Clinical Implications.
Recent immunoglobulin preparations contain high anti-SARS-CoV-2–specific antibodies resulting in passive COVID immunization. The steady-state anti-spike-IgG serum levels in recipients achieve pharmacokinetically predictable serum levels and virus neutralization capacities comparable with those of mRNA-vaccinated subjects.
Patients with antibody deficiency are at risk for bacterial and viral infections.1 , 2 Regular immunoglobulin replacement therapy by intravenous or subcutaneous infusions reduces infection susceptibility. Immunoglobulin preparations containing highly enriched polyclonal IgG are derived from pooled plasma from greater than 103 donors to cover a broad range of pathogen-specific antibodies. The amounts of pathogen-specific IgG within immunoglobulin preparations depend on the seroprevalence in the plasma donor population and are influenced by high pathogen circulation or high vaccination rates against a given pathogen. SARS-CoV-2, the causative agent of COVID-19, encountered an immunologically naive population in late 2019 and rapidly spread worldwide. The high number of SARS-CoV-2 infections and, from 2021 on, the vaccination campaign continuously built up population immunity. Hence, immunoglobulin preparations are expected to contain substantial anti-SARS-CoV-2 antibodies cumulatively. This potentially allows for passive immunization in patients with severe antibody deficiency without the need for additional therapeutic monoclonal anti-SARS-CoV-2 antibodies. Here, we tested for the presence, function, and composition of anti-SARS-CoV-2 antibodies in different commercially available immunoglobulin preparations collected over time during the pandemic. In a proof-of-concept study, we then investigated whether the anti-SARS-CoV-2 antibodies in immunoglobulin preparations are sufficient to build up assumed protective anti-SARS-CoV-2 serum levels in a patient with severe antibody deficiency who failed to mount humoral anti-SARS-CoV-2 after repeated mRNA vaccinations.
First, we measured anti-SARS-CoV-2 spike antibodies (anti-S-IgG) using the Roche Elecsys anti-SARS-CoV-2-Spike-IgG/M assay (Basel, Switzerland) in 14 immunoglobulin preparations (Table I ). Anti-S-IgG levels were above assay positivity threshold in nine of 14 immunoglobulin preparations (64.3%). In seven of nine, the measured concentrations were greater than 150 IU/mL. In addition, we measured anti-nucleoprotein (anti-NP-IgG) and anti-S1-receptor-binding domain (RBD)-IgG using Luminex technology (Austin, Texas) (Table I). The antibody levels in these assays highly correlated with the anti-S-IgG levels in the Roche assay: anti-S1-RBD-IgG (P < .001, Spearman r = 0.94) and anti-NP-IgG (P < .001, r = 0.95). Anti-S-IgM antibodies were absent in all tested products.
Table I.
Anti-SARS-CoV-2-IgG in intravenous immunoglobulin (IVIG) and subcutaneous immunoglobulin (SCIG) preparations
| Batch ID | Production date∗ | Expiration date | Product (manufacturer) | % | Lot no. | Spike IgG/M (U/mL)† | S1-IgG (MFI)‡ | S1 receptor-binding domain-IgG (MFI)‡ | Nucleoprotein-IgG (MFI)‡ |
|---|---|---|---|---|---|---|---|---|---|
| SCIG 3 | October 2021 | 03/24 | Hizentra (CSL Behring) | 20 | P100388734 | 39,783 | 27,506 | 27,103 | 5,860 |
| SCIG 2 | August 2021 | 01/24 | Hizentra (CSL Behring) | 20 | P100360850 | 12,413 | 10,273 | 9,590 | 3,182 |
| IVIG 10 | April 2021 | 03/24 | Privigen (CSL Behring) | 10 | P100331140 | 538 | 3,664 | 2,950 | 1,136 |
| IVIG 11 | May 2021 | 04/24 | Privigen (CSL Behring) | 10 | P100340496 | 283 | 2,192 | 2,108 | 621 |
| IVIG 9 | April 2021 | 03/24 | Privigen (CSL Behring) | 10 | P100328004 | 158 | 698 | 658 | 146 |
| IVIG 7 | March 2021 | 02/24 | Privigen (CSL Behring) | 10 | P100316724 | 151 | 482 | 331 | 194 |
| IVIG 8 | n/a | 03/24 | Intratect (Biotest, Switzerland) | 10 | C791461P01 | 172 | 431 | 405 | 105 |
| IVIG 2 | n/a | 04/23 | Octagam (Octapharma, Switzerland) | 10 | K119A8564 | 96 | 232 | 200 | 55 |
| IVIG 3 | July 2020 | 06/23 | Privigen (CSL Behring) | 10 | P100271163 | 0.9 | 30 | 25 | 27 |
| SCIG 1 | December 2020 | 05/23 | Hizentra (CSL Behring)∗ | 20 | P100292459 | <0.7 | 29 | 22 | 10 |
| IVIG 6 | n/a | 10/23 | Intratect (Biotest) | 10 | C792500P01 | 3.2 | 27 | 23 | 11 |
| IVIG 5 | n/a | 08/23 | Intratect (Biotest) | 10 | C792210P02 | 0.8 | 18 | 18 | 14 |
| IVIG 4 | September 2020 | 08/23 | Privigen (CSL Behring) | 10 | P100270791 | <0.7 | 17 | 18 | 7 |
| IVIG 1 | n/a | 07/22 | Kiovig (Takeda, Switzerland) | 10 | LE12W155AD | 0.8 | 18 | 18 | 16 |
IVIG, intravenous immunoglobulin; MFI, median fluorescence intensity; n/a, not applicable; SCIG, subcutaneous immunoglobulin.
The three batches used in the in vivo study are indicated in bold.
Production date indicates the date the pooled plasma was bottled. Collection of the plasma samples occurred more than 7 to 12 months before this date. Exact dates are not released by manufacturers.
All values were measured at the same time in the Roche Elecsys Anti-SARS-CoV-2 assay (lower level of detection <0.7 U/mL; upper level of detection >2,500 U/ml).
Luminex assay in MFI (background level <50).
There were no substantial differences between immunoglobulin manufacturers. However, the time of plasma pooling of intravenous (IVIG) and subcutaneous (SCIG) preparations, indirectly reflected by the expiration date, was associated with the presence of anti-S-IgG in the products (Table I).
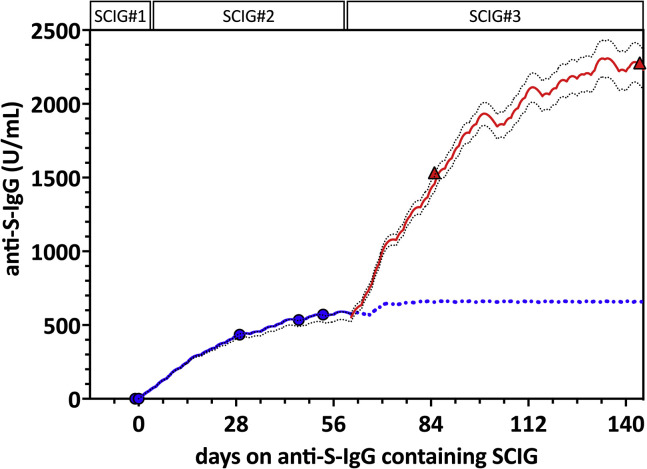
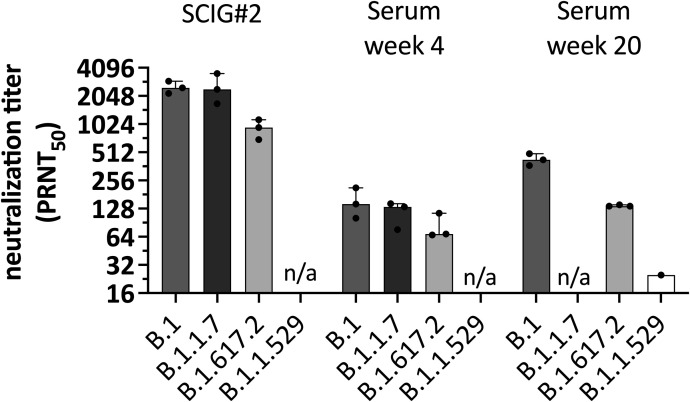
To assess whether the detected anti-S-IgG would result in potentially protective serum antibody levels in vivo, we next performed a proof-of-concept study in a 34-year-old man with severe antibody deficiency resulting from nuclear-κB factor insufficiency. Details about the patient, including the NFκB1 genotype (heterozygote frameshift mutation c.1071-1074del AGAA), were published elsewhere in a cohort study.3 The patient had almost absent peripheral B cells (4/μL) and low T cell and natural killer cell numbers. After the first two doses of an mRNA COVID-19 vaccine (Spikevax, Moderna, Switzerland), he had an undetectable immune response to the spike and nucleocapsid antigen defined by negative SARS-CoV-2–specific antibodies and virus-specific B- and T-cell activation in a commercial lymphocyte activation assay (ADR-AC GmbH, Bern, Switzerland). Four weeks after a third dose of Spikevax, CD8 T cell reactivity to the spike protein could be detected in the commercial T cell assay, but virus-specific antibodies were still absent on October 12, 2021. At that time, he was receiving SCIG replacement therapy (16-20 g weekly) with an SCIG preparation without detectable anti-S-IgG (SCIG 1 in Table I). We switched to an SCIG product from the same company (Hizentra, CSL Behring, Switzerland) with what was then the highest levels of anti-S-IgG (ie, SCIG 2 = 12,413 IU/mL) in our study (Table I) and longitudinally assessed serum anti-S-IgG levels in the patient. After 4, 6, and 7 weeks, anti-S-IgG serum antibody levels rose to 435, 534, and 571 IU/mL, respectively (Figure 1 ). Total IgG levels remained stable between 11.7 and 11.9 g/L after the switch to the SCIG product. Luminex testing confirmed that anti-S-IgG, anti-RBD-IgG, and anti-NP-IgG increased in parallel. Virus-specific antibodies were predominantly of the IgG1 subclass, as measured by IgG subtype-specific Luminex (not shown). To assess the functional capacity of the serum antibody levels, we performed SARS-CoV-2 neutralization assays against the Wuhan-Hu-1–like original strain (B.1) and the Alpha (B.1.1.7), and Delta variants (B.1.617.2) (see text in this article’s Online Repository at www.jaci-inpractice.org). The SCIG 2 preparation demonstrated high neutralization titers against the B.1 and B1.1.7. strains (median NT50 2519 and 2436, respectively) and reduced but measurable neutralization against the Delta variant (NT50 909) (see Figure E1 in this article’s Online Repository at www.jaci-inpractice.org). After 4 weeks of treatment with SCIG 2, the patient’s serum neutralized all three SARS-CoV-2 variants (NT50 146, 114, and 81, respectively) in the range of convalescent plasma.4
Figure 1.
Anti-spike antibodies and pharmacokinetic modeling of anti-S-IgG immunity. Observed and predicted anti-S-IgG serum concentrations using subcutaneous immunoglobulin 2 (SCIG#2) with 12,413 IU/mL anti-S-IgG (blue line) (days 0-56) and SCIG#3 with 39,783 IU/mL (red line) (days 57-144). Blue dotted line indicates predicted anti-S-IgG steady-state through levels with SCIG#2 (predicted Ctrough about 665 IU/mL). Simulations show a predicted further increase in anti-S-IgG serum concentration after switching to SCIG#3 (red line) (predicted Ctrough approximately 2,100 IU/mL). Serum anti-S-IgG measurements on days 53 (under SCIG#2) (blue dots), 85, and 144 (under SCIG#3) (red triangles) confirmed good agreement with the model. Black dotted lines indicate upper and lower prediction intervals (dotted black lines).
Figure E1.
Virus neutralization against different SARS-CoV-2 variants of concern. Virus neutralization capacity of the subcutaneous immunoglobulin 2 (SCIG#2) product and patient's serum 4 and 20 weeks after immunoglobulin substitution with products containing increasing anti-S-IgG are shown. Data represent mean and SD of three independent experiments. Subcutaneous immunoglobulin 2 shows high neutralization NT50 against the original strain (B.1) and B.1.1.7 (Alpha), and about threefold reduced neutralization against B.1.617.2 (Delta). The patient's serum showed virus neutralization against all three SARS-CoV-2 variants, albeit at lower NT50. In steady-state after 20 weeks, neutralization capacity increased further. However, the newly emerging Omicron variant was only poorly neutralized. Data are derived from three independent experiments. Bars indicate median and range. n/a, respective variant was not tested for the time point; PRNT50, 50% reduction in plaque reduction neutralization test.
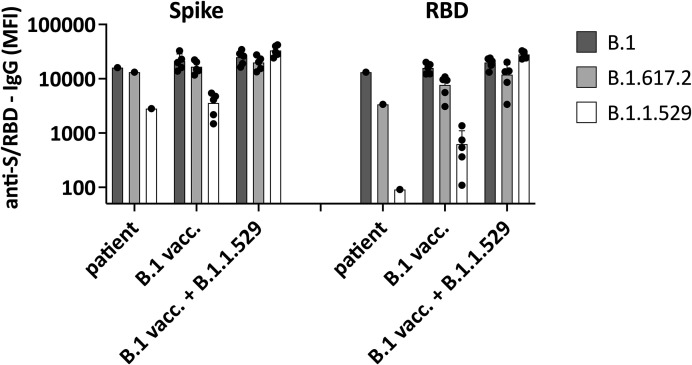
We then applied pharmacokinetic (PK) modeling to predict steady-state anti-S-IgG serum antibody levels based on the total IgG and anti-S-IgG concentrations in the SCIG preparations. In the first step, we build a linear one-compartment PK model parameterized with IgG clearance, volume at steady state, and absorption parameter, which is required only for subcutaneous administration. Further details can be found in the text in the Online Repository. Typical PK parameter values were derived from the literature.5 , 6 The clearance was calculated from the patient’s total IgG trough concentration (Ctrough) at steady state by dividing the total weekly dose of SCIG with the measured IgG Ctrough at steady-state multiplied by 7 days. Using this base PK model, we conducted and compared simulations of the anti-S-IgG serum levels with the measured in vivo data. The PK parameters of the base model were updated to match the observed data accordingly. The refined PK model predicted Ctrough levels at steady state for anti-S-IgG to reach about 665 IU/mL with SCIG 2 (Figure 1). At 23 days after the switch to SCIG 3 containing 39,783 IU/mL anti-S-IgG (Table I), we observed a further increase in the anti-S-IgG serum level to 1,533 IU/mL, which was in good agreement with the model predicting a level of 1,425 IU/mL (Figure 1). Using SCIG 3, we predicted a steady-state in vivo serum level of approximately 2,100 U/mL after about 4 weeks. In a follow-up sample after 20 weeks with SCIG 3, the serum anti-S-IgG level increased to 2,278 IU/mL. We repeated neutralization assays on this serum sample against B.1, B.1.617.2, and the by then dominating B.1.1.529 Omicron variant. We observed a further increase in the serum neutralization capacity against B.1 and B.1.617.2 compared with the sample taken after 4 weeks (NT50 426 vs 146 for B.1 and 137 vs 81 for B.1.617.2). Neutralization against B.1.1.529 Omicron was poor (NT50 25), as expected based on the literature on serum of convalescent and vaccinated subjects.7 However, we detected cross-reactive non-neutralizing antibodies against Omicron using Luminex (see Figure E2 in this article’s Online Repository at www.jaci-inpractice.org).
Figure E2.
Non-neutralizing strain-specific antibody response. Antibody binding to spike protein (S1, left) and receptor-binding domain (RBD) of spike protein (right) of the B.1 (Wuhan), B1.1.617.2 (Delta), and B.1.1.529 (Omicron) were measured. We compared the binding of patient serum (20 weeks on the anti-SARS-CoV-2–containing subcutaneous immunoglobulin) and serum of five mRNA-vaccinated (B.1 vacc.) subjects collected 4 weeks after the second dose of an mRNA vaccine (Spikevax, Moderna) and of five subjects with Omicron breakthrough infection despite two doses of an mRNA vaccine (B.1 vacc. + B.1.1.529). Receptor-binding domain IgG is considered a surrogate for virus neutralization, and total anti-S1-IgG also contains non-neutralizing antibodies. Data indicate cross-reactive anti-S1-IgG that recognize Omicron in all tested subjects, whereas only Omicron breakthrough infection is associated with high anti-RBD-IgG against Omicron. MFI, median fluorescence intensity.
Our data indicate that more recently collected IVIG and SCIG preparations contain substantial levels of anti-SARS-CoV-2 IgG. These levels are sufficient to increase the serum antibody levels in severely antibody-deficient patients to values found in vaccinated or convalescent individuals associated with protection from severe COVID-19 disease. Because immunoglobulin preparations also contain high non-neutralizing antibodies against the nucleoprotein and cross-reactive non-neutralizing antibodies against the Omicron spike protein, protection from SARS-CoV-2 may expand beyond neutralizing just antibodies.
Our data confirm recent reports of anti-SARS-CoV-2 antibodies against the spike or nucleocapsid protein in immunoglobulin preparations.8 , 9 However, compared with the most recent SCIG preparations used in our study, the reported antiviral antibody levels were substantially lower, and those studies did not address whether the antibody levels in the IVIG or SCIG preparations translated into high anti-SARS-CoV-2 IgG in recipient serum. Currently, manufacturers provide no information about the anti-S-IgG content in their immunoglobulin preparations or the time of plasma collection. Plasma pools are collected 7 to 12 months before the production date indicated in Table I. Thus, plasma of the products tested in our study was collected before the COVID mass-vaccination campaign. A further increase in anti-S-IgG levels in immunoglobulin preparations can be expected for the near future.
The proposed PK modeling approach is suited to predict the anticipated steady-state levels of anti-S-IgG at a given level in the SCIG preparation. However, this approach implies that there are negligible endogenous IgG levels and that the clearance derived from the steady-state concentration is in the same range as the clearance of the anti-S-IgG. Further studies will be required to define whether our data can be directly applicable to patients with higher endogenous IgG levels. Once IVIGs and SCIGs with high anti-S-IgG are broadly available, the method can be refined to enable applicability further to immunosuppressed patients with relevant endogenous IgG levels.
Our findings suggest that immunodeficient patients who fail to develop vaccine-induced anti-SARS-CoV-2 antibodies may be passively immunized by immunoglobulin replacement therapy. The achievable antiviral antibody levels in vivo may be pharmacokinetically predicted and may guide personalized dosing. The observed reduced neutralization against the Omicron variant suggests that patients with inborn errors of immunity may still need to receive prophylactic monoclonal antibodies with retained neutralization capacity against all circulating SARS-CoV-2 variants to be optimally protected.
Acknowledgments
This study was approved by the ethical committee of the Northwest and Central Switzerland (EKNZ 2015-187) as part of the prospective cohort study of the functional and genetic architecture of primary immunodeficiencies. The datasets generated during and analyzed during this study are available from the corresponding author on reasonable request. All authors contributed to the study. Material preparation, data collection and analysis were performed by J.R. Hirsiger, S. Weigang, A.-C. Walz, J. Fuchs, M.-L. Daly, S. Eggimann, O. Hausmann, M. Recher, and C.T. Berger. Study design, conception, interpretation, and funding were performed by M. Schwemmle, G. Kochs, M. Panning, K. Warnatz, M. Recher, and C.T. Berger. The first draft of the manuscript was written by J.R. Hirsiger, A.-C. Walz, and C.T. Berger, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Footnotes
This work was supported by the Uniscientia Foundation (to C.T. Berger), the Swiss National Science Foundation (Grant No. 310030_192652 to M. Recher), the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation, Grant No. PA 2274/4-1 to M. Panning), and the Bundesministerium für Bildung und Forschung through the Deutsches Zentrum für Luft- und Raumfahrt, Germany (Grant No. 01KI2077 to G. Kochs). K. Warnatz has received funding from the Deutsche Forschungsgemeinschaft (WA 1597/6-1 and WA 1597/7-1). We thank Roman Woelfel (Bundeswehr Institute of Microbiology) for providing the B.1 (Muc-IMB-1) isolate, Donata Hoffmann and Martin Beer (Friedrich-Loeffler-Institut, Insel Riems) for providing the B.1.1.7 isolate, and Marek Widera and Sandra Ciesek for providing the B.1.1.529 isolate. We thank Dr Estelle Marrer-Berger for the inspiration and critical feedback for this work.
Conflicts of interest: A.C. Walz is an employee of Roche, Switzerland. O. Hausmann is an employee of ADR-AC GmbH, Switzerland. K. Warnatz has received speakers’ fees from CSL Behring. The rest of the authors declare that they have no relevant conflicts of interest.
Online Repository. Luminex Anti-SARS-CoV-2-Antibody Binding Testing
Magnetic MagPlex-microspheres (Luminex Corporation, Austin, Texas) were coated using 10 ng SARS-CoV-2 spike protein (eENZYME LLC, Gaithersburg, Md) according to xMAP cookbook (Luminex Corporation). Bovine serum albumin (BSA)-coated beads were the negative control. To measure anti-S–specific-IgG, M, and IgG subclass-specific antibodies, serum or the subcutaneous or intravenous immunoglobulin (SCIG/IVIG) preparations were mixed with spike protein–coated or bovine serum albumin–coated control beads for 1 hour at room temperature on a plate shaker at 800 rpm. The beads were washed twice with PBS-0.05% Tween, 1% BSA, 0.1% Sodium Azide (TBN) buffer and then incubated with a phycoerythrin-labeled mouse anti-human detection antibody (anti-IgG, -IgM, -IgA, and -IgG1-4; all from SouthernBiotech, Birmingham, Ala) for another 45 minutes. Binding of spike-specific detection antibodies was measured on a Luminex 100 analyzer running on xPonent software (Luminex Corporation). The extent of antibody-binding is represented by the phycoerythrin median fluorescence intensity (MFI).
We found that serum after 4, 6, and 7 weeks on SCIG 2 showed increasing amounts of anti-S1(B.1)-receptor-binding domain (RBD)-IgG (MFI: 2,740, 3,692, and 4,212) and anti-nucleoprotein (median MFI: 1,107, 1,503 and 1,834) levels. Using different recombinant spike proteins of the B.1 (Wuhan), B.1.617.2 (Delta), and B.1.1.529 (Omicron) variants, we also tested for variant-specific binding antibodies, as shown in Figure E2.
Immunoglobulin IgG1 to 4 subset–specific anti-S-IgG measurement in the SCIG products showed that the anti-S-IgGs were predominantly of the IgG1 subset (70% to 80%) and less of the IgG2 subset. Similarly, the patient’s serum contained predominantly anti-S-IgG of the IgG1 subclass. Interestingly, the RBD response showed a higher amount of IgG2 subclass antibodies in the serum.
SARS-CoV-2 in Vitro Neutralization Assay
Virus neutralization experiments with SARS-CoV-2 were performed under Biosafety level 3 protocols at the Institute of Virology, Freiburg. Adherent African green monkey kidney VeroE6 cells (ATCC CRL-1586) were cultured in 1× Dulbecco’s modified Eagle medium containing 5% or 10% fetal calf serum. The SARS-CoV-2 isolates used to test for neutralization were Delta variant B.1.617.2 (EPI_ISL_2535433) isolated from a patient in Freiburg, Germany; Muc-IMB-1, lineage B.1 (EPI_ISL_406862 Germany/BavPat1/2020); Alpha variant B.1.1.7 (EPI_ISL_751799); and Omicron variant BA.1 (EPI_ISL_6959868). Neutralizing antibody titers were determined by a plaque reduction assay. Serial twofold dilutions of the sera or the SCIG preparations were incubated for 1 hour with 100 pfu of the respective SARS-CoV-2 isolates. The serum–virus mixture was then used to infect VeroE6 for 90 minutes at 37°C. The inoculum was removed and cells were overlaid with 0.6 % oxoid agar for 48 to 72 hours at 37°C. Cells were fixed with 3.7% formaldehyde and stained with crystal violet. The reduction in counted plaque numbers was determined compared with an untreated mock-infected control without serum. Each experiment was performed in three independent replicates. A least-squares nonlinear regression was calculated (constraints: 0% and 100%) and NT50 values were determined based on the curve fits.
Pharmokinetic Modeling
The patient received an average total dose of about 20 g/wk SCIG, resulting in a trough concentration at steady state (Css,trough) of 11.5 g/L IgG. From this observation, an estimated clearance of 0.25 L/d was derived according to the formula: CL = total weekly dose / (Css,trough × 7 d). Two different batches were administered: batch 1 (charge P100360850) had a concentration of 12,413 IU/mL, and batch 2 (charge P100388734) had a concentration of 39,783 IU/mL of the anti-S-IgG. For pharmacokinetic prediction, a one-compartmental model was used with a first-order absorption rate constant ka of 0.34 1/d (E1) a central volume Vc of 7.4 L, a clearance of 0.22 L/d, and an estimated half-life of about 25 days in the range of reported values for total IgGs (E2). The simulations were conducted in Berkeley Madonna software version 8.3.18 (Berkeley Madonna Inc., Albany, CA, USA).
References
- 1.Gathmann B., Mahlaoui N., Ceredih, Gerard L., Oksenhendler E., Warnatz K., et al. Clinical picture and treatment of 2212 patients with common variable immunodeficiency. J Allergy Clin Immunol. 2014;134:116–126. doi: 10.1016/j.jaci.2013.12.1077. [DOI] [PubMed] [Google Scholar]
- 2.Mallick R., Divino V., Smith B.D., Jolles S., DeKoven M., Vinh D.C. Infections in secondary immunodeficiency patients treated with Privigen® or Hizentra®: a retrospective US administrative claims study in patients with hematological malignancies. Leuk Lymphoma. 2021;62:3463–3473. doi: 10.1080/10428194.2021.1961233. [DOI] [PubMed] [Google Scholar]
- 3.Lorenzini T., Fliegauf M., Klammer N., Frede N., Proietti M., Bulashevska A., et al. Characterization of the clinical and immunologic phenotype and management of 157 individuals with 56 distinct heterozygous NFKB1 mutations. J Allergy Clin Immunol. 2020;146:901–911. doi: 10.1016/j.jaci.2019.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kaleta T., Kern L., Hong S.L., Holzer M., Kochs G., Beer J., et al. Antibody escape and global spread of SARS-CoV-2 lineage A.27. Nat Commun. 2022;13:1152. doi: 10.1038/s41467-022-28766-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang Y., Baheti G., Chapdelaine H., Hofmann J., Rojavin M., Tortorici M., et al. Population pharmacokinetic analysis of weekly and biweekly IgPro20 (Hizentra®) dosing in patients with primary immunodeficiency. Int Immunopharmacol. 2020;81 doi: 10.1016/j.intimp.2019.106005. [DOI] [PubMed] [Google Scholar]
- 6.Alyanakian M.A., Bernatowska E., Scherrmann J.M., Aucouturier P., Poplavsky J.L. Pharmacokinetics of total immunoglobulin G and immunoglobulin G subclasses in patients undergoing replacement therapy for primary immunodeficiency syndromes. Vox Sang. 2003;84:188–192. doi: 10.1046/j.1423-0410.2003.00278.x. [DOI] [PubMed] [Google Scholar]
- 7.Schmidt F., Muecksch F., Weisblum Y., Da Silva J., Bednarski E., Cho A., et al. Plasma neutralization of the SARS-CoV-2 Omicron variant. N Engl J Med. 2022;386:599–601. doi: 10.1056/NEJMc2119641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Karbiener M., Farcet M.R., Schwaiger J., Powers N., Lenart J., Stewart J.M., et al. Plasma from post-COVID-19 and COVID-19-vaccinated donors results in highly potent SARS-CoV-2 neutralization by intravenous immunoglobulins. J Infect Dis. 2021;224:1707–1711. doi: 10.1093/infdis/jiab482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miller A.L., Rider N.L., Pyles R.B., Judy B., Xie X., Shi P.Y., et al. The arrival of SARS-CoV-2-neutralizing antibodies in a currently available commercial immunoglobulin. J Allergy Clin Immunol. 2022;149:1958–1959. doi: 10.1016/j.jaci.2022.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
References
- Zhang Y., Baheti G., Chapdelaine H., Hofmann J., Rojavin M., Tortorici M., et al. Population pharmacokinetic analysis of weekly and biweekly IgPro20 (Hizentra(R)) dosing in patients with primary immunodeficiency. Int Immunopharmacol. 2020;81:106005. doi: 10.1016/j.intimp.2019.106005. [DOI] [PubMed] [Google Scholar]
- Alyanakian M.A., Bernatowska E., Scherrmann J.M., Aucouturier P., Poplavsky J.L. Pharmacokinetics of total immunoglobulin G and immunoglobulin G subclasses in patients undergoing replacement therapy for primary immunodeficiency syndromes. Vox Sang. 2003;84:188–192. doi: 10.1046/j.1423-0410.2003.00278.x. [DOI] [PubMed] [Google Scholar]