 ± 13.40 years. The median and mean Lp(a) level was 13.41 mg/dl (25th percentile–75th percentile: 7.14–31.05 mg/dl) and 26.04 ± 31.41 mg/dl. Lp(a) levels ranged from 0.1 to 237.0 mg/dl (Fig. 1).
Fig. 1.
A histogram displaying the range and frequency of Lp(a) levels. Lp(a) levels range from 0.1 mg/dl to 237.0 mg/dl. Lp(a), lipoprotein(a).
The demographical, socioeconomic, clinical and medical characteristics of the study population at baseline were listed in Table 1. Patients in the high-Lp(a) group had higher prevalence of STEMI and multivessel diseases; moreover, they were presented with lower level of triglyceride. Apart from that, there were no significant differences in age, sex, smoker, pre-existing conditions, clinical symptoms, blood pressure, heart rate, other results of laboratory measurements, PCI, medicine at discharge, days in hospital and costs between the two groups (P > 0.05).
Table 1.
Baseline clinical characteristics grouped by lipoprotein(a) levels
| Variable | Low-Lp(a) group (<30 mg/dl) (n = 562) |
High-Lp(a) group (≥30 mg/dl) (n = 203) |
P value |
|---|---|---|---|
| Demographic | |||
| Age, years | 65.74 ± 13.24 | 65.48 ± 13.88 | 0.813 |
| Male, n (%) | 418 (74.4) | 156 (76.6) | 0.486 |
| Social benefit | |||
| Hospital stay, days | 9.00 (7.00, 12.00) | 9.00 (7.00, 11.00) | 0.722 |
| Hospitalized cost, 10 000 yuan | 3.52 (1.29, 4.86) | 3.83 (2.03, 5.28) | 0.058 |
| Medical history | |||
| Smoking, n (%) | 229 (40.7) | 80 (39.4) | 0.739 |
| Diabetes, n (%) | 159 (28.3) | 46 (22.7) | 0.120 |
| Hypertension, n (%) | 331 (58.9) | 126 (62.1) | 0.430 |
| Dyslipidemia, n (%) | 62 (11.0) | 24 (12.0) | 0.710 |
| Prior CHD, n (%) | 91 (16.2) | 36 (17.7) | 0.613 |
| Prior MI, n (%) | 33 (5.9) | 15 (7.4) | 0.145 |
| Prior PCI, n (%) | 39 (7.0) | 16 (7.9) | 0.665 |
| Prior stroke, n (%) | 26 (4.6) | 9 (4.5) | 0.917 |
| Chronic obstructive pulmonary disease, n (%) | 20 (3.6) | 13 (6.4) | 0.088 |
| Peripheral arterial disease, n (%) | 8 (1.4) | 2 (1.0) | 0.919 |
| Type of ACS, n (%) | |||
| STEMI | 304 (54.1) | 127 (62.6) | 0.037 |
| NSTEMI | 148 (26.3) | 42 (20.7) | 0.111 |
| UAP | 110 (19.6) | 34 (16.7) | 0.378 |
| Clinical presentation | |||
| Chest pain/chest tightness | 534 (95.0) | 189 (93.1) | 0.249 |
| Dyspnea | 17 (3.0) | 10 (4.9) | 0.208 |
| Nausea and vomiting | 41 (7.3) | 14 (6.9) | 0.852 |
| Sweat | 124 (22.1) | 43 (21.2) | 0.797 |
| Systolic blood pressure, mmHg | 130.0 (115.0, 148.0) | 130.0 (115.0, 150.0) | 0.701 |
| Heart rate, beats per minute | 78.0 (68.0, 91.0) | 80.0 (69.0, 89.0) | 0.941 |
| Laboratory measurements | |||
| Lp(a), mg/dl | 9.76 (5.62, 16.23) | 55.40 (40.46, 84.79) | <0.001 |
| Total cholesterol, mmol/l | 4.32 (3.59, 5.17) | 4.23 (3.55, 5.08) | 0.727 |
| LDL-C, mmol/l | 2.60 (1.98, 3.27) | 2.52 (2.05, 3.15) | 0.915 |
| HDL-C, mmol/l | 1.13 (0.94, 1.36) | 1.14 (0.96, 1.40) | 0.324 |
| Triglyceride, mmol/l | 1.44 (1.01, 2.17) | 1.15 (0.84, 1.78) | <0.001 |
| Serum creatinine, μmol/l | 77.3 (64.5, 94.7) | 77.9 (66.0, 97.2) | 0.633 |
| eGFR, ml/(minutes × 1.73 m2) | 85.36 (65.22, 98.74) | 84.98 (65.47, 97.46) | 0.779 |
| Fibrinogen, g/l | 3.18 (2.60, 4.02) | 3.35 (2.69, 4.43) | 0.095 |
| Hemoglobin, g/l | 133.0 (121.0, 145.0) | 131.0 (116.8, 146.3) | 0.377 |
| Multiple coronary artery lesions, n (%) | 159 (36.2) | 76 (45) | 0.047 |
| PCI, n (%) | 401 (71.4) | 156 (76.8) | 0.132 |
| Postdischarge medication | |||
| Antiplatelet drugs, n (%) | 193 (99.0) | 526 (97.4) | 0.318 |
| Statins, n (%) | 189 (96.9) | 518 (95.9) | 0.533 |
| Β-blocker, n (%) | 142 (72.8) | 384 (71.1) | 0.650 |
| ACEI/ARB, n (%) | 99 (50.8) | 270 (50.0) | 0.854 |
| Diuretics, n (%) | 43 (22.1) | 97 (18.0) | 0.213 |
ACEI, angiotensin converting enzyme inhibitor; ACS, acute coronary syndrome; ARB, angiotensin receptor blocker; CHD, coronary heart disease; eGFR, estimated glomerular filtration rate; HDL-C, HDL-cholesterol; LDL-C, LDL-cholesterol; Lp(a), lipoprotein(a); MI, myocardial infarction; NSTEMI, non-ST-segment elevation myocardial infarction; PCI, percutaneous coronary intervention; STEMI, ST-segment elevation myocardial infarction; UAP, unstable angina pectoris.
Clinical outcomes and Kaplan–Meier analysis
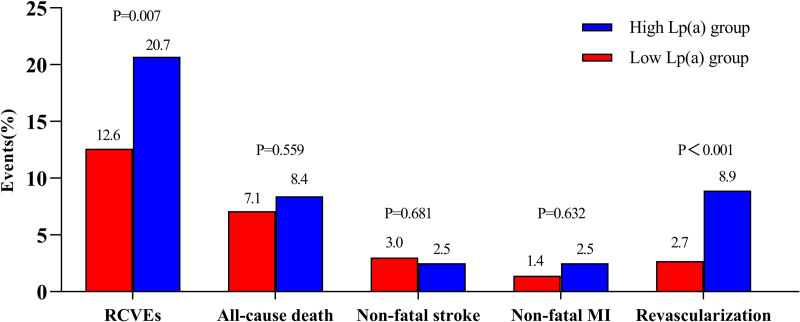
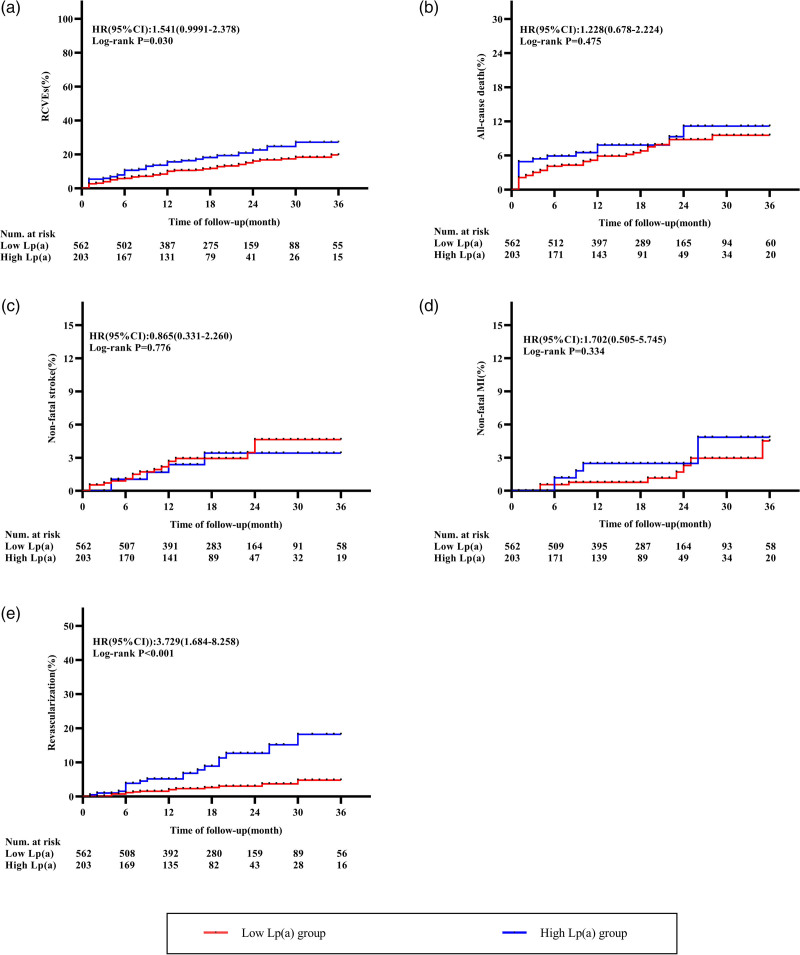
During the 17 months (10 and 24 months) follow-up period, 113 RCVEs (14.8%) were occurred, including 57 (7.5%) all-cause death, 22 (2.9%) nonfatal stroke, 13 (1.7%) nonfatal MI and 33 (4.3%) coronary revascularization. The prevalence of RCVEs was higher in the high-Lp(a) group than in the low-Lp(a) group (20.7% vs. 12.6%; P = 0.006), and this was mainly driven by revascularization (8.9% vs. 2.7%; P < 0.001) (Fig. 2). Additionally, the Kaplan–Meier survival curves confirmed the same trend. The curves for primary endpoint and revascularization showed a significant difference between two groups (Fig. 3a, Log-rank P = 0.002; Fig. 3e, Log-rank P < 0.001), whereas subjects in higher Lp(a) levels failed to show statistical distinction of all-cause death, nonfatal sroke and MI (Fig. 3b–d, Log-rank P = 0.475; P = 0.776; P = 0.252, respectively).
Fig. 2.
Prevalence of RCVEs and components of RCVEs in the high- and low-Lp(a) group. The rate of RCVEs and revascularization in high-Lp(a) group was significantly higher than that in the low-Lp(a) group. Lp(a), lipoprotein(a); RCVEs, recurrent cardiovascular events; MI, myocardial infarction.
Fig. 3.
Kaplan–Meier curves for the composite endpoint of RCVEs and endpoint events that RCVEs included. (a) Kaplan–Meier curves for primary endpoint (RCVEs); (b) Kaplan–Meier curves for all-cause death; (c) Kaplan–Meier curves for nonfatal stroke; (d) Kaplan–Meier curves for nonfatal MI and (e) Kaplan–Meier curves for revascularization. Lp(a), lipoprotein(a); MI, myocardial infarction; RCVEs, recurrent cardiovascular events.
Cox proportional hazard regression analyses to evaluate the association of lipoprotein(a) with recurrent cardiovascular events
Based on the univariate regression analysis for Lp(a), three models constructed by using Cox proportion hazard analyses (model 1–model 3, as above) were applied to evaluate the relationship between Lp(a) and RCVEs. Whether Lp(a) was a nominal variable or continuous variable, univariate Cox regression analysis (Crude Model) showed that Lp(a) was significantly correlated with RCVES [as a nominal variable: HR (95% CI), 1.819 (1.241–2.666); P = 0.002, and as a continuous variable: HR (95% CI), 1.255 (1.094–1.439); P = 0.001]. After adjusting for sex, age, type of ACS, smoking, diabetes, hypertension, LDL-C, serum creatinine and PCI in model 3, this association still existed [as a nominal variable: HR (95% CI), 2.068 (1.366–3.132); P < 0.001, and as a continuous variable: HR (95% CI), 1.285 (1.112–1.484); P < 0.001] (Table 2).
Table 2.
Different models to evaluate association of lipoprotein(a) with recurrent cardiovascular events
| Model | LP(a) as a nominal variablea | LP(a) as a continuous variableb | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P value | HR | 95% CI | P value | |
| Crude model | 1.819 | 1.241–2.666 | 0.002 | 1.255 | 1.094–1.439 | 0.001 |
| Model 1 | 1.850 | 1.262–2.713 | 0.002 | 1.264 | 1.100–1.453 | <0.001 |
| Model 2 | 1.937 | 1.313–2.856 | <0.001 | 1.280 | 1.115–1.470 | <0.001 |
| Model 3 | 2.068 | 1.366–3132 | <0.001 | 1.285 | 1.112–1.484 | <0.001 |
Model 1: adjusted for age, sex and type of ACS.
Model 2: adjusted for variables in model 1 and smoking, diabetes and hypertension.
Model 3: adjusted for variables in model 2 and LDL-C, serum creatinine and PCI.
ACS, acute coronary syndrome; CI, confidence interval; HR, hazard ratio; Lp(a), lipoprotein(a); PCI, percutaneous coronary intervention.
The HR was examined regarding low-Lp(a) group as reference.
The HR was examined by per 1-SD increase of Lp(a).
Furthermore, we used model 3 to evaluate the association of Lp(a) with each component of primary endpoint. When the RCVEs were considered separately, we observed that only the rate of revascularization rose significantly with elevated Lp(a) levels (P < 0.001), with a 4.387-fold higher risk in high-Lp(a) group compared with the reference group and a 1.588-fold higher risk 1-SD increment in Lp(a). The results showed that higher Lp(a) levels were independently associated with both RCVEs and revascularization, but not with nonfatal stroke and MI (Table 3).
Table 3.
Association of lipoprotein(a) with recurrent cardiovascular events and components of recurrent cardiovascular events by using model 3
| End point | Univariate analysis | Multivariate analysisc | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P value | HR | 95% CI | P value | |
| Lp(a) as a nominal variablea | ||||||
| Primary endpoint | 1.819 | 1.241–2.666 | 0.002 | 2.068 | 1.366–3.132 | <0.001 |
| All-cause death | 1.230 | 0.697–2.170 | 0.475 | 1.370 | 0.737–2.550 | 0.320 |
| Nonfatal stroke | 0.866 | .0319–2.349 | 0.778 | 0.770 | 0.253–2.346 | 0.646 |
| Nonfatal MI | 1.901 | 0.621–5.813 | 0.260 | 2.239 | 0.699–7.172 | 0.175 |
| Unplanned revascularization | 3.765 | 1.896–7.476 | <0.001 | 4.387 | 2.052–9.382 | <0.001 |
| Lp(a) as a continuous variableb | ||||||
| Primary endpoint | 1.255 | 1.094–1.439 | 0.001 | 1.285 | 1.112–1.484 | <0.001 |
| All-cause death | 1.082 | 0.855–1.369 | 0.513 | 1.104 | 0.852–1.431 | 0.453 |
| Nonfatal stroke | 0.976 | 0.631–1.510 | 0.912 | 0.897 | 0.526–1.527 | 0.688 |
| Nonfatal MI | 1.034 | 0.625–1.711 | 0.897 | 1.091 | 0.662–1.799 | 0.733 |
| Unplanned revascularization | 1.539 | 1.279–1.851 | <0.001 | 1.588 | 1.305–1.932 | <0.001 |
CI, confidence interval; HR, hazard ratio; Lp(a), lipoprotein(a); MI, myocardial infarction.
The HR was examined regarding low-Lp(a) group as reference.
The HR was examined by per 1-SD increase of Lp(a).
The multivariate analysis was performed by using model 3.
Discussion
This multicenter, observational study retrospectively investigated the predictive significance of Lp(a) levels obtained at admission for RVCEs in patients with ACS. We demonstrated that elevated Lp(a) mass concentration was a strong independent predictor of RVCEs and revascularization (as PCI or CABG), but not significantly associated with all-cause death, nonfatal stroke and MI irrespective of appropriate lipid-lowering therapy.
To the best of our knowledge, a large number of studies have demonstrated that Lp(a) plays an important role in the risk of CVD as primary prevention in the general people with different races, such as White, Black, Asian and mixed [3–5,20–22]. As for secondary prevention, the association between Lp(a) levels and the risk of subsequent CVEs has been recognized in the patients with established coronary artery diseases (CAD), even they were complicated with diabetes [6,7,19,23]. However, among studies that were targeted at subjects who had ever-experienced CVEs revealed inconsistent results [11–15,24]. As early as 2012, Zhou et al. [11] have described the positive correlation between elevated Lp(a) levels and the incidence of consequent major adverse cardiovascular events combined with definite or suspected cardiac death, nonfatal MI, revascularization and fatal or nonfatal stroke within 713 patients. The HR (95% CI) was 1.51 (1.20–1.91) and 1.38 (1.08–1.77) for patients with ACS and those who underwent PCI, respectively [11]. Besides, several studies that enrolled ACS patients who underwent PCI also revealed patients with higher Lp(a) levels (≥20 mg/dl or ≥118 mmol/l) were associated with poor prognosis [12,15]. However, they did not find any significant distinction in death, stroke and MI between the two groups, whereas the occurrence of revascularization was obviously higher when Lp(a) concentrations were elevated. In contrast, the dal-outcomes randomized clinical trial that pooled 969 ACS patients’ and 3170 control patients’ data from 27 countries indicated that there was no association between baseline Lp(a) and ischemic CVEs defined as CAD death, nonfatal coronary event and ischemic stroke [13]. Recently, Xu et al. [24] reported that among 6714 patients who received PCI with an average of 874 days follow-up, plasma Lp(a) was not an independent predictor of long-term cardiovascular outcomes. The present study indeed found higher Lp(a) level was a useful marker for the prognosis of RCVEs. The discrepancy among the existed studies might be probably attributed to multiple confounding factors. For example, the conflicting results between our study and dal-outcomes trial could be explained by Lp(a) and potential LDL-C concentrations. Previous studies have already demonstrated that the risk ratio was continuously elevated with an increasing Lp(a) concentration, and management of LDL-C was of great importance in CVD prevention [4,11,14,19,23,25,26], although Lp(a) seems to play a more important role in causing CAD [15,27,28]. The median Lp(a) and LDL-C levels of our study (13.41 mg/dl and 2.70 mmol/l) were relatively higher than those in the dal-outcomes trial (12.30 mg/dl and 1.94 mmol/l), which may properly explain the inconsistent effects. Another interference factor could be different characteristics in the enrolled patients. In a Chinese prospective study with a large sample size, patients who received PCI without a history of MI or PCI/CABG were included [24], whereas the present study enrolled subjects with ACS and did not exclude those with prior MI, PCI or CABG, causing more prevalence of adverse events. In addition, the ACS population of our study is characterized by the high use of medication [aspirin, P2Y12 inhibitors (94%) and statin (92.4%)] at discharge, which was unlikely to be the point of the disparity, although we have no information about the medication compliance during the follow-up.
In addition, we found that unplanned revascularization drove the composite outcome, which was to some extent in agreement with the former researchers [12,15]. This could be explained by the pathogenicity of Lp(a), which included atherosclerosis, inflammation and thrombosis [9]. Another possible reason may be that patients with high-Lp(a) levels were more likely to have lesions in multiple coronary arteries, and the lesions usually need to be treated several times. If these lesions were not addressed through planned revascularization, they were probably those that were addressed in unplanned revascularization.
Furthermore, several studies indicated that high-Lp(a) levels were related to the occurrence of MI, CVD death or stroke [14,16,17]. A meta-analysis enrolled CAD patients, though, showed negative findings [7]. It is concordant with what we observed in the present study, even though we found there were still stepwise increments in the HR for all-cause death and nonfatal MI, but not for nonfatal stroke [1.147, 1.096 and 0.898 for continuous Lp(a), respectively]. Notably, elevated Lp(a) was independent of the increased risk of CVD mortality and all-cause death that were reported in the preceding mentioned meta-analysis [29], implying higher Lp(a) probably was not predictive for all-cause death. Allocating fatal-MI/stroke to all-cause death may cause the predictive value of nonfatal MI/stroke to be NS. What is more, subjects with previous cerebrovascular issues had greater RCVEs risk than those with a previous ACS [14]. As the proportion of patients who had ever experienced stroke in the present study was only 4.6%, this low rate may also be part of the reason why our results differed. Because of the unified conclusions about the controversies, further studies should be explored by standardizing the assay methods of Lp(a), extending follow-up time and expanding sample’s quantities.
As is well known, plasma Lp(a) levels were genetically determined (by Lp(a) gene located on chromosome 6q26) and not reduced by any intervention, such as diet, age and exercise. By contrast, having diet in obese individuals whether with diabetes or not led to an increase in Lp(a) levels, despite improvement in LDL-C levels [30]. Likewise, statins that lower lipid-like LDL-C also cause a slight increase in Lp(a) concentrations, which could probably be explained by apo(a) expression increment and a new buffer for oxidized phospholipid in the circulation due to the decrease of LDL-C [31,32]. In order to modify Lp(a) levels, some drugs have been tested to figure out whether they did work. Niacin and proprotein convertase subtilisin/kexin type 9 (PCSK9) were reported to be associated with a significant reduction in Lp(a) levels (about 20–30%), and simultaneously in LDL-C levels, but the clinical effects were not identical [33–38]. Niacin did not reduce the risk of major CVEs and, even worse, increased it [33–35]. Instead, PCSK9, such as evolocumab and alirocumab, made it possible to achieve favorable effects on clinical outcomes [36–38]. Recently, antisense therapy was also been developed. Several randomized, double-blind and placebo-controlled trials investigated that antisense DNA oligonucleotides, like ISIS-APO(a)Rx, IONIS-APO(a)Rx and AKCEA-APO(a)-LRx, were indeed a novel, dose-dependent and potent way that benefited Lp(a) lowering without safety concerns [39–41], and this was achieved by targeting apo(a), which was by binding to the complementary apo(a) mRNA sequence to lead to the reduction of translation of apo(a) [42]. What was interesting was that the proportion of Lp(a) reduction by using AKCEA-APO(a)-LRx in CVD patients was significantly larger than that by using PCSK9 (47% vs. 16%). More importantly, the former one reduced the proinflammatory activation of circulating monocytes as well [43], which may indicate a better treatment for patients with elevated Lp(a) levels. Whether isolated Lp(a)-lowering therapy would contribute to a decreased risk of CVD or not still needs to be explored.
Our findings were limited by several factors. First, the present study used retrospective data with a modest sample size, which might potentially result in selection and recall bias. Due to the limitation of sample size, we did not classify patients into more groups based on different Lp(a) levels, which may provide more information on the relation between Lp(a) concentrations and RCVEs. Second, plasma Lp(a) levels were only measured on admission, but since Lp(a) was inherently determined, it may remain relatively stable in the whole life if no Lp(a)-lowering drugs were taken. Third, we were not able to obtain the information about medication use during the follow-up time, and the adherence was unknown. The lack of aspirin, P2Y12, or statin use might influence our results to a certain degree. Fourth, since multivessel CAD probably could be found in patients with high-Lp(a) levels, unplanned revascularization may be conducted for the lesions that were already present at baseline. The missing data of coronary angiography might also affect our results. In addition, Lp(a) may be differently measured in each hospital, and that would probably bring systematic errors.
Conclusion
Our study found that a rise in Lp(a) levels is an independent risk factor of RCVEs in patients with ACS. Among components that made up the RCVEs, interestingly, only revascularization showed the positive correlation with Lp(a). These findings provided additional information about potentially important role of Lp(a), which is not currently widely screened, but maybe should be done in the general population.
Acknowledgements
The work was supported by the Science and Technology Department of Sichuan, China (Grant numbers 2021YJ0215 and 2020YJ0483). The authors would like to thank all the participants of the 11 hospitals for their great contributions. The authors would also like to thank the Science and Technology Department of Sichuan, China for the support.
Conflicts of interest
The authors declare no conflict of interest.
References
- 1.Jernberg T, Hasvold P, Henriksson M, Hjelm H, Thuresson M, Janzon M. Cardiovascular risk in post-myocardial infarction patients: nationwide real world data demonstrate the importance of a long-term perspective. Eur Heart J. 2015; 36:1163–1170. [DOI] [PubMed] [Google Scholar]
- 2.Virani SS, Alonso A, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP, et al. Heart disease and stroke statistics-2020 update: a report from the American Heart Association. Circulation. 2020; 141:e139–e596. [DOI] [PubMed] [Google Scholar]
- 3.Welsh P, Welsh C, Celis-Morales CA, Brown R, Ho FK, Ferguson LD, et al. Lipoprotein(a) and cardiovascular disease: prediction, attributable risk fraction, and estimating benefits from novel interventions. Eur J Prev Cardiol. 2020; zwaa063. [DOI] [PubMed] [Google Scholar]
- 4.Guo C, Cao H, Shan G, Zhao W, Zhang H, Niu K, et al. Elevated lipoprotein(a) and risk of coronary heart disease according to different lipid profiles in the general Chinese community population: the CHCN-BTH study. Ann Transl Med. 2021; 9:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kamstrup PR, Benn M, Tybjaerg-Hansen A, Nordestgaard BG. Extreme lipoprotein(a) levels and risk of myocardial infarction in the general population: the Copenhagen City Heart Study. Circulation. 2008; 117:176–184. [DOI] [PubMed] [Google Scholar]
- 6.Jin JL, Cao YX, Zhang HW, Sun D, Hua Q, Li YF, et al. Lipoprotein(a) and cardiovascular outcomes in patients with coronary artery disease and prediabetes or diabetes. Diabetes Care. 2019; 42:1312–1318. [DOI] [PubMed] [Google Scholar]
- 7.Nestel PJ, Barnes EH, Tonkin AM, Simes J, Fournier M, White HD, et al. Plasma lipoprotein(a) concentration predicts future coronary and cardiovascular events in patients with stable coronary heart disease. Arterioscler Thromb Vasc Biol. 2013; 33:2902–2908. [DOI] [PubMed] [Google Scholar]
- 8.McLean JW, Tomlinson JE, Kuang WJ, Eaton DL, Chen EY, Fless GM, et al. cDNA sequence of human apolipoprotein(a) is homologous to plasminogen. Nature. 1987; 330:132–137. [DOI] [PubMed] [Google Scholar]
- 9.Liu T, Yoon WS, Lee SR. Recent updates of lipoprotein(a) and cardiovascular disease. Chonnam Med J. 2021; 57:36–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mach F, Baigent C, Catapano AL, Koskinas KC, Casula M, Badimon L, et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J. 2020; 41111–188. [DOI] [PubMed] [Google Scholar]
- 11.Zhou J, Cui X, Jin X, Zhou J, Fu M, Zhong C, et al. Association between Lipoprotein (a) level on admission and the incidence of subsequent cardiovascular events in patients with acute coronary syndrome. Int J Cardiol. 2012; 158:464–466. [DOI] [PubMed] [Google Scholar]
- 12.Matsushita K, Hibi K, Komura N, Kimura Y, Matsuzawa Y, Konishi M, et al. Impact of serum lipoprotein (a) level on coronary plaque progression and cardiovascular events in statin-treated patients with acute coronary syndrome: a yokohama-acs substudy. J Cardiol. 2020; 76:66–72. [DOI] [PubMed] [Google Scholar]
- 13.Schwartz GG, Ballantyne CM, Barter PJ, Kallend D, Leiter LA, Leitersdorf E, et al. Association of lipoprotein(a) with risk of recurrent ischemic events following acute coronary syndrome: analysis of the dal-outcomes randomized clinical trial. JAMA Cardiol. 2018; 3:164–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu HH, Cao YX, Jin JL, Zhang HW, Hua Q, Li YF, et al. Association of lipoprotein(a) levels with recurrent events in patients with coronary artery disease. Heart. 2020; 106:1228–1235. [DOI] [PubMed] [Google Scholar]
- 15.Liu Y, Zeng Z, Yu X, Li T, Yao Y, Chen R, Zheng J. Impact of lipoprotein(a) on long-term outcomes after percutaneous coronary intervention in patients with reduced low-density lipoprotein cholesterol. Rev Cardiovasc Med. 2020; 21:147–153. [DOI] [PubMed] [Google Scholar]
- 16.Paré G, Çaku A, McQueen M, Anand SS, Enas E, Clarke R, et al. Lipoprotein(a) levels and the risk of myocardial infarction among 7 ethnic groups. Circulation. 2019; 139:1472–1482. [DOI] [PubMed] [Google Scholar]
- 17.Cui FM, Fang F, He YM, Cai DP, He J, Yang XJ. Establishing age and sex dependent upper reference limits for the plasma lipoprotein (a) in a Chinese health check-up population and according to its relative risk of primary myocardial infarction. Clin Chim Acta. 2018; 484:232–236. [DOI] [PubMed] [Google Scholar]
- 18.Collet JP, Thiele H, Barbato E, Barthélémy O, Bauersachs J, Bhatt DL, et al. 2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur Heart J. 2021; 42:1289–1367. [DOI] [PubMed] [Google Scholar]
- 19.Bigazzi F, Minichilli F, Sbrana F, Pino BD, Corsini A, Watts GF, et al. Gender difference in lipoprotein(a) concentration as a predictor of coronary revascularization in patients with known coronary artery disease. Biochim Biophys Acta Mol Cell Biol Lipids. 2021; 1866:158869. [DOI] [PubMed] [Google Scholar]
- 20.Finneran P, Pampana A, Khetarpal SA, Trinder M, Patel AP, Paruchuri K, et al. Lipoprotein(a) and coronary artery disease risk without a family history of heart disease. J Am Heart Assoc. 2021; 10:e017470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Virani SS, Brautbar A, Davis BC, Nambi V, Hoogeveen RC, Sharrett AR, et al. Associations between lipoprotein(a) levels and cardiovascular outcomes in black and white subjects: the Atherosclerosis Risk in Communities (ARIC) Study. Circulation. 2012; 125:241–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mehta A, Virani SS, Ayers CR, Sun W, Hoogeveen RC, Rohatgi A, et al. Lipoprotein(a) and family history predict cardiovascular disease risk. J Am Coll Cardiol. 2020; 76:781–793. [DOI] [PubMed] [Google Scholar]
- 23.Dai W, Long J, Cheng Y, Chen Y, Zhao S. Elevated plasma lipoprotein(a) levels were associated with increased risk of cardiovascular events in Chinese patients with stable coronary artery disease. Sci Rep. 2018; 8:7726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xu N, Tang XF, Yao Y, Jia SD, Liu Y, Zhao XY, et al. Lipoprotein(a) levels are associated with coronary severity but not with outcomes in Chinese patients underwent percutaneous coronary intervention. Nutr Metab Cardiovasc Dis. 2020; 30:265–273. [DOI] [PubMed] [Google Scholar]
- 25.Lloyd-Jones DM, Wilson PW, Larson MG, Beiser A, Leip EP, D’Agostino RB, Levy D. Framingham risk score and prediction of lifetime risk for coronary heart disease. Am J Cardiol. 2004; 94:20–24. [DOI] [PubMed] [Google Scholar]
- 26.Chen Q, Ding D, Zhang Y, Yang Y, Li Q, Chen X, et al. Prediction of the risk of mortality using risk score in patients with coronary heart disease. Oncotarget. 2016; 7:81680–81690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Willeit P, Yeang C, Moriarty PM, Tschiderer L, Varvel SA, McConnell JP, Tsimikas S. Low-density lipoprotein cholesterol corrected for lipoprotein(a) cholesterol, risk thresholds, and cardiovascular events. J Am Heart Assoc. 2020; 9:e016318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Willeit P, Ridker PM, Nestel PJ, Simes J, Tonkin AM, Pedersen TR, et al. Baseline and on-statin treatment lipoprotein(a) levels for prediction of cardiovascular events: individual patient-data meta-analysis of statin outcome trials. Lancet. 2018; 392:1311–1320. [DOI] [PubMed] [Google Scholar]
- 29.Wang Z, Zhai X, Xue M, Cheng W, Hu H. Prognostic value of lipoprotein (a) level in patients with coronary artery disease: a meta-analysis. Lipids Health Dis. 2019; 18:150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Berk KA, Yahya R, Verhoeven AJM, Touw J, Leijten FP, van Rossum EF, et al. Effect of diet-induced weight loss on lipoprotein(a) levels in obese individuals with and without type 2 diabetes. Diabetologia. 2017; 60:989–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tsimikas S, Gordts PLSM, Nora C, Yeang C, Witztum JL. Statin therapy increases lipoprotein(a) levels. Eur Heart J. 2020; 41:2275–2284. [DOI] [PubMed] [Google Scholar]
- 32.Vavuranakis MA, Jones SR, Cardoso R, Gerstenblith G, Leucker TM. The role of lipoprotein(a) in cardiovascular disease: current concepts and future perspectives. Hellenic J Cardiol. 2020; 61:398–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Albers JJ, Slee A, O’Brien KD, Robinson JG, Kashyap ML, Kwiterovich PO, Jr, et al. Relationship of apolipoproteins A-1 and B, and lipoprotein(a) to cardiovascular outcomes: the AIM-HIGH trial (Atherothrombosis Intervention in Metabolic Syndrome with Low HDL/High Triglyceride and Impact on Global Health Outcomes). J Am Coll Cardiol. 2013; 62:1575–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sahebkar A, Reiner Ž, Simental-Mendía LE, Ferretti G, Cicero AF. Effect of extended-release niacin on plasma lipoprotein(a) levels: a systematic review and meta-analysis of randomized placebo-controlled trials. Metabolism. 2016; 65:1664–1678. [DOI] [PubMed] [Google Scholar]
- 35.Landray MJ, Haynes R, Hopewell JC, Parish S, Aung T, Tomson J, et al. ; HPS2-THRIVE Collaborative Group. Effects of extended-release niacin with laropiprant in high-risk patients. N Engl J Med. 2014; 371:203–212. [DOI] [PubMed] [Google Scholar]
- 36.Bittner VA, Szarek M, Aylward PE, Bhatt DL, Diaz R, Edelberg JM, et al. ; ODYSSEY OUTCOMES Committees and Investigators. Effect of alirocumab on lipoprotein(a) and cardiovascular risk after acute coronary syndrome. J Am Coll Cardiol. 2020; 75:133–144. [DOI] [PubMed] [Google Scholar]
- 37.Szarek M, Bittner VA, Aylward P, Baccara-Dinet M, Bhatt DL, Diaz R, et al.; ODYSSEY OUTCOMES Investigators. Lipoprotein(a) lowering by alirocumab reduces the total burden of cardiovascular events independent of low-density lipoprotein cholesterol lowering: ODYSSEY OUTCOMES trial. Eur Heart J. 2020; 41:4245–4255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sabatine MS, Giugliano RP, Keech AC, Honarpour N, Wiviott SD, Murphy SA, et al. ; FOURIER Steering Committee and Investigators. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med. 2017; 376:1713–1722. [DOI] [PubMed] [Google Scholar]
- 39.Viney NJ, van Capelleveen JC, Geary RS, Xia S, Tami JA, Yu RZ, et al. Antisense oligonucleotides targeting apolipoprotein(a) in people with raised lipoprotein(a): two randomised, double-blind, placebo-controlled, dose-ranging trials. Lancet. 2016; 388:2239–2253. [DOI] [PubMed] [Google Scholar]
- 40.Tsimikas S, Viney NJ, Hughes SG, Singleton W, Graham MJ, Baker BF, et al. Antisense therapy targeting apolipoprotein(a): a randomised, double-blind, placebo-controlled phase 1 study. Lancet. 2015; 386:1472–1483. [DOI] [PubMed] [Google Scholar]
- 41.Tsimikas S, Karwatowska-Prokopczuk E, Xia S. Lipoprotein(a) reduction in persons with cardiovascular disease. Reply. N Engl J Med. 2020; 382:e65. [DOI] [PubMed] [Google Scholar]
- 42.Graham MJ, Viney N, Crooke RM, Tsimikas S. Antisense inhibition of apolipoprotein (a) to lower plasma lipoprotein (a) levels in humans. J Lipid Res. 2016; 57:340–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stiekema LCA, Prange KHM, Hoogeveen RM, Verweij SL, Kroon J, Schnitzler JG, et al. Potent lipoprotein(a) lowering following apolipoprotein(a) antisense treatment reduces the pro-inflammatory activation of circulating monocytes in patients with elevated lipoprotein(a). Eur Heart J. 2020; 41:2262–2271. [DOI] [PMC free article] [PubMed] [Google Scholar]