Background:
Transdermal delivery is very important in pharmaceutics. However, the barrier function of the stratum corneum hinders drugs absorption. How to improve transdermal delivery efficiency is a hot topic. The key advantages of physical technologies are their wide application for the delivery of previously nonappropriate transdermal drugs, such as proteins, peptides, and hydrophilic drugs. Based on the improved permeation of drugs delivered via multiple physical techniques, many more diseases may be treated, and transdermal vaccinations become possible. However, their wider application depends on the related convenient and portable devices. Combined products comprising medicine and devices represent future commercial directions of artificial intelligence and 3D printing.
Methods:
A comprehensive search about transdermal delivery assisted by physical techniques has been carried out on Web of Science, EMBASE database, PubMed, Wanfang Database, China National Knowledge Infrastructure, and Cochrane Library. The search identified and retrieved the study describing multiple physical technologies to promote transdermal penetration.
Results:
Physical technologies, including microneedles, lasers, iontophoresis, sonophoresis, electroporation, magnetophoresis, and microwaves, are summarized and compared. The characteristics, mechanism, advantages and disadvantages of physical techniques are clarified. The individual or combined applicable examples of physical techniques to improve transdermal delivery are summarized.
Conclusion:
This review will provide more useful guidance for efficient transdermal delivery. More therapeutic agents by transdermal routes become possible with the assistance of various physical techniques.
Keywords: electroporation, iontophoresis, laser, magnetophoresis, microneedles, microwaves
1. Introduction
Transdermal delivery has clear advantages over injection and oral routes in terms of increasing patient compliance and avoiding first-pass metabolism. The skin, as one of the largest organs in the human body, has a surface area close to 1–2 m2 available for drug delivery. Moreover, the application of a patch-like device to the skin surface can be controlled manually, for example, by removal, replacement, or repositioning. The major advantages of a transdermal drug delivery system (TDDS) include:
Patients can self-administer at home;
The continuous release of the drug can ensure that the drug concentration in the body remains at a stable level;
Avoids first-pass hepatic metabolism and enzymatic degradation in the gastrointestinal tract and also avoids gastrointestinal irritation;
Less frequent dosing improves patient compliance;
Alternative to other routes of administration;
Dose delivery unaffected by vomiting or diarrhea;
The drug release can be stopped at any time by removing the TDDS.
Previously, TDDSs developed slowly because of the many limitations of drugs. Molecular weight (MW) is the major limiting factor. In general, drugs with an MW < 500 Da[1] are the best choice for transdermal permeability. However, increasing numbers of bioactive macromolecules have been developed or identified with the rapid development of genomics and biotechnology. Improving the transdermal permeability of bioactive macromolecules is important.[2]
2. Skin structure
TDDSs can achieve systemic therapeutic effects through topical administration on the skin. Consequently, it is critical to understand the barrier function, biochemical properties, and structure of skin, and the rate at which drugs enter the body through the skin.
The skin is generally divided into 3 layers: The stratum corneum (SC), the epidermis, and the dermis, which are penetrated by sweat glands and hair follicles. The SC is located on the outside and is composed of dead cells. Therefore, the epidermis without the SC is often referred to as the viable epidermis. The SC is the most important rate-limiting barrier for most molecules during transdermal penetration. From a dry to a wet state, the thickness of the SC swells from 10–15 to 40 μm. Keratin-rich corneocytes in the SC structure are often compared with bricks embedded in a lipid-rich matrix (the “mortar”). This SC arrangement has about 15–20 layers.
The epidermis is located on the dermis and is a superficial structure of the skin. The dermis mainly comprises fibroblasts, and the fibers and stroma they produce, and has nerves, lymphatic vessels, blood vessels, skin appendages, and other cellular components. The epidermal layer is located on the outermost layer of the skin and is the protective layer of the body skin, with an average thickness of about 0.1 mm, which can protect the skin from sun, microorganisms, and other external environments.[3,4] The extracellular matrix lipid is different in many ways.[5]
It is the only continuous phase (and diffusion pathway) from the skin surface to the SC base.
In contrast to other biofilms, it does not contain phospholipids.
SC lipids exhibit a multilayered sheet shape in the absence of polar bilayer-forming lipids.
Compared with the usual liquid crystalline membrane, the highly ordered, intersecting configuration, and gel-phase membrane domains are more favorable for its formation under conditions of saturated long-chain hydrocarbon tails.
3. Major Transdermal Routes
The multiple advantages of TDDSs have made them a research hot spot, and many commercial formulations are currently available (Table 1).
Table 1.
Drugs approved by FDA for transdermal administration.
| Drugs | Company | Indication | Approval year |
|---|---|---|---|
| Rivastigmine | Novartis | Dementia | 2007 |
| Rotigotine | UCB, Inc. | Parkinson disease | 2007 |
| Granisetron | Prostrakan, Inc. | Chemotherapy-induced nausea and vomiting | 2008 |
| Oxybutynin | Watson | Urinary incontinence | 2009 |
| Chloride | Allergan | Overactive bladder | 2009 |
| Buprenorphine | Purdue Pharma L. P | Pain and opioid dependence | 2010 |
| Scopolamine | Glaxosmithkline CON | Motion thickness | 1979 |
| Nitroglycerin | Hospira | Angina pectori | 1981 |
| Clonidine | Boehringer Ingelheim | Hype tension | 1984 |
| Estradio | Novartis | Menopausal symptoms | 1986 |
| Fentanyl | JANSSEN PHARMS | Chronic pain | 1990 |
| Nicotine | Sanofi-Aventis US | Smoking cessation | 1991 |
| Testosterone | Alza Pharmaceuticals | Testosterone deficiency | 1993 |
| Estradiol/norethisterone | Parke Davis | Menopausal symptoms | 1996 |
| Ethinylestradiol/norelgestromin | JANSSEN PHARMS | Contraception | 2001 |
| Estradiol/levonorgestrel | BAYER HLTHCARE | Menopausal symptoms | 2003 |
| Lidocaine with tetracaine | Local dermal analgesia | 2004 | |
| Methylphenidate | NOVEN PHARMS INC. | Hyperactivity disorder | 2006 |
| Selegiline | Somerset | Depressive disorder | 2006 |
| Diclofenac epolamine | Inst. Biochem. | Acute pain | 2007 |
| Capsaicin | Neuropathy pain | 2009 | |
| Influenza-virus vaccine | Influenza virus | 2011 |
FDA = Food and Drug Administration.
There are 3 critical methods by which drugs can pass through an intact SC: via the intercellular lipid domains, the skin appendages, or the transcellular route. The transdermal flux of a drug through a specific pathway is determined by the physicochemical properties of the molecule.[6]
3.1. The appendageal route
The transdermal routes include permeation through the sweat glands and across the hair follicles with their associated sebaceous glands. Skin appendages are an important continuous channel across the SC barrier. The opening diameter and follicular number volume are important in drug delivery. The forehead, as the follicular infundibula, occupies 13.7 mm2/cm2 (approximately 0.1%) of SC of forearm skin.[5]
3.2. Transcellular route
Keratinocytes containing highly hydrated keratin can increase the permeability of hydrophilic drugs that cross the skin through the transcellular route. The transcellular pathway requires that drugs can distribute and diffuse into the keratin bricks and the intercellular lipids.
3.3. Intercellular route
The intercellular route is a challenging pathway because it requires the drug to diffuse through the continuous lipid matrix.
Compared with the relatively direct pathway of the transcellular pathway, the interdigitating nature of corneocytes results in a tortuous pathway for intercellular drug permeation.
Drugs need to be sequentially assigned to the water and lipid domains of the intercellular domain and repeatedly diffused through them. This approach is only suitable for transdermal delivery of uncharged small molecules.
4. Physical Techniques To Improve Transdermal Delivery
There are a number of solutions to overcome the barrier function of the SC, such as chemical enhancers, novel formulations, and physical improvement techniques. Chemical penetrators have been developed to improve drug dermal penetration, such as azone[7] and cyclodextrins.[8] However, besides their limited transdermal penetration enhancement efficiency, they are also associated with side effects, such as rash, irritation, and hypersensitivity. Thus, novel formulations have been developed to improve transdermal absorption, including nanoparticles,[9] liposomes,[10,11] transferosomes,[12] microemulsion,[13,14] hydrogels,[15,16] invasomes,[17] and ethomes.[18]
Physical penetration technologies have developed quickly along with the emergence of technologies, such as microelectromechanical systems, artificial intelligence, microneedles (MNs),[19,20] sonophoresis,[21,22] iontophoresis,[23,24] electroporation (EP),[25,26] microwave,[27] magnetophoresis,[28] and lasers.[29] They can change the skin surface structure directly and reversibly to enhance transdermal absorption.[30–32] Moreover, these technologies are safe and efficient, show high bioavailability, and have been used widely, making them a better choice to promote the transdermal absorption of drugs (Table 2).[33]
Table 2.
Companies developing and commercializing skin permeability technologies.
| Technologies | Company | Product | Mechanism |
|---|---|---|---|
| Low-frequency sonophoresis/Sensing | Echo Therapeutics, Inc. | Symphony Continuous | Glucose monitoring by interstitial fluid after skin |
| Glucose Monitor | Permeability | ||
| Microneedles | Becton Dickinson/Sanofi Pasteur | Intanza | Prefillable injection system utilizing the microneedle for delivery of vaccine against seasonal influenza |
| Corium International, Inc. | MicroCor | Dissolvable microneedle device | |
| NanoPassTechnologies, Ltd. | MicronJet | Hollow microneedle device | |
| Seventh Sense Biosystems, Inc | TAP 20C | Microneedle penetration of the skin followed by application of vacuum for blood extraction | |
| TheraJect | TheraJect Patch | Dissolvable microneedle device | |
| Valeritas, Inc. | Micro-Trans | Microneedle device | |
| Vaxxas, Inc. | The Nanopatch | Vaccine-coated microneedle device | |
| ZosanoPharma | ZP Patch | Drug-coated microneedle patch | |
| Electrical techniques | Ichor Medical Systems | TriGrid Delivery System | Electroporation platform for vaccination |
| Inovio Pharmaceuticals, Inc. | Cellectr | Electroporation platform for vaccination | |
| NuPathe, Inc. | Zecuit | Iontophoresis-facilitated delivery of sumatriptan for acute treatment of migraines | |
| OncoSec Medical, Inc. | OncoSec Medical System | Electroporation platform for vaccination | |
| NB Therapeutics | Iontophoresis Platform | Iontophoresis-facilitated delivery of terbinafine HCl for the treatment of toenail fungus |
4.1. Microneedles
MNs are commonly used to minimally disrupt the outermost layer of the skin to enhance transdermal drug delivery. MN devices rely on a large number of micron-level needles to penetrate the epidermal layer of the skin to achieve skin penetration. MNs can be divided into hollow and solid MNs according to their structure (Fig. 1). According to the type of materials, MNs can also be divided into metal and soluble polymer MNs. MNs promote transdermal drug penetration and accumulation in specific sites by piercing into the skin to provide permeation channels.[34] MNs are suitable for many drugs, including macromolecules, such as peptides, proteins, small interfering RNAs, oligonucleotides, and vaccines. However, the disadvantages of MNs are that they can deliver only a limited drug dose, and they have insufficient mechanical strength. Further research is required to overcome these disadvantages.
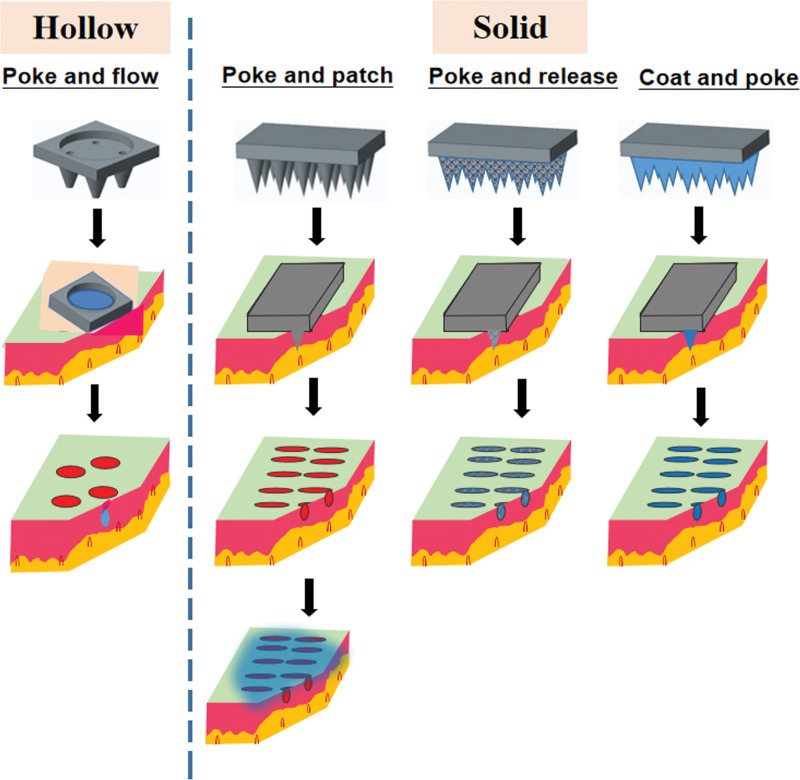
Figure 1.
Schematic illustration of hollow and solid microneedles.
Hollow MNs pierce the skin and release the drug solution through the internal pores, which is similar to subcutaneous injection.[35] Hollow MNs bypass the SC to directly deposit the drug into the live epidermis or dermis in a manner that is particularly suitable for high MW drugs.[36] Hollow MNs consist of a catheter structure, particularly for a fluid or liquid formulation, driven by the external pressure, which can be adjusted to vary the drug delivery speed.[37]
Solid MNs are commonly used in the “poke, separate, and diffuse” format and can be applied on the skin surface to destroy the SC physically. Removal of solid MNs from the skin results in a brief microchannel in the SC, after which the pharmaceutical preparation can be applied, such as solution, suspension, gel, cream, or patch. The delivery of drug formulations penetrating into those microchannels mainly depends on passive diffusion. Systemic delivery or local effects of therapeutics depend on absorption first and then on transportation. Solid MNs can increase the transdermal permeability of nanoparticles by up to 4 times.[38]
MN rollers are also a method of improving drug delivery efficiency. An MN roller is a cylindrical stainless-steel MN that is rolled over the surface of the skin to create micropores. It could improve transdermal delivery of antiallergic drugs through simulation experiments using pig skin. Commercial formulations combined with MNs are a relatively mature technology.
The hydrogel MNs containing donepezil hydrochloride were prepared and applied to pig skin. At 24 hours after the MNs were removed, donepezil hydrochloride had a higher in vitro permeability and plasma concentration, demonstrating its success delivery.[39]
During drug/vaccine loading, the bioinspired use of biomimetic methods can help orient fluid transport. This design will benefit the drug/vaccine loading process on the needle surface by improving efficiency and reducing drug/vaccine waste during the MN coating process.[40]
Capsaicin-loaded dissolving MNs (DMNs) were prepared to investigate the analgesic effect of capsaicin on skin. The dimensions of each MN were as follows: diameter of the base, 17 mm; length, 500 μm; and width, 300 μm. The average capsaicin content in the DMNs loaded with a low and high dose of capsaicin was 8.8 ± 0.5 and 12.5 ± 0.4 mg, respectively. Almost all the capsaicin, 99.3 ± 4.1% and 99.7 ± 2.2% for low-dose and high-dose DMNs, respectively, were released within 20 minutes. The pharmacological activity of capsaicin DMNs was compared with that of capsaicin cream as a positive control, by measuring the idiospasm of depilated rat skin. The time required to achieve 50% idiospasm suppression was 26.3 ± 1.9 and 53.0 ± 2.3 minutes for low-dose and high-dose DMNs, respectively. The different loading of capsaicin leads to the difference in the time to suppress spasticity. A pharmacokinetic study showed high tissue capsaicin levels of 660.2 ± 120.6 and 1805.3 ± 218.1 μg/g wet weight for low-dose and high-dose DMNs, respectively, at 5 minutes after administration. The results suggested that DMNs could exert a rapid local analgesic action on the skin.[41]
Compared with the traditional H1N1 influenza vaccine microneedles with stabilizers, the stabilizer-free H1N1 microneedles prepared in a 10 °C production environment induced the obvious protective immune response. In animal experiments, mice showed a strong antibody response with an array of low-temperature H1N1 microneedles without a stabilizer (LT-MN). Compared with the traditional intramuscular immunization, low-temperature H1N1 microneedles with a stabilizer (LT-MN-T), and room-temperature H1N1 microneedles with a stabilizer (RT-MN-T), LT-MN produced comparable results in inducing protective immunity. A plaque reduction neutralization test found that LT-MN and LT-MN-T provided greater immunity compared with intramuscular immunization and RT-MN-T. This stabilizer-free low temperature H1N1 microneedles are suitable for a variety of temperature-sensitive biological agents.[42]
4.2. Lasers
Lasers are an important way to increase transdermal permeability (Fig. 2). Lasers can ablate the SC and thus enhance drug delivery. Laser-induced skin damage recovers quickly after several days. Lasers can directly damage the skin barrier through the photothermal effect to increase the transdermal absorption of drugs. This method can increase bioavailability and shorten the treatment period.[43] The transdermal effects of various drugs can be enhanced using lasers.[44,45] Despite the low energy output of the lasers used, laser safety remains a controversial issue.[46] However, one of the problems that limits laser application is that specialized laser equipment is very large and not appropriate for personal use.
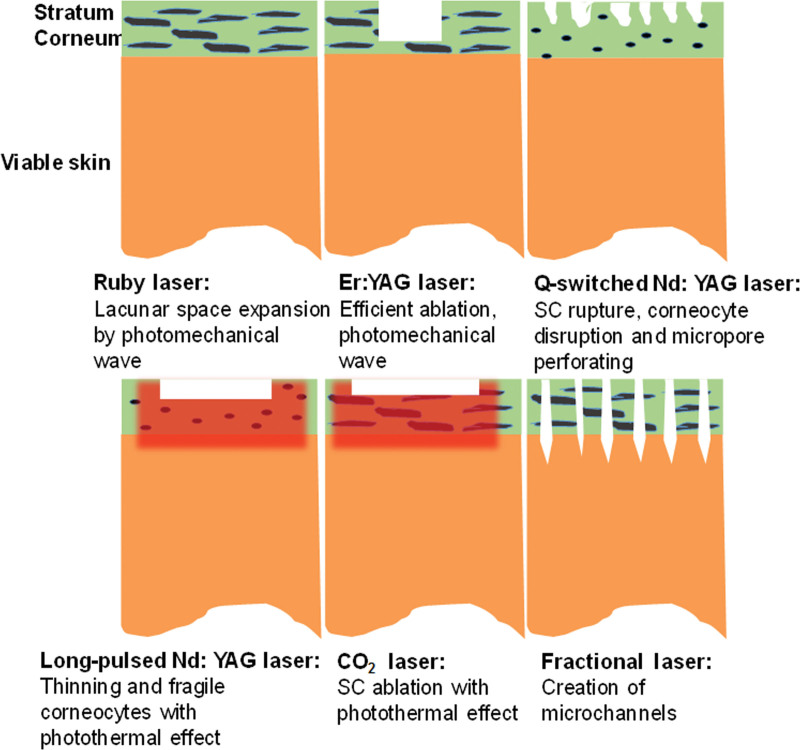
Figure 2.
Overview of the different mechanisms of laser-facilitated drug delivery. SC = stratum corneum.
Laser irradiation can interact with skin tissues, such as SC, epidermis, and dermis. Skin is a semipermeable barrier that protects the body from external damage and prevents moisture loss.[47] Laser has not only reduced the topical dose required but also allows drugs to penetrate deeper into the soft tissues.
Different types of lasers have different wavelengths and mechanisms, including ruby (694 nm), yttrium-scandium-gallium-garnet (2790 nm), erbium: yttrium-gallium-garnet (Er:YAG, 2940 nm), neodymium-doped yttrium-gallium-garnet (Nd:YAG, 355, 532, 1064, and 1320 nm), and CO2 (10600 nm) lasers.
The ruby laser emits laser light at 694 nm and is used in clinical dermatology to treat freckles, pigmented skin lesions, macula, and chloasma.[48] This laser can produce optomechanical waves that facilitate local/transdermal drug administration. The application of optomechanical waves occurs in a very short time of 10 ns-1 μs. The permeabilized photomechanical wave on the SC is temporary and returns to normal after a few minutes of irradiation.[49] The penetration of photomechanical wave-assisted dextran (5 J/cm2, 490 ns) reached 400 μm into the rat dermis. Extensive and sustained distribution of dextran in live skin indicated that the delivery route was intercellular or intracellular. Another study investigated the application of a single photomechanical wave from ruby laser to rat skin for the local delivery of nanoparticles of about 100 nm. Exposure of skin to the photomechanical wave allowed the nanoparticles to diffuse into the epidermis. This demonstrated that the photomechanical wave can promote the local delivery of very large permeates.[43]
An Er:YAG laser emits light with a wavelength of 2940 nm and is used as a surface repair tool for microdermabrasion, in which the emitted light is effectively absorbed by water molecules. Its shallow radiation into the skin can precisely ablate the SC to reduce thermal damage. Er:YAG lasers used for ablation have a shorter healing time, induce less erythema, and cause fewer pigmentation problems.[50]
The use of an Nd:YAG laser is another resurfacing technique with skin ablation ability that facilitates drug delivery. Nd:YAG lasers are effective for dermatological treatment and cosmetic regeneration in clinical applications, such as pigmentation, scar repair, hair removal, skin remodeling, and lipolysis.[51] Nd:YAG lasers with a long pulse (LP, 15 J/cm2) and Q-switched (0.5 J/cm2) lasers were evaluated for their ability to improve glycerol transdermal delivery in rats.[52] Lasers delivering an LP could attenuate SC barriers, which may be adversely affected by SC interruption caused by the photothermal effects. The QS laser destroyed the SC, possibly because of its high energy output and the enormous pressure in the ultrashort range. This enhancement effect may last for at least 1 week without infection.
CO2 lasers have an emission wavelength of 10,600 nm. They produce instantaneous heating of water and cause tissue vaporization. The residual thermal effect causes coagulated skin and necrosis. CO2 lasers usually ablate the SC, and the skin thickness is thinner than that resulting from ER:YAG treatment. In addition, the thermal effect can rejuvenate the skin for a cosmetic effect after optimizing the viable laser energy. Transdermal delivery of 2 vitamin C derivatives, 3-O-ethyl ascorbic acid and ascorbic acid 2-glucoside, with or without Er:YAG and CO2 lasers, was compared. The results showed that the permeability of the 2 vitamin C derivatives pretreated with Er: YAG or CO2 lasers increased markedly.[53]
Three vitamin C derivatives were used as model drugs, and the transdermal ability of 2 Er:YAG and CO2 lasers was compared. The results showed that the transdermal volume of all 3 drugs increased by several tens of times. This showed that these 2 lasers have a significant effect on increasing the transdermal absorption of drugs.[54]
Studies have shown that 1064–nm Nd:YAG lasers with an LP (15 J/cm2) can loosen keratin and make the keratinocytes brittle or detached, whereas the output mode of Q-switched (0.5 J/cm2) completely destroys keratin or keratin cells, forming perforations on the SC. It was concluded that the degree of enhanced transdermal delivery is different for different output modes.[55]
Currently, nanotherapy combined with stimulation-activated activators has become a focus of research attention. For example, Fe3O4 nanoparticles were used as the core of a drug-loaded hydrogel coating, and folic acid was grafted onto the surface of the composite material to construct laser-sensitive magnetic nanoparticles. This method could not only perform targeted therapy but also improved the transdermal absorption of the drug, thereby improving the effect of the drug to treat the disease.[56]
During a scalp test using a fractional Er:YAG laser, it was found that the transmitted pulse energy might affect the size and/or depth of the microchannels created by the laser, and the duration of the pulse might affect the amount of thermally changed tissue. This meant that the tissue ablation threshold was lowered with shorter pulse durations and higher power. For smaller molecules, transdermal delivery efficiency is increased by increasing the size of the microchannels created by the laser and can partition easily into the skin when less thermal damage occurs. In comparison, the transportation of larger molecules through the skin becomes increasingly difficult.[44]
4.3. Iontophoresis
Iontophoresis is the application of a small electrical current (< 5 mA/cm2) to increase the transdermal penetration of a drug, especially the dissociation of drugs into or through the skin.[57] The transdermal enhancement mechanism of iontophoresis depends on electric current, which leads to differences of potential and concentration.[58]
The advantages of iontophoresis-assisted transdermal delivery include easy application, safety, high transdermal efficiency, and miniaturized instruments. Limitations include the limited application range and longer operation time. If iontophoresis could be applied for peptides, proteins, and other macromolecules, they would have a promising and wider application.
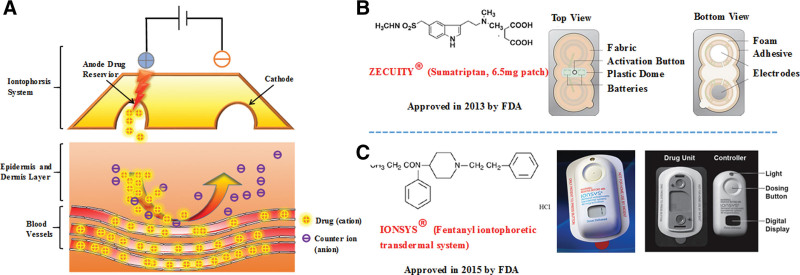
Currently, there are 2 commercial formulations composed of iontophoresis (Fig. 3). The sumatriptan iontophoresis system was approved by Food and Drug Administration (FDA) for the acute treatment of adult migraine in 2013. The transdermal system delivers sumatriptan with a low level of current and bypasses the gastrointestinal tract. Pharmacokinetic studies indicated that iontophoretic delivery of sumatriptan reached a maximum serum concentration of 22 ng/mL at 15 minutes after activation. A linear relationship was found between the total applied current and the sumatriptan delivery ratio. Patches with 6 and 12 mA per hour produced a favorable plasma level of sumatriptan that exceeded 10 ng/mL for more than 7 hours. In addition, patients with the sumatriptan patch had a painless rate of 18% at 2 hours after administration with iontophoresis compared with only 9% (P = 0.0092) for the placebo. Oral sumatriptan may cause severe nausea or vomiting for migraine patients. This sumatriptan transdermal system may be a very good choice to avoid such adverse events.[59,60]
Figure 3.
Iontophoresis for transdermal permeation. (A) The principle of iontophoresis using an Ag/AgCl electrode system. (B), (C) Commercial formulations using iontophoresis. A− = anionic drugs, Cl− = chloride ions in the drug reservoir, D+ = cationic drugs, FDA = Food and Drug Administration, Na+ = sodium ions in the drug reservoir.
Estradiol-loaded nanoparticles combined with iontophoresis can significantly increase the permeability of estradiol, which is advantageous for the treatment of osteoporosis. Iontophoresis facilitated the permeation of nanoparticles through follicles into capillary vessel and finally reached the effective blood concentration. The bone mineral density with iontophoresis was significantly higher than that of the passive diffusion and the control groups.[61]
Postoperative pain is usually controlled by patient-controlled analgesia.[62] The use of noninvasive transdermal analgesics (such as lidocaine, fentanyl, and morphine) overcomes the problems associated with patient-controlled analgesia. For example, iontophoresis can drive fentanyl molecules through the skin into the systemic circulation.[63] The fentanyl iontophoretic transdermal system is generally effective in the well-tolerated acute postoperative management of hospitalized patients with severe pain in the abdomen and chest, or in orthopedics. Overall, fentanyl iontophoretic transdermal system provides the same analgesic effect compared to morphine but is considered more convenient and easier for patients to use.[64]
Transdermal enhancement by iontophoresis on the penetration of anti-inflammation drugs was studied. Iontophoresis could disrupt the arrangement of lipids between cells and increase their fluidity. Moreover, the intercellular space is enlarged, and the SC structure is loosened, which promotes transdermal administration. In addition, the degree of drug dissociation has a significant effect on the enhancement by iontophoresis. The higher the degree of drug dissociation, the more obvious the transdermal enhancement effect.[65] The effects of various technologies on the transdermal delivery of 3-fluoroamphetamine hydrochloride were compared, and the results showed that iontophoresis had the most significant effect in increasing the transdermal effect of the drug. Thus, iontophoresis was considered superior to the other transdermal enhancement techniques.[66]
Experiments have shown that neurotensin-loaded silk fibroin films not only release neurotensin quickly to regulate inflammation after being stimulated by iontophoresis but also that anode iontophoresis has a certain inhibitory effect on gram-positive bacteria. Thus, stimulation of peptide fibrin membranes by iontophoresis has broad prospects for treating wounds.[67] A study used the prodrug ketoprofen choline chloride as a model drug for nonsteroidal anti-inflammatory drugs. After treatment with iontophoresis, the transdermal delivery rate of ketoprofen choline chloride could be increased by several times.[68] Another study has proved that the permeability of nanoparticles treated with iontophoresis is higher than that of pure nanoparticles.[69]
4.4. Sonophoresis
Sonophoresis is a physical technique that uses ultrasound (US) to promote transdermal absorption. The advantages of sonophoresis include its noninvasive nature, little damage to the skin, simple operation, high universality, and suitability for almost all drugs. However, sonophoresis requires an ultrasonic coupling agent, commonly hydrogels, to transmit US. Therefore, active chemical agents could be added to the ultrasonic coupling agent, which could achieve physical therapy and transdermal drug delivery simultaneously.
Current research suggests that the mechanism of increased transdermal delivery by sonophoresis mainly includes a cavitation effect, thermal effect, and mechanical effect.
4.4.1. Cavitation effect.
When the ultrasonic wave propagates in the medium, the average distance of the molecules varies with the molecular vibration. When it exceeds the critical molecular distance, a cavitation effect is formed.[70] Cavitation can instantly produce high temperature and pressure, accompanied by strong shock wave or ray flow, resulting in disorder of the lipid bilayer structure in the SC. The cavitation effect is considered as the main mechanism by which sonophoresis facilitates transdermal drug delivery.
4.4.2. Thermal effect.
The thermal effect refers to the phenomenon that the medium absorbs energy that is attenuated by the ultrasonic wave in the propagation process, which causes increased temperature.[71] The higher the temperature, the higher the molecular diffusion rate. Therefore, rates of blood flow and drug dissolution are accelerated. This is advantageous for drug permeation.
4.4.3. Mechanical effects.
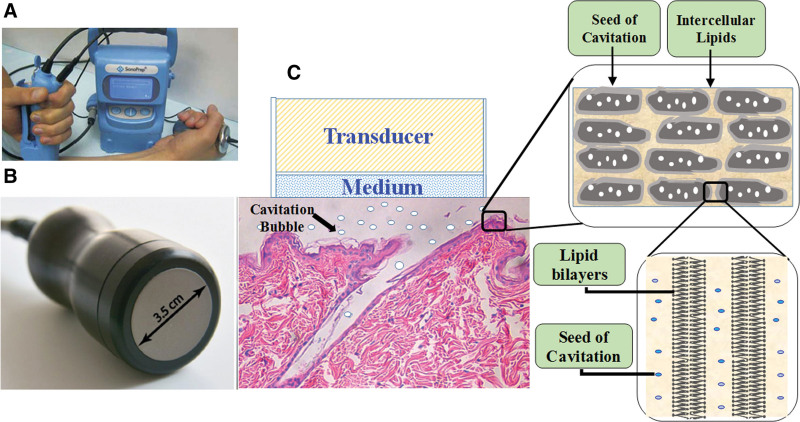
US can transfer energy through mechanical vibration. During high-speed ultrasonic vibration, the structure of the lipid layer in the SC changes, and its permeability increases. When the mechanical intensity is high enough, pores in the cell membrane form, and thus, the permeability of the drug increases. This effect is also called the “acoustic pore effect”[72] (Fig. 4).
Figure 4.
Sonophoresis for transdermal permeation. (A) Low-frequency ultrasound treatment in a clinical setting with the SonoPrep® device (Echo Therapeutics, Franklin, MA). (B) Ultrasound transducer, 3.5-cm diameter. (C) Mechanistic overview of sonophoresis-facilitated drug delivery
It is generally believed that frequencies of 20–100 kHz comprise low-frequency sonophoresis (LFS), and frequencies of 0.7–16 MHz comprise high-frequency sonophoresis (HFS).[73] According to previous research, LFS seems to be more effective in increasing skin permeability and delivering a wide range of molecules and formulations, such as proteins, biopolymers, and vaccines, compared with that of HFS.[74–76]
Transient cavitation is the most important mechanism by which LFS enhances skin permeability. The enhanced skin permeability by cavitation activity on the skin surface was evaluated using porous resin as a cavitation nuclear coupling medium.[77] The microjets produced by LFS could penetrate into the skin or collapsed near the skin surface to cause structure disorders. In addition, the collision of the cavitation foam on the surface had an important effect on enhanced skin permeability.
Cavities may play an important role in transdermal delivery by HFS.[78] Histological examination revealed that voids are formed in the skin after an HFS of 10 and 16 MHz. The voids in the keratinocytes led to direct interaction with the oscillating cavitation bubbles, which induced a disordered lipid bilayer and increased skin permeability.
Some studies have used ketoprofen as a model drug and found that both poly(amidoamine) dendrimers and US treatment could increase the transdermal absorption of the drug, but combining the 2 further improved the transdermal ability of ketoprofen.[79,80] Experiments have compared the transdermal effects of dual-frequency US (20 kHz + 1 MHz) and single-frequency US (20 kHz), and found that dual-frequency US could expand the pores to increase the permeability of the drug to a greater extent, while reducing the treatment time, without causing increased damage to the skin.[81] Over a decade ago, LFS was combined with chemical penetration enhancers (CPE) for the first time and was found to have a synergistic effect in increasing the permeability of the skin to drugs.[82]
4.5. Electroporation
EP is a technique used to increase the permeability of the cell membrane. Nowadays, it is used to promote drug transdermal or transmucosal absorption. EP uses an instantaneous high-voltage pulsed electric field to form a temporary, reversible hydrophilic channel in a lipid bilayer of the cell membrane. The size and duration of the channels are related to the voltage, pulse number, and pulse time.[83] Many studies have indirectly proposed the formation of “holes” or “water channels” formed via EP. These holes are small (<10 nm), temporary, and sparse (about 0.1% of the surface area).
The advantages of EP are its versatility. First, it can strictly control the transdermal permeation rate. Second, the application range of EP is wide, being especially suitable for biomacromolecules delivery, such as peptides, proteins, and vaccines. Consequently, there is no selectivity for drugs and the other therapeutic agents through skin using EP.
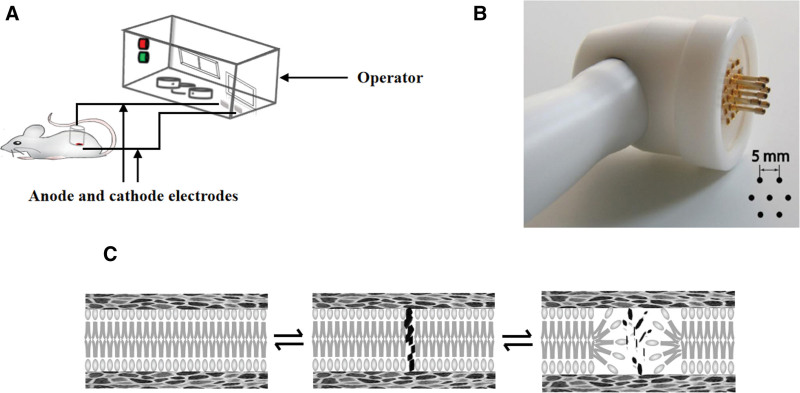
Transdermal EP occurs when the skin is exposed to high electric field pulses, also known as electroosmosis.[84] EP is a physical transfer mechanism that applies electrical impulses directly to cells or tissues, which transiently permeabilizes the plasma membrane, increasing the permeability of mammalian cell membranes and allowing macromolecules to enter cells, thereby effectively delivering exogenous molecules.[85] According to the hole formation theory of water thermodynamics, water molecules penetrate into the lipid bilayer of the membrane, resulting in rearrangement of adjacent lipids and their polar head groups. The EP mechanisms include the following steps. First, a complete phospholipid bilayer before the EP is required. Second, because of the interaction between the water dipoles and the electric field after EP, changes in the ultrastructure of SC lipids cause water molecules to penetrate the bilayer to form a water filament, and the ingress of water increases the likelihood of pore formation, resulting in the formation of many pores per unit area and per unit time in the phospholipid bilayer. Finally, the lipids adjacent to the water begin to be realigned with their polar heads, stabilizing the pores and allowing more water and other polar molecules to enter (Fig. 5).
Figure 5.
Electroporation for transdermal permeation. (A) The components of an electroporation device. (B) Electroporation pin electrodes arranged in a honeycomb configuration. (C) Mechanistic overview of electroporation facilitated drug delivery.
Low-dose cisplatin delivered by EP was evaluated as a new treatment strategy for osteosarcoma on 2 osteosarcoma cell lines.[86] The results indicated that the metabolic regulators delivered by EP were more effective in inhibiting the cell cycle of osteosarcoma cells and had a negative impact on their ability to recover and proliferate.[86]
Arthritis in the right knee joint of rats was established to evaluate the anti-inflammatory and analgesic effects of diclofenac sodium hydrogel delivered via EP. EP was applied using a high-voltage pulse of 900 V for 8 minutes with 5 ms on and 20 ms off. The results indicated that EP significantly increased the plasma concentration of diclofenac. The therapeutic benefit provided by EP was comparable with that of oral diclofenac.[87]
Cisplatin EP toxicity toward human pancreatic ductal adenocarcinoma cell lungs in vitro showed that cisplatin EP has a higher cytostatic effect than cisplatin alone.[88] Studies have shown that EP and reverse iontophoresis have synergistic effects in promoting skin penetration.[89]
A new EP method that could effectively deliver DNA and small interfering RNA was proposed. Nucleic acid molecules could be delivered through mouse skin by combining MNs and flexible interdigitated electroporation arrays. This technology has laid the foundation for dermatological or intradermal vaccination.[90]
4.6. Magnetophoresis
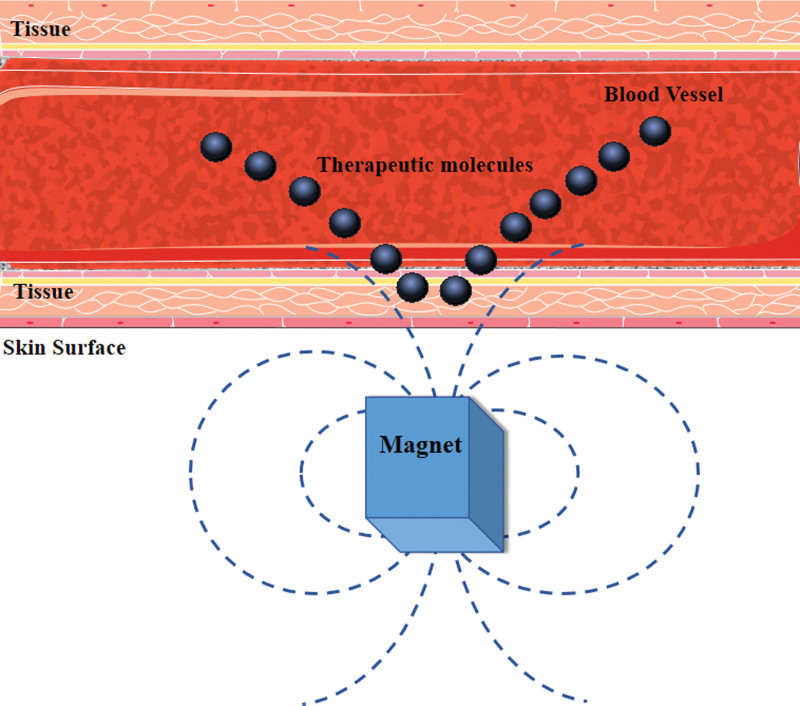
There are few studies of magnetophoresis in transdermal delivery. Magnetophoresis works by applying a magnetic field to enhance the passage of drugs across a biological barrier. The stronger the magnetic field, the better the transdermal effect (Fig. 6).[91] The fixed magnetic field could increase permeation flux enhancement factor, and the alternating magnetic field could enhance drug penetration. Both of the combined use has a massage-like effect on skin. The fluorescence signal was detected in deeper regions of the skin with a larger area with the combination application of the stationary and the alternating magnetic fields.[92]
Figure 6.
Schematic representation of a magnetic nanoparticle-based drug delivery system. The steps for magnetic NP-based drug delivery are: Coupling the drug to the MNP; applying an external magnetic field to attract the MNP to the desired location, such as a tumor; and the release of the drugs from the NP under the influence of an external alternating magnetic field once it reaches the target. MNP = magnetic nanoparticle, NP = nanoparticle.
4.7. Microwave-assisted transdermal delivery
In recent years, microwave technology has received widespread attention in the transdermal field. Microwave technology promotes transdermal drug delivery by allowing the lipid structure of the SC to enter the nondomain.[28,93] In addition, microwaves have a certain synergistic effect with CPEs.[94]
A study combined chitosan nanoparticles with microwave technology. Microwave and chitosan nanoparticles affected the ceramide, palmitic acid, and keratin domains of the skin, thus achieving synergistic promotion of transdermal administration.[95]
Microwaves are considered one of the most promising transdermal delivery technologies. However, further research is needed to explore their value in the medical field.
4.8. Combined technologies to improve topical delivery
4.8.1. Iontophoresis and chemical enhancers.
Both CPEs and iontophoresis have good transdermal promotion effects. Thus, experiments have combined both techniques, with lidocaine HCl, nicotine hydrogen tartrate, and diltiazem HCl as model drugs. The in vitro percutaneous treatment of each drug in pig skin and buccal tissue was compared, together with enhanced buccal delivery. The results demonstrated that the enhancement of transdermal delivery by iontophoresis was more pronounced than the enhancement achieved by buccal delivery of the drugs.[96]
4.8.2. Lasers and microneedles.
There is an urgent need to substitute noninvasive or minimally invasive delivery methods for the injections currently used for low MW heparin multidose therapy. In a study, a laser-engineered dissolution MN (DMN) array was fabricated. This technique does not reduce the strength of the MN or the biological activity of the drug. This method is not only a relatively low-cost functional delivery system but also has great potential for the transdermal delivery of large molecules.[97] A study showed that silica-coated lanthanum hexaboride (LaB6 @ SiO2) nanostructures could be incorporated into polycaprolactone MNs as infrared absorbers. Under infrared light, the light and heat generated by the infrared absorbers were transmitted to cause the MNs to melt, thereby releasing the drug. This near-infrared, light-triggerable device has excellent reproducibility, low state leakage, and noninvasive trigger ability, and thus represents an advance in transdermal drug delivery technology.[98]
4.8.3. Microneedles with electroporation and sonophoresis.
MNs, EP, and US therapy are 3 transdermal methods for physically enhancing drug delivery. Some studies have used fluorescein isothiocyanate-dextran as a model drug and studied combinations of the 3 technologies (i.e., microneedle–electroporation [MN-EP], microneedle–ultrasound, electroporation-ultrasound [EP-US], and microneedle–electroporation–ultrasound [MN-EP-US]) for skin penetration in vitro. The experimental results showed that the combination of all 3 technologies (MN-EP-US) caused the most significant increase in the permeability of fluorescein isothiocyanate-dextran.[99] The permeability of bovine serum albumin increased to 1 μm/s with the microneedles patch combined with 15-W US output, which is about 10 times higher than the that of the passive diffusion. This technique provides the possibility of transdermal absorption of large molecules with high efficiency.[100]
4.8.4. 3D printing.
3D printing technology appeared in the mid-1990s. First, a blueprint is drawn by a computer according to demand; then, the “printing material” is superimposed layer by layer using technologies such as light curing and paper lamination under computer control. Finally, the blueprint on the computer becomes a physical object. Here, we review 3D printing in transdermal drug delivery.
As a technology precisely controlled by a computer, 3D printing can print a transdermal patch that perfectly fits the human skin according to the precise and complete contours of the human body, which could increase the transdermal delivery rate of the drug by improving its bioavailability. Researchers obtained a 3D computer-aided design model of human volunteers’ heads and faces through a handheld 3D scanner and then produced a proprietary nasal patch using 3D printing.[101] The use of specific nose patches that fitted the patient’s skin perfectly provided a more accurate dose of the medicine.
One of the important applications of 3D printing in transdermal delivery is MN fabrication, which can create micropores in the skin through a large number of micron-sized needle tips, thereby allowing macromolecular drugs to penetrate the skin, such as insulin[102] and melanostatin.[103] MNs on a personalized curved surface meant that each MN is perpendicular to the skin surface and can fully insert into the skin. The stereolithography 3D-printing technique was used to fabricate the hollow MNs interfaced with the microfluidic structures. Compared with the previous methods, the method was with lower cost, higher printing speed, and higher throughput.[104]
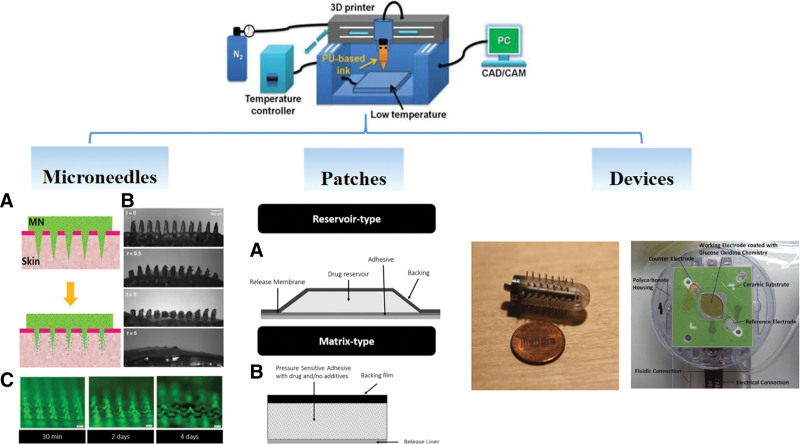
3D printing allows rapid optimization of materials and changes to the geometry to fabricate personalized MNs. In addition, the advantages of 3D printing in transdermal delivery are represented by personalized electrical devices (Fig. 7).
Figure 7.
3D printing in transdermal delivery. CAD = computer-aided design, CAM = computer aided manufacturing, MN = microneedle, PC = personal computer.
5. Conclusion
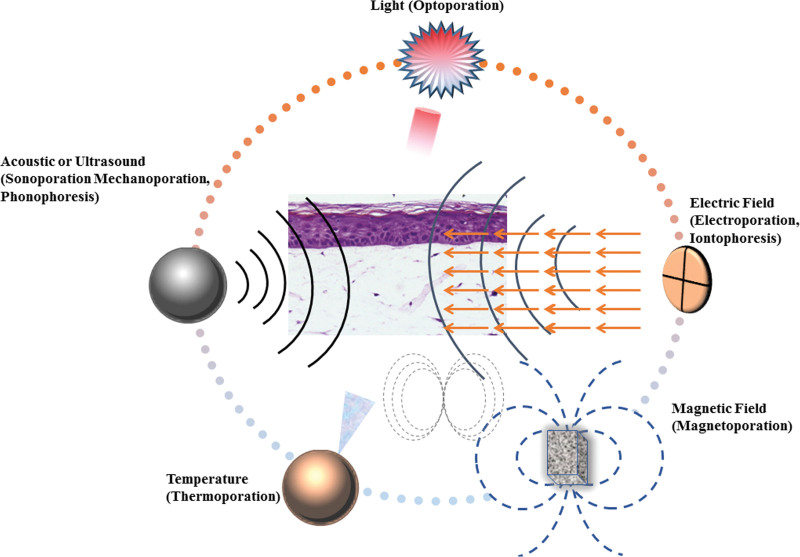
TDDSs have many advantages; therefore, they have attracted more and more attention in the field of medicine, especially from pharmaceutical companies. There are more than 20 FDA-approved transdermal drugs according to the published reports. Most of these drugs have been delivered via other administration routes before being used for transdermal drug delivery, emphasizing the growing interest in transdermal administration. Most drugs currently approved for transdermal administration rely on passive administration or moderate levels of permeability. However, the multiple physical techniques may be highly effective to improve transdermal efficiency (Fig. 8). Applying these technologies to the preparation of miniaturized wearable electronic devices has broad commercial prospects. The advantages and disadvantages of different physical technologies are diverse. Overall, the properties of a drug determine which is the best method to improve its transdermal efficiency, such as MW, pKa, and logP.
Figure 8.
Different physical techniques to improve transdermal delivery.
About half of the currently available commercial products have the potential to be tested as a vaccine, and many vaccines are currently undergoing clinical trials for various incurable diseases, such as various cancers and HIV. Some transdermal techniques can also be applied to vaccination of any disease, and as research continues, the goal of vaccine self-management could be achieved. The use of live vectors that can trigger an immune response in current viral vaccines for injections has limited the ability to immunize to some extent. However, the use of vaccines that apply EP for DNA is now feasible. This has the potential to achieve immunization against various cancers and viruses. MN technology also has potential in this respect. These attractive features will continue to attract research and commercial interest.
Electrical-based drug delivery may be more restricted. Like LFS, they require electrical input and are more complex and expensive compared with MN technology, making them more difficult to implement than MN. In addition, during treatment, both EP and iontophoresis can cause discomfort or pain to the patient, especially iontophoresis. Finally, it may take several hours for the drug to reach the effective site, which makes the kinetics of drug delivery clinically meaningless. Until these devices are further optimized, the combination of the 2 methods will only complicate the treatment plan.
The key advantages of physical technologies are their wide application for previously nonappropriate transdermal drugs, such as proteins, peptides, and hydrophilic drugs. Based on the improved permeation of drugs via multiple physical techniques, many more diseases and conditions may be treated, such as tumors, dementia, Parkinson’s disease, chemotherapy-related nausea/vomiting, urinary incontinence, pain and opioid dependence, contraception, hypertension, and motion sickness. The selection depends on the advantages, disadvantages, and characteristics of the specialized permeation technologies (Table 3).
Table 3.
Comparison of different physical technologies used for drug delivery.
| Physical energy | Electric field | Magnetic field | Temperature | Ultrasound | Light |
|---|---|---|---|---|---|
| Poration | Electroporation | MNP | Thermoporation | Sonoporation | Optoporation |
| Limitations | Narrow range of clinically safe electric field parameters (refer to current standards for safety levels) | Limited drug carrying capacity of magnetic field due to their biodistribution | Low penetration depth (since only applied topically so far) | Sonoporation devices have poor calibration in terms of the amount of ultrasound energy emitted | Limited time duration between optoporation and drug delivery |
| Narrow range of magnetic field | |||||
| Disadvantages | Irreversible electroporation, cell death with high fields | Aggregation of MNP can cause embolization | Excess heat can induce thermohemolysis | Shear forces may induce rupture of cells | Excessive inflammation, postinflammatory, and hyperpigmentation |
| Electromechanical coupling effect | Cytotoxicity increases with the increased concentrations of MNP | Relies on electric field to heat up the filaments; therefore, the disadvantages of electric field applies | Temperature increases as a function of frequency and eventually disrupts cells | Laser usage and ultrastructural changes in epidermis | |
| Advantages | Inexpensive and simple to perform | Noninvasive nature of the magnetic field | Noninvasive nature of low heat compared to EP | Less invasive compared to EP | Remote operation with less cellular damage |
| Drugs are easy to overcome the cell membrane barrier | Field modulated externally without electrode contacts unlike EP | Selective irreparable cellular damage | Instant impermeabilization after ultrasound exposure | Enhanced optofection efficiency compared with regular gene delivery | |
| High efficiency of drugs delivery compared with that without the magnetic field | Deep penetration; key nanosurgical tool to the microscopist |
EP = electroporation, MNP = magnetoporation.
However, the extensive application of the physical technologies combined with therapeutic agents is dependent on the related convenient and portable devices. Most of the traditional transdermal technologies are assisted by equipment. In addition to improving the equipment, more new technologies such as MNs should be explored. In the development of the device, while blindly increasing the permeability of the drug, it should be considered that the doctor and patient can use it together and take advantage of transdermal drug delivery.
Combined products comprising medicine and devices are future commercial directions associated with artificial intelligence and 3D printing. Further research should break through the limitations of traditional transdermal drugs and explore more drug potential for transdermal absorption.
As a newly developed drug delivery system, the TDDS has its unique advantages over other drug delivery methods. However, there is still much room for development and implementation of TDDSs. Research directed at unmet clinical needs will be particularly promoted by the benefits provided by specific methods, which will drive innovation. In view of its more humanized drug treatment characteristics and the continuous development of transdermal technology, it will have broader prospects in the future.
Author contribution
Conceptualization: Yan Gao, Lina Du, Shan Ma, Xiu Wang, Bonian Zhao.
Data curation: Yan Gao, Qian Li, Qi Li.
Formal analysis: Yan Gao, Qian Li, Lin Zhu.
Investigation: Yan Gao, Lina Du.
Methodology: Yan Gao, Qian Li, Lina Du
Project administration: Meiyan Yang.
Software: Lina Du, Shan Ma, Xiu Wang.
Writing - original draft: Yan Gao, Qian Li, Lin Zhu.
Writing - review and editing: Yan Gao, Qian Li, Qi Li, Lina Du
Acknowledgments
We are thankful for the Key Technologies for Quality Identification and Control of Traditional Chinese Medicine Formula Granules and their Industrial Application (2021CXGC010511), and Bengbu city and Bengbu Medical College Jointed Science and Technology Key Projects (BYLK201823).
Abbreviations:
- CPE =
- chemical penetration enhancers
- DMNs =
- capsaicin-loaded dissolving microneedles
- EP =
- electroporation
- Er:YAG =
- Erbium: yttrium-gallium-garnet
- FD-4 =
- fluorescein isothiocyanate-dextran
- HFS =
- high-frequency sonophoresis
- KCC =
- ketoprofen choline chloride
- LFS =
- low-frequency sonophoresis
- LP =
- long pulse
- MN =
- microneedle,
- MW =
- molecular weight
- Nd:YAG =
- neodymium-doped yttrium-gallium-garnet
- NT =
- neurotensin
- SC =
- stratum corneum
- TDDS =
- transdermal drug delivery system
- US =
- ultrasound
The authors have no funding and conflicts of interest to disclose.
How to cite this article: Gao Y, Li Q, Li Q, Zhu L, Yang M, Wang X, Ma S, Zhao B, Du L. How physical techniques improve the transdermal permeation of therapeutics: A review. Medicine 2022;101:26(e29314).
Yan Gao and Lina Du contributed equally.
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
Funding acquisition: The Key Technologies for Quality Identification and Control of Traditional Chinese Medicine Formula Granules and their Industrial Application (2021CXGC010511) and Bengbu city and Bengbu Medical College Jointed Science and Technology Key Projects (BYLK201823).
Contributor Information
Yan Gao, Email: gaoyaningyes@163.com.
Lina Du, Email: dulina@188.com.
Qian Li, Email: qili1029@163.com.
Qi Li, Email: qili1029@163.com.
Lin Zhu, Email: ajolina@yeah.net.
Meiyan Yang, Email: ymyzi@163.com.
Xiu Wang, Email: bbwx1016@163.com.
Bonian Zhao, Email: bonianzh@163.com.
References
- [1].Bos JD, Meinardi MM. The 500 Dalton rule for the skin penetration of chemical compounds and drugs. Exp Dermatol. 2000;9:165–9. [DOI] [PubMed] [Google Scholar]
- [2].Münch S, Wohlrab J, Neubert RHH. Dermal and transdermal delivery of pharmaceutically relevant macromolecules. Eur J Pharm Biopharm. 2017;119:5–42. [DOI] [PubMed] [Google Scholar]
- [3].Naik A, Kalia YN, Guy RH. Transdermal drug delivery: overcoming the skin’s barrier function. Pharm Sci Technol Today. 2000;3:318–26. [DOI] [PubMed] [Google Scholar]
- [4].Ita K. Transdermal drug delivery: progress and challenges. J Drug Delivery Sci Technol. 2014;24:245–50. [Google Scholar]
- [5].Rastogi V, Yadav P. Transdermal drug delivery system: an overview. Asian J Pharm. 2012;6:161–70. [Google Scholar]
- [6].Singhal M, Lapteva M, Kalia YN. Formulation challenges for 21st century topical and transdermal delivery systems. Expert Opin on Drug Deliv. 2017;14:705–8. [DOI] [PubMed] [Google Scholar]
- [7].Lane ME. Skin penetration enhancers. Int J Pharm. 2013;447:12–21. [DOI] [PubMed] [Google Scholar]
- [8].Sridevi S, Diwan P VR. Optimized transdermal delivery of ketoprofen using pH and hydroxypropyl-b-cyclodextrin as co-enhancers. Eur J Pharm Biopharm. 2002;54:1–4. [DOI] [PubMed] [Google Scholar]
- [9].Wiraja C, Zhu Y, Lio LDS, et al. Framework nucleic acids as programmable carrier for transdermal drug delivery. Nat Commun. 2019;10:1147–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Madhavia N, Sudhakara B, Reddyb S, et al. Design by optimization and comparative evaluation of vesicular gels of etodolac for transdermal delivery. Drug Dev Ind Pharm. 2019;45:611–28. [DOI] [PubMed] [Google Scholar]
- [11].Franzè S, Musazzi U M, Minghetti P, et al. Drug-in-micelles-in-liposomes (DiMiL) systems as a novel approach to prevent drug leakage from deformable liposomes. Eur J Pharm Sci. 2019;130:27–35. [DOI] [PubMed] [Google Scholar]
- [12].Shamshiri MK, Momtazi-Borojeni AA, Shahraky MK, et al. Lecithin soybean phospholipid nano-transfersomes as potential carriers for transdermal delivery of the human growth hormone. J Cell Biochem. 2019;120:9023–33. [DOI] [PubMed] [Google Scholar]
- [13].Sintov AC. Transdermal delivery of curcumin via microemulsion. Int J Pharm. 2015;481:7–103. [DOI] [PubMed] [Google Scholar]
- [14].Zhang J, Michniak-Kohn B. Investigation of microemulsion microstructures and their relationship to transdermal permeation of model drugs: ketoprofen, lidocaine, and caffeine. Int J Pharm. 2011;421:4–44. [DOI] [PubMed] [Google Scholar]
- [15].Sun Y, Du L, Liu Y, et al. Transdermal delivery of the in situ hydrogels of curcumin and its inclusion complexes of hydroxypropyl-b-cyclodextrin for melanoma treatment. Int J Pharm. 2014;469:1–9. [DOI] [PubMed] [Google Scholar]
- [16].Lin R-Z, Chen Y-C, Moreno-Luna R, et al. Transdermal regulation of vascular network bioengineering using a photopolymerizable methacrylated gelatin hydrogel. Biomaterials. 2013;34:5–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Ahmed OAA, Badr-Eldin SM. Development of an optimized avanafil-loaded invasomal transdermal film: ex vivo skin permeation and in vivo evaluation. Int J Pharm. 2019;570:118657. [DOI] [PubMed] [Google Scholar]
- [18].Niu X-Q, Zhang D-P, Bian Q, et al. Mechanism investigation of ethosomes transdermal permeation. Int J Pharm. 2019;X1:100027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Ita K. Dissolving microneedles for transdermal drug delivery: advances and challenges. Biomed Pharmacother. 2017;93:6–27. [DOI] [PubMed] [Google Scholar]
- [20].Wang M, Hu L, Xu C. Recent advances in the design of polymeric microneedles for transdermal drug delivery and biosensing. Lab Chip. 2017;17:1373–87. [DOI] [PubMed] [Google Scholar]
- [21].Park J, Lee H, Lim G-S, et al. Enhanced transdermal drug delivery by sonophoresis and simultaneous application of sonophoresis and iontophoresis. AAPS PharmSciTech. 2019;20:96–103. [DOI] [PubMed] [Google Scholar]
- [22].Nguyen HX, Banga AK. Electrically and ultrasonically enhanced transdermal delivery of methotrexate. Pharmaceutics. 2018;10:117–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Tokumoto S, Higo N, Todo H, et al. Effect of combination of low-frequency sonophoresis or electroporation with iontophoresis on the mannitol flux or electroosmosis through excised skin. Biol Pharm Bull. 2016;39:1206–10. [DOI] [PubMed] [Google Scholar]
- [24].Liu K-C, Green CR, Alany RG, et al. Synergistic effect of chemical penetration enhancer and iontophoresis on transappendageal transport of oligodeoxynucleotides. Int J Pharm. 2013;441:687–92. [DOI] [PubMed] [Google Scholar]
- [25].Prausnitz M, Langer R. Transdermal drug delivery. Nat Biotechnol. 2008;26:1261–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Rizwan M, Aqil M, Talegaonkar S, et al. Enhanced transdermal drug delivery techniques: an extensive review of patents. Recent Pat Drug Deliv Formul. 2009;3:105–24. [DOI] [PubMed] [Google Scholar]
- [27].Nawaz A, Wong TW. Chitosan-carboxymethyl-5-fluorouracil-folate conjugate particles: microwave modulated uptake by skin and melanoma cells. J Invest Dermatol. 2018;138:2412–22. [DOI] [PubMed] [Google Scholar]
- [28].Wong TW. Electrical, magnetic, photomechanical and cavitational waves to overcome skin barrier for transdermal drug delivery. J Control Release. 2014;193:257–69. [DOI] [PubMed] [Google Scholar]
- [29].Wenande E, Tam J, Bhayana B, et al. Laser-assisted delivery of synergistic combination chemotherapy in in vivo skin. J Control Release. 2018;275:242–53. [DOI] [PubMed] [Google Scholar]
- [30].Ulashchik VS. The physical and chemical properties of the skin and the action of therapeutic physical factors. Vopr Kurortol Fizioter Lech Fiz Kult. 2018;95:4–13. [DOI] [PubMed] [Google Scholar]
- [31].Mitragotri S. Devices for overcoming biological barriers: the use of physical forces to disrupt the barriers. Adv Drug Deliv Rev. 2013;65:100–3. [DOI] [PubMed] [Google Scholar]
- [32].Puri A, Murnane KS, Blough BE, et al. Effects of chemical and physical enhancement techniques on transdermal delivery of 3-fluoroamphetamine hydrochloride. Int J Pharm. 2017;528:452–62. [DOI] [PubMed] [Google Scholar]
- [33].Benson H, Namjoshi S. Proteins and peptides: strategies for delivery to and across the skin. J Pharm Sci. 2008;97:3591–610. [DOI] [PubMed] [Google Scholar]
- [34].Jain AK, Lee CH, Gill HS. 5-aminolevulinic acid coated microneedles for photodynamic therapy of skin tumors. J Control Release. 2016; 239:72–81. [DOI] [PubMed] [Google Scholar]
- [35].Niu L, Chu LY, Burton SA, et al. Intradermal delivery of vaccine nanoparticles using hollow microneedle array generates enhanced and balanced immune response. J Control Release. 2019;294:8–78. [DOI] [PubMed] [Google Scholar]
- [36].Mansoor I, Lai J, Ranamukhaarachchi S, et al. A microneedle-based method for the characterization of diffusion in skin tissue using doxorubicin as a model drug. Biomed Microdevices. 2015;17:9967. [DOI] [PubMed] [Google Scholar]
- [37].Chen J, Cheng P, Sun Y, et al. A minimally invasive hollow microneedle with a cladding structure: ultra-thin but strong, batch manufacturable. IEEE Trans Biomed Eng. 2019;66:3480–5. [DOI] [PubMed] [Google Scholar]
- [38].Mcallister DV, Wang PM, Davis SP, et al. Microfabricated needles for transdermal delivery of macromolecules and nanoparticles: fabrication methods and transport studies. Proc Natl Acad Sci USA. 2003;100:13755–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Kearney M-C, Caffarel-Salvador E, Fallows SJ, et al. Microneedle-mediated delivery of donepezil: Potential for improved treatment options in Alzheimer’s disease. Eur J Pharm Biopharm. 2016;103:3–50. [DOI] [PubMed] [Google Scholar]
- [40].Plamadeala C, Gosain SR, Hischen F, et al. Bio-inspired microneedle design for efficient drug/vaccine coating. Biomed Microdevices. 2020;22:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Ito Y, Kobuchi S, Inoue G, et al. Dissolving microneedles for enhanced local delivery of capsaicin to rat skin tissue. J Drug Target. 2017;25:420–4. [DOI] [PubMed] [Google Scholar]
- [42].Jeong H-R, Park S, Park J-H, et al. Preparation of H1N1 microneedles by a low-temperature process without a stabilizer. Eur J Pharm Biopharm. 2019;143:1–7. [DOI] [PubMed] [Google Scholar]
- [43].Lin C-H, Aljuffali IA, Fang J-Y. Lasers as an approach for promoting drug delivery via skin. Expert Opin Drug Deliv. 2014;11:599–614. [DOI] [PubMed] [Google Scholar]
- [44].Zorec B, Škrabelj D, Marincek M, et al. The effect of pulse duration, power and energy of fractional Er:YAG laser for transdermal delivery of differently sized FITC dextrans. Int J Pharm. 2017;516:204–13. [DOI] [PubMed] [Google Scholar]
- [45].Lee W-R, Hsiao C-Y, Huang T-H, et al. Post-irradiation recovery time strongly influences fractional laser-facilitated skin absorption. Int J Pharm. 2019;564:48–58. [DOI] [PubMed] [Google Scholar]
- [46].Hsiao CY, Sung HC, Hu S, et al. Fractional carbon dioxide laser treatment to enhance skin permeation of ascorbic acid 2-glucoside with minimal skin disruption. Dermatology. 2015;230:9–75. [DOI] [PubMed] [Google Scholar]
- [47].Sadick NS, Cardona A. Laser treatment for facial acne scars: a review. J Cosmet Laser Ther. 2018;20:424–35. [DOI] [PubMed] [Google Scholar]
- [48].Raulin C, Schönermark MP, Greve B, et al. Q-switched ruby laser treatment of tattoos and benign pigmented skin lesions: a critical review. Ann Plast Surg. 1998;41:555–65. [DOI] [PubMed] [Google Scholar]
- [49].Lee S, Mcaulffe DJ, Kollias N, et al. Permeabilization and recovery of the stratum corneum in vivo: the synergy of photomechanical waves and sodium lauryl sulfate. Lasers Surg Med. 2001;29:145–50. [DOI] [PubMed] [Google Scholar]
- [50].Kauvar ANB. Successful treatment of melasma using a combination of microdermabrasion and Q-switched Nd:YAG lasers. Lasers Surg Med. 2012;44:117–24. [DOI] [PubMed] [Google Scholar]
- [51].Alexiades M. Randomized, double-blind, split-face study evaluating fractional ablative erbium:YAG laser-mediated trans-epidermal delivery of cosmetic actives and a novel acoustic pressure wave ultrasound technology for the treatment of skin aging, melasma, and acne scars. J Drugs Dermatol. 2015;14:1191–8. [PubMed] [Google Scholar]
- [52].Liu C, Zhang J, Yue Y, et al. 1064 nm-Nd:YAG lasers with different output modes enhancing transdermal delivery: physical and physiological mechanisms. J Biomed Opt. 2013;18:61228. [DOI] [PubMed] [Google Scholar]
- [53].Hsiao C-Y, Huang C-H, Hu S, et al. Skin pretreatment with lasers promotes the transdermal delivery of vitamin C derivatives. Lasers Med Sci. 2011;26:369–76. [DOI] [PubMed] [Google Scholar]
- [54].Huang C-H, Sung H-C, Hsiao C-Y, et al. Transdermal delivery of three vitamin C derivatives by Er:YAG and carbon dioxide laser pretreatment. Lasers Med Sci. 2013;28:7–14. [DOI] [PubMed] [Google Scholar]
- [55].Liu C, Zhang J, Yue Y, et al. 1064 nm-Nd:YAG lasers with different output modes enhancing transdermal delivery: physical and physiological mechanisms. J Biomed Opt. 2013;18:061228. [DOI] [PubMed] [Google Scholar]
- [56].Zhang L-K, Du S, Wang X, et al. Bacterial cellulose based composites enhanced transdermal drug targeting for breast cancer treatment. Chem Eng J. 2019;370:749–59. [Google Scholar]
- [57].Du L, Liu X, Jia J, et al. Improvement effect of iontophoresis on transdermal permeation of ferulic acid. J Int Pharm Res. 2014;41:595–7. [Google Scholar]
- [58].Malinovskaja-Gomez K, Labouta HI, Schneider M. Transdermal iontophoresis of flufenamic acid loaded PLGA nanoparticles. Eur J Pharm Sci. 2016;89:4–62. [DOI] [PubMed] [Google Scholar]
- [59].Vikelis M, Mitsikostas DD, Rapoport AM. Sumatriptan iontophoretic transdermal system for the acute treatment of migraine. Pain Manag. 2014;4:123–8. [DOI] [PubMed] [Google Scholar]
- [60].Vikelis M, Mitsikostas DD, Rapoport AM. Sumatriptan transdermal iontophoretic patch (NP101-Zelrix™): review of pharmacology, clinical efficacy, and safety in the acute treatment of migraine. Neuropsychiatr Dis Treat. 2012;8:9–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Takeuchi I, Fukuda K, Kobayashi S, et al. Transdermal delivery of estradiol-loaded PLGA nanoparticles using iontophoresis for treatment of osteoporosis. BioMed Mater Eng. 2016;27:475–83. [DOI] [PubMed] [Google Scholar]
- [62].Abrolat M, Eberhart LHJ, Kalmus G, et al. Patient-controlled Analgesia (PCA): An overview about methods, handling and new modalities. Anasthesiol Intensivmed Notfallmed Schmerzther. 2018;53:270–80. [DOI] [PubMed] [Google Scholar]
- [63].Power I. Fentanyl HCl iontophoretic transdermal system (ITS): clinical application of iontophoretic technology in the management of acute postoperative pain. Br J Anaesth. 2007;98:4–11. [DOI] [PubMed] [Google Scholar]
- [64].Grond S, Radbruch L, Lehmann KA. Clinical pharmacokinetics of transdermal opioids: focus on transdermal fentanyl. Clin Pharmacokinet. 2000;38:59–89. [DOI] [PubMed] [Google Scholar]
- [65].Zuo J, Du L, Li M, et al. Transdermal enhancement effect and mechanism of iontophoresis for non-steroidal anti-inflammatory drugs. Int J Pharm. 2014;466:6–82. [DOI] [PubMed] [Google Scholar]
- [66].Puri A, Murnane KS, Blough BE, et al. Effects of chemical and physical enhancement techniques on transdermal delivery of 3-fluoroamphetamine hydrochloride. Int J Pharm. 2017;528:2–62. [DOI] [PubMed] [Google Scholar]
- [67].Lemos CN, Cubayachi C, Dias K, et al. Iontophoresis-stimulated silk fibroin films as a peptide delivery system for wound healing. Eur J Pharm Biopharm. 2018;128:7–55. [DOI] [PubMed] [Google Scholar]
- [68].Lobo S, Yan G. Improving the direct penetration into tissues underneath the skin with iontophoresis delivery of a ketoprofen cationic prodrug. Int J Pharm. 2018;535:8–36. [DOI] [PubMed] [Google Scholar]
- [69].Tomoda K, Terashima H, Suzuki K, et al. Enhanced transdermal delivery of indomethacin using combination of PLGA nanoparticles and iontophoresis in vivo. Colloids Surf B. 2012;92:0–4. [DOI] [PubMed] [Google Scholar]
- [70].Ma P, Chen X, Chen W, et al. Effect of physical techniques on in vitro transdermal permeability of sinapine thiocyanate. Int J Pharm Res. 2017;44:432–6. [Google Scholar]
- [71].Ahmadi F, Mcloughlin IV, Chauhan S. Bio-effects and safety of low-intensity, low-frequency ultrasonic exposure. Prog Biophys Mol Biol. 2012;108:119–38. [DOI] [PubMed] [Google Scholar]
- [72].Cock ID, Zagato E, Braeckmans K. Ultrasound and microbubble mediated drug delivery: acoustic pressure as determinant for uptake via membrane pores or endocytosis. J Control Release. 2015;197:20–8. [DOI] [PubMed] [Google Scholar]
- [73].Zorec B, Jelenc J, Miklavcic D, et al. Ultrasound and electric pulses for transdermal drug delivery enhancement: ex vivo assessment of methods with in vivo oriented experimental protocols. Int J Pharm. 2015;490:5–73. [DOI] [PubMed] [Google Scholar]
- [74].Park D, Park H, Seo J, et al. Sonophoresis in transdermal drug deliveries. Ultrasonics. 2014;54:56–65. [DOI] [PubMed] [Google Scholar]
- [75].Polat BE, Blankschtein D, Langer R. Low-frequency sonophoresis: application to the transdermal delivery of macromolecules and hydrophilic drugs. Expert Opin Drug Deliv. 2010;7:5–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Smith NB. Perspectives on transdermal ultrasound mediated drug delivery. Int J Nanomed. 2007;2:585–94. [PMC free article] [PubMed] [Google Scholar]
- [77].Terahara T, Mitragotri S, Langer R. Porous resins as a cavitation enhancer for low-frequency sonophoresis. J Pharm Sci. 2002;91:753–9. [DOI] [PubMed] [Google Scholar]
- [78].Rich KT, Hoerig CL, Rao MB, et al. Relations between acoustic cavitation and skin resistance during intermediate- and high-frequency sonophoresis. J Control Release. 2014;194:6–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Manikkath J, Manikkath A, Shavi GV, et al. Low frequency ultrasound and PAMAM dendrimer facilitated transdermal delivery of ketoprofen. J Drug Deliv Sci Tech. 2017;41:334–43. [Google Scholar]
- [80].Manikkath J, Hegde AR, Kalthur G, et al. Influence of peptide dendrimers and sonophoresis on the transdermal delivery of ketoprofen. Int J Pharm. 2017;521:0–9. [DOI] [PubMed] [Google Scholar]
- [81].Schoellhammer CM, Srinivasan S, Barman R, et al. Applicability and safety of dual-frequency ultrasonic treatment for the transdermal delivery of drugs. J Control Release. 2015;202:93–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Polat BE, Deen WM, Langer R, et al. A physical mechanism to explain the delivery of chemical penetration enhancers into skin during transdermal sonophoresis - insight into the observed synergism. J Control Release. 2012;158:250–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Denet AR, Vanbever R, Préat V. Skin electroporation for transdermal and topical delivery. Adv Drug Deliv Rev. 2004;56:659–74. [DOI] [PubMed] [Google Scholar]
- [84].Prausnitz MR, Corbett JD, Gimm JA, et al. Millisecond measurement of transport during and after an electroporation pulse. Biophys J. 1995;68:1864–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Jakutavičiūtė M, Ruzgys P, Tamošiūnas M, et al. Physical methods for drug and gene delivery through the cell plasma membrane. Adv Anat Embryol Cell Biol. 2017;227:73–92. [DOI] [PubMed] [Google Scholar]
- [86].Gill KS, Fernandes P, Bird B, et al. Combination of electroporation delivered metabolic modulators with low-dose chemotherapy in osteosarcoma. Oncotarget. 2018;9:31473–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Hartmann P, Butt E, Fehér A, et al. Electroporation-enhanced transdermal diclofenac sodium delivery into the knee joint in a rat model of acute arthritis. Drug Des Devel Ther. 2018;12:7–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Michel O, Kulbacka J, Saczko J, et al. Electroporation with cisplatin against metastatic pancreatic cancer: in vitro study on human primary cell culture. Biomed Res Int. 2018;2018:7364539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Lee C-K, Ching CT, Sun T-P, et al. Non-invasive and transdermal measurement of blood uric acid level in human by electroporation and reverse iontophoresis. Int J Nanomedicine. 2010;5:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Huang D, Zhao D, Wang X, et al. Efficient delivery of nucleic acid molecules into skin by combined use of microneedle roller and flexible interdigitated electroporation array. Theranostics. 2018;8:2361–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Murthy SN, Sammeta SM, Bowers C. Magnetophoresis for enhancing transdermal drug delivery: mechanistic studies and patch design. J Control Release. 2010;148:197–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Chen A-Z, Chen L-Q, Wang S-B, et al. Study of magnetic silk fibroin nanoparticles for massage-like transdermal drug delivery. Int J Nanomedicine. 2015;10:4639–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Wong TW, Anuar NK. Physicochemical modulation of skin barrier by microwave for transdermal drug delivery. Pharm Res. 2013;30:90–103. [DOI] [PubMed] [Google Scholar]
- [94].Wong T. Microwave technology enabled transdermal nanocarrier and drug delivery. Asian J Pharm Sci. 2016;11:3–4. [Google Scholar]
- [95].Nawaz A, Wong TW. Microwave as skin permeation enhancer for transdermal drug delivery of chitosan-5-fluorouracil nanoparticles. Carbohydr Polym. 2017;157:6–19. [DOI] [PubMed] [Google Scholar]
- [96].Hu L, Silva S MC, Damaj BB, et al. Transdermal and transbuccal drug delivery systems: enhancement using iontophoretic and chemical approaches. Int J Pharm. 2011;421:3–62. [DOI] [PubMed] [Google Scholar]
- [97].Gomaa YA, Garland MJ, Mcinnes F, et al. Laser-engineered dissolving microneedles for active transdermal delivery of nadroparin calcium. Eur J Pharm Biopharm. 2012;82:9–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Chen M-C, Ling M-H, Wang K-W, et al. Near-infrared light-responsive composite microneedles for on-demand transdermal drug delivery. Biomacromolecules. 2015;16:8–607. [DOI] [PubMed] [Google Scholar]
- [99].Petchsangsai M, Rojanarata T, Opanasopit P, et al. The combination of microneedles with electroporation and sonophoresis to enhance hydrophilic macromolecule skin penetration. Biol Pharm Bull. 2014;37:1373–82. [DOI] [PubMed] [Google Scholar]
- [100].Han T, Das DB. Permeability enhancement for transdermal delivery of large molecule using low-frequency sonophoresis combined with microneedles. J Pharm Sci. 2013;102:3614–22. [DOI] [PubMed] [Google Scholar]
- [101].Goyanes A, Det-Amornrat U, Wang J, et al. 3D scanning and 3D printing as innovative technologies for fabricating personalized topical drug delivery system. J Control Release. 2016;234:41–8. [DOI] [PubMed] [Google Scholar]
- [102].Ling MH, Chen MC. Dissolving polymer microneedle patches for rapid and efficient transdermal delivery of insulin to diabetic rats. Acta Biomater. 2013;9:2–61. [DOI] [PubMed] [Google Scholar]
- [103].Mohammed YH, Yamada M, Lin LL, et al. Microneedle enhanced delivery of cosmeceutically relevant peptides in human skin. PLoS One. 2014;9:e101956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Yeung C, Chen S, King B, et al. A 3D-printed microfluidic-enabled hollow microneedle architecture for transdermal drug delivery. Biomicrofluidics. 2019;13:064125. [DOI] [PMC free article] [PubMed] [Google Scholar]