Abstract
Background
Respiratory cultures are often obtained as part of a “pan-culture” in mechanically ventilated patients in response to new fevers or leukocytosis, despite an absence of clinical or radiographic evidence suggestive of pneumonia.
Methods
This was a propensity score–stratified cohort study of hospitalized mechanically ventilated adult patients between 2014 and 2019, with a new abnormal temperature or serum white blood cell count (NATW), but without radiographic evidence of pneumonia, change in ventilator requirements, or documentation of purulent secretions. Two patient groups were compared: those with respiratory cultures performed within 36 hours after NATW and those without respiratory cultures performed. The co-primary outcomes were the proportion of patients receiving >2 days of total antibiotic therapy and >2 days of broad-spectrum antibiotic therapy within 1 week after NATW.
Results
Of 534 included patients, 113 (21.2%) had respiratory cultures obtained and 421 (78.8%) did not. Patients with respiratory cultures performed were significantly more likely to receive antibiotics for >2 days within 1 week after NATW than those without respiratory cultures performed (total antibiotic: adjusted odds ratio [OR], 2.57; 95% CI, 1.39–4.75; broad-spectrum antibiotic: adjusted OR, 2.47, 95% CI, 1.46–4.20).
Conclusions
Performance of respiratory cultures for fever/leukocytosis in mechanically ventilated patients without increasing ventilator requirements, secretion burden, or radiographic evidence of pneumonia was associated with increased antibiotic use within 1 week after incident abnormal temperature and/or white blood cell count. Diagnostic stewardship interventions targeting performance of unnecessary respiratory cultures in mechanically ventilated patients may reduce antibiotic overuse within intensive care units.
Keywords: antimicrobial stewardship, diagnostic stewardship, pneumonia, ventilator-associated pneumonia
Indiscriminate antibiotic use is a leading cause of adverse drug events and a catalyst for the emergence of multidrug-resistant organisms (MDROs), which account for 2.8 million infections and 37 000 deaths annually in the United States [1, 2]. Antimicrobial stewardship programs are instrumental in curbing antibiotic overuse, but their reach into intensive care units (ICUs)—where antibiotic resistance is most prevalent and problematic—has been limited [3–5]. Treatment for suspected respiratory infection—in particular, ventilator-associated pneumonia (VAP)—accounts for 50%–70% of antibiotic use within intensive care units [6–9]. Studies using multidisciplinary expert case review or autopsy findings as reference gold standards for pneumonia diagnosis demonstrate that a substantial number of ICU patients treated for VAP are misdiagnosed, resulting in excessive antimicrobial exposures, adverse drug events, and generation of MDROs [10–13].
Efforts to reduce VAP antibiotic overuse have predominantly focused on therapeutic processes—specifically antibiotic de-escalation or discontinuation strategies—in established VAP cases [14–22]. Comparatively little attention has been given to stewardship approaches targeting the diagnostic testing pathway for VAP, particularly the practice of obtaining respiratory cultures. Positive respiratory cultures inform the diagnostic probability of VAP but are not synonymous with infection, owing to the high burden of bacterial colonization of endotracheal tubing and distal lung parenchyma in critically ill patients [23–26]. In mechanically ventilated patients, respiratory cultures are frequently ordered indiscriminately and in clinical scenarios where their benefit is uncertain [27, 28].
A common example of indiscriminate culture collection among mechanically ventilated patients is as part of a “pan-culture” workup for new fever or leukocytosis, in which respiratory cultures are obtained as part of a packaged workup for infection even in the absence of radiographic or clinical evidence of pneumonia [29, 30]. Recovery of bacteria from the respiratory tract in this context is unlikely to represent pneumonia and may motivate unnecessary antibiotic treatment of positive-culture results in patients otherwise lacking localizing pulmonary features of infection. We hypothesized that, among clinically stable, mechanically ventilated patients without localizing clinical/radiographic features of pulmonary infection, the performance of respiratory cultures as part of workup for an isolated new fever or leukocytosis is associated with excess antibiotic use.
METHODS
Study Design and Setting
This was a retrospective cohort study conducted at Michigan Medicine University Hospital, a facility housing 7 intensive care units (ICUs) and 108 ICU beds. This study included patients distributed among postsurgical cardiothoracic, medical, surgical, neurosurgical, and trauma-burn ICUs, as well as ICU-level patients in other locations. The Institutional Review Board of the University of Michigan approved this study for waiver of informed consent.
All study ICUs have clinical pharmacists embedded within ICU care teams. The study institution has a robust Antimicrobial Stewardship program with institutional guidelines recommending 7 days of antibiotic therapy for VAP cases without complications; procalcitonin was not routinely utilized within study ICUs to guide VAP treatment durations. No major changes to ICU VAP antimicrobial protocols were instituted during the study period. The Clinical Microbiology Laboratory at the University of Michigan performs quantitative and semiquantitative gram stains and cultures depending on whether respiratory samples were obtained proximally via endotracheal tube or distally via bronchoscopic or nonbronchoscopic bronchoalveolar lavage. Respiratory cultures that grow oral flora are reported with a “nudge” comment indicating the presence of commensal flora and the absence of methicillin-resistant Staphylococcus aureus and Pseudomonas aeruginosa.
Cohort Description and Data Collection
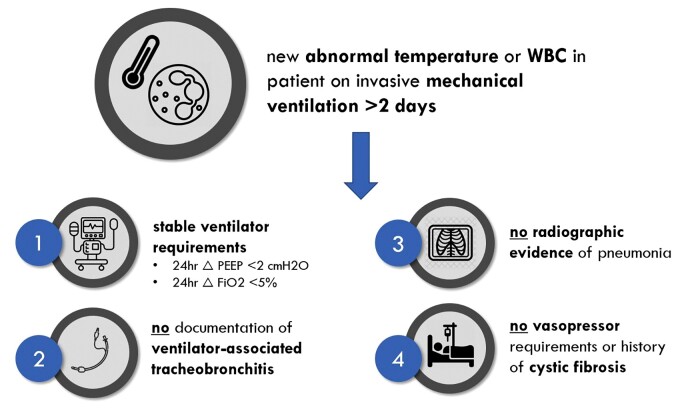
A structured query through the institutional electronic medical record was used to retrospectively identify all mechanically ventilated adult patients hospitalized between January 1, 2014, and December 31, 2019, in Michigan Medicine ICUs who met the following prespecified inclusion criteria (Figure 1): (1) >2 consecutive calendar days of invasive mechanical ventilation, (2) new abnormal temperature or white blood cell (WBC) count (temperature <36°C or >38°C, WBC <4 or >12 × 109/mL; henceforth referred to as NATW), (3) no change in ventilator requirements during the 24 hours before NATW, (4) chest imaging within 24 hours of NATW without radiographic evidence of pneumonia, and (5) no clinical documentation of increased or purulent sputum within physician progress notes. Patients with vasopressor requirements or an established diagnosis of cystic fibrosis were excluded. Keyword abstraction from radiologist chest imaging transcripts was utilized to screen for radiographic evidence of pneumonia—a methodology that has been previously validated at this institution [31]. The charts of 30 study subjects were reviewed to ensure validity of the structured query and automated text abstraction. Full details on inclusion criteria for the structured EMR query are detailed in Supplementary Table 1.
Figure 1.
Study schema. Abnormal temperature ≤36°C or >38°C; abnormal WBC ≤4 or >12 × 109/mL. Abbreviations: FiO2, fraction of inspired oxygen; PEEP, positive end-expiratory pressure; WBC, white blood cell count.
Patients were stratified into 2 groups: those with respiratory cultures performed and those without respiratory cultures performed within 36 hours of NATW. All modalities of respiratory culture collection—including expectorated sputum, endotracheal aspiration, and bronchoscopic or nonbronchoscopic bronchoalveolar lavage—were included. Relevant demographics, comorbidities, health care exposures, and measures of acute severity of illness were collected at the time of NATW for all patients [32, 33].
The co-primary study outcomes were (1) the proportion of patients who received any antibiotics for >2 calendar days within 1 week of NATW and (2) the proportion of patients who received broad-spectrum antibiotics for >2 calendar days within 1 week of NATW. A categorical primary outcome of antibiotic use >2 calendar days was chosen as many mechanically ventilated patients are started on broad-spectrum antibiotics in response to NATW, which are then de-escalated or discontinued within 48 hours after subsequent workup. This study’s hypothesis was that respiratory culture performance would identify clinically irrelevant bacteria from respiratory specimens that would interrupt antibiotic de-escalation or discontinuation. A 1-week time frame was selected for this study, as antimicrobial management decisions made by treatment teams beyond this time window were felt to be unlikely to be uniquely related to the performance of index respiratory cultures and their subsequent results. The antimicrobial agents included for study purposes are listed in Supplementary Table 2.
Secondary study outcomes included total and broad-spectrum antibiotic days of therapy (DOT; defined as the aggregate number of calendar days a patient received any prespecified antibiotic) within 1 week of NATW, all-cause mortality, a composite outcome of alive and ventilator free at 30 days, 30 ICU-free days, and total inpatient length of stay [34]. Adverse drug events related to antimicrobial use (including acute kidney injury, anaphylaxis, cytopenias, diarrhea, drug fever, drug rash including Stevens Johnson syndrome and toxic epidermal necrolysis, and yeast infection) were captured using International Classification of Diseases, Tenth Revision, codes related to the patient’s hospitalization. Lastly, subsequent isolation of multidrug-resistant organisms (MDROs), including methicillin-resistant Staphylococcus aureus, vancomycin-resistant Enterococci, extended-spectrum β-lactamase-producing Enterobacterales, carbapenem-resistant Enterobacterales, carbapenemase-producing organisms, and Clostridioides difficile, was measured within both 30- and 90-day time periods subsequent to the patient’s NATW.
An exploratory descriptive analysis was performed on all patients with respiratory cultures ordered in response to NATW to ensure diagnostic validity of the automated EMR query in capturing non-VAP cases. Chart and imaging review were conducted by a committee of infectious diseases physicians to adjudicate VAP diagnosis, causes of NATW in patients without VAP, and microbiological results of respiratory cultures. VAP was evaluated using Infectious Diseases Society of America/American Thoracic Society (IDSA/ATS) diagnostic criteria of a “new lung infiltrate plus clinical evidence that the infiltrate is of infectious origin, which include the new onset of fever, purulent sputum, leukocytosis and decline in oxygenation” [35]. Patients not meeting IDSA/ATS diagnostic criteria for pneumonia were categorized as non-VAP, and causes of NATW in this group were adjudicated via clinical judgment. Patients without respiratory cultures performed were not subjected to chart review as VAP is only rarely diagnosed at our institution without respiratory culture performance and because inclusion of patients with VAP among those without respiratory cultures performed would bias study outcomes toward the null hypothesis.
Data Analysis
For the primary analysis, outcomes were compared among patients with and without respiratory cultures performed after NATW. For bivariate analysis, categorical variables were compared using the Fisher exact test, and continuous variables were compared using the t test or Wilcoxon rank-sum test as indicated by normality of variable distribution. Missing data were supplanted by nonparametric random forest-based imputation using the missForest package in R, version 3.4.2. All remaining statistical analysis was performed in SAS, version 9.4 (SAS Institute Inc., Cary, NC, USA).
A propensity score–stratified analysis of the cohort was used to evaluate the independent impact of respiratory culture performance on antimicrobial usage. Three distinct multivariable logistic regression models were created for the purposes of propensity score generation. In each model, the dependent variable was the exposure of interest—performance of respiratory culture. Model 1 predicted respiratory culture performance from a large selection of variables with plausible clinical relevance to study outcomes (based on published literature/expert opinion), as well as significant association with the study’s primary outcome in unadjusted bivariate analysis. Model 2 predicted respiratory culture from only those variables significantly (P < .05) associated with the study’s primary outcome of total antibiotic use >2 days in unadjusted bivariate analysis. Model 3 included all variables from Model 2 along with only those additional variables from Model 1 determined to be confounders, defined by a change in the β-coefficient of the respiratory culture performance covariate of >10%. The models were evaluated based on Akaike Information Criteria (AIC) and the c-statistic. Model 2 was determined to have the best balance in model fit and predictive power and was selected for generation of propensity scores predictive of respiratory culture performance. The after variables were included in the final propensity score model: gender, hemiplegia, myocardial infarction, antecedent mechanical ventilation >3 days, use of antibiotics before NATW, bronchoscopy within the prior 90 days, P/F ratio >250, abnormal temperature, and subject residence within surgical or cardiac ICUs. Propensity score distributions were compared across those who did and did not have respiratory cultures performed, and nonoverlapping regions were trimmed. A stratified analysis of study outcomes was performed based on propensity score quartiles, analysis of binary outcomes was performed using stratified logistic regression, and analysis of continuous outcomes was performed using stratified linear regression.
RESULTS
Patient Characteristics
A total of 4467 patients met initial study inclusion criteria of NATW after requirement for invasive mechanical ventilation for >2 days. Of these 4467 patients, 534 (12.0%) met the remaining study inclusion criteria (stable ventilator requirements, no documentation of purulent/increased sputum, no radiographic evidence of new pneumonia, no history of cystic fibrosis or vasopressor requirements). Of these 534 patients, 113 (21.2%) had respiratory cultures obtained after NATW, and 421 (78.8%) did not. Male patients, patients with selected comorbidities (cerebrovascular disease, paraplegia, myocardial infarction), those with abnormal temperatures (as opposed to abnormal WBC count), and those residing in cardiac or neurosurgical ICUs (rather than general surgical or medical ICUs) were more likely to have respiratory cultures obtained (Table 1; Supplementary Table 3). For the propensity score–stratified cohort analysis, 365 of the initial 421 patients who did not have respiratory cultures performed after NATW were included in the trimmed population. Demographic characteristics, prevalence of comorbidities (including chronic lung disease and tracheostomy dependence), measures of health care exposure, and acute severity of illness metrics were similar between groups in the propensity score–stratified cohort.
Table 1.
Patient Characteristics
| Total Cohort | Propensity Score–Stratified Cohort | ||||
|---|---|---|---|---|---|
| Respiratory Culture Performed (n = 113) | Respiratory Culture Not Performed (n = 421) | P Value | Respiratory Culture Not Performed (n = 365) | P Value | |
| Demographics | |||||
| Male gender, No. (%) | 75 (66.4) | 239 (56.8) | .07 | 216 (59.2) | .19 |
| Age, mean (SD), y | 58.2 (14.6) | 57.7 (16.1) | .73 | 57.8 (16.1) | .76 |
| Caucasian race, No. (%) | 90 (79.7) | 309 (73.4) | .18 | 269 (73.7) | .22 |
| Comorbidities | |||||
| Charlson composite score, mean (SD) | 8.10 (4.23) | 7.93 (4.74) | .78 | 8.05 (4.23) | .92 |
| Congestive heart failure, No. (%) | 63 (55.8) | 223 (53.0) | .67 | 200 (54.8) | .91 |
| Cerebrovascular disease, No. (%) | 56 (49.6) | 156 (37.0) | .017 | 140 (38.4) | .04 |
| Chronic pulmonary disease, No. (%) | 50 (44.3) | 167 (39.7) | .39 | 143 (39.2) | .38 |
| Diabetes with chronic complications, No. (%) | 25 (22.1) | 95 (22.6) | 1 | 84 (23.0) | .9 |
| Paraplegia, No. (%) | 25 (22.1) | 56 (13.3) | .03 | 55 (15.1) | .08 |
| Moderate–severe liver disease, No. (%) | 10 (8.9) | 44 (10.5) | .73 | 38 (10.4) | .72 |
| Myocardial infarction, No. (%) | 45 (39.8) | 112 (26.6) | .008 | 110 (30.1) | .07 |
| Renal disease, No. (%) | 50 (44.3) | 205 (48.7) | .46 | 177 (48.5) | .45 |
| Tracheostomy, No. (%) | 31 (27.4) | 118 (28.0) | 1 | 94 (25.8) | .71 |
| Immunocompromised, No. (%) | 18 (15.9) | 75 (17.8) | .68 | 65 (17.8) | .78 |
| Health care exposures | |||||
| Length of stay before NATW, median (IQR), d | 4 (3–11) | 5 (3–12) | .43 | 4 (3–11) | .95 |
| Time on vent before NATW, median (range), d | 2 (2–29) | 2 (2–26) | .054 | 2 (2–26) | .12 |
| On antibiotics before event, No. (%) | 80 (70.8) | 328 (77.9) | .13 | 277 (75.9) | .32 |
| Prior pneumonia in last 3 mo, No. (%) | 9 (8.0) | 51 (12.1) | .24 | 40 (11.0) | .48 |
| MDRO in 1 y before NATW, No. (%) | 12 (10.6) | 64 (15.2) | .28 | 64 (15.2) | .28 |
| Acuity of illness | |||||
| APACHE score, mean (SD) | 21.8 (6.4) | 21.1 (6.8) | .28 | 21.4 (6.9) | .49 |
| P/F ratio, median (IQR) | 239.4 (176.6–325.6) | 249.6 (188.8–327.5) | .24 | 241.7 (183.88–315.3) | .55 |
| Abnormal temp, No. (%) | 84 (74.3) | 248 (58.9) | .003 | 239 (65.5) | .09 |
| Abnormal WBC, No. (%) | 79 (69.9) | 342 (81.2) | .013 | 292 (80.0) | .03 |
| Abnormal temp and WBC, No. (%) | 50 (44.3) | 169 (40.1) | .45 | 166 (45.5) | .83 |
NATW was defined as abnormal white blood cell count (>12 × 109/L or <4 × 109/L) OR temperature (>38°C or <36°C) with normal value in the preceding 36 hours. Immunocompromised was defined as history of solid organ transplant, hematopoietic stem cell transplant, hematologic malignancy within the past year, or receipt of immunosuppressing medications (steroids, anti–tumor necrosis factor therapy, mammalian target of rapamycin inhibitors, calcineurin inhibitors, antiproliferative agents). MDRO was defined as methicillin-resistant Staphylococcus aureus (on clinical or screening culture), vancomycin-resistant Enterococci (on clinical or screening culture), extended-spectrum β-lactamase-producing Enterobacterales, carbapenem-resistant Enterobacterales, carbapenemase-producing organisms, and Clostridioides difficile.
Abbreviations: APACHE, Acute Physiology and Chronic Health Evaluation; IQR, interquartile range; MDRO, multidrug-resistant organism; NATW, new abnormal temperature or white blood cell count; P/F ratio, partial pressure of oxygen/fraction of inspired oxygen; WBC, white blood cell.
Outcomes
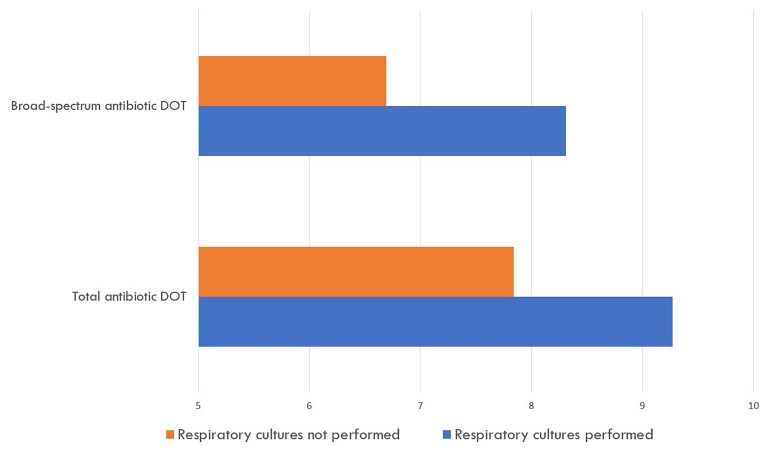
Primary and secondary study outcomes are shown in Table 2. Within the entire study cohort, 416 patients (77.9%) received antibiotics for >2 calendar days within 1 week of NATW, and 371 patients (69.5%) received broad-spectrum antibiotics for >2 calendar days within 1 week of NATW. Patients with respiratory cultures performed were significantly more likely than those without respiratory cultures performed to receive both total and broad-spectrum antibiotics for >2 calendar days (total antibiotic use odds ratio [OR], 2.32; 95% CI, 1.23–4.24; broad-spectrum antibiotic use OR, 2.23; 95% CI, 1.33–3.73). Patients with respiratory cultures performed received 1.43 more total antibiotic DOTs per patient (0.01) and 1.52 more broad-spectrum antibiotic DOTs (P < .01) within 1 week of NATW than those without respiratory cultures performed (Figure 2).
Table 2.
Adjusted Primary/Secondary Outcomes in Total and Propensity Score–Stratified Cohort
| Total Cohort | Propensity Score–Stratified Cohort | ||||
|---|---|---|---|---|---|
| Respiratory Culture Performed (n = 113) | Respiratory Culture Not Performed (n = 421) | P Value | Respiratory Culture Not Performed (n = 365) | P Value | |
| Primary outcomes | |||||
| >2 d all antibiotics, No. (%) | 99 (87.6) | 317 (75.3) | .005 | 272 (74.5) | .002 |
| >2 d broad-spectrum antibiotics, No. (%) | 92 (81.4) | 279 (66.3) | .002 | 238 (65.2) | <.001 |
| Secondary outcomes | |||||
| Antibiotic DOT within 7 d of NATW, mean (SD) | |||||
| Total antibiotic DOT | 9.27 (5.36) | 7.84 (5.34) | .01 | 7.86 (5.43) | .02 |
| Broad-spectrum DOT | 8.31 (5.36) | 6.79 (5.41) | .008 | 6.79 (5.49) | .009 |
| Safety outcomes | |||||
| 30-d mortality, No. (%) | 34 (30.1) | 119 (28.3) | .70 | 107 (29.3) | .95 |
| Alive and ventilator free at 30 d, No. (%) | 77 (68.1) | 294 (69.8) | .73 | 251 (68.8) | .92 |
| Total inpatient length of stay, median (IQR), d | 24 (16–38) | 24 (14–41) | .76 | 23 (14–40) | .73 |
| 30 d ICU free, mean (SD), d | 18.6 (9.3) | 19.6 (8.8) | .33 | 19.7 (8.80) | .29 |
| Antibiotic toxicity/resistance, No. (%) | |||||
| Adverse drug event | 74 (65.5) | 288 (68.4) | .57 | 247 (67.7) | .62 |
| New MDRO within 30 d after NATW | 12 (10.6) | 50 (11.9) | .71 | 46 (12.6) | .62 |
NATW was defined as abnormal white blood cell count (>12 × 109/L or <4 × 109/L) OR temperature (>38°C or <36°C) with a normal value in the preceding 36 hours. Antibiotic DOT was defined as the aggregate sum of days for which any amount of a specific antimicrobial agent was received by a patient. Adverse drug event was defined as diarrhea, neutropenia, thrombocytopenia, AKI, yeast infection, rash, anaphylaxis, Stevens-Johnson syndrome/toxic epidermal necrolysis attributed to antibiotics. MDRO was defined as methicillin-resistant Staphylococcus aureus (on clinical or screening culture), vancomycin-resistant Enterococci (on clinical or screening culture), extended-spectrum β-lactamase-producing Enterobacterales, carbapenem-resistant Enterobacterales, carbapenemase-producing organisms, and Clostridioides difficile.
Abbreviations: AKI, acute kidney injury; DOT, days of therapy; ICU, intensive care unit; IQR, interquartile range; MDRO, multidrug-resistant organism; NATW, new abnormal temperature or white blood cell count.
Figure 2.
Total and broad-spectrum antibiotic days of therapy within 1 week of new abnormal temperature or white blood cell count. Antibiotic days of therapy was defined as the aggregate sum of days for which any amount of a specific antimicrobial agent was received by a patient. For example, if a patient obtained 2 days of vancomycin and 2 days of piperacillin-tazobactam within the 1-week time frame, this would equate to 4 antibiotic days of therapy. Abbreviation: DOT, days of therapy.
Analysis of the propensity score–stratified cohort demonstrated similar results: Respiratory culture performance remained strongly associated with antibiotic use >2 days (total antibiotic use: adjusted OR, 2.57; 95% CI, 1.39–4.75; broad-spectrum antibiotic use: adjusted OR, 2.47; 95% CI, 1.46–4.20). Secondary outcomes, including rates of mortality, ventilator dependence, ICU and hospital length of stay, adverse drug events, and incidence of novel MDROs after NATW were similar between groups in the propensity score–stratified cohort.
Descriptive Analysis of Cases With Respiratory Cultures Performed
Of the 113 study cohort patients with respiratory cultures performed, only 6 (5.3%) patients met IDSA/ATS criteria for possible VAP after chart and imaging review by a committee of infectious diseases physicians. Among the 107 remaining patients, the most common identifiable causes of NATW are shown in Table 3. Respiratory cultures grew a pathogenic organism in 41 (38.3%) patients. Sixty-one percent of respiratory cultures were obtained via endotracheal aspiration, while 39% were obtained via bronchoalveolar lavage. Among patients with organisms recovered, the most common organisms were Staphylococcus aureus, Enterobacterales, and nonfermenting gram-negative bacilli (Pseudomonas aeruginosa and Stenotrophomonas maltophilia). Owing to limitations of documentation, it was not possible to discern how many patients had antibiotics continued specifically in response to positive respiratory cultures. A sensitivity analysis was performed excluding the 6 patients with possible VAP from the group of patients with respiratory cultures performed and showed no difference in the study’s primary outcomes (total antibiotic use >2 days: adjusted OR, 2.41; 95% CI, 1.30–4.48; broad-spectrum antibiotic use >2 days: adjusted OR, 2.31; 95% CI, 1.36–3.94).
Table 3.
Causes of New Abnormal Fever or Leukocytosis in Patients With Respiratory Cultures Obtained Without Clinical/Radiographic Evidence of Pneumonia
| Cause of Abnormal Temperature/White Blood Cell Count | Total No. (n = 107) | Percentage of Cases |
|---|---|---|
| Postoperative fever/leukocytosis | 32 | 29.9 |
| Central process | 24 | 22.4 |
| Extrapulmonary infection | 24 | 22.4 |
| Noninfectious pulmonary decompensation | 17 | 15.9 |
| Other | 11 | 10.3 |
| Steroids/leukemoid stress reaction | 10 | 9.3 |
| Ischemia | 6 | 5.6 |
| DVT/PE | 6 | 5.6 |
| Hematoma | 5 | 4.7 |
| Cancer/tumor fever | 4 | 3.7 |
| Autoimmune condition | 1 | 0.9 |
| Drug fever | 1 | 0.9 |
Abbreviation: DVT/PE, deep vein thrombosis/pulmonary embolism.
Abnormal white blood cell count was defined as abnormal white blood cell count (>12 × 109/L or <4 × 109/L) with a normal value in the preceding 36 hours. Abnormal temperature was defined as >38°C or <36°C with a normal value in the preceding 36 hours. Central process = stroke, intraparenchymal hemorrhage, seizures, and global cerebral ischemia secondary to cardiac arrest. Noninfectious decompensation = flash pulmonary edema, aspiration pneumonitis, mucous plugging. Leukemoid stress reaction = leukocytosis attributable to non–septic shock physiology (ie, cardiogenic shock).
DISCUSSION
In this study of clinically stable mechanically ventilated ICU patients with new abnormal WBC or temperature measurements but without localizing features of pulmonary infection, performance of respiratory cultures was independently associated with significant increases in antibiotic use. While abnormal temperature and WBC often accompany VAP, they are nonspecific findings in critically ill patients, even in the presence of positive respiratory cultures [23]. Asymptomatic colonization of both the proximal and distal respiratory tract with pathogenic organisms occurs in >50% of critically ill patients, particularly those with indwelling endotracheal prosthetic material [24]. If one assumes a VAP prevalence of 10% among mechanically ventilated patients [35], before respiratory culture, those patients with fever but without new radiographic infiltrates, alterations in gas exchange, or purulent sputum have a pretest probability of VAP of only 2.6%. A positive respiratory culture in this context increases the post-test probability of VAP to only 3.7%, rendering the test marginally beneficial at best and, in many cases, a precipitant of unnecessary antibiotic use.
For the vast majority of patients in this study, recovery of microorganisms from the respiratory tract likely reflected clinically irrelevant colonization of the respiratory tract, unrelated to the index fever or leukocytosis that prompted culture collection. Our group has previously characterized this scenario as “asymptomatic bacterisputia” and argued that contemporary respiratory culturing practices in critically ill patients may precipitate antibiotic overuse [28]. Obtaining respiratory cultures to evaluate a new fever or WBC count in mechanically ventilated patients regardless of the presence of signs or symptoms localizing infection to the respiratory tract is commonplace in modern clinical practice and is endorsed in multiple heavily referenced expert guidelines [36, 37]. Our study suggests that this practice of “pan-culturing” to evaluate fever or leukocytosis in mechanically ventilated patients may motivate unnecessary antibiotic use.
While the effect size on antimicrobial use observed in this study was modest—1.5 DOT/week, applying to only 12% of screened patients—we expect that the clinical impact of targeting ordering practices for respiratory cultures would be markedly higher in clinical practice for the following reasons: First, patients requiring prolonged mechanical ventilation often have multiple episodes of NATW throughout their clinical course, each of which may precipitate unnecessary antibiotic exposures; and second, our prior published experience suggests that at least 30% of patients excluded from this study because of the presence of radiographic keywords in chest imaging transcripts would not have met diagnostic criteria for pneumonia after chart review and thus could have benefitted from diagnostic stewardship interventions targeting the ordering of respiratory cultures [28, 31].
Most efforts to reduce VAP antibiotic overuse have focused on therapeutic processes, namely antibiotic de-escalation or discontinuation strategies in established VAP cases [14–22]. Few efforts have instead focused on the diagnostic process for VAP—interventions targeting the pathway of ordering, collection, and reporting of diagnostic tests to proactively avert unnecessary antibiotic use in patients with a low pretest probability of infection. Such approaches, known as diagnostic stewardship approaches, have demonstrated considerable success in preventing unnecessary treatment of asymptomatic bacteriuria and Clostridioides difficile colonization but remain relatively unexplored in respiratory infections [38–46]. Our study suggests that diagnostic stewardship efforts targeting indiscriminate respiratory culture performance among mechanically ventilated patients may therefore represent an untapped avenue to reduce unnecessary ICU antibiotic use.
This study has several limitations. First, its retrospective nature comes with inherent limitations. Second, chest imaging interpretation is subjective, and our use of automated keyword abstraction from chest radiograph transcripts may have captured patients with legitimate radiographic evidence of VAP despite absence of pneumonia-specific keywords in interpretive reports. Importantly, we have previously validated automated keyword abstraction from chest radiograph transcripts for pneumonia diagnosis: Among a pneumonia-enriched cohort with positive respiratory cultures, the absence of pneumonia-specific radiographic keywords utilized for this study had a sensitivity of 97.2% and a negative predictive value of 80% in excluding pneumonia [31]. Furthermore, our manual chart and imaging review of all cases in which respiratory cultures were performed found only 6 cases out of 113 reviewed that met IDSA/ATDS criteria for VAP [35]. Another potential limitation of this study is the high prevalence of antecedent antibiotic use in both groups (>70%). While diagnostic considerations differ in patients on existing antibiotics vs antibiotic-naïve ICU patients, crafting a study of only antibiotic-naïve ICU patients is infeasible: A recent multinational point prevalence study of ICUs in 1150 hospitals in 88 countries demonstrated that 70% of patients within a 24-hour time period received at least 1 antibiotic [47]. While we did not capture the indications for antecedent antibiotic use in this study population, we did incorporate antecedent antibiotic use into our propensity score modeling. Finally, it is possible that respiratory culture performance is a surrogate marker for a more aggressive therapeutic approach to antimicrobial use among certain intensivists, rather than the catalyst in and of itself for antibiotic use.
Given the precipitous rise of antimicrobial resistance within ICUs and the relatively limited inroads made by antimicrobial stewardship programs among critically ill patients, novel approaches to ICU stewardship are required. This study suggests that diagnostic VAP stewardship approaches—particularly interventions targeting the ordering phase of respiratory cultures—merit prospective evaluation as methods to limit ICU antibiotic overuse.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Acknowledgments
Financial support. This research received no funding from any agency in the public, commercial, or non-for-profit sectors. K.S.K. is funded by the National Institute of Allergy and Infectious Diseases (DMID Protocol Number: 10-0065 and R01-AI119446-01). J.G.P. is funded by the National Institute of Allergy and Infectious Diseases (K01-AI141579).
Potential conflicts of interest. All authors: no reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Patient consent. This study was approved for a waiver of informed consent by the Institutional Review Board of the University of Michigan.
Contributor Information
Owen R Albin, Department of Internal Medicine, University of Michigan Medical School, Ann Arbor, Michigan, USA.
Louis Saravolatz, Department of Internal Medicine, University of Michigan Medical School, Ann Arbor, Michigan, USA.
Joshua Petrie, Department of Epidemiology, University of Michigan School of Public Health, Ann Arbor, Michigan, USA.
Oryan Henig, Department of Infectious Diseases, Unit of Infection Control, Tel Aviv Sourasky Medical Center, Tel Aviv, Israel.
Keith S Kaye, Department of Internal Medicine, University of Michigan Medical School, Ann Arbor, Michigan, USA.
References
- 1. CDC . Antibiotic Resistance Threats in the United States, 2019. US Department of Health and Human Services, CDC; 2019. [Google Scholar]
- 2. Tamma PD, Avdic E, Li DX, Dzintars K, Cosgrove SE. Association of adverse events with antibiotic use in hospitalized patients. JAMA Intern Med 2017; 177:1308–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Denny KJ, De Waele J, Laupland KB, Harris PNA, Lipman J. When not to start antibiotics: avoiding antibiotic overuse in the intensive care unit. Clin Microbiol Infect 2020; 26:35–40. [DOI] [PubMed] [Google Scholar]
- 4. Chiotos K, Tamma PD, Gerber JS. Antibiotic stewardship in the intensive care unit: challenges and opportunities. Infect Control Hosp Epidemiol 2019; 40:693–8. [DOI] [PubMed] [Google Scholar]
- 5. Doernberg SB, Chambers HF. Antimicrobial stewardship approaches in the intensive care unit. Infect Dis Clin North Am 2017; 31:513–34. [DOI] [PubMed] [Google Scholar]
- 6. Bergmans DC, Bonten MJ, Gaillard CA, et al. Indications for antibiotic use in ICU patients: a one-year prospective surveillance. J Antimicrob Chemother 1997; 39:527–35. [DOI] [PubMed] [Google Scholar]
- 7. Rimawi RH, Mazer MA, Siraj DS, Gooch M, Cook PP. Impact of regular collaboration between infectious diseases and critical care practitioners on antimicrobial utilization and patient outcome. Crit Care Med 2013; 41:2099–107. [DOI] [PubMed] [Google Scholar]
- 8. Vincent JL, Rello J, Marshall J, et al. International study of the prevalence and outcomes of infection in intensive care units. JAMA 2009; 302:2323–9. [DOI] [PubMed] [Google Scholar]
- 9. Thomas Z, Bandali F, Sankaranarayanan J, Reardon T, Olsen KM. A multicenter evaluation of prolonged empiric antibiotic therapy in adult ICUs in the United States. Crit Care Med 2015; 43:2527–34. [DOI] [PubMed] [Google Scholar]
- 10. Nussenblatt V, Avdic E, Berenholtz S, et al. Ventilator-associated pneumonia: overdiagnosis and treatment are common in medical and surgical intensive care units. Infect Control Hosp Epidemiol 2014; 35:278–84. [DOI] [PubMed] [Google Scholar]
- 11. Swoboda SM, Dixon T, Lipsett PA. Can the clinical pulmonary infection score impact ICU antibiotic days? Surg Infect (Larchmt) 2006; 7:331–9. [DOI] [PubMed] [Google Scholar]
- 12. Petersen IS, Aru A, Skødt V, et al. Evaluation of pneumonia diagnosis in intensive care patients. Scand J Infect Dis 1999; 31:299–303. [DOI] [PubMed] [Google Scholar]
- 13. Fagon JY, Chastre J, Hance AJ, Domart Y, Trouillet JL, Gibert C. Evaluation of clinical judgment in the identification and treatment of nosocomial pneumonia in ventilated patients. Chest 1993; 103:547–53. [DOI] [PubMed] [Google Scholar]
- 14. Akagi T, Nagata N, Wakamatsu K, et al. Procalcitonin-guided antibiotic discontinuation might shorten the duration of antibiotic treatment without increasing pneumonia recurrence. Am J Med Sci 2019; 358:33–44. [DOI] [PubMed] [Google Scholar]
- 15. Bouadma L, Luyt CE, Tubach F, et al. Use of procalcitonin to reduce patients’ exposure to antibiotics in intensive care units (PRORATA trial): a multicentre randomised controlled trial. Lancet 2010; 375:463–74. [DOI] [PubMed] [Google Scholar]
- 16. de Jong E, van Oers JA, Beishuizen A, et al. Efficacy and safety of procalcitonin guidance in reducing the duration of antibiotic treatment in critically ill patients: a randomised, controlled, open-label trial. Lancet Infect Dis 2016; 16:819–27. [DOI] [PubMed] [Google Scholar]
- 17. Luyt CE, Combes A, Trouillet JL, Chastre J. Value of the serum procalcitonin level to guide antimicrobial therapy for patients with ventilator-associated pneumonia. Semin Respir Crit Care Med 2011; 32:181–7. [DOI] [PubMed] [Google Scholar]
- 18. Stolz D, Smyrnios N, Eggimann P, et al. Procalcitonin for reduced antibiotic exposure in ventilator-associated pneumonia: a randomised study. Eur Respir J 2009; 34:1364–75. [DOI] [PubMed] [Google Scholar]
- 19. Tabah A, Cotta MO, Garnacho-Montero J, et al. A systematic review of the definitions, determinants, and clinical outcomes of antimicrobial de-escalation in the intensive care unit. Clin Infect Dis 2016; 62:1009–17. [DOI] [PubMed] [Google Scholar]
- 20. Tabah A, Bassetti M, Kollef MH, et al. Antimicrobial de-escalation in critically ill patients: a position statement from a task force of the European Society of Intensive Care Medicine (ESICM) and European Society of Clinical Microbiology and Infectious Diseases (ESCMID) Critically Ill Patients Study Group (ESGCIP). Intensive Care Med 2020; 46:245–65. [DOI] [PubMed] [Google Scholar]
- 21. Musgrove MA, Kenney RM, Kendall RE, et al. Microbiology comment nudge improves pneumonia prescribing. Open Forum Infect Dis 2018; 5:XXX–XX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Klompas M, Li L, Menchaca JT, Gruber S; Centers for Disease Control and Prevention Epicenters Program . Ultra-short-course antibiotics for patients with suspected ventilator-associated pneumonia but minimal and stable ventilator settings. Clin Infect Dis 2017; 64:870–6. [DOI] [PubMed] [Google Scholar]
- 23. Klompas M. Does this patient have ventilator-associated pneumonia? JAMA 2007; 297:1583–93. [DOI] [PubMed] [Google Scholar]
- 24. Jongerden IP, Speelberg B, Satizábal CL, et al. The role of systemic antibiotics in acquiring respiratory tract colonization with gram-negative bacteria in intensive care patients: a nested cohort study. Crit Care Med 2015; 43:774–80. [DOI] [PubMed] [Google Scholar]
- 25. Feldman C, Kassel M, Cantrell J, et al. The presence and sequence of endotracheal tube colonization in patients undergoing mechanical ventilation. Eur Respir J 1999; 13:546–51. [DOI] [PubMed] [Google Scholar]
- 26. Lepainteur M, Ogna A, Clair B, et al. Risk factors for respiratory tract bacterial colonization in adults with neuromuscular or neurological disorders and chronic tracheostomy. Respir Med 2019; 152:32–6. [DOI] [PubMed] [Google Scholar]
- 27. Kenaa B, Richert ME, Claeys KC, et al. Ventilator-associated pneumonia: Diagnostic test stewardship and relevance of culturing practices. Curr Infect Dis Rep 2019; 21:50. [DOI] [PubMed] [Google Scholar]
- 28. Albin OR, Petty LA, Pogue JM, Kaye KS. Asymptomatic bacterisputia: rethinking diagnostic stewardship in pneumonia. Infect Control Hosp Epidemiol 2021; 42:737–9. [DOI] [PubMed] [Google Scholar]
- 29. Wald HL. Challenging the “culture of culturing”: the case for less testing and more clinical assessment. JAMA Intern Med 2016; 176:587–8. [DOI] [PubMed] [Google Scholar]
- 30. Vaughn VM, Chopra V. Revisiting the panculture. BMJ Qual Saf 2017; 26:236–9. [DOI] [PubMed] [Google Scholar]
- 31. Albin OR, Kaye KS. Utility of radiographic keyword abstraction for identification of misdiagnosed pneumonia. Infect Control Hosp Epidemiol 2021;42:1500–2. [DOI] [PubMed] [Google Scholar]
- 32. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987; 40:373–83. [DOI] [PubMed] [Google Scholar]
- 33. Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med 1985; 13:818–29. [PubMed] [Google Scholar]
- 34. Polk RE, Fox C, Mahoney A, Letcavage J, MacDougall C. Measurement of adult antibacterial drug use in 130 US hospitals: comparison of defined daily dose and days of therapy. Clin Infect Dis 2007; 44:664–70. [DOI] [PubMed] [Google Scholar]
- 35. Kalil AC, Metersky ML, Klompas M, et al. Management of adults with hospital-acquired and ventilator-associated pneumonia: 2016 clinical practice guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin Infect Dis 2016; 63:e61–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Maclaren G, Spelman D.. Fever in the intensive care unit. UptoDate. Available at: https://www.uptodate.com/contents/fever-in-the-intensive-care-unit. Accessed 21 May 2021.
- 37. O’Grady NP, Barie PS, Bartlett JG, et al. Guidelines for evaluation of new fever in critically ill adult patients: 2008 update from the American College of Critical Care Medicine and the Infectious Diseases Society of America. Crit Care Med 2008; 36:1330–49. [DOI] [PubMed] [Google Scholar]
- 38. Morgan DJ, Croft LD, Deloney V, et al. Choosing wisely in healthcare epidemiology and antimicrobial stewardship. Infect Control Hosp Epidemiol 2016; 37:755–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Claeys KC, Blanco N, Morgan DJ, et al. Advances and challenges in the diagnosis and treatment of urinary tract infections: the need for diagnostic stewardship. Curr Infect Dis Rep 2019; 21:11. [DOI] [PubMed] [Google Scholar]
- 40. Trautner BW, Grigoryan L, Petersen NJ, et al. Effectiveness of an antimicrobial stewardship approach for urinary catheter-associated asymptomatic bacteriuria. JAMA Intern Med 2015; 175:1120–7. [DOI] [PubMed] [Google Scholar]
- 41. Keller SC, Feldman L, Smith J, Pahwa A, Cosgrove SE, Chida N. The use of clinical decision support in reducing diagnosis of and treatment of asymptomatic bacteriuria. J Hosp Med 2018; 13:392–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Shirley D, Scholtz H, Osterby K, Musuuza J, Fox B, Safdar N. Optimizing inpatient urine culture ordering practices using the electronic medical record: a pilot study. Infect Control Hosp Epidemiol 2017; 38:486–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. McNulty CA, Lasseter GM, Charlett A, et al. Does laboratory antibiotic susceptibility reporting influence primary care prescribing in urinary tract infection and other infections? J Antimicrob Chemother 2011; 66:1396–404. [DOI] [PubMed] [Google Scholar]
- 44. Chow SK, Naderpour A, Van Enk J. It is not about the assay: preanalytical screening is the key to reducing. J Clin Microbiol 2019; 57:e01553-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Yen C, Holtom P, Butler-Wu SM, Wald-Dickler N, Shulman I, Spellberg B. Reducing Clostridium difficile colitis rates via cost-saving diagnostic stewardship. Infect Control Hosp Epidemiol 2018; 39:734–6. [DOI] [PubMed] [Google Scholar]
- 46. Rock C, Maragakis LL. Diagnostic stewardship for Clostridiodes difficile testing: from laxatives to diarrhea and beyond. Clin Infect Dis 2020; 71:1479–80. [DOI] [PubMed] [Google Scholar]
- 47. Vincent JL, Sakr Y, Singer M, et al. Prevalence and outcomes of infection among patients in intensive care units in 2017. JAMA 2020; 323:1478–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.