Abstract
A veterinarian in Thailand was diagnosed with COVID-19 after being sneezed on by an infected cat owned by an infected patient. Genetic study supported the hypothesis of SARS-CoV-2 transmission from the owner to the cat, and then from the cat to the veterinarian.
Keywords: COVID-19, coronavirus disease, SARS-CoV-2, severe acute respiratory syndrome coronavirus 2, viruses, respiratory infections, zoonoses, vaccine-preventable diseases, cat, feline, Thailand
COVID-19, caused by SARS-CoV-2, has been suspected to be a zoonosis because of its link to a live animal market in Wuhan, China (1). In addition, several countries in the Americas, Africa, Europe, and Asia have reported the occurrence of COVID-19 in various animal species, including minks, cats, dogs, lions, and tigers (2). However, most of these infections primarily originated from humans and were transmitted to the animal (i.e., reverse zoonosis), with numerous reports in domestic cats (2,3). A recent report describes a possible animal-to-human transmission of SARS-CoV-2 from infected farm minks to farmworkers in the Netherlands (4). We describe a suspected zoonotic SARS-CoV-2 transmission from a cat to a human.
Case Report
During July–September 2021, the COVID-19 pandemic was shifting from the Alpha variant to the Delta variant. On August 15, 2021, in Songkhla, a business province in southern Thailand, patient A, a 32-year-old previously healthy female veterinarian who lived alone in a dormitory on campus visited the hospital of Prince of Songkla University, located in Hatyai District, Songkhla Province, with a history of fever, clear nasal discharge, and productive cough of 2 days’ duration. Results of a physical examination, including a chest radiograph, were otherwise unremarkable. When questioned about her history, she said that 5 days earlier, she and 2 other veterinarians (patients E and F) had examined a cat belonging to 2 men (patients B and C).
Patients B and C, a 32- and 64-year-old son and father, were from Bangkok, the capital city of Thailand. They were confirmed positive for SARS-CoV-2 infection by reverse transcription PCR (RT-PCR) a day earlier and were transferred to Prince of Songkla University hospital because of the unavailability of hospital beds in Bangkok. Together with their cat, patients B and C were transported by an ambulance in a 20-hour, 900-km trip on August 8, 2021 (Figure 1). On arrival, the patients were immediately admitted to an isolation ward. The cat that had been sleeping on the same beds as the patients was sent to the university veterinarian hospital for an examination by patient A on August 10, 2021, and found to be clinically normal. Patient A retrieved nasal and rectal swab specimens from the cat while patients E and F restrained it. During the nasal swabbing, the sedated cat sneezed in the face of patient A. All 3 veterinarians were wearing disposable gloves and N95 respirator masks without face shields or eye goggles at the time. The entire veterinarian–cat encounter lasted ≈10 minutes.
Figure 1.
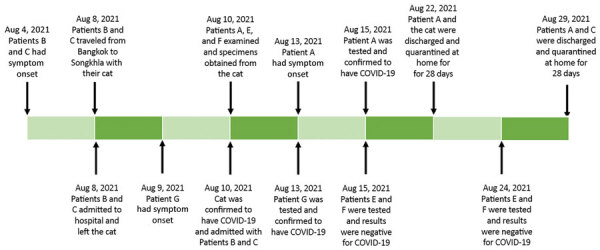
Timeline of suspected cat-to-human transmission of SARS-CoV-2, Thailand, August 2021.
Three days after exposure to the cat, patient A became symptomatic but did not seek medical consultation until August 15, when the RT-PCR test results of the cat returned COVID-19–positive (Table). On investigation, nasopharyngeal swab specimens from patient A showed detectable SARS-CoV-2 (Table). Patients A, B and C and the cat were admitted for isolation in the hospital. Test results for the swab specimens from patients E and F were negative.
Table. Sample metadata of SARS-CoV-2 genome derived from feline and human patients after suspected cat-to-human transmission of SARS-CoV-2, Thailand, July–September 2021*.
| Patient | Sequence ID | Type of sample | Cycle threshold |
PANGO lineage | Pairwise distance, bp | |
|---|---|---|---|---|---|---|
| ORF1ab gene | N gene | |||||
| A | Patient_A | Nasopharyngeal swab | 20 | 16 | B.1.167.2 | 0.00 |
| B | Patient_B | Nasopharyngeal swab | 19 | 22 | B.1.167.2 | 0.00 |
| C | Patient_C | Nasopharyngeal swab | 15 | 20 | B.1.167.2 | 0.00 |
| G | Patient_G | Nasopharyngeal swab | 24 | 26 | B.1.167.2.30 | 40.00 |
| Cat | Throat_cat | Throat swab | 17 | 16 | B.1.167.2 | 0.00 |
| Cat | Rectal_cat | Rectal swab | 21 | 15 | B.1.167.2 | 0.00 |
*PANGO lineages identified by using Pangolin and Pangolearn (https://cov-lineages.org/resources/pangolin/pangolearn.html). N, nucleoprotein; ORF, open reading frame.
No close contacts of patient A were diagnosed with COVID-19. Contact tracing investigations of all the 30 personnel working at the Veterinary Hospital identified 1 additional contact with COVID-19, a veterinarian who worked in the Department of Large Animals (patient G). Patient G had fever onset 1 day before the cat’s arrival and had tested positive for COVID-19 on August 13, 2021. He reported no direct or indirect contact with the cat or patients A, E, or F.
Before genotyping, we tested viral RNA from the cat, patients A, B, C, and G, and other patients in Songkhla Province for SARS-CoV-2 by using RT-PCR. Primer sets were designed to target the nucleoprotein and open reading frame 1ab genes (Table). For viral whole-genome sequencing, we performed library preparation for the SARS-CoV-2 genome by using QIAseq DIRECT SARS-CoV-2 kits (QIAGEN, https://www.qiagen.com) on an Illumina NextSeq 550 (Illumina, https://www.illumina.com) at the Translational Medicine Research Center at Prince of Songkla University. We identified the PANGO lineages by using Pangolin and Pangolearn (https://cov-lineages.org/resources/pangolin/pangolearn.html) (Table). We generated maximum-likelihood phylogenetic trees of the representative cases and others from aligned consensus sequences by using an IQ-TREE (http://www.iqtree.org) with 1,000 bootstraps. We visualized the phylogenetic tree by using FigTree (http://tree.bio.ed.ac.uk/software/figtree). The genomes of patients B and C and the cat were identical to that obtained from patient A, but they were distinct from those of other patients in the same province (Figure 2). The pairwise distance between patient A and the cat was shown to be similar (5) by MEGA 11 (https://www.megasoftware.net) with 1,000 bootstraps (Table).
Figure 2.
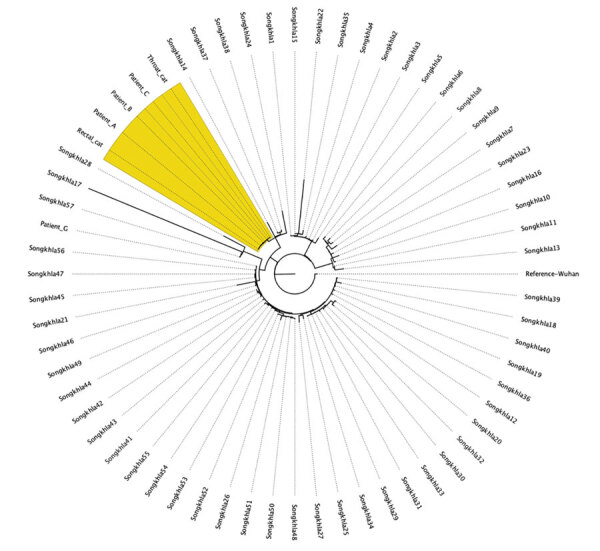
Phylogenetic tree of SARS-CoV-2 genome sequences retrieved from patients A, B, and C and the cat belonging to patients B and C (yellow shading) compared with reference sequences from COVID-19 patients from Songkhla Province, Thailand, July–September 2021. Tree constructed with IQ-TREE (http://www.iqtree.org) by using the maximum-likelihood method and 1,000 bootstrap replicates.
Conclusions
The identical SARS-CoV-2 genome sequences obtained from patient A and the sequences derived from the cat and its 2 owners, together with the temporal overlapping of the animal and human infections, indicated that their infections were epidemiologically related. Because patient A had no prior meetings with patients B or C, she probably acquired SARS-CoV-2 from the cat when it sneezed in her face. The genome sequences were distinct from that of patient G and other sequences circulating in the same province, and by using the pairwise distance formula, we were able to rule out external transmission (5). The Alpha variant was widely spread until the end of July 2021 in Songkhla Province; on the other hand, in Bangkok, the Delta variant has been widespread since the beginning of July 2021 (6).
The transmission chain of SARS-CoV-2 infections in this cluster probably began in Bangkok. Cats are known to be susceptible to SARS-CoV-2 infection (8–10), especially during close interactions with humans with symptomatic SARS-CoV-2 infections (7). Because infected cats have relatively short incubation and contagious periods (8–10), this cat probably had acquired its SARS-CoV-2 infection no longer than a week before possibly transmitting the disease to patient A.
Although direct or indirect (fomites) contacts are also potential routes of transmission to patient A, these possibilities are less likely because she wore gloves and washed her hands before and after examining the cat. Transmission from the cat sneeze is hypothesized because of this brief but very close encounter. The relatively low RT-PCR cycle thresholds (11) in the nasal swab obtained from the cat suggest that the viral load was high and infectious (12,13). Because patient A wore an N95 mask without a face shield or goggles, her exposed ocular surface was vulnerable to infection by droplets expelled from the cat. Her infection signifies the possibility of ocular transmission and the importance of wearing protective goggles or face shields in addition to a mask during close-range interactions with high-risk humans or animals.
In summary, we have provided evidence that cats can transmit the SARS-CoV-2 infection to humans. However, the incidence of this transmission method is relatively uncommon because of the short (median 5 days) duration of cats shedding viable viruses (8–10). Nevertheless, to prevent transmission of SARS-CoV-2 from humans to cat, persons with suspected or confirmed COVID-19 should refrain from contact with their cat. Eye protection as part of the standard personal protection is advisable for caregivers during close interactions with cats suspected to be infected.
Acknowledgments
The authors thank David Patterson for manuscript revision.
T.S., W.L., J.S., and S.S. performed experiments and data interpretation. C.K. and T.I. contributed to epidemiologic data collection, analysis, and manuscript editing. T.S., W.L., and S.S. provided access to the crucial experimental components. P.S., N.K., S.K., T.H., B.C., and S.C. contributed to clinical data collection and interpretation. S.C. drafted the manuscript and provided scientific content of the manuscript. O.F.N. provided grammatical revision of the manuscript. S.C. conceived the idea and experimental design of the study. All authors read and approved the final manuscript.
Biography
Mr. Sila is a graduate student at Prince of Songkla University’s Department of Health Science and Medical Research in the Faculty of Medicine. His primary research interests include genomics, evolutionary microbiology, bioinformatics, sequence and genome analysis, and viral culture.
Footnotes
Suggested citation for this article: Sila T, Sunghan J, Laochareonsuk W, Surasombatpattana S, Kongkamol C, Ingviya T, et al. Suspected cat-to-human transmission of SARS-CoV-2, Thailand, July–September 2021. Emerg Infect Dis. 2022 Jul [date cited]. https://doi.org/10.3201/eid2807.212605
References
- 1.Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, et al. ; China Novel Coronavirus Investigating and Research Team. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727–33. 10.1056/NEJMoa2001017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Organisation for Animal Health. COVID-19 portal [cited 2021 October 13]. https://www.oie.int/app/uploads/2021/09/sars-cov-2-situation-report-4.pdf
- 3.Hobbs EC, Reid TJ. Animals and SARS-CoV-2: Species susceptibility and viral transmission in experimental and natural conditions, and the potential implications for community transmission. Transbound Emerg Dis. 2021;68:1850–67. 10.1111/tbed.13885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Oude Munnink BB, Sikkema RS, Nieuwenhuijse DF, Molenaar RJ, Munger E, Molenkamp R, et al. Transmission of SARS-CoV-2 on mink farms between humans and mink and back to humans. Science. 2021;371:172–7. 10.1126/science.abe5901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Worby CJ, Chang HH, Hanage WP, Lipsitch M. The distribution of pairwise genetic distances: a tool for investigating disease transmission. Genetics. 2014;198:1395–404. 10.1534/genetics.114.171538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.World Health Organization. Classification of Omicron (B1.1.529): SARS-CoV-2 variant of concern [cited 2022 Mar 26]. https://reliefweb.int/sites/reliefweb.int/files/resources/2022_01_19_tha-sitrep-219-covid-19_th_r02.pdf
- 7.Goryoka GW, Cossaboom CM, Gharpure R, Dawson P, Tansey C, Rossow J, et al. One Health investigation of SARS-CoV-2 infection and seropositivity among pets in households with confirmed human COVID-19 cases—Utah and Wisconsin, 2020. Viruses. 2021;13:1813. 10.3390/v13091813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bosco-Lauth AM, Hartwig AE, Porter SM, Gordy PW, Nehring M, Byas AD, et al. Experimental infection of domestic dogs and cats with SARS-CoV-2: Pathogenesis, transmission, and response to reexposure in cats. Proc Natl Acad Sci U S A. 2020;117:26382–8. 10.1073/pnas.2013102117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Halfmann PJ, Hatta M, Chiba S, Maemura T, Fan S, Takeda M, et al. Transmission of SARS-CoV-2 in domestic cats. N Engl J Med. 2020;383:592–4. 10.1056/NEJMc2013400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shi J, Wen Z, Zhong G, Yang H, Wang C, Huang B, et al. Susceptibility of ferrets, cats, dogs, and other domesticated animals to SARS-coronavirus 2. Science. 2020;368:1016–20. 10.1126/science.abb7015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Segalés J, Puig M, Rodon J, Avila-Nieto C, Carrillo J, Cantero G, et al. Detection of SARS-CoV-2 in a cat owned by a COVID-19-affected patient in Spain. Proc Natl Acad Sci U S A. 2020;117:24790–3. 10.1073/pnas.2010817117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bullard J, Dust K, Funk D, Strong JE, Alexander D, Garnett L, et al. Predicting infectious severe acute respiratory syndrome coronavirus 2 from diagnostic samples. Clin Infect Dis. 2020;71:2663–6. 10.1093/cid/ciaa638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Singanayagam A, Patel M, Charlett A, Lopez Bernal J, Saliba V, Ellis J, et al. Duration of infectiousness and correlation with RT-PCR cycle threshold values in cases of COVID-19, England, January to May 2020. Euro Surveill. 2020;25:2001483. 10.2807/1560-7917.ES.2020.25.32.2001483 [DOI] [PMC free article] [PubMed] [Google Scholar]


