Abstract
Introduction
Musculoskeletal (MSK) symptoms, including arthritis and arthralgia, are common manifestations of systemic lupus erythematosus (SLE); definitions of activity patterns in SLE differ across studies. This study described clinical characteristics and treatment patterns of patients with SLE-MSK over time and by disease activity patterns from a real-world setting.
Methods
This retrospective descriptive analysis includes a subset of patients with SLE from the Hopkins Lupus Cohort with identified MSK involvement by scores on the arthritis domain of the Safety of Estrogens in Systemic Lupus Erythematosus National Assessment-Systemic Lupus Erythematosus Disease Activity Index (SELENA-SLEDAI) or Lupus Activity Index. Clinical characteristics and treatment patterns were described for patients with at least two visits over the observation period (2010–2019) for the SLE-MSK population based on three disease activity patterns: chronically active (MSK-CA), relapsing–remitting (MSK-RR), and long quiescence (MSK-LQ).
Results
The SLE-MSK subpopulation included 664 patients (4069 person-years). The most frequently used medications over the observation period were antimalarials (95%), corticosteroids (92%), immunosuppressants (58%), and nonsteroidal anti-inflammatory drugs (NSAIDs) (48%); 7% of patients used biologics. The highest use of corticosteroids was in the MSK-CA group (90.5% of follow-up time), followed by MSK- RR (83.9%), and MSK-LQ (46.5%). Mean prednisone dose was significantly higher in MSK-RR (8.5 mg) compared to MSK-CA (6.5 mg).
Conclusions
This descriptive analysis highlights the impact of prevalent manifestations such as arthritis on the chronic use of corticosteroids, immunosuppressants, and NSAIDs to manage disease activity in patients with SLE, suggesting there is a need for new therapeutic options that enable a lower use of medication when treating lupus.
Supplementary Information
The online version contains supplementary material available at 10.1007/s12325-022-02148-8.
Keywords: Systemic lupus erythematosus, Musculoskeletal symptoms, Corticosteroids, Treatment patterns
Key Summary Points
| Why carry out this study? |
| Musculoskeletal (MSK) symptoms, including arthritis and arthralgia, are common manifestations of systemic lupus erythematosus (SLE). |
| The natural history of SLE has been described in longitudinal cohorts of patients with SLE; however, definitions of the activity patterns have differed across studies and the nature of flaring patterns compared with chronic or quiescent patterns specific to MSK has not been characterized in the literature, nor has it been reported how these patterns impact therapeutic approaches. |
| The primary objective of this study was to describe the clinical characteristics and treatment patterns of patients with SLE-MSK over time and by disease activity patterns (chronically active, relapsing–remitting, long quiescence) in a 10-year follow-up period from the real-world setting of the Hopkins Lupus Cohort. |
| What was learned from this study? |
| This descriptive analysis highlights the impact of prevalent manifestations, such as arthritis on the use of corticosteroids, immunosuppressants, and nonsteroidal anti-inflammatory drugs (NSAIDs) to manage disease activity in patients with SLE. Half of the patients in our analysis presented in an active disease activity pattern, most commonly relapsing–remitting disease activity measured by Safety of Estrogens in Systemic Lupus Erythematosus National Assessment-Systemic Lupus Erythematosus Disease Activity Index. |
| The presence of the active patterns (despite high use of combination therapy, corticosteroids, and NSAIDs) suggests there is a need for new therapeutic options to manage disease activity in patients with SLE, permitting better efficacies to be achieved and diminishing the chronic use of these drugs and their long-term side effects. |
Introduction
Musculoskeletal (MSK) symptoms, including arthritis and arthralgia, are common manifestations of systemic lupus erythematosus (SLE); 53–95% of patients report MSK manifestations and joint involvement over the disease course [1] and up to 60% during disease flares [2]. Patients with SLE usually have non-deforming, non-erosive arthritis with synovitis detectable in some, as well as tenosynovitis [3]. The occurrence of arthralgia without clinically detectable synovitis in SLE is notable [2].
The natural history of SLE has been described in longitudinal cohorts of patients; however, definitions of activity patterns differed across studies. Previous data from the Hopkins Lupus Cohort identified the following patterns of SLE disease activity: chronically active (CA), relapsing–remitting (RR), and long quiescence (LQ) each defined using a modified Systemic Lupus Erythematosus Disease Activity Index (mSLEDAI) score and Physician Global Assessment (PGA) [4]. Chronically active patients have consistently active disease (mSLEDAI > 0; PGA > 0 at all visits); during an RR pattern, patients have active disease (mSLEDAI > 0; PGA > 0) that alternates with inactive disease (mSLEDAI = 0; PGA = 0); LQ describes a period of time where patients have inactive disease (mSLEDAI = 0; PGA = 0 at all visits) [4]. Among 1886 patients from the Hopkins Lupus Cohort (10,792 patient-years of follow-up), RR was the most common pattern (54% of patients) followed by LQ (31%) and CA (15%) [4].
While specific symptoms involved in flaring patterns can vary across patients, data from the Hopkins Lupus Cohort showed the MSK domain to be one of the most common organ systems associated with flares, reported in almost 60% of patients [5]. However, the nature of flaring patterns compared with chronic or quiescent patterns specific to MSK has not been characterized in the literature, nor has it been reported how these patterns impact therapeutic approaches.
The 2019 European League Against Rheumatism recommendations for the management of SLE describe MSK symptoms in the mild to moderate category with treatment options including hydroxychloroquine (HCQ), corticosteroids, methotrexate, azathioprine, and nonsteroidal anti-inflammatory drugs (NSAIDs). Alternative treatments for more severe disease include calcineurin inhibitors, mycophenolate, and belimumab [6]. How MSK symptoms are being managed in real-world practice and if/how treatment patterns of MSK symptoms vary across disease activity patterns have not been reported.
The primary objective of this study was to describe the clinical characteristics and treatment patterns of patients with SLE-MSK (patient level) over time and by disease activity patterns (person-years, PY) in a 10-year follow-up period from a real-world setting.
Methods
This was a retrospective descriptive analysis of patients with SLE followed in the Hopkins Lupus Cohort, a longitudinal cohort established in 1987 that includes over 2000 patients classified per revised American College of Rheumatology (ACR) 1997 or Systemic Lupus International Collaboration Clinics 2012 criteria [7]. The study was conducted in accordance with the Declaration of Helsinki and cohort is approved by the Johns Hopkins University School of Medicine Institutional Review Board (study number NA 00039294) on a yearly basis, and all patients gave written informed consent. Data for the current analysis include patients in the cohort from January 1, 2010 through December 31, 2019.
Study Design and Population
This analysis focused on a subpopulation of the Hopkins Lupus Cohort, patients with MSK involvement identified by scores on the arthritis domain of the Safety of Estrogens in Systemic Lupus Erythematosus National Assessment-Systemic Lupus Erythematosus Disease Activity Index (SELENA-SLEDAI) scale [8] and/or the joint domain of the Lupus Activity Index (LAI) [9], as recorded by rheumatologists during routine clinical care. As such, the analyses were focused on patients who experienced arthritis/arthralgia, referred to as SLE-MSK throughout the paper. Patients with rheumatoid arthritis were excluded from inclusion into the Hopkins Cohort and X-rays were not performed routinely, but would have been obtained in patients presenting with Jaccoud’s arthropathy. The SLEDAI was defined using the SELENA-SLEDAI dichotomous scale (present/absent) of arthritis in at least three joints (scored as 1 for present in the cohort) or LAI joint domain that assesses symptoms (including inflammatory arthralgia) related to joints using a 0–3 visual analog (0 = no symptoms, 1 = mild, 2 = moderate, and 3 = severe), using a score of 1 or more for inclusion in the study. Adult patients (at least 18 years old at the start of the observation period) were included if they met the following criteria: presence of active MSK involvement during any follow-up year of the observation period (January 1, 2010 to December 31, 2019) and history of MSK involvement before January 2010 or before the time entering the cohort if patient was enrolled after 2010. For any year of observation, patients needed to have at least two annual visits to be included for analysis.
Clinical characteristics and treatment patterns were described over the total observation period (2010–2019) at the patient level and for the SLE-MSK population based on disease activity patterns in PY. Three disease activity patterns (CA, RR, and LQ), adapted from previously described patterns in patients with SLE from the Hopkins Lupus cohort (Supplementary Material Table S1) [4, 10], were classified for each year for patients with at least two visits of data collected each year during the observation period.
As previously described, during a 10-year observation period, patients with SLE experienced different disease activity patterns [4, 5, 10]. To account for the potential variability in disease activity patterns experienced by each patient over time, the three disease activity patterns were evaluated by PY, a combination of the time spent across all patients with the same disease-activity pattern.
Data were collected at enrolment, and patients in the cohort were monitored per protocol with routine follow-up every 3–6 months. Disease activity was assessed using the SELENA-SLEDAI, LAI, and PGA. Antinuclear antibody positivity was defined as a titre of at least 1:80, and anti-double-stranded DNA positivity was defined as a titre of at least 1:10 on the Crithidia luciliae indirect immunofluorescence test. Antimalarial drugs were exclusive for HCQ; immunosuppressive treatments included methotrexate, azathioprine, mycophenolate, tacrolimus, and leflunomide; biologics were represented by belimumab and rituximab; NSAIDs included several drugs in this class, mostly naproxen; and corticosteroids included oral treatment of prednisone, intravenously administered methylprednisolone, and intramuscularly administered triamcinolone.
Statistical Analysis
Demographics and baseline characteristics of the patients with SLE-MSK were described at the patient level with frequency and percentages for categorical variables and with means and standard deviations for continuous variables. Baseline demographics were defined at the first visit of the observation period, while the baseline period for clinical characteristics and medication history was defined over the first calendar year (index year) of the observation period.
For variables derived from a period of time (a year or overall observation period), patients were defined as having a manifestation (dichotomized variables) or a treatment if there was presence at any visit. For each year of a patient’s follow-up, if a manifestation or treatment was present at any time during that year, the patient was determined to have had that manifestation or treatment for that year. If a manifestation or treatment was present at any follow-up year, the patient was determined to have ever had the manifestation or treatment during the observation period. The presence was then summarized with frequency and percentage by patients and by disease activity pattern using PY. For clinical scores (continuous variables), means and maximum scores of each time period (calendar year or overall observation period) were derived.
Treatment doses were only calculated for prednisone; systemic corticosteroid treatments were not considered in dose calculations. The mean and maximum prednisone dose for each calendar year were derived by calculating the mean and the maximum of non-zero doses. The association of prednisone dosage with SELENA-SLEDAI total score was explored by comparing prednisone mean doses between SELENA-SLEDAI mild disease activity (0–6), moderate disease activity (7–11), and severe disease activity (12 or more) using Wilcoxon rank tests. The combination of medications was defined as patients taking this combination of medications at a year, exclusively or not exclusively. If a patient had a combination pattern in any year, the patient was defined as having this combination pattern present during the observation period. The combination pattern was then summarized over the observation period as frequency and percentages (number and percentage of patients who ever had this combination pattern in any calendar year).
For comparison between the three disease activity patterns, t tests and Wilcoxon rank tests were used for continuous variables, and Fisher’s exact tests were used for categorical variables. No multicomparison adjustments were used. A p value less than 0.05 was considered statistically significant. All analyses were done with SAS Enterprise Guide V7.1.
Results
Of the 1540 patients with SLE in the Hopkins Lupus cohort during the observation period (January 1, 2010 to December 30, 2019), 664 patients (4069 PY) met the inclusion criteria and were included in the total population of patients with SLE-MSK (Supplementary Material Fig. S1). The majority of patients (91.4%) had at least 2 years of follow-up, and 81.2% of patients had at least 3 years of follow-up. There were 8.6% and 10.2% of patients who had only 1 year and 2 years of follow-up, respectively; some of these patients, however, could have been patients who entered the cohort in 2018 or 2019 and did not have an opportunity for longer follow-up. Over the 4069 PY, the greatest proportion of follow-up time was spent in an inactive disease pattern of MSK symptoms (MSK-LQ; 2360 PY; 58.0% of total follow-up time). In those with active MSK symptoms, the majority of time was spent in an MSK-RR pattern (1363 PY; 33.5% of follow-up time), followed by MSK-CA (346 PY; 8.5% of follow-up time) (Supplementary Material Fig. S2).
Demographics and Baseline Patient Characteristics
Of the 664 patients with SLE-MSK, 94% were female, mean age was 43.3 years, and mean time since diagnosis was 11.3 years. Cumulative ACR manifestations of SLE showed that almost all patients (97.9%) had positive antinuclear antibody, 82.7% had non-erosive arthritis (per ACR classification criteria), and 80.9% reported immunological disorder by ACR classification criteria with mild disease activity over the first year of the observation period. Medication use at baseline is outlined in Table 1. Orally administered prednisone, used by 46.4% of patients, had a mean dose of 9.6 mg and mean maximum dose of 13.3 mg. Median prednisone dose at baseline was higher in patients with moderate (10.0 mg, n = 41) and severe (20.0 mg, n = 4) disease activity, as defined by SELENA-SLEDAI, versus patients with mild disease activity (6.3 mg, n = 619; p = 0.007 and 0.041, respectively).
Table 1.
Baseline demographics and clinical characteristics
| All patients with SLE-MSK (n = 664) | |
|---|---|
| At baseline/index date | |
| Age, years, mean (SD) | 43.3 (13.2) |
| Female, n (%) | 622 (93.7) |
| Race, n (%) | |
| Asian | 24 (3.6) |
| Black | 312 (47.0) |
| Caucasian | 297 (44.7) |
| Hispanic | 1 (0.2) |
| Other | 30 (4.5) |
| Time since diagnosis, years, mean (SD) | 11.3 (8.9) |
| ACR criteria for diagnosis, n (%) | |
| Malar rash | 313 (47.1) |
| Discoid rash | 126 (19.0) |
| Photosensitive rash | 342 (51.5) |
| Oral ulcers | 405 (61.0) |
| Non-erosive arthritis | 548 (82.7) |
| Pleuritis or pericarditis | 347 (52.3) |
| Renal disorder | 300 (45.3) |
| Neurological disorder | 64 (9.6) |
| Hematological disorder | 444 (67.0) |
| Immunological disorder | 537 (80.9) |
| Positive antinuclear antibody | 650 (97.9) |
| Average follow-up time per person, years, mean (SD) | 6.1 (3.1) |
| Number of visits annually, years, mean (SD) | 3.1 (1.2) |
| Index year | |
| SELENA-SLEDAI total score, mean (SD) | 2.7 (2.6) |
| SELENA-SLEDAI total score max, mean (SD) | 4.2 (3.8) |
| SELENA SLEDAI organ involvement | |
| MSK | 112 (16.7) |
| Skin | 336 (50.6) |
| Renal | 96 (14.5) |
| Immunologic | 299 (45.0) |
| Constitutional | 4 (0.6) |
| Central nervous system | 8 (1.2) |
| Vascular | 23 (3.5) |
| Hematology | 57 (8.6) |
| Serositis | 41 (6.2) |
| PGA, mean (SD) | 0.7 (0.5) |
| PGA max, mean (SD) | 1.1 (0.7) |
| Autoantibodies | |
| Patients with anti-dsDNA (≥ 1:10)a, n (%) | 225 (34.0) |
| Patients with complement 3 < LLN 0.9 g/dL, n (%) | 176 (26.6) |
| Patients with complement 4 < LLN 0.1 g/dL, n (%) | 151 (22.8) |
| Medication history, n (%) | |
| Antimalarial | 602 (90.7) |
| Hydroxychloroquine | 602 (90.7)b |
| Corticosteroids | 447 (67.3) |
| Prednisone | 308 (46.4) |
| Immunosuppressants | 287 (43.2) |
| Methotrexate | 49 (7.4) |
| Azathioprine | 93 (14.0) |
| Mycophenolate | 145 (21.8) |
| Leflunomide | 12 (1.8) |
| Tacrolimus | 28 (4.2) |
| Cyclophosphamide | 5 (0.8) |
| NSAIDS | 178 (26.8) |
| Biologics | 19 (2.9) |
| Rituximab | 9 (1.4) |
| Etanercept | 1 (0.2) |
| Abatacept | 0 |
| Adalimumab | 0 |
| Belimumab | 9 (1.4) |
Baseline defined as the first observation analyzed for demographics and over the first year of the observation period for clinical characteristics
ACR American College of Rheumatology, LLN lower limit of normal, n number of patients in a cohort, MSK musculoskeletal, NSAIDs nonsteroidal anti-inflammatory drugs, PGA Physician Global Assessment, SD standard deviation, SELENA SLEDAI Safety of Estrogens in Lupus National Assessment Systemic Lupus Erythematosus Disease Activity Index, SLE systemic lupus erythematosus
aDefined as a titre ≥ 1:10 on the C. luciliae indirect immunofluorescence test
bHydroxychloroquine was the only antimalarial used by patients
Treatment Patterns over the Observation Period (Overall SLE-MSK Population)
For the total SLE-MSK population over the 10-year observation period, the frequency of medications used at the class level was antimalarials (95.0%), corticosteroids (oral and parenteral; 92.2%), immunosuppressants (57.8%), and NSAIDs (47.6%); 6.9% of patients used biologics (Table 2). The most frequently used corticosteroids were triamcinolone (83.9%) and orally administered prednisone (56.6%) (mean dose 9.6 mg, and a mean maximum dose of 19.6 mg). Patients with moderate disease activity, as defined by SELENA-SLEDAI, had a higher median dose of prednisone (12.1 mg/day, Q1–Q3 9.3–16.3) compared to patients with mild disease activity (6.8 mg/day, Q1–Q3 4.8–10.1; p = 0.002). The most frequently used immunosuppressant was mycophenolate (30.9%) and the most frequently used biologics was rituximab (4.2%) (Table 2).
Table 2.
Treatment patterns over the observation period (2010–2019) in the overall SLE-MSK population (N = 664)
| Medication names | Medication n (%)b |
|---|---|
| Antimalarial | 631 (95.0) |
| Hydroxychloroquine | 631 (95.0) |
| Corticosteroids | 612 (92.2) |
| Prednisone | 376 (56.6) |
| Prednisone dose, mean (SD) | 9.6 (8.8) |
| Triamcinolone | 557 (83.9) |
| Methylprednisolone | 25 (3.8) |
| Immunosuppressants | 384 (57.8) |
| Methotrexate | 83 (12.5) |
| Azathioprine | 138 (20.8) |
| Mycophenolate (mofetil or mycophenolic acid) | 205 (30.9) |
| Leflunomide | 19 (2.9) |
| Tacrolimus | 55 (8.3) |
| Cyclophosphamide | 15 (2.3) |
| NSAIDs | 316 (47.6) |
| Biologics | 46 (6.9) |
| Rituximab | 28 (4.2) |
| Etanercept | 2 (0.3) |
| Abatacept | 3 (0.5) |
| Adalimumab | 0 |
| Belimumab | 17 (2.6) |
| Combination therapiesa | |
| Non-mutually exclusive categories | |
| Corticosteroids + antimalarials | 581 (87.5%) |
| Corticosteroids + immunosuppressants | 353 (53.2%) |
| Corticosteroids + immunosuppressants + antimalarials | 333 (50.2%) |
| Immunosuppressants + antimalarials | 364 (54.8%) |
| Mutually exclusive categories | |
| Corticosteroids + antimalarials only | 288 (43.4) |
| Corticosteroids + immunosuppressants only | 28 (4.2) |
| Corticosteroids + immunosuppressants + antimalarials only | 271 (40.8) |
| Immunosuppressants + antimalarials only | 129 (19.4) |
MSK musculoskeletal, n number of patients in a cohort, NSAID nonsteroidal anti-inflammatory drug, SD standard deviation, SLE systemic lupus erythematosus
aCombination of drugs used in at least 1 year during the observation period
bUnless otherwise indicated
Medication Combination
Table 3 summarizes combination medications used during the observed period with data presented for medications used either exclusive of others or as part of a larger treatment regimen. The most frequent combination was HCQ and corticosteroids (87.5% of patients) and approximately half of the patients (50.2%) used three medications (HCQ, corticosteroids, and immunosuppressants). Few patients used one medication only in at least 1 year during the study period. One exception was for antimalarial drugs: about one-third of patients (30.9%) used HCQ only, which is consistent with previous publications [11].
Table 3.
Treatment patterns by disease activity pattern
| Number of person-years (%) | p value | ||||||
|---|---|---|---|---|---|---|---|
| CA (PY = 346) | RR (PY = 1363) | LQ (PY = 2360) | Overall (PY = 4069) | (CA vs RR) | (CA vs LQ) | (LQ vs RR) | |
| Antimalarial | 307 (88.73%) | 1262 (92.59%) | 2135 (90.47%) | 3704 (91.03%) | 0.0275 | 0.3314 | 0.0301 |
| Hydroxychloroquine | 307 (88.73%) | 1262 (92.59%) | 2135 (90.47%) | 3704 (91.03%) | 0.0275 | 0.3314 | 0.0301 |
| Corticosteroids | 313 (90.46%) | 1144 (83.93%) | 1098 (46.53%) | 2555 (62.79%) | 0.0017 | < 0.0001 | < 0.0001 |
| Prednisone | 131 (37.86%) | 554 (40.65%) | 832 (35.25%) | 1517 (37.28%) | 0.3574 | 0.3672 | 0.0012 |
| Triamcinolone | 293 (84.68%) | 976 (71.61%) | 462 (19.58%) | 1731 (42.54%) | < 0.0001 | < 0.0001 | < 0.0001 |
| Methylprednisolone | 1 (0.29%) | 8 (0.59%) | 18 (0.76%) | 27 (0.66%) | 0.6963 | 0.4983 | 0.6837 |
| Immunosuppressants | 158 (45.66%) | 651 (47.76%) | 959 (40.64%) | 1768 (43.45%) | 0.5074 | 0.0795 | < 0.0001 |
| Methotrexate | 51 (14.74%) | 139 (10.20%) | 125 (5.30%) | 315 (7.74%) | 0.0212 | < 0.0001 | < 0.0001 |
| Azathioprine | 53 (15.32%) | 201 (14.75%) | 236 (10.0%) | 490 (12.04%) | 0.7997 | 0.0038 | < 0.0001 |
| Mycophenolate mofetil or mycophenolic acid | 38 (10.98%) | 302 (22.16%) | 567 (24.03%) | 907 (22.29%) | < 0.0001 | < 0.0001 | 0.1984 |
| Leflunomide | 12 (3.47%) | 20 (1.47%) | 22 (0.93%) | 54 (1.33%) | 0.0236 | 0.0006 | 0.1484 |
| Tacrolimus | 3 (0.87%) | 48 (3.52%) | 96 (4.07%) | 147 (3.61%) | 0.0071 | 0.0011 | 0.4283 |
| Cyclophosphamide | 0 | 8 (0.59%) | 19 (0.81%) | 27 (0.66%) | 0.3711 | 0.1584 | 0.5498 |
| NSAIDs | 108 (31.21%) | 375 (27.51%) | 558 (23.64%) | 1041 (25.58%) | 0.1813 | 0.0032 | 0.0096 |
| Biologics | 15 (4.34%) | 45 (3.30%) | 41 (1.74%) | 101 (2.48%) | 0.3304 | 0.0038 | 0.0030 |
| Rituximab | 6 (1.73%) | 21 (1.54%) | 18 (0.76%) | 45 (1.11%) | 0.8093 | 0.1129 | 0.0295 |
| Etanercept | 1 (0.29%) | 0 | 2 (0.08%) | 3 (0.07%) | 0.2025 | 0.3367 | 0.5357 |
| Abatacept | 2 (0.58%) | 1 (0.07%) | 0 | 3 (0.07%) | 0.1062 | 0.0163 | 0.3661 |
| Belimumab | 6 (1.73%) | 25 (1.83%) | 22 (0.93%) | 53 (1.30%) | 1.0000 | 0.1595 | 0.0217 |
A p-value < 0.05 was considered statistically significant
CA chronically active, LQ long quiescence, NSAIDs nonsteroidal anti-inflammatory drugs, PY person-years, RR relapsing–remitting
Treatment Patterns by Musculoskeletal Disease Activity Pattern
Antimalarial use was high across all disease activity patterns. The highest use (greatest percentage of follow-up time) was in MSK-RR (1262 PY; 92.6%) and lowest in MSK-CA (307 PY; 88.7%), with statistically significant differences between MSK-RR and both MSK-CA (p = 0.0275) and MSK-LQ (p = 0.0301) (Table 3). Overall corticosteroid use was statistically significantly different across all pairwise comparisons, with the highest use in the MSK-CA group (313 PY; 90.5%), followed by MSK-RR (1144 PY; 83.9%), and MSK-LQ (1098 PY; 46.5%) primarily driven by triamcinolone use in the CA (84.7%) and RR (71.6%) groups (Table 3).
Across MSK disease activity patterns, the mean prednisone dose was significantly higher in MSK-RR (8.5 mg) compared to MSK-CA (6.5 mg; p = 0.0063). The mean prednisone dose in MSK-LQ was 7.6 mg, which was not statistically significantly different from the other two MSK disease activity patterns.
Overall immunosuppressant use was similar between MSK-CA (158 PY; 45.7% of follow-up time) and MSK-RR (651 PY; 47.8%) but was statistically significantly lower in MSK-LQ (959 PY; 40.6%) compared with MSK-RR (p < 0.0001) and some significant differences were seen with individual immunosuppressants (Table 3). Overall, biologic use was low (101 PY, 2.5%) with a higher use in MSK-CA (15 PY; 4.3%) and MSK-RR (45 PY; 3.3%) compared to MSK-LQ (41 PY; 1.7%) (p = 0.003, 0.004) with a similar use of both rituximab (45 PY; 1.1%) and belimumab (53 PY; 1.3%) during follow-up time (Table 3).
Clinical Characteristics over the Observation Period (Overall SLE-MSK Population)
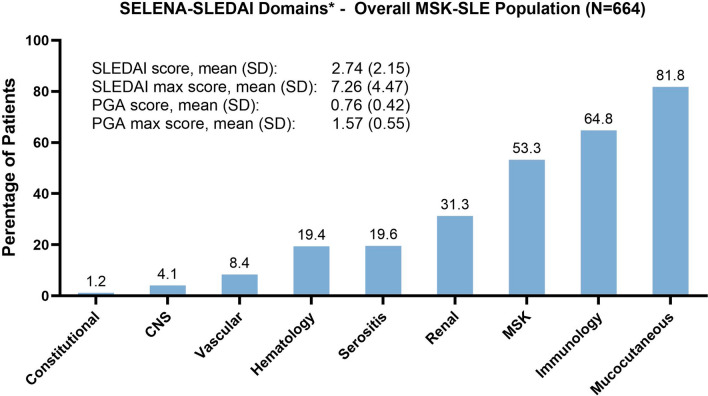
For the total SLE-MSK population, over the observation period, 53.3% of patients had presence of MSK involvement by the SELENA-SLEDAI MSK domain (Fig. 1), (52.9% with arthritis, 2.7% with myositis). On the basis of the joint domain of the LAI (LAI joints ≥ 1), 98.2% of patients had presence of MSK symptoms over the observational period. Other SELENA-SLEDAI domains that occurred in more than 30% of patients were mucocutaneous (81.8%) with 30.4% presenting lupus rash and 63.3% alopecia; immunology (64.8%) with 48.6% of the patients presenting positive anti-dsDNA and 51.7% low complement, and renal (31.3%) where proteinuria was the most common manifestation (27.1%). The mean SELENA-SLEDAI total score and PGA remained mild over the observation period with maximum levels reaching the moderate range (Fig. 1).
Fig. 1.
Presence of manifestations* by SELENA-SLEDAI domains over the observation period for the total SLE-MSK population and mean and mean max SLEDAI and PGA scores. *Percentage with SELENA-SLEDAI > 0 at least once for each of the domains over the observation period. CNS central nervous system, MSK musculoskeletal, PGA Physician Global Assessment, SELENA SLEDAI Safety of Estrogens in Lupus National Assessment Systemic Lupus Erythematosus Disease Activity Index, SLE systemic lupus erythematosus
Clinical Characteristics by Disease Activity Pattern
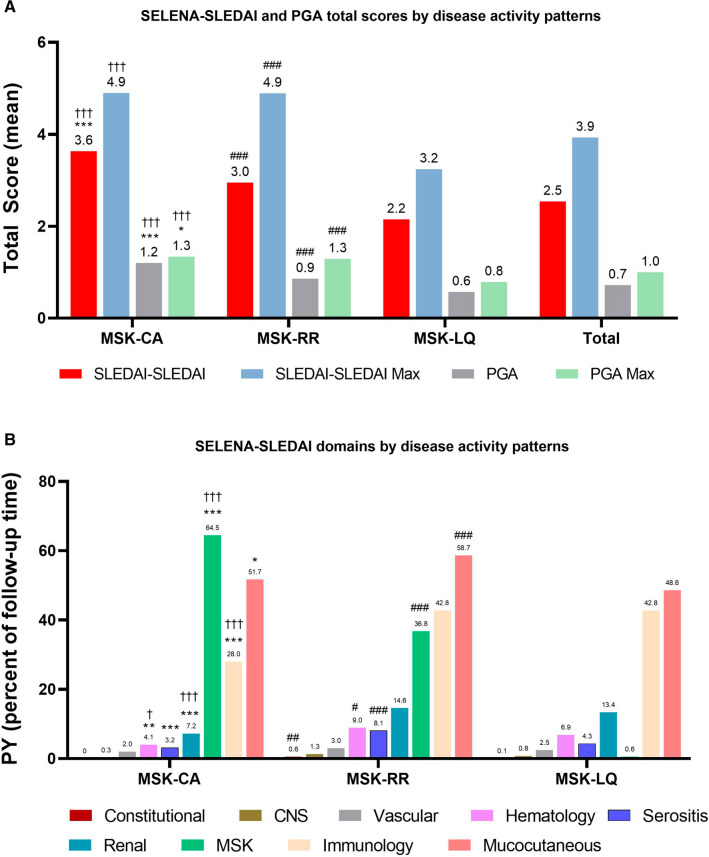
Disease activity scores (total SELENA-SLEDAI and PGA) were highest in the MSK-CA pattern, followed by MSK-RR and MSK-LQ with disease activity remaining mild (Fig. 2a). For organ manifestations, frequency of MSK involvement was greatest in the MSK-CA pattern (64.5%), and significantly higher than MSK-RR (36.8%; p < 0.001) and MSK-LQ (0.6%; p < 0.001) (Fig. 2b). Mucocutaneous involvement was frequent across the three disease activity patterns, though significantly greater in MSK-RR (58.7%) compared to MSK-CA (51.7%, p < 0.05 and MSK-LQ (48.6%, p < 0.001). Renal involvement had a higher percentage of PY follow-up time in the MSK-RR pattern (14.6%) relative to MSK-CA (7.2%, p < 0.001) and MSK-LQ (13.4%, p < 0.001).
Fig. 2.
a SELENA-SLEDAI and PGA scores by disease activity patterns over the observation period and b organ manifestations by SELENA-SLEDAI domains over the observation period by disease activity pattern. ***p < 0.001 MSK-CA vs MSK-RR; **p < 0.01 MSK-CA vs MSK-RR; *p < 0.05 MSK-CA vs MSK-RR; †††p < 0.001 MSK-CA vs MSK-LQ; ††p < 0.01 MSK-CA vs MSK-LQ; †p < 0.05 MSK-CA vs MSK-LQ; ###p < 0.001 MSK-RR vs MSK-LQ; ##p < 0.001 MSK-RR vs MSK-LQ; and #p < 0.05 MSK-RR vs MSK-LQ. CA chronically active, LQ long quiescence, MSK musculoskeletal, PGA Physician Global Assessment, RR relapsing–remitting, SELENA SLEDAI Safety of Estrogens in Lupus National Assessment Systemic Lupus Erythematosus Disease Activity Index
Discussion
Involvement of the MSK system is common in the clinical course of SLE, occurring in almost all patients [1]. The most common MSK manifestations are inflammatory arthralgia (occurring in up to 90% of patients) and non-erosive, non-deforming arthritis (occurring in up to 85% of patients) [12]. In this observational study, we described the clinical characteristics and treatment patterns together with disease activity patterns of patients with SLE, specifically those experiencing MSK involvement, over a 10-year observational period from a real-world cohort. The patients with SLE-MSK in our study required higher use of antimalarials and corticosteroids (oral and systemic) (over 90% each), immunosuppressants (almost 60%), NSAIDs (48%) and biologics (7%) compared to non-MSK specific patients from an inception lupus cohort that reported corticosteroid use in a total of 46.8% of patients over the course of their study [11]. Data from cross-sectional, real-world studies from United States (US) insurance claims databases have reported antimalarial use ranging from 43% to 59%, corticosteroids 48% to 69%, immunosuppressants 18% to 26%, NSAIDs 35% to 38%, and biologics at approximately 3% [13–16]. The negative effect that corticosteroids [17] and NSAIDs [18] have on the cardiovascular system is now well studied. Evidence from research has demonstrated that patients with relatively stable, mild-to-moderate SLE disease activity accrued damage, especially in patients with chronic use of NSAIDs commonly taken to reduce MSK pain, which resulted in an increased risk of cardiovascular damage accrual [18].
Considering the MSK-specific patients in our study, 42% (PY 1709) of the follow-up time was spent in an active disease activity pattern (RR, CA), with the flaring pattern being the most common. Both active SLE-MSK patterns had statistically higher use of immunosuppressants compared to the inactive SLE-MSK pattern, with no usage differences between CA and RR. A chronically active pattern of MSK symptoms warranted greater intensity and statistically significant higher use of corticosteroids (oral and systemic) over time compared to a flaring or LQ pattern of SLE-MSK symptoms. A remarkable finding was that the mean prednisone dose was numerically higher for patients in a quiescent SLE-MSK disease activity compared to patients in a CA pattern possibly because of other manifestations present such as mucocutaneous, where there was 49% of follow-up time in the LQ pattern. Despite these findings about corticosteroid use, biologic use was low across all disease activity patterns. Drug combinations that included corticosteroids were the most frequently used consistent with previous data reported [11]. Combinations of HCQ + corticosteroids or triple therapy of HCQ + corticosteroids + immunosuppressants were used at least once by over 87.5% and 50.2% of patients with SLE-MSK, respectively. While use of these combinations is consistent with current treatment recommendations, it does highlight the intensity of therapy used in patients experiencing active SLE-MSK involvement, in particular the extent of corticosteroids and NSAIDs use. It is well known that corticosteroids are heavily relied on for SLE therapy; however, numerous adverse effects may result. Evidence has shown that chronic and sustained use of corticosteroids in patients with SLE contributes to permanent organ damage and an increased rate of morbidity (mainly related to long-term cardiovascular complications and infections), and it is one of the most important predictors of damage accrual [19]. One of the treatment goals in SLE should be reducing corticosteroid use to the lowest possible dose [6]. Lastly, while not evaluated in this study, patient preference studies indicate that multimodal treatment regimens should also be a consideration as patient satisfaction could have an impact on adherence. Data from a US survey assessing the treatment satisfaction of patients with SLE found that triple therapy comprising antimalarial + corticosteroid + immunosuppressant was a significant driver of patient dissatisfaction with therapy [20].
The fluctuating nature of disease activity over time in SLE has been recognized for many years.
We based the present study on the observations of Barr et al., who first published results in 1999 on 204 patients with SLE from the Hopkins Lupus Cohort, which described three major patterns of SLE disease activity over time, with CA being the most frequent and LQ being the least common [10]. Over the observed period of our study in the SLE-MSK population, just over half of the time was spent in a long quiescent pattern of MSK activity (58%) with the other half of patients distributed in active MSK disease activity patterns. Over one-third of our patients were in an RR pattern of MSK activity, the most prevalent pattern described in more recent studies [4, 21].
Clinical characteristics were also identified during the observed period and among the three SLE-MSK disease activity patterns. For the overall SLE-MSK subpopulation, disease activity remained mild with high mean and maximum corticosteroid use, together with high use of combination therapy, which may presumably explain the disease activity scores we found. These patients presented with additional manifestations including mucocutaneous (82%), immunological (almost half of whom were positive for anti-dsDNA antibody [47%]), and renal manifestations (31%), consistent with previous reports of organ involvement and symptoms in SLE [22].
Renal domain involvement across the SLE-MSK disease activity patterns had significantly greater PY follow-up time in MSK-RR versus MSK-CA disease patterns. This may also be reflected by the high use of mycophenolate mofetil in this subpopulation and specifically in the RR pattern compared to CA.
Our study highlights the almost universal impact of arthritic symptoms, likely leading to an increased use of corticosteroids, immunosuppressants, and NSAIDs in this group of patients with SLE with active MSK disease activity, suggesting there is a need for new therapeutic options that enable a less chronic use of these therapies when treating lupus. While other manifestations are more likely to lead to organ failure and early mortality, MSK symptoms are the main determinant of disease impact that effects a greater number of patients [23]. Apart from fatigue, in a recent study on the burden of SLE the majority of patients reported joint involvement as the most predominant manifestation they would like to get rid of [22]. Additional research has demonstrated the strong correlation of SLE-MSK symptoms with reduced health-related quality of life, with only arthralgia being significantly associated with work disability [24–26] and at the same time the most important modifiable factor in disability in patients with SLE.
A limitation of the current study is that it is an analysis of patients from a single tertiary academic center treated by a single rheumatologist, making it difficult to extrapolate our results to a general lupus population. Additionally, groups were not balanced using methods such as propensity scores, which limits comparisons between outcomes and specific reasons for treatments being given were not determined; there could be dual indications for steroid use due to other organ manifestations such as mucocutaneous involvement in addition to the MSK symptoms. As a result of the descriptive nature of our study, no comparisons were made to a cohort of patients without MSK manifestations; 82% of the patients in this cohort had a non-erosive arthritis at baseline. One of the strengths of this study is that the nature of flaring patterns compared with chronic or quiescent patterns specific to SLE-MSK has not been previously characterized, nor has current literature addressed how these patterns impact therapeutic approaches. Additionally, we add to the literature how SLE-MSK involvement is being managed in a real-world practice and if/how treatment patterns of MSK involvement in patients with SLE vary across different disease activity.
Conclusions
Over a 10-year observational period in an SLE-MSK subpopulation, half of the patients presented in an active disease activity pattern, most commonly RR with disease activity measured by SELENA-SLEDAI. Our results showed a high reliance on NSAIDS and combination therapy comprising high mean doses of oral corticosteroids and immunosuppressants with a lower use of biologics, all of which remain prominent in the therapeutic armamentarium of active SLE-MSK symptoms. Our study highlights the impact of arthritic symptoms as the contributing manifestation to the intense use of these therapies. Greater and earlier use of newly approved biologic therapies may offer a better path forward. The presence of the active patterns (despite high use of combination therapy, corticosteroids, and NSAIDs) suggests there is a need for new therapeutic options to manage disease activity in patients with SLE, permitting better efficacies to be achieved and diminishing the chronic use of these drugs and their long-term side effects.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
Funding
The Hopkins Lupus Cohort is funded by NIH R01 AR069572. The Rapid Service and Open Access fees from the journal were funded by Eli Lilly and Company.
Medical Writing, Editorial, and Other Assistance
Medical writing support, under the guidance of the authors, was provided by Kathy Oneacre, MA, and editing support by Cynthia Rae Abbott of Syneos Health (Morrisville, NC USA) and was funded by Eli Lilly and Company (Indianapolis, IN, USA) in accordance with Good Publication Practice guidelines.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
Natalia Bello participated in the conception and design of the study, interpretation of data, and drafting and critical revision of the manuscript. Julie A. Birt participated in the conception and design of the study, interpretation of data, and drafting and critical revision of the manuscript. Jennifer Workman participated in the conception and design of the study, interpretation of data, and drafting and critical revision of the manuscript. Xian Zhou participated in the design of the study, analysis and interpretation of data, and drafting and critical revision of the manuscript. Jorge A. Ross-Terres participated in the conception and design of the study, interpretation of data, and critical revision of the manuscript. Michelle Petri participated in the conception and design of the study, acquisition and interpretation of data, and critical revision of the manuscript.
Disclosures
Natalia Bello, Julie A. Birt, Jennifer Workman, and Jorge A. Ross-Terres are employees and stockholders of Eli Lilly and Company. Xian Zhou is an employee of Syneos Health, which received funding from Eli Lilly and Company. Michelle Petri is a consultant to Eli Lilly and received grant support from Eli Lilly.
Compliance with Ethics Guidelines
The study was conducted in accordance with the Declaration of Helsinki and cohort is approved by the Johns Hopkins University School of Medicine Institutional Review Board (study number NA 00039294) on a yearly basis, and all patients gave written informed consent.
Data Availability
The datasets generated during and/or analyzed during the current study are not publicly available due to protected health information.
References
- 1.Zoma A. Musculoskeletal involvement in systemic lupus erythematosus. Lupus. 2004;13:851–853. doi: 10.1191/0961203303lu2021oa. [DOI] [PubMed] [Google Scholar]
- 2.Torrente-Segarra V, Monte TCS, Corominas H. Musculoskeletal involvement and ultrasonography update in systemic lupus erythematosus: new insights and review. Eur J Rheumatol. 2018;5:127–130. doi: 10.5152/eurjrheum.2017.17198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grossman JM. Lupus arthritis. Best Pract Res Clin Rheum. 2009;23:495–506. doi: 10.1016/j.berh.2009.04.003. [DOI] [PubMed] [Google Scholar]
- 4.Györi N, Giannakou I, Chatzidionysiou K, et al. Disease activity patterns over time in patients with SLE: analysis of the Hopkins Lupus Cohort. Lupus Sci Med. 2017;4:e000192. doi: 10.1136/lupus-2016-000192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Petri M, Genovese M, Engle E, et al. Definition, incidence, and clinical description of flare in systemic lupus erythematosus. A prospective cohort study. Arthritis Rheum. 1991;34:937–944. doi: 10.1002/art.1780340802. [DOI] [PubMed] [Google Scholar]
- 6.Fanouriakis A, Kostopoulou M, Alunno A, et al. 2019 update of the EULAR recommendations for the management of systemic lupus erythematosus. Ann Rheum Dis. 2019;78:736–745. doi: 10.1136/annrheumdis-2019-215089. [DOI] [PubMed] [Google Scholar]
- 7.Fangtham M, Petri M. 2013 update: Hopkins lupus cohort. Curr Rheumatol Rep. 2013;15:360. doi: 10.1007/s11926-013-0360-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Petri M, Kim MY, Kalunian KC, et al. OC-SELENA Trial. Combined oral contraceptives in women with systemic lupus erythematosus. N Engl J Med. 2005;353:2550–2558. doi: 10.1056/NEJMoa051135. [DOI] [PubMed] [Google Scholar]
- 9.Petri M, Hellman D, Hochberg M. Validity and reliability of lupus activity measures in the routine clinic setting. J Rheumatol. 1992;19:53–59. [PubMed] [Google Scholar]
- 10.Barr SG, Zonana-Nacach A, Magder L, et al. Patterns of disease activity in systemic lupus erythematosus. Arthitis Rheum. 1999;42:2682–2688. doi: 10.1002/1529-0131(199912)42:12<2682::AID-ANR26>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 11.Hanly JG, Sayani A, Doucette S, Iczkovitz S, Terres JAR. Treatment pathways in an inception lupus cohort over the first three years. Lupus. 2017;26(2):119–124. doi: 10.1177/0961203316655213. [DOI] [PubMed] [Google Scholar]
- 12.Tani C, Carli L, Stagnaro C, et al. Imaging of joints in systemic lupus erythematosus. Clin Exp Rheumatol. 2018;114:68–73. [PubMed] [Google Scholar]
- 13.Chen SY, Choi CB, Li Q, et al. Glucocorticoid use in patients with systemic lupus erythematosus: association between dose and health care utilization and Costs. Arthritis Care Res. 2015;67:1086–1094. doi: 10.1002/acr.22574. [DOI] [PubMed] [Google Scholar]
- 14.Kariburyo F, Xie L, Sah J, et al. Real-world medication use and economic outcomes in incident systemic lupus erythematosus patients in the United States. J Med Econ. 2019;23:1–9. doi: 10.1080/13696998.2019.1678170. [DOI] [PubMed] [Google Scholar]
- 15.Birt JA, Wu J, Griffing K, et al. Corticosteroid dosing and opioid use are high in patients with SLE and remain elevated after belimumab initiation: a retrospective claims database analysis. Lupus Sci Med. 2020;7:e000435. doi: 10.1136/lupus-2020-000435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bell CF, Priest J, Stott-Miller M, et al. Real-world treatment patterns, healthcare resource utilisation and costs in patients with systemic lupus erythematosus treated with belimumab: a retrospective analysis of claims data in the USA. Lupus Sci Med. 2020;26(7):e000357. doi: 10.1136/lupus-2019-000357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Magder LS, Petri M. Incidence of and risk factors for adverse cardiovascular events among patients with systemic lupus erythematosus. Am J Epidemiol. 2012;176:708–719. doi: 10.1093/aje/kws130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hill DD, Eudy AM, Egger PJ, et al. Impact of systemic lupus erythematosus disease activity, hydroxychloroquine and NSAID on the risk of subsequent organ system damage and death: analysis in a single US medical centre. Lupus Sci Med. 2021;8:e000446. doi: 10.1136/lupus-2020-000446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Petri M. Musculoskeletal complications in systemic lupus erythematosus in the Hopkins Lupus cohort: an update. Arthritis Care Res. 1995;8:137–145. doi: 10.1002/art.1790080305. [DOI] [PubMed] [Google Scholar]
- 20.Pascoe K, Lobosco S, Bell D, et al. Patient- and physician-reported satisfaction with systemic lupus erythematosus treatment in US clinical practice. Clin Ther. 2017;39:1811–1826. doi: 10.1016/j.clinthera.2017.07.039. [DOI] [PubMed] [Google Scholar]
- 21.Tselios K, Gladman DD, Touma Z, et al. Disease course patterns in systemic lupus erythematosus. Lupus. 2019;28:114–122. doi: 10.1177/0961203318817132. [DOI] [PubMed] [Google Scholar]
- 22.Cornet A, Andersen J, Myllys K, et al. Living with systemic lupus erythematosus in 2020: a European patient survey. Lupus Sci Med. 2021;8:e000469. doi: 10.1136/lupus-2020-000469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mahmoud K, Zayat A, Vital EM. Musculoskeletal manifestations of systemic lupus erythmatosus. Curr Opin Rheumatol. 2017;29:486–492. doi: 10.1097/BOR.0000000000000421. [DOI] [PubMed] [Google Scholar]
- 24.Baker K, Pope J. Employment and work disability in systemic lupus erythematosus: a systematic review. Rheumatology (Oxford) 2009;48:281–284. doi: 10.1093/rheumatology/ken477. [DOI] [PubMed] [Google Scholar]
- 25.Baker K, Pope J, Fortin P, 1000 Faces of Lupus Investigators. CaNIOS (Canadian Network for Improved Outcomes in SLE) et al. Work disability in systemic lupus erythematosus is prevalent and associated with socio-demographic and disease related factors. Lupus. 2009;18:1281–1288. doi: 10.1177/0961203309345784. [DOI] [PubMed] [Google Scholar]
- 26.Golder V, Kandane-Rathnayake R, Hoi AY, et al. Association of the lupus low disease activity state (LLDAS) with health-related quality of life in a multinational prospective study. Arthritis Res Ther. 2017;19:62. doi: 10.1186/s13075-017-1256-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analyzed during the current study are not publicly available due to protected health information.