Abstract
Objective
Routine vaccinations are associated with an increased risk of gout flares. We examined the association between COVID-19 vaccination, an immunization program implemented to a large proportion of population, and the risk of gout flares.
Methods
We conducted a time-stratified case-crossover study among patients with gout who experienced gout flares between December 2020 and September 2021, using data from The Health Improvement Network. We compared the risk of gout flares on each of the seven days on and after the day of COVID-19 vaccination vs. no vaccination during that period using conditional logistic regression. In addition, we performed subgroup analyses stratified by different COVID-19 vaccines (i.e., BNT162b2, hereafter referred to as BNT, and ChAdOx1 nCov-19, hereafter referred to as ChAd).
Results
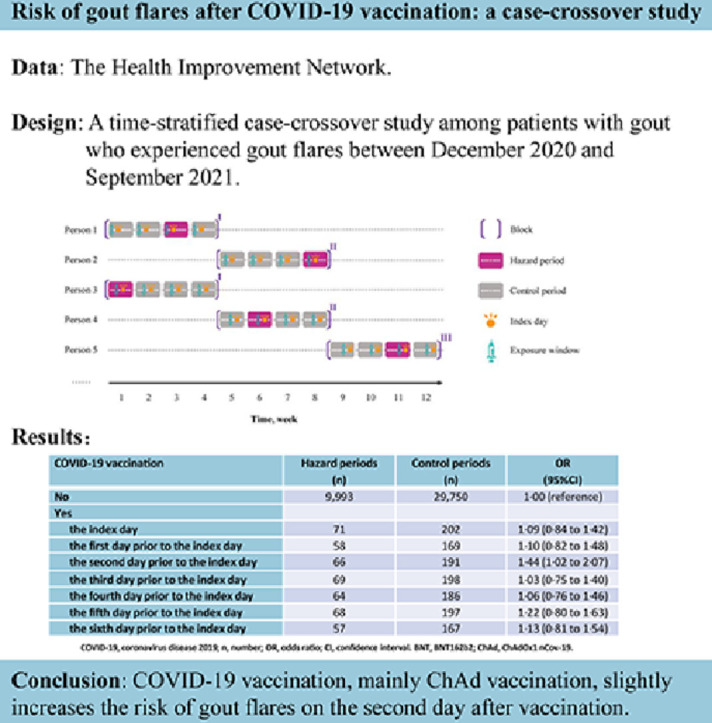
Among 5,904 patients with gout (mean age: 63·1 years; 85·5% male) who experienced gout flares within one month, the risk of gout flares slightly increased on the second day after COVID-19 vaccination (odds ratio: 1·44; 95% CI: 1·02 to 2·07). The risk of gout flares also slightly increased after receiving COVID-19 vaccine on other remaining days (ORs ranged from 1·03 to 1·22); however, none of them was statistically significant. An increased risk of gout flares on the second day after vaccination was mainly observed for the ChAd vaccine (odds ratio: 1·44; 95% CI: 1·00 to 2·05), but not for BNT vaccine (odds ratio: 1·18; 95% CI: 0·67 to 2·02).
Conclusion
COVID-19 vaccination, mainly ChAd vaccination, slightly increases the risk of gout flares on the second day after vaccination. This finding reassures the safety of COVID-19 vaccination for patients with gout.
Keywords: COVID-19, Vaccination, Gout flare
Graphical abstract
Abbreviations
- messenger RNA
(mRNA);
- BNT162b2
(BNT);
- ChAdOx1 nCov-19
(ChAd);
- odds ratio
(OR);
- The coronavirus disease 2019
(COVID-19);
- the United Kingdom
(UK);
- the Health Improvement Network
(THIN);
- general practitioners
(GPs);
- nonsteroidal anti-inflammatory drugs
(NSAIDs);
- confidence interval
(CI).
Introduction
The coronavirus disease 2019 (COVID-19) pandemic has had tremendous social and economic impacts, especially for people living with inflammatory disorders like gout [1]. COVID-19 vaccines are effective against severity of SARS-COV-2 infection, such as hospitalization and death, but there has been a concern regarding their effect on the risk of flares in patients with gout [2,3], which are associated with health-related quality of life, healthcare resource utilization, and work productivity [4]. Indeed, previous studies have reported that some routine vaccinations were associated with an increased risk of gout flares [5], [6], [7]. These findings have prompted the implementation of an enhanced post-marketing surveillance program for gout flares following vaccination [8]. Recently, a study showed that the odds of gout flares increased by almost 6-fold after receiving the inactivated COVID-19 vaccines [9]. However, whether the risk of gout flares increases after receiving other COVID-19 vaccines, such as messenger RNA (mRNA) vaccines BNT162b2 (Pfizer–BioNtech, hereafter referred to as BNT) or adenovirus vector vaccines ChAdOx1 nCov-19 (Oxford–AstraZeneca; hereafter referred to as ChAd) is unknown.
In the United Kingdom (UK), total number of people who have received the first, the second, and a booster or third dose of COVID-19 vaccination was 53, 50, and 39 million, respectively [10]. Considering COVID-19 vaccine has been given to a large proportion of population and approximately one in 40 adults are living with gout [11], assessment of the potential risk of gout flares following vaccination among patients with gout has important clinical and public health implications. Using the data collected from The Health Improvement Network (THIN) in the UK, we examined the association of COVID-19 vaccine developed using other platforms and risk of gout flares among patients with gout.
Methods
Data source
THIN (now called IQVIA Medical Research Database) is an electronic medical record database including general practitioners’ (GPs) records in the UK and represents the UK population regarding demographics and medical conditions. THIN contains anonymized medical records from 790 general practices with approximately 17 million patients. Health care information is recorded at each practice on socio-demographics, anthropometrics, lifestyle factors, visits to GPs, diagnoses from specialists and hospital admissions, and laboratory test results. Details of THIN database have been described previously [12]. The scientific review committee for THIN database and the institutional review board at Xiangya Hospital approved this study, with waiver of informed consent. This study followed the recommendations of the STROBE initiative for reporting observational studies in epidemiology [13].
Study design and cohort definition
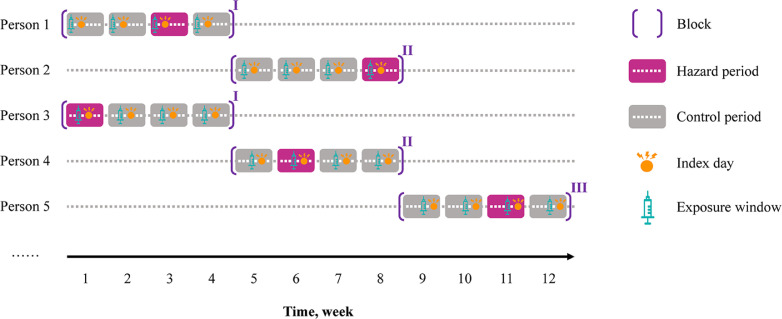
The study was conducted from December 1, 2020 (i.e., when public vaccination began) through September 30, 2021. Eligible individuals included people between 20 and 90 years of age with more than one year of continuous enrollment with a general practice prior to December 1, 2020, had a history of gout diagnosis, and experienced at least one gout flare during the study period. Individuals were excluded if they had a history of cancer. People with gout were identified based on the presence of at least one gout Read code, which has been used in previous studies in THIN database [14], [15], [16], [17]. A previous study showed a validity of 90% for gout diagnoses recorded in the General Practice Research Database (GPRD) when Read code and anti-gout medication were used [18]. Approximately 60% of patients in THIN are overlapped with those in GPRD. We used similar algorithm as part of the operational definition for gout flares (described in Exposure and outcome section). We conducted a time-stratified case-crossover study to examine the association between COVID-19 vaccination and gout flares. Specifically, we divided the calendar days into blocks, with each block consists of 28 days starting on December 1, 2020. Each 28-day time block comprises four 7-day periods. The day on which gout flare occurred was used as the index day to anchor the 7-day hazard period. If a participant experienced two or more gout flares within a 28-day time block, only the first gout flare related 7-day hazard period was included, and the latter gout flares related 7-day periods were excluded from this time block. Thus, the hazard period and up to three control periods were matched to the same day of the week within the same block [19]. The time-stratified case-crossover study design can control for cyclical variation of the underlying hazard of gout attacks according to day of the week. For example, individuals may have different behaviors on weekdays versus weekends that could have bearing on the risk of gout attacks, such as alcohol drinking. By restricting the control periods to the same day of the week within a short period of time (e.g., 35 days), potential control selection bias and time trend of exposure are minimized [20,21]. The study design is depicted in Fig. 1 .
Fig. 1.
Study design of exposure measurement on the second day prior to the index day. A white dot represents one weekday in hazard and control periods.
Exposure and outcome
COVID-19 vaccination was ascertained using Read codes on each day during the hazard and control periods, respectively. The primary outcome was the occurrence of gout flares. The operational definition of gout flare was defined as follows: having a recorded Read code of gout together with a recorded prescription of colchicine [22]; or having a recorded Read code of gout together with at least one of the following treatment patterns within one week: intra-articular corticosteroids, prescription of nonsteroidal anti-inflammatory drugs (NSAIDs), corticosteroid or adrenocorticotropic hormone [22]; or having Read codes specific for gout flare. The date of prescription of colchicine or other treatment or gout flare diagnosis was taken as the date of gout flare. Ascertainment of recurrent gout flares was performed recursively adding in every new follow-up a period of grace of 30 days to the date of the gout flare detected in each consecutive search (to account for full remission of that attack).
Statistical analysis
We examined the relation of COVID-19 vaccination on each day over 7-day period to the risk of gout flares using a conditional logistic regression. No vaccination over a 7-day period was the referent category. In addition, we performed subgroup analyses stratified by different COVID-19 vaccines (i.e., BNT and ChAd vaccines). We also performed an analysis to examine whether the relation of the first dose of COVID-19 vaccine to the risk of gout flares different from that of the second dose of COVID-19 vaccine.
All P values were 2-sided and P<0·05 was considered significant for all tests. All statistical analyses were performed with SAS software, version 9·4 (SAS Institute, Cary, North Carolina, USA).
Results
During the study period 5904 individuals experienced gout flares. Of them, 3748 (63·5%) had one gout flare, 1125 (19·1%) had two gout flares, and the remaining 1031 (17·4%) had three or more gout flares. The majority of diagnosis of gout flares (87·9%) were based on Read code of gout plus colchicine, followed by Read code of gout plus other treatment (11·5%), and then Read code of gout flare (0·6%). As shown in Table 1 , more than 80% individuals with gout flare were men, the mean age was 63·1 years, and mean BMI was 31 kg/m2. Two-thirds of individuals received ChAd vaccine and one third received BNT vaccine.
Table 1.
Patient characteristics.
| Characteristics | Patients (n = 5904) |
|---|---|
| Age, mean (SD), y | 63·1 (14·7) |
| Female (%) | 14·5 |
| BMI, mean (SD), kg/m2 | 31·0 (5·9) |
| Socioeconomic deprivation index, mean (SD)* | 2·9 (1·3) |
| Region (%) | |
| England | 12·9 |
| Northern Ireland | 14·0 |
| Scotland | 31·9 |
| Wales | 41·2 |
| Type of vaccination (%) | |
| ChAd | 66·9 |
| BNT | 31·1 |
| Others | 2·0 |
| Gout flares per person, mean (SD) | 1·9 (1·3) |
n, number; BMI, body mass index; SD, standard deviation; COVID-19, coronavirus disease 2019.
The Socio-Economic Deprivation Index was measured by the Townsend Deprivation Index, which was grouped into quintiles from 1 (least deprived) to 5 (most deprived).
The relation of COVID-19 vaccination on each day over 7-day period to the risk of gout flares is presented in Table 2 . Compared with no COVID-19 vaccination over 7-day period, the risk of gout flares was statistically significantly higher (odds ratio [OR]: 1·44; 95% Confidence interval [CI]: 1·02–2·07) on the second day after vaccination. The risk of gout flares also slightly increased after receiving COVID-19 vaccine on other remaining days (ORs ranged from 1·03 to 1·22); however, none of them was statistically significant.
Table 2.
COVID-19 vaccination in the six days that prior to the index day and risk of gout flares.
| COVID-19 vaccination | Hazard periods (n) | Control periods (n) | OR (95%CI) |
|---|---|---|---|
| No | 9993 | 29750 | 1·00 (reference) |
| Yes | |||
| the index day | 71 | 202 | 1·09 (0·84 to 1·42) |
| the first day prior to the index day | 58 | 169 | 1·10 (0·82 to 1·48) |
| the second day prior to the index day | 66 | 191 | 1·44 (1·02 to 2·07) |
| the third day prior to the index day | 69 | 198 | 1·03 (0·75 to 1·40) |
| the fourth day prior to the index day | 64 | 186 | 1·06 (0·76 to 1·46) |
| the fifth day prior to the index day | 68 | 197 | 1·22 (0·80 to 1·63) |
| the sixth day prior to the index day | 57 | 167 | 1·13 (0·81 to 1·54) |
COVID-19, coronavirus disease 2019; n, number; OR, odds ratio; CI, confidence interval.
Results from subgroup analysis stratified by the different COVID-19 vaccines (i.e., BNT and ChAd vaccines) were showed in Table 3 . Consistent with the primary analysis, we observed an increased risk of gout flares after COVID-19 vaccination among individuals who received ChAd vaccine. Compared with those who did not receive ChAd vaccine over 7-day period, the OR of gout flares on the second day after ChAd vaccination was 1·44 (95% CI: 1·00 to 2·05). However, no statistically significant association was found between BNT vaccination and the risk of gout flares (ORs ranged from 0·90 to 1·18). In addition, an increased risk of gout flares was mainly observed on the second day after receiving either the first dose of COVID-19 vaccine or the second dose of COVID-19 vaccine (Supplementary Table 1). However, the sample size was reduced when the stratified analyses by the dose of COVID-19 vaccine were performed; thus, none of the ORs was statistically significant.
Table 3.
Vaccination with the BNT or ChAd in the six days that prior to the index day and risk of gout flares.
| COVID-19 vaccination | Hazard periods (n) | Control periods (n) | OR (95%CI) |
|---|---|---|---|
| No | 9993 | 29750 | 1·00 (reference) |
| BNT | |||
| the index day | 20 | 58 | 1·09 (0·84 to 1·42) |
| the first day prior to the index day | 19 | 55 | 1·15 (0·66 to 1·99) |
| the second day prior to the index day | 16 | 47 | 1·18 (0·67 to 2·02) |
| the third day prior to the index day | 22 | 66 | 0·99 (0·61 to 1·61) |
| the fourth day prior to the index day | 21 | 63 | 0·90 (0·54 to 1·50) |
| the fifth day prior to the index day | 18 | 52 | 1·15 (0·34 to 2·84) |
| the sixth day prior to the index day | 14 | 40 | 1·12 (0·47 to 2·36) |
| ChAd | |||
| the index day | 50 | 141 | 1·10 (0·83 to 1·44) |
| the first day prior to the index day | 37 | 109 | 1·01 (0·70 to 1·46) |
| the second day prior to the index day | 49 | 142 | 1·44 (1·00 to 2·05) |
| the third day prior to the index day | 46 | 130 | 1·22 (0·85 to 1·77) |
| the fourth day prior to the index day | 41 | 118 | 1·34 (0·91 to 1·96) |
| the fifth day prior to the index day | 49 | 143 | 1·39 (0·97 to 1·99) |
| the sixth day prior to the index day | 41 | 121 | 1·15 (0·81 to 1·62) |
COVID-19, coronavirus disease 2019; n, number; OR, odds ratio; CI, confidence interval. BNT, BNT162b2; ChAd, ChAdOx1 nCov-19.
Discussion
In this population-based case-crossover study of individuals with gout, risk of gout flares slightly increased on the second day after COVID-19 vaccination, and an increased risk of gout flares was mainly found among individuals who received ChAd vaccine.
Recently, a cross-sectional study reported that inactivated COVID-19 vaccination was associated with a 6-fold higher odds of gout flares [9]. The inactivated COVID-19 vaccine in that study contains aluminum hydroxide adjuvant, and previous studies have shown that adjuvant could activate the inflammatory cascade, resulting in an increased risk of gout flares [23,24]. In the UK, nearly 98% individuals received two doses of either mRNA vaccines BNT or adenovirus vector vaccines ChAd, neither of these vaccines contains aluminum hydroxide adjuvant. While studies have shown that the administration of BNT and ChAd vaccines could lead to an inflammatory response [25,26], the magnitude of the association between these two vaccines and the risk of gout flares was much smaller than that reported for inactivated COVID-19 vaccines.
Results from subgroup analysis stratified by BNT and ChAd vaccines showed that a significantly increased risk of gout flares was only observed among individuals who received ChAd vaccines, but not among those who received BNT vaccine. Previous study found that ChAd vaccines induce a more pronounced increase in several inflammatory markers than mRNA vaccine [27], which may explain why ChAd vaccine was a trigger of gout flares. Moreover, both ChAd and BNT vaccines could generate interferon. Even though previous studies found that there was no difference of the effect of generation of interferon between adenovirus vaccine and the mRNA vaccine three weeks after the first dose [28], and 2–12 weeks following secondary dose [29], the differences in interferon production between adenovirus vaccine and the mRNA vaccine within one week remain unknown, which may be another possible biologic reason for the results we found. Future studies are needed to understand the potential mechanisms.
Our study has several strengths. We conducted a time-stratified case-crossover study. Self-matching of each individual on the same day of the week not only eliminates the confounding bias from the time constant risk factors for gout, such as body mass index [30], but also minimizes the potential bias from some lifestyle factors that may vary by the day of the week, such as alcohol consumption. The study also has several limitations. First, owing to lack of data on short-term time-varying confounders, such as medication takes during the past seven days, we are unable to adjust for these risk factors and thus cannot rule out their potential confounding effects. However, these biases, if they occurred, are likely to be non-differential; thus, resulting in an underestimating effect. Second, the ascertainment of gout flares based on a pragmatic approach could have led to a misclassification of gout flares. In the current study, approximately 86% of patients with gout had a recorded code for gout on the same date as that of prescription of NSAIDs or corticosteroids, and the remaining patients had a recorded code for gout within seven days before the prescription. Thus, we believe these medications were likely to be used for therapeutic purposes of gout flares. Although the therapeutic use of colchicine has extended to other disorders such as cardiovascular disease [31], colchicine is more often prescribed to the patients who experienced gout flares, particularly a new prescription for a discrete episode [32,33]. Patients taking colchicine for flares prophylaxis or cardiovascular disease prevention would be expected to be on this medication continuously. Furthermore, if gout flares were misclassified, in general, such non-differential misclassification is likely to bias the results towards the null. Finally, the date of gout flare diagnosis may not be the exact date of gout flare occurs. However, we believe that individuals are unlikely to receive COVID-19 vaccination during a gout flare. Any delay of gout diagnosis date is likely to dilute the effect estimate of COVID-19 vaccination on the risk of gout flares.
Conclusion
In conclusion, COVID-19 vaccines, mainly ChAd vaccine, only slightly increases the risk of gout flares. This finding reassures the safety of COVID-19 vaccination for patients with gout.
Author contributions
YZ and JW had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. All authors have read, provided critical feedback on intellectual content, and approved the final manuscript. Concept and design: YZ and JW. Acquisition, analysis, or interpretation of data: All authors. Drafting of the manuscript: HL, JW, and YZ. Critical revision of the manuscript for important intellectual content: All authors. Statistical analysis: YZ, JW and XL. Obtained funding: ZSW and JAS. Administrative, technical, or material support: YZ and JW. Supervision: YZ and JW.
Funding
This work was supported by the National Institutes of Health and the National Institute of Arthritis and Musculoskeletal and Skin Diseases (K23 AR073334, R03 AR078938, R01 AR077607, P30 AR070253, and P30 AR072577), and the R. Bruce and Joan M. Mickey Research Scholar Fund. The funders of the study had no role in study design, data analysis, data collection, data interpretation, manuscript preparation, or decision to publish.
Data availability
The data that support the findings of this study are available within the article and its supplementary information files or from the corresponding author upon reasonable request.
Ethical approval
This study received approval from the medical ethical committee at Xiangya Hospital (2018091077), with waiver of informed consent.
Scientific approval
This study was approved by the THIN Scientific Review Committee (22SRC008).
Statement
THIN is a registered trademark of Cegedim SA in the United Kingdom and other countries. Reference made to the THIN database is intended to be descriptive of the data asset licensed by IQVIA. This work uses de-identified data provided by patients as a part of their routine primary care.
Declaration of Competing Interest
JAS has received research support from Bristol Myers Squibb and performed consultancy for AbbVie, Amgen, Boehringer Ingelheim, Bristol Myers Squibb, Gilead, Inova Diagnostics, Janssen, Optum, and Pfizer unrelated to this work. ND has received consulting fees, speaker fees or grants from AstraZeneca, Dyve Biosciences, Horizon, Amgen, Selecta, Arthrosi, JW Pharmaceutical Corporation, PK Med, PTC Therapeutics, Protalix, Cello Health, Abbvie, and Janssen, outside the submitted work. No conflict of interest for other authors.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.semarthrit.2022.152059.
Appendix. Supplementary materials
References
- 1.World Health Organization Coronavirus (COVID-19) Dashboard, https://covid19.who.int/; 2022 [accessed 14 June 2022].
- 2.Vasileiou E., Simpson C.R., Shi T., Kerr S., Agrawal U., Akbari A., Bedston S., Beggs J., Bradley D., Chuter A., et al. Interim findings from first-dose mass COVID-19 vaccination roll-out and COVID-19 hospital admissions in Scotland: a national prospective cohort study. Lancet. 2021;397(10285):1646–1657. doi: 10.1016/S0140-6736(21)00677-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lopez Bernal J., Andrews N., Gower C., Robertson C., Stowe J., Tessier E., Simmons R., Cottrell S., Roberts R., O'Doherty M., et al. Effectiveness of the Pfizer-BioNTech and Oxford-AstraZeneca vaccines on covid-19 related symptoms, hospital admissions, and mortality in older adults in England: test negative case-control study. BMJ. 2021;373:n1088. doi: 10.1136/bmj.n1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Khanna P.P., Nuki G., Bardin T., Tausche A.K., Forsythe A., Goren A., Vietri J., Khanna D. Tophi and frequent gout flares are associated with impairments to quality of life, productivity, and increased healthcare resource use: results from a cross-sectional survey. Health Qual Life Outcomes. 2012;10:117. doi: 10.1186/1477-7525-10-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lal H., Cunningham A.L., Godeaux O., Chlibek R., Diez-Domingo J., Hwang S.J., Levin M.J., McElhaney J.E., Poder A., Puig-Barbera J., et al. Efficacy of an adjuvanted herpes zoster subunit vaccine in older adults. N Engl J Med. 2015;372(22):2087–2096. doi: 10.1056/NEJMoa1501184. [DOI] [PubMed] [Google Scholar]
- 6.Cunningham A.L., Lal H., Kovac M., Chlibek R., Hwang S.J., Diez-Domingo J., Godeaux O., Levin M.J., McElhaney J.E., Puig-Barbera J., et al. Efficacy of the herpes zoster subunit vaccine in adults 70 years of age or older. N Engl J Med. 2016;375(11):1019–1032. doi: 10.1056/NEJMoa1603800. [DOI] [PubMed] [Google Scholar]
- 7.Yokose C., McCormick N., Chen C., Neogi T., Chaisson C., Terkeltaub R., Hunter D.J., Zhang Y., Choi H. Risk of gout flares after vaccination: a prospective case cross-over study. Ann Rheum Dis. 2019;78(11):1601–1604. doi: 10.1136/annrheumdis-2019-215724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Paula E.A., Rebecca Reindel M.D. 2017. Fdabiologics license application clinical review memorandum: shingrix. [Google Scholar]
- 9.Lu J., He Y., Terkeltaub R., Sun M., Ran Z., Xu X., Wang C., Li X., Hu S., Xue X., et al. Colchicine prophylaxis is associated with fewer gout flares after COVID-19 vaccination. Ann Rheum Dis. 2022 doi: 10.1136/annrheumdis-2022-222199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vaccinations inUnited Kingdom, https://coronavirus.data.gov.uk/details/vaccinations; 2022 [accessed 14 June 2022].
- 11.Kuo C.F., Grainge M.J., Mallen C., Zhang W., Doherty M. Rising burden of gout in the UK but continuing suboptimal management: a nationwide population study. Ann Rheum Dis. 2015;74(4):661–667. doi: 10.1136/annrheumdis-2013-204463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zeng C., Dubreuil M., LaRochelle M.R., Lu N., Wei J., Choi H.K., Lei G., Zhang Y. Association of tramadol with all-cause mortality among patients with osteoarthritis. JAMA. 2019;321(10):969–982. doi: 10.1001/jama.2019.1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.von Elm E., Altman D.G., Egger M., Pocock S.J., Gotzsche P.C., Vandenbroucke J.P., Initiative S. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370(9596):1453–1457. doi: 10.1016/S0140-6736(07)61602-X. [DOI] [PubMed] [Google Scholar]
- 14.Wei J., Choi H.K., Neogi T., Dalbeth N., Terkeltaub R., Stamp L.K., Lyu H., McCormick N., Niu J., Zeng C., et al. Allopurinol initiation and all-cause mortality among patients with gout and concurrent chronic kidney disease: a population-based cohort study. Ann Intern Med. 2022 doi: 10.7326/M21-2347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schlesinger N., Lu N., Choi H.K. Gout and the risk of incident erectile dysfunction: a body mass index-matched population-based study. J Rheumatol. 2018;45(8):1192–1197. doi: 10.3899/jrheum.170444. [DOI] [PubMed] [Google Scholar]
- 16.Vargas-Santos A.B., Peloquin C.E., Zhang Y., Neogi T. Association of chronic kidney disease with allopurinol use in gout treatment. JAMA Intern Med. 2018;178(11):1526–1533. doi: 10.1001/jamainternmed.2018.4463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang Y., Peloquin C.E., Dubreuil M., Roddy E., Lu N., Neogi T., Choi H.K. Sleep apnea and the risk of incident gout: a population-based, body mass index-matched cohort study. Arthritis Rheumatol. 2015;67(12):3298–3302. doi: 10.1002/art.39330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meier C.R., Jick H. Omeprazole, other antiulcer drugs and newly diagnosed gout. Br J Clin Pharmacol. 1997;44(2):175–178. doi: 10.1046/j.1365-2125.1997.00647.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Levy D., Lumley T., Sheppard L., Kaufman J., Checkoway H. Referent selection in case-crossover analyses of acute health effects of air pollution. Epidemiology. 2001;12(2):186–192. doi: 10.1097/00001648-200103000-00010. [DOI] [PubMed] [Google Scholar]
- 20.Janes H., Sheppard L., Lumley T. Case-crossover analyses of air pollution exposure data: referent selection strategies and their implications for bias. Epidemiology. 2005;16(6):717–726. doi: 10.1097/01.ede.0000181315.18836.9d. [DOI] [PubMed] [Google Scholar]
- 21.Mittleman M.A. Optimal referent selection strategies in case-crossover studies: a settled issue. Epidemiology. 2005;16(6):715–716. doi: 10.1097/01.ede.0000183170.92955.25. [DOI] [PubMed] [Google Scholar]
- 22.Rothenbacher D., Primatesta P., Ferreira A., Cea-Soriano L., Rodriguez L.A. Frequency and risk factors of gout flares in a large population-based cohort of incident gout. Rheumatology (Oxford) 2011;50(5):973–981. doi: 10.1093/rheumatology/keq363. [DOI] [PubMed] [Google Scholar]
- 23.Eisenbarth S.C., Colegio O.R., O'Connor W., Sutterwala F.S., Flavell R.A. Crucial role for the Nalp3 inflammasome in the immunostimulatory properties of aluminium adjuvants. Nature. 2008;453(7198):1122–1126. doi: 10.1038/nature06939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Heineman T.C., Cunningham A., Levin M. Understanding the immunology of Shingrix, a recombinant glycoprotein E adjuvanted herpes zoster vaccine. Curr Opin Immunol. 2019;59:42–48. doi: 10.1016/j.coi.2019.02.009. [DOI] [PubMed] [Google Scholar]
- 25.Greinacher A., Thiele T., Warkentin T.E., Weisser K., Kyrle P.A., Eichinger S. Thrombotic Thrombocytopenia after ChAdOx1 nCov-19 Vaccination. N Engl J Med. 2021;384(22):2092–2101. doi: 10.1056/NEJMoa2104840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McGonagle D., De Marco G., Bridgewood C. Mechanisms of immunothrombosis in vaccine-induced thrombotic thrombocytopenia (VITT) compared to natural SARS-CoV-2 infection. J Autoimmun. 2021;121 doi: 10.1016/j.jaut.2021.102662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ostrowski S.R., Sogaard O.S., Tolstrup M., Staerke N.B., Lundgren J., Ostergaard L., Hvas A.M. Inflammation and platelet activation after COVID-19 vaccines - possible mechanisms behind vaccine-induced immune thrombocytopenia and thrombosis. Front Immunol. 2021;12 doi: 10.3389/fimmu.2021.779453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang J., Ko J.H., Baek J.Y., Hong J., Ha S., Lee B., Huh K., Cho S.Y., Kang C.I., Chung D.R., et al. Effects of short-term corticosteroid use on reactogenicity and immunogenicity of the first dose of ChAdOx1 nCoV-19 vaccine. Front Immunol. 2021;12 doi: 10.3389/fimmu.2021.744206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hollstein M.M., Münsterkötter L., Schön M.P., Bergmann A., Husar T.M., Abratis A., Eidizadeh A., Schaffrinski M., Zachmann K., Schmitz A., et al. Interdependencies of cellular and humoral immune responses in heterologous and homologous SARS-CoV-2 vaccination. Allergy. 2022 doi: 10.1111/all.15247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.The case-crossover design: a method for studying transient effects on the risk of acute events. Am J Epidemiol. 2017;185(11):1174–1183. doi: 10.1093/aje/kwx105. [DOI] [PubMed] [Google Scholar]
- 31.Leung Y.Y., Yao H.L.L., Kraus V.B. Colchicine–Update on mechanisms of action and therapeutic uses. Semin Arthritis Rheum. 2015;45(3):341–350. doi: 10.1016/j.semarthrit.2015.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.FitzGerald J.D., Dalbeth N., Mikuls T., Brignardello-Petersen R., Guyatt G., Abeles A.M., Gelber A.C., Harrold L.R., Khanna D., King C., et al. 2020 American college of rheumatology guideline for the management of gout. Arthritis Rheumatol. 2020;72(6):879–895. doi: 10.1002/art.41247. [DOI] [PubMed] [Google Scholar]
- 33.Liu C.H., Lin Y.S., Sung P.S., Wei Y.C., Chang T.Y., Lee T.H., Lee C.Y., Li Y.R. Colchicine use and risks of stroke recurrence in acute non-cardiogenic ischemic stroke patients: a population-based cohort study. J Pers Med. 2021;11(9) doi: 10.3390/jpm11090935. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available within the article and its supplementary information files or from the corresponding author upon reasonable request.