Abstract
Objective
The coronavirus disease 2019 (COVID-19) vaccination coverage, willingness, and safety profiles in patients with epilepsy remain poorly understood. We aimed to summarize the available evidence of COVID-19 vaccination coverage, willingness, and safety profiles among patients with epilepsy.
Methods
We performed a literature search in the Pubmed, EMBASE, and Cochrane Central Register database between 1 January 2020 and 30 April 2022. We included eligible studies that provided information on the COVID-19 vaccination coverage, willingness, and safety profiles among patients with epilepsy. We investigated the association between baseline characteristics of patients with epilepsy and unvaccination status using a fixed-effect model. We calculated the pooled overall willingness to be vaccinated against COVID-19. We systematically reviewed the safety profiles after COVID-19 vaccination in patients with epilepsy.
Results
Ten eligible observational studies and two case reports yielded 2589 participants with epilepsy or their caregivers. Among 2145 participants that provided the information of vaccination status, 1508 (70.3%) patients with epilepsy were not administered COVID-19 vaccine, and 58% (95%CI 40–75%) of respondents were willing to be vaccinated against COVID-19. Seizure status (active versus inactive, OR 1.84 95%CI 1.41–2.39, I2 = 0%) rather than seizure type (focal versus non-focal, OR 1.22 95%CI 0.94–1.58, I2 = 0%) was associated with COVID-19 unvaccination status. Vaccines were well-tolerated; epilepsy-related problems such as increase in seizure frequency and status epilepticus after COVID-19 vaccination were uncommon.
Conclusions
Our findings suggest a low COVID-19 vaccination coverage and willingness in patients with epilepsy. Vaccination against COVID-19 appears to be well-tolerated and safe in patients with epilepsy, supporting a positive outlook toward vaccination in this population.
Abbreviations: COVID-19, coronavirus disease 2019; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; PRISMA, Systematic Review and Meta-Analysis; EMBASE, Excerpta Medica database; AHRQ, Agency for Healthcare Research and Quality; ORs, odds ratios; DS, Dravet syndrome; DSUK, National Hospital for Neurology and Neurosurgery; CCE, Chalfont Centre for Epilepsy; ILAE, International League Against Epilepsy; HRQOL, Health-related quality of life; ASM, antiseizure medication
Keywords: COVID-19, Vaccines, Epilepsy, Seizure, Adverse effect
1. Introduction
As of 30 April 2022, coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection had affected more than 500 million confirmed cases and over six million fatalities worldwide [1]. The increased infectivity of the delta and omicron mutations of the SARS-CoV-2 exacerbates the public panic [2], [3]. Vaccines remain the most promising approach for controlling COVID-19 pandemic and reestablishing pre-pandemic routines.
Emerging COVID-19 vaccines protects from symptomatic SARS-CoV-2 infection. Neurologic complications of COVID-19 vaccines have been reported, including strokes, cranial neuropathies, peripheral neuropathies, acute disseminated encephalomyelitis, transverse myelitis, and acute idiopathic demyelinating polyneuropathy [4], [5], [6], [7], [8], [9], [10], [11], [12]. There are substantial concerns regarding the potential risks after vaccination in those with preexisting neurologic disorders. Epilepsy is one of the most common neurological disorders, affecting more than 70 million people worldwide [13]. A previous study suggested that patients with epilepsy were at a higher risk of experiencing unfavorable COVID-19 outcomes [14]. Takinginto account the COVID-19 pandemic, it is urgent to know the benefits and risks of vaccination for patients with epilepsy. To our knowledge, lines of evidence of the COVID-19 vaccination coverage, willingness, and safety profiles in patients with epilepsy were limited. In this systematic review and meta-analysis, we aimed to summarize the currently available evidence regarding the COVID-19 vaccination coverage, willingness, or hesitancy in patients with epilepsy. Moreover, we systematically reviewed the safety and tolerability of COVID-19 vaccines among patients with epilepsy.
2. Methods
This systematic review and meta-analysis was prospectively registered in the international prospective register of systematic reviews (PROSPERO: CRD42021293066) based on the Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA) guidelines [15]. We applied the methods that are recommended in the Meta-analysis of Observational Studies in Epidemiology proposal [16].
2.1. Search strategy
We conducted a literature search up to 30 April 2022 for relevant publications with no language restriction in Pubmed, Excerpta Medica database (EMBASE), and Cochrane Central Register database. Our search strategy included the following set of terms: (“COVID-19” OR “coronavirus disease 2019” OR “SARS-CoV-2” OR “nCoV” OR “severe acute respiratory syndrome coronavirus 2”) AND (“vaccin*”) AND (“seizure” OR “epilepsy”). We also manually screened references for additional studies. Additionally, the official websites of the vaccine developers were also searched.
2.2. Inclusion and exclusion criteria
Eligible studies included randomized controlled trials, non-randomized controlled trials, case-control studies, cohort studies, case series, case-reports, and cross-sectional studies. We included studies that provided information on the COVID-19 vaccination status and willingness (hesitancy) toward COVID-19 vaccination among patients with epilepsy. We applied the following exclusion criteria: (1) Insufficient data information provided; (2) Review articles, meta-analyses, literature reviews, editorials, and commentaries; (3) Abstracts or posters from conference proceedings before the full-text paper was formally published in a peer-reviewed journal. Disagreements regarding inclusion or exclusion criteria were settled by team discussion.
2.3. Screening and data extraction
Two trained authors (K.L. and S.F.) screened the title and abstract of the identified publications to retrieve potentially eligible articles for a full-text review. These two authors (K.L. and S.F.) blindly assessed study inclusion and study quality, and extracted data on study characteristics (i.e., authors, date of publication, study design, setting, and sample size), participants' characteristics (i.e., mean/median age, gender, seizure types), inclusion and exclusion criteria, and outcome measures using standardized data collection sheets.
2.4. Definitions and outcomes
Active epilepsy was defined as having seizure occurrence within the preceding year, and inactive epilepsy was defined as reaching seizure-free status in the preceding year, regardless of whether antiseizure medications were administered [17]. The primary outcome was vaccination coverage. Secondary outcomes included the COVID-19 vaccination willingness and unwillingness. Additional outcomes included impact of vaccination on epilepsy-related problems, and adverse effects after COVID-19 vaccination.
2.5. Assessment of publication bias and study quality assessment (Risk of Bias)
All included literatures were evaluated using the Agency for Healthcare Research and Quality (AHRQ) scale [18]. The AHRQ scale is rated based on the overall score of its 11 items. For each item, one score is awarded if the quality of the study meets the methodological standard. A score of 0–4 indicates a high risk of bias, 5–7 indicates a moderate risk of bias, and 8–11 indicates a low risk of bias [19]. Publication bias tests for funnel plot asymmetry and the Egger test were not performed due to the limited number of studies.
2.6. Statistical analysis
We summarized dichotomous primary outcome of interest as odds ratios (ORs) using a fixed-effect model (Mantel–Haenszel approach). We conducted a sensitivity analysis using a random-effect model. We evaluated heterogeneity by inspecting forest plots, and with tests for heterogeneity after calculating the Q statistic and I2 values. We considered an I2 statistic threshold of 50% as a low and high heterogeneity [20]. Based on the International League Against Epilepsy (ILAE) criteria, seizures are classified into focal onset, generalized onset, and unknown onset. Epilepsy is classified into four subtypes: combined generalized and focal epilepsy, generalized epilepsy, focal epilepsies, and unknown category [21]. To investigate the association between the seizure type and unvaccination status, if the original study gave epileptic classification rather than seizure classification, we added unclassified seizure in epilepsy classification to the non-focal group in seizure type classification. We combined the generalized and unknown types into a group to reflect non-focal onset versus focal onset type. We also performed a single-arm meta-analysis to determine the pooled overall willingness and unwillingness to be vaccinated against COVID-19. We performed a separate analysis by excluding patients' caregivers to investigate vaccination willingness in patients with epilepsy. We did not perform the predefined subgroup analysis because of the small number of included studies. All analyses were performed using the STATA 15.0 (Stata Corp LP, College Station, TX) and the Cochrane Collaboration’s Review Manager (Rev Man 5.3) Software Package (2014; Nordic Cochrane Centre, Cochrane Collaboration, Copenhagen, Denmark). Statistical significance was set at α = 0.05 for all analyses.
3. Results
3.1. Study selection
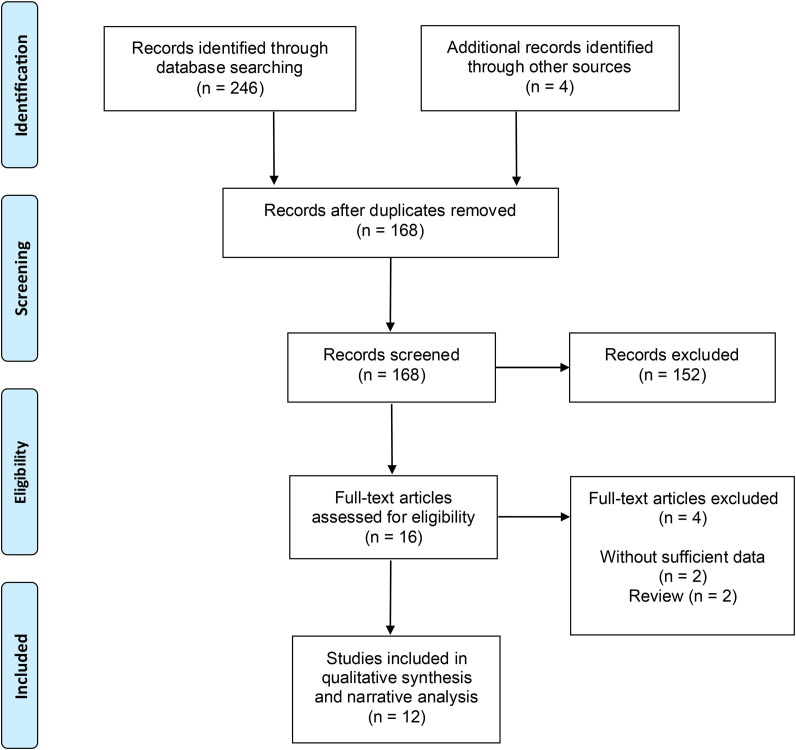
An initial literature search yielded 250 articles. After screening 82 duplicates and 152 papers through titles and abstracts, we retrieved the full texts of the remaining 16 studies. Ten observational studies and two case report studies [17], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32] that met the inclusion criteria were included in this systematic review and meta-analysis (Fig. 1 ).
Fig. 1.
Flow diagram of study.
3.2. Study characteristics
Table 1 summarizes the baseline characteristics of the included studies. The sample size of eligible participants in ten observational studies ranged from 38 to 557, yielding a total of 2587 participants. No studies provided propensity score matching analysis results. Seven studies [17], [29], [30], [23], [24], [25], [26] provided the information of primary outcome (vaccination status), and six studies [22], [28], [29], [24], [25], [26] reported the secondary outcomes of vaccination willingness and unwillingness. Seven studies [17], [30], [23], [24], [25], [26], [27] reported the incidence of adverse effects after COVID-19 vaccination. Supplemental Table 1 summarizes the inclusion, exclusion criteria and definition of seizure free in the included studies. Supplemental Table 2 lists the timing of the studies in the respected nations with regard to their vaccination programs. Notably, two studies [22], [28] were performed before the start of the vaccination campaign in the respected nations.
Table 1.
Baseline characteristics of the included studies.
| Study | Country | Research type | Setting | Study duration | Participants | Number of patients | Male n (%) | Age (y) | Seizure type classification (n) | Epilepsy classification (n) | Epilepsy frequency (n) | Outcomes | Vaccine platform |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (Respondents) | (Number of vaccinations) | ||||||||||||
| Li et al. [17] | China | Cross-sectional | Single-center | July 1 – July 21, 2021 | Patients with epilepsy | 357 | 193 (54.1%) | 33.1 | Focal aware motor seizure (22) | Not mentioned | Active epilepsy (203) | Vaccination status (yes or no) | Inactivated vaccine first dose (n = 38) |
| (online survey) | Focal aware nonmotor seizure (30) | Inactive epilepsy (154) | Adverse effects of vaccines | Inactivated vaccine second dose (n = 22) | |||||||||
| Focal impaired awareness motor seizure (12) | |||||||||||||
| Focal impaired awareness nonmotor seizure (35) | |||||||||||||
| Focal to bilateral tonic-clonic seizure (97) | |||||||||||||
| General motor seizure (6) | |||||||||||||
| Asadi et al. [22] | Iran | Single-center | Late 2020 | Patients with epilepsy | 153 | Not mentioned | Not mentioned | Not mentioned | Not mentioned | Not mentioned | Willing to be vaccinated | / | |
| Clayton et al. [23] | UK | (survey) | Multi- | February 2 – June, 2021 | Caregivers | 38 | Not mentioned | Under 5 years (n = 6) | Not mentioned | Not mentioned | Not mentioned | Adverse effects of vaccines | Viral-vector vaccine first dose (n = 15) |
| center | 5–11 (n = 7) | Vaccination status (yes or no) | Viral-vector vaccine second dose (n = 10) | ||||||||||
| 12–17 (n = 12) | |||||||||||||
| 18–24 (n = 5) | |||||||||||||
| 25–34 (n = 4) | |||||||||||||
| 35–44 (n = 4) | |||||||||||||
| Hood et al. [24] | USA/Canada | Cross-sectional | Multi-center | May 17, 2021 – August 2, 2021 | Caregivers | 278 | 111 (40.0%) | <12 (n = 122) | Not mentioned | Not mentioned | Not mentioned | Vaccination status (yes or no) | mRNA vaccine (n = 115) |
| (online survey) | 12–19 (n = 86) | Adverse effects of vaccines | Viral-vector vaccine (n = 5) | ||||||||||
| >20 (n = 70) | Willing to be vaccinated | ||||||||||||
| Lu et al. [25] | China | Cross-sectional | Multi-center | July 24 – August 31, 2021 | Patients with epilepsy | 491 | 243 (49.5%) | 30.4 | Focal onset (245) | Not mentioned | Over 1 year seizure-free (145) | Vaccination status (yes or no) | Inactivated vaccine (n = 187) |
| (face-to-face survey) | Generalized onset (124) | Once per year (77) | Adverse effects of vaccines | Subunit vaccine (n = 14) | |||||||||
| Unknown onset (92) | Once every 6 months (47) | Willing to be vaccinated | Viral-vector vaccine (n = 2) | ||||||||||
| Unclassified (30) | Once every 3 months (61) | mRNA vaccine (n = 1) | |||||||||||
| Once every month (97) | |||||||||||||
| Once every week (53) | |||||||||||||
| Once every day (11) | |||||||||||||
| Massoud et al. [26] | Kuwait | Cross-sectional | Single-center | April 4, 2020 – April 18, 2021 | Patients with epilepsy | 111 | 46 (41.4%) | 33.2 | Not mentioned | Generalized tonic-clonic (35) | A mean of 1.547 seizure | Vaccination status (yes or no) | mRNA vaccine first dose (n = 15) |
| (survey) | Focal with loss awareness (28) | per month | Adverse effects of vaccines | mRNA vaccine second dose (n = 35) | |||||||||
| Focal without loss awareness (24) | Willing to be vaccinated | Viral-vector vaccine first dose (n = 32) | |||||||||||
| Absence (10) | |||||||||||||
| Myoclonus (14) | |||||||||||||
| Özdemir et al. [27] | Turkey | Cross-sectional | Single-center | January 7 – January 9, 2021 | Patients with epilepsy | 178 | 87 (48.9%) | 29 | Not mentioned | Focal epilepsy (27) | Seizure free (105) | Adverse effects of vaccines | mRNA vaccine (n = 136) |
| (survey) | Generalized epilepsy (111) | Lower seizure frequency (34) | Inactivated vaccine (n = 35) | ||||||||||
| Combined epilepsy (40) | Higher seizure frequency (39) | Combination (n = 7) | |||||||||||
| Puteikis et al. [28] | Lithuania | Cross-sectional | Single-center | December 7 – December 31, 2020 | Patients (n = 58), | 111 | 44 (39.6%) | 25 | Focal (49) | Not mentioned | Several times per day (17) | Willing to be vaccinated | / |
| (online survey) | Caregiver (n = 53) | Generalized (“whole-body” seizures) (37) | Several times per week (14) | ||||||||||
| Generalized (absence or myoclonic seizures) (10) | Several times per month (27) | ||||||||||||
| Other (10) | Once per month (6) | ||||||||||||
| Unknown (5) | Once (13) | ||||||||||||
| None (34) | |||||||||||||
| Qiao et al. [29] | China | Cross-sectional | Multi-center | Jun-21 | Patients with epilepsy | 557 | 298 (53.5%) | 42 | Focal onset (367) | Not mentioned | Seizure freedom (334) | Vaccination status (yes or no) | / |
| (face-to-face survey) | Generalized onset (159) | Uncontrolled (223) | Willing to be vaccinated | ||||||||||
| Unknown onset (31) | |||||||||||||
| Wrede et al. [30] | Germany | Cross-sectional | Single-center | March 19 – April 20, 2021 | Patients with epilepsy | 54 | 27 (50.0%) | 47.9 | Focal (40) | Not mentioned | Seizure free (11) | Adverse effects of vaccines | mRNA vaccine (n = 34) |
| (survey) | Generalized (6) | ≤ 10 seizures per year (16) | Viral-vector vaccine (n = 18) | ||||||||||
| Unknown (8) | >10 seizures per year (27) | Unable to remember (n = 2) | |||||||||||
| Additional psychogenic seizures (3) | |||||||||||||
| Šín R et al. [31] | Czech Republic | Case report | Single-center | / | Patient with well-compensated secondary epilepsy | 1 | 1 | 56 | Not mentioned | Not mentioned | No record of an epileptic seizure within the last 2 years | Adverse effects of vaccines | Viral-vector vaccine (n = 1) |
| Odincova et al. [32] | Russian | Case report | Single-center | / | Patient with focal pharmacoresistant epilepsy | 1 | 1 | 59 | Not mentioned | Not mentioned | 3-year seizure remission after surgical management | Adverse effects of vaccines | mRNA vaccine (n = 1) |
3.3. Association between the baseline characteristics of epilepsy and unvaccination
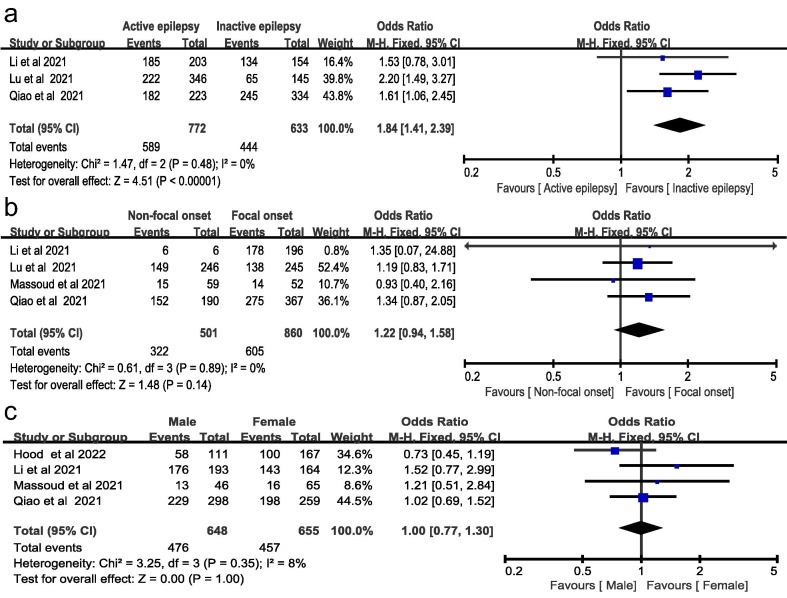
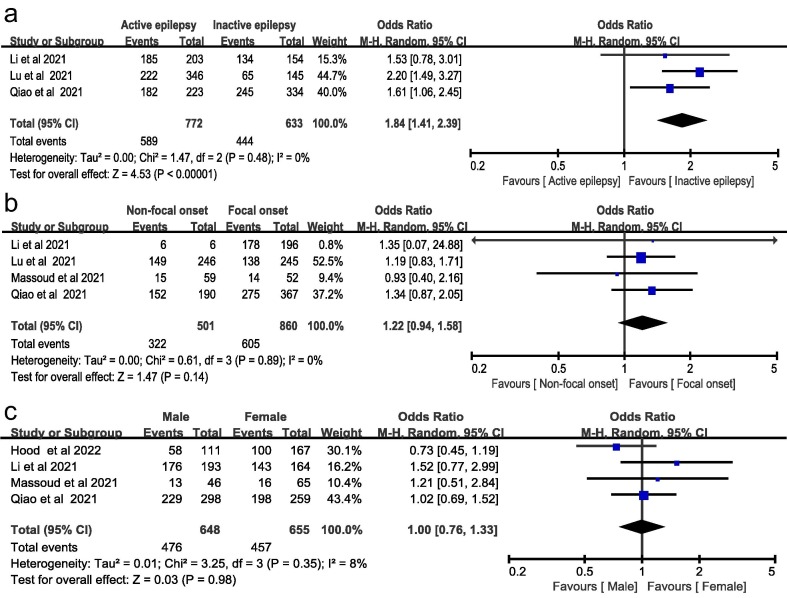
Supplemental Table 3 summarizes the baseline characteristics among vaccinated and unvaccinated patients. Of 2154 participants that provided the information of vaccination status, 1508 (70.3%) patients with epilepsy were not administered COVID-19 vaccines [17], [29], [30], [23], [24], [25], [26]. A meta-analysis showed that patients with active epilepsy were more likely to be unvaccinated than those with inactive epilepsy (OR 1.84, 95%CI 1.41–2.39, I 2 = 0%, fixed-effects model, Fig. 2 A). The unvaccination status was not significantly different among different seizure types (focal versus non-focal onset, OR 1.22, 95%CI 0.94–1.58, I 2 = 0%, fixed-effects model, Fig. 2B) and gender (male versus female, OR 1.00, 95%CI 0.77–1.30, I 2 = 8%, fixed-effects model, Fig. 2C). Sensitivity analyses using the random-effects model yielded similar results (Fig. 3 ).
Fig. 2.
Association between the baseline characteristics of epilepsy and unvaccination status using a fixed-effect model. (A) Seizure frequency; (B) seizure type; (C) Gender.
Fig. 3.
Association between the baseline characteristics of epilepsy and unvaccination status using a random-effects model. (A) Seizure frequency; (B) seizure type; (C) Gender.
3.4. Willingness and unwillingness to be vaccinated against COVID-19
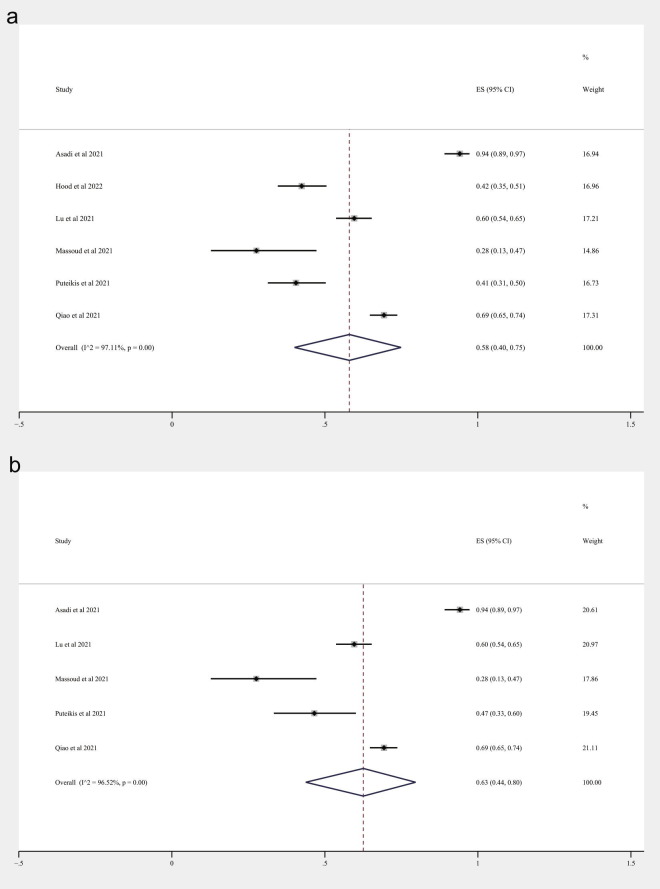
Table 2 summarizes the COVID-19 vaccination willingness, unwillingness, or hesitancy among patients with epilepsy or their caregivers. The percentage of respondents who were willing to be vaccinated ranged from 27.59% to 94.12%. Fear of aggravating epilepsy and concern about the potential adverse effects were the most common reasons for unwillingness. Fig. 4 A shows that 58% (95%CI 40–75%, random-effects models) of respondents were willing to be vaccinated against COVID-19. A sensitivity analysis by excluding their caregivers shows that 63% (95%CI 44% − 80%, random-effects models) of patients with epilepsy were willing to be vaccinated against COVID-19 (Fig. 4B).
Table 2.
Willingness and unwillingness to be vaccinated against COVID-19 among patients with epilepsy and their caregivers.
| Study | Willingness | Unwillingness or hesitancy (n) | Total | Willing percentage | Unwilling or hesitate percentage (%) | Factors for unwillingness (n [%]) | Factors for willingness (n [%]) |
|---|---|---|---|---|---|---|---|
| (n) | (n) | (%) | |||||
| Li et al. [17] | NA | NA | NA | NA | NA | Fear of aggravating epilepsy (185 [58]) | NA |
| Discouragement from health workers for epilepsy (70 [22]) | |||||||
| Fear of other unknown serious side effects (42 [13]) | |||||||
| Other diseases (12 [3.8]) | |||||||
| Age limit (4 [1.3]) | |||||||
| Breast-feeding (1 [0.3]) | |||||||
| Recent human papillomavirus vaccination (1 [0.3]) | |||||||
| Vaccine shortage (1 [0.3]) | |||||||
| Others (3 [0.9]) | |||||||
| Asadi et al. [22] | 144 | 9 | 153 | 144 (94.12%) | 9 (5.88%) | NA | NA |
| Clayton et al. [23] | NA | NA | NA | NA | NA | NA | NA |
| Hood et al. [24] | 67 | 91 | 158 | 67 (42.4%) | 91(57.6%) | Risk of increased seizures or status | NA |
| epileticus (71[78.0]) | |||||||
| Vaccine is not necessary (29[32.0]) | |||||||
| Vaccine is not safe (41[45.0]) | |||||||
| Other (27[30.0]) | |||||||
| Lu et al. [25] | 171 | 116 | 287 | 171 (59.58%) | 116 (40.42%) | Worried about the potential adverse effects (153 [53.3]) | NA |
| Unsatisfied with seizure/disease control (135 [47.0]) | |||||||
| Worried about the interaction between current medication and the vaccine (16 [5.6]) | |||||||
| Other comorbidity (42 [14.6]) | |||||||
| Local administration suggested delaying | |||||||
| the injection (12 [4.2]) | |||||||
| Others (15 [5.2]) | |||||||
| Massoud et al. [26] | 8 | 21 | 29 | 8(27.59%) | 21(72.41%) | Fear of vaccine adverse events (9 [42.9]) | NA |
| Fear of interaction with ASMs (4 [19.0]) | |||||||
| Fear of epilepsy worsening (5 [23.8]) | |||||||
| Already got a COVID-19 (3 [14.3]) | |||||||
| Özdemir et al. [27] | NA | NA | NA | NA | NA | NA | NA |
| Puteikis et al. [28] | 27 | 31 | 58 | 27(46.55%) | 31(53.44%) | Respondents thought it could cause the infection (OR = 0.14, 95% CI = 0.04–0.49) | Influenza shot in 2020 (OR = 9.17, 95%CI = 1.15–73.47) |
| (patient responses) | (patient responses) | The beliefs that vaccines are generally safe (OR = 7.90, 95% CI = 2.43–25.74) | |||||
| 18 | 35 | 53 | 18(33.96%) | 35(66.04%) | Only convenient way to gain immunity (OR = 3.91, 95% CI = 1.02–15.05) | ||
| (caregiver responses) | (caregiver responses) | ||||||
| Qiao et al. [29] | 296 | 131 | 427 | 296(69.32%) | 131(30.68%) | Worried about aggravating seizures by vaccination (108 [82.4]) | Junior high school and below (OR = 2.611, 95% CI = 1.222–5.579) |
| Worried about vaccine safety (59 [45.0]) | Urban residents (OR = 3.821, 95% CI = 2.043–7.149) | ||||||
| Lack of vaccine knowledge (43 [32.8]) | Seizure freedom (OR = 0.181, 95% CI = 0.075–0.437) | ||||||
| Vaccination contraindications (severe medical disease/acute phase of disease) (37 [28.2]) | |||||||
| Less than 18 years old (32 [24.4]) | |||||||
| Medical workers do not recommend vaccination (25 [19.1]) | |||||||
| Lack of vaccination channels (22 [16.8]) | |||||||
| The domestic epidemic situation is stable, there is no need for vaccination (17 [13.0]) | |||||||
| Breastfeeding, preparing for pregnancy (12 [9.2]) | |||||||
| Others (7 [5.3]) | |||||||
| Wrede et al. [30] | NA | NA | NA | NA | NA | NA | NA |
Abbreviations: COVID = coronavirus disease 2019; NA = not applicable; OR = odds ratio;
Fig. 4.
Willingness to be vaccinated against COVID-19. (A) In patients with epilepsy and their caregivers; (B) in patients with epilepsy.
3.5. Impact of COVID-19 vaccination on epilepsy-related problems
Eight studies [17], [30], [31], [23], [24], [25], [26], [27] provided information on the impact of COVID-19 vaccination on epilepsy-related problems (Supplemental Table 4). Five studies [27], [30], [23], [24], [25] provided information on the changes in the seizure frequency. Clayton et al. [23] showed three (20%) of patients with Dravet syndrome (a severe, early-onset, developmental, and epileptic encephalopathy) experienced an increase in seizure frequency after the first dose of Oxford/AstraZeneca vaccine, whereas there was no increase in seizure frequency or duration after the second dose. Hood et al. [24] showed that 11 (9%) patients with epilepsy had increased seizure after dose one, and 10 (11%) had increased seizure activity after dose two. Lu et al. [25] showed that 19 (9.3%) participants with epilepsy experienced increased seizure frequency after vaccination. Özdemir et al. [27] showed that vaccination did not affect the monthly number of seizures, and only four (2.2%) patients had more seizures than normal after vaccination. Wrede et al. [30] showed that two (3.7%) patients with epilepsy reported increased seizure frequency after COVID-19 vaccination. Changes in seizure type were reported in two studies. Lu et al. [25] reported two (1.0%) participants had a first-ever convulsive seizure. Wrede et al. [30] showed that one (1.9%) patient reported a new seizure type four days after COVID-19 vaccination. Two studies reported the occurrence of status epilepticus [26], [31], both after mRNA vaccination. No occurrence of status epilepticus was reported in four studies [24], [25], [27], [30]. Additionally, four studies [17], [25], [26], [30] reported the outcome of seizure worsening or seizure exacerbation. However, only one study [26] provided a clear definition of seizure worsening [33] (defined as an increase in total average monthly seizure frequency, average monthly generalized tonic-clonic seizures (GTCS), new-onset GTCS, or new-onset status epilepticus). Their results showed that among 82 patients who received COVID-19 vaccination, five (6.1%) experienced seizure worsening [26]. Other three studies reported that there was no evidence of seizure exacerbation or aggravated epilepsy [17], [25], [30]. Generally, epilepsy-related problems after COVID-19 vaccination were uncommon.
3.6. Adverse effects after COVID-19 vaccination in patients with epilepsy
Table 3 shows that the solicited local and systemic adverse effects after COVID-19 vaccination were generally mild to moderate. Clayton et al. [23] showed that 11 (73%) patients with Dravet syndrome experienced at least one side effect after the first dose of SARS-CoV-2 vaccination, with the most common adverse effects being fatigue (40%), fever (40%), and injection site (33%). Li et al. [17] reported non-severe adverse effects after the first dose (79%) and the second dose (68%), including injection site pain, fatigue, headache, and nausea. Lu et al. [25] showed that local injection site skin adverse events and muscle pain were common after COVID-19 vaccination. Massoud et al. [26] reported the solicited local adverse effects after the first dose of COVID-19 vaccine and solicited systemic adverse effects after mRNA-based vaccination. Özdemir et al. [27] summarized the difference in side effects between two doses of mRNA vaccine (BNT162b2) and inactivated vaccine. Their data showed that patients who received BNT162b2 were more likely to experience local adverse events than those who received CoronaVac after the first and second doses (p = 0.003 and p = 0.001, respectively). Wrede et al. [30] reported different solicited systemic adverse effects after vector-based and mRNA-based vaccination. In total, no serious adverse effects or mortality were reported.
Table 3.
Adverse effects of COVID-19 vaccine in patients with epilepsy.
| Study | Vaccine platform | Adverse effects (frequency with percentage) |
|---|---|---|
| Li et al. [17] | First dose of inactivated vaccine (n = 38) | Pain at the injection site 1 (2.6%), fatigue 3 (7.9%), nausea 2 (5.3%), headache 2 (5.3%), fever 0 (0.0%) |
| Second dose of inactivated vaccine (n = 22) | Pain at the injection site 3 (13.6%), fatigue 2 (9.1%), nausea 1 (4.5%), headache 1 (4.5%), fever 0 (0.0%) | |
| Clayton et al. [23] | viral vector and nucleic acid-based vaccines (n = 15) | Pain at the injection site 5 (33%), fatigue 6 (40%), nausea or vomiting 0 (0%), headache 2 (13.3%), fever 5 (33%), allergic reaction 0 (0%), aching 4 (26.7%), other 1 (6.7%) |
| Veronica Hood et al. [24] | mRNA vaccine (n = 115) | Dose one: No side effects 66(55%), injection site score 32 (27%), fever 6 (5%), allergic reaction 1(1%), lethargy 23(19%), headache 10(8%), chills 1(1%), muscle pain 2(2%), nausea 1(1%) |
| Viral-vector vaccine (n = 5) | Dose two: No side effects 47(50%), injection site score 18 (19%), fever 18 (19%), allergic reaction 1(1%), lethargy 26(28%), headache 10(10%), chills 4(4%), muscle pain 7(7%), nausea 6(6%). | |
| Lu et al. [25] | First dose of inactivated vaccine (n = 204) | Local injection site skin adverse events 12 (5.9%), muscle pain 6 (2.9%), fatigue 4 (2.0%), headache 4 (2.0%), drowsiness 1 (0.5%), fever 1 (0.5%), others 9 (0.0%) |
| Second dose of inactivated vaccine (n = 139) | Local injection site skin adverse events 5 (3.6%), muscle pain 3 (2.2%), fatigue 2 (1.4%), headache 4 (2.9%), drowsiness 1 (0.7%), fever 0 (0.0%), others 9 (6.5%) | |
| Massoud et al. [26] | First dose of mRNA vaccine (n = 15) | Injection site pain 43 (52.4%), injection site redness 14 (17.1%), injection site swelling 23 (28.0%), headache 28 (34.1%), fatigue 33 (40.2%), fever 30 (36.6%), gastrointestinal symptoms 13 (15.9%), myalgia 28 (34.1%), chills 17 (20.7%), arthralgia 13 (15.9%), sore throat 12 (14.6%) |
| Second dose of mRNA vaccine (n = 35) | ||
| First dose of viral-vector vaccine (n = 32) | ||
| Özdemir et al. [27] | First dose of mRNA vaccine (n = 136) | Injection site redness/pain 49 (36.0%), fatigue 25 (18.3%), headache 19 (13.9%), fever 16 (11.7%), myalgia 11 (8.1%), chills 9 (6.6%), nausea 2 (1.4%), dizziness 2 (1.4%), diarrhea 2 (1.4%) |
| First dose of inactivated vaccine (n = 42) | Injection site redness/pain 3 (7.1%), fatigue 4 (9.5%), headache 5 (11.9%), fever 3 (7.1%), myalgia 2 (4.7%), chills 3 (7.1%), nausea 1 (2.3%), dizziness 2 (4.47%), diarrhea 1 (2.3%) | |
| Second dose of mRNA vaccine (n = 136) | Injection site redness/pain 41 (30.1%), fatigue 16 (11.7%), headache 18 (12.5%), fever 10 (7.3%), myalgia 11 (8.1%), chills 7 (5.1%), nausea 2 (1.4%), dizziness 3 (2.2%), diarrhea 1 (0.7%) | |
| Second dose of inactivated vaccine (n = 42) | Injection site redness/pain 2 (4.7%), fatigue3 (7.1%), headache 4 (9.5%), fever 1 (2.3%), myalgia 2 (4.7%), chills 2 (4.7%), nausea 1 (2.3%), dizziness 1 (2.3%), diarrhea 0 | |
| Wrede et al. [30] | mRNA vaccine (n = 34) | Headache 9 (16.7%), fatigue 8 (14.8%), fever/shivering 5 (9.3%), gastrointestinal symptoms 2 (3.7%) |
| Viral-vector vaccine (n = 18) | ||
| Unable to remember (n = 2) | ||
Abbreviations: COVID = coronavirus disease 2019.
3.7. Assessment of the qualities of the studies
All included studies were survey-based cross-sectional studies, including face-to-face interview [25], [29] and online survey. All included studies did not provide the information regarding the external validation of the questionnaire or survey. Table 4 shows that two studies [26], [29] had a low risk of bias and eight studies [17], [27], [28], [30], [22], [23], [24], [25] had a moderate risk of bias assessed using the AHRQ method.
Table 4.
Risk of bias of the cross-sectional studies assessed using AHRQ.
| Study | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | Score |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Li et al. [17] | Y | Y | Y | Y | N | N | N | Y | N | Y | N | 6 |
| Asadi et al. [22] | Y | Y | Y | Y | N | N | N | Y | N | Y | N | 6 |
| Clayton et al. [23] | Y | Y | Y | Y | N | N | N | Y | N | Y | N | 6 |
| Hood et al. [24] | Y | Y | Y | Y | N | N | N | Y | N | Y | Y | 7 |
| Lu et al. [25] | Y | Y | Y | Y | N | N | N | Y | N | Y | N | 6 |
| Massoud et al. [26] | Y | Y | Y | Y | N | Y | Y | Y | N | Y | N | 8 |
| Özdemir et al. [27] | Y | Y | Y | Y | N | Y | N | Y | N | Y | N | 7 |
| Puteikis et al. [28] | Y | Y | Y | Y | N | N | N | Y | Y | Y | N | 7 |
| Qiao et al. [29] | Y | Y | Y | Y | N | Y | Y | Y | Y | Y | N | 9 |
| Wrede et al. y[30] | Y | Y | Y | Y | N | N | N | Y | N | Y | N | 6 |
NOTE: 1.Define the source of information (survey, record review). 2. List inclusion and exclusion criteria for exposed and unexposed subjects (cases and controls) or refer to previous publications. 3. Indicate time period used for identifying patients. 4. Indicate whether or not subjects were consecutive if not population-based. 5. Indicate if evaluators of subjective components of study were masked to other aspects of the status of the participants. 6. Describe any assessments undertaken for quality assurance purposes (e.g., test/retest of primary outcome measurements). 7. Explain any patient exclusions from analysis. 8. Describe how confounding was assessed and/or controlled. 9. If applicable, explain how missing data were handled in the analysis. 10. Summarize patient response rates and completeness of data collection. 11. Clarify what follow-up, if any, was expected and the percentage of patients for which incomplete data or follow-up was obtained.
Abbreviations: AHRQ = Agency for Healthcare Research and Quality; Y = Yes; N = No.
4. Discussion
The present study showed a low COVID-19 vaccination coverage and willingness among patients with epilepsy. However, the uncommon incidence of epilepsy worsening after vaccination and no reports of severe COVID-19 vaccine-related adverse effects suggest that COVID-19 vaccines were safe and well-tolerated in patients with epilepsy.
Public health is under the threat of the unprecedented COVID-19 pandemic, urging the need for large-scale safe and effective vaccine coverage to achieve herd immunity. The COVID-19 vaccination coverage ranges from 10.6% to 73.9% in the included studies. The vaccine uptake rate in people with epilepsy was lower than in their same-age controls [25]. Possible reasons for the low COVID-19 vaccination coverage in patients with epilepsy might include population priority, doubts about the necessity, and availability of vaccines and vaccination services [34], [35]. Moreover, our findings showed that patients with active epilepsy were more likely to be unvaccinated than those with inactive epilepsy (OR 1.84, 95%CI 1.41–2.39, I 2 = 0%). However, the unvaccination status was not significantly different among different seizure types (focal versus non-focal onset, OR 1.22, 95%CI 0.94–1.58, I 2 = 0%) and gender (male versus female, OR 1.00, 95%CI 0.77–1.30, I 2 = 8%). The ILAE recommends vaccination for patients with epilepsy based on the low risk of increased seizure frequency after vaccination [36]. The risk of COVID-19 infection and potential complications might outweigh the risk of adverse effects caused by COVID-19 vaccine. However, different countries had different regulations to vaccination for patients with epilepsy, adapting to the local situation and culture. According to the Technical Guidelines for COVID-19 vaccination issued by the Chinese Center for Disease Control and Prevention (first edition), uncontrolled seizures are considered contraindications for vaccination in China [37]. The Chinese branch of ILAE published a Chinese consensus on the issue of COVID-19 vaccine and epilepsy in July 2021, updating that epilepsy is not a contraindication for vaccination and defining patients with six months seizure-free could be vaccinated [38]. Nevertheless, clinicians and healthcare workers may remain cautious about vaccination for patients with active epilepsy discouraging vaccination for patients with active epilepsy.
Our analysis showed that a considerable proportion of patients with epilepsy or their caregivers were unwilling to receive COVID-19 vaccination. Notably, there is substantial heterogeneity (26.1–89.3%) regarding the unwillingness or hesitancy among included studies. For example, Asadi et al. [22] showed a low rate of vaccine hesitancy among patients with epilepsy (9 [5.9%]), which was not different from that in healthy individuals (8 [7.4%]). However, two studies [26], [28] reported higher rates of vaccine hesitancy. Fear of seizure worsening, and concerns about the adverse effects and efficacy rank among the leading causes of vaccination hesitancy. Cases of seizure onset after COVID-19 vaccination [31], [39], [40] might influence the clinicians’ decision to recommend vaccination in patients with epilepsy. However, our analysis showed that epilepsy-related problems after COVID-19 vaccination such as increased seizure frequency and status epilepticus were uncommon in most of the included studies. For example, Lu et al. [25] showed no evidence of seizure exacerbation, and less than 10% of patients had seizure increase after COVID-19 vaccination. Özdemir et al. found that only four out of 178 patients with epilepsy had more seizures than normal [27]. Notably, Clayton et al. [23] reported that three (20%) patients with Dravet syndrome experienced an increase in seizure frequency after the first dose of Oxford/AstraZeneca vaccine, whereas there was no increase in seizure frequency or duration after the second dose. Since different types of vaccines (i.e., mRNA vaccines, viral vector vaccines, and inactivated vaccines) have different principles for immunogenicity, whether COVID-19 vaccine types influence the seizure worsening in patients with epilepsy needs to be investigated in further studies.
A previous study showed that a considerable proportion (302 [54.2%]) of patients with epilepsy believed there were differences in safety and efficacy among different vaccines [29]. Concern about potential vaccine-related adverse effects is a critical factor influencing vaccination coverage and willingness. The currently available vaccines have been shown to be safe and tolerable in the general population. However, limited evidence of vaccination in patients with epilepsy might influence the attitudes of health workers, caregivers, and patients with epilepsy towardCOVID-19 vaccination. Although the limited information in the included studies does not permit quantitative analysis, most reported adverse effects were self-limited mild to moderate. Moreover, adverse effects were rarely severe, suggesting a considerable safety profile of COVID-19 vaccines in patients with epilepsy. However, caution needs to be addressed because long-term evidence is still scarce; future studies with long-term follow-up are needed.
Social and educational factors like insufficient publicity and education, and lack of knowledge about vaccination might also account for a low vaccination coverage and willingness. Healthcare workers cannot give suggestive answers on whether or not to receive COVID-19 vaccination without adequate education and training. For example, Clayton et al. [23] showed that 27 (77%) caregivers did not receive any professional healthcare advice on COVID-19 vaccination in patients with epilepsy. Other factors may include education background, occupation, residence, and economic status. Comprehensive education programs are needed to improve the attitudes toward COVID-19 vaccination.
Our study has limitations. First, the survey-based nature of the included studies contributes to both measurement bias and selection bias. For example, administration of surveys on a cross-sectional basis inevitably introduces measurement bias through inaccurate reporting of vaccine-related adverse effects. Second, the survey tools in the included studies had not been validated. Future studies with well-validated survey tools are needed. Moreover, publication bias tests and subgroup analysis stratified by vaccine types and dosage were not performed due to the limited number of studies with insufficient information. Future larger sample sized studies with long follow-up are necessary to evaluate the safety and tolerability of COVID-19 vaccines among patients with epilepsy. Lastly, lack of a control group in most included studies does not permit direct comparisons in patients with epilepsy to the general population.
5. Conclusion
The present study suggests that the COVID-19 vaccination coverage and willingness were still low in patients with epilepsy. However, vaccination against COVID-19 appears to be well-tolerated and safe in patients with epilepsy, supporting a positive outlook towardvaccination in this population.
Acknowledgments
Acknowledgements
Not applicable.
Conflicts of interests statement
The authors declare that they have no conflict of interests.
Funding
None.
Ethics approval
The ethics approval was waived because this is a systemic review and meta-analysis.
Availability of data and materials
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Authors' contributions
K.L. and H.D. designed the study. K.L., H.H., and S.F. drafted the manuscript and shared the first-author. K.L. and S.F. contributed to data extraction. K.L. and H.D. performed the statistical analysis. G.Z., K.F. and N.L. made the critical revision of the manuscript for important intellectual content. H.D had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.yebeh.2022.108822.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Hopkins J. Johns Hopkins Coronavirus Resource Center. Https://coronavirus.Jhu.Edu/map.Html (2022, accessed 30 April 2022).
- 2.Mahase E. Covid-19: Omicron and the need for boosters. BMJ. 2021;375:n3079. doi: 10.1136/bmj.n3079. [DOI] [PubMed] [Google Scholar]
- 3.Lopez Bernal J., Andrews N., Gower C., Gallagher E., Simmons R., Thelwall S., et al. Effectiveness of covid-19 vaccines against the B.1.617.2 (Delta) Variant N Engl J Med. 2021;385(7):585–594. doi: 10.1056/NEJMoa2108891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wolf M.E., Luz B., Niehaus L., Bhogal P., Bäzner H., Henkes H. Thrombocytopenia and intracranial venous sinus thrombosis after “COVID-19 vaccine AstraZeneca” exposure. J Clin Med. 2021;10(8):1599. doi: 10.3390/jcm10081599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Krzywicka K, van de Munckhof A, Sánchez van Kammen M, Heldner MR, Jood K, Lindgren E, et al. Age-Stratified Risk of Cerebral Venous Sinus Thrombosis After SARS-CoV-2 Vaccination. Neurology. 2022 Feb 15;98(7):e759-e768. [DOI] [PubMed]
- 6.Waheed W., Carey M.E., Tandan S.R., Tandan R. Post COVID-19 vaccine small fiber neuropathy. Muscle Nerve. 2021;64(1):E1–E2. doi: 10.1002/mus.27251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Márquez Loza A.M., Holroyd K.B., Johnson S.A., Pilgrim D.M., Amato A.A. Guillain-Barré syndrome in the placebo and active arms of a COVID-19 vaccine clinical trial: temporal associations do not imply causality. Neurology. 2021;96(22):1052–1054. doi: 10.1212/WNL.0000000000011881. [DOI] [PubMed] [Google Scholar]
- 8.Havla J., Schultz Y., Zimmermann H., Hohlfeld R., Danek A., Kümpfel T. First manifestation of multiple sclerosis after immunization with the Pfizer-BioNTech COVID-19 vaccine. J Neurol. 2022;269(1):55–58. doi: 10.1007/s00415-021-10648-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Khayat-Khoei M., Bhattacharyya S., Katz J., Harrison D., Tauhid S., Bruso P., et al. COVID-19 mRNA vaccination leading to CNS inflammation: a case series. J Neurol. 2022;269(3):1093–1106. doi: 10.1007/s00415-021-10780-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Faissner S., Richter D., Ceylan U., Schneider-Gold C., Gold R. COVID-19 mRNA vaccine induced rhabdomyolysis and fasciitis. J Neurol. 2022;269:1774–1775. doi: 10.1007/s00415-021-10768-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Khan E., Shrestha A.K., Colantonio M.A., Liberio R.N., Sriwastava S. Acute transverse myelitis following SARS-CoV-2 vaccination: a case report and review of literature. J Neurol. 2022;269:1121–1132. doi: 10.1007/s00415-021-10785-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zuhorn F., Graf T., Klingebiel R., Schäbitz W.R., Rogalewski A. Postvaccinal encephalitis after ChAdOx1 nCov-19. Ann Neurol. 2021;90(3):506–511. doi: 10.1002/ana.26182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thijs R.D., Surges R., O'Brien T.J., Sander J.W. Epilepsy in adults. Lancet. 2019;393:689–701. doi: 10.1016/S0140-6736(18)32596-0. [DOI] [PubMed] [Google Scholar]
- 14.Siahaan Y.M.T., Ketaren R.J., Hartoyo V., Hariyanto T.I. Epilepsy and the risk of severe coronavirus disease 2019 outcomes: A systematic review, meta-analysis, and meta-regression. Epilepsy Behav. 2021;125:108437. doi: 10.1016/j.yebeh.2021.108437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6(7):e1000100. [DOI] [PMC free article] [PubMed]
- 16.Stroup D.F., Berlin J.A., Morton S.C., Olkin I., Williamson G.D., Rennie D., et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283(15):2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 17.Li N., Chu C., Lin W. A survey of hesitancy and response to the COVID-19 vaccine among patients with epilepsy in Northeast China. Front Neurol. 2021;12:778618. doi: 10.3389/fneur.2021.778618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zeng X., Zhang Y., Kwong J.S.W., Zhang C., Li S., Sun F., et al. The methodological quality assessment tools for preclinical and clinical studies, systematic review and meta-analysis, and clinical practice guideline: a systematic review. J Evid Based Med. 2015;8(1):2–10. doi: 10.1111/jebm.12141. [DOI] [PubMed] [Google Scholar]
- 19.Chen D., Zhi Q., Zhou Y., Tao Y., Wu L., Lin H. Association between dental caries and BMI in children: A systematic review and meta-analysis. Caries Res. 2018;52(3):230–245. doi: 10.1159/000484988. [DOI] [PubMed] [Google Scholar]
- 20.Higgins J.P., Thompson S.G., Deeks J.J., Altman D.G. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Scheffer I.E., Berkovic S., Capovilla G., Connolly M.B., French J., Guilhoto L., et al. ILAE classification of the epilepsies: Position paper of the ILAE Commission for Classification and Terminology. Epilepsia. 2017;58(4):512–521. doi: 10.1111/epi.13709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Asadi-Pooya A.A., Barzegar Z., Sadeghian S., Nezafat A., Shahisavandi M., Nabavizadeh S.A. COVID-19 vaccine hesitancy among patients with epilepsy or other chronic conditions. Disaster Med Public Health Prep. 2021:1–3. doi: 10.1017/dmp.2021.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Clayton L.M., Balestrini S., Cross J.H., Wilson G., Eldred C., Evans H., et al. The impact of SARS-CoV-2 vaccination in Dravet syndrome: A UK survey. Epilepsy Behav. 2021;124:108258. doi: 10.1016/j.yebeh.2021.108258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hood V, Berg AT, Knupp KG, Koh S, Laux L, Meskis MA, et al. COVID-19 vaccine in patients with Dravet syndrome: Observations and real-world experiences. Epilepsia. 2022.doi: 10.1111/epi.17250. [DOI] [PMC free article] [PubMed]
- 25.Lu L.u., Zhang Q.i., Xiao J., Zhang Y., Peng W., Han X., et al. COVID-19 vaccine take-up rate and safety in adults with epilepsy: Data from a multicenter study in China. Epilepsia. 2022;63(1):244–251. doi: 10.1111/epi.17138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Massoud F., Ahmad S.F., Hassan A.M., Alexander K.J., Al-Hashel J., Arabi M. Safety and tolerability of the novel 2019 coronavirus disease (COVID-19) vaccines among people with epilepsy (PwE): A cross-sectional study. Seizure. 2021;92:2–9. doi: 10.1016/j.seizure.2021.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Özdemir H.N., Dere B., Gökçay F., Gökçay A. Are COVID-19 vaccines safe for people with epilepsy? A cross-sectional study. Neurol Sci. 2022;43(6):3489–3496. doi: 10.1007/s10072-022-05956-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Puteikis K., Mameniškienė R. Factors associated with COVID-19 vaccine hesitancy among people with epilepsy in Lithuania. Int J Environ Res Public Health. 2021;18:4374. doi: 10.3390/ijerph18084374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Qiao S., Zhang R.-R., Yang T.-T., Wang Z.-H., Fang X.-Q., Fang C.-Y., et al. Attitudes to being vaccinated against COVID-19: a survey of people with epilepsy in China. Front Neurol. 2021;12:743110. doi: 10.3389/fneur.2021.743110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.von Wrede R., Pukropski J., Moskau-Hartmann S., Surges R., Baumgartner T. COVID-19 vaccination in patients with epilepsy: First experiences in a German tertiary epilepsy center. Epilepsy Behav. 2021;122:108160. doi: 10.1016/j.yebeh.2021.108160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Šín R., Štruncová D. Status epilepticus as a complication after COVID-19 mRNA-1273 vaccine: A case report. World J Clin Cases. 2021;9(24):7218–7223. doi: 10.12998/wjcc.v9.i24.7218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Odincova G.V., Nezdorovin O.V., Nezdorovina V.G. Vliyanie COVID-19 na techenie epilepsii [An impact of COVID-19 on the course of epilepsy] Zh Nevrol Psikhiatr Im S S Korsakova. 2022;122(2):101–106. doi: 10.17116/jnevro2022122021101. [DOI] [PubMed] [Google Scholar]
- 33.Sarkis R.A., Jehi L., Bingaman W., Najm I.M. Seizure worsening and its predictors after epilepsy surgery. Epilepsia. 2012;53(10):1731–1738. doi: 10.1111/j.1528-1167.2012.03642.x. [DOI] [PubMed] [Google Scholar]
- 34.Goldman R.D., Staubli G., Cotanda C.P., Brown J.C., Hoeffe J., Seiler M., et al. Factors associated with parents' willingness to enroll their children in trials for COVID-19 vaccination. Hum Vaccin Immunother. 2021;17(6):1607–1611. doi: 10.1080/21645515.2020.1834325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Szilagyi PG, Shah MD, Delgado JR, Thomas K, Vizueta N, Cui Y, et al. Parents' Intentions and Perceptions About COVID-19 Vaccination for Their Children: Results From a National Survey. Pediatrics. 2021;148(4):e2021052335. [DOI] [PMC free article] [PubMed]
- 36.International League Against Epilepsy. COVID-19 vaccines and people with epilepsy. https://www.ilae.org/patient-care/covid-19-and-epilepsy/covid-19-vaccines-and-people-with-epilepsy. (Last updated: 19 November 2021, accessed 24 December 2021).
- 37.Chinese Center for Disease Control and Prevention. Technical guidelines for COVID-19 vaccination (first edition). http://www.nhc.gov.cn/xcs/yqfkdt/202103/c2febfd04fc5498f916b 1be080905771.shtml (2021, accessed 24 Dec 2021).
- 38.Lu L., Mu J., Zhou D., Jiang W., Li S.Z., Hong Z. Epilepsy patients and COVID-19 vaccine: expert recommendations. J Epilepsy. 2021;7(4):323–326. [Google Scholar]
- 39.Makhlouf A.T., Van Alphen M.U., Manzano G.S., Freudenreich O. A seizure after COVID-19 vaccination in a patient on clozapine. J Clin Psychopharmacol. 2021;41(6):689–690. doi: 10.1097/JCP.0000000000001488. [DOI] [PubMed] [Google Scholar]
- 40.Aladdin Y., Shirah B. New-onset refractory status epilepticus following the ChAdOx1 nCoV-19 vaccine. J Neuroimmunol. 2021;357:577629. doi: 10.1016/j.jneuroim.2021.577629. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.