Abstract
The condition of collateral pathways is an important predictor of stroke prognoses; however the major determinants of collaterals are still unknown. The purpose of this study is to identify potentially determinants for collateral circulation status in patients with chronic occlusion of cerebral arterial circle.
All patients with chronic occlusion of either unilateral internal carotid artery or middle cerebral artery M1 or M2 segment, diagnosed by digital subtraction angiography at the neurology department of the First Medical Centre of Chinese PLA General Hospital from January 2015 to December 2017, were retrospectively collected in our sample. After screening according to inclusion and exclusion criteria, the patients’ relevant clinical data were collected and analyzed. Collateral circulations were assessed by 2 independent raters using the American society of interventional and therapeutic neuroradiology/society of interventional radiology flow-grading system.
Baseline characteristics (n = 163): our sample consists of 116 (71.2%) male and 47 (28.8%) female patients with an average age of 57.5 ± 11.9 years. Cerebral collateral flow was poor in 59 (36.2%) patients. Our univariate analyses showed that poor collateral circulation was associated with lower high-density lipoproteins cholesterol (HDL), elevated homocysteine levels, aging and hyperlipidemia. A multivariate analysis identified HDL, homocysteine levels and ageing as major predictors for collateral circulation status. In the subgroup analysis, the HDL contributed to collateral angiogenesis internal carotid artery occlusion group. In the middle cerebral artery occlusion group, the homocysteine and ageing were related to the poor collateral status.
Low HDL, high levels of homocysteine and ageing are identified as possible risk factors for a poor collateral vessel blood flow in patients with chronic anterior circulation occlusion.
Keywords: ageing, chronic anterior circulation occlusion, collateral circulation, high density lipoproteins, homocysteine
1. Introduction
Leptomeningeal collaterals are part of a preexisting[1] network that cross-connect a small number of the most distant arterioles of the intracerebral system.[1,2] These collaterals are endogenous bypasses capable of maintaining blood flow in case of a primary blood flow disruption, guarding against ischemic brain damage.[2] Cerebral collaterals are important to cerebrovascular reserve, especially for patients with artery obstruction. Additionally, collaterals is an important predictor of clinical outcome and response to acute stroke therapy.[3–5] Measures of collateral flow on noninvasive modalities and angiography might help to define specific regions of brain at risk of infarction and hemorrhage. Moreover, both ischemia and reperfusion, determinants of outcome and hemorrhagic transformation, are influenced by collaterals.[6] Good collateral status is associated with higher rates of favorable functional outcome, and lower rates of intracranial hemorrhage and mortality, in patients with acute ischemic stroke receiving endovascular therapies.[7]
However, factors that dictate the collateral vascular distribution and perfusion are still largely unknown. Animal studies point to genetic polymorphisms in genes that control the formation and postobstruction remodeling of collateral circulation,[8,9] as well as milieu interne factors, such as age[1,10,11] and blood pressure. The Rabep2, a novel gene involved in VEGFA-Flk1 signaling and required for formation of collaterals during development, is crucial to the formation of collateral and collateral formation is abolished in mice lacking Rabep2.[12] In mice, ageing, hypertension, metabolic syndrome, and other cardiovascular-stroke risk factors cause pruning away of pial collaterals and a smaller diameter in those that remain.[13–16] Findings for pial collateral grading in humans are consistent with those for aging and metabolic syndrome.[1,17]Hypertension[18,19] and the use of statin[10] are both associated with thinning of collaterals. Many factors likely contribute to the observed phenotypic variation in cerebral collaterals, and the role played by age and various risk factors for cardiovascular diseases in the primitive formation of collateral vessels is yet to be investigated.
Our study was to determine which factors are good indicators for the state of collateral vasculature in patients with the chronic occlusion of cerebral arterial circle. The grade scale of collateral filling is American Society of Intervention and Therapeutic Neuroradiology/ Society of Interventional Radiology (ASITN/SIR), which describes collateral filling of the ischemic territory, measuring arterial vigor and could be used in any territorial occlusion.[6] By the investigation for the determinants of cerebral collaterals, we hope to find the method to improve the function and extend of collaterals.
2. Materials and methods
2.1. Patients
Our sample was taken from the list of patients diagnosed with the occlusion of cerebral arterial circle who were admitted into the neurology department of the First Medical Centre of Chinese People Liberation Army (PLA) General Hospital. This is a retrospective observational study carried out from January 2015 to December 2017. The study was approved by Ethics Committee of Chinese PLA General Hospital and informed consent was obtained from all patients. The following criteria need to be fulfilled for patients to be included in this study: (a) The occlusion of either unilateral internal carotid artery or middle cerebral artery M1 or M2 segment was confirmed by digital subtraction angiography (DSA); (b) Patient information were completed after admission; (c) The entire episode of disease lasts for at least 4 weeks (to be characterized as a chronic illness). Patients were excluded if: (a) Occlusion was located in the posterior circulation, including occlusion of the basilar and vertebral arteries; (b) Patient had previously experienced subarachnoid hemorrhage or has had intracranial artery malformation or tumors; (c) Patient was diagnosed by Multiple arteritis(diagnosed by the American College of Rheumatology classification criteria[20]); (d) There were bilateral occlusions of internal carotid artery or middle cerebral artery; (e)Patient was diagnosed with coexisting Moyamoya disease(diagnosed by the Japanese research committee diagnostic criteria[21]).
2.2. Clinical variables
Age, gender, BMI, the presence or absence of hypertension, diabetes, and hyperlipidemia, whether there is a medical history of coronary heart disease or ischemic stroke, or a history of tobacco or alcohol abuse were all recorded. Laboratory parameters (including hematocrit, blood uric acid, triglyceride, cholesterol, homocysteine, low-density lipoproteins (LDL), high density lipoproteins (HDL) levels) upon admission were collected as individual baseline values. Diagnoses of hypertension, diabetes, hyperlipidemia, coronary heart disease, or ischemic stroke was made either in a tertiary hospital prior to admission by our hospital or at our own facility. A smoking history of was at least 10 cigarettes per day for over 5 years. An excessive drinking habit, more than 5 years, of consuming over 50 g of alcohol per day was recorded.
2.3. Imaging variables
A Philips Allure X-per FD20 Biplane DSA machine (Philips Medical Systems, The Netherlands) was used to perform each patient’s DSA. The radiographs were taken at 4 inches per seconds. Vascular distributions of the internal carotid artery, middle cerebral artery, external carotid artery and the vertebral artery, as well as the structural integrity of the circle of Willis, were noted in details. Any hypoplasia (vessel diameter <1.0 mm) or missing of either the posterior cerebral artery or the anterior cerebral artery was recorded. The anterior circle of Willis pathway was considered incomplete when the anterior communicating artery could not be observed or when the A1 segment of any of the anterior cerebral arteries was hypoplastic, absent or both. Likewise, its posterior circle was considered incomplete when the posterior communicating artery was not visible or when there was any hypoplastic or missing P1 segment of one of posterior cerebral arteries. An external carotid or vertebral stenosis was considered severe if the degree of obstruction was >70%.
All DSA images were evaluated by American society for interventional and therapeutic neuroradiology/interventional radiology(ASITN/SIR) collateral flow grading system[22]:
Grade 0: No collateral to the ischemic site.
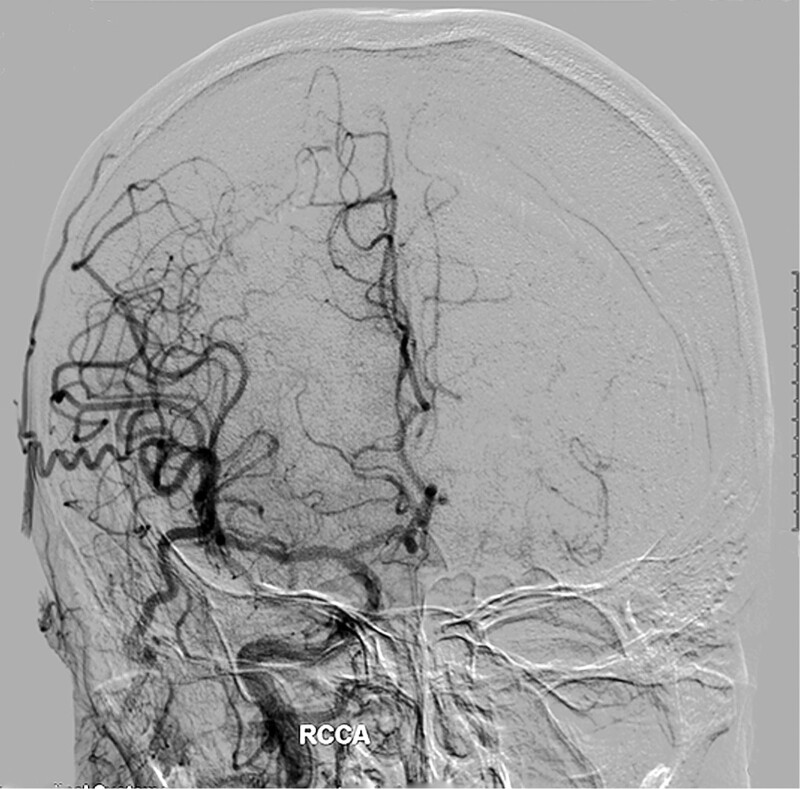
Grade 1: Slow collaterals to the periphery of the ischemic site with persistence of some of the defect (Fig. 1).
Figure 1.
A 58-year-old man with left internal carotid artery occlusions, the compensation of collaterals from the right internal carotid artery, external carotid artery and the left vertebral artery to the left hemisphere is insufficient and slow. ASITN/SIR collateral flow grading system: 1.
Grade 2: Rapid collaterals to the periphery of ischemic site with persistence of some of the defect and to only a portion of the ischemic territory.
Grade 3: Collaterals with slow but complete angiographic blood flow of the ischemic bed by the late venous phase.
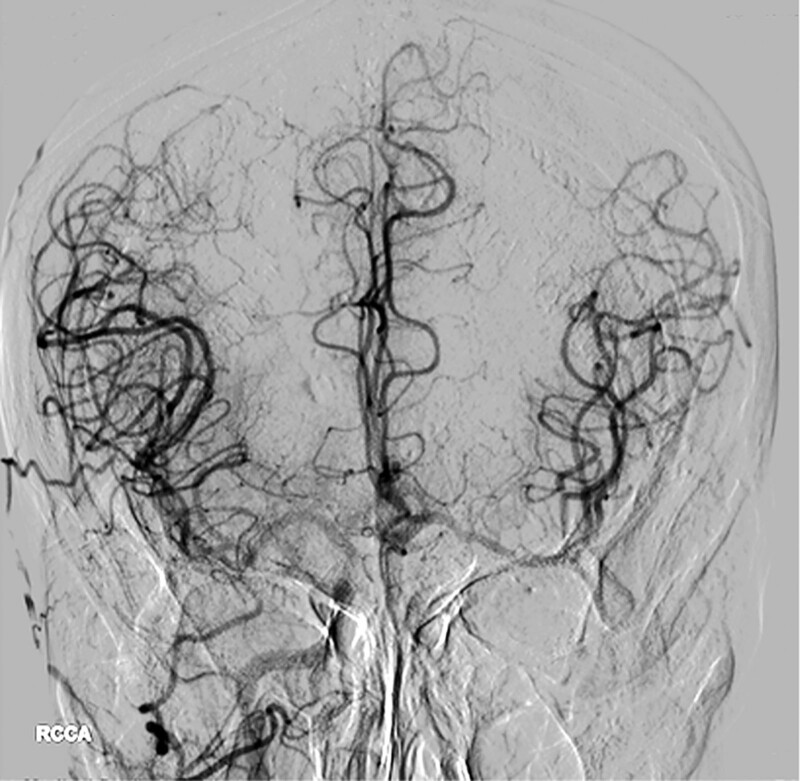
Grade 4: Complete and rapid collateral blood flow to the vascular bed in the entire ischemic territory by retrograde perfusion (Fig. 2).
Figure 2.
A 79-year-old man with left internal carotid artery occlusions, the compensation of collaterals from right hemisphere to left hemisphere is full and rapid. ASITN/SIR collateral flow grading system: 4.
A score of 0–2 was perceived as a poor grading, and a collateral flow that received a score of 3–4 was considered good, respectively.
Two physicians (PCH and ZDF) independently grade collaterals status to minimize the bias. If there was any disagreement between 2 reviewers that not solved by discussion, a third reviewer (Wang) decided the final grade.
2.4. Statistical analyses
Statistical analyses were performed with SPSS 24. The outcome was the collateral grade dichotomized as 0–2 (poor) vs 3–4 (good). Less than 3% of data were missing and the median values were imputed to fill the gaps.
Univariate analyses were first conducted for all the candidates. Statistically significant variables in univariate analyses were included in the multivariate logistic regression model. In addition, whether there is multi-collinearity among the independent variables was assessed using Spearman correlation for multi-comparisons. Potentially correlated variables (R > .5) were not included in the same model. Our specific model was built by the means of forward selection until a simple model incorporating only statistically significant variables (P < .05) was obtained. A 2-sided P value of <.05 was considered statistically significant.
3. Results
A total of 181 patients were diagnosed with chronic occlusions of anterior circulation. After excluding 15 patients with acute arterial occlusion and another 3 patients missing their necessary relevant personal information, we included 163 patients in the final analyses, among whom 116 (71.2%) patients were male. Their mean age was 57.5 (SD: ±11.9). Majority of the patients had hypertension (66.2%) and most of them suffered the incomplete Willis (73%). There is no patient was diagnosed Moyamoya disease or Multiple arteritis according to the diagnostic criteria. There were no patients with grade 0 and only 7 with grade1. In order to reduce the bias of the results, they were divided into poor collateral circulation (grade 0–2) and good collateral circulation (grades 3–4) for comparative analysis. Collateral flow was poor in 59 (36.2%) patients and good in 104 (63.8%) of them. Baseline characteristics of our sample and research outcomes are summarized in Table 1.
Table 1.
Variables stratified by good vs poor collateral status on DSA.
| Poor collateral | Good collateral | P value | |
|---|---|---|---|
| n | 59 | 104 | |
| Age [mean (sd)] | 61.07 (9.74) | 55.46 (12.52) | 0.003* |
| Smoking (%) | 31 (52.5) | 42 (40.4) | 0.181 |
| Alcohol (%) | 18 (30.5) | 26 (25.0) | 0.563 |
| Hypertension (%) | 40 (67.8) | 68 (65.4) | 0.754 |
| Hyperlipidemia (%) | 15 (25.4) | 12 (11.5) | 0.038* |
| Coronary heart disease (%) | 17 (28.8) | 23 (22.1) | 0.444 |
| diabetes (%) | 18 (30.5) | 36 (34.6) | 0.717 |
| Stroke (%) | 23 (39.0) | 30 (28.8) | 0.249 |
| BMI (median [IQR]) | 27.45 | 27 | 0.253 |
| ECA = 1 (%) | 3 (5.1) | 12 (11.5) | 0.277 |
| Willis = 1 (%) | 46 (78.0) | 73 (70.2) | 0.373 |
| ACA = 1 (%) | 7 (11.9) | 15 (14.4) | 0.825 |
| PCA = 1 (%) | 5 (8.5) | 13 (12.5) | 0.598 |
| Vertebral = 1 (%) | 13 (22.0) | 34 (32.7) | 0.206 |
| Gender = 2 (%) | 12 (20.3) | 35 (33.7) | 0.104 |
| SP (mean (sd)) | 133.64 (18.31) | 130.34 (15.27) | 0.219 |
| DP (mean (sd)) | 78.31 (9.45) | 78.46 (10.86) | 0.926 |
| homocysteine (mean (sd)) | 18.46 (9.86) | 14.72 (6.52) | 0.004* |
| uric acid (mean (sd)) | 306.69 (95.93) | 304.90 (77.21) | 0.897 |
| Triglyceride (mean (sd)) | 1.45 (0.78) | 1.38 (0.56) | 0.536 |
| Cholesterol (mean (sd)) | 3.47 (0.93) | 3.66 (0.85) | 0.203 |
| LDL (mean (sd)) | 2.09 (0.80) | 2.19 (0.75) | 0.424 |
| HDL (mean (sd)) | 0.94 (0.23) | 1.08 (0.34) | 0.009* |
| Platelet (mean (sd)) | 218.04 (60.93) | 220.82 (55.98) | 0.768 |
Abbreviations: ACA = 1: the A1 segment of one of anterior cerebral arteries was hypoplasia, absent, DP: diastolic pressure, ECA = 1: stenosis of external carotid artery was >70%, HDL: high density lipoprotein, LDL: low density lipoprotein, PCA = 1: the P1 segment of one of posterior cerebral arteries was hypoplasia, absent, SP: systolic pressure, Vertebral = 1: stenosis of vertebral artery were considered was >70%, Willis = 1: the circle of Willis was incomplete. *P < .05.
Univariate analyses showed that patients who had relatively poor collateral circulation were older. Potential medical issues like hyperlipidemia, elevated homocysteine levels, and low levels of HDL were also more prevalent in poor collateral population. The relationships among age, hyperlipidemia, homocysteine, and HDL levels were also explored by the Spearman correlation analysis.
The binary logistic regression model with forward selection demonstrated age as a factor of interest in addition to HDL and homocysteine levels. In addition, low level of HDL, elevated homocysteine level in serum, and old age were identified as relevant factors associated with a poor collateral circulation status (Table 2).
Table 2.
Final model predicting poor collateral status using binary logistic regression.
| variable | OR | 95% of CI | P value |
|---|---|---|---|
| Age | 1.046 | (1.012, 1.081) | 0.007 |
| Homocysteine | 1.055 | (1.006, 1.106) | 0.027 |
| HDL | 0.273 | (0.075, 0.996) | 0.049 |
Abbreviation: HDL = high density lipoprotein.
Because internal carotid artery occlusion and middle cerebral artery occlusion are different case scenarios by which collateral circulation could be affected, subgroup analyses were carried out. Under internal carotid artery occlusion, HDL level in serum was significant indicators for collateral circulation status according to a univariate analysis. Only high level of HDL was proven to be the protective factor (Table 3), as later confirmed by a binary regression. As for the middle cerebral artery occlusion group, age, HDL, homocysteine levels, blood pressure, history of stroke, and tobacco use were all predictors of collateral flow in univariate analysis. Based on additional binary regression analysis, high levels of homocysteine and ageing were associated with a poor collateral flow (Table 4).
Table 3.
Internal carotid artery occlusion group final model predicting poor collateral status using logistic regression.
| variable | OR | 95% of CI | P value |
|---|---|---|---|
| HDL | 0.189 | (0.038, 0.951) | 0.043 |
Abbreviation: HDL: high density lipoprotein.
Table 4.
Middle cerebral artery occlusion group final model predicting poor collateral status using logistic regression.
| variable | OR | 95% of CI | P value |
|---|---|---|---|
| Age | 1.063 | (1.010, 1.118) | 0.019 |
| Homocysteine | 1.087 | (1.020, 1.159) | 0.010 |
4. Discussion
In this study, elevated homocysteine levels and ageing were independently associated with an inadequate collateral flow, and high HDL levels were correlated with a relatively better collateral blood flow among patients with the occlusion of cerebral arterial circle. Furthermore, in the internal carotid artery occlusion group, the HDL contributed to collateral angiogenesis. While in the middle cerebral artery occlusion group, the homocysteine and ageing were related to the poor collateral status.
The primitive conformation of collateral vessels is contingent on genetic and environmental determinants. Animal experiments demonstrated that variants in different genes may govern collateral formation and its remodeling.[8] Previous studies have shown that VEGF,[23] CLIC4,[24] along with the Rabep2[25] gene were the critical determinants. Despite the aforementioned progress, studies in search for corresponding factors in human were rather retarded and had been suffered from problems, such as small sample sizes.[10] Although clinical studies in the coronary vascular have demonstrated that cardiovascular risk factors (including dyslipidemia, hyperglycemia, hypertension, and ageing) may contribute to vulnerability in collateral vascular, the causal mechanisms remain unknown.[26,27] Menon et al found that metabolic syndrome, hyperuricemia, and age are associated with poor collaterals in patients with acute occlusion of anterior circulation.[1] Besides, Faber et al showed that age and hypertension is related to poor posterior communicating collaterals, but hyperlipidemia and metabolic syndrome had no effect.[28] Those studies indicated age was a common factor that related with poor collaterals, but other vascular risk factors and collateral vasculature were not consisted.
4.1. HDL
Low HDL levels remains a powerful independent predictor of elevated cardiovascular risks in patients with ischemic heart disease,[29] even though several clinical trials have demonstrated that substantially raising HDL levels could not lead to an effective reduction in cardiovascular event rates.[30,31] The HDL number may partly represent the function of HDL, but it probably does not represent other protein and lipid in HDL that play important roles in cardio-protect.[32] Thus, the HDL number may be not precise enough to estimate its effectiveness on vessels.
Besides lipid transportation, HDL has clear antiinflammatory traits and antioxidative properties. Furthermore, HDL can be found in myeloid cells, which leads to a suppression of cytokine and chemokine productions, a down-regulation of costimulatory molecules and an inhibition of antigen presentation.[33,34] It also has been documented to dampen endothelial inflammatory activation and to induce the repair by increasing nitric oxide(NO) generation.[35] Moreover, HDL-associated enzymes that have been reported to hydrolyze oxidized phospholipids into lysophosphatidylcholine played a pivotal role in its antioxidative properties.[36] HDL has been shown to prevent the oxidative modification of LDL, thus inhabiting of the macrophage foam-cell generation in the vessels.[37]
The results of a series of preclinical experiments show that the antiinflammatory and antioxidant effects of HDL on endothelial cells ensure the integrity of endothelial cells and then protect blood vessels. The vascular protective effect of HDL may be more obvious in patients with internal carotid artery occlusion. However, in the future, the vascular protective effect of HDL needs to be verified by different HDL proteins.
4.2. Hyperhomocysteine
Homocysteinemia was an independent risk factor for stroke associated with atherosclerotic vascular diseases.[38,39] Previous study found that a decline in serum homocysteine was strongly linked to a reduction in stroke risks in Chinese adults with hypertension.[39]
High plasma concentration of homocysteine leaves patients more susceptible to small and large vessel disease.[40] Moreover, homocysteine can result in an accumulation of endothelial NO synthase (eNOS) inhibitor asymmetric dimethylarginine,[38] which reduces the amount of eNOS. Endothelial dysfunction is considered the first step of atherogenesis, an underlying mechanism for which could be the attenuation of eNOS. The eNOS destruction leads to a reduced-bioavailability of NO and increased inflammation. Not only can homocysteine reduce the bioavailability of NO, it can also directly give rise to endothelial incompleteness. Thiolactone generated by homocysteine attracts lysine-rich proteins and potentially triggers an endoplasmic reticulum stress-related endothelial apoptotic response.[41] In addition, homocysteine was a potent excitatory neurotransmitter that promotes oxidative stress and facilitates calcium influx at the cellular level,[42]which could directly lead to endothelial apoptotic.
The decrease of eNOS and the injury of endothelial cells caused by homocysteine led to vasodilation dysfunction and atherogenesis, resulting in the decrease of collateral vessel density and diameter. Therefore, for patients with chronic vascular occlusion, it is especially necessary to control homocysteine to a lower level.
5. Ageing
Ageing was associated with increased ischemic mortality and atherosclerotic incidence.[43] Focal narrowing of the arteries, one of the most important characteristics of arterial atherosclerosis, makes carotid artery stenosis highly likely to evolve into an ischemic event. The incidence of carotid artery stenosis increased from 1% in the population that falls into the age range from 50 to 59 to as high as 10% in the population aged 70 and above.[44] With the increasing age, accelerated cellular degeneration, brought about by ageing, may exacerbate the sole aging of vessel walls in atherosclerosis. Previous studies have proven senior as an established risk factor in progressing atherosclerosis,[43,45,46] which has been classified as a type of geriatric diseases. In addition, aging of the vessel walls could reduce the number of vascular smooth muscle cells (VSMC), increase collagen deposition, and render elastin lamellae more vulnerable to fracture, leading to a reduced flow-mediated dilation and increased stiffness.[46]
Aged endothelial cells (ECs) show attenuated eNO production and increased susceptibility to apoptosis, and monocyte migration into the vessel wall is enhanced.[43] Moreover, as ECs under laminar shear stress upregulate antioxidant enzymes,[47] turbulent flow at the plaque formation sites can in turn reduce antioxidant capacity. In vitro studies have shown that ageing VSMCs has more oxidative stress-induced damages, probably due to increased reactive oxygen species (ROS) generation and antioxidant defense impairment.[48–50] VSMC senescence leads to its reduced proliferation and makes the cell more susceptible to apoptosis, thus promoting atherosclerosis progression and inhibiting plaque repair.[43] Increased oxidative phosphorylation and mitochondrial deoxyribonucleic acid damage can increase ROS generation. Both vascular ageing and cellular senescence are associated with increased expression of pro-inflammatory cytokines and adhesion molecules, further promoting persistent inflammation, which can aggravate the whole atherosclerotic crisis.[45]
The ageing of ECs and VSMC will cause the increase of inflammation and apoptosis, the decrease of antioxidant capacity and vasodilation dysfunction will lead to the increase of collateral vascular atherosclerosis and vascular wall injury, resulting in the decrease of collateral density.
Our study has limitations. The “HDL cholesterol hypothesis” has been substituted by the “HDL function/flux hypothesis”.[51] Samia Mora et al found that the number of HDL particles (HDL-P) may be a better marker of residual risk than the chemically-measured HDL level,[52] thus HDL-P could a better independent variable to gauge the function of HDL. Furthermore, a binary classification of the dependent variable (i.e., collateral circulation status) was adapted, which may oversimplify the complex pathophysiology of collateral hemodynamics. Lastly, there are only Eastern Asian included in our analysis, so the population bias is inevitable. As such, our finding may only apply to population we studied. Nevertheless, our study provides an insight to understanding the factors effected the formation of collateral vascular and the controllable factors, such as HDL and homocysteine, could be the appropriate therapeutic target to enhance collaterals.
6. Conclusions
In conclusion, low HDL level, high level of homocysteine and ageing were found to retard a sophisticated development of cerebral collaterals in our study. The clinical physicians need to take more attention to the level of HDL and homocysteine for patients with chronic occlusion of internal carotid artery or middle cerebral artery. The elevated HDL and low homocysteine may be beneficial to the formation of cerebral collaterals. As stated above, more studies on clinical trials and therapeutic strategies in enhancing collateral maturation and its functional augmentation are still needed.
Acknowledgment
The authors thank the professor Xinyuan Tong, Department of Statistics, the First Medical Centre, Chinese PLA General Hospital, for his contribution in statistical analysis.
Author contributions
Pi Chenghui: the design of research protocol, data collection, the grade of collateral flow, data analysis and article composition
Wang Jun: the correction and guidance of the protocol and article and provide the data resources
Zhao Dengfa: the grade of collateral flow
Yu Shengyuan: the guidance and correction of the protocol
Abbreviations:
- ASITN/SIR =
- American society of interventional and therapeutic neuroradiology/society of interventional radiology
- DSA =
- digital subtraction angiography
- ECs =
- endothelial cells
- eNOS =
- endothelial NO synthase
- HDL =
- high density lipoproteins
- HDL-P =
- HDL particles
- LDL =
- low-density lipoproteins
- ROS =
- reactive oxygen species
- SD =
- standard deviation
- VSMCs =
- vascular smooth muscle cells
Funding: This work was supported by the National Natural Science Foundation of China (grants: 82071226, 81901145, 82171208, 81471147, and 81671077).
Statement of ethics: The study was approved by the Ethics Committee of Chinese PLA General Hospital.
Ethics approval and consent to participate: All experimental protocols for this study were approved by Ethics Committee of Chinese PLA General Hospital.
Consent for publication: All authors gave their consent for publication.
The authors declare no conflict of interest.
Data availability statement: The data that support the findings of this study are available from the corresponding authors upon reasonable request.
How to cite this article: Pi C, Wang J, Zhao D, Yu S. The determinants of collateral circulation status in patients with chronic cerebral arterial circle occlusion: A STROBE Study. Medicine 2022;101:26(e29703).
Contributor Information
Chenghui Pi, Email: m13521765304@163.com.
Jun Wang, Email: wjsjnk@126.com.
Dengfa Zhao, Email: dengfa1314@163.com.
Reference
- [1].Menon BK, Smith EE, Coutts SB, et al. Leptomeningeal collaterals are associated with modifiable metabolic risk factors. Ann Neurol. 2013;74:241–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].van Seeters T, Biessels GJ, Kappelle LJ, et al. ; Dutch Acute Stroke Study I. Determinants of leptomeningeal collateral flow in stroke patients with a middle cerebral artery occlusion. Neuroradiol. 2016;58:969–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Liebeskind DS, Jahan R, Nogueira RG, et al. Impact of collaterals on successful revascularization in Solitaire FR with the intention for thrombectomy. Stroke. 2014;45:2036–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Shin NY, Kim KE, Park M, et al. Dual-phase CT collateral score: a predictor of clinical outcome in patients with acute ischemic stroke. PLoS One. 2014;9:e107379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].de Havenon A, Haynor DR, Tirschwell DL, et al. Association of collateral blood vessels detected by arterial spin labeling magnetic resonance imaging with neurological outcome after ischemic stroke. JAMA Neurol. 2017;74:453–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Liebeskind DS, Sanossian N. How well do blood flow imaging and collaterals on angiography predict brain at risk? Neurology. 2012;79(13 Suppl 1):S105–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Leng X, Fang H, Leung TW, et al. Impact of collaterals on the efficacy and safety of endovascular treatment in acute ischaemic stroke: a systematic review and meta-analysis. J Neurol Neurosurg Psychiatry. 2016;87:537–44. [DOI] [PubMed] [Google Scholar]
- [8].Zhang H, Prabhakar P, Sealock R, et al. Wide genetic variation in the native pial collateral circulation is a major determinant of variation in severity of stroke. J Cereb Blood Flow Metab. 2010;30:923–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Faber JE, Zhang H, Rzechorzek W, et al. Genetic and environmental contributions to variation in the posterior communicating collaterals of the circle of willis. Transl Stroke Res. 2019;10:189–203. [DOI] [PubMed] [Google Scholar]
- [10].Malik N, Hou Q, Vagal A, et al. Demographic and clinical predictors of leptomeningeal collaterals in stroke patients. J Stroke Cerebrovasc Dis. 2014;23:2018–22. [DOI] [PubMed] [Google Scholar]
- [11].Agarwal S, Scoffings DJ, Jones PS, et al. Interaction of age with the ischaemic penumbra, leptomeningeal collateral circulation and haemodynamic variables in acute stroke: a pilot study. J Neurol Neurosurg Psychiatry. 2013;84:271–6. [DOI] [PubMed] [Google Scholar]
- [12].Zhang H, Rzechorzek W, Aghajanian A, et al. Hypoxia induces de novo formation of cerebral collaterals and lessens the severity of ischemic stroke. J Cereb Blood Flow Metab. 2020;40:1806–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Hecht N, He J, Kremenetskaia I, et al. Cerebral hemodynamic reserve and vascular remodeling in C57/BL6 mice are influenced by age. Stroke. 2012;43:3052–62. [DOI] [PubMed] [Google Scholar]
- [14].Faber JE, Zhang H, Lassance-Soares RM, et al. Aging causes collateral rarefaction and increased severity of ischemic injury in multiple tissues. Arterioscler Thromb Vasc Biol. 2011;31:1748–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Dai X, Faber JE. Endothelial nitric oxide synthase deficiency causes collateral vessel rarefaction and impairs activation of a cell cycle gene network during arteriogenesis. Circ Res. 2010;106:1870–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Moore SM, Zhang H, Maeda N, et al. Cardiovascular risk factors cause premature rarefaction of the collateral circulation and greater ischemic tissue injury. Angiogenesis. 2015;18:265–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Arsava EM, Vural A, Akpinar E, et al. The detrimental effect of aging on leptomeningeal collaterals in ischemic stroke. J Stroke Cerebrovasc Dis. 2014;23:421–6. [DOI] [PubMed] [Google Scholar]
- [18].Lima FO, Furie KL, Silva GS, et al. The pattern of leptomeningeal collaterals on CT angiography is a strong predictor of long-term functional outcome in stroke patients with large vessel intracranial occlusion. Stroke. 2010;41:2316–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Romano JG, Liebeskind DS. Revascularization of collaterals for hemodynamic stroke: insight on pathophysiology from the carotid occlusion surgery study. Stroke. 2012;43:1988–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Craven A, Robson J, Ponte C, et al. ACR/EULAR-endorsed study to develop Diagnostic and Classification Criteria for Vasculitis (DCVAS). Clin Exp Nephrol. 2013;17:619–21. [DOI] [PubMed] [Google Scholar]
- [21].Fukui M. Guidelines for the diagnosis and treatment of spontaneous occlusion of the circle of willis (“moyamoya” disease). research committee on spontaneous occlusion of the circle of willis (Moyamoya Disease) of the ministry of health and welfare, Japan. Clin Neurol Neurosurg. 1997;99(Suppl 2):S238–40. [PubMed] [Google Scholar]
- [22].Higashida RT, Furlan AJ, Roberts H, et al. Trial design and reporting standards for intra-arterial cerebral thrombolysis for acute ischemic stroke. Stroke. 2003;34:e109–37. [DOI] [PubMed] [Google Scholar]
- [23].Clayton JA, Chalothorn D, Faber JE. Vascular endothelial growth factor-A specifies formation of native collaterals and regulates collateral growth in ischemia. Circ Res. 2008;103:1027–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Chalothorn D, Zhang H, Smith JE, et al. Chloride intracellular channel-4 is a determinant of native collateral formation in skeletal muscle and brain. Circ Res. 2009;105:89–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Lucitti JL, Sealock R, Buckley BK, et al. Variants of Rab GTPase-effector binding protein-2 cause variation in the collateral circulation and severity of stroke. Stroke. 2016;47:3022–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Teunissen PF, Horrevoets AJ, van Royen N. The coronary collateral circulation: genetic and environmental determinants in experimental models and humans. J Mol Cell Cardiol. 2012;52:897–904. [DOI] [PubMed] [Google Scholar]
- [27].de Marchi SF, Gloekler S, Meier P, et al. Determinants of preformed collateral vessels in the human heart without coronary artery disease. Cardiology. 2011;118:198–206. [DOI] [PubMed] [Google Scholar]
- [28].Faber JE, Zhang H, Rzechorzek W, et al. Genetic and environmental contributions to variation in the posterior communicating collaterals of the circle of willis. Transl Stroke Res. 2019;10:189–203. [DOI] [PubMed] [Google Scholar]
- [29].Shishehbor MH, Venkatachalam S, Sun Z, et al. A direct comparison of early and late outcomes with three approaches to carotid revascularization and open heart surgery. J Am Coll Cardiol. 2013;62:1948–56. [DOI] [PubMed] [Google Scholar]
- [30].Investigators A-H, Boden WE, Probstfield JL, et al. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N Engl J Med. 2011;365:2255–67. [DOI] [PubMed] [Google Scholar]
- [31].Schwartz GG, Olsson AG, Abt M, et al. Effects of dalcetrapib in patients with a recent acute coronary syndrome. N Engl J Med. 2012;367:2089–99. [DOI] [PubMed] [Google Scholar]
- [32].Shah AS, Tan L, Long JL, et al. Proteomic diversity of high density lipoproteins: our emerging understanding of its importance in lipid transport and beyond. J Lipid Res. 2013;54:2575–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Saemann MD, Poglitsch M, Kopecky C, et al. The versatility of HDL: a crucial anti-inflammatory regulator. Eur J Clin Invest. 2010;40:1131–43. [DOI] [PubMed] [Google Scholar]
- [34].Norata GD, Catapano AL. HDL and adaptive immunity: a tale of lipid rafts. Atherosclerosis. 2012;225:34–5. [DOI] [PubMed] [Google Scholar]
- [35].Marsche G, Saemann MD, Heinemann A, et al. Inflammation alters HDL composition and function: implications for HDL-raising therapies. Pharmacol Ther. 2013;137:341–51. [DOI] [PubMed] [Google Scholar]
- [36].Mackness B, Mackness M. The antioxidant properties of high-density lipoproteins in atherosclerosis. Panminerva Med. 2012;54:83–90. [PubMed] [Google Scholar]
- [37].Navab M, Hama SY, Anantharamaiah GM, et al. Normal high density lipoprotein inhibits three steps in the formation of mildly oxidized low density lipoprotein: steps 2 and 3. J Lipid Res. 2000;41:1495–508. [PubMed] [Google Scholar]
- [38].Lai WK, Kan MY. Homocysteine-Induced Endothelial Dysfunction. Ann Nutr Metab. 2015;67:1–12. [DOI] [PubMed] [Google Scholar]
- [39].Huang X, Li Y, Li P, et al. Association between percent decline in serum total homocysteine and risk of first stroke. Neurol. 2017;89:2101–7. [DOI] [PubMed] [Google Scholar]
- [40].Jeon SB, Kang DW, Kim JS, et al. Homocysteine, small-vessel disease, and atherosclerosis: an MRI study of 825 stroke patients. Neurol. 2014;83:695–701. [DOI] [PubMed] [Google Scholar]
- [41].Austin RC, Lentz SR, Werstuck GH. Role of hyperhomocysteinemia in endothelial dysfunction and atherothrombotic disease. Cell Death Differ. 2004;11(Suppl 1):S56–64. [DOI] [PubMed] [Google Scholar]
- [42].McCully KS. Homocysteine and the pathogenesis of atherosclerosis. Expert Rev Clin Pharmacol. 2015;8:211–9. [DOI] [PubMed] [Google Scholar]
- [43].Uryga AK, Bennett MR. Ageing induced vascular smooth muscle cell senescence in atherosclerosis. J Physiol. 2016;594:2115–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Touze E. Natural history of asymptomatic carotid artery stenosis. Rev Neurol (Paris). 2008;164:793–800. [DOI] [PubMed] [Google Scholar]
- [45].Wang JC, Bennett M. Aging and atherosclerosis: mechanisms, functional consequences, and potential therapeutics for cellular senescence. Circ Res. 2012;111:245–59. [DOI] [PubMed] [Google Scholar]
- [46].Gupta A, Nair S, Schweitzer AD, et al. Neuroimaging of cerebrovascular disease in the aging brain. Aging Dis. 2012;3:414–25. [PMC free article] [PubMed] [Google Scholar]
- [47].Mowbray AL, Kang DH, Rhee SG, et al. Laminar shear stress up-regulates peroxiredoxins (PRX) in endothelial cells: PRX 1 as a mechanosensitive antioxidant. J Biol Chem. 2008;283:1622–7. [DOI] [PubMed] [Google Scholar]
- [48].Mahmoudi M, Gorenne I, Mercer J, et al. Statins use a novel Nijmegen breakage syndrome-1-dependent pathway to accelerate DNA repair in vascular smooth muscle cells. Circ Res. 2008;103:717–25. [DOI] [PubMed] [Google Scholar]
- [49].Moon SK, Thompson LJ, Madamanchi N, et al. Aging, oxidative responses, and proliferative capacity in cultured mouse aortic smooth muscle cells. Am J Physiol Heart Circ Physiol. 2001;280:H2779–88. [DOI] [PubMed] [Google Scholar]
- [50].Guntani A, Matsumoto T, Kyuragi R, et al. Reduced proliferation of aged human vascular smooth muscle cells—role of oxygen-derived free radicals and bubR1 expression. J Surg Res. 2011;170:143–9. [DOI] [PubMed] [Google Scholar]
- [51].Larach DB, deGoma EM, Rader DJ. Targeting high density lipoproteins in the prevention of cardiovascular disease? Curr Cardiol Rep. 2012;14:684–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Mora S, Glynn RJ, Ridker PM. High-density lipoprotein cholesterol, size, particle number, and residual vascular risk after potent statin therapy. Circulation. 2013;128:1189–97. [DOI] [PMC free article] [PubMed] [Google Scholar]