Whole-virion inactivated SARS-CoV-2 vaccines are one of the most widely used vaccines worldwide. However, compared with the mRNA-based and adenovirus-based platforms,1 little information is available about the immune response that is induced by inactivated virus vaccines2 and the convenience of applying heterologous boosters to reach an improved response against variants of concern, including omicron (B.1.1.529). Particularly scarce are data for older people (ie, age >60 years).
In this study, we performed a longitudinal analysis of serum samples from an older population of volunteers (n=26 for prime vaccination and n=98 for booster vaccination; mean age 79 years [SD 11·8]), obtained 21 days, 100 days, 160 days, and 220 days after the second dose of a two-dose primary immunisation schedule with the inactivated virus BBIBP-CorV (Sinopharm) vaccine, and 21 days and 90 days after application of a booster with ChAdOx1 nCoV-19 (Oxford-AstraZeneca), Sputnik V (Gamaleya Research Institute of Epidemiology and Microbiology), or BNT162b2 (Pfizer-BioNTech). Because of the low seroconversion rates observed after BBIBP-CorV primary vaccination, a homologous booster dose was not included in this study. We evaluated serum concentrations of IgG anti-spike antibodies3 and neutralising capacity against the original B.1 lineage and the omicron variant of concern.4
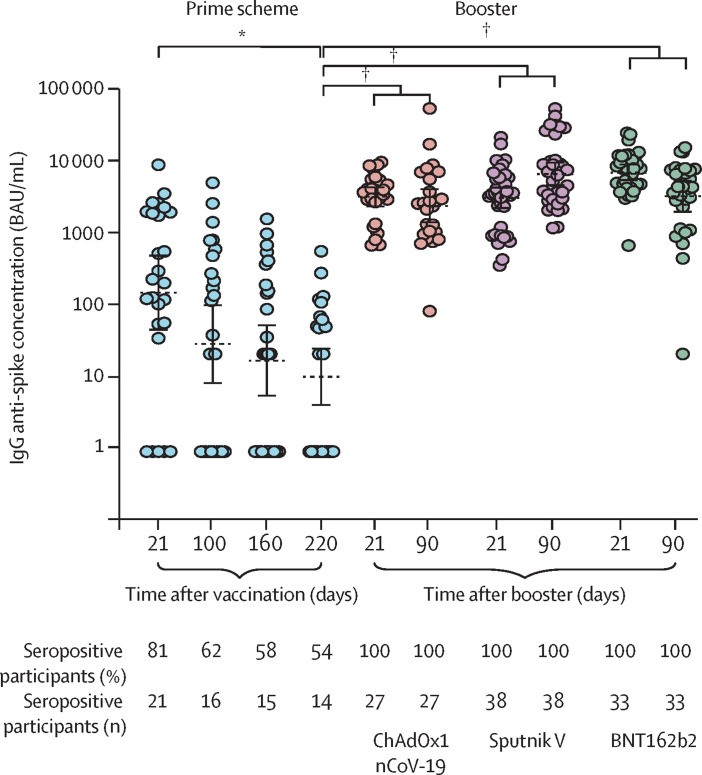
Both the concentration of IgG anti-spike antibodies and the seropositivity rate greatly declined over time after vaccination with two doses of BBIBP-CorV (figure ). After 220 days, the seropositivity rate was reduced from 81% to 54%. Application of a booster dose of ChAdOx1 nCoV-19, Sputnik V, or BNT162b2 raised the concentrations of IgG anti-spike antibodies on day 21 more than 350-fold (from 11·8 binding antibody units [BAU]/mL to 4397 BAU/mL for ChAdOx1 nCoV-19, 4285 BAU/mL for Sputnik V, and 9391 BAU/mL for BNT162b2) and seropositivity was detected in 98 (100%) participants (figure). This response was sustained at 90 days after the booster dose (figure).
Figure.
Humoral response over time after two-dose scheme with BBIBP-CorV and heterologous booster with ChAdOx1 nCoV-19, Sputnik V, or BNT162b2
IgG anti-spike antibody concentrations are quantified according to the WHO International Antibody Standard. Antibodies were measured at days 21, 100, 160, and 220 after primary immunisation in 26 participants and at days 21 and 90 after a booster dose in 98 participants. 27 volunteers received ChAdOx1 nCoV-19, 38 volunteers received Sputnik V, and 33 volunteers received BNT162b2. Geometric means with 95% CIs are indicated. Circles indicate individual participants. The Mann-Whitney U test was used. BAU=binding antibody units. *p=0·0003. †p<0·0001.
Neutralising antibodies against B.1 and omicron also decreased over time since primary immunisation (appendix p 1). Neutralising activity against the B.1 virus was detected in six (23%) of 26 participants at 220 days after vaccination with two doses of BBIBP-CorV (appendix p 1). Application of a heterologous booster dose of ChAdOx1 nCoV-19, Sputnik V, or BNT162b2 greatly increased neutralising activity against B.1, with activity detected in 97–100% of participants who received a booster. Only two (8%) of 26 participants showed detectable concentrations of neutralising antibodies against omicron 220 days after the application of the primary BBIBP-CorV scheme. This percentage increased to 74–91% after a booster dose of ChAdOx1 nCoV-19, Sputnik V, or BNT162b2 (appendix p 1).
Few data are available on effectiveness of a booster dose for individuals who are immunised with inactivated COVID-19 vaccines.2 The results presented here indicate that a heterologous booster dose with ChAdOx1 nCoV-19, Sputnik V, or BNT162b2 vaccines markedly increases the neutralising activity against the omicron variant in older people who have received two doses of BBIBP-CorV.
We declare no competing interests. SOR, PER, EAM, PR, and MMGLL contributed equally.
Supplementary Material
References
- 1.Menni C, May A, Polidori L, et al. COVID-19 vaccine waning and effectiveness and side-effects of boosters: a prospective community study from the ZOE COVID Study. Lancet Infect Dis. 2022;22:1002–1010. doi: 10.1016/S1473-3099(22)00146-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Costa Clemens SA, Weckx L, Clemens R, et al. Heterologous versus homologous COVID-19 booster vaccination in previous recipients of two doses of CoronaVac COVID-19 vaccine in Brazil (RHH-001): a phase 4, non-inferiority, single blind, randomised study. Lancet. 2022;399:521–529. doi: 10.1016/S0140-6736(22)00094-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ojeda DS, Gonzalez Lopez Ledesma MM, Pallarés HM, et al. Emergency response for evaluating SARS-CoV-2 immune status, seroprevalence and convalescent plasma in Argentina. PLoS Pathog. 2021;17 doi: 10.1371/journal.ppat.1009161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sanchez L, Oviedo Rouco S, Pifano M, et al. Antibody durability at 1 year after Sputnik V vaccination. Lancet Infect Dis. 2022;22:589–590. doi: 10.1016/S1473-3099(22)00176-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.