Abstract
To investigate the feasibility of readout-segmented diffusion-weighted (rsDW) magnetic resonance (MR) in assessing nerve invasion of soft tissue tumor. Forty-four patients with soft tissue mass in upper leg suspected of nerve invasion underwent rsDW MR. Nerve invasion by tumor was rated by 2 radiologists, respectively. Sensitivity and specificity of rsDW MR in identifying nerve invasion were calculated, with operation findings as reference of standard. Apparent diffusion coefficient and fraction of anisotropy of nerve were obtained using DW MR, and then were compared between invaded nerves and noninvasion cases. Inter-reader agreement in using rsDW MR to rate nerve invasion was excellent (kappa = 0.891 ± 0.043, P < 0.001). Sensitivity and specificity of rsDW MR in identifying nerve invasion were 89% and 88%, respectively. Apparent diffusion coefficient was significantly higher in invaded nerves versus normal nerves (1.45 ± 0.67 × 10−3 mm2/s vs 1.39 ± 0.46 × 10−3 mm2/s, P < 0.05). Fraction of anisotropy was significantly lower in invaded nerves versus normal nerves (0.22 ± 0.11 vs 0.37 ± 0.13, P < .05).
Readout-segmented DW MR was feasible in assessing sciatic nerve invasion by soft tissue tumor in selected patients.
Keywords: diffusion, invasion, peripheral nerve, MR, soft tissue tumor
1. Introduction
Soft tissue tumors of lower extremity may invade adjacent vessels or nerves.[1,2] Computed tomography angiography (CTA) serves as the first-line tests in revealing vascular invasion by soft tissue tumor, and magnetic resonance angiography is an alternative to CTA. However, they both have difficulty in identifying peripheral nerve invasion of soft tissue mass.[3] Compared with CTA and magnetic resonance angiography, fast spin echo (FSE) T2-weighted imaging (T2WI) is more feasible in depicting peripheral nerve.[4,5] However, FSE T2WI does not provide any quantitative parameters correlating with intrinsic characteristics of nerve.[6] Quantitative magnetic resonance (MR) sequences that are able to objectively assess nerve invasion are still urgently required.
Diffusion-weighted (DW) MR, such as diffusion-weighted imaging (DWI), diffusion tensor imaging (DTI), are widely used quantitative sequences. However, conventional DW MR is limited in depicting small lesions in extremities due to image distortion or susceptible artifacts.[7] Fortunately, in recent years, the readout-segmented method was applied to DWI, resulting in better image quality[8,9] and improved depiction of small lesions in limbs.[10,11]
It is well-established water molecular motion is more restricted in nerve than in muscle. Based on the diffusion difference, DW MR is able to identify peripheral nerves from background muscles.[12,13] Compared with DWI, DTI uses more diffusion directions, so is more feasible for the identification of peripheral nerves. In addition, fraction of anisotropy (FA) can be provided by DTI, as well as apparent diffusion coefficient (ADC). FA is a frequently used parameter in the assessment of central nervous diseases but is seldom used to evaluate peripheral nerves in patients with soft tissue tumors. The purpose of the study is thus to investigate the feasibility of readout-segmented diffusion-weighted (rsDW) MR in assessing sciatic nerve invasion by soft tissue mass.
2. Methods
2.1. Patients
This prospective study was approved by the IRB of university. Each patient signed an informed consent before inclusion of study. Inclusion criterion was as follows: patients with a plan of surgical operation for soft tissue mass in upper leg suspected of sciatic nerve invasion. Exclusion criteria were as follows: (1) standard contraindication to MR examination (such as claustropibia); (2) metal internal fixator in upper leg caused significant artifacts, making identification of nerves impossible; (3) chemotherapy or radiotherapy was performed before MR examination, changing the characteristics of nerves; and (4) clinically confirmed peripheral nerve disease that may make FA or ADC of nerve deviate from normality. From September 2016 to January 2021, 55 patients with plans of operation for soft tissue mass in upper leg were enrolled in the study. Eleven patients were excluded by us according to the following reasons: claustrophobia (n = 1); metal internal fixator (n = 3); and chemotherapy/radiotherapy (n = 7). Thus, a total of 44 patients underwent rsDW MR before operation and were finally analyzed.
2.2. MRI examinations
All MR examinations were performed on a 3T whole-body scanner (Skyra, Siemens, Germany). The upper leg was covered with an 18-element body coil. For the purpose of localizing soft tissue mass, fat-suppressed FSE T2WI was performed with the following parameters: TR/TE, 4000/80 ms; matrix, 320; field of view, 320 mm or greater; slice thickness, 4 mm; slice number, 20 or more. rsDW MR was performed in the transverse plane covering the soft tissue mass with the following parameters: TR/TE, 5500/78 ms; matrix, 240; field of view, 240 mm × 240 mm or greater to fit of mass size; phase direction, anterior-posterior; slice thickness, 4 mm; slice number, 24 or more; b value, 0 and 800; average for b = 0, 3; average for b = 800, 2; directions, 20; readout segments, 5; scanning time, 7–9 minutes. ADC map and FA map were automatically generated by the scanner.
2.3. Data analysis
Two radiologists with 7 and 9 years’ experience in diagnosing peripheral nerve diseases in consensus rated the depiction of sciatic nerve on b = 0 map, DW map, ADC map, and FA map. Subjective score was given to each map according to the following scale: 0 = poor depiction of nerve, identification from background impossible; 1 = moderate depiction, with nerve identification and region of interest (ROI) assessment possible; 2 = good depiction of nerve, adequate nerve-to-background contrast; 3 = excellent depiction of nerve.
Two radiologists with 8 and 11 years’ experience in diagnosing peripheral nerve diseases separately rated the invasion of sciatic nerve by using all DW MR maps. A subjective score was given to each case according to the following scale: 0 = nerve was free from tumor; 1 = tumor was close to nerve, but nerve boundary was clear; 2 = no space was seen between tumor and nerve, and part of nerve boundary was not clear; 3 = nerve boundary was not clear, and separation of nerve from tumor was impossible. In the current study, invasion score 0 and 1 were considered as nerve noninvasion, whereas score 2 and 3 were considered as nerve invasion. The 2 radiologists separately measured FA value and ADC value of sciatic nerve by drawing ROI on the DW MR maps. ROI was drawn at 5 consecutive slices where soft tissue mass and sciatic nerve were the closest. ROI was drawn on DW map first, and then transferred to FA map and ADC map. The ROI was asked to be round or oval, similar to nerve shape on the transverse section. The diameter of ROI was asked to be slightly smaller than that of nerve. ROI analysis of nerve was abandoned if the separation of sciatic nerve from tumor was impossible (nerve invasion score = 3). The area of ROI in nerve ranged from 3.8 to 9.4 mm2, with an average of 6.4 mm2. The 2 readers also measured FA and ADC value of soft tissue mass by drawing ROI at the slices where mass size was the largest. ROI of soft tissue mass had irregular shape, and avoided necroses, vessels, and fat. Values from multiple slices were averaged.
2.4. Statistical analysis
All statistical analysis was performed using SPSS 22.0 (IBM, USA). The nerve depiction score was compared among MR maps using a Wilcoxon sign-bank test. Cohen kappa coefficient was used to determine the inter-reader agreement in rating nerve invasion. Intraclass correlation coefficient (ICC) was calculated to determine the inter-reader reproducibility in measurement of ADC and FA. ICC > 0.7 was considered excellent inter-reader reproducibility, thus data from 2 readers could be averaged. A Mann-Whitney test was used to compare parameters of sciatic nerve between nerve invasion cases and noninvasion cases. Receiver operator characteristic curve was constructed for further evaluation. P value <0.5 was considered as statistical significance.
3. Results
From September 2016 to January 2021, 44 patients (male:female = 24:20; mean age = 43.4 years; age range = 17–71) underwent rsDW MR to reveal nerve invasion of tumor before surgical operation. The interval between operation and MR ranged from 1 to 5 days. Nerve invasion was found during operation in 18 of 44 cases. For the other 26 cases, nerve invasion was not seen in operation.
Soft tissue sarcoma (n = 17) was the most in the study, including fibrosarcoma, n = 4; synovial sarcoma, n = 2; myofibrosarcoma, n = 2; leiomyosarcoma, n = 2; epitheliosarcoma, n = 2; rhabdomyosarcoma, n = 2; and liposarcoma, n = 3. Soft tissue sarcoma was followed by vascular anomalies (n = 9) and lipoma (n = 5). Main clinical information of the patients was provided in Table 1.
Table 1.
Clinical information patients with soft tissue mass in upper leg
| Nerve invasion (n = 18) |
Noninvasion (n = 26) |
Total (n = 44) |
|
|---|---|---|---|
| Age and gender | |||
| Age, range (year) | 19–71 | 17–69 | 17–71 |
| Mean age (year) | 44.6 ± 17.4 | 42.5 ± 15.8 | 43.4 ± 16.3 |
| Male:female | 10:8 | 14:12 | 24:20 |
| Symptoms, n | |||
| Limb pain | 16 | 5 | 21 |
| Limb numbness | 13 | 2 | 15 |
| Muscle weakness | 8 | 2 | 10 |
| Pathology results, n | |||
| Soft tissue sarcoma | 15 | 2 | 17 |
| Vascular anomalies | 0 | 9 | 9 |
| Lipoma | 0 | 5 | 5 |
| Schwannoma | 2 | 0 | 2 |
| Neurofibroma | 3 | 0 | 3 |
| Leiomyoma | 0 | 2 | 2 |
| Angioleiomyoma | 0 | 1 | 1 |
| Tenosynovial giant cell tumor | 0 | 1 | 1 |
| Metastasis | 1 | 1 | 2 |
| Fibroma | 0 | 2 | 2 |
Nerve invasion was confirmed by operation.
On DW map, sciatic nerve depiction was rated score = 3 in 20 cases, whereas score = 2 in 21 cases. On b = 0 map, sciatic nerve depiction was rated score = 0 in 14 cases, whereas score = 1 in 15 cases. Sciatic nerve was better depicted with DW map versus b = 0 map (mean score 2.3 vs 1.0, P < 0.05, see Table 2).
Table 2.
Depiction of sciatic nerve on each map of MR was assessed by 2 radiologists in consensus according to the following scale: score 0 = poor depiction; score 1 = moderate depiction; score 2 = good depiction; score 3 = excellent depiction
| Score 0, n | Score 1, n | Score 2, n | Score 3, n | Mean score | Versus b = 0 map, P |
Versus DW map, P |
Versus ADC map, P | Versus FA map, P | |
|---|---|---|---|---|---|---|---|---|---|
| b = 0 map | 14 | 15 | 14 | 1 | 1.0 ± 0.9 | <0.001 | 0.46 | 0.04 | |
| DW map | 2 | 1 | 21 | 20 | 2.3 ± 0.7 | <0.001 | <0.001 | <0.001 | |
| ADC map | 10 | 16 | 16 | 2 | 1.2 ± 0.9 | 0.46 | <0.001 | 0.03 | |
| FA map | 20 | 23 | 1 | 0 | 0.6 ± 0.5 | 0.04 | <0.001 | 0.03 |
A Wilcoxon sign-bank test was used to compare depiction score.
The inter-reader agreement in rating sciatic nerve invasion with DW MR was excellent (kappa = 0.891 ± 0.043, P < 0.001, see Table 3). Divergence occurred in 4 of 44 cases (see Table 3). The nerves invasion rated by 1 reader was as follows: score = 0 (n = 14); score = 1 (n = 11); score = 2 (n = 17); score = 3 (n = 2). The nerves invasion rated by another reader was as follows: score = 0 (n = 13); score = 1 (n = 12); score = 2 (n = 16); score = 3 (n = 3). In the study, score 0 and 1 were considered as negative (of nerve invasion), whereas 2 and 3 were considered as positive. Thus, there were 19 positives and 25 negatives, for both readers.
Table 3.
Sciatic nerve invasion was assessed using readout-segmented diffusion-weighted MR according to the following scale: 0 = nerve free from tumor; 1 = nerve close to tumor, nerve boundary clear; 2 = no space between nerve and tumor, part of nerve boundary not clear; 3 = separation of nerve from tumor impossible
| Reader 1 | |||||
|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | ||
| Reader 2 | 0 | 13 | 0 | 0 | 0 |
| 1 | 1 | 10 | 1 | 0 | |
| 2 | 0 | 1 | 15 | 0 | |
| 3 | 0 | 0 | 1 | 2 | |
DTI = diffusion tensor imaging.
Nineteen positives included 16 true-positives (score 2 or 3, operation-confirmed invasion) and 3 false-positives (score 2, operation-confirmed noninvasion). Twenty-five negatives included 23 true-negatives (score 0 or 1, operation-confirmed noninvasion) and 2 false-negatives (score 1, operation-confirmed invasion). The sensitivity and specificity of rsDW MR for identification of sciatic nerve invasion were 89% (16/18) and 88% (23/26), respectively.
ICC for ADC and FA of sciatic nerve were 0.91 and 0.88, respectively. ICC for ADC and FA of soft tissue mass were 0.93 and 0.91, respectively. As all ICC above 0.7, data from the 2 readers were averaged. ADC of sciatic nerve was significantly different between noninvasion cases and nerve invasion cases (1.39 ± 0.46 × 10−3mm2/s vs 1.45 ± 0.67 × 10−3mm2/s, P < 0.05). Nerve FA was significantly lower in nerve invasion cases versus noninvasion cases (0.22 ± 0.11 vs 0.37 ± 0.13, P < 0.05). Area under curve for ADC and FA in identifying nerve invasion was 0.67 and 0.85, respectively.
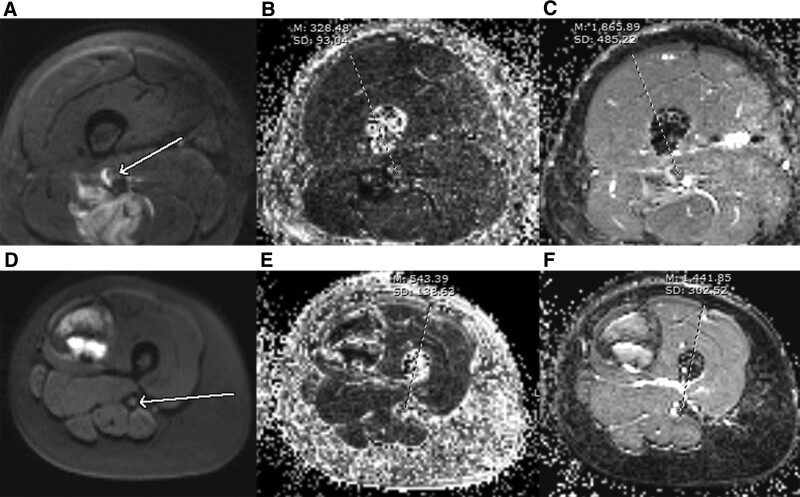
ADC and FA were significantly lower in soft tissue sarcoma versus vascular anomalies (see Table 4). Area under curve for ADC and FA were 0.72 and 0.84, respectively, in discrimination of soft tissue tumor and vascular anomalies. Figure 1 is a comparison of ADC value and FA value between nerve invasion case and noninvasion case.
Table 4.
ADC and FA of nerve were compared between nerve invasion cases and noninvasion cases using a Mann-Whitney test
| Noninvasion (n = 26) |
Nerve invasion n = 15) |
P | Soft tissue sarcoma (n = 17) |
Vascular anomalies (n = 9) |
P | |
|---|---|---|---|---|---|---|
| Age | 42.5 | 44.5 | 0.12 | 48.6 | 38.3 | <0.001 |
| ADC(10-3mm2/s) | 1.39 ± 0.46 | 1.45 ± 0.67 | 0.04 | 1.34 ± 0.42 | 1.57 ± 0.55 | 0.03 |
| FA | 0.37 ± 0.13 | 0.22 ± 0.11 | 0.004 | 0.14 ± 0.06 | 0.35 ± 0.16 | 0.01 |
ADC and FA of mass were compared between soft tissue sarcoma and vascular anomalies.
ADC = apparent diffusion coefficient; FA = fraction of anisotropy.
Figure 1.
A comparison in ADC and FA between invasion and noninvasion. (A–C) were respectively diffusion-weighted map, FA map, and ADC map for a nerve invasion case. (D–F) were respectively diffusion-weighted map, FA map, and ADC map for a case without nerve invasion. The FA value was significantly lower in the invaded nerve versus the normal nerve (B, D, red arrows). The ADC value was significantly higher in the invaded nerve versus the nerve free from tumor (C, F, red arrows). ADC = apparent diffusion coefficient; FA = fraction of anisotropy.
4. Discussions
In the current study, we investigated the feasibility of rsDW MR in assessing nerve invasion of soft tissue tumor. The most important findings were as follows: (1) the nerve could be well depicted with DW map; (2) rsDW MR had 89% sensitivity and 88% specificity in identification of nerve invasion.
It is of importance to assess peripheral nerve invasion by soft tissue tumor before producing correct treatment plan.[14–16] Compared with CT, MR with excellent soft tissue contrast is more feasible for the depiction of peripheral nerve. In recent years, several MR sequences have been tried for the assessment of peripheral nerve diseases.[17–19] However, these sequences failed to provide quantitative parameters that could reflect intrinsic characteristics of nerve. Readout-segmented DW MR is able to provide quantitative parameters including FA and ADC[20–23] so was introduced by us as a new way to assess peripheral nerve.
In the current study, b = 0 map failed to well depict nerves in some cases, because the signal intensity was similar in nerve and background tissue. DW map provided better depiction of nerve, due to substantially different signal intensity of nerve and background. It is well-established water molecular diffusion is more restricted in nerve.[24,25] Thus, nerve is generally brighter than background soft tissue on DW map. In the study, DW MR had excellent inter-reader agreement in assessment of nerve invasion. A possible explanation was tumor and nerve were both hyperintensity on the DW map. In the study, FA was lower in invaded nerves versus normal nerves. The decrease in FA might be cause by the destroy of myelin sheath.[26,27]
A comparison of soft tissue sarcoma and vascular anomalies was performed in the study. We found soft tissue sarcoma had significantly lower ADC versus vascular anomalies, which was in consist with previous research.[28] It is interesting that soft tissue sarcoma also had lower FA value, which is likely caused by more disordered tissue structure.
Our study had some limitations. First, the sample size was small. We only collected 44 operation-confirmed cases during 4 years. Multicenter large-size study is required to validate our results. Second, readout-segmented DW map was not compared with conventional DW map. The latter always provided inadequate depiction of nerve, so was not performed in the study in consideration of scanning time. Third, DW MR was not compared to other MR sequences in the assessment of nerve. The study focused on the feasibility of DW MR in assessing the nerve invasion by the soft tissue tumor. In further study, DW MR should be compared with other sequences, such as contrast-enhanced T1.
In conclusion, readout-segmented DW MR was feasible to assess sciatic nerve invasion by soft tissue tumor in selected patients.
Author contributions
Gang Wu and Liangjin Liu have equally contributed to this article.
Abbreviations:
- ADC =
- apparent diffusion coefficient
- CTA =
- computed tomography angiography
- DTI =
- diffusion tensor imaging
- DW =
- diffusion weighted
- FA =
- fraction of anisotropy
- FSE =
- fast spin echo
- ICC =
- intraclass correlation coefficient
- MRA =
- magnetic resonance angiography
- ROC =
- receiver operator characteristic
- rsDW =
- readout-segmented diffusion weighted
- SI =
- signal intensity
- T2WI =
- T2-weighted imaging
How to cite this article: Wu G, Liu L, Mei Z, Li X. Diffusion-weighted MR is useful to assess peripheral nerve invasion of soft tissue tumor. Medicine 2022;101:26(29779).
This study has received funding by National Natural Scientific foundation of China (Number: 31630025, and 81571643).
The authors have no conflicts of interest to disclose.
The data sets generated during and/or analyzed during the current study are publicly available.
Contributor Information
Gang Wu, Email: tongjiwugang1984@qq.com.
Liangjin Liu, Email: 42292815@qq.com.
References
- [1].Cetinkaya OA, Celik SU, Kalem M, et al. Clinical characteristics and surgical outcomes of limb-sparing surgery with vascular reconstruction for soft tissue sarcomas. Ann Vasc Surg. 2019;56:73–80. [DOI] [PubMed] [Google Scholar]
- [2].Jin T, Wu G, Li X, et al. Evaluation of vascular invasion in patients with musculoskeletal tumors of lower extremities: use of time-resolved 3D MR angiography at 3-T. Acta Radiol. 2018;59:586–92. [DOI] [PubMed] [Google Scholar]
- [3].Chen Y, Haacke EM, Li J, et al. Peripheral nerve magnetic resonance imaging. F1000Res. 2019;8:1803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Kronlage M, Knop KC, Schwarz D, et al. Amyotrophic lateral sclerosis versus multifocal motor neuropathy: utility of MR neurography. Radiology. 2019;292:149–56. [DOI] [PubMed] [Google Scholar]
- [5].Andreisek G. Can MR neurography differentiate between amyotrophic lateral sclerosis and multifocal motor neuropathy? Radiology. 2019;292:157–8. [DOI] [PubMed] [Google Scholar]
- [6].Kollmer J, Hilgenfeld T, Ziegler A, et al. Quantitative MR neurography biomarkers in 5q-linked spinal muscular atrophy. Neurology. 2019;93:e653–64. [DOI] [PubMed] [Google Scholar]
- [7].Park SY, Lee MH, Jeon JY, et al. MRI evaluation of suspected pathologic fracture at the extremities from metastasis: diagnostic value of added diffusion-weighted imaging. Korean J Radiol. 2019;20:812–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Kim TH, Baek MY, Park JE, et al. Comparison of DWI methods in the pediatric brain: PROPELLER turbo spin-echo imaging versus readout-segmented echo-planar imaging versus single-shot echo-planar imaging. Am J Roentgenol. 2018;210:1352–8. [DOI] [PubMed] [Google Scholar]
- [9].Wu G, Morelli J, Xiong Y, et al. Diffusion weighted cardiovascular magnetic resonance imaging for discriminating acute from non-acute deep venous Thrombus. J Cardiovasc Magn Reson. 2019;21:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Wengler K, Tank D, Fukuda T, et al. Diffusion tensor imaging of human Achilles tendon by stimulated echo readout-segmented EPI (ste-RS-EPI). Magn Reson Med. 2018;80:2464–74. [DOI] [PubMed] [Google Scholar]
- [11].Ho CY, Deardorff R, Kralik SF, et al. Comparison of multi-shot and single shot echo-planar diffusion tensor techniques for the optic pathway in patients with neurofibromatosis type 1. Neuroradiology. 2019;61:431–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Kim HS, Yoon YC, Choi BO, et al. Diffusion tensor imaging of the sciatic nerve in Charcot-Marie-Tooth disease type I patients: a prospective case-control study. Eur Radiol. 2019;29:3241–52. [DOI] [PubMed] [Google Scholar]
- [13].Farinas AF, Pollins AC, Stephanides M, et al. Diffusion tensor tractography to visualize axonal outgrowth and regeneration in a 4-cm reverse autograft sciatic nerve rabbit injury model. Neurol Res. 2019;41:257–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Jia X, Chen C, Chen L, et al. Large malignant granular cell tumor with suprascapular nerve and brachial plexus invasion: a case report and literature review. Medicine. 2017;96:e8531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Muratori F, De Gori M, Campo FR, et al. Giant schwannoma of the foot: a case report and literature review. Clin Cases Miner Bone Metab. 2017;14:265–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Chowdhury D, Chudy M, Bhattacharya S, et al. Soft tissue mesenchymal tumour - a case report with review of literature. Int J Surg Case Rep. 2017;31:89–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Su X, Kong X, Liu D, et al. Multimodal magnetic resonance imaging of peripheral nerves: establishment and validation of brachial and lumbosacral plexi measurements in 163 healthy subjects. Eur J Radiol. 2019;117:41–8. [DOI] [PubMed] [Google Scholar]
- [18].Agarwal A, Chandra A, Jaipal U, et al. Can imaging be the new yardstick for diagnosing peripheral neuropathy? A comparison between high resolution ultrasound and MR neurography with an approach to diagnosis. Insights Imaging. 2019;10:104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Martín Noguerol T, Barousse R. Update in the evaluation of peripheral nerves by MRI, from morphological to functional neurography. Radiologia. 2020;62:90–101. [DOI] [PubMed] [Google Scholar]
- [20].Elliott CA, Danyluk H, Aronyk KE, et al. Intraoperative acquisition of DTI in cranial neurosurgery: readout-segmented DTI versus standard single-shot DTI. J Neurosurg. 2019;16:1–10. [DOI] [PubMed] [Google Scholar]
- [21].Ho MJ, Ciritsis A, Manoliu A, et al. Diffusion tensor imaging of the brachial plexus: a comparison between readout-segmented and conventional single-shot echo-planar imaging. Magn Reson Med Sci. 2019;18:150–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Yamaguchi K, Nakazono T, Egashira R, et al. Diagnostic performance of diffusion tensor imaging with readout-segmented echo-planar imaging for invasive breast cancer: correlation of ADC and FA with pathological prognostic markers. Magn Reson Med Sci. 2017;16:245–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Manoliu A, Ho M, Nanz D, et al. Diffusion tensor imaging of lumbar nerve roots: comparison between fast readout-segmented and selective-excitation acquisitions. Invest Radiol. 2016;51:499–504. [DOI] [PubMed] [Google Scholar]
- [24].Holzgrefe RE, Wagner ER, Singer AD, et al. Imaging of the peripheral nerve: concepts and future direction of magnetic resonance neurography and ultrasound. J Hand Surg Am. 2019;44:1066–79. [DOI] [PubMed] [Google Scholar]
- [25].Vilanova JC, García-Figueiras R, Luna A, et al. Update on whole-body MRI in musculoskeletal applications. Semin Musculoskelet Radiol. 2019;23:312–23. [DOI] [PubMed] [Google Scholar]
- [26].Kronlage M, Pitarokoili K, Schwarz D, et al. Diffusion tensor imaging in chronic inflammatory demyelinating polyneuropathy: diagnostic accuracy and correlation with electrophysiology. Invest Radiol. 2017;52:701–7. [DOI] [PubMed] [Google Scholar]
- [27].Heckel A, Weiler M, Xia A, et al. Peripheral nerve diffusion tensor imaging: assessment of axon and myelin sheath integrity. PLoS One. 2015;10:e0130833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Wu G, Liu X, Xiong Y, et al. Intravoxel incoherent motion and diffusion kurtosis imaging for discriminating soft tissue sarcoma from vascular anomalies. Medicine. 2018;97:e13641. [DOI] [PMC free article] [PubMed] [Google Scholar]