Background:
Clinically relevant postoperative pancreatic fistula (CR-POPF) is a common and troublesome complication after pancreatoduodenectomy (PD). We conducted a systematic review and meta-analysis to identify the risk factors of CR-POPF after PD.
Methods:
We searched PubMed, EMBASE, and Cochrane Library databases for studies related to risk factors of CR-POPF after PD. Odds ratios (ORs) with corresponding 95% confidence intervals (CIs) were extracted from the included studies, then a meta-analysis was conducted. If necessary, sensitivity analysis would be performed by changing the effect model or excluding 1 study at a time. Publication bias was assessed by funnel plot and Begg test and Egger test.
Results:
A total of 27 studies with 24,740 patients were included, and CR-POPF occurred in 3843 patients (incidence = 17%, 95% CI: 16%–19%). Male (OR = 1.56, 95% CI: 1.42–1.70), body mass index >25 kg/m2 (OR = 1.98, 95% CI: 1.23–3.18), pancreatic duct diameter <3 mm (OR = 1.87, 95% CI: 1.66–2.12), soft pancreatic texture (OR = 3.49, 95% CI: 2.61–4.67), and blood transfusion (OR = 3.10, 95% CI: 2.01–4.77) can significantly increase the risk of CR-POPF. Pancreatic adenocarcinoma (OR = 0.54, 95% CI: 0.47–0.61), vascular resection (OR = 0.57, 95% CI: 0.39–0.83), and preoperative chemoradiotherapy (OR = 0.68, 95% CI: 0.57–0.81) can significantly decrease the factor of CR-POPF. Diabetes mellitus was not statistically associated with CR-POPF (OR = 0.66, 95% CI: 0.40–1.08). However, the analysis of body mass index, pancreatic texture, and diabetes mellitus had a high heterogeneity, then sensitivity analysis was performed, and the result after sensitivity analysis showed diabetes mellitus can significantly decrease the risk of CR-POPF. There was no significant publication bias in this meta-analysis.
Conclusions:
The current review assessed the effects of different factors on CR-POPF. This can provide a basis for the prevention and management of CR-POPF. Effective interventions targeting the above risk factors should be investigated in future studies for decreasing the occurrence of CR-POPF.
Keywords: clinically relevant pancreatic fistula, meta-analysis, pancreaticoduodenectomy, risk factor, systematic review
1. Introduction
Pancreatoduodenectomy (PD) is a classic surgery for tumors originating from the pancreatic head, lower common bile duct, and periampullary region.[1] PD has always been a challenging surgery with high mortality and morbidity. Although the mortality of PD has reduced to about 2%, the postoperative morbidity is as high as 30% to 50%. Pancreatic fistula (PF) is one of the most important complications after PD, with an incidence of about 10% to 28%.[2] According to the definition of the International Study Group on Pancreatic Fistula (ISGPF) in 2016, grade A PF was replaced by biochemical leak because of no clinical impact, and grades B and C PF are also named as clinically relevant postoperative PF (CR-POPF).[3] Compared with grade A PF, CR-POPF not only can increase the length and cost of hospitalization, but can also increase the risk of other morbidities such as postoperative bleeding, abdominal infection, and even death.[4–6] Therefore, it has an essential significance to prevent the occurrence of CR-POPF after PD. Identification of risk factors for CR-POPF can better understand the potential mechanisms of CR-POPF and is also conducive to the prevention and management of CR-POPF. Many studies have extensively investigated the risk factors of PF, such as increased body mass index (BMI), smaller pancreatic duct diameter, soft pancreatic texture, lower serum albumin, surgical techniques, etc.[7–9] However, most previous studies related to risk factors of PF did not exclude grade A PF, and the effects of different factors on CR-POPF were still unclear. In the current article, we conducted a systematic review and meta-analysis to identify the risk factors for CR-POPF after PD, thereby providing a basis for the prevention and management of CR-POPF.
2. Methods
Our protocol was registered in the International Prospective Register of Systematic Reviews on December 9, 2020 (PROSPERO CRD42020219814). This systematic review and meta-analysis was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis guidelines.[10]
2.1. Search strategy
Relevant studies were searched from PubMed, EMBASE, and Cochrane Library databases from inception to November 2019 with no limitation of language. We used the following combined Medical Subject Heading terms which included “risk factors”, “pancreatic fistula”, and “pancreaticoduodenectomy”. Furthermore, we also scanned the references from included studies in order not to miss relevant studies.
2.2. Eligibility criteria
Studies that met all the following conditions were considered for eligibility: patients underwent PD; studies analyzed the risk factors of CR-POPF; the diagnosis of CR-POPF met the definition of ISGPF; studies provided the result of multivariate logistic regression analysis, odds ratio (OR) with corresponding 95% confidence interval (CI), P values, or relevant values could be calculated based on the original data reported in the study; retrospective or prospective studies.
Studies with duplicate data were excluded.
2.3. Data extraction
Two researchers independently conducted data extraction according to a standardized data collection form, and the disagreement would be resolved by discussion. The extracted data included first author, publication year, nation, source of patients, recruitment period, study design, number of patients, number of CR-POPF, risk factors, and OR with corresponding 95% CI.
2.4. Quality assessment
The quality of the included studies was assessed by 2 researchers according to the Newcastle-Ottawa Scale (NOS).[11] The NOS contains 3 aspects and 8 items: selection (4 items, 1 star each); comparability (1 item, up to 2 stars); and outcome (3 items, 1 star each) for a cohort study or exposure (3 items, 1 star each) for case–control study. The score of NOS is on a scale of between 0 and 9 stars, and the study with NOS score >6 stars was considered as a high-quality study.[12]
2.5. Data synthesis and analysis
We used Review Manager software (version 5.3; The Cochrane Collaboration, Copenhagen, Denmark) and Stata statistical software (version 14.0) for the statistical processing. The pooled OR with corresponding 95% CI was calculated, and P value of <.05 was considered statistically significant. Heterogeneity was assessed by Cochran’s Q test and the inconsistency (I2) statistic. I2 <50% represented a low heterogeneity, and the fixed effects model would be used. If not, the random effects model would be used. Sensitivity analysis was performed through changing the effect model or excluding 1 study at a time. If >10 studies were included, publication bias would be assessed by funnel plot and Begg and Egger tests, and P value of <.05 indicated significant publication bias.
3. Results
3.1. Search result
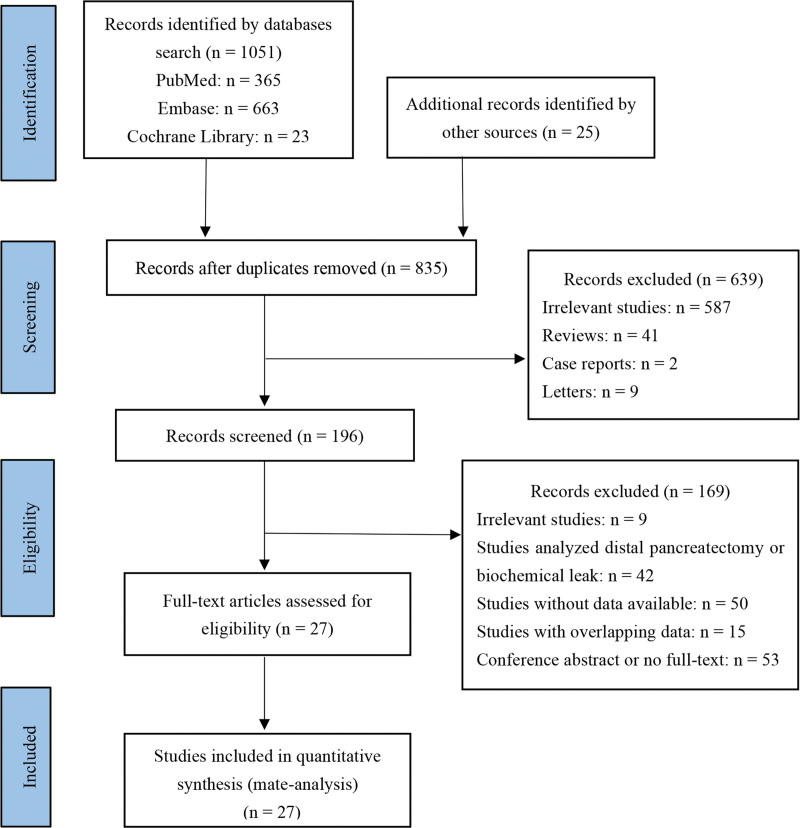
One thousand seventy-six studies were acquired by initial search, then 241 duplicate studies were excluded, 639 studies were excluded by scanning title and abstract, 169 studies were excluded by reading full text, and finally 27 studies[13–39] were included. The process of study exclusion and inclusion is shown in Figure 1. Of the 27 included studies, 3 studies were from the United States, 2 studies were from Italy, 3 studies were from France, 1 study was from Sweden, 6 studies were from China, 11 studies were from Japan, and 1 study was from Korea. Three studies were prospective and 24 studies were retrospective. The NOS scores of 27 studies were between 7 and 9 stars. The characteristics of the 27 included studies are shown in Table 1.
Figure 1.
Flow diagram of study inclusion and exclusion.
Table 1.
Characteristics of the included studies.
| Study | Nation | Study period | Study design | Patients number | Number of CR-POPF | Score of NOS | Risk factors |
|---|---|---|---|---|---|---|---|
| Pratt et al[13] (2008) | USA | 2001.10–2007.03 | Prospective | 233 | 31 | 8 | 4. 5 |
| Kawai et al[14] (2011) | Japan | 2005.07–2009.06 | Retrospective | 1239 | 178 | 8 | 1. 4 |
| Hiyoshi et al[15] (2013) | Japan | 2002.03–2010.10 | Retrospective | 176 | 30 | 8 | 1 |
| Malleo et al[16] (2013) | Italy | 2000.01–2011.12 | Prospective | 602 | 63 | 8 | 1. 3. 4. 6 |
| Kanda et al[17] (2014) | Japan | 2008.01–2013.03 | Retrospective | 246 | 57 | 7 | 4 |
| Chen et al[18] (2015) | China | 2008.01–2013.12 | Retrospective | 921 | 89 | 8 | 4. 7 |
| Fujiwara et al[19] (2015) | Japan | 2000.05–2013.05 | Retrospective | 247 | 43 | 8 | 1 |
| Nagai et al[20] (2015) | Japan | 2007–2013 | Retrospective | 254 | 44 | 9 | 4. 8. 9 |
| Nishida et al[21] (2016) | Japan | 2010.01–2014.12 | Retrospective | 266 | 43 | 8 | 2. 4. 5. 8 |
| Yamashita et al[22] (2016) | Japan | 2007.01–2012.12 | Retrospective | 174 | 18 | 8 | 1. 2. 6 |
| Kantor et al[23] (2017) | USA | 2011–2012 | Retrospective | 1731 | 270 | 8 | 1. 2 |
| Partelli et al[24] (2017) | Italy | 2013–2015 | Retrospective | 463 | 64 | 8 | 1. 7 |
| Xia et al[25] (2018) | China | 2009.01–2015.12 | Retrospective | 225 | 40 | 9 | 4 |
| Ke et al[26] (2018) | China | 2015.09–2017.08 | Retrospective | 170 | 44 | 9 | 4. 6 |
| Ellis et al[27] (2019) | USA | 2014–2017 | Retrospective | 10,022 | 1658 | 8 | 1. 3. 5. 9 |
| Kang et al[28] (2019) | Korea | 2007–2014 | Prospective | 1898 | 275 | 9 | 1. 4. 5. 6. 8 |
| Li et al[29] (2019) | China | 2011.01–2016.12 | Retrospective | 189 | 38 | 7 | 3. 4 |
| Zarzavadjian Le Bian et al[30] (2019) | France | 2004.02–2016.12 | Retrospective | 270 | 74 | 8 | 2. 7 |
| Abe et al[31] (2020) | Japan | 2006–2018 | Retrospective | 136 | 37 | 9 | 1 |
| Bardol et al[32] (2020) | France | 2009.01–2018.04 | Retrospective | 195 | 58 | 8 | 3. 4. 5. 8. 9 |
| Ohgi et al[33] (2020) | Japan | 2010.01–2014.12 | Retrospective | 346 | 116 | 8 | 2. 3. 4. 5. 8 |
| Tabchouri et al[34] (2020) | France | 2012–2017 | Retrospective | 448 | 103 | 8 | 1. 4. 5. 9 |
| Utsumi et al[35] (2020) | Japan | 2008.04–2018.09 | Retrospective | 108 | 32 | 7 | 5 |
| Williamsson et al[36] (2020) | Sweden | 2010.01–2018.06 | Retrospective | 2503 | 245 | 8 | 1. 6. 8 |
| Zou et al[37] (2020) | China | 2005.01–2016.12 | Retrospective | 707 | 75 | 7 | 2 |
| Huang et al[38] (2020) | China | 2010.01–2018.05 | Retrospective | 762 | 82 | 8 | 3. 4. 5 |
| Nakamura et al[39] (2020) | Japan | 2013.05–2018.05 | Retrospective | 209 | 36 | 8 | 3. 4. 5. 9 |
Remarks = 1: gender, 2: BMI (25 kg/m2), 3: pancreatic duct diameter (3 mm), 4: pancreatic texture, 5: pathology type, 6: diabetes mellitus, 7: Blood transfusion, 8: vascular resection, 9: preoperative chemoradiotherapy.
BMI = body mass index, CR-POPF = clinically relevant postoperative pancreatic fistula, NOS = Newcastle-Ottawa Scale.
3.2. Risk factors of CR-POPF
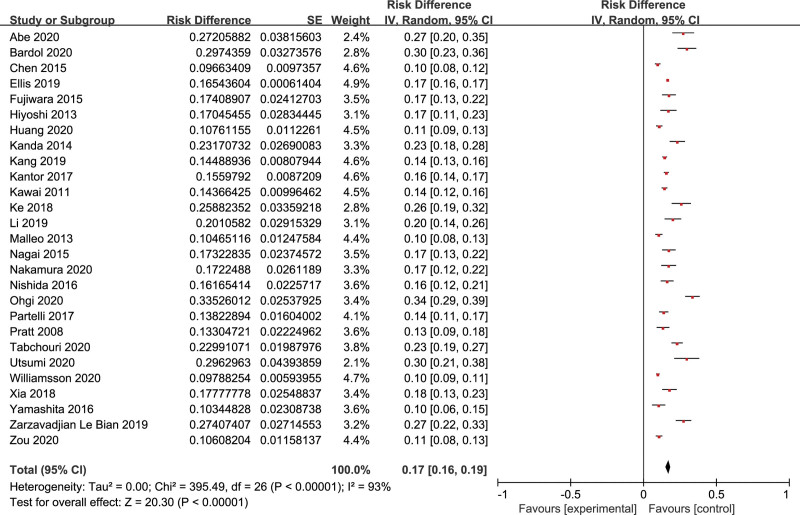
Twenty-seven studies included 24,740 patients who underwent PD, and CR-POPF occurred in 3843 patients; the pooled incidence was 17% (95% CI: 16%–19%) (Fig. 2). Nine factors had sufficient data and were reported in at least 3 studies. A meta-analysis was conducted for these factors: gender, BMI (25 kg/m2), pancreatic duct diameter (3 mm), pancreatic texture, pathology type, diabetes mellitus, blood transfusion, vascular resection, and preoperative chemoradiotherapy. And the meta-analysis results of risk factors are summarized in Table 2.
Figure 2.
The pooled incidence of CR-POPF after PD. CR-POPF = clinically relevant postoperative pancreatic fistula, PD = pancreatoduodenectomy.
Table 2.
The summarized results of risk factors.
| Risk factors | Number of studies | OR | 95% CI | P | I 2 |
|---|---|---|---|---|---|
| Gender | 12 | 1.56 | 1.42–1.70 | <.001 | 29% |
| BMI (25 kg/m2) | 6 | 1.98 | 1.23–3.18 | <.001 | 80% |
| Pancreatic duct diameter (3 mm) | 7 | 1.87 | 1.66–2.12 | <.001 | 28% |
| Pancreatic texture | 16 | 3.49 | 2.61–4.67 | <.001 | 57% |
| Pathology type | 10 | 0.54 | 0.47–0.61 | <.001 | 33% |
| Diabetes mellitus | 5 | 0.66 | 0.40–1.08 | 0.10 | 62% |
| Blood transfusion | 3 | 3.10 | 2.01–4.77 | <.001 | 0% |
| Vascular resection | 6 | 0.57 | 0.39–0.83 | .003 | 0% |
| Preoperative chemoradiotherapy | 5 | 0.68 | 0.57–0.81 | <.001 | 49% |
BMI = body mass index, CI = confidence interval, OR = odds ratio.
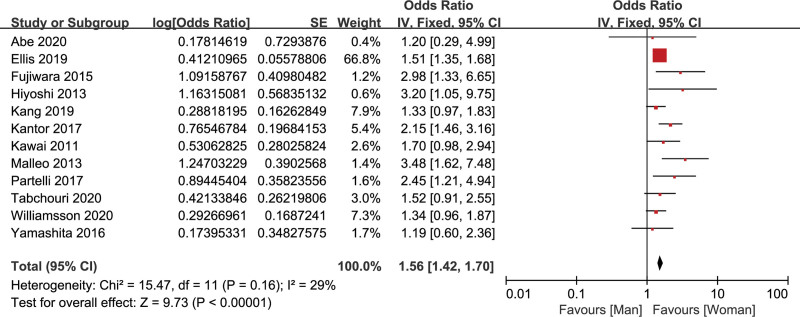
3.2.1. Gender
Twelve studies[14–16,19,22–24,27,28,31,34,36] analyzed the relationship of gender and CR-POPF, containing 19,636 patients. There was a low heterogeneity (I2 = 29%), so the fixed effects model was used for data synthesis. The pooled data showed that male was a significant risk factor of CR-POPF (OR = 1.56, 95% CI: 1.42–1.70, P < .001) (Fig. 3).
Figure 3.
Forest plot of the relationship between CR-POPF and gender. CR-POPF = clinically relevant postoperative pancreatic fistula.
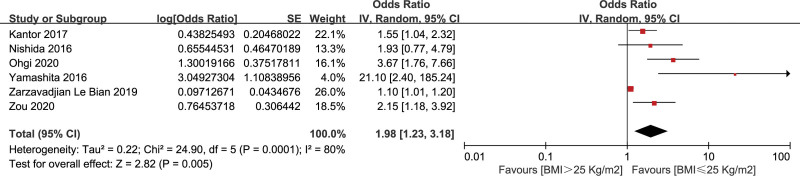
3.2.2. Body mass index (25 kg/m2)
Six studies[21–23,30,33,37] reported the effect of BMI >25 kg/m2 on CR-POPF, including 3494 patients. There was a high heterogeneity (I2 = 80%), so the random effects model was adopted. The result indicated that BMI >25 kg/m2 was a significant risk factor of CR-POPF (OR = 1.98, 95% CI: 1.23–3.18, P < .001) (Fig. 4).
Figure 4.
Forest plot of the relationship between CR-POPF and BMI (25 kg/m2). BMI = body mass index, CR-POPF = clinically relevant postoperative pancreatic fistula.
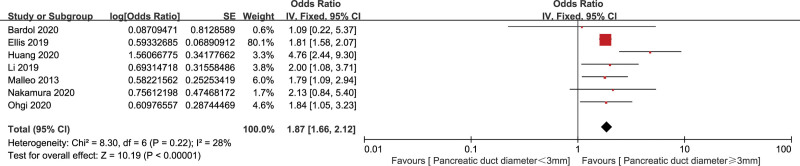
3.2.3. Pancreatic duct diameter (3 mm)
Seven studies[16,27,29,32,33,38,39] analyzed the relationship of pancreatic duct diameter <3 mm and CR-POPF, involving 12,325 patients. The fixed effects model was used for data synthesis because of a low heterogeneity (I2 = 28%) among the 7 studies. The pooled data showed that pancreatic duct diameter <3 mm was a significant risk factor of CR-POPF (OR = 1.87, 95% CI: 1.66–2.12, P < .001) (Fig. 5).
Figure 5.
Forest plot of the relationship between CR-POPF and pancreatic duct diameter (3 mm). CR-POPF = clinically relevant postoperative pancreatic fistula.
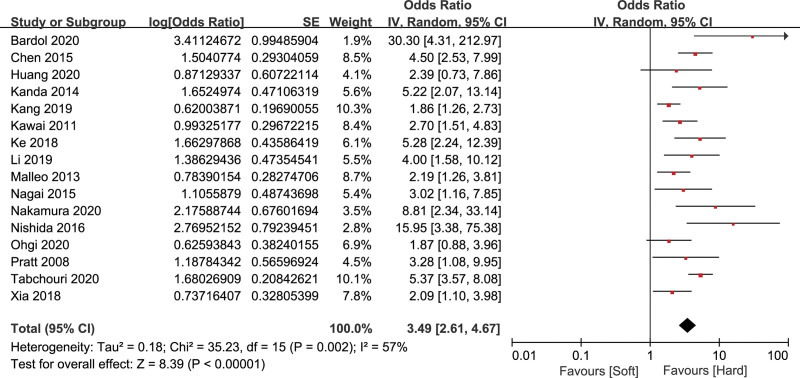
3.2.4. Pancreatic texture
Sixteen studies[13,14,16–18,20,21,25,26,28,29,32–34,38,39] evaluated the relationship of pancreatic texture and CR-POPF, including 8203 patients. There was a high heterogeneity (I2 = 57%), so the random effects model was adopted. The result indicated that soft pancreatic texture was a significant risk factor of CR-POPF (OR = 3.49, 95% CI: 2.61–4.67, P < .001) (Fig. 6).
Figure 6.
Forest plot of the relationship between CR-POPF and pancreatic texture. CR-POPF = clinically relevant postoperative pancreatic fistula.
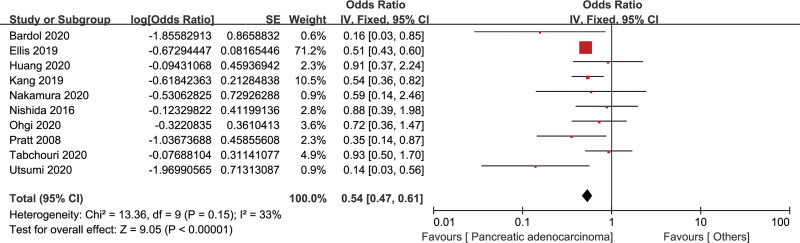
3.2.5. Pathology type
The relationship of pathology type and CR-POPF was assessed by 10 studies with 14,487 patients.[13,21,27,28,32–35,38,39] There was a low heterogeneity (I2 = 33%), and the fixed effects model was used. The pooled data indicated that compared with other pathology types, pancreatic adenocarcinoma was a significant protective factor of CR-POPF (OR = 0.54, 95% CI: 0.47–0.61, P < .001) (Fig. 7).
Figure 7.
Forest plot of the relationship between CR-POPF and pathology type. CR-POPF = clinically relevant postoperative pancreatic fistula.
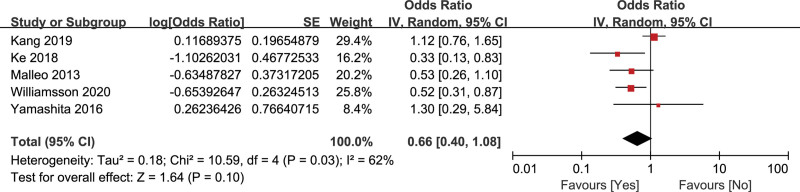
3.2.6. Diabetes mellitus
Five studies[16,22,26,28,36] evaluated the relationship of diabetes mellitus and CR-POPF, containing 5247 patients. The random effects model was adopted because of a high heterogeneity (I2 = 62%). The result showed that diabetes mellitus could reduce the incidence of CR-POPF, but was not statistically significant (OR = 0.66, 95% CI: 0.40–1.08, P = .10) (Fig. 8).
Figure 8.
Forest plot of the relationship between CR-POPF and diabetes mellitus. CR-POPF = clinically relevant postoperative pancreatic fistula.
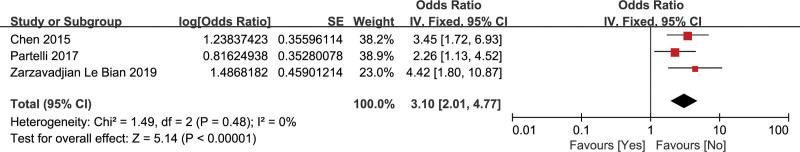
3.2.7. Blood transfusion
Three studies[18,24,30] evaluated the relationship of blood transfusion and CR-POPF, including 1654 patients. The fixed effects model was used for data synthesis because no heterogeneity (I2 = 0%). The pooled data indicated that blood transfusion was a significant risk factor of CR-POPF (OR = 3.10, 95% CI: 2.01–4.77, P < .001) (Fig. 9).
Figure 9.
Forest plot of the relationship between CR-POPF and blood transfusion. CR-POPF = clinically relevant postoperative pancreatic fistula.
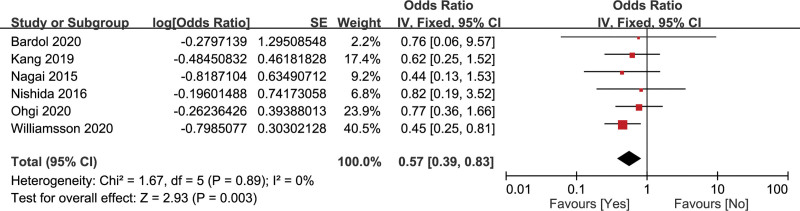
3.2.8. Vascular resection
The association of vascular resection and CR-POPF was assessed by 6 studies with 5462 patients.[20,21,28,32,33,36] There was no heterogeneity (I2 = 0%), so the fixed-effects model was adopted. The result showed that intraoperative vascular resection was a significant protective factor of CR-POPF (OR = 0.57, 95% CI: 0.39–0.83, P = .003) (Fig. 10).
Figure 10.
Forest plot of the relationship between CR-POPF and vascular resection. CR-POPF = clinically relevant postoperative pancreatic fistula.
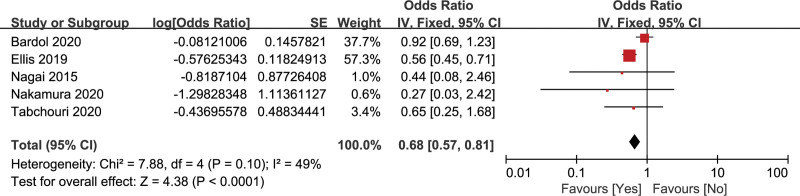
3.2.9. Preoperative chemoradiotherapy
The relationship of preoperative chemoradiotherapy and CR-POPF was assessed by 5 studies with 11,128 patients.[20,27,32,34,39] There was a low heterogeneity (I2 = 49%), so the fixed effects model was adopted. The pooled data showed that preoperative chemoradiotherapy was a significant protective factor of CR-POPF (OR = 0.68, 95% CI: 0.57–0.81, P < .001) (Fig. 11).
Figure 11.
Forest plot of the relationship between CR-POPF and preoperative chemoradiotherapy. CR-POPF = clinically relevant postoperative pancreatic fistula.
3.3. Sensitivity analysis
There was a high heterogeneity in the analysis of BMI, pancreatic texture, and diabetes mellitus, so further sensitivity analysis was conducted. The effects of BMI and pancreatic texture on CR-POPF did not change by converting the effects model (Supplementary Figures 1 and 2, http://links.lww.com/MD/G805). Diabetes mellitus went from not statistically significant to a significant protective factor of CR-POPF by converting the random-effects model to the fixed effects model (Supplementary Figure 3, http://links.lww.com/MD/G805). Furthermore, the sources of heterogeneity were identified by excluding 1 study at a time. The heterogeneity decreased from I2 = 62% to I2 = 0% by excluding the study by Kang et al[28] in the analysis of diabetes mellitus. The result after excluding the sources of heterogeneity showed that diabetes mellitus was a significant protective factor of CR-POPF (OR = 0.51, 95% CI: 0.35–0.74, P < .001) (Supplementary Figure 4, http://links.lww.com/MD/G805).
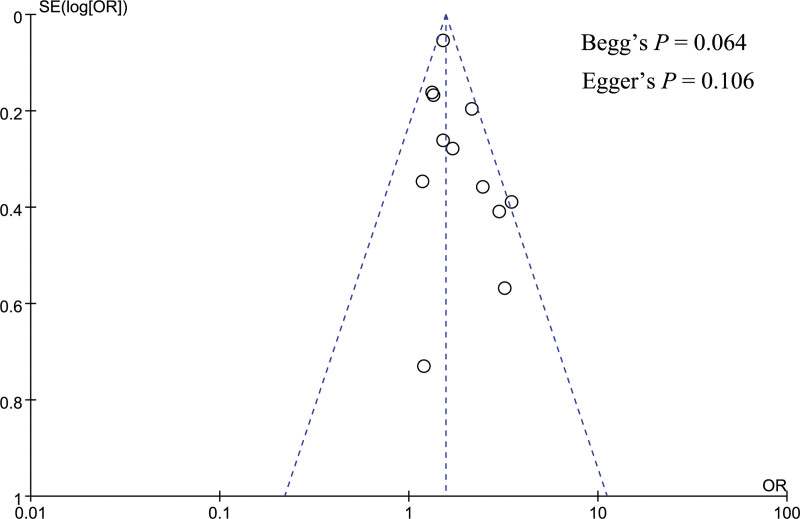
3.4. Publication bias analysis
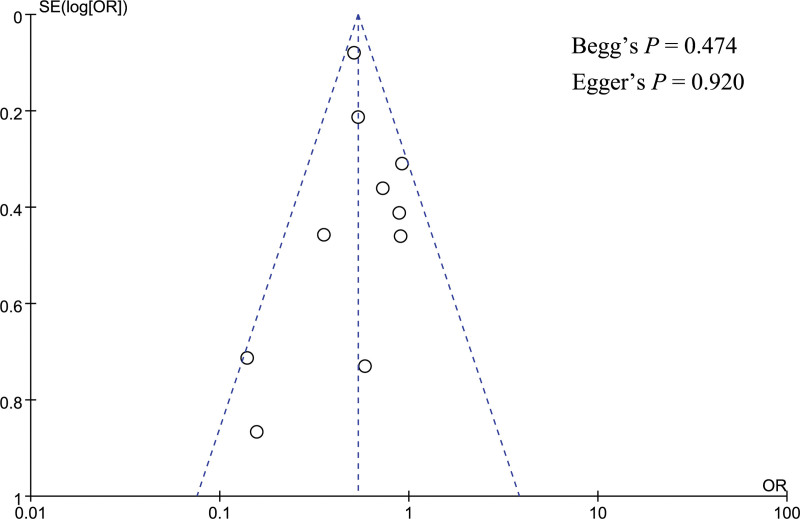
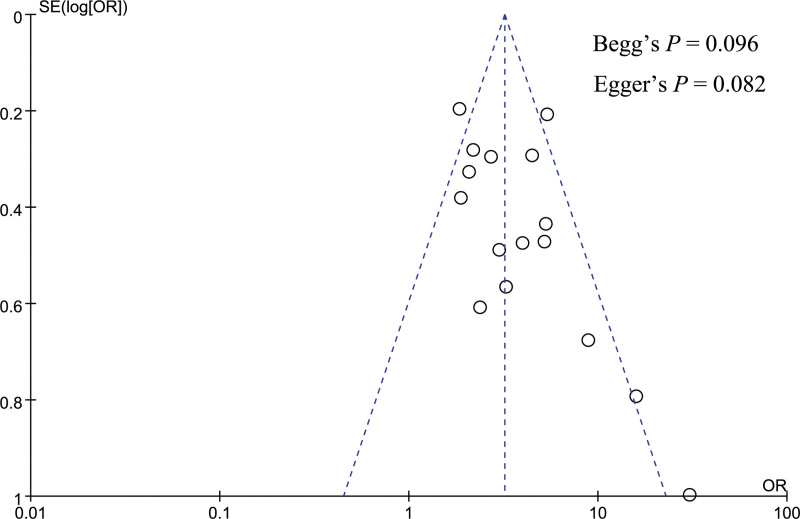
Publication bias was performed for gender, pancreatic texture, and pathology type. A funnel plot was utilized to visually assess the publication bias, and the funnel plot looked symmetrical in the analysis of gender, pancreatic texture, and pathology type (Figs. 12–14). Besides, Begg and Egger tests were utilized to quantitatively assess publication bias, and the result showed no significant publication bias for gender (Begg test P = .064, Egger test P = .106), pancreatic texture (Begg test P = .096, Egger test P = .082), and pathology type (Begg test P = .474, Egger test P = .920).
Figure 12.
Funnel plot showed no significant publication bias in the analysis of gender.
Figure 14.
Funnel plot showed no significant publication bias in the analysis of pathology type.
Figure 13.
Funnel plot showed no significant publication bias in the analysis of pancreatic texture.
4. Discussion
CR-POPF is a very common and troublesome complication after PD and is also associated with other complications such as postoperative bleeding, abdominal infection, and delayed gastric emptying. Therefore, it has an essential significance to prevent the occurrence of CR-POPF after PD. Identifying the risk factors of CR-POPF can provide a basis for the prevention and management of CR-POPF. The current meta-analysis showed males, BMI >25 kg/m2, pancreatic duct diameter <3 mm, soft pancreatic texture, and blood transfusion were significant risk factors of CR-POPF. Pancreatic adenocarcinoma, vascular resection, and preoperative chemoradiotherapy were significant protective factors of CR-POPF. Diabetes mellitus appears to reduce the incidence of CR-POPF, but more studies are needed to determine whether it is statistically significant.
As we know, BMI is a measure of overweight and obesity, and increased BMI is associated with perioperative adverse events such as longer operation time and postoperative incision infection.[37] Our meta-analysis indicated that BMI >25 kg/m2 could significantly increase the risk of CR-POPF after PD (OR = 1.98, 95% CI: 1.23–3.18, P < .001). The study by Ellis et al[27] demonstrated that compared with BMI <25 kg/m2, higher BMI could increase the risk of CR-POPF (OR = 1.40 for BMI = 25–30 kg/m2, OR = 1.97 for BMI >30 kg/m2). Increased BMI is associated with fatty pancreas and soft pancreatic remnant; studies[40,41] showed that patients with POPF usually had significantly increased pancreatic fat and decreased pancreatic fibrosis.
Pancreatic duct diameter <3 mm and soft pancreatic texture were generally recognized risk factors of CR-POPF. The current meta-analysis showed that pancreatic duct diameter <3 mm could increase the risk by 1.87 times, and soft pancreatic texture could increase the risk of CR-POPF by 3.49 times. A smaller pancreatic duct diameter usually means a preserved exocrine function and more challenges for the reconstruction of the anastomosis, all of these can increase the risk of CR-POPF. Akamatsu et al[42] found that POPF was associated with the ratio of main pancreatic duct diameter to pancreatic body (MPD index), and compared with MPD index ≥0.4, MPD index <0.4 was a significant risk factor (OR = 7.3 for MPD index = 0.2–0.4; OR = 50 for MPD index <0.2). There are several reasons why soft pancreatic texture can increase the risk of CR-POPF: the soft pancreas usually has a larger pancreatic body and a smaller pancreatic duct diameter: the soft pancreas generally has a smaller fibrosis ratio and a larger lobular ratio, which is associated with increased exocrine function; soft pancreas is more likely to be damaged by intraoperative dissection, suturing, and knotting; soft pancreas is more difficult to rebuild the anastomosis.[25,26,42,43]
The current meta-analysis showed pancreatic adenocarcinoma was a significant protective factor of CR-POPF (OR = 0.54, 95% CI: 0.47–0.61, P < .001). Besides, studies[13,44] showed that chronic pancreatitis was also a protective factor of POPF. Nonpancreatic adenocarcinomas such as biliary, periampullary, and neuroendocrine tumors were usually characterized by soft and nonfibrous pancreas. However, pancreatic adenocarcinoma and chronic pancreatitis generally had a hard fibrotic pancreas and poor exocrine function, which can decrease the risk of CR-POPF.[13,44,45]
Diabetes mellitus is generally associated with postoperative adverse events. However, the effect of diabetes mellitus on CR-POPF remains controversial. Mathur et al[41] found that patients with diabetes mellitus had more fibrosis and less fat in their pancreas and had less incidence of POPF compared with those without diabetes mellitus. Our meta-analysis showed that diabetes mellitus could reduce the incidence of CR-POPF, but was not statistically significant (OR = 0.66, 95% CI: 0.40–1.08, P = .10). Five studies were included in the analysis of diabetes mellitus, of which 2 studies showed diabetes mellitus was a significant protective factor of CR-POPF, and 3 studies showed no significant association between diabetes mellitus and CR-POPF. However, the analysis of diabetes mellitus had a high heterogeneity, and a further sensitivity analysis was performed. The result after sensitivity analysis showed that diabetes mellitus could significantly decrease the risk of CR-POPF. Therefore, further studies are necessary to identify the relationship between diabetes mellitus and CR-POPF. The effect of diabetes mellitus on CR-POPF may be related to its types and onset time.
Blood transfusion was a significant risk factor for CR-POPF in this meta-analysis. The reason that blood transfusion could increase the risk of CR-POPF was related to a large amount of intraoperative blood loss. Studies[13,14] showed that intraoperative blood loss ≥1000 mL could significantly increase the risk of CR-POPF. Chen et al[18] found that intraoperative blood loss ≥800 mL and blood transfusion were associated with an increased risk of CR-POPF (OR = 3.45, P < .001). Therefore, surgeons should minimize intraoperative bleeding in order to reduce the risk of CR-POPF.
The current meta-analysis showed that intraoperative vascular resection was a protective factor of CR-POPF after PD. The study by Williamsson et al[36] showed that vascular resection was associated with neoadjuvant therapy and a large tumor, and a large tumor that required vascular resection was more likely to block the pancreatic duct and increase the stiffness of the pancreas. Besides, a recent study by Zettervall et al[46] showed that vascular reconstruction in PD could successfully achieve microscopically negative margins (R0) resection, and venous reconstruction was safe without increasing the 30-day morbidity and mortality, but arterial reconstruction significantly increased 30-day morbidity and mortality.
Neoadjuvant therapy that preoperative chemoradiotherapy for malignant tumors has increased significantly over the last decades. A study[47] showed preoperative chemoradiotherapy was an independent risk factor for symptomatic anastomotic leakage following anterior resection of the rectum. However, preoperative chemoradiotherapy appears to be a protective factor for CR-POPF after PD. And our meta-analysis showed that preoperative chemoradiotherapy could significantly decrease the risk of CR-POPF (OR = 0.68, 95% CI: 0.57–0.81, P < .001). Preoperative chemoradiotherapy was associated with increased pancreatic fibrosis and decreased exocrine function, which can decrease the risk of CR-POPF.[27,34]
5. Strengths and limitations
The current meta-analysis assessed the effect of different factors on CR-POPF after PD, and grade A PF with no clinical impact was excluded. This can help clinicians better judge the risk of CR-POPF after PD, thus providing a basis for the prevention and management of CR-POPF. For high-risk patients, we should be vigilant and take effective measures to prevent the occurrence of CR-POPF. Vuorela et al[48] found that pasireotide could reduce the occurrence of CR-POPF in high-risk patients with small pancreatic duct, soft pancreatic texture, biliary, or neuroendocrine tumors. The study by Lyonell et al[49] showed that duct-to-mucosa or invagination techniques were not statistically associated with CR-POPF for patients with mean size of pancreatic duct, but duct-to-mucosa was preferable for patients with pancreatic duct diameter of >6 mm.
There were also some limitations in this meta-analysis. First, most included studies were retrospective, and more prospective studies were necessary for further assessing the risk factors of CR-POPF. Second, the association between some factors and CR-POPF were not discussed in this meta-analysis because the cutoff value of some factors was inconsistent in different studies or some factors were reported in <3 studies, such as age, hypoproteinemia, surgical techniques, etc.
6. Conclusion
The current meta-analysis indicated that males, BMI >25 kg/m2, pancreatic duct diameter <3 mm, soft pancreatic texture, and blood transfusion were significant risk factors of CR-POPF. Pancreatic adenocarcinoma, vascular resection, and preoperative chemoradiotherapy were significant protective factors of CR-POPF. Diabetes mellitus appeared to decrease the incidence of CR-POPF, but this still needs further studies to assess. Effective interventions targeting the above-mentioned risk factors should be investigated in future studies for decreasing the occurrence of CR-POPF.
Author contributions
Conceptualization: Biao Zhang and Dong Shang.
Data curation: Biao Zhang and Qihang Yuan.
Funding acquisition: Dong Shang.
Investigation: Shuang Li and Zhaohui Xu.
Methodology: Xu Chen and Lunxu Li.
Project administration: Dong Shang.
Supervision: Biao Zhang.
Visualization: Biao Zhang and Qihang Yuan.
Writing – original draft: Biao Zhang, Qihang Yuan, Shuang Li, and Zhaohui Xu.
Writing – review and editing: Dong Shang.
Supplementary Material
Abbreviations:
- BMI =
- body mass index
- CI =
- confidence interval
- CR-POPF =
- clinically relevant postoperative pancreatic fistula
- ISGPF =
- International Study Group on Pancreatic Fistula
- NOS =
- Newcastle-Ottawa Scale
- OR =
- odds ratios
- PD =
- pancreatoduodenectomy
- PF =
- pancreatic fistula
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
How to cite this article : Zhang B, Yuan Q, Li S, Xu Z, Chen X, Li L, Shang D. Risk factors of clinically relevant postoperative pancreatic fistula after pancreaticoduodenectomy: A systematic review and meta-analysis. Medicine 2022;101:26(e29757).
Supplemental Digital Content is available for this article.
The authors have no conflicts of interest to disclose.
The type of study is systematic review and meta-analysis, the whole process of research does not involve human trials, the data used in the institute are obtained through published literature, so ethical approval is not necessary for this study.
Contributor Information
Biao Zhang, Email: afn199505@163.com.
Qihang Yuan, Email: qihangdy@163.com.
Shuang Li, Email: 253609295@qq.com.
Zhaohui Xu, Email: dmuzhaohui@163.com.
Xu Chen, Email: 1169777928@qq.com.
Lunxu Li, Email: 253609295@qq.com.
References
- [1].Ausania F, Landi F, Martinez-Perez A, et al. A meta-analysis of randomized controlled trials comparing laparoscopic vs open pancreaticoduodenectomy. HPB (Oxford). 2019;21:1613–20. [DOI] [PubMed] [Google Scholar]
- [2].Allen PJ, Gönen M, Brennan MF, et al. Pasireotide for postoperative pancreatic fistula. N Engl J Med. 2014;370:2014–22. [DOI] [PubMed] [Google Scholar]
- [3].Bassi C, Marchegiani G, Dervenis C, et al. The 2016 update of the international study group (ISGPS) definition and grading of postoperative pancreatic fistula: 11 years after. Surgery. 2017;161:584–91. [DOI] [PubMed] [Google Scholar]
- [4].Huang R, Liu B, Chen H, et al. Risk factors and medico-economic effect of pancreatic fistula after pancreaticoduodenectomy. Gastroenterol Res Pract. 2015;2015:917689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Liu Q-Y. Analysis of risk factors for postoperative pancreatic fistula following pancreaticoduodenectomy. World J Gastroenterol. 2014;20:17491–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Liu R, Cai Y, Cai H, et al. Dynamic prediction for clinically relevant pancreatic fistula: a novel prediction model for laparoscopic pancreaticoduodenectomy. BMC Surg. 2021;21:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Lin JW, Cameron JL, Yeo CJ, et al. Risk factors and outcomes in postpancreaticoduodenectomy pancreaticocutaneous fistula. J Gastrointest Surg. 2004;8:951–9. [DOI] [PubMed] [Google Scholar]
- [8].Hu BY, Wan T, Zhang WZ, et al. Risk factors for postoperative pancreatic fistula: analysis of 539 successive cases of pancreaticoduodenectomy. World J Gastroenterol. 2016;22:7797–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Akgul O, Merath K, Mehta R, et al. Postoperative pancreatic fistula following pancreaticoduodenectomy-stratification of patient risk. J Gastrointest Surg. 2019;23:1817–24. [DOI] [PubMed] [Google Scholar]
- [10].Moher D, Liberati A, Tetzlaff J, et al. PRISMA group. preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535–b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25:603–5. [DOI] [PubMed] [Google Scholar]
- [12].Peng YP, Zhu XL, Yin LD, et al. Risk factors of postoperative pancreatic fistula in patients after distal pancreatectomy: a systematic review and meta-analysis. Sci Rep. 2017;7:185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Pratt WB, Callery MP, Vollmer CM, Jr. Risk prediction for development of pancreatic fistula using the ISGPF classification scheme. World J Surg. 2008;32:419–28. [DOI] [PubMed] [Google Scholar]
- [14].Kawai M, Kondo S, Yamaue H, et al. Predictive risk factors for clinically relevant pancreatic fistula analyzed in 1,239 patients with pancreaticoduodenectomy: multicenter data collection as a project study of pancreatic surgery by the Japanese society of hepato-biliary-pancreatic surgery. J Hepatobiliary Pancreat Sci. 2011;18:601–8. [DOI] [PubMed] [Google Scholar]
- [15].Hiyoshi M, Chijiiwa K, Fujii Y, et al. Usefulness of drain amylase, serum C-reactive protein levels and body temperature to predict postoperative pancreatic fistula after pancreaticoduodenectomy. World J Surg. 2013;37:2436–42. [DOI] [PubMed] [Google Scholar]
- [16].Malleo G, Mazzarella F, Malpaga A, et al. Diabetes mellitus does not impact on clinically relevant pancreatic fistula after partial pancreatic resection for ductal adenocarcinoma. Surgery. 2013;153:641–50. [DOI] [PubMed] [Google Scholar]
- [17].Kanda M, Fujii T, Suenaga M, et al. Estimated pancreatic parenchymal remnant volume accurately predicts clinically relevant pancreatic fistula after pancreatoduodenectomy. Surgery. 2014;156:601–10. [DOI] [PubMed] [Google Scholar]
- [18].Chen JY, Feng J, Wang XQ, et al. Risk scoring system and predictor for clinically relevant pancreatic fistula after pancreaticoduodenectomy. World J Gastroenterol. 2015;21:5926–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Fujiwara Y, Shiba H, Shirai Y, et al. Perioperative serum albumin correlates with postoperative pancreatic fistula after pancreaticoduodenectomy. Anticancer Res. 2015;35:499–503. [PubMed] [Google Scholar]
- [20].Nagai M, Sho M, Akahori T, et al. Impact of preoperative asymptomatic renal dysfunction on clinical course after pancreatoduodenectomy. J Hepatobiliary Pancreat Sci. 2015;22:810–8. [DOI] [PubMed] [Google Scholar]
- [21].Nishida Y, Kato Y, Kudo M, et al. Preoperative sarcopenia strongly influences the risk of postoperative pancreatic fistula formation after pancreaticoduodenectomy. J Gastrointest Surg. 2016;20:1586–94. [DOI] [PubMed] [Google Scholar]
- [22].Yamashita Y, Yoshizumi T, Fukuzawa K, et al. Surgical results of pancreaticoduodenectomy for pancreatic ductal adenocarcinoma: a multi-institutional retrospective study of 174 patients. Anticancer Res. 2016;36:2407–12. [PubMed] [Google Scholar]
- [23].Kantor O, Talamonti MS, Pitt HA, et al. Using the NSQIP pancreatic demonstration project to derive a modified fistula risk score for preoperative risk stratification in patients undergoing pancreaticoduodenectomy. J Am Coll Surg. 2017;224:816–25. [DOI] [PubMed] [Google Scholar]
- [24].Partelli S, Pecorelli N, Muffatti F, et al. Early postoperative prediction of clinically relevant pancreatic fistula after pancreaticoduodenectomy: usefulness of c-reactive Protein. HPB (Oxford). 2017;19:580–6. [DOI] [PubMed] [Google Scholar]
- [25].Xia W, Zhou Y, Lin Y, et al. A predictive risk scoring system for clinically relevant pancreatic fistula after pancreaticoduodenectomy. Med Sci Monit. 2018;24:5719–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Ke Z, Cui J, Hu N, et al. Risk factors for postoperative pancreatic fistula: analysis of 170 consecutive cases of pancreaticoduodenectomy based on the updated ISGPS classification and grading system. Medicine (Baltimore). 2018;97:e12151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Ellis RJ, Brock Hewitt D, Liu JB, et al. Preoperative risk evaluation for pancreatic fistula after pancreaticoduodenectomy. J Surg Oncol. 2019;119:1128–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Kang JS, Park T, Han Y, et al. Clinical validation of scoring systems of postoperative pancreatic fistula after pancreatoduodenectomy: applicability to Eastern cohorts? Hepatobiliary Surg Nutr. 2019;8:211–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Li Y, Zhou F, Zhu DM, et al. Novel risk scoring system for prediction of pancreatic fistula after pancreaticoduodenectomy. World J Gastroenterol. 2019;25:2650–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Zarzavadjian Le Bian A, Fuks D, Montali F, et al. Predicting the severity of pancreatic fistula after pancreaticoduodenectomy: overweight and blood loss as independent risk factors: retrospective analysis of 277 patients. Surg Infect (Larchmt). 2019;20:486–91. [DOI] [PubMed] [Google Scholar]
- [31].Abe T, Amano H, Kobayashi T, et al. Preoperative anthropomorphic and nutritious status and fistula risk score for predicting clinically relevant postoperative pancreatic fistula after pancreaticoduodenectomy. BMC Gastroenterol. 2020;20:264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Bardol T, Delicque J, Hermida M, et al. Neck transection level and postoperative pancreatic fistula after pancreaticoduodenectomy: a retrospective cohort study of 195 patients. Int J Surg. 2020;82:43–50. [DOI] [PubMed] [Google Scholar]
- [33].Ohgi K, Okamura Y, Sugiura T, et al. Pancreatic attenuation on computed tomography predicts pancreatic fistula after pancreaticoduodenectomy. HPB (Oxford). 2020;22:67–74. [DOI] [PubMed] [Google Scholar]
- [34].Tabchouri N, Bouquot M, Hermand H, et al. A novel pancreatic fistula risk score including preoperative radiation therapy in pancreatic cancer patients. J Gastrointest Surg. 2021;25:991–1000. [DOI] [PubMed] [Google Scholar]
- [35].Utsumi M, Aoki H, Nagahisa S, et al. Preoperative nutritional assessment using the controlling nutritional status score to predict pancreatic fistula after pancreaticoduodenectomy. In Vivo. 2020;34:1931–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Williamsson C, Stenvall K, Wennerblom J, et al. Predictive factors for postoperative pancreatic fistula—a Swedish nationwide register-based study. World J Surg. 2020;44:4207–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Zou SY, Wang WS, Zhan Q, et al. Higher body mass index deteriorates postoperative outcomes of pancreaticoduodenectomy. Hepatobiliary Pancreat Dis Int. 2020;19:163–8. [DOI] [PubMed] [Google Scholar]
- [38].Huang XT, Huang CS, Liu C, et al. Development and validation of a new nomogram for predicting clinically relevant postoperative pancreatic fistula after pancreatoduodenectomy. World J Surg. 2021;45:261–269. [DOI] [PubMed] [Google Scholar]
- [39].Nakamura K, Sho M, Kinoshita S, et al. New insight into the association between bile infection and clinically relevant pancreatic fistula in patients undergoing pancreatoduodenectomy. J Hepatobiliary Pancreat Sci. 2020;27:992–1001. [DOI] [PubMed] [Google Scholar]
- [40].Gaujoux S, Cortes A, Couvelard A, et al. Fatty pancreas and increased body mass index are risk factors of pancreatic fistula after pancreaticoduodenectomy. Surgery. 2010;148:15–23. [DOI] [PubMed] [Google Scholar]
- [41].Mathur A, Pitt HA, Marine M, et al. Fatty pancreas: a factor in postoperative pancreatic fistula. Ann Surg. 2007;246:1058–64. [DOI] [PubMed] [Google Scholar]
- [42].Akamatsu N, Sugawara Y, Komagome M, et al. Risk factors for postoperative pancreatic fistula after pancreaticoduodenectomy: the significance of the ratio of the main pancreatic duct to the pancreas body as a predictor of leakage. J Hepatobiliary Pancreat Sci. 2010;17:322–8. [DOI] [PubMed] [Google Scholar]
- [43].Callery MP, Pratt WB, Kent TS, et al. A prospectively validated clinical risk score accurately predicts pancreatic fistula after pancreatoduodenectomy. J Am Coll Surg. 2013;216:1–14. [DOI] [PubMed] [Google Scholar]
- [44].Büchler M, Friess H, Klempa I, et al. Role of octreotide in the prevention of postoperative complications following pancreatic resection. Am J Surg. 1992;163:125–30. [DOI] [PubMed] [Google Scholar]
- [45].Aoki S, Miyata H, Konno H, et al. Risk factors of serious postoperative complications after pancreaticoduodenectomy and risk calculators for predicting postoperative complications: a nationwide study of 17,564 C in Japan. J Hepatobiliary Pancreat Sci. 2017;24:243–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Zettervall SL, Ju T, Holzmacher JL, et al. Arterial, but not venous, reconstruction increases 30-day morbidity and mortality in pancreaticoduodenectomy. J Gastrointest Surg. 2019;24:578–84. [DOI] [PubMed] [Google Scholar]
- [47].Matthiessen P, Hallböök O, Andersson M, et al. Risk factors for anastomotic leakage after anterior resection of the rectum. Colorectal Dis. 2004;6:462–9. [DOI] [PubMed] [Google Scholar]
- [48].Vuorela T, Harri M, Arto K, et al. Pasireotide administration after pancreaticoduodenectomy may decrease clinically relevant postoperative pancreatic fistula in high-risk patients with small pancreatic ducts, soft pancreatic parenchyma and cystic or neuroendocrine neoplasia. Pancreatology. 2020;20:757–61. [DOI] [PubMed] [Google Scholar]
- [49].Kone LB, Maker VK, Banulescu M, et al. A propensity score analysis of over 12,000 pancreaticojejunal anastomoses after pancreaticoduodenectomy: does technique impact the clinically relevant fistula rate? HPB (Oxford). 2020;22:1394–401. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.