Abstract
Objectives
This study assessed the roles of various exposures and personal protective equipment (PPE) use on healthcare workers' (HCWs) risk of COVID-19 working in primary care, long-term-care facilities or hospitals.
Methods
We conducted a matched case-control (1:1) study (10 April through 9 July 2021). Cases (HCWs with confirmed COVID-19) and controls (HCWs without any COVID-19-positive test or symptoms) were invited by E-mail to complete an online questionnaire on their exposures and PPE use over the 10-day period preceding inclusion. Risk factors were analysed using multivariable conditional logistic regression.
Results
A total of 2076 cases and 2076 matched controls were included. The analysis retained exposure to an infected person outside work (adjusted OR 19.9 (95% CI, 12.4–31.9)), an infected colleague (OR 2.26 (95% CI, 1.53–3.33)) or COVID-19 patients (OR 2.37 (95% CI, 1.66–3.40)), as independent predictors of COVID-19 in HCWs, while partial (OR 0.30 (95% CI, 0.22–0.40)) or complete (OR 0.19 (95% CI, 0.14–0.27)) immunisation was protective. Eye protection (OR 0.57 (95% CI, 0.37–0.87)) and wearing a gown (OR 0.58 (95% CI, 0.34–0.97)) for COVID-19 patient care were protective, while wearing an apron slightly increased the risk of infection (OR 1.47 (95% CI, 1.00–2.18)). Protection of N95 respirators and surgical face masks did not differ. Compared to medical professions, being a nurse (OR 3.79 (95% CI, 2.50–5.76)) or a nurse's aide (OR 9.08 (95% CI, 5.30–15.5)) was associated with COVID-19. Results were consistent across all healthcare settings.
Discussion
HCWs were more likely to get COVID-19 in their personal sphere than during occupational activities. Our results suggest that eye protection for HCWs during patient care should be actively promoted.
Keywords: COVID-19, Healthcare workers, Infection control and prevention, Risk factors, Personal protective equipments
Introduction
Protecting healthcare workers (HCWs) from COVID-19 is critical to ensure their own safety and maintain continuity and quality of care. HCWs have been estimated to have a 1.6- to 3.4-fold higher risk of infection compared to the general population in some studies [1,2]. High on-site involvement of HCWs during the acute phases of the pandemic, including during lockdown periods (implying multiple interactions with colleagues and patients at work and with other individuals in public transports), and lack of access to personal protective equipment (PPE) likely contributed to higher exposure. The WHO estimated that between 80 000 and 180 000 HCWs have died from COVID-19 between January 2020 and May 2021 [3]. In France, from March 2020 to September 2021, 87 647 (9%) laboratory-confirmed infections and 19 (<0.1%) attributable deaths were reported among 935 732 HCWs from healthcare facilities [4]. The emergence of highly transmissible variants further affected the healthcare-workforce capacity.
Like in the general population, younger male HCWs with comorbidities, in contact with an infected household member or who participated in gathering events, have higher risk for COVID-19 [[5], [6], [7], [8]]. The following specific occupational exposures were identified in HCWs: regular patient-facing activities and contacts with infected colleagues [2,5,6,9,10]. Protection conferred by PPE was mainly studied for influenza, severe acute respiratory syndrome (SARS) and middle east respiratory syndrome (MERS), but evidence is controversial for COVID-19 [[11], [12], [13]]. Moreover, very few data have been published on HCWs in long-term care facilities (LTCFs) and primary care [14,15], despite intense involvement in the pandemic response. Most studies focused on the hospital setting, although exposures, organization of care, and access to infection prevention and control (IPC) expertise vary greatly across facilities.
This study aimed to identify occupational and non-occupational exposures, and PPE use associated with COVID-19 risk for HCWs working in primary care, LTCFs, or hospitals.
Methods
Study design and participants
We conducted a matched case-control study from an ongoing national survey (ComCor) led by Institut Pasteur (Paris, France) since October 2020 [[16], [17], [18]]. The ComCor survey aims at identifying COVID-19 risk factors in the general population through a case-control study on community and occupational exposures to SARS-CoV-2.
Participants were included between 10 April and 9 July 2021. Over the study period, alpha variant was the main strain circulating in France [19].
All laboratory-confirmed cases of COVID-19 (either nasopharyngeal RT-PCR or antigenic test) compiled by the French National Health Insurance were contacted by e-mail within a week after notification of the positive test and invited to complete a questionnaire as soon as possible. Respondents who selected the “healthcare worker or working within health field” criterion in the questionnaire were included as cases in this study.
Controls were recruited during the same period through two different sources: 1) Ipsos, a French marketing research and public opinion specialist, selected controls from a panel representative of the French population using frequency-matching with cases for age, sex, region, population density, and week of inclusion for the Comcor survey; and 2) 24 professional corporations, scientific associations, and medical platforms were asked to forward the questionnaire to their members in April and May 2021. Participants declaring to be HCWs using the above-described criterion and reporting no previous symptoms or positive test were enrolled as controls. Controls were free to complete the questionnaire whenever they decided.
The date of inclusion was defined as the date of positive test for cases and the date of questionnaire completion for controls.
After recruitment, the final study population was obtained by randomly selecting cases and controls with a 1:1 ratio by exact case-control matching for 10-year age-category distribution, sex, and residential region.
Data collection
Participants received online information about the study and gave consent for participation by completing the self-administered questionnaire. They opted-in without any incentives or reminders. Questionnaires covered the 10 days preceding symptoms onset for cases (or testing if asymptomatic) and the 10 days preceding questionnaire completion for controls. It included sociodemographic characteristics (age, sex, residential region, household composition) and health condition (prior medical history, COVID-19–immunisation status). The full list of variables is available in a previous report [16]. Occupational activities were assessed for cases and controls: professional category, size and type of healthcare setting, frequency of contacts with patients and COVID-19 patients, contacts with colleagues at work, and PPE use for COVID-19 patient care during the previous 10 days. HCW professions were grouped in four categories: medical staff (physicians, residents, dentists, pharmacists, and clinical biologists), nurses, nurse's aides, and other professions (including, among others, laboratory or imaging technicians, administrative staff, speech or physical therapists, social workers, and opticians). To account for previous immunisation against SARS-CoV-2, we classified participants as either “not immunised,” “partially immunised,” or “fully immunised” [17,20]. Participants without any documented previous COVID-19 and either not vaccinated or first-dose vaccinated within the 21 days preceding inclusion were considered “not immunised.” Participants included 14 days to 6 months after laboratory-confirmed COVID-19 infection or >7 days after a second vaccine dose (28 days for 1-dose regimen) were classified as fully immunised. Other participants were considered partially immunised.
Statistical analyses
Categorical variables are described by number (percentage). All statistical analysis were computed with R Studio version 4.1.0 (R Foundation for Statistical Computing, Vienna, Austria). Cases and controls were matched with the Matching package. Association between variables and the outcome (COVID-19) were assessed through univariable and multivariable conditional logistic regression to account for the matching strategy. All variables tested in univariable analyses were included in the multivariable analysis, and both analyses were adjusted on the week of inclusion. Missing data were managed with multiple imputations by chained equations using the MICE (Multivariate Imputation by Chained Equations) package. To evaluate imputation effects on our results, supplementary analysis was done on a sample of fully completed questionnaires only, excluding individuals with missing data. To compare risk factors within healthcare setting categories (hospitals, LTCFs, and primary care), subgroup analyses were conducted on three population samples using the same 1:1 matching strategy for age, sex, and residential region. Strengthening of Reporting of Observational Studies in Epidemiology (STROBE) reporting guidelines were followed.
Ethical considerations
The ComCor study was approved by the Comité de Protection des Personnes (CPP) Sud Ouest et Outre Mer-1 on 21 September 2020. The data protection authority Commission Nationale de l'Informatique et des Libertés (CNIL) authorized data processing on 21 October 2020. CPP and CNIL accorded authorizations for substantial modification to recruit controls through professional societies and associations on 31 March 2021. Informed consent was obtained from all participants. The study is registered with ClinicalTrials.gov under the identifier NCT04607941.
Results
Participants
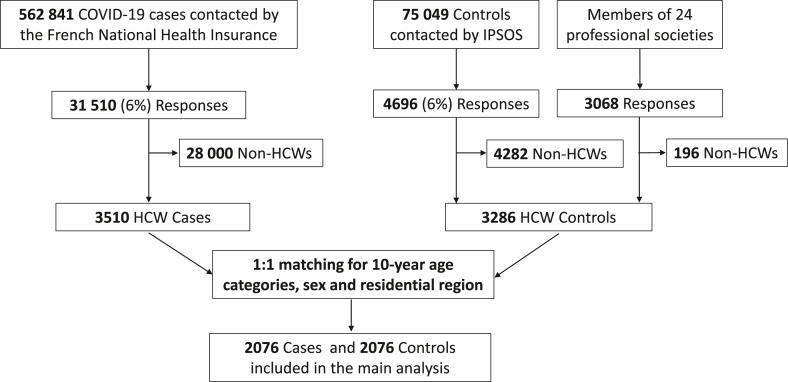
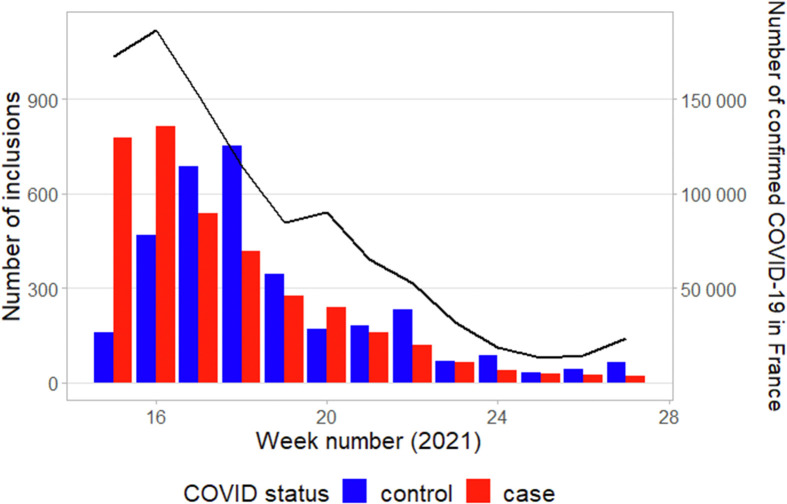
Among 562 841 individuals with confirmed COVID-19 contacted by the French National Health Insurance (10 April through 9 July 2021), 31 510 (6%) completed the questionnaire, including 3510 (11%) HCWs, and 1:1 matching paired 2076 cases to 2076 controls for the analysis. The study flow-chart is displayed in Fig.1 . Overall, data were missing for 126 (6%) cases and 25 (1%) controls. The weekly number of inclusions and confirmed COVID-19 cases reported in France throughout the study period are reported in Fig. 2 .
Fig. 1.
Flow chart of study participants. Abbreviations:HCWs, healthcare workers; Ipsos, French marketing-research and public-opinion specialist.
Fig. 2.
Weekly number of inclusions of cases (red bars) and controls (blue bars). The black line shows the weekly number of laboratory-confirmed cases reported in France throughout the study period (source: Santé Publique France).
Table 1 reports study population characteristics. Most participants were female, mostly working in primary care. Overall, 1770/4152 (43%) HCWs were classified as either partially or fully immunised against COVID-19; 678/4152 (16%) declared being posted in a COVID-19-dedicated unit or mostly caring for COVID-19 patients. In the subgroup of HCWs in contact with COVID-19 patients in the 10 previous days (n = 2086), 1616 (77%) declared to consistently wear a gown, 1608 (77%) gloves, 1490 (71%) a N95 respirator, 1345 (64%) goggles/face shield, 1146 (55%) an apron, and 584 (28%) a surgical face mask for patient care. Overall, 564/1088 (52%) cases and 465/998 (47%) controls declared to consistently wear both a gown and an apron.
Table 1.
Study population and infection determinants: description and results of the univariable and multivariable conditional logistic regression analyses adjusted to the week of inclusion
| Cases (n = 2076) | Controls (n = 2076) | aOR (95% CI) |
||
|---|---|---|---|---|
| Univariable analysis | Multivariable analysis | |||
| Characteristic | ||||
| Age category, y | ||||
| 18–28 | 281 (14) | 281 (14) | ||
| 29–38 | 639 (31) | 639 (31) | ||
| 39–48 | 616 (30) | 616 (30) | ||
| 49+ | 540 (26) | 540 (26) | ||
| Female sex | 1762 (85) | 1762 (85) | ||
| At least one comorbiditya | 305 (15) | 235 (11) | 1.38 (1.10–1.74) | 1.28 (0.92–1.78) |
| Smoker | 367 (18) | 337 (16) | 1.21 (0.98–1.49) | 0.82 (0.60–1.11) |
| COVID-19 immunisation | ||||
| None | 1552 (75) | 817 (39) | Reference | Reference |
| Partial | 312 (15) | 532 (26) | 0.30 (0.24–0.38) | 0.30 (0.22–0.40) |
| Complete | 206 (10) | 720 (35) | 0.21 (0.16–0.27) | 0.19 (0.14–0.27) |
| Healthcare sector | ||||
| Hospital | 694 (33) | 800 (39) | Reference | Reference |
| Long-term care facility | 372 (18) | 291 (14) | 1.58 (1.25–2.01) | 1.11 (0.77–1.61) |
| Primary care | 1010 (49) | 985 (47) | 1.14 (0.96–1.36) | 1.70 (1.28–2.26) |
| HCWs professional category | ||||
| Medical professions | 174 (8) | 552 (27) | Reference | Reference |
| Nurses | 451 (22) | 401 (19) | 5.87 (4.30–8.02) | 3.79 (2.50–5.76) |
| Nurse's aides | 357 (17) | 126 (6) | 14.2 (9.81–20.4) | 9.08 (5.30–15.5) |
| Others | 1094 (53) | 997 (48) | 4.22 (3.23–5.51) | 2.16 (1.52–3.08) |
| Exposures during the 10 days preceding inclusion | ||||
| Regular COVID-19 patient-facing activities | 393 (19) | 285 (14) | 1.63 (1.31–2.03) | 2.37 (1.66–3.40) |
| Exposure to an infected colleagueb | 339 (17) | 111 (5) | 3.31 (2.48–4.43) | 2.26 (1.53–3.33) |
| Exposure to an infected person outside of workb | 434 (22) | 47 (2) | 11.3 (7.74–16.6) | 19.9 (12.4–31.9) |
| Professional cluster (patients and/or HCWs)b | 376 (19) | 172 (8) | 2.70 (2.09–3.49) | 2.14 (1.50–3.06) |
| For COVID-19 patient carec, consistent use of | ||||
| Mask type | ||||
| Surgical face mask | 331 (30) | 253 (25) | — | — |
| Cloth mask | 8 (<1) | 4 (<1) | 1.46 (0.35–6.14) | 1.67 (0.18–15.8) |
| N95 respirator | 749 (69) | 741 (74) | 0.64 (0.50–0.83) | 0.85 (0.55–1.29) |
| Gloves | 883 (81) | 725 (73) | 1.37 (1.06–1.78) | 1.44 (0.87–2.39) |
| Eye protection (goggles or face shield) | 653 (60) | 692 (69) | 0.58 (0.46–0.73) | 0.57 (0.37–0.87) |
| Gown | 813 (75) | 803 (81) | 0.68 (0.52–0.89) | 0.58 (0.34–0.97) |
| Apron | 625 (57) | 521 (52) | 1.29 (1.03–1.62) | 1.47 (1.00–2.18) |
| Did not care for COVID-19 patients | 988 (48) | 1078 (52) | — | — |
Results are presented as number (%) and adjusted odds ratios (aOR) (95% CI).
Abbreviations: HCW, healthcare worker.
Comorbidity among: diabetes, arterial hypertension, myocardial infarction, and/or chronic pulmonary disease.
Analysis computed with multiple imputations for missing data.
For personal protective equipment use, percentages were calculated based on the number of HCWs who cared to COVID-19 patients during the 10 past days (1088 cases and 998 controls).
Association between exposures and COVID-19 status
According to the multivariable analysis, the strongest predictor of contracting COVID-19 was exposure to an infected person outside work, while complete or partial immunisation was protective (Table 1). Occupational exposure to an infected colleague, to COVID-19 patients, or working in a unit harbouring a cluster of nosocomial cases increased the risk of HCW infection. Compared to medical staff, being a nurse or a nurse's aide was significantly associated with the risk of contracting COVID-19. Eye protection (goggles or face shield) and gowning for COVID-19–patient care were associated with lower risk, while wearing an apron posed a higher risk. No significant difference was found for protection of N95 respirators as compared to surgical face masks. The supplementary analysis of cases with fully completed questionnaires (see Supplementary material, Table S1) yielded similar results.
Subgroup analyses
The subgroup analyses by healthcare sector are reported in the Supplementary material, Tables S2 and S3. After 1:1 matching, 1388 HCWs from hospitals, 558 from LTCFs, and 1842 from primary care were included. When caring for COVID-19 patients, HCWs declared more frequently wearing N95 respirators in hospitals and primary care than in LTCFs. Adherence to eye protection was particularly poor in primary care (46% of cases and 53% of controls). According to the multivariable analyses, partial or complete immunisation was protective in all three settings, while exposure to an infected person outside work was consistently the main risk factor for infection.
Discussion
In this large case-control study, the strongest predictor of HCW COVID-19 infection was exposure to an infected person outside work. Contact with an infected colleague and regular COVID-19 patient-facing activities were also significantly, but to a lesser extent, associated with infection. Eye protection and gowning during patient care decreased the risk, while N95 respirators did not confer better protection than surgical masks. These results were consistent across healthcare settings (hospitals, LTCFs, and primary care).
As also shown by others, our results suggest that direct contact with infected household members, relatives, or, to a lesser degree, colleagues were the main sources of HCW acquisition of COVID-19 [5,6,9]. Exposure in the community was consistently and strongly associated with COVID-19 in HCWs across all different healthcare settings, which adds considerable epidemiologic strength to the association. Nevertheless, COVID-19 patient-facing activities seem to further enhance the risk, although previous results were heterogenous [2,5,7,9,21,22]. One explanation for this heterogeneity across settings and wards may be the various degrees of HCWs education and training to follow IPC protocols and best practices.
Correct PPE use by HCWs is essential to avoid contaminations during patient care. Since the start of the pandemic, French guidelines have recommended universal masking with surgical facemasks for patient care and N95 respirator use for aerosol-generating procedures [23]. Eye protection, gowns, or aprons must be worn for confirmed COVID-19 patient with aerosol-generating procedure or direct contacts. Gloves are restricted to activities carrying a risk of exposure to body fluids. Our findings highlighted marked divergence of PPE use from French guidelines, since the majority of HCWs declared to consistently wear a N95 respirator and gloves when caring for COVID-19 patients. However, N95 respirators were not superior to surgical face masks in the main analysis, which was consistent across all healthcare settings. This is consistent with the results of a recent meta-analysis of four randomised controlled trials on other viral respiratory infections [11]. In a multicentre observational study in Switzerland, an institutional policy of systematic N95 respirator use was not associated with a lower HCWs' seroconversion rate for COVID-19 [24]. Although additional safety conferred by eye protection was also suggested in a recent meta-analysis [25], most clinical workers find face shields and goggles uncomfortable and to impair vision and interfere with work, probably contributing to poor adherence [26]. Unexpectedly, apron use was associated with a heightened risk of contamination, while gowns were protective. In both cases and controls, many HCWs declared to consistently wear both apron and gown simultaneously, which illustrates that PPE are worn in bundle, making difficult to estimate the individual effect of each PPE. Nevertheless, all PPE were included in our multivariable analysis and OR adjusted consequently. The pandemic led to a widespread use or reuse of homemade aprons during COVID-19-patient care, owed to gown shortage for which donning and doffing might be easier. These observed misuses of aprons and possible lack of personal protection suits may have led to an increased risk of cross contamination during care. Immunisation was protective in our analysis, but only 43% of participants were partially or completely immunised. In France, vaccination was available to all HCWs from 6 February 2021, but by 30 April 2021, only 39% were estimated to be completely immunised [27]. The low immunisation rate in our sample is consistent with the national vaccine coverage at that time and further explained by the fact that half were cases, hence with a lower vaccine coverage.
HCWs from different healthcare settings have participated in the COVID-19 response. In France, 72% of nursing homes had at least one COVID-19–infected resident in 2020 [28], and numerous devastating outbreaks were described worldwide [29]. Herein, HCWs from LTCFs and primary care tended to be at higher risk of infection, which probably reflects a globally lower awareness on the infectious risk and prevention measures in addition to large-scale staff turnover and limited access to PPE. Support from hospitals and regional health authorities should be encouraged to continue staff training and ensure PPE supply. The risk of contracting COVID-19 was also influenced by the professional category: being a nurse or a nurse's aide was more closely associated with COVID-19 than medical staff. Although those associations might be biased by unbalanced case and control populations for professions, they might also reflect that nurses and nurse's aides were engaged in more prolonged and closer patient care than other professions, as well as a lack of training in IPC. This higher risk of infection was described for domestic cleaners and porters, but not for nurses and nurse's aides, to our knowledge [5,10,22].
The strengths of this study are the large sample size, enabling exact matching of cases and controls for age, sex, and residential region, adjustment to the week of inclusion, and the nationwide distribution of study participants. Notably, sources of infection according to healthcare-facility category have not previously been assessed. The main limitations of the study are the low response rate of cases and controls and the use of an online questionnaire, which may have resulted in selection biases toward younger participants, more comfortable with internet and French language. Second, the under-representation of some professional categories impaired subgroup analyses, despite specific occupational exposures, e.g. physiotherapists or speech therapists. Third, the data used relied upon HCW declarations, potentially influenced by social desirability or recall bias, and by the time between symptoms onset and questionnaire completion. Fourth, we did not rule out past or current asymptomatic infections among controls [30]. Nevertheless, our population was composed of HCWs, more likely to recognize COVID-19 symptoms and with 3 to 5-fold higher access to tests than the general population, then lowering the risk of misclassification bias [1]. Finally, the study took place between April and July 2021, during the third COVID-19 wave in France. HCWs might have been better prepared and protected than during the first wave, especially regarding PPE, and the delta and omicron variants emerged in France after the end of the study period. Omicron transmissibility is much higher than previous variants, which may affect the relative weights of transmission sources and appropriate PPE [31].
In conclusion, our study results indicated that, for HCWs, occupational exposures increase the risk of getting infected, but community exposures appear to represent higher risks. Moreover, our results suggest that, when caring for COVID-19 patients, HCWs should wear a surgical face mask (apart from aerosols-generating procedures), eye protection, and a gown. The protection conferred by gloving should be further explored.
Transparency declaration
All authors have no conflict of interest to declare
The study was funded by Institut Pasteur and Research, Action Emerging Infectious Diseases (REACTing), and the French Agency ANRS- Maladies Infectieuses Emergentes (ComCor project). MB is funded by the ARS Grand Est. AF's laboratory receives support from the Labex IBEID (ANR-10-LABX-62-IBEID) and the INCEPTION project (PIA/ANR-16-CONV-0005) for studies on emerging viruses. Tetracycline is funded by the Fondation de France (Alliance “Tous unis contre le virus”).
Author's contributions
Conceptualisation: MB, JCL, GB, SK; Methodology: MB, TC, LS, ST, XD, JCL, AF, GB, SK; Formal analysis: MB, LS; Writing original draft: MB, JCL, GB, SK; Writing review editing: MB, TC, LS, ST, XD, JCL, AF, GB, SK.
Editor: J. Bielicki
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cmi.2022.05.038.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Nguyen L.H., Drew D.A., Graham M.S., Joshi A.D., Guo C., Ma W., et al. Risk of COVID-19 among front-line health-care workers and the general community: a prospective cohort study. Lancet Public Health. 2020;5:e475–e483. doi: 10.1016/S2468-2667(20)30164-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Iversen K., Bundgaard H., Hasselbalch R.B., Kristensen J.H., Nielsen P.B., Pries-Heje M., et al. Risk of COVID-19 in health-care workers in Denmark: an observational cohort study. Lancet Infect Dis. 2020;20:1401–1408. doi: 10.1016/S1473-3099(20)30589-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization The impact of COVID-19 on health and care workers: a closer look at deaths. https://www.who.int/publications-detail-redirect/WHO-HWF-WorkingPaper-2021.1 Available at: [Accessed 11 May 2022]
- 4.France Santé Publique. Recensement national des cas de COVID-19 chez les professionnels en établissements de santé. https://www.santepubliquefrance.fr/etudes-et-enquetes/recensement-national-des-cas-de-covid-19-chez-les-professionnels-en-etablissements-de-sante [Accessed 11 May 2022]
- 5.Eyre D.W., Lumley S.F., O’Donnell D., Campbell M., Sims E., Lawson E., et al. Differential occupational risks to healthcare workers from SARS-CoV-2 observed during a prospective observational study. ELife. 2020;9:e60675. doi: 10.7554/eLife.60675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lentz R.J., Colt H., Chen H., Cordovilla R., Popevic S., Tahura S., et al. Assessing coronavirus disease 2019 (COVID-19) transmission to healthcare personnel: the global ACT-HCP case-control study. Infect Control Hosp Epidemiol. 2021;42:381–387. doi: 10.1017/ice.2020.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weinberger T., Steffen J., Osterman A., Mueller T.T., Muenchhoff M., Wratil P.R., et al. Prospective longitudinal serosurvey of health care workers in the first wave of the SARS-CoV-2 pandemic in a quaternary care hospital in Munich, Germany. Clin Infect Dis. 2021;73:e3055–e3065. doi: 10.1093/cid/ciaa1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li J., Huang D.Q., Zou B., Yang H., Hui W.Z., Rui F., et al. Epidemiology of COVID-19: a systematic review and meta-analysis of clinical characteristics, risk factors, and outcomes. J Med Virol. 2021;93:1449–1458. doi: 10.1002/jmv.26424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Contejean A., Leporrier J., Canouï E., Fourgeaud J., Mariaggi A., Alby-Laurent F., et al. Transmission routes of severe acute respiratory syndrome coronavirus 2 among healthcare workers of a French University hospital in Paris, France. Open Forum Infect Dis. 2021;8 doi: 10.1093/ofid/ofab054. ofab054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Howard-Anderson J.R., Adams C., Sherman A.C., Dube W.C., Smith T.C., Edupuganti N., et al. Occupational risk factors for severe acute respiratory coronavirus virus 2 (SARS-CoV-2) infection among healthcare personnel: a cross-sectional analysis of subjects enrolled in the COVID-19 Prevention in Emory Healthcare Personnel (COPE) study. Infect Control Hosp Epidemiol. 2022;43:381–386. doi: 10.1017/ice.2021.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bartoszko J.J., Farooqi M.A.M., Alhazzani W., Loeb M. Medical masks vs N95 respirators for preventing COVID-19 in healthcare workers: a systematic review and meta-analysis of randomized trials. Influenza Other Respir Virus. 2020;14:365–373. doi: 10.1111/irv.12745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chu D.K., Akl E.A., Duda S., Solo K., Yaacoub S., Schünemann H.J., et al. Physical distancing, face masks, and eye protection to prevent person-to-person transmission of SARS-CoV-2 and COVID-19: a systematic review and meta-analysis. Lancet. 2020;395:1973–1987. doi: 10.1016/S0140-6736(20)31142-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abbas M., Goto M., Tartari E., Perencevich E., Pittet D. Revisiting the evidence for physical distancing, face masks, and eye protection. Lancet. 2021;398:661–663. doi: 10.1016/S0140-6736(21)01739-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Al-Kuwari M.G., AbdulMalik M.A., Al-Nuaimi A.A., Abdulmajeed J., Al-Romaihi H.E., Semaan S., et al. Epidemiology characteristics of COVID-19 infection amongst primary health care workers in Qatar: March-October 2020. Front Public Health. 2021;9:679254. doi: 10.3389/fpubh.2021.679254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ladhani S.N., Chow J.Y., Janarthanan R., Fok J., Crawley-Boevey E., Vusirikala A., et al. Increased risk of SARS-CoV-2 infection in staff working across different care homes: enhanced CoVID-19 outbreak investigations in London care Homes. J Infect. 2020;81:621–624. doi: 10.1016/j.jinf.2020.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Galmiche S., Charmet T., Schaeffer L., Paireau J., Grant R., Chény O., et al. Exposures associated with SARS-CoV-2 infection in France: a nationwide online case-control study. Lancet Reg Health Eur. 2021;7:100148. doi: 10.1016/j.lanepe.2021.100148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Charmet T., Schaeffer L., Grant R., Galmiche S., Chény O., Von Platen C., et al. Impact of original, B.1.1.7, and B.1.351/P.1 SARS-CoV-2 lineages on vaccine effectiveness of two doses of COVID-19 mRNA vaccines: results from a nationwide case-control study in France. Lancet Reg Health Eur. 2021;8:100171. doi: 10.1016/j.lanepe.2021.100171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grant R., Charmet T., Schaeffer L., Galmiche S., Madec Y., Von Platen C., et al. Impact of SARS-CoV-2 delta variant on incubation, transmission settings and vaccine effectiveness: results from a nationwide case-control study in France. Lancet Reg Health Eur. 2021;13:100278. doi: 10.1016/j.lanepe.2021.100278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.France Santé Publique. https://www.santepubliquefrance.fr/dossiers/coronavirus-covid-19/coronavirus-circulation-des-variants-du-sars-cov-2 Coronavirus: circulation des variants du SARS-CoV-2 [updated 2022 Jul 12]. Available at:
- 20.Hall V.J., Foulkes S., Saei A., Andrews N., Oguti B., Charlett A., et al. COVID-19 vaccine coverage in health-care workers in England and effectiveness of BNT162b2 mRNA vaccine against infection (SIREN): a prospective, multicentre, cohort study. Lancet. 2021;397:1725–1735. doi: 10.1016/S0140-6736(21)00790-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sims M.D., Maine G.N., Childers K.L., Podolsky R.H., Voss D.R., Berkiw-Scenna N., et al. COVID-19 seropositivity and asymptomatic rates in healthcare workers are associated with job function and masking. Clin Infect Dis. 2021;73:S154–S162. doi: 10.1093/cid/ciaa1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tian C., Lovrics O., Vaisman A., Chin K.J., Tomlinson G., Lee Y., et al. Risk factors and protective measures for healthcare worker infection during highly infectious viral respiratory epidemics: a systematic review and meta-analysis. Infect Control Hosp Epidemiol. 2022;43:639–650. doi: 10.1017/ice.2021.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.SF2H Covid-19, infection par le SARS-CoV-2. https://www.sf2h.net/publications/coronavirus-2019-ncov Available at:
- 24.Szajek K., Fleisch F., Hutter S., Risch M., Bechmann T., Luyckx V.A., et al. Healthcare institutions’ recommendation regarding the use of FFP-2 masks and SARS-CoV-2 seropositivity among healthcare workers: a multicenter longitudinal cohort study. Antimicrob Resist Infect Control. 2022;11:6. doi: 10.1186/s13756-021-01047-x. [Accessed 18 August 2021] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Byambasuren O., Beller E., Clark J., Collignon P., Glasziou P. The effect of eye protection on SARS-CoV-2 transmission: a systematic review. Antimicrob Resist Infect Control. 2021;10:156. doi: 10.1186/s13756-021-01025-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alzunitan M.A., Perencevich E.N., Edmond M.B. Assessing health care worker perceptions of face coverings during the COVID-19 pandemic. Am J Infect Control. 2021;49:521–522. doi: 10.1016/j.ajic.2020.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.France Santé Publique. COVID-19: point épidémiologique du 29 avril 2021. https://www.santepubliquefrance.fr/maladies-et-traumatismes/maladies-et-infections-respiratoires/infection-a-coronavirus/documents/bulletin-national/covid-19-point-epidemiologique-du-29-avril-2021 Available at:
- 28.Francaise Republique. En 2020, trois Ehpad sur quatre ont eu au moins un résident infecté par la Covid-19:Direction de la recherche, des études, de l’évaluation et des statistiques. https://drees.solidarites-sante.gouv.fr/publications/etudes-et-resultats/en-2020-trois-ehpad-sur-quatre-ont-eu-au-moins-un-resident-infecte Available from: [Accessed 26 April 2022]
- 29.Hashan M.R., Smoll N., King C., Ockenden-Muldoon H., Walker J., Wattiaux A., et al. Epidemiology and clinical features of COVID-19 outbreaks in aged care facilities: a systematic review and meta-analysis. EClinicalMedicine. 2021;33:100771. doi: 10.1016/j.eclinm.2021.100771. [Accessed 18 August 2021] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.He J., Guo Y., Mao R., Zhang J. Proportion of asymptomatic coronavirus disease 2019: a systematic review and meta-analysis. J Med Virol. 2021;93:820–830. doi: 10.1002/jmv.26326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nishiura H., Ito K., Anzai A., Kobayashi T., Piantham C., Rodríguez-Morales A.J. Relative reproduction number of SARS-CoV-2 omicron (B.1.1.529) compared with delta variant in South Africa. J Clin Med. 2022;11:30. doi: 10.3390/jcm11010030. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.