Abstract
Recent observations that viruses are very abundant and biologically active components in marine ecosystems suggest that they probably influence various biogeochemical and ecological processes. In this study, the population dynamics of the harmful bloom-forming phytoplankton Heterosigma akashiwo (Raphidophyceae) and the infectious H. akashiwo viruses (HaV) were monitored in Hiroshima Bay, Japan, from May to July 1998. Concurrently, a number of H. akashiwo and HaV clones were isolated, and their virus susceptibilities and host ranges were determined through laboratory cross-reactivity tests. A sudden decrease in cell density of H. akashiwo was accompanied by a drastic increase in the abundance of HaV, suggesting that viruses contributed greatly to the disintegration of the H. akashiwo bloom as mortality agents. Despite the large quantity of infectious HaV, however, a significant proportion of H. akashiwo cells survived after the bloom disintegration. The viral susceptibility of H. akashiwo isolates demonstrated that the majority of these surviving cells were resistant to most of the HaV clones, whereas resistant cells were a minor component during the bloom period. Moreover, these resistant cells were displaced by susceptible cells, presumably due to viral infection. These results demonstrated that the properties of dominant cells within the H. akashiwo population change during the period when a bloom is terminated by viral infection, suggesting that viruses also play an important role in determining the clonal composition and maintaining the clonal diversity of H. akashiwo populations. Therefore, our data indicate that viral infection influences the total abundance and the clonal composition of one host algal species, suggesting that viruses are an important component in quantitatively and qualitatively controlling phytoplankton populations in natural marine environments.
Viruses are now recognized as the most abundant and biologically active components of marine ecosystems (1, 24). Field studies indicate that the majority are probably bacterial viruses, i.e., bacteriophages (5, 33), but viruses and viruslike particles have been observed in many phytoplankton species and in a wide range of natural seawater samples (8, 28, 32). These observations have led to increased interest in the impact of viral infection on the population dynamics and community structure of marine phytoplankton. Several studies have suggested that viruses can be significant agents of phytoplankton mortality. For example, an addition of native virus concentrates reduced phytoplankton biomass and primary productivity under experimental laboratory conditions (28, 29). Also, electron microscopic observations have shown that the proportion of cells harboring viruslike particles or the abundance of viruslike particles within the water column increases in the final stages of blooms (2, 3, 16, 17). In contrast to these postulates, a few reports have demonstrated that viruses are probably not responsible for a large proportion of host mortality (33, 36). Waterbury and Valois (33) observed that some isolated cyanobacteria, Synechococcus spp., tended to be resistant to co-occurring viruses and concluded that cyanophages were not significant in controlling host abundance but were likely to influence the clonal composition of Synechococcus populations in Woods Hole harbor.
Heterosigma akashiwo (Raphidophyceae) is one of the harmful bloom-forming phytoplankton which often cause mortality of caged fishes, such as salmon and yellowtail, in coastal waters of subtropical, temperate, and subarctic areas of the world (10, 27). A noteworthy feature of H. akashiwo blooms is that they often disintegrate suddenly. Nagasaki et al. (17) observed by transmission electron microscopy that the proportion of virus-harboring cells increased along with the sudden decrease in cell density of H. akashiwo. These data strongly suggest that viral infection contributes to the disintegration of H. akashiwo blooms. Recently, lytic viruses infecting H. akashiwo (H. akashiwo virus [HaV]) have been isolated from natural seawaters and cultured, and their characteristics have been investigated in the laboratory (18–22). HaV is a large, double-stranded DNA virus and is most likely specific for H. akashiwo because the potential for other phytoplankton species to become infected by this virus has not been confirmed (18). Other important points are that the specificity of infection of H. akashiwo is significantly diverse even among HaV clones and that the viral susceptibilities of H. akashiwo isolates can also be diverse (19, 22). This fact suggests that the interaction between H. akashiwo and HaV in natural environments is more than a simple host-virus interaction.
In this study, we monitored the population dynamics of H. akashiwo and HaV during the formation and decay of an H. akashiwo bloom in Hiroshima Bay, Japan. Concurrently, a number of H. akashiwo and HaV clones were isolated and the development in virus susceptibility of the host species and the host range of the virus were investigated through laboratory cross-reactivity tests. Our data provide evidence that viral infection influences the total abundance and the clonal composition of one host algal species in natural environments.
MATERIALS AND METHODS
Sampling.
Surface water was collected from once to three times a week from a semienclosed basin (Itsukaichi Fishing Port; 34°21.400′N, 132°21.864′E) located in northern Hiroshima Bay, the Seto Inland Sea of Japan, from mid-May through July 1998. In this area, an H. akashiwo bloom is an annual event that occurs around June to July (11). Sampling was conducted between 9:00 and 10:00 a.m. because this species migrates towards the surface in the early morning (35). Water samples were also collected from several depths, including 0.2 m above the sediment-water interface after 3 June.
Abundance of phytoplankton species and lytic viruses.
Cell counts and taxonomic identification of H. akashiwo and other phytoplankton species were carried out with a Sedgewick-Rafter chamber under optical microscopy on the sampling day without fixation of the sample waters.
The abundance of HaV in seawater was estimated by the most probable number (MPN) technique (7, 30). Water samples were passed through a glass fiber filter (Whatman GF/F) and diluted with modified SWM3 medium (4, 12) in a series of 10-fold dilution steps. Aliquots (100 μl) of each dilution were added to 8 wells in cell culture plates with 96 round-bottom wells and mixed with 150 μl of exponentially growing culture of H. akashiwo. As a host strain, a clonal strain (H93616) isolated from the northern part of Hiroshima Bay in June 1993 was used. In previous experiments (19, 22), this strain was infected and lysed by all HaV isolates, and we considered it to be the most suitable host strain. The cell culture plates were incubated under a 14- and 10-h light-dark cycle of ca. 50 μmol of photons m−2 s−1 at 20°C and were monitored for 10 to 14 days for the occurrence of culture lysis. The abundance of lytic viruses was calculated with a BASIC program from the number of wells in which lysis occurred (23).
Isolation of H. akashiwo and HaV clones.
Ten cells of H. akashiwo, as a rule, were randomly isolated from each water sample on the day of sampling by the micropipetting method. These clonal but not axenic isolates were grown in modified SWM3 medium as described above.
One or two HaV clones were isolated from the most diluted wells of each sample when HaV abundance was determined by the MPN method. The clonal isolation was carried out with two cycles of the extinction dilution procedure (6, 18).
Virus susceptibility.
The virus susceptibilities of H. akashiwo isolates were examined by using a range of HaV clonal isolates. Each HaV clone was inoculated into a host culture of the H. akashiwo H93616 strain, and the resultant fresh lysates were used as inocula. Aliquots (50 μl) of each lysate were added to 1 ml of exponentially growing culture of each H. akashiwo isolate. The cultures were incubated under the conditions of light and temperature described above and monitored for 10 to 14 days for the occurrence of cell lysis. Cultures that were not lysed 14 days after viral inoculation were considered to be resistant to the virus clone.
RESULTS
H. akashiwo dynamics.
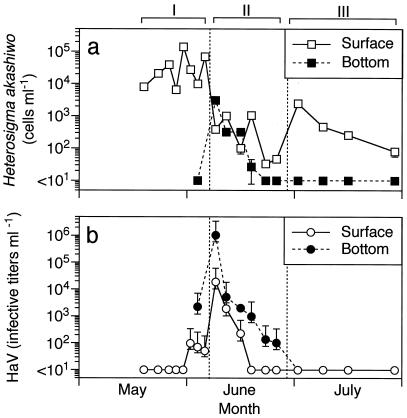
On 18 May, H. akashiwo had already formed a moderate bloom (8.0 × 103 cells ml−1) and maintained high densities ranging from 6.5 × 103 to 1.4 × 105 ml−1 in the surface water from 22 May through 5 June (Fig. 1a). During this period, H. akashiwo constituted up to 90% of the phytoplankton community in terms of cell abundance (data not shown). On 8 June, the cell density of H. akashiwo suddenly decreased to 3.8 × 102 ml−1. This value is 2 orders of magnitude lower than that on 5 June, indicating that the bloom disintegrated within a few days. Another interesting observation was that cells were present at a higher density in the bottom water than in the surface water on 8 June. This vertical distribution pattern was the opposite of that during the bloom period. On 1 July, H. akashiwo exhibited a small peak (2.5 × 103 cells ml−1) but did not reach the densities of a massive bloom formation.
FIG. 1.
Temporal changes in abundances of H. akashiwo (a) and HaV (b) in surface and bottom waters in northern Hiroshima Bay, the Seto Inland Sea of Japan, during the period mid-May to July 1998. Based on the population dynamics of H. akashiwo, the investigation period was tentatively divided into three periods (I, II, and III).
With respect to the variability of H. akashiwo dynamics described above, we can distinguish three major periods (I, II, and III [Fig. 1]). Period I, from 18 May to 5 June, was characterized by a dense bloom formation; during period II (from 8 to 25 June), the bloom disintegrated and the cell density remained at relatively low levels; period III, from 1 to 28 July, contained a smaller but significant peak, i.e., a secondary bloom.
Virus dynamics.
Infectious HaV were not detectable (less than 1.3 ml−1) in the surface water at the beginning of period I and were first observed at 102 ml−1 on 1 June (Fig. 1b). Coincident with the decline in host abundance, the concentration of infectious HaV drastically increased and reached maximum values in both surface (1.9 × 104 ml−1) and bottom (1.0 × 106 ml−1) waters on 8 June. The latter concentration was approximately 10 to 100 times higher than the algal abundance in the surface water during period I. The abundance exponentially decreased but was present above 102 ml−1 in the bottom water during period II. Despite the significant abundance of H. akashiwo during period III, the HaV concentration was extremely low and decreased to a nondetectable level even in the bottom water by 15 July.
Virus susceptibility and host range.
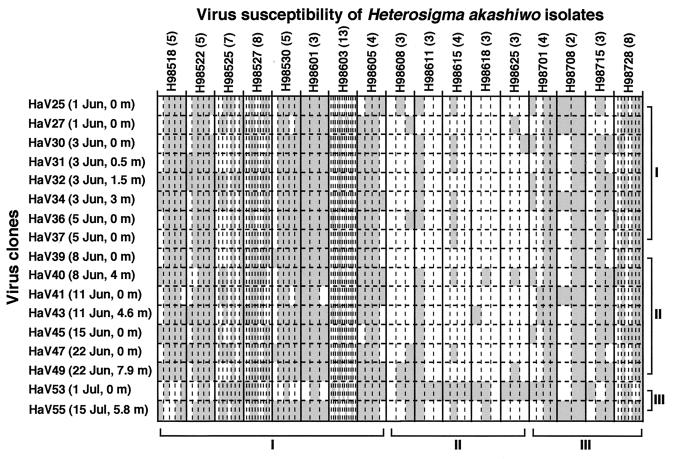
A total of 89 H. akashiwo isolates were obtained from surface water during the investigation period. The virus susceptibility of each isolate was examined against 17 clones of HaV isolated during the same period. The susceptibility spectra showed various patterns among the H. akashiwo isolates (Fig. 2). Different spectral patterns were recognized even among those from the same water sample, indicating that multiple cells with different viral infection characteristics coexisted in the water column. There were significant differences in virus susceptibility (expressed as a percentage of HaV isolates which caused cell lysis) among H. akashiwo isolates during the three periods described above. A Kruskal-Wallis test showed that the susceptibility of H. akashiwo isolates during period II (average ± standard deviation, 18% ± 23%) was significantly lower than those of isolates during periods I and III (59% ± 40% and 61% ± 37%, respectively; n = 3; P < 0.001), indicating that H. akashiwo populations during period II were dominated by cells resistant to the viruses.
FIG. 2.
Viral susceptibility spectrum of H. akashiwo isolates to co-occurring HaV isolates from water samples in northern Hiroshima Bay, the Seto Inland Sea of Japan, during the period mid-May to July 1998. Both types of isolate are shown in order of isolation date. Each clonal isolate of H. akashiwo was named according to the isolation date (e.g., H98518 was isolated on 18 May 1998), and the number of cultured isolates obtained from the same water sample is indicated in parentheses. The shaded and open columns indicate susceptibility (with cells lysed) and resistance (with cell growth equal to that of the controls) to each HaV clone, respectively. The roman numerals correspond to the periods shown in Fig. 1.
Significant diversity in host ranges was also found among HaV clones (Fig. 2). Similar to the susceptibility spectra of H. akashiwo isolates, the host ranges were also slightly different even among HaV clones isolated from the same water (HaV25 and HaV27; HaV36 and HaV37). It was especially remarkable that HaV53, which was isolated at the beginning of period III, had a unique host range. This virus clone infected and lysed 14 of the 16 H. akashiwo isolates during period II, which was much more infective than other HaV clones (which infected only 1 to 4 of the 16 H. akashiwo isolates). In contrast, HaV53 was less infectious to H. akashiwo isolates during periods I and III than the other virus clones.
DISCUSSION
Virus impact on host mortality.
In order to measure infectious HaV abundance, we used the MPN method, in which a single strain of H. akashiwo (H93616) was used as the host strain. Titer determination methods (MPN or plaque assay) are the most sensitive assays for specific viruses in water samples, but their detection capabilities depend on the host strain used as the assay organism. Thus, these methods can unpredictably give underestimated results, especially in a case where different host strains can be lysed only by selected virus clones. In fact, several studies have found that the detected viral titers were markedly different depending on the host strains used (25, 31, 36). Nonetheless, it is most likely that our MPN assay detected the majority of the infectious HaV in the water samples, as the host strain we used in this study (H93616) has been found to be infected and lysed by all HaV clones we have isolated to date (19, 22).
The data presented in this paper demonstrate that infectious HaV are an abundant and dynamic component of natural marine environments and that their temporal and spatial dynamics are closely related to host algal dynamics. The highest concentration of infectious HaV was found when the abundance of H. akashiwo cells suddenly decreased (Fig. 1). The comparative population dynamics of the viruses and the hosts indicate that most of the H. akashiwo cells probably suffered from viral infection and that numerous progeny viruses were released into the water column by fatal bursting of the cells. The vertical distribution pattern of H. akashiwo cells in the decaying phase of the bloom (period II [Fig. 1]) also suggests that viral infection was the predominant factor in the mortality. H. akashiwo generally exhibits diel vertical migrations characterized by daytime ascent and dark descent (27, 35). Actually, in the morning, high concentrations were observed in the surface water during the bloom period (period I). In contrast, cells were present at a higher concentration in the bottom water than in the surface water in the decaying phase of the bloom (period II), indicating that most cells lost their ability to migrate upward. This was similar to the response of H. akashiwo cells to HaV inoculation under laboratory conditions; the cells lost motility within 24 h after HaV inoculation and consequently sank to the bottoms of culture vessels (21). It has been suggested that viruses can be significant agents of phytoplankton mortality (2, 3, 16, 17, 24, 26, 28, 29, 32). Most of the studies were based on transmission electron microscopic observations of field-collected phytoplankton cells. For H. akashiwo, a high percentage of cells containing viruslike particles has also been observed in the final stage of blooms (16, 17). However, investigations of the temporal dynamics of phytoplankton species and their infectious viruses in host-virus systems are scarce (7, 31, 33, 36). Bratbak et al. (2) showed that the termination of an Emiliania huxleyi bloom was accompanied by a simultaneous increase in large viruslike particles in mesocosm experiments. However, there exists no direct evidence that these viruslike particles were specific to E. huxleyi, although several lines of evidence (e.g., morphology and distribution) suggested that they were probably produced by E. huxleyi. As far as we are aware, this is the first study reporting a significant inverse relationship between the abundances of an algal host and its specific virus in natural seawater, and it provides strong evidence that viral infections contribute greatly to the disintegration of blooms as mortality agents.
Viral impact on clonal composition within host population.
Despite the high concentration of infectious HaV, H. akashiwo populations did not completely disappear from the water column and remained at levels of 101 to 102 cells ml−1 during period II (Fig. 1). The viral susceptibilities of H. akashiwo isolates indicated that the majority of the isolates during period II were resistant to most of the HaV clonal isolates (Fig. 2), suggesting that the H. akashiwo population during period II was dominated by cells resistant to co-occurring viruses. In contrast, the fact that the viral susceptibilities of the H. akashiwo isolates during period I were much higher than those during period II suggests that the resistant cells were a minor component during the bloom period. These observations indicate that the properties of dominant cells within the H. akashiwo population change during a period when a bloom is terminated by viral infection. The development of resistance to viral infection has been shown to occur in the marine cyanobacteria Synechococcus spp. (33). Since most Synechococcus isolates were resistant to co-occurring cyanophages, Waterbury and Valois (33) concluded that cyanophages were probably not responsible for a large proportion of the cyanobacterial mortality but were important in determining the clonal composition of these populations. Our data also suggest that viruses can have a significant effect on determining the clonal composition within one host species. However, there is a marked difference between the present study and that of Waterbury and Valois (33). Waterbury and Valois concluded that these cyanobacterial communities are dominated by cells resistant to their co-occurring phages and that these phages are maintained by scavenging on the relatively few susceptible cells in the communities through the annual cycle. In contrast, our data demonstrated that H. akashiwo populations were dominated by cells susceptible to viruses and that the resistant cells were only minor components during the bloom period.
A possible explanation is that host species may pay a cost in reduced competitive fitness for the increased resistance. It has been demonstrated that resistance is often developed through the alteration or loss of some important receptor (13). This suggests that resistant cells may confer a competitive disadvantage against susceptible ones. Unfortunately there are no studies of viral resistance related to physiological strategy for any phytoplankton species, including H. akashiwo. Thus, further study is needed to explain why H. akashiwo cells susceptible to viruses dominated over the resistant ones during the bloom period.
In period III, H. akashiwo populations were dominated by cells susceptible to HaV clones again (Fig. 2). One possibility is that lytic viruses decreased in abundance. As viruses must diffuse randomly from host to host, viral infection is density dependent (15). Thus, a decrease in the abundance of lytic viruses might allow the growth of susceptible cells. Another possibility is that the resistant cells which dominated during period II might also be infected and lysed by viruses. Indeed, the virus clone HaV53 infecting these resistant cells was isolated from the water sample at the beginning of period III. This also shows that natural H. akashiwo populations do not consist simply of two distinct groups, i.e., susceptible and resistant groups, suggesting that the relationship between H. akashiwo and HaV in natural environments is very complex.
Implications.
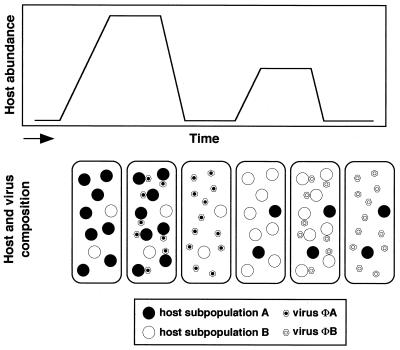
Our data provide evidence that viral infection influences not only the total abundance but also the clonal composition of one host algal species in natural seawater. This leads us to propose a hypothetical model describing interactions between one host algal species and its infectious viruses (Fig. 3). In this model, it is assumed that there are two distinct subpopulations (A and B) with different viral infection characteristics within one phytoplankton species and that viruses ΦA and ΦB specifically infect subpopulations A and B, respectively. Under favorable growth conditions for one subpopulation (A), subpopulation A quickly increases in population density and subpopulation B remains at a low level of abundance. If no viral infection occurs, subpopulation A maintains dominance and eventually will eliminate subpopulation B. On the other hand, the presence of virus ΦA specifically decreases subpopulation A, and subpopulation B, which is resistant to virus ΦA, then dominates. Consequently, the total host abundance decreases but subpopulation succession occurs, and the clonal diversity within one phytoplankton species is maintained under constant environmental conditions. We acknowledge that this conceptual model oversimplifies the very complex series of host-virus interactions in natural environments. Nonetheless, the model is useful in generalizing viral impacts on the total abundance and strain composition of one host algal species on the basis of the data on the temporal dynamics of H. akashiwo and its infectious viruses empirically obtained in the present study.
FIG. 3.
Conceptual model describing interactions between one host algal species and its infectious virus. The model hypothesizes changes in the total abundance and clonal composition of one host algal species in response to viral infection. It is assumed that there are two distinct subpopulations (A and B) with different viral infection characteristics within one phytoplankton species and that viruses ΦA and ΦB specifically infect subpopulations A and B, respectively. For a detailed explanation, see Discussion.
It has been demonstrated that some procaryotic and eucaryotic phytoplankton species comprise genetically and/or physiologically different strains (9, 14). Although we do not have any information about the genetic and/or physiological differences among H. akashiwo cells, the diversity of their viral susceptibilities probably reflects some genetic or physiological differences. Therefore, viral infection may contribute to the maintenance of genetic and physiological diversity within one phytoplankton species. Moreover, as genetic and physiological diversity presumably allows populations to thrive under a broad range of environmental conditions, viral infection might function as an advantageous strategy in the ecological success of a host species.
ACKNOWLEDGMENTS
This work was supported by grants from the Fisheries Agency of Japan and from the Japan Science and Technology Corporation.
We thank K. Tamai (National Research Institute of Fisheries and Environment of Inland Sea) for many helpful comments on the manuscript.
REFERENCES
- 1.Bergh O, Børsheim K Y, Bratbak G, Heldal M. High abundance of viruses found in aquatic environments. Nature. 1989;340:467–468. doi: 10.1038/340467a0. [DOI] [PubMed] [Google Scholar]
- 2.Bratbak G, Egge J K, Heldal M. Viral mortality of the marine alga Emiliania huxleyi (Haptophyceae) and termination of algal blooms. Mar Ecol Prog Ser. 1993;93:39–48. [Google Scholar]
- 3.Brussaard C P D, Kempers R S, Kop A J, Riegman R, Heldal M. Virus-like particles in a summer bloom of Emiliania huxleyi in the North Sea. Aquat Microb Ecol. 1996;10:105–113. [Google Scholar]
- 4.Chen L C M, Edelstein T, McLachlan J. Bonnemaisonia hamifera Hariot in nature and in culture. J Phycol. 1969;5:211–220. doi: 10.1111/j.1529-8817.1969.tb02605.x. [DOI] [PubMed] [Google Scholar]
- 5.Cochlan W P, Wikner J, Steward G F, Smith D C, Azam F. Spatial distribution of viruses, bacteria and chlorophyll a in neritic, oceanic and estuarine environments. Mar Ecol Prog Ser. 1993;92:77–87. [Google Scholar]
- 6.Cottrell M T, Suttle C A. Wide-spread occurrence and clonal variation in viruses which cause lysis of a cosmopolitan, eukaryotic marine phytoplankter, Micromonas pusilla. Mar Ecol Prog Ser. 1991;78:1–9. [Google Scholar]
- 7.Cottrell M T, Suttle C A. Dynamics of a lytic virus infecting the photosynthetic marine picoflagellate Micromonas pusilla. Limnol Oceanogr. 1995;40:730–739. [Google Scholar]
- 8.Dodds J A. Viruses of marine algae. Experientia. 1979;35:440–442. [Google Scholar]
- 9.Gallagher J C. Genetic variation in harmful algal bloom species: an evolutionary ecology approach. In: Anderson D M, Cembella A D, Hallegraeff G M, editors. Physiological ecology of harmful algal blooms. Berlin, Germany: Springer; 1998. pp. 225–242. [Google Scholar]
- 10.Honjo T. Overview on bloom dynamics and physiological ecology of Heterosigma akashiwo. In: Smayda T J, Shimizu Y, editors. Toxic phytoplankton blooms in the sea. Amsterdam, The Netherlands: Elsevier; 1993. pp. 33–41. [Google Scholar]
- 11.Imai I, Itakura S. Importance of cysts in the population dynamics of the red tide flagellate Heterosigma akashiwo (Raphidophyceae) Mar Biol. 1999;133:755–762. [Google Scholar]
- 12.Itoh K, Imai I. Japan Fisheries Resource Conservation Association (ed.), A guide for studies of red tide organisms. Shuwa, Tokyo, Japan. (In Japanese.) 1987. Rafido so (Raphidophyceae) pp. 122–130. [Google Scholar]
- 13.Lenski R E. Dynamics of interactions between bacteria and virulent bacteriophage. Adv Microb Ecol. 1988;10:1–44. [Google Scholar]
- 14.Moore L R, Chisholm S W. Photophysiology of the marine cyanobacterium Prochlorococcus: ecotypic differences among cultured isolates. Limnol Oceanogr. 1999;44:628–638. [Google Scholar]
- 15.Murray A G, Jackson G A. Viral dynamics: a model of the effects of size, shape, motion and abundance of single-celled planktonic organisms and other particles. Mar Ecol Prog Ser. 1992;89:103–116. [Google Scholar]
- 16.Nagasaki K, Ando M, Itakura S, Imai I, Ishida Y. Virus-like particles in Heterosigma akashiwo (Raphidophyceae): a possible red tide disintegration mechanism. Mar Biol. 1994;119:307–312. [Google Scholar]
- 17.Nagasaki K, Ando M, Itakura S, Imai I, Ishida Y. Viral mortality in the final stage of Heterosigma akashiwo (Raphidophyceae) red tide. J Plankton Res. 1994;16:1595–1599. [Google Scholar]
- 18.Nagasaki K, Yamaguchi M. Isolation of a virus infectious to the harmful bloom causing microalga Heterosigma akashiwo (Raphidophyceae) Aquat Microb Ecol. 1997;13:135–140. [Google Scholar]
- 19.Nagasaki K, Yamaguchi M. Intra-species host specificity of HaV (Heterosigma akashiwo virus) clones. Aquat Microb Ecol. 1998;14:109–112. [Google Scholar]
- 20.Nagasaki K, Yamaguchi M. Effect of temperature on the algicidal activity and stability of HaV (Heterosigma akashiwo virus) Aquat Microb Ecol. 1998;15:211–216. [Google Scholar]
- 21.Nagasaki K, Tarutani K, Yamaguchi M. Growth characteristics of Heterosigma akashiwo virus and its possible use as a microbiological agent for red tide control. Appl Environ Microbiol. 1999;65:898–902. doi: 10.1128/aem.65.3.898-902.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nagasaki K, Tarutani K, Yamaguchi M. Cluster analysis on algicidal activity of HaV clones and virus sensitivity of Heterosigma akashiwo (Raphidophyceae) J Plankton Res. 1999;21:2219–2226. [Google Scholar]
- 23.Nishihara T, Kurano N, Shinoda S. Calculation of most probable number for enumeration of bacteria on microcomputer. Eisei Kagaku. 1986;32:226–228. . (In Japanese with English abstract.) [Google Scholar]
- 24.Proctor L M, Fuhrman J A. Viral mortality of marine bacteria and cyanobacteria. Nature. 1990;343:60–62. [Google Scholar]
- 25.Sahlsten E. Seasonal abundance in Skagerrak-Kattegat coastal waters and host specificity of viruses infecting the marine photosynthetic flagellate Micromonas pusilla. Aquat Microb Ecol. 1998;16:103–108. [Google Scholar]
- 26.Sieburth J M, Johnson P W, Hargraves P E. Ultrastructure and ecology of Aureococcus anophagefferens gen. et sp. nov. (Chrysophyceae): the dominant picoplankter during a bloom in Narragansett Bay, Rhode Island, summer 1985. J Phycol. 1988;24:416–425. [Google Scholar]
- 27.Smayda T J. Ecophysiology and bloom dynamics of Heterosigma akashiwo (Raphidophyceae) In: Anderson D M, Cembella A D, Hallegraeff G M, editors. Physiological ecology of harmful algal blooms. Berlin, Germany: Springer; 1998. pp. 113–131. [Google Scholar]
- 28.Suttle C A, Chan A M, Cottrell M T. Infection of phytoplankton by viruses and reduction of primary production. Nature. 1990;347:467–469. [Google Scholar]
- 29.Suttle C A. Inhibition of photosynthesis in phytoplankton by the submicron size fraction concentrated from seawater. Mar Ecol Prog Ser. 1992;87:105–112. [Google Scholar]
- 30.Suttle C A. Enumeration and isolation of viruses. In: Kemp P F, Sherr B, Sherr E, Cole J J, editors. Current methods in aquatic microbial ecology. London, United Kingdom: Lewis Publishers; 1993. pp. 135–138. [Google Scholar]
- 31.Suttle C A, Chan A M. Dynamics and distribution of cyanophages and their effect on marine Synechococcus spp. Appl Environ Microbiol. 1994;60:3167–3174. doi: 10.1128/aem.60.9.3167-3174.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Van Etten J L, Lane L C, Meints R H. Viruses and viruslike particles of eukaryotic algae. Microbiol Rev. 1991;55:586–620. doi: 10.1128/mr.55.4.586-620.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Waterbury J B, Valois F W. Resistance to co-occurring phages enables marine Synechococcus communities to coexist with cyanophages abundant in seawater. Appl Environ Microbiol. 1993;59:3393–3399. doi: 10.1128/aem.59.10.3393-3399.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wommack K E, Hill R T, Kessel M, Russek-Cohen E, Colwell R R. Distribution of viruses in the Chesapeake Bay. Appl Environ Microbiol. 1992;58:2965–2970. doi: 10.1128/aem.58.9.2965-2970.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yamochi S, Abe T. Mechanisms to initiate a Heterosigma akashiwo red tide in Osaka Bay. II. Diel vertical migration. Mar Biol. 1984;83:225–261. [Google Scholar]
- 36.Zingone A, Sarno D, Forlani G. Seasonal dynamics in the abundance of Micromonas pusilla (Prasinophyceae) and its viruses in the Gulf of Naples (Mediterranean Sea) J Plankton Res. 1999;21:2143–2159. [Google Scholar]