Abstract
Aims
The adapter protein p130Cas, encoded by the Bcar1 gene, is a key regulator of cell movement, adhesion, and cell cycle control in diverse cell types. Bcar1 constitutive knockout mice are embryonic lethal by embryonic days (E) 11.5–12.5, but the role of Bcar1 in embryonic development remains unclear. Here, we investigated the role of Bcar1 specifically in cardiovascular development and defined the cellular and molecular mechanisms disrupted following targeted Bcar1 deletions.
Methods and results
We crossed Bcar1 floxed mice with Cre transgenic lines allowing for cell-specific knockout either in smooth muscle and early cardiac tissues (SM22-Cre), mature smooth muscle cells (smMHC-Cre), endothelial cells (Tie2-Cre), second heart field cells (Mef2c-Cre), or neural crest cells (NCC) (Pax3-Cre) and characterized these conditional knock outs using a combination of histological and molecular biology techniques. Conditional knockout of Bcar1 in SM22-expressing smooth muscle cells and cardiac tissues (Bcar1SM22KO) was embryonically lethal from E14.5–15.5 due to severe cardiovascular defects, including abnormal ventricular development and failure of outflow tract (OFT) septation leading to a single outflow vessel reminiscent of persistent truncus arteriosus. SM22-restricted loss of Bcar1 was associated with failure of OFT cushion cells to undergo differentiation to septal mesenchymal cells positive for SMC-specific α-actin, and disrupted expression of proteins and transcription factors involved in epithelial-to-mesenchymal transformation (EMT). Furthermore, knockout of Bcar1 specifically in NCC (Bcar1PAX3KO) recapitulated part of the OFT septation and aortic sac defects seen in the Bcar1SM22KO mutants, indicating a cell-specific requirement for Bcar1 in NCC essential for OFT septation. In contrast, conditional knockouts of Bcar1 in differentiated smooth muscle, endothelial cells, and second heart field cells survived to term and were phenotypically normal at birth and postnatally.
Conclusion
Our work reveals a cell-specific requirement for Bcar1 in NCC, early myogenic and cardiac cells, essential for OFT septation, myocardialization and EMT/cell cycle regulation and differentiation to myogenic lineages.
Keywords: Bcar1, Outflow tract septation, Epithelial-to-mesenchymal transformation, Heart development
Graphical Abstract
Graphical Abstract.
1. Introduction
The adapter protein p130Cas, encoded by the Bcar1 gene, plays a critical role in chemotactic signalling in diverse cell types, through regulation of the actin cytoskeleton and its function in multimolecular complexes of focal adhesions.1,2 Tyrosine phosphorylation of Bcar1/p130Cas at multiple sites in its substrate domain is thought to play a key role in mediating protein–protein interactions, downstream effector activation, signalling and cell migration in response to cytokines, and growth factors.1–7 However, information about the in vivo role of Bcar1/p130Cas is very limited. Bcar1-null embryos died in utero between embryonic days (E) 11.5–12.5, exhibiting severe defects in development of the heart and prominent dilation of major blood vessels.8 These findings showed that Bcar1/p130Cas is essential for normal embryonic development, but did not indicate whether defects observed in Bcar1-null embryos arose from a specific role of Bcar1/p130Cas in cardiovascular development or were an indirect effect of complete organismal loss of Bcar1, and provided no indication of the molecular or cellular mechanisms involved. Mutant mice with a global deletion of the Bcar1/p130Cas exon 2-encoded SH3 domain died in utero at E12.5–E13.5, with severe liver degeneration, but display no defects in development of the heart or other major organs.9 Furthermore, other conditional tissue-specific ablations of Bcar1/p130Cas all produced live viable progeny and did not cause developmental defects.10–12 Thus far, studies of conditional knock outs for Bcar1 do not account for the embryonic lethality displayed by Bcar1-null mice.
Division of the common outflow tract (OFT) into the pulmonary and aortic trunks, is a fundamental process in development of the mammalian heart, occurring in the mouse embryo from E11.5–E15.13 OFT septation into the pulmonary and aortic trunks occurs progressively in a distal to proximal order towards the ventricles and involves the coordinated migration of progenitors, including cardiac neural crest cells (CNCC),14 smooth muscle progenitor cells, and myocardial cells of the second heart field.15 Initially the aortic sac (AS) is invaded by progenitors, then the distal portion of the OFT (the truncus) is divided by the aortico-pulmonary septum, which is purely neural crest cells (NCC) in origin, followed by septation of the proximal OFT (the conus) by fusion of the OFT cushions (OTCs) (also called the bulbar/conotruncal ridges). Essential to septation of the proximal OFT is the differentiation of progenitors into mesenchymal and myocardial cells, resulting from the endocardial epithelial-to-mesenchymal transformation (EMT).13–15
The role of Bcar1/p130Cas in cardiovascular development was investigated herein by generating conditional cell type-specific deletions of Bcar1 targeting the major cell types in the developing cardiovascular system. 0Mice with conditional knock out of Bcar1/p130Cas in smooth muscle cells (SMC) (Bcar1fl/fl cross with smMHC-Cre mice) or endothelial cells (Tie2-Cre) were phenotypically normal. In contrast, deletion of Bcar1 in early smooth muscle and cardiac cells using SM22-Cre mice caused severe defects in cardiovascular development from E11.5, including severely impaired OFT septation, aberrant remodelling of the ventricular myocardium, ventricular septal defects, and a grossly dilated AS. Furthermore, NCC-specific knockout, using the Pax3-Cre transgenic line,16 replicated key features of the Bcar1SM22KO mutant phenotype, including defective OFT septation and an abnormally dilated AS, indicating a specific role for Bcar1 in NCC essential for OFT septation and remodelling of the AS. SM22- and Pax3-restricted loss of Bcar1 was also associated with disruption of mechanisms important for myogenic differentiation and EMT. These findings demonstrate an essential role for Bcar1 in migration and differentiation of cells required for OFT septation and development of the ventricular myocardium.
2. Materials and methods
2.1 Generation and genotyping of Bcar1 conditional knockout mice
Bcar1fl/fl mice were generated by flanking exons 7 and 8 of the Bcar1 gene by loxP sites to allow their removal upon Cre-mediated action. Exons 7 and 8, encode most of the C-terminal Bcar1/p130Cas region and the 3′UTR, containing the endogenous polyadenylation site. The recombined embryonic stem cells were injected into blastocysts derived from an albino C57BL/6 strain (C57BL/6J-Tyrc-2J/J) to produce chimaeras (genOway, Lyon, France). Male chimaeras were subsequently bred with C57BL/6 Flp-deleter females to generate the F1 generation of Bcar1fl/fl mice (Supplementary material online, Figure S1). Targeted deletion of Bcar1 was achieved by crossing female Bcar1fl/fl mice to either heterozygous male smMHC-Cre,17SM22-Cre,18Tie2-Cre,19Mef2c-Cre,20or Pax3-Cre16 mice to produce Bcar1 knockouts in, respectively, SMC (Bcar1fl/fl; smMHC-Cre+/−, hereafter Bcar1SMKO), SMC and cardiac tissues (Bcar1fl/fl; SM22-Cre+/−; hereafter Bcar1SM22KO), endothelial cells (Bcar1fl/fl; Tie2-Cre+/−; hereafter Bcar1TIE2KO), second heart field cells (Bcar1fl/fl; Mef2c-Cre+/−; hereafter Bcar1MEF2CKO), or NCC (Bcar1fl/fl; Pax3-Cre+/−; hereafter Bcar1PAX3KO). Some lines were crossed to a Rosa26 reporter strain to allow detection of Cre activity by X-gal staining.21 Mice were backcrossed to the C57BL/6 background for at least five generations, and all mutants were compared with wild-type littermates. Genotyping of genomic DNA extracted from ear biopsies (adult mice) or tail tips (neonates and embryos) was performed by polymerase chain reaction using the primers and conditions listed in Supplementary material online, Table S1.
2.1.1 Ethics statement
No anaesthetic/analgesic agents were used. Euthanasia was performed by overexposure to CO2 gas in a closed chamber followed by cervical dislocation. Animal maintenance, husbandry, and procedures conform to the guidelines from Directive 2010/63/EU of the European Parliament and were conducted in accordance with the Animal Care and Ethics Guidelines of University College London (UK) and the United Kingdom Home Office Animals (Scientific Procedures) Act of 1986.
2.2 Western blotting
Aortic tissue from adult Bcar1SMKO mice, or right ventricle (RV) and OFT tissue from Bcar1SM22KO embryos was homogenized in RIPA lysis buffer supplemented with protease inhibitors (Sigma #P2714). Equivalent amounts of protein were blotted with the primary antibodies listed in Supplementary material online, Table S2. Protein bands were visualized with horseradish peroxidase-conjugated secondary antibodies (Santa Cruz) and ECL 2 Western Blotting Substrate kit (Thermo Scientific) according to the manufacturers’ instructions.
2.3 X-Gal staining
Tissues were stained for β-galactosidase activity using a kit from InvivoGen (catalog #rep-lz-t), according to the protocol provided. Briefly, embryos were dissected, fixed with 0.5% glutaraldehyde in phosphate buffered saline (PBS)/MgCl2 solution on ice, and then stained with X-Gal staining solution overnight at 37°C followed by extensive washes with PBS. Samples were then embedded in paraffin wax, and 7 μm sections were cut and counterstained with Nuclear Fast Red.
2.4 Histological and immunohistochemical staining
Embryos were dissected at the specified ages and fixed with 1× zinc fixative (BD Biosciences #552658) overnight at room temperature. Samples were then processed to paraffin and embryos were embedded on their backs to provide sections in the frontal orientation, which offers a good view of the vessels entering and leaving the heart, and also gives a clear view of OFT septation. Serial tissue sections, 7 μm thickness, were either stained with haematoxylin and eosin (H&E) or subjected to immunohistochemistry/immunofluorescence with primary and secondary antibodies (Supplementary material online, Table S3).
2.5 Ink injections
Ink injections were performed on embryos, which had been fixed in 4% paraformaldehyde overnight. The OFT was injected with Indian ink (Pelican) diluted 1:5 in PBS using a microinjection glass capillary needle. Images were acquired with a camera attached to a Leica stereo microscope.
2.6 Amira reconstructions and HREM imaging
The 7 μm-thick serial sections from E14.5 control, Bcar1SM22KO, and Bcar1PAX3KO embryos were stained with H&E and scanned with the NanoZoomer HT slide scanner (Hamamatsu Photonics Ltd.). Image files were uploaded to the Amira software where they were aligned and processed to produce animations illustrating septation of the entire OFT. For High Resolution Episcopic Microscopy (HREM) analysis, embryos were fixed in 4% PFA overnight, dehydrated through an acetone series (50%, 80%, 100%, 100%, and 100%), then infiltrated with Technovit 8100 resin (Taab Laboratories) through a series of 50:50 (Technovit 8100: Acetone) for 24 h, 75:25 (Technovit 8100: acetone) for 24 h, and 100% Technovit 8100 for a final 24 h. All solutions had the addition of 1 mg/ml Eosin B (Sigma) and were performed at 4°C with constant agitation. Finally, embryos were embedded in Technovit 8100 under vacuum at 4°C. After 48 h, samples were imaged using the Optical HREM system (Indigo Scientific) using 405/495 nm Ex/Em filter set, at appropriate resolution to ensure the entire embryo was in the field of view, and using a slice thickness to provide approximately isotropic resolution. Image segmentation was performed by manual annotation. Both segmentation and volume rendering were performed with Amira and ImageJ software.
2.7 RNASeq analysis
Whole RNA was extracted from dissected OFTs from E11.5 Bcar1SM22KO mutants and littermate controls using the QIAGEN RNeasy Micro Kit, according to the manufacturers’ instructions. Samples were processed for RNASeq analysis by the UCL Genomics facility using the NextSeq. Differential expression analysis was performed using Galaxy software with the local fit parametric. Raw P-values were corrected using the Benjamini–Hochberg method to account for multiple testing.
2.8 Statistical analysis
Data are presented as means ± SEM. Statistical significance was determined using the 2-tailed Student’s t-test and P ≤ 0.05 was considered statistically significant. When quantifying the pharyngeal arch artery (PAA) diameter, the diameter across the widest part of the vessel was taken, n = 3 embryos were used for each genotype and at least six measurements taken from each embryo.
3. Results
3.1 Bcar1 is essential for embryonic heart development but is not required in smooth muscle cells or endothelial cells
To investigate the role of Bcar1 in early cardiovascular development, we generated a targeted deletion of Bcar1 in smooth muscle and cardiac cells by crossing Bcar1fl/fl mice with the SM22-Cre mouse line (Supplementary material online, Figures S1–S3). SM22α is transiently expressed in the presumptive RV (bulbus cordis) and OFT myocardium of the mouse heart between E8.0 and E12.5,22–24 and in vascular smooth muscle cells (VSMC) from E9.5. SM22-Cre promoter activity was detected in the heart and OFT at E8.5–E9 as determined by mapping β-galactosidase expression in Bcar1SM22KO mice crossed to a Rosa26 reporter strain (Supplementary material online, Figure S2A). Recombination of the floxed Bcar1 allele was confirmed by genotyping (Supplementary material online, Figure S2B), and immunoblotting of cardiac tissue from Bcar1SM22KO mice including the RV and OFT verified that excision of the conditional Bcar1 allele resulted in depletion of the Bcar1-encoded p130Cas protein compared with wild-type littermates (Supplementary material online, Figure S2C). To distinguish possible functions of Bcar1 in cardiomyocytes and SMC, we generated mice with conditional Bcar1 knock out restricted to SMC by crossing Bcar1fl/fl with smMHC-Cre mice to produce the Bcar1SMKO line (Supplementary material online, Figure S2D–F). SMC-specific Myosin Heavy Chain (smMHC or Myh11) is a highly specific marker of smooth muscle, detected in the early aorta (Ao) from E10.5, in aortic arch arteries from E12.5, and in the immature coronary artery plexuses from E14.5.25 SMC-specific loss of Bcar1 was verified by SMC-restricted β-galactosidase expression in the heart, the presence of the recombined allele, and by SMC-specific loss of Bcar1/p130Cas protein in Bcar1SMKO mice as assessed by immunoblot of aortic tissue (Supplementary material online, Figure S2D–F). We also assessed the developmental role of Bcar1 in endothelial cells by generating mice with Tie2-restricted Bcar1 deletion (Bcar1TIE2KO).
Bcar1SM22KO mutants, in which Bcar1 was ablated in both SMCs and early cardiac tissues, were embryonic lethal by E14.5–E15.5, and by E14.5 ∼50% of Bcar1SM22KO embryos were starting to be reabsorbed while the remainder showed signs of severe morbidity including evidence of haemorrhaging and severely abnormal heart morphology (Supplementary material online, Figure S3A and B). This phenotype was fully penetrant and no live Bcar1SM22KO progeny was produced (Supplementary material online, Figure S3A). In contrast, Bcar1SMKO and Bcar1TIE2KO progeny were healthy, viable, and born at the expected Mendelian ratios (Supplementary material online, Figure S3C), with no overt phenotype, nor detectable peri- or early post-natal mortality. Histological examination of the vasculature of the heart and lungs of neonatal Bcar1SMKO mice (9 days old) also revealed no apparent vessel abnormalities (Supplementary material online, Figure S3D).
3.2 Bcar1 expression in the heart and OFT
Cardiovascular Bcar1/p130Cas expression was previously reported at E11.5–E12.5,8 but the detailed pattern of embryonic Bcar1/p130Cas expression is unclear. To gain insight into regions of the developing heart, where Bcar1/p130Cas is most prominent, the expression pattern of Bcar1/p130Cas in the heart and large vessels was examined by immunostaining from E10.5 up to E13.5. Bcar1/p130Cas protein expression was not apparent in the myocardium or OFT at E10.5 (Supplementary material online, Figure S4A and B), and was first detected at E11.5 in the cushion mesenchyme of the OFT, developing ventricular septum and endocardium (Supplementary material online, Figure S4C, D and I), expression becoming more pronounced in the OTC mesenchyme by E12.5. Bcar1/p130Cas was also expressed strongly in the epicardium, endocardium, and more weakly in the cardiomyocytes at E12.5 (Supplementary material online, Figure S4E, F and J). By E13.5 Bcar1/p130Cas expression was no longer seen in the OFT septum but was localized to the aortic and pulmonary valve leaflets and remained strongly expressed in the epicardium (Supplementary material online, Figure S4G and H).
3.3 Bcar1SM22KO mice exhibit severe defects in the remodelling of the ventricular myocardium, and OFT septation
The underlying causes of the embryonic lethality of the Bcar1SM22KO mice were investigated by histological analysis of the embryonic heart. From E11.5 Bcar1SM22KO mice exhibited defects in ventricular development, particularly the RV (Figure 1A and D). A ventricular septal defect was also observed in the Bcar1SM22KO embryos (Figure 1B and E). By E14.5, Bcar1SM22KO embryos had defects in the remodelling of both the right and left ventricular myocardium, including fewer trabeculations compared to littermate controls and a significantly thinner myocardial wall (Figure 1C, F, G, H and I). In addition, ventricular septation, compaction of the ventricular myocardium, and formation of the ‘spongy layer’ between the compact and trabeculated ventricular tissue, which normally occur between E13 and E14,26 were all impaired in Bcar1SM22KO mutants (Figure 1E and F). Defects in trabeculation have been associated with ventricular compaction defects,27 which may account for the thinned myocardial walls seen in the Bcar1SM22KO mutants. Ventricular defects in Bcar1SM22KO embryos were not associated with observable effects on either cardiomyocyte proliferation or apoptosis at E13.5 (Supplementary material online, Figure S5).
Figure 1.
Ventricular defects in Bcar1SM22KO embryos. Ventricular development in control (A–C and G) and Bcar1SM22KO embryos (D–F and H). At E11.5 Bcar1SM22KO embryos (D) had an under-developed RV compared to control littermates (A). By E14.5 Bcar1SM22KO embryos (F) exhibited defects in the remodelling of the ventricular myocardium, which included thinning of the myocardial wall (arrowhead) and fewer ventricular trabeculations compared to littermate controls (C). An interventricular septal defect was seen in the mutants at E12.5 and E14.5 (asterisk in E and F) compared with littermate controls (B and C). MF20 staining, which labels the cardiac myosin heavy chain, indicates thinning of the ventricular myocardium (arrowheads) in E14.5 Bcar1SM22KO embryos (H) compared to littermate controls (G); higher magnification views are shown in boxed regions, arrowheads indicate the ventricular wall and hollow arrowheads highlight the ventricular trabeculations. (I) Quantification of LV and RV wall thickness in Bcar1+/+ (n=3, white bar) and Bcar1SM22KO mice (n=4, black bar); *P=0.0155 and **P=0.0032 as determined by 2-tailed t-test. Scale bars are 900 μm. Ao, aorta; PT, pulmonary trunk; PTA, persistent truncus arteriosus.
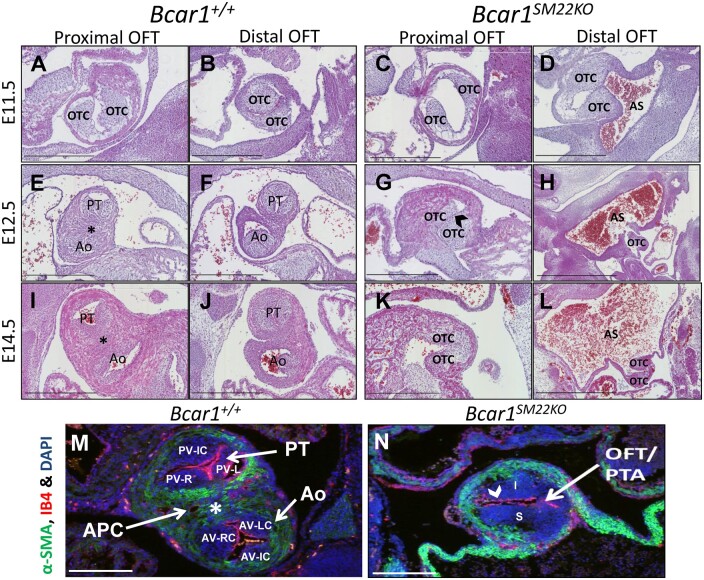
A failure in OFT septation occurred in Bcar1SM22KO mutants with 100% penetrance (Figures 2 and 3 and Supplementary material online, Figure S6). These defects were apparent from E11.5 (Figure 3 A–D) and became progressively more severe with age. In wild-type E12.5 embryos, the mesenchymal cushions in the proximal OFT had fused and a fibrous raphe [the aortico-pulmonary complex (APC)] had formed between the pulmonary and aortic roots (asterisk in Figure 3E, I and M). In contrast, the OTCs in the Bcar1SM22KO mutants failed to fuse, and remained clearly demarcated by layers of endothelial cells (arrowheads in Figure 3G and N), preventing formation of the APC and septum (Figure 3G, K and N). In the distal OFT, compared to wild-type embryos (Figure 3F and J), separation of the aortic and pulmonary trunks in Bcar1SM22KO embryos from E12.5 had failed, and the distal OFT was fused with a severely dilated AS (Figure 3H and L). By E14.5, septation of the entire OFT had failed in the Bcar1SM22KO mutants leading to a single outflow vessel resembling persistent truncus arteriosus (PTA),28 whereas OFT septation in littermate control embryos was complete (Figure 2 and Supplementary material online, Figure S6).
Figure 2.
Amira false coloured rendering of OFT septation and HREM volume renderings at E14.5. H&E sections of Bcar1+/+ and Bcar1SM22KO embryos (A–D), are presented in false coloured renderings (E–H), highlighting the OFT (blue), heart tissue (red), and AS (purple); the Ao and pulmonary trunk (PT) are also indicated. (I and J) 3D grey-scale renderings of E14.5 embryonic hearts, with cut-through to show interior structure, and pseudocoloured surface renderings from HREM data. Ao, aorta; PT, pulmonary trunk. Asterisk in (A) and (E) highlights the aortico-pulmonary septum. Scale bars are 1 mm.
Figure 3.
OFT septation defects in Bcar1SM22KO embryos. (A–D) OFT septation at E11.5 in littermate controls (A and B) and Bcar1SM22KO embryos (C and D). Proximally, the OTCs in the mutants have migrated less compared to controls; distally the cushions have failed to fuse and the OFT is connected to an abnormally dilated AS. (E–N) OFT septation at E12.5 (E–H) and E14.5 (I–N). Defects in OFT septation in Bcar1SM22KO embryos were seen throughout the length of the OFT (G, H, K, L, and N) as compared to littermate controls (E, F, I, J, and M). In control embryos septation of the OFT is complete and the proximal OTC have fused to form the APC (E, I, and M). At the site of fusion, a fibrous raphe is seen separating the pulmonary and aortic roots (asterisks in E and I). This fibrous raphe is transformed into a muscularized septum by E14.5, staining positive for SMA (asterisk in M). In Bcar1SM22KO mutants (G, K, and N), full separation of the aortic and pulmonary roots and development of the fibrous raphe are not seen, indicated by lack of SMA staining and PTA. Demarcation of the OTC by a layer of endothelial cells is seen in the Bcar1SM22KO mutants (arrowheads in G and N). Distally, in the mutants (H and L), the OFT remains open and connected to an abnormally dilated AS. Whereas in the controls, the Ao and PT are fully separated (F, J, and M). Immunofluorescent staining of endothelial cells (IB4) and SMA with nuclear DAPI counterstaining is shown in (M and N). PV‐IC, pulmonary valve intercalated cushion; AV‐IC, aortic valve intercalated cushion; APC, aortico-pulmonary complex; AV‐RC, aortic valve right coronary cusp; AV‐LC, aortic valve left coronary cusp; PV‐R, pulmonary valve right cusp; PV‐L, pulmonary valve left cusp; I, inferior septal cushion; S, superior septal cushion. Scale bars are 400 μm except for (H and L), which are 900 μm and (M and N), which are 200 μm.
3.4 Bcar1 deficiency does not impact on PAA development in Bcar1SM22KO mice but disrupts vascular remodelling
Given the aneurysmal dilatation of the AS in Bcar1SM22KO mutants and its failure to remodel into the aortic and pulmonary channels, we performed intra-cardiac ink injections to investigate a possible effect on development of the pharyngeal arches and their derivatives, including the aortic arch arteries, which arise from the AS. At E10.5, the third, fourth, and sixth pharyngeal arch arteries were seen in both the Bcar1SM22KO mutants and their wild-type littermates (Supplementary material online, Figure S7A–D), indicating no impairment of PAA development. However, the vessels were significantly dilated in the Bcar1SM22KO mutants compared to controls (Supplementary material online, Figure S7E–G), possibly due to altered blood flow in the mutants. Examination of the vasculature at E13.5 revealed aberrant PAA remodelling evidenced by the presence of the abnormally dilated AS and lack of the carotid and subclavian arteries (Supplementary material online, Figure S7H and I).
3.5 Effect of Bcar1 knock out on cell proliferation and migration in the OFT
At E13.5, cell proliferation, detected by immunostaining for the proliferation marker Ki67, was seen throughout the heart and other organs in both wild-type and Bcar1SM22KO embryos. However, whereas a zone of mainly non-proliferating cells was apparent in wild-type OTCs in the region of the developing septum (asterisk in Supplementary material online, Figure S8A), the Bcar1SM22KO OTCs exhibited strong and generalized Ki67 staining, and lacked a clearly defined area of hypoproliferation (Supplementary material online, Figure S8B). Given the known role of myocardialization in OFT septation, we determined whether loss of Bcar1 could affect cardiomyocyte survival and/or migration into the mesenchymal outlet septum during OTC fusion and septation. We addressed this by immunostaining cardiomyocytes using MF20, which specifically recognizes cardiomyocytes but not VSMC or NCC, and using cleaved caspase-3 as a marker of apoptosis. In wild-type embryos, MF20-positive cardiomyocytes infiltrated deep into the developing septal bridge, whereas Bcar1SM22KO embryos displayed reduced cardiomyocyte migration into the septum (Supplementary material online, Figure S8E–H). No clear change in apoptosis was observed in Bcar1SM22KO embryos compared with littermate controls (Supplementary material online, Figure S8C and D). Quantitation of invasion of septal MF20-positive cells into the OFT revealed a significant decrease in OFT invasion in Bcar1SM22KO compared with wild-type littermates (Supplementary material online, Figure S8I).
We next investigated whether loss of Bcar1 could affect migration by impacting on expression of key components of the actin cytoskeleton-associated macromolecular machinery that regulates cell migration. Consistent with this possibility, we observed a reduction of F-actin-specific phalloidin staining in cells of the OTCs in the Bcar1SM22KO mutants compared with littermate controls (Supplementary material online, Figure S9A and B), suggesting a disruption in actin cytoskeleton organization in these cells. However, we were unable to detect significantly altered protein expression of Talin, FAK, or Rac1 in protein lysates of OFTs from E11.5 Bcar1SM22KO embryos compared with wild-type littermate controls (Supplementary material online, Figure S9C and D). We also examined expression of Cdc42 (cell division cycle 42), a member of the Rho family of GTPases that acts as a molecular switch to regulate cytoskeleton remodelling and cell movement in cardiovascular development.29–31 At E13.5, Cdc42 expression was largely limited to the wild-type OTCs, which had fused and were remodelling to form the muscularized septum separating the aortic and pulmonary channels (arrow in Supplementary material online, Figure S9E and F). Strong Cdc42 expression was also present throughout the unfused OTCs of Bcar1SM22KO embryos (arrows in Supplementary material online, Figure S9G and H).
3.6 Sm22-restricted Bcar1 deletion blocks myogenic differentiation and EMT in the OFT
The OTCs are populated by mesenchymal cells that migrate from the pharyngeal mesoderm and dorsal neural tube, and subsequently differentiate to smooth muscle actin-expressing cells that form the septum separating the aortic and pulmonary vessels. We hypothesized that the failure in OFT septation in Bcar1SM22KO mice could arise from an effect of Bcar1 deficiency on myogenic cell differentiation or EMT. To investigate this possibility, we determined the appearance of mesenchymal cells in the OFT by immunostaining for SMC-specific α-actin (SMA). In wild-type embryos, SMA staining was abundant in the OTCs, localized to the periphery of cells differentiating to form the septum (Figure 4A and B), whereas SMA-positive cells were sparse in the Bcar1SM22KO OTCs and failed to form a septum (Figure 4C and D).
Figure 4.
Myogenic differentiation in OTC mesenchyme. Compared to Bcar1+/+ controls (A), E12.5 SMA protein expression in the OTC mesenchyme in Bcar1SM22KO mutants (C) was reduced and a muscular septum failed to develop in the distal OFT (D vs. B). (E) Heat-map of differentially regulated genes involved in myogenic differentiation, in RNA lysates from whole OFTs from E11.5 embryos: red represents increased expression, blue decreased expression. (F) mRNA expression of Bcar1, Twist1, and Msx1 from OFT RNA lysates from E11.5 Bcar1SM22KO mutants compared to littermate controls (Bcar1+/+ n=5, Bcar1SM22KO n=4; **P<0.001, *P<0.05, as determined by 2-tailed t-test). Scale bars are 100 µm.
To determine the molecular pathways disrupted following Bcar1 ablation, we performed RNASeq analysis of dissected OFTs from E11.5 Bcar1SM22KO mutants and littermate controls (Supplementary material online, Table S4). This revealed significant up-regulation of several genes encoding transcription factors, including two implicated in inhibition of myogenic differentiation, the basic helix-loop-helix (HLH) transcription factor, Twist-1, and the homeobox gene Msx-1 (Supplementary material online, Table S4 and Figure 4E and F). RNASeq analysis also revealed significant down-regulation of Endothelin Receptor Type B, a signalling pathway which regulates actin cytoskeletal dynamics and is centrally involved in NCC migration in development32 (Supplementary material online, Table S4 and Figure 4E). We also observed down-regulation of the mesenchymal differentiation markers Sm22/Transgelin and Myh11 (Supplementary material online, Table S4 and Figure 4E). Gene ontology analysis of RNAseq data revealed an enrichment of genes involved in mesenchymal cell differentiation and cell migration (Supplementary material online, Table S5), indicating a role for Bcar1 in these processes.
The effect of Bcar1 deficiency on EMT was examined by determining the expression of phospho(p)-Smad2, which is a key mediator of TGF-β-induced EMT, and of Slug, an EMT-promoting transcription factor, at E14.5. A marked reduction in pSmad2 expression was seen in the Bcar1SM22KO embryos compared to the controls, which was particularly apparent in the OTCs (Figure 5A and B). Expression of Slug in wild-type embryos was observed in the OTCs (arrowheads in Figure 5C), and, concomitant with reduction of pSmad2, was also markedly reduced in the Bcar1SM22KO mutants (arrowheads in Figure 5D).
Figure 5.
Expression of markers of EMT. Immunostaining of pSmad2 (A and B), Slug (C and D), Bcar1 (E and F), and N-cadherin (G and H) in OFTs in Bcar1+/+ wild-type and Bcar1SM22KO littermates (pSmad2 and Slug at E14.5; N-cadherin and Bcar1 at E12.5), magnified views of boxed regions are shown. At E12.5 immunostaining for Bcar1/p130Cas in WT embryos revealed a distinctive whorl-like pattern in the central region of the OTCs (red arrowheads in E), which was absent in the Bcar1SM22KO mutants (F). (I) Representative western blots of N-cadherin and pSmad2 expression from protein lysates from whole dissected OFTs at E12.5. (J) Quantitation of western blot protein bands from E12.5 OFT tissue lysates (**P<0.01, ***P<0.005; Bcar1+/+ n≥5, Bcar1SM22KO n≥5). (A–D) Scale bars are 200 µm; (E–H) scale bars are 400 µm.
N-cadherin is expressed in the dorsal region of the neural tube from which CNCCs migrate, and is essential for remodelling of the OFT once CNCCs reach the heart.33 Marked N-cadherin expression occurred in the central region of wild-type OTCs very similar to that for Bcar1/p130Cas in serial sections (Figure 5E and G and Supplementary material online, Figure S4E). Bcar1/p130Cas and N-cadherin immunostaining was completely absent in Bcar1SM22KO OFTs (Figure 5F and H), indicating loss of Bcar1 targeted to cells of the developing OFT also expressing N-cadherin, and suggesting a possible role for Bcar1 in the regulation of N-cadherin expression in CNCCs affecting their ability to differentiate to SMC. Concordant with immunostaining data, western blotting of N-cadherin and pSmad2 expression in OFT lysates from E12.5 embryos revealed a significant reduction in the Bcar1SM22KO mutants compared to their littermate Bcar1+/+ controls (Figure 5I and J). Slug expression was also reduced in the Bcar1SM22KO mutants but this did not reach statistical significance, possibly due to low Slug expression and the mixed cell population in the tissue lysates analysed.
3.7 Bcar1 deletion in NCC causes defective OFT septation and abnormal AS dilatation
To identify more precisely the role of Bcar1 in heart development, we generated conditional Bcar1 knockouts using the Mef2c-Cre (Bcar1MEF2CKO) and Pax3-Cre (Bcar1PAX3KO) transgenic mouse lines, which generate genetic deletions targeted, respectively, to cells that give rise to the second heart field, and to NCC. Bcar1MEF2cKO mice were born at expected Mendelian ratios, developed to adulthood, and displayed no overt defects (Supplementary material online, Figure S10A). In contrast, crossing Bcar1fl/fl with Pax3-Cre mice produced no live homozygous knockout progeny (Supplementary material online, Figure S10B).
Bcar1PAX3KO embryos exhibited aberrant OFT septation, which appeared to be limited to septation of the proximal OFT (Supplementary material online, Figures S10C–L and S11 and Figure 6A–H). An abnormally dilated AS was also observed in Bcar1PAX3KO mutants. Distally, the OTC mesenchyme in the Bcar1PAX3KO mutants formed a muscularized septum, indicated by the presence of α-SMA expressing cells (Figure 6I and J). Myocardialization of the OFT, examined by MF20 immunostaining, was also apparent in the Bcar1PAX3KO mutants (arrows in Figure 6K and L). Immunostaining of the Bcar1PAX3KO mutants revealed a reduction in Bcar1/p130Cas and N-cadherin expression in the OTC mesenchyme whereas no loss of CD31-positive endothelial cells was detected (Figure 6M–R).
Figure 6.
AMIRA false coloured rendering of OFT septation at E12.5 and protein expression in Bcar1PAX3KO mutant OFTs at E12.5. At E12.5, the APC (asterisk) has formed in the proximal OFT (A) and the distal OFT has separated into the Ao and PT (B) in the Bcar1+/+ controls, whereas septation of the proximal OFT is incomplete in the Bcar1PAX3KO mutants (C) and distally, the OFT has separated although the Ao remains connected to an abnormally dilated AS (D). (E–H) are false coloured renderings of the tissue sections, highlighting the OFT (blue), heart tissue (red), and the AS (purple). Scale bars in (A and B) are 500 µm and in (C and D) are 1 mm. Alpha-SMA expression in the distal OFTs in Bcar1PAX3KO (J) and control littermates (I). MF20 immunostaining reveals invasion of cardiomyocytes into the OTC mesenchyme in the Bcar1PAX3KO mutants (L) and littermate controls (K). A reduction in Bcar1 (N) and N-Cadherin (P) expression was seen in the OTC mesenchyme of Bcar1PAX3KO mutants compared to controls (M and O). CD31 immunostaining revealed an increase in CD31 +ve cells in the OFTs of the Bcar1PAX3KO mutants (R) compared to littermate controls (Q). (I–L) Scale bars are 500 µm; (M–R) scale bars are 400 µm.
4. Discussion
This study demonstrates for the first time that targeted deletion of Bcar1/p130Cas either in early smooth muscle and cardiac cells, or specifically in NCC, causes severe abnormalities in cardiovascular development, most strikingly in OFT septation and remodelling of the AS. Conditional knock out of Bcar1/p130Cas in both SMC and cardiac cells using SM22-Cre mice caused an array of developmental cardiovascular anomalies, including a hypoplastic myocardial wall, impaired ventricular development and ventricular septation, a failure of OFT septation resulting in PTA, and abnormal dilatation and remodelling of the AS. The conclusion that these cardiovascular abnormalities are due to direct effects of Bcar1 deficiency in cardiac cells is supported by the correlation between areas of the heart where the Bcar1SM22KO mutant embryos show severe defects and with both the regions of prominent Bcar1 expression in the normal developing heart, namely the ventricles and the OTCs, and the regions of cardiac expression of SM22 during embryogenesis, such as the presumptive right and left ventricles (LV) and OFT myocardium.23,24 Whole mouse Bcar1 knockouts (Bcar1-null mutants) displayed a ‘poorly developed heart, consisting of thin myocardium, and prominently dilated blood vessels that retained blood cells’, with prominent expression of Bcar1 in the wild-type heart and blood vessels from E11.5–12.5.8 Our findings of a significantly thinned myocardial wall, dilated AS, and Bcar1/p130Cas expression from E11.5 are consistent with the phenotype of Bcar1-null mice, though the latter study reported no OFT defects.
SM22α transcripts are also expressed in vascular SMC from E9.5 and continue to be expressed in all SMC into adulthood.22–24 However, Bcar1 knock out specifically in SMC using smMHC-Cre mice, gave rise to no cardiovascular defects or any overt developmental phenotype and generated viable homozygous mice. It is noteworthy that conditional deletion of Bcar1/p130Cas in skeletal muscle using the muscle creatine kinase (Ckmm)-Cre transgenic line also produced phenotypically normal, viable, and fertile mice in normal Mendelian ratios.10 Targeted ablation of Bcar1 in endothelial cells and second heart field cells also had no developmental or post-natal effects. In contrast, NCC-specific Bcar1 deletion in Bcar1PAX3KO mice partially recapitulated the cardiac defects in Bcar1SM22KO mutants, including proximal OFT septation defects and abnormal dilatation of the AS. The overlap in defects seen in the Bcar1SM22KO and Bcar1PAX3KO mutants highlight a role for Bcar1 specifically in NCC relevant for OFT septation and septum formation. The results also indicate that other cell types, including epicardial, endocardial, and other cardiomyocyte progenitor cells, may be responsible for the ventricular remodelling and proximal OFT septation defects observed in the Bcar1SM22KO mutants but not the Bcar1PAX3KO mutants. Identifying these other specific cell types will be the focus of future experimental work. In contrast, Bcar1 is not required for SMC or endothelial cell development or maturation, in second heart field cells, or for adult SMC function in the Ao or other heart vessels.
The conclusion that Bcar1 plays a key role in OFT septation and ventricular development is supported by the spatiotemporal correlation between these processes and dynamic changes in Bcar1/p130Cas protein expression in the embryonic heart. In wild-type embryos, Bcar1/p130Cas expression was first observed in the OTCs at E11.5, coincident with the onset of distal OFT septation, formation of the spiralling OTCs, and NCC insertion into the AS to divide the aortic and pulmonary channels.26 Bcar1/p130Cas expression in OTCs was prominent at E12.5, when septation of the proximal OFT progresses, but had markedly declined by E13.5 when OFT septation is complete, consistent with a dynamic and time-specific role for Bcar1 in the cardiac cell contributions to OFT septation. A similar spatiotemporal correlation is evident between ventricular Bcar1/p130Cas expression and ventricular septation, myocardial compaction, trabeculation and increased ventricular wall thickness, phenotypic characteristics impaired in Bcar1SM22KO mice, and all of which occur between E11.5 and E13.5. Given that NCC-specific N-cadherin deficiency generated using Wnt1-Cre mice also causes a lethal failure of normal OFT septation,33 the loss of N-Cadherin expression caused by Bcar1 deletion in Bcar1SM22KO and Bcar1PAX3KO mice is a possible mechanism underlying the failure of OFT septation. This is supported by the marked similarity in the immunostaining for N-cadherin and Bcar1/p130Cas in the OTCs, which strongly resemble the infiltrating columns of CNCCs during OFT septation, and the OFT septation defects seen in the NCC-specific Bcar1PAX3KO mutants. These findings suggest that SM22-Cre and Pax3-Cre may drive conditional Bcar1 deletion in overlapping NCC populations, though confirmation of this will require further experimental work.
The embryonic lethality of Bcar1SM22KO and Bcar1PAX3KO mice from E14.5 to 15.5 probably arises from the failure of OFT septation and abnormal AS dilatation leading to inadequate blood supply to the developing embryo. Another notable feature of Bcar1SM22KO mutants likely contributing to their embryonic lethality was defective compaction of the ventricular myocardium, associated with impaired development of the network of trabeculations in the ‘spongy layer’ during compaction, which increases myocardial oxygenation in the absence of the coronary vessels.34 Compaction of the ventricular myocardium, occurring between E13 and E14 in the mouse, is important for function of the ventricles during the later foetal stages, and its disruption is linked to heart failure and sudden cardiac death.26
Differentiation of myogenic and mesenchymal cells and endocardial EMT play essential roles in heart development, particularly in the heart valves and the OTCs.35 Septation of the proximal OFT requires both the transformation of endocardial cells into mesenchymal cells (endocardial EMT) that populate the cardiac jelly, and invasion of the OTCs by neural crest cells.14,26,36,37 This study presents several lines of evidence that Bcar1 plays a key role in mesenchymal differentiation and EMT in OFT septation. The absence of SMA-positive OTC cells in Bcar1SM22KO mutants demonstrates a marked impairment in mesenchymal differentiation. The increase in proliferation indicated by increased Ki67 staining in Bcar1SM22KO OTC cells and the lack of a clear septal zone of hypoproliferation, suggests that loss of Bcar1 resulted in deregulation of cell proliferation possibly arising from a failure to arrest proliferation precedent to differentiation. Disrupted EMT is also supported by the marked reduction in Smad2 phosphorylation and in Slug expression in the OTCs observed in mutants compared with wild-type embryos. TGF-β, an essential inducer of EMT in cardiac development, induces SMA expression in NCC, while Smad2 phosphorylation is a key mediator in TGF-β signalling.38,39 Slug transcription factor also plays a central role in EMT and in neural crest emigration.40 For example, silencing of Slug expression in chick embryos causes mesodermal malformation and neural crest emigration failure.40 Reduced SMA expression, and loss of Smad2 phosphorylation and downstream Slug expression are therefore likely to result in suppression of EMT. RNASeq analysis of Bcar1SM22KO OFTs at E11.5 showed that ablation of Bcar1 resulted in up-regulation of Twist1 and Msx1, transcription factors implicated in negative regulation of myogenic cell differentiation. During myogenesis, down-regulation of Twist1 in developing somites occurs co-ordinately with up-regulation of the myogenic transcription factors MyoD and Myf5, which play key roles in the later stages of muscle development,41 and Twist1 inhibited muscle cell differentiation in mouse C2C12 mouse myoblasts and in embryoid bodies derived from human embryonic stem cells.42,43 During development, Msx1 expression is associated with regions of highly proliferative and multipotent cells, and inversely correlated with cell differentiation and exit from the cell cycle, in limb buds, e.g.44 Furthermore, Msx1 overexpression inhibits myogenic differentiation in cell culture models and in vivo, and represses expression of MyoD and other myogenic transcription factors.45,46 Targeted Bcar1 deletion also resulted in down-regulation of Endothelin Receptor Type B, which has a role in embryonic NCC migration and in signalling pathways that regulate actin cytoskeletal organization,32 as well as markers of smooth muscle differentiation, such as Sm22/transgelin. These changes in gene expression taken together with the severe impairment of mesenchymal cell maturation and decreased expression of markers of EMT in Bcar1SM22KO, suggest that during normal heart development, Bcar1 plays a key role in maintaining a microenvironment permissive of myogenic and mesenchymal differentiation, in part by negatively regulating transcriptional networks involving Twist1 and Msx1, and may also play a role in NCC migration through the positive regulation of pro-migratory pathways. Though it is unclear how Bcar1/p130Cas regulates OFT gene expression, it is noteworthy that the C-terminal Bcar1/p130Cas region contains a HLH domain,1,47 and a 31 kDa Bcar1/p130Cas C-terminal cleavage product containing the HLH domain heterodimerized with the basic HLH transcription factor, E2A, translocated to the nucleus and inhibited E2A-mediated p21(Waf1/Cip1) transcription.48,49 Further work is warranted to elucidate whether Bcar1/p130Cas and/or its cleavage products could similarly mediate direct or indirect effects on gene expression programmes during cardiac OFT remodelling.
A dilated AS was a striking feature of Bcar1SM22KO and Bcar1PAX3KO mice that may result from an impact on SMC progenitors, particularly NCC-derived SMC. For example, SM22-restricted ablation of Integrin-linked kinase caused aortic aneurysm associated with perinatal lethality between E18.5 and P1, with no defects in OFT septation or ventricular development.50 This suggests that SM22-specific knock out can affect AS remodelling independently of effects on the progenitor cell populations contributing to OFT and ventricular remodelling, most likely through an impact on SMC and their progenitors. The lack of phenotype in SMC-specific Bcar1SMKO mice indicates that the aortic aneurysm in Bcar1SM22KO mice may not be due to SMC-specific loss, a conclusion supported by our observation that loss of Bcar1 did not prevent migration or subsequent differentiation of SMC progenitors essential for formation of the pharyngeal arch arteries. However, the dilated AS in Bcar1SM22KO mice may reflect impaired function of a subpopulation of SMC progenitors unaffected by smMHC-restricted ablation and distinct from those responsible for remodelling of pharyngeal arch arteries. AS dilatation may also be secondary to haemodynamic changes arising from earlier aberrant ventricular and/or OFT development due to Bcar1 deficiency in NCC and other cardiac cells.
The finding that conditional deletion of Bcar1 impairs heart development in mice has implications for the aetiology of human heart defects. A marked feature of Bcar1SM22KO mice was a failure of fusion of the OTCs and of the development of the fibrous raphe, resulting in formation of a single outflow vessel. This is a salient feature of PTA, which is a hallmark of several human congenital heart diseases, including DiGeorge syndrome (also known as del22q11).28 DiGeorge syndrome results from heterozygous deletions within human chromosome 22q11, and it is of interest for this study that one of the genes most strongly involved in these deletions is v-crk sarcoma virus CT10 oncogene homolog (avian)-like (CrkL), closely related to Crk, which is known to associate with p130Cas (Cas denotes Crk-associated substrate). Bcar1 is located on human chromosome 16, and not predicted to be perturbed by heterozygous deletions within 22q11. However, many congenital heart defects are of unknown aetiology, and may result from interactions between rare and largely unknown genetic defects. Tetralogy of Fallot (TOF) is the most common human cyanotic heart malformation, but only 20% of TOF patients have defined genetic causes, such as DiGeorge syndrome. A recent study of single-gene variants in a well-characterized TOF patient cohort, identified several novel null variants including a deletion of exons 2–7 in Bcar1, and a stopgain variant of IQGAP1,51 a gene encoding for an adapter protein that associates with Bcar1/p130Cas in endothelial cells.52 Another study reported a loss-of-function variant in Bcar1 in patients with conotruncal defects.53 These findings indicate that functional loss of Bcar1 and associated signalling networks plays a role in the aetiology of some human congenital heart defects, and suggest that mice with conditional genetic disruption of Bcar1 will prove useful for dissecting underlying mechanisms involved in the causation of such congenital heart abnormalities.
Data availability
The data underlying this article are available in the article and in its Supplementary material online.
Supplementary material
Supplementary material is available at Cardiovascular Research online.
Supplementary Material
Acknowledgements
The authors thank the UCL Biological Services Staff for assistance with animal husbandry and maintenance. We thank Professor Adrian Hobbs, Queen Mary University London, for the Tie2-Cre mouse line.
Conflict of interest: none declared.
Funding
This work was supported by British Heart Foundation project grant PG/17/20/32864 to I.Z., M.M., and P.S. British Heart Foundation grant RG/15/14/31880 and Leducq Foundation grant 15CVD01 to P.S. P.F. and L.W. were supported by a British Heart Foundation Doctoral training grant awarded to P.F. for L.W. (FS/16/41/32235 to L.W.).
Contributor Information
Marwa Mahmoud, Centre for Cardiometabolic and Vascular Science, BHF Laboratories, UCL Division of Medicine, 5 University Street, London WC1E 6JF, UK.
Ian Evans, Centre for Cardiometabolic and Vascular Science, BHF Laboratories, UCL Division of Medicine, 5 University Street, London WC1E 6JF, UK.
Laura Wisniewski, Centre for Cardiometabolic and Vascular Science, BHF Laboratories, UCL Division of Medicine, 5 University Street, London WC1E 6JF, UK.
Yuen Tam, Centre for Cardiometabolic and Vascular Science, BHF Laboratories, UCL Division of Medicine, 5 University Street, London WC1E 6JF, UK.
Claire Walsh, UCL Centre for Advanced Biomedical Imaging, Paul O'Gorman Building, 72 Huntley Street, London WC1E 6DD, UK.
Simon Walker-Samuel, UCL Centre for Advanced Biomedical Imaging, Paul O'Gorman Building, 72 Huntley Street, London WC1E 6DD, UK.
Paul Frankel, Institute of Cardiovascular Science, University College London, 5 University Street, London WC1E 6JF, UK.
Peter Scambler, Developmental Biology of Birth Defects Section, UCL Institute of Child Health, 30 Guilford Street, London WC1N 1EH, UK.
Ian Zachary, Centre for Cardiometabolic and Vascular Science, BHF Laboratories, UCL Division of Medicine, 5 University Street, London WC1E 6JF, UK.
Translational perspective.
The molecular pathways coordinating cardiogenesis and the remodelling of the OFT are complex, and dysregulation of these pathways causes human heart defects. Our findings highlight a specific requirement for Bcar1 essential for cardiogenesis. Furthermore, the failure of OFT septation in Bcar1SM22KO mice resembles PTA, a feature of several human congenital heart diseases, including DiGeorge syndrome. Our findings have implications for the mechanisms underlying the pathogenesis of congenital heart disease, and suggest that mice with conditional Bcar1 deletions may be useful models for dissecting mechanisms involved in the pathogenesis of human heart defects.
References
- 1. Barrett A, Pellet-Many C, Zachary IC, Evans IM, Frankel P.. p130Cas: a key signalling node in health and disease. Cell Signal 2013;25:766–777. [DOI] [PubMed] [Google Scholar]
- 2. Defilippi P, Di Stefano P, Cabodi S.. p130Cas: a versatile scaffold in signaling networks. Trends Cell Biol 2006;16:257–263. [DOI] [PubMed] [Google Scholar]
- 3. Pellet-Many C, Frankel P, Evans IM, Herzog B, Jünemann-Ramírez M, Zachary IC.. Neuropilin-1 mediates PDGF stimulation of vascular smooth muscle cell migration and signalling via p130Cas. Biochem J 2011;435:609–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Evans IM, Yamaji M, Britton G, Pellet-Many C, Lockie C, Zachary IC, Frankel P.. Neuropilin-1 signaling through p130Cas tyrosine phosphorylation is essential for growth factor-dependent migration of glioma and endothelial cells. Mol Cell Biol 2011;31:1174–1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Brugnera E, Haney L, Grimsley C, Lu M, Walk SF, Tosello-Trampont AC, Macara IG, Madhani H, Fink GR, Ravichandran KS.. Unconventional Rac-GEF activity is mediated through the Dock180-ELMO complex. Nat Cell Biol 2002;4:574–582. [DOI] [PubMed] [Google Scholar]
- 6. Gumienny TL, Brugnera E, Tosello-Trampont AC, Kinchen JM, Haney LB, Nishiwaki K, Walk SF, Nemergut ME, Macara IG, Francis R, Schedl T, Qin Y, Van Aelst L, Hengartner MO, Ravichandran KS.. CED-12/ELMO, a novel member of the CrkII/Dock180/Rac pathway, is required for phagocytosis and cell migration. Cell 2001;107:27–41. [DOI] [PubMed] [Google Scholar]
- 7. Klemke RL, Leng J, Molander R, Brooks PC, Vuori K, Cheresh DA.. CAS/Crk coupling serves as a "molecular switch" for induction of cell migration. J Cell Biol 1998;140:961–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Honda H, Oda H, Nakamoto T, Honda Z, Sakai R, Suzuki T, Saito T, Nakamura K, Nakao K, Ishikawa T, Katsuki M, Yazaki Y, Hirai H.. Cardiovascular anomaly, impaired actin bundling and resistance to Src-induced transformation in mice lacking p130Cas. Nat Genet 1998;19:361–365. [DOI] [PubMed] [Google Scholar]
- 9. Tazaki T, Sasaki T, Uto K, Yamasaki N, Tashiro S, Sakai R, Tanaka M, Oda H, Honda Z, Honda H.. p130Cas, Crk-associated substrate plays essential roles in liver development by regulating sinusoidal endothelial cell fenestration. Hepatology 2010;52:1089–1099. [DOI] [PubMed] [Google Scholar]
- 10. Akimoto T, Okuhira K, Aizawa K, Wada S, Honda H, Fukubayashi T, Ushida T.. Skeletal muscle adaptation in response to mechanical stress in p130cas-/- mice. Am J Physiol Cell Physiol 2013;304:C541–C547. [DOI] [PubMed] [Google Scholar]
- 11. Riccomagno MM, Sun LO, Brady CM, Alexandropoulos K, Seo S, Kurokawa M, Kolodkin AL.. Cas adaptor proteins organize the retinal ganglion cell layer downstream of integrin signaling. Neuron 2014;81:779–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nagai Y, Osawa K, Fukushima H, Tamura Y, Aoki K, Ohya K, Yasuda H, Hikiji H, Takahashi M, Seta Y, Seo S, Kurokawa M, Kato S, Honda H, Nakamura I, Maki K, Jimi E.. p130Cas, Crk-associated substrate, plays important roles in osteoclastic bone resorption. J Bone Miner Res 2013;28:2449–2462. [DOI] [PubMed] [Google Scholar]
- 13. Kirby ML. Cardiac Development. New York: Oxford University Press; 2007. [Google Scholar]
- 14. Waldo K, Miyagawa-Tomita S, Kumiski D, Kirby ML.. Cardiac neural crest cells provide new insight into septation of the cardiac outflow tract: aortic sac to ventricular septal closure. Dev Biol 1998;196:129–144. [DOI] [PubMed] [Google Scholar]
- 15. Dyer LA, Kirby ML.. The role of secondary heart field in cardiac development. Dev Biol 2009;336:137–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Li J, Chen F, Epstein JA.. Neural crest expression of Cre recombinase directed by the proximal Pax3 promoter in transgenic mice. Genesis 2000;26:162–164. [DOI] [PubMed] [Google Scholar]
- 17. Xin HB, Deng KY, Rishniw M, Ji G, Kotlikoff MI.. Smooth muscle expression of Cre recombinase and eGFP in transgenic mice. Physiol Genomics 2002;10:211–215. [DOI] [PubMed] [Google Scholar]
- 18. Holtwick R, Gotthardt M, Skryabin B, Steinmetz M, Potthast R, Zetsche B, Hammer RE, Herz J, Kuhn M.. Smooth muscle-selective deletion of guanylyl cyclase-A prevents the acute but not chronic effects of ANP on blood pressure. Proc Natl Acad Sci USA 2002;99:7142–7147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kisanuki YY, Hammer RE, Miyazaki J, Williams SC, Richardson JA, Yanagisawa M.. Tie2-Cre transgenic mice: a new model for endothelial cell-lineage analysis in vivo. Dev Biol 2001;230:230–242. [DOI] [PubMed] [Google Scholar]
- 20. Verzi MP, McCulley DJ, De Val S, Dodou E, Black BL.. The right ventricle, outflow tract, and ventricular septum comprise a restricted expression domain within the secondary/anterior heart field. Dev Biol 2005;287:134–145. [DOI] [PubMed] [Google Scholar]
- 21. Soriano P. Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat Genet 1999;21:70–71. [DOI] [PubMed] [Google Scholar]
- 22. Moessler H, Mericskay M, Li Z, Nagl S, Paulin D, Small JV.. The SM 22 promoter directs tissue-specific expression in arterial but not in venous or visceral smooth muscle cells in transgenic mice. Development 1996;122:2415–2425. [DOI] [PubMed] [Google Scholar]
- 23. Li L, Miano JM, Mercer B, Olson EN.. Expression of the SM22alpha promoter in transgenic mice provides evidence for distinct transcriptional regulatory programs in vascular and visceral smooth muscle cells. J Cell Biol 1996;132:849–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Li L, Miano JM, Cserjesi P, Olson EN.. SM22 alpha, a marker of adult smooth muscle, is expressed in multiple myogenic lineages during embryogenesis. Circ Res 1996;78:188–195. [DOI] [PubMed] [Google Scholar]
- 25. Miano JM, Cserjesi P, Ligon KL, Periasamy M, Olson EN.. Smooth muscle myosin heavy chain exclusively marks the smooth muscle lineage during mouse embryogenesis. Circ Res 1994;75:803–812. [DOI] [PubMed] [Google Scholar]
- 26. Savolainen SM, Foley JF, Elmore SA.. Histology atlas of the developing mouse heart with emphasis on E11.5 to E18.5. Toxicol Pathol 2009;37:395–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zhang W, Chen H, Qu X, Chang CP, Shou W.. Molecular mechanism of ventricular trabeculation/compaction and the pathogenesis of the left ventricular noncompaction cardiomyopathy (LVNC). Am J Med Genet C Semin Med Genet 2013;163C:144–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Jacobs ML. Congenital heart surgery nomenclature and database project: truncus arteriosus. Ann Thorac Surg 2000;69:S50–S55. [DOI] [PubMed] [Google Scholar]
- 29. Fuchs S, Herzog D, Sumara G, Buchmann-Moller S, Civenni G, Wu X, Chrostek-Grashoff A, Suter U, Ricci R, Relvas JB, Brakebusch C, Sommer L.. Stage-specific control of neural crest stem cell proliferation by the small rho GTPases Cdc42 and Rac1. Cell Stem Cell 2009;4:236–247. [DOI] [PubMed] [Google Scholar]
- 30. Gamell C, Osses N, Bartrons R, Ruckle T, Camps M, Rosa JL, Ventura F.. BMP2 induction of actin cytoskeleton reorganization and cell migration requires PI3-kinase and Cdc42 activity. J Cell Sci 2008;121:3960–3970. [DOI] [PubMed] [Google Scholar]
- 31. Liu Y, Jin Y, Li J, Seto E, Kuo E, Yu W, Schwartz RJ, Blazo M, Zhang SL, Peng X.. Inactivation of Cdc42 in neural crest cells causes craniofacial and cardiovascular morphogenesis defects. Dev Biol 2013;383:239–252. [DOI] [PubMed] [Google Scholar]
- 32. Bondurand N, Dufour S, Pingault V.. News from the endothelin-3/EDNRB signaling pathway: role during enteric nervous system development and involvement in neural crest-associated disorders. Dev Biol 2018;444(Suppl. 1):S156–S169. [DOI] [PubMed] [Google Scholar]
- 33. Luo Y, High FA, Epstein JA, Radice GL.. N-cadherin is required for neural crest remodeling of the cardiac outflow tract. Dev Biol 2006;299:517–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wessels A, Sedmera D.. Developmental anatomy of the heart: a tale of mice and man. Physiol Genomics 2003;15:165–176. [DOI] [PubMed] [Google Scholar]
- 35. von Gise A, Pu WT.. Endocardial and epicardial epithelial to mesenchymal transitions in heart development and disease. Circ Res 2012;110:1628–1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Markwald RR, Fitzharris TP, Manasek FJ.. Structural development of endocardial cushions. Am J Anat 1977;148:85–119. [DOI] [PubMed] [Google Scholar]
- 37. Waldo KL, Lo CW, Kirby ML.. Connexin 43 expression reflects neural crest patterns during cardiovascular development. Dev Biol 1999;208:307–323. [DOI] [PubMed] [Google Scholar]
- 38. Chen S, Lechleider RJ.. Transforming growth factor-beta-induced differentiation of smooth muscle from a neural crest stem cell line. Circ Res 2004;94:1195–1202. [DOI] [PubMed] [Google Scholar]
- 39. Wurdak H, Ittner LM, Lang KS, Leveen P, Suter U, Fischer JA, Karlsson S, Born W, Sommer L.. Inactivation of TGFbeta signaling in neural crest stem cells leads to multiple defects reminiscent of DiGeorge syndrome. Genes Dev 2005;19:530–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Nieto MA, Sargent MG, Wilkinson DG, Cooke J.. Control of cell behavior during vertebrate development by Slug, a zinc finger gene. Science 1994;264:835–839. [DOI] [PubMed] [Google Scholar]
- 41. Hebrok M, Wertz K, Fuchtbauer EM.. M-twist is an inhibitor of muscle differentiation. Dev Biol 1994;165:537–544. [DOI] [PubMed] [Google Scholar]
- 42. Rohwedel J, Horak V, Hebrok M, Fuchtbauer EM, Wobus AM.. M-twist expression inhibits mouse embryonic stem cell-derived myogenic differentiation in vitro. Exp Cell Res 1995;220:92–100. [DOI] [PubMed] [Google Scholar]
- 43. Koutalianos D, Koutsoulidou A, Mastroyiannopoulos NP, Furling D, Phylactou LA.. MyoD transcription factor induces myogenesis by inhibiting Twist-1 through miR-206. J Cell Sci 2015;128:3631–3645. [DOI] [PubMed] [Google Scholar]
- 44. Bendall AJ, Abate-Shen C.. Roles for Msx and Dlx homeoproteins in vertebrate development. Gene 2000;247:17–31. [DOI] [PubMed] [Google Scholar]
- 45. Bendall AJ, Ding J, Hu G, Shen MM, Abate-Shen C.. Abate-Shen C. Msx1 antagonizes the myogenic activity of Pax3 in migrating limb muscle precursors. Development 1999;126:4965–4976. [DOI] [PubMed] [Google Scholar]
- 46. Woloshin P, Song K, Degnin C, Killary AM, Goldhamer DJ, Sassoon D, Thayer MJ.. MSX1 inhibits myoD expression in fibroblast x 10T1/2 cell hybrids. Cell 1995;82:611–620. [DOI] [PubMed] [Google Scholar]
- 47. Law SF, Zhang YZ, Fashena SJ, Toby G, Estojak J, Golemis EA.. Dimerization of the docking/adaptor protein HEF1 via a carboxy-terminal helix-loop-helix domain. Exp Cell Res 1999;252:224–235. [DOI] [PubMed] [Google Scholar]
- 48. Kim W, Kook S, Kim DJ, Teodorof C, Song WK.. The 31-kDa caspase-generated cleavage product of p130cas functions as a transcriptional repressor of E2A in apoptotic cells. J Biol Chem 2004;279:8333–8342. [DOI] [PubMed] [Google Scholar]
- 49. Kim W, Seok Kang Y, Soo Kim J, Shin N-Y, Hanks SK, Song WK, Song WK.. The integrin-coupled signaling adaptor p130Cas suppresses Smad3 function in transforming growth factor-β signaling. Mol Biol Cell 2008;19:2135–2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Shen D, Li J, Lepore JJ, Anderson TJ, Sinha S, Lin AY, Cheng L, Cohen ED, Roberts JD Jr, Dedhar S, Parmacek MS, Gerszten RE.. Aortic aneurysm generation in mice with targeted deletion of integrin-linked kinase in vascular smooth muscle cells. Circ Res 2011;109:616–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Reuter MS, Jobling R, Chaturvedi RR, Manshaei R, Costain G, Heung T, Curtis M, Hosseini SM, Liston E, Lowther C, Oechslin E, Sticht H, Thiruvahindrapuram B, Mil SV, Wald RM, Walker S, Marshall CR, Silversides CK, Scherer SW, Kim RH, Bassett AS.. Haploinsufficiency of vascular endothelial growth factor related signaling genes is associated with tetralogy of Fallot. Genet Med 2019;21:1001–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Evans IM, Kennedy SA, Paliashvili K, Santra T, Yamaji M, Lovering RC, Britton G, Frankel P, Kolch W, Zachary IC.. Vascular endothelial growth factor (VEGF) promotes assembly of the p130Cas interactome to drive endothelial chemotactic signaling and angiogenesis. Mol Cell Proteomics 2017;16:168–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Jin SC, Homsy J, Zaidi S, Lu Q, Morton S, DePalma SR, Zeng X, Qi H, Chang W, Sierant MC, Hung WC, Haider S, Zhang J, Knight J, Bjornson RD, Castaldi C, Tikhonoa IR, Bilguvar K, Mane SM, Sanders SJ, Mital S, Russell MW, Gaynor JW, Deanfield J, Giardini A, Porter GA Jr, Srivastava D, Lo CW, Shen Y, Watkins WS, Yandell M, Yost HJ, Tristani-Firouzi M, Newburger JW, Roberts AE, Kim R, Zhao H, Kaltman JR, Goldmuntz E, Chung WK, Seidman JG, Gelb BD, Seidman CE, Lifton RP, Brueckner M.. Contribution of rare inherited and de novo variants in 2,871 congenital heart disease probands. Nat Genet 2017;49:1593–1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article are available in the article and in its Supplementary material online.