Abstract
Coronavirus disease 2019 (COVID-19) has caused a historic pandemic of respiratory disease. COVID-19 also causes acute and post-acute neurological symptoms, which range from mild, such as headaches, to severe, including hemorrhages. Current evidence suggests that there is no widespread infection of the central nervous system (CNS) by SARS-CoV-2, thus what is causing COVID-19 neurological disease? Here, we review potential immunological mechanisms driving neurological disease in COVID-19 patients. We begin by discussing the implications of imbalanced peripheral immunity on CNS function. Next, we examine the evidence for dysregulation of the blood-brain barrier during SARS-CoV-2 infection. Last, we discuss the role myeloid cells may play in promoting COVID-19 neurological disease. Combined, we highlight the role of innate immunity in COVID-19 neuroinflammation and suggest areas for future research.
Introduction
The severe acute respiratory syndrome coronavirus (SARS-CoV)-2 is associated with neurologic impairments during acute infection and after recovery (reviewed in Klein, 2022 [1]). Common acute symptoms include headache, anosmia, and dysgeusia. Less commonly, patients present with hallucinations, delusions, and behavioral changes, while meningitis, encephalitis, inflammatory demyelination, coagulopathy with infarction, cerebrovascular disease, and acute hemorrhagic necrotizing encephalopathy are rare presentations of acute coronavirus disease 2019 (COVID-19). Common acute neurological symptoms may persist for weeks to years after recovery from the initial infection [2]. Additional post-acute sequela of SARS-CoV-2 infection (PASC) that occur even in patients with mild COVID-19 include fatigue, confusion, dysexecutive function, and memory impairments that worsen over time [3]. The neuropathogenesis of SARS-CoV-2-mediated neurologic disease is unclear, especially regarding direct vs. indirect effects of the virus itself.
SARS-CoV-2 has a linear, positive-sense, single-stranded RNA genome encoding four structural proteins, known as the spike (S), envelope, membrane, and nucleocapsid (N) proteins. SARS-CoV-2 S protein mediates binding to the receptor angiotensin-converting enzyme 2 (ACE2). During binding, host serine proteases, including transmembrane serine protease (TMPRSS)2, promote membrane fusion via proteolysis of the S protein into the subunits S1 and S2, allowing entry of the virus into the cytosol (reviewed in Jackson et al., 2022 [4]). ACE2 is expressed by cells in many organs and tissues, including the lungs, gastrointestinal tract, kidneys, heart, adipose, and gonads, consistent with the clinical presentation of COVID-19 as multi-organ infectious disease [5]. ACE2 has also been detected in brain endothelial cells, the choroid plexus, and the ventral posterior nucleus of the thalamus, albeit in very low levels [6,7]. TMPRSS2 is not expressed by cells of the CNS. However, neuropilin-1, which is expressed in the brain, is a membrane-bound coreceptor to a tyrosine kinase receptor for both vascular endothelial growth factor (VEGF) and semaphorin family members, and it may serve as a host factor for SARS-CoV-2 infection [8]. Despite this, there are limited data supporting neuroinvasive routes for SARS-CoV-2 and lack of evidence for productive infection of the CNS parenchyma (reviewed in Klein, 2022). Emerging evidence suggests that SARS-CoV-2-mediates dysregulation of innate immune responses, leading to a cytokine release syndrome (CRS) with hyper-elevation of pro-inflammatory cytokines, including interleukin(IL)-1β, IL-6, and tumor necrosis factor (TNF), as well as a delayed or muted type I interferon (IFN) responses [9, 10, 11]. Excess IL-6 and IL-1β, with simultaneous subdued type I IFN levels, are positively correlated with disease severity and could drive neurological effects and alter the blood-brain barrier (BBB) [12, 13, 14, 15]. This review will provide a detailed discussion of peripheral and neuroimmune responses that contribute to BBB instability and neuroinflammation in the setting of acute and post-acute COVID-19.
Dysregulated peripheral immunity and implications for the CNS
Cytokine imbalance during COVID-19
Serum cytokines induced by systemic inflammation can impact BBB function (as reviewed in Galea, 2021); thus, CRS observed in severe COVID-19 patients could contribute to BBB disruption and the initiation of neurological disease. The BBB includes the specializations of CNS endothelial cells that occur at capillaries and post-capillary venules, including tight and adherens junctions, restricted entry of cells, solutes, and molecules, reduced transcytosis ability, and associated pericytes and astrocytes that maintain and participate in barrier function (reviewed in Klein et al., 2019 [17]). Junctional integrity is regulated by the activation of nucleotide guanosine triphosphate (GTP) family members via hydrolysis to guanosine diphosphate (GDP) that alters cytoskeletal structures. Studies in animal models of viral infections indicate that cytokines differentially activate GTPases, leading to increased or decreased BBB integrity [18]. Type I IFNs promote BBB integrity through direct action on endothelial cells or indirect signaling through astrocytes, which then promotes barrier function specifically in the hindbrain [18,19]. In contrast, pro-inflammatory cytokines such as IL-6 or IL-1β disrupt the BBB via the disruption of GTPases in the endothelial cells [18,20,21]. This suggests that elevated IL-6 and IL-1β combined with muted type I IFN levels observed in COVID-19 patients may be uniquely poised to promote BBB disruption. In addition to effects on the BBB, serum cytokines may directly impact neuronal functions. Recent work found serum CCL11 levels were positively correlated with post-acute cognitive deficits in COVID-19 patients, and that in a mouse model, the injection of CCL11 was sufficient to impair the generation of new neurons in the hippocampus [22]. Understanding the serum cytokines associated with cognitive impairment will be an important step in identifying biomarkers for patients at risk of PASC.
Pro-inflammatory cytokines are also key mediators of both sickness behaviors and post-acute CNS function. Current evidence suggests that SARS-CoV-2 infection may promote pro-inflammatory cytokine release in the CNS, as the analysis of cerebrospinal fluid (CSF) from COVID-19 patients with neurological symptoms found elevated levels of IL-1β, TNF-α, IL-8, IL-6, IL-15, MCP-1, and MIP-1β [23,24]. Even during mild SARS-CoV-2 infection in a mouse model, inflammatory cytokines (IL-6, CXCL5, and CCL11) were elevated at acute and post-acute timepoints in the CSF [22]. This could directly impact CNS function as many of these cytokines are known to target various neural cell types. For example, IL-1β targets neural stem cells to reduce adult neurogenesis, which is required for certain forms of learning and memory, as well as neurons and astrocytes, leading to glutamate excitotoxicity [25,26]. Of note, COVID-19 patients exhibited elevated CSF cytokine levels despite the lack of detection of CSF SARS-CoV-2 RNA [23,27]. The source of CSF pro-inflammatory cytokines during COVID-19 and their long-term implications are pressing questions for future studies.
Peripheral immune cells and the CNS during COVID-19
Expansion of pro-inflammatory monocytes during COVID-19 is necessary for viral control but also helps drive immunopathology, and the persistence of these monocytes is correlated with the development of PASC [28, 29, 30, 31, 32, 33]. Recent work has demonstrated that monocytes are susceptible to abortive infection by SARS-CoV-2, which causes inflammasome activation and pyroptotic cell death, thus promoting immunopathology [34]. Pro-inflammatory monocytes are not found in significant numbers in the homeostatic CNS; however, they can infiltrate across the BBB under inflammatory conditions. The analysis of border regions in post-mortem COVID-19 patients identified increases in activated (CD68+) monocytes/macrophages and perivascular macrophages [35,36]. Additionally, in a hamster model, SARS-CoV-2 infection increased parenchymal CD68+ IBA-1+ cells, which are either infiltrating monocytes/macrophages or activated microglia [37]. Infiltration of pro-inflammatory, classical monocytes into the CNS during acute injury often exacerbates neuroinflammation, and entry of infected monocytes into the CNS could promote pathology, as pyroptotic cell death is highly inflammatory and would release non-infectious viral RNA into the brain parenchyma [34]. However, differentiation of monocytes into non-classical, wound-healing myeloid cells can promote the resolution of inflammation and is beneficial after sterile injury of the CNS [38]. Thus, myeloid cells in the CNS during COVID-19 could be playing dual roles in the initiation and resolution of inflammation. Further studies are needed to confirm these findings and dissect the mechanisms of myeloid cell CNS recruitment, location, and impact on brain function.
Adaptive immune cells have come under scrutiny for their potential role in promoting PASC due to the long lifespan of memory B and T cells, and indeed, elevated levels of cytotoxic T cells in the serum and lung are correlated with the development of gastrointestinal and respiratory PASC [33,39]. The recruitment and impact of T cells into the CNS during COVID-19 is less clear. Immunohistochemical analyses of post-mortem tissue found small increases in CD3+ T cells in the brain parenchyma, while RNA-sequencing and mass cytometry analyses identified a moderate increase in both cytotoxic CD8+ and helper CD4+ T cell frequencies [36,40, 41, 42, 43]. T cells in the CNS of COVID-19 patients were activated based on increased expression of markers of exhaustion (programmed cell death protein 1 and T cell immunoglobulin and mucin domain-containing protein 3), cytotoxic granules (granzyme B), and recently undergone proliferation [36]. To date, there is very little evidence examining the role of B cells in the CNS during SARS-CoV-2 infection. Mass cytometry analysis found a minor increase in B cell numbers in the brain parenchyma and analysis of the CSF from COVID-19 patients found elevated IgG levels, some of which were autoantibodies against CNS epitopes [36,44,45]. Together, these data find some evidence for a role of adaptive immunity in neurological PASC; however, further studies are needed to clearly define if T and B cells enter the CNS after SARS-CoV-2 infection.
The blood–brain barrier during COVID-19
One clear finding that has emerged is evidence of cerebral vascular dysfunction during COVID-19 neurological disease. Studies of post-mortem CNS tissues derived from >80 COVID-19 patients confirmed that hemorrhages and ischemia were a common feature of CNS pathology [40, 41, 42]. Imaging studies on patients recovering from COVID-19 also identified abnormalities in cerebral blood flow and frontoparietal hypometabolism, suggesting BBB disruption [46,47]. SARS-CoV-2 infection of Syrian Golden Hamsters confirmed arterial level dysfunction, as well as venous/capillary level dysfunction, as demonstrated by an Evans Blue dye assay indicating increased BBB permeability after infection [37]. Together, these data identify BBB disruption as a hallmark of COVID-19-mediated neurologic disease.
One of the most pressing questions about COVID-19 and the BBB is whether SARS-CoV-2 can directly infect brain endothelial cells. Evidence for in vivo infection of the brain parenchyma is lacking; however, the detection of SARS-CoV-2 RNA was observed in perivascular spaces or in some endothelial cells of the vasculature, which suggests either breakdown of the BBB allowing virus in the blood to enter or direct infection of the endothelium [36,42]. In vitro human-induced pluripotent stem cell-derived brain capillary endothelial cells and hamster brain microvascular endothelial cell (BMEC) models can be productively infected with SARS-CoV-2 [37,48]. In contrast, in vitro BBB models using human cerebral endothelial cells grown on a membrane over pericytes reported extremely low levels of apical virus and no detectable basolateral virus [49]. Analysis of SARS-CoV-2 infection of a variety of primary human endothelial cells, including those derived from brain, reported that infection was only possible if ACE-2 was overexpressed [50]. Combined, these data find conflicting evidence for SARS-CoV-2 infection of endothelial cells, and the lack of evidence for robust infection in vivo suggests that if infection occurs, it is likely a rare event.
Although SARS-CoV-2 may not productively infect the BBB endothelium, several studies have reported direct effects of the S protein on BBB function. To determine if S impacts the BBB, Rhea et al. administered radiolabeled S1 to outbred mice and measured S1 accumulation within the brain parenchyma via regression analysis of radioactivity in the blood vs. the brain. They found that intranasal or intravenously delivered S1 can cross the BBB via adsorptive transcytosis in an ACE-2-dependent manner [51]. This interaction may disrupt the BBB, as the addition of S protein to in vitro BBB models resulted in a decrease of trans-endothelial electrical resistance [37,52]. However, tight-junction protein expression did not change in response to S protein, suggesting intracellular signaling mechanisms of disruption [37,49]. SARS-CoV-2 also induces endothelial cell activation, as the administration of S or N proteins to in vitro cultures initiated NFkB signaling that upregulated inflammatory cytokines, including IL-6, TNF, IL-1β, and adhesion molecules, such as intercellular cell adhesion molecule (ICAM)-1 and vascular cell adhesion molecule (VCAM)-1 [37,52,53]. This phenomenon was unique to SARS-CoV-2 and did not occur after exposure to SARS-CoV-1, MERS-CoV, or HKU.1 [52,53]. In vivo evidence for S protein interaction with the BBB is lacking; however, Nanostring technology was able to identify increased expression of antiviral (interferon-induced transmembrane protein (IFITM) 1 and 2) and antigen presentation genes (B2M, HLA-B) on blood vessels in the brains of COVID-19 patients [48]. Thus, the BBB endothelium is altered after COVID-19; however, further experiments are needed to determine if this is due to exposure to S protein.
Metabolic dysfunction or apoptosis of BMECs may also play a role in BBB disruption during COVID-19. String vessels are collapsed blood vessels no longer capable of blood flow and are associated with vascular dysfunction during neurodegenerative diseases. Analysis of post-mortem COVID-19 brain tissue identified increased string vessel formation and vessel apoptosis [54]. Investigation using an in vitro endothelial cell line and intravenous delivery to mice of an adenoviral vector identified SARS-CoV-2 main protease (Mpro), as a potential inducer of string vessel formation. Mechanistically, the addition of Mpro to endothelial cells degraded the NFkB modulator, Nemo, which induced a receptor-interacting serine/threonine kinase 3 (RIPK3)-dependent apoptotic pathway [54]. The addition of S protein to in vitro endothelial cells did not cause changes in viability, but it did induce morphological alterations toward a contractile state, which is a sign of cell stress, and it inhibited mitochondrial function in a pericyte cell line [55,56]. While it is still unclear if Mpro or S directly impacts the BBB in vivo, SARS-CoV-2 viremia during acute infection was found to be a strong predictor of post-acute memory deficits [39]. Together, these data demonstrate a link between SARS-CoV-2 viral proteins and disruption of endothelial cell or pericyte function and highlight a need to better define these interactions using more complete in vitro BBB models or in vivo systems.
CNS resident immunity and SARS-CoV-2
Microglial activation during COVID-19
Microglia are yolk-sac-derived CNS-resident myeloid cells that play essential roles in homeostasis and immune responses to injury or infection. One common finding in analyses of post-mortem brain tissue from COVID-19 patients is the presence of activated, parenchymal myeloid cells, as identified by increased numbers of CD68+ or IBA-1+ cells, both of which are markers for myeloid cells engaged in phagocytosis [35,42,43,56,57]. Single nucleus RNA-sequencing analyses of the CNS confirmed that SARS-CoV-2 infection alters the microglial transcriptome, with ∼178 differently expressed genes (DEGs) between COVID-19 patients and healthy controls [57]. Many of these top DEGs were related to innate immune signaling, such as interferon regulatory factor 8 (IRF8) and complement protein C1QC, or cell stress pathways, such as ATF5, which regulates transcriptional responses to stress, and RIPK1, which promotes cell death [43,57]. High-dimensional spatial profiling of post-mortem brain tissue from COVID-19 patients also identified significant increases in the number of activated microglial nodules [36]. Together, these data demonstrate the presence of activated microglia in humans after SARS-CoV-2 infection.
Microglia are potent pathogen sensing and antigen presentation cells. During a typical neurotropic viral infection microglia sense virus via pattern recognition receptors (PRRs), which detect molecular patterns unique to viral RNA and initiate the secretion of inflammatory cytokines. However, SARS-CoV-2 may not productively infect the CNS, thus posing the question: what is causing microglial activation during COVID-19? S protein can activate the transmembrane PRR, TLR4, and since S1 may cross the BBB, microglial sensing of S1, even in the absence of replication, could trigger inflammation [58]. Direct injection of S1 into the meninges of rats led to increased and sustained expression of molecules involved in antigen presentation, such as major histocompatibility complex (MHC) II and CD11b, as well as inflammasome signaling, a pathway in which a PRR initiates cleavage of pro-IL-1β into the active, secreted form of the cytokine, in the hippocampus, hypothalamus, and frontal cortex [59]. The addition of S protein to human microglia in vitro also induced cytokine production and metabolic changes [60]. These data suggest that any S1 that crosses the BBB during COVID-19 could cause a sustained microglial inflammatory response. Microglial activation can also occur in response to danger-associated molecular patterns (DAMPs), such as extracellular ATP or reactive oxygen species release [61]. Studies of ischemic stroke have found that sterile disruption of the BBB results in the release of DAMPs that lead to the rapid activation of microglia [62]. Thus, microglial activation during COVID-19 could be due to either release of DAMPs after disruption of the BBB and/or the detection of S1 that has crossed the BBB.
Microglial activation is necessary to respond to CNS insults; however, sustained activation can promote pathogenic outcomes [63]. A key question is whether microglial activation in COVID-19 patients promotes disease. Little evidence is currently available, but one study noted that cortical microglia from COVID-19 patients had a similar transcriptomic profile to “disease-associated microglia” observed in neurodegenerative disease [43]. Additionally, tissue regions with microglial nodules had increased axonal damage as measured by amyloid precursor protein deposits, which is a biomarker used to identify persistent damage after traumatic brain injury [36,64]. Microglial activation induced by injection of S1 into the meninges of rats correlated with sickness behavior, anxiety, and cognitive deficits [59,65]. A mouse model of mild COVID-19 found that activated microglia persisted for up to seven weeks after infection, suggesting that microglia could be partly responsible for neurological PASC [22]. However, detailed mechanistic studies are needed to define how SARS-CoV-2-induced microglial activation impacts patient outcomes.
Neurons and astrocytes
The role of other CNS resident cells that can participate in immune responses, including neurons and astrocytes, during COVID-19 has been poorly defined. The analysis of longitudinal blood samples from COVID-19 patients identified increased concentrations of neurofilament light chain, a marker of neurodegeneration, and glial fibrillary acidic protein, a marker of glial activation, during the acute phase of disease [66]. Single nucleus RNA-sequencing of cortices from deceased COVID-19 patients found evidence of decreased synaptic activity in excitatory neurons, via downregulation of genes-mediating neurotransmission such as VAMP2 and SNAP25 [43]. Histopathological analyses of SARS-CoV-2 infected primates identified evidence of neuronal death [67]. Two publications demonstrated that rodent models of acute COVID-19 exhibit decreased neurogenesis in the dentate gyrus region of the hippocampus and a loss of myelination in subcortical white matter tracts, with the former study confirming the loss of neurogenesis in humans [22,68]. Multiple post-mortem studies of human and primate tissue noted increased GFAP staining, but more data are needed to understand alterations in astrocyte function and the sources of astrocyte activation [41,42]. Together, these data suggest that neuronal function may be altered after SARS-CoV-2 infection, but many more studies are needed to confirm and extend upon these findings, such as microscopy analyses of synapse formation, neurogenesis, and glial activation in humans.
Conclusions and implications
Research into COVID-19-associated neurological disease is still in its infancy; however, a few key conclusions can be made. First, cognitive deficits are relatively common during acute infection and in PASC, despite a lack of evidence for productive infection of the CNS. Second, SARS-CoV-2 infection disrupts the BBB and induces endothelial cell activation. Last, COVID-19 patients have increased numbers of activated myeloid cells within the brain parenchyma. The significant research effort is needed to understand how these events are linked, the mechanisms that induce BBB and CNS dysfunction after SARS-CoV-2, and their long-term impact. However, we can speculate based on other models of CNS disease that even in the absence of productive infection of the CNS, viremia could result in the delivery of viral particles or S1 protein to the BBB. Combined with the systemic cytokine imbalances observed during acute COVID-19, this could initiate endothelial innate immune responses and BBB disruption. Even transient disruption of the BBB can result in neuroinflammation and microglial activation due to the leakage of viral PAMPs into the parenchyma or direct release of DAMPs by injured endothelial cells (See Figure 1 ). Microgliosis can promote cognitive deficits, as shown in other viral infections, where activated microglia engulf synapses in a complement protein (C1q)-dependent manner, driving memory deficits [63]. Understanding the mechanisms behind COVID-19-mediated neurological disease is a pressing matter for the scientific community, as current data estimates that 30–60% of individuals infected with SARS-CoV-2 develop at least one post-acute symptom. As over 500 million people are already infected, and a the continuing high rate of infection, understanding the mechanisms behind COVID-19-induced neurological disease is a public health emergency.
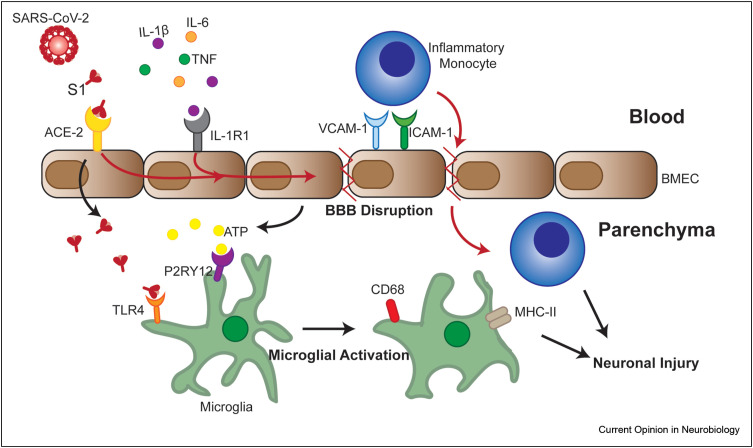
Figure 1.
Model of hypothesis for mechanisms of neuroinflammation during COVID-19. SARS-CoV-2 infection of the respiratory tract results in the release of pro-inflammatory cytokines (IL-1β, IL-6, and TNF) into the serum. Cytokines, such as IL-1β, can bind to receptors (IL-1R1) expressed by brain microvascular endothelial cells (BMECs) and promote loss of tight junction proteins in other viral models [18]. Some COVID-19 patients develop viremia, and SARS-CoV-2 or S1 protein in the serum can bind to ACE-2 expressed by BMECs and transcytoses into the brain parenchyma [51,52]. S1 binding on BMECs in combination with IL-1R1-induced signaling could promote barrier disruption and upregulation of adhesion molecules. Barrier disruption results in the release of danger-associated molecular proteins, such as ATP, which can bind to receptors, such as P2RY12, on microglia and promote activation. S1 that crosses the barrier can bind to TLR4 and promote microglial activation, indicated by increased phagocytosis (CD68) and antigen presentation capability (MHC-II) [58]. Increased transcellular permeability observed in in vitro models after S1 treatment could also promote the entry of inflammatory myeloid cells [37,51,52]. Together activated microglial and infiltrating myeloid cells could promote neuronal injury. Black arrows indicate known processes described in the literature, whereas red arrows promote hypothesized processes during COVID-19.
Funding sources
This work was supported by NIH grants R35NS122310, R01NS116788, R01 AI160188 (to RSK), and F32 NS128065-01 (to AV).
Conflict of interest statement
None declared.
This review comes from a themed issue on Crosstalk between neural and immune systems
Edited by Kelly Jordan-Sciutto and Anthony Filiano
References
- 1.Klein R.S. Mechanisms of coronavirus infectious disease 2019-related neurologic diseases. Curr Opin Neurol. 2022 doi: 10.1097/WCO.0000000000001049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Taquet M., Dercon Q., Luciano S., Geddes J.R., Husain M., Harrison P.J. Incidence, co-occurrence, and evolution of long-COVID features: a 6-month retrospective cohort study of 273,618 survivors of COVID-19. PLoS Med. 2021;18 doi: 10.1371/journal.pmed.1003773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Méndez R., Balanzá-Martínez V., Luperdi S.C., Estrada I., Latorre A., González-Jiménez P., Bouzas L., Yépez K., Ferrando A., Reyes S., et al. Long-term neuropsychiatric outcomes in COVID-19 survivors: a 1-year longitudinal study. J Intern Med. 2022;291:247–251. doi: 10.1111/joim.13389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jackson C.B., Farzan M., Chen B., Choe H. Mechanisms of SARS-CoV-2 entry into cells. Nat Rev Mol Cell Biol. 2022;23:3–20. doi: 10.1038/s41580-021-00418-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hamming I., Timens W., Bulthuis M.L.C., Lely A.T., Navis G.J., van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol. 2004;203:631–637. doi: 10.1002/path.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pellegrini L., Albecka A., Mallery D.L., Kellner M.J., Paul D., Carter A.P., James L.C., Lancaster M.A. SARS-CoV-2 infects the brain choroid plexus and disrupts the blood-CSF barrier in human brain organoids. Cell Stem Cell. 2020;27:951–961. doi: 10.1016/j.stem.2020.10.001. e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen R., Wang K., Yu J., Howard D., French L., Chen Z., Wen C., Xu Z. The spatial and cell-type distribution of SARS-CoV-2 receptor ACE2 in the human and mouse brains. Front Neurol. 2020;11 doi: 10.3389/fneur.2020.573095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Davies J., Randeva H.S., Chatha K., Hall M., Spandidos D.A., Karteris E., Kyrou I. Neuropilin-1 as a new potential SARS-CoV-2 infection mediator implicated in the neurologic features and central nervous system involvement of COVID-19. Mol Med Rep. 2020;22:4221–4226. doi: 10.3892/mmr.2020.11510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arunachalam P.S., Wimmers F., Mok C.K.P., Perera R.A.P.M., Scott M., Hagan T., Sigal N., Feng Y., Bristow L., Tak-Yin Tsang O., et al. Systems biological assessment of immunity to mild versus severe COVID-19 infection in humans. Science. 2020;369:1210–1220. doi: 10.1126/science.abc6261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blanco-Melo D., Nilsson-Payant B.E., Liu W.-C., Uhl S., Hoagland D., Møller R., Jordan T.X., Oishi K., Panis M., Sachs D., et al. Imbalanced host response to SARS-CoV-2 drives development of COVID-19. Cell. 2020;181:1036–1045. doi: 10.1016/j.cell.2020.04.026. e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vanderheiden A., Ralfs P., Chirkova T., Upadhyay A.A., Zimmerman M.G., Bedoya S., Aoued H., Tharp G.M., Pellegrini K.L., Manfredi C., et al. Type I and type III interferons restrict SARS-CoV-2 infection of human airway epithelial cultures. J Virol. 2020:94. doi: 10.1128/JVI.00985-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bastard P., Rosen L.B., Zhang Q., Michailidis E., Hoffmann H.-H., Zhang Y., Dorgham K., Philippot Q., Rosain J., Béziat V., et al. Autoantibodies against type I IFNs in patients with life-threatening COVID-19. Science. 2020;370 doi: 10.1126/science.abd4585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fajnzylber J., Regan J., Coxen K., Corry H., Wong C., Rosenthal A., Worrall D., Giguel F., Piechocka-Trocha A., Atyeo C., et al. SARS-CoV-2 viral load is associated with increased disease severity and mortality. Nat Commun. 2020;11:5493. doi: 10.1038/s41467-020-19057-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liao M., Liu Y., Yuan J., Wen Y., Xu G., Zhao J., Cheng L., Li J., Wang X., Wang F., et al. Single-cell landscape of bronchoalveolar immune cells in patients with COVID-19. Nat Med. 2020;26:842–844. doi: 10.1038/s41591-020-0901-9. [DOI] [PubMed] [Google Scholar]
- 15.Zhang Q., Bastard P., Liu Z., Le Pen J., Moncada-Velez M., Chen J., Ogishi M., Sabli I.K.D., Hodeib S., Korol C., et al. Inborn errors of type I IFN immunity in patients with life-threatening COVID-19. Science. 2020;370 doi: 10.1126/science.abd4570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Klein R.S., Garber C., Funk K.E., Salimi H., Soung A., Kanmogne M., Manivasagam S., Agner S., Cain M. Neuroinflammation during RNA viral infections. Annu Rev Immunol. 2019;37:73–95. doi: 10.1146/annurev-immunol-042718-041417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Daniels B.P., Holman D.W., Cruz-Orengo L., Jujjavarapu H., Durrant D.M., Klein R.S. Viral pathogen-associated molecular patterns regulate blood-brain barrier integrity via competing innate cytokine signals. mBio. 2014;5 doi: 10.1128/mBio.01476-14. e01476-01414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Daniels B.P., Jujjavarapu H., Durrant D.M., Williams J.L., Green R.R., White J.P., Lazear H.M., Gale M., Diamond M.S., Klein R.S. Regional astrocyte IFN signaling restricts pathogenesis during neurotropic viral infection. J Clin Invest. 2017;127:843–856. doi: 10.1172/JCI88720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Skelly D.T., Hennessy E., Dansereau M.-A., Cunningham C. A systematic analysis of the peripheral and CNS effects of systemic LPS, IL-1β, [corrected] TNF-α and IL-6 challenges in C57BL/6 mice. PLoS One. 2013;8 doi: 10.1371/journal.pone.0069123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wong R., Lénárt N., Hill L., Toms L., Coutts G., Martinecz B., Császár E., Nyiri G., Papaemmanouil A., Waisman A., et al. Interleukin-1 mediates ischaemic brain injury via distinct actions on endothelial cells and cholinergic neurons. Brain Behav Immun. 2019;76:126–138. doi: 10.1016/j.bbi.2018.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fernández-Castañeda A., Lu P., Geraghty A.C., Song E., Lee M.-H., Wood J., O’Dea M.R., Dutton S., Shamardani K., Nwangwu K., et al. Mild respiratory COVID can cause multi-lineage neural cell and myelin dysregulation. Cell. 2022;185:2452–2468. doi: 10.1016/j.cell.2022.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Normandin E., Holroyd K.B., Collens S.I., Shaw B.M., Siddle K.J., Adams G., Rudy M., Solomon I.H., Anahtar M.N., Lemieux J.E., et al. Intrathecal inflammatory responses in the absence of SARS-CoV-2 nucleic acid in the CSF of COVID-19 hospitalized patients. J Neurol Sci. 2021;430 doi: 10.1016/j.jns.2021.120023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pilotto A., Masciocchi S., Volonghi I., De Giuli V., Caprioli F., Mariotto S., Ferrari S., Bozzetti S., Imarisio A., Risi B., et al. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) encephalitis is a cytokine release syndrome: evidences from cerebrospinal fluid analyses. Clin Infect Dis. 2021;73 doi: 10.1093/cid/ciaa1933. e3019–e3026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Garber C., Vasek M.J., Vollmer L.L., Sun T., Jiang X., Klein R.S. Astrocytes decrease adult neurogenesis during virus-induced memory dysfunction via IL-1. Nat Immunol. 2018;19:151–161. doi: 10.1038/s41590-017-0021-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pozzi D., Menna E., Canzi A., Desiato G., Mantovani C., Matteoli M. The communication between the immune and nervous systems: the role of IL-1β in synaptopathies. Front Mol Neurosci. 2018;11:111. doi: 10.3389/fnmol.2018.00111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jiao L., Yang Y., Yu W., Zhao Y., Long H., Gao J., Ding K., Ma C., Li J., Zhao S., et al. The olfactory route is a potential way for SARS-CoV-2 to invade the central nervous system of rhesus monkeys. Signal Transduct Targeted Ther. 2021;6:169. doi: 10.1038/s41392-021-00591-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Grant R.A., Morales-Nebreda L., Markov N.S., Swaminathan S., Querrey M., Guzman E.R., Abbott D.A., Donnelly H.K., Donayre A., Goldberg I.A., et al. Circuits between infected macrophages and T cells in SARS-CoV-2 pneumonia. Nature. 2021;590:635–641. doi: 10.1038/s41586-020-03148-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Patterson B.K., Francisco E.B., Yogendra R., Long E., Pise A., Rodrigues H., Hall E., Herrera M., Parikh P., Guevara-Coto J., et al. Persistence of SARS CoV-2 S1 protein in CD16+ monocytes in post-acute sequelae of COVID-19 (PASC) up to 15 Months post-infection. Front Immunol. 2021;12 doi: 10.3389/fimmu.2021.746021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Utrero-Rico A., González-Cuadrado C., Chivite-Lacaba M., Cabrera-Marante O., Laguna-Goya R., Almendro-Vazquez P., Díaz-Pedroche C., Ruiz-Ruigómez M., Lalueza A., Folgueira M.D., et al. Alterations in circulating monocytes predict COVID-19 severity and include chromatin modifications still detectable six months after recovery. Biomedicines. 2021;9:1253. doi: 10.3390/biomedicines9091253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vanderbeke L., Van Mol P., Van Herck Y., De Smet F., Humblet-Baron S., Martinod K., Antoranz A., Arijs I., Boeckx B., Bosisio F.M., et al. Monocyte-driven atypical cytokine storm and aberrant neutrophil activation as key mediators of COVID-19 disease severity. Nat Commun. 2021;12:4117. doi: 10.1038/s41467-021-24360-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vanderheiden A., Thomas J., Soung A.L., Davis-Gardner M.E., Floyd K., Jin F., Cowan D.A., Pellegrini K., Shi P.-Y., Grakoui A., et al. CCR2 signaling restricts SARS-CoV-2 infection. mBio. 2021;12 doi: 10.1128/mBio.02749-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vijayakumar B., Boustani K., Ogger P.P., Papadaki A., Tonkin J., Orton C.M., Ghai P., Suveizdyte K., Hewitt R.J., Desai S.R., et al. Immuno-proteomic profiling reveals aberrant immune cell regulation in the airways of individuals with ongoing post-COVID-19 respiratory disease. Immunity. 2022;55:542–556. doi: 10.1016/j.immuni.2022.01.017. e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Junqueira C., Crespo Â., Ranjbar S., de Lacerda L.B., Lewandrowski M., Ingber J., Parry B., Ravid S., Clark S., Schrimpf M.R., et al. FcγR-mediated SARS-CoV-2 infection of monocytes activates inflammation. Nature. 2022 doi: 10.1038/s41586-022-04702-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Khan M., Yoo S.-J., Clijsters M., Backaert W., Vanstapel A., Speleman K., Lietaer C., Choi S., Hether T.D., Marcelis L., et al. Visualizing in deceased COVID-19 patients how SARS-CoV-2 attacks the respiratory and olfactory mucosae but spares the olfactory bulb. Cell. 2021;184:5932–5949. doi: 10.1016/j.cell.2021.10.027. e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schwabenland M., Salié H., Tanevski J., Killmer S., Lago M.S., Schlaak A.E., Mayer L., Matschke J., Püschel K., Fitzek A., et al. Deep spatial profiling of human COVID-19 brains reveals neuroinflammation with distinct microanatomical microglia-T-cell interactions. Immunity. 2021;54:1594–1610. doi: 10.1016/j.immuni.2021.06.002. e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang L., Zhou L., Bao L., Liu J., Zhu H., Lv Q., Liu R., Chen W., Tong W., Wei Q., et al. SARS-CoV-2 crosses the blood–brain barrier accompanied with basement membrane disruption without tight junctions alteration. Signal Transduct Targeted Ther. 2021;6:1–12. doi: 10.1038/s41392-021-00719-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Russo M.V., Latour L.L., McGavern D.B. Distinct myeloid cell subsets promote meningeal remodeling and vascular repair after mild traumatic brain injury. Nat Immunol. 2018;19:442–452. doi: 10.1038/s41590-018-0086-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Su Y., Yuan D., Chen D.G., Ng R.H., Wang K., Choi J., Li S., Hong S., Zhang R., Xie J., et al. Multiple early factors anticipate post-acute COVID-19 sequelae. Cell. 2022;185:881–895. doi: 10.1016/j.cell.2022.01.014. e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jensen M.P., Le Quesne J., Officer-Jones L., Teodòsio A., Thaventhiran J., Ficken C., Goddard M., Smith C., Menon D., Allinson K.S.J. Neuropathological findings in two patients with fatal COVID-19. Neuropathol Appl Neurobiol. 2021;47:17–25. doi: 10.1111/nan.12662. [DOI] [PubMed] [Google Scholar]
- 41.Matschke J., Lütgehetmann M., Hagel C., Sperhake J.P., Schröder A.S., Edler C., Mushumba H., Fitzek A., Allweiss L., Dandri M., et al. Neuropathology of patients with COVID-19 in Germany: a post-mortem case series. Lancet Neurol. 2020;19:919–929. doi: 10.1016/S1474-4422(20)30308-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Thakur K.T., Miller E.H., Glendinning M.D., Al-Dalahmah O., Banu M.A., Boehme A.K., Boubour A.L., Bruce S.S., Chong A.M., Claassen J., et al. COVID-19 neuropathology at Columbia university irving medical center/New York presbyterian hospital. Brain. 2021 doi: 10.1093/brain/awab148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yang A.C., Kern F., Losada P.M., Agam M.R., Maat C.A., Schmartz G.P., Fehlmann T., Stein J.A., Schaum N., Lee D.P., et al. Dysregulation of brain and choroid plexus cell types in severe COVID-19. Nature. 2021;595:565–571. doi: 10.1038/s41586-021-03710-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Franke C., Ferse C., Kreye J., Reincke S.M., Sanchez-Sendin E., Rocco A., Steinbrenner M., Angermair S., Treskatsch S., Zickler D., et al. High frequency of cerebrospinal fluid autoantibodies in COVID-19 patients with neurological symptoms. Brain Behav Immun. 2021;93:415–419. doi: 10.1016/j.bbi.2020.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jarius S., Pache F., Körtvelyessy P., Jelčić I., Stettner M., Franciotta D., Keller E., Neumann B., Ringelstein M., Senel M., et al. Cerebrospinal fluid findings in COVID-19: a multicenter study of 150 lumbar punctures in 127 patients. J Neuroinflammation. 2022;19:19. doi: 10.1186/s12974-021-02339-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hosp J.A., Dressing A., Blazhenets G., Bormann T., Rau A., Schwabenland M., Thurow J., Wagner D., Waller C., Niesen W.D., et al. Cognitive impairment and altered cerebral glucose metabolism in the subacute stage of COVID-19. Brain. 2021;144:1263–1276. doi: 10.1093/brain/awab009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Qin Y., Wu J., Chen T., Li J., Zhang G., Wu D., Zhou Y., Zheng N., Cai A., Ning Q., et al. Long-term microstructure and cerebral blood flow changes in patients recovered from COVID-19 without neurological manifestations. J Clin Invest. 2021;131 doi: 10.1172/JCI147329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Krasemann S., Haferkamp U., Pfefferle S., Woo M.S., Heinrich F., Schweizer M., Appelt-Menzel A., Cubukova A., Barenberg J., Leu J., et al. The blood-brain barrier is dysregulated in COVID-19 and serves as a CNS entry route for SARS-CoV-2. Stem Cell Rep. 2022;17:307–320. doi: 10.1016/j.stemcr.2021.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Constant O., Barthelemy J., Bolloré K., Tuaillon E., Gosselet F., Chable-Bessia C., Merida P., Muriaux D., Van de Perre P., Salinas S., et al. SARS-CoV-2 poorly replicates in cells of the human blood-brain barrier without associated deleterious effects. Front Immunol. 2021;12 doi: 10.3389/fimmu.2021.697329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nascimento Conde J., Schutt W.R., Gorbunova E.E., Mackow E.R. Recombinant ACE2 expression is required for SARS-CoV-2 to infect primary human endothelial cells and induce inflammatory and procoagulative responses. mBio. 2020;11 doi: 10.1128/mBio.03185-20. e03185-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rhea E.M., Logsdon A.F., Hansen K.M., Williams L.M., Reed M.J., Baumann K.K., Holden S.J., Raber J., Banks W.A., Erickson M.A. The S1 protein of SARS-CoV-2 crosses the blood-brain barrier in mice. Nat Neurosci. 2021;24:368–378. doi: 10.1038/s41593-020-00771-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Buzhdygan T.P., DeOre B.J., Baldwin-Leclair A., Bullock T.A., McGary H.M., Khan J.A., Razmpour R., Hale J.F., Galie P.A., Potula R., et al. The SARS-CoV-2 spike protein alters barrier function in 2D static and 3D microfluidic in-vitro models of the human blood-brain barrier. Neurobiol Dis. 2020;146 doi: 10.1016/j.nbd.2020.105131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Qian Y., Lei T., Patel P.S., Lee C.H., Monaghan-Nichols P., Xin H.-B., Qiu J., Fu M. Direct activation of endothelial cells by SARS-CoV-2 nucleocapsid protein is blocked by simvastatin. J Virol. 2021;95 doi: 10.1128/JVI.01396-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wenzel J., Lampe J., Müller-Fielitz H., Schuster R., Zille M., Müller K., Krohn M., Körbelin J., Zhang L., Özorhan Ü., et al. The SARS-CoV-2 main protease Mpro causes microvascular brain pathology by cleaving NEMO in brain endothelial cells. Nat Neurosci. 2021;24:1522–1533. doi: 10.1038/s41593-021-00926-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Khaddaj-Mallat R., Aldib N., Bernard M., Paquette A.-S., Ferreira A., Lecordier S., Saghatelyan A., Flamand L., ElAli A. SARS-CoV-2 deregulates the vascular and immune functions of brain pericytes via Spike protein. Neurobiol Dis. 2021;161 doi: 10.1016/j.nbd.2021.105561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lei Y., Zhang J., Schiavon C.R., He M., Chen L., Shen H., Zhang Y., Yin Q., Cho Y., Andrade L., et al. SARS-CoV-2 spike protein impairs endothelial function via downregulation of ACE 2. Circ Res. 2021;128:1323–1326. doi: 10.1161/CIRCRESAHA.121.318902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fullard J.F., Lee H.-C., Voloudakis G., Suo S., Javidfar B., Shao Z., Peter C., Zhang W., Jiang S., Corvelo A., et al. Single-nucleus transcriptome analysis of human brain immune response in patients with severe COVID-19. Genome Med. 2021;13:118. doi: 10.1186/s13073-021-00933-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhao Y., Kuang M., Li J., Zhu L., Jia Z., Guo X., Hu Y., Kong J., Yin H., Wang X., et al. SARS-CoV-2 spike protein interacts with and activates TLR41. Cell Res. 2021;31:818–820. doi: 10.1038/s41422-021-00495-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Frank M.G., Nguyen K.H., Ball J.B., Hopkins S., Kelley T., Baratta M.V., Fleshner M., Maier S.F. SARS-CoV-2 spike S1 subunit induces neuroinflammatory, microglial and behavioral sickness responses: evidence of PAMP-like properties. Brain Behav Immun. 2022;100:267–277. doi: 10.1016/j.bbi.2021.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Clough E., Inigo J., Chandra D., Chaves L., Reynolds J.L., Aalinkeel R., Schwartz S.A., Khmaladze A., Mahajan S.D. Mitochondrial dynamics in SARS-COV2 spike protein treated human microglia: implications for neuro-COVID. J Neuroimmune Pharmacol. 2021;16:770–784. doi: 10.1007/s11481-021-10015-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gülke E., Gelderblom M., Magnus T. Danger signals in stroke and their role on microglia activation after ischemia. Ther Adv Neurol Disord. 2018;11 doi: 10.1177/1756286418774254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dong R., Huang R., Wang J., Liu H., Xu Z. Effects of microglial activation and polarization on brain injury after stroke. Front Neurol. 2021:12. doi: 10.3389/fneur.2021.620948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Vasek M.J., Garber C., Dorsey D., Durrant D.M., Bollman B., Soung A., Yu J., Perez-Torres C., Frouin A., Wilton D.K., et al. A complement-microglial axis drives synapse loss during virus-induced memory impairment. Nature. 2016;534:538–543. doi: 10.1038/nature18283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Johnson V.E., Stewart W., Smith D.H. Traumatic brain injury and amyloid-β pathology: a link to Alzheimer's disease? Nat Rev Neurosci. 2010;11:361–370. doi: 10.1038/nrn2808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Oh J., Cho W.-H., Barcelon E., Kim K.H., Hong J., Lee S.J. SARS-CoV-2 spike protein induces cognitive deficit and anxiety-like behavior in mouse via non-cell autonomous hippocampal neuronal death. Sci Rep. 2022;12:5496. doi: 10.1038/s41598-022-09410-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kanberg N., Simrén J., Edén A., Andersson L.-M., Nilsson S., Ashton N.J., Sundvall P.-D., Nellgård B., Blennow K., Zetterberg H., et al. Neurochemical signs of astrocytic and neuronal injury in acute COVID-19 normalizes during long-term follow-up. EBioMedicine. 2021:70. doi: 10.1016/j.ebiom.2021.103512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rutkai I., Mayer M.G., Hellmers L.M., Ning B., Huang Z., Monjure C.J., Coyne C., Silvestri R., Golden N., Hensley K., et al. Neuropathology and virus in brain of SARS-CoV-2 infected non-human primates. Nat Commun. 2022;13:1745. doi: 10.1038/s41467-022-29440-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Klein R., Soung A., Sissoko C., Nordvig A., Canoll P., Mariani M., Jiang X., Bricker T., Goldman J., Rosoklija G., et al. COVID-19 induces neuroinflammation and loss of hippocampal neurogenesis. Res Sq. 2021 doi: 10.21203/rs.3.rs-1031824/v1. [DOI] [Google Scholar]