Abstract
High-density lipoprotein cholesterol (HDL-C) is not a homogenous lipid fraction, but it can be further divided into subfractions. It is well-known that the Roma population has a high prevalence of reduced HDL-C levels and cardiovascular diseases (CVDs). However, it is unknown how this reduction affects different HDL subfractions, and whether changes in their quantity/representation are associated with an increased cardiovascular risk among them. In the present study, the HDL subfraction profile of the Hungarian general (HG) and the Roma populations were compared, and the subfractions showing a significant difference between the two populations were identified. The association of HDL subfractions with CVD risk estimated by the Framingham risk score (FRS) and the Systematic COronary Risk Evaluation (SCORE) algorithms were also defined. The present study is the first to find a significant association between HDL subfractions and cardiovascular risk estimated by FRS and SCORE. Ten HDL subfractions were investigated on small but carefully selected samples comprising 100 control subjects (with normal lipid profile) and 277 case subjects (with reduced HDL-C levels) from HG and Roma populations of a complex health survey. The level of HDL-1 to 3 subfractions and HDL-L showed a significant inverse association with cardiovascular risk estimated by both SCORE and FRS algorithms, whereas HDL-4 to 6 and HDL-I only for FRS. A higher representation (in %) of HDL-1 to 3 has a significant risk-reducing effect, while HDL-8 to 10 has a risk-increasing effect estimated by FRS. Our results confirmed that reduced levels of HDL-6 and -7 expressed in mmol/L were significantly associated with Roma ethnicity.
Subject terms: Biomarkers, Cardiology, Risk factors
Introduction
In 2019, an estimated 17.9 million people died from cardiovascular diseases (CVDs), representing 32% of all global deaths and making it the leading cause of death worldwide (85% of which were due to stroke or heart attack)1. CVDs were also responsible for 38% of the 17 million premature (under 70 years) deaths due to non-communicable diseases in the same year. The development of these diseases can be prevented by addressing lifestyle and environmental risk factors such as unhealthy diet and obesity, harmful alcohol consumption, smoking, and the lack of physical activity1. In addition to these changeable risk factors, unchangeable ones, such as age, sex, race, and genetic predisposition, also have a significant effect on an individual's cardiovascular risk2.
Numerous cardiovascular risk estimation algorithms are known which use changeable and unchangeable factors to estimate the probability of a cardiovascular event within a defined period3. The best known and most widely used of these algorithms are the Framingham Risk Score (FRS)4 and the Systematic COronary Risk Evaluation (SCORE) calculations5. Both models estimate the risk of various cardiovascular events within 10 years and consider an individual's total cholesterol (TC) and high-density lipoprotein cholesterol levels (HDL-C), among many other CVD risk factors.
The lipid profile including TC, low-density lipoprotein cholesterol (LDL-C), HDL-C, and triglyceride (TG) is strongly associated with the risk of developing CVDs6. Concerning the lipid metabolic pathways, the serum levels of these lipids are highly correlated with each other, and a stronger association is obtained when their effects on cardiovascular risk are considered together. Epidemiological studies have shown that high levels of LDL-C and TG combined with low levels of HDL-C are associated with an increased risk for the development of CVDs6 and can, therefore, be used as a risk predictor, as well as to detect cardiovascular diseases earlier and treat them more effectively.
People on low incomes often have less access to preventive services and do not benefit from primary health care programs aimed at early detection and treatment of CVDs. As a result, these people are frequently diagnosed late and die at a younger age from CVDs or other non-communicable diseases, often in their most productive years1,7. Moreover, in addition to individual health risks, at a macroeconomic level, CVDs impose a heavy burden on the economy of several countries1,8,9.
The socio-economic situation of the Roma population is significantly less favorable compared to general ones regardless of their host country10–14. Evidence shows that the life expectancy at birth of the Roma population is significantly lower than that of non-Roma populations15. The prevalence of cardiovascular risk factors16–20 and, thus, the overall estimated cardiovascular risk are significantly higher among the Roma than the host country average21. Previous studies have shown that reduced HDL-C levels are very common in the Roma population22–25 and that the estimated 10-year cardiovascular risk is also significantly higher in the case of both sexes among them than in the Hungarian general population21.
It is a well-known fact that elevated HDL-C levels in the blood show an inverse relationship with the incidence of CVDs26 and in addition to being involved in the mechanism of reverse cholesterol transport, it is also known to have several beneficial health effects (anti-thrombotic27, anti-inflammatory28, anti-oxidative29, and pro-vasodilatory30).
In recent years, our research team has found that genetic factors, in addition to lifestyle and environmental ones, play a very important role in the high prevalence of reduced HDL-C levels among the Roma31–34. In the interpretation of the high frequency of low HDL-C levels among the Roma, it has to be also considered that HDL-C particles do not show a uniform pattern; HDL can be further divided into subfractions which differ in size, density, and components and have different effects on cardiovascular risk35–39. HDL subfractions are classified into three major subclasses: large HDLs (HDL-L), intermediate HDLs (HDL-I), and small HDLs (HDL-S). A higher concentration of large HDL-C but not that of intermediate, small, or total HDL-C is associated with lower cardiovascular risk37,38, whereas the HDL-S shows a relationship with an elevated risk for CVDs40.
In a single study published in 2018 by Hubková and her colleagues, the serum lipoprotein profiles of Roma and non-Roma populations from Slovakia were compared and the concentrations of the small HDL subfractions (8 to 10 by Lipoprint test) were described as significantly lower among the Roma41. However, whether this phenomenon is a unique feature of the Roma population, or a general characteristic consequence of the reduced HDL-C level found very frequently among them was not investigated. Furthermore, the reduced HDL-S level (and the associated reduced cardiovascular risk) contradicts the numerous publications describing an increased cardiovascular risk among the Roma in general16–21.
Currently, there is only a limited number of publications that describe the association of HDL subfractions with cardiovascular risk estimated by algorithms37,42. What is more, no study has been published so far on the association of the SCORE risk assessment, the most widely used cardiovascular risk algorithm in Europe, with HDL subfraction profile.
Therefore, the major goal of our study is to try and answer the following questions: (1) Is there a difference in the quantity (in mmol/L) or representation (in %) of HDL subfractions between Hungarian general and Roma individuals? (2) Is there a correlation between the cardiovascular risk estimated by SCORE or FRS and the profile of HDL subfractions?
Material and methods
Study design and populations
Study design and data collection were described in detail in our previous study43. In brief, a complex health survey was designed and performed to create a database for comparative and association studies to better understand the background of the very unfavorable health of Roma people in comparison with the Hungarian general population, with a special emphasis on the high burden of cardiometabolic diseases. This cross-sectional study had three main pillars including questionnaire-based, physical, and laboratory investigations involving adults aged 20–64 years from the Hungarian general (HG) and Hungarian Roma (Roma) populations. Altogether, 832 participants were recruited for the study including 417 HG (185 male and 232 female) and 415 Roma (108 male and 307 female) subjects. In addition to anthropometric (to define BMI), demographic (sex, age), socioeconomic, and health-related data (among them blood pressure measurement, use of antihypertensive medication, diabetes diagnosed), fasting blood samples were also collected for routine laboratory tests (among them TC, LDL-C, HDL-C, TG, apolipoprotein A1 (ApoAI) and apolipoprotein B (ApoB)). Using the routine laboratory data TG/HDL-C and ApoB/ApoAI ratios were also calculated.
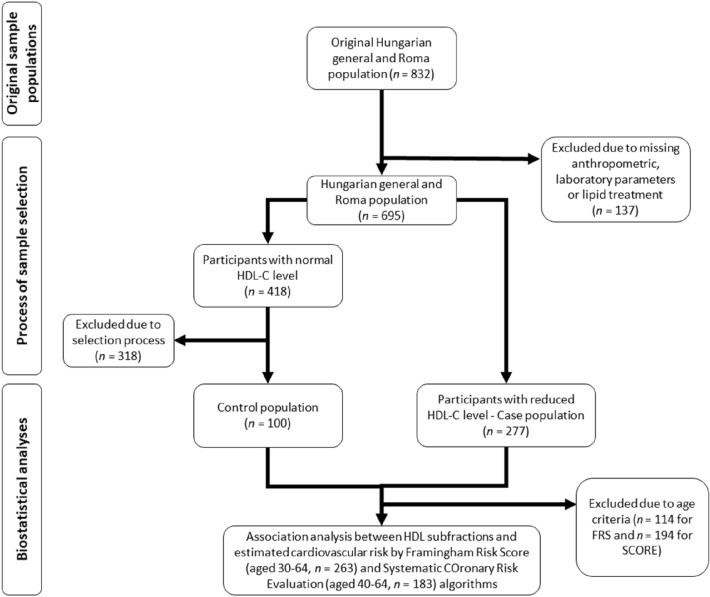
Participants whose anthropometric and/or laboratory parameters had been missing (20 HG and 47 Roma) and those who had received any lipid treatment (27 HG and 43 Roma) were excluded from further analysis. The remaining 695 persons (370 HG and 325 Roma) were divided into two groups based on their HDL-C levels. The first group included people who had normal HDL-C levels (≥ 1.03 mmol/L in males and ≥ 1.29 mmol/L in females) and had no other lipid abnormality (126 HG and 87 Roma). One hundred persons (25 HG male, 25 Roma male, 25 HG female, and 25 Roma female) were randomly selected from among them to represent the control group of the present study. The second group included people who had reduced HDL-C levels (115 HG and 162 Roma). To examine the association of HDL subfractions with the estimated risk of developing CVDs within 10 years, individuals belonging to the age group defined to the calculation of FRS (30–75 years; 124 HG and 139 Roma) and SCORE (40–65 years; 89 HG and 94 Roma) were studied. For more details, see Fig. 1.
Figure 1.
Flowchart showing the process of sample selection and biostatistical analyses.
Analysis of HDL subfractions
HDL is a highly heterogeneous class of lipoproteins that are considered and discussed as a group based solely on the hydrated density of its particles. Several methods are known to subfractionate HDL into subfractions. Most published prospective and clinical studies evaluating the use of HDL subfractions to predict results have used one of the proprietary laboratory tests or proprietary in-house systems available to clinicians: Lipoprint HDL® (gel electrophoresis), Cardio IQ® (ion mobility), NMR LipoProfile® (nuclear magnetic resonance) and, until recently, Vertical auto profile (VAP) ® (ultracentrifugation).
For the present study, HDL subfractions were determined using an electrophoretic method on polyacrylamide gel with the Lipoprint HDL Subfractions Test (Quantimetrix Corp., CA, USA) according to the manufacturer’s instructions. This commercially available test based on the linear polyacrylamide gel electrophoresis method separates and quantifies up to 10 HDL subfractions in serum or plasma.
Concisely, 25 μL serum was added to the 3% polyacrylamide gel tubes along with a 300 μL Lipoprint HDL Loading Gel solution. The tubes contained Sudan Black as a lipophilic dye and were photopolymerized at room temperature for 30 min. Electrophoresis with tubes containing sera samples and the manufacturer’s quality controls were performed at a constant of 3 mA/tube for 50 min. Subfraction bands were identified by their mobility (Rf) using very-LDL (VLDL) + LDL as the starting (Rf 0.0) and albumin as the ending (Rf 1.0) reference point and were scanned with an ArtixScan M1 digital scanner (Microtek International Inc., CA, USA).
Ten HDL subfractions were differentiated between VLDL + LDL and albumin peaks and were grouped into three major classes: HDL-L (from HDL-1 to 3), HDL-I (from HDL-4 to 7), and HDL-S (from HDL-8 to 10) HDL subfractions. Cholesterol concentrations of the HDL particle subsets were calculated with Lipoware software (Quantimetrix Corp., CA, USA) by multiplying the total cholesterol concentration of the samples by the relative area under the curve of the subfraction bands.
Estimation of the cardiovascular risk by FRS and SCORE in Study Populations
In the present study, we estimated the cardiovascular risk of HG and Roma populations by applying the two most used risk estimation models (FRS and SCORE) in Europe. Both algorithms are sex-specific and estimate the risk of a cardiovascular event occurring within 10 years.
The first version of FRS was developed based on data obtained from the Framingham Heart Study, to estimate the 10-year risk of developing a coronary heart disease, which was later revised to calculate also the 10-year risk of developing CVDs in general. In the present study, we used both versions of the FRS developed for hard coronary heart disease (FRSCHD)44 and cardiovascular disease (FRSCVD)4. Both versions consider age, sex, total cholesterol, HDL-C levels, systolic blood pressure, high blood pressure treatment, and smoking status; for FRSCVD, addition, diabetes status is also included in the algorithm. Analyses for FRS were performed on participants of the study populations aged 30–64.
An estimated risk based on SCORE, which is the algorithm recommended by the 2007 European Society of Cardiology guidelines on cardiovascular disease prevention in clinical practice—was also calculated for both study populations5. The model has been calibrated according to each European country’s mortality statistics. In the present study, the SCORE algorithm used for countries in the high-risk group was applied to both study populations. All analyses for SCORE were performed for participants of the study populations aged 40–64.
A more detailed explanation of the cardiovascular risk models used in the present study is described in our previous publication21.
Statistical analyses
All statistical analyses were conducted by using SPSS (version 26) software (IBM Company, Armonk, NY, USA). Prevalence data were compared by the χ2 test. Comparisons between subgroups were performed by Student’s unpaired t-test in case of normally distributed variables and by Mann–Whitney U-test in case of variables with non-normal distribution. Correlations between continuous variables were assessed by linear regression analysis, while logistic regression was used for binary outcome variables. Bonferroni correction was applied for multiple analyses of the same dependent variable to avoid type I error and the p value determined by Bonferroni correction were considered as the threshold for statistical significance.
Ethics declarations
All subjects gave their informed consent for inclusion before they participated in the study. The study was conducted following the Declaration of Helsinki, and the protocol was approved by the Ethics Committee of the Hungarian Scientific Council on Health (61327-2017/EKU).
Results
Characteristics of study populations by factors used to estimate cardiovascular risk and lipid profile of the study populations by HDL-C status
When examining the characteristics of the study populations with a normal HDL-C level, a significant difference between the Roma and the HG population was observed in the prevalence of current smokers (HG: 32.00% vs. 62.22%, p = 0.004).
In the group with reduced HDL-C levels, there was a significant difference in systolic blood pressure (HG: 127.90 mmHg vs. Roma: 119.75 mmHg, p < 0.001), distribution of sex (36.52% male/63.48% female in the HG vs. 20.37% male/79.63% female in the Roma; p = 0.003) and the prevalence of current smokers (HG: 39.13% vs. Roma 70.19%, p < 0.001) between the study populations. See Supplementary Table 1 for more details.
For the groups with normal and reduced HDL-C levels, there were no significant differences in either lipid or apoprotein profiles between the study populations. See Table 1 for more details.
Table 1.
Lipid and apolipoprotein profiles of study populations by HDL-C status (normal vs. reduced HDL-C levels).
| Normal HDL-C levels | Reduced HDL-C levels | |||||
|---|---|---|---|---|---|---|
| Hungarian general (n = 50) |
Roma (n = 50) |
p value | Hungarian general (n = 115) |
Roma (n = 162) |
p value | |
| Mean (95% CI) | Mean (95% CI) | |||||
| HDL-C (mmol/L) | 1.62 (1.53–1.71) | 1.59 (1.49–1.70) | 0.290 | 1.02 (0.99–1.05) | 1.01 (0.98–1.03) | 0.678 |
| LDL-C (mmol/L) | 2.50 (2.33–2.67) | 2.63 (2.50–2.77) | 0.359 | 3.04 (2.86–3.23) | 3.17 (3.02–3.32) | 0.186 |
| TG (mmol/L) | 0.90 (0.80–0.99) | 0.88 (0.80–0.96) | 0.814 | 2.06 (1.84–2.28) | 1.88 (1.71–2.06) | 0.169 |
| TG/HDL-C ratio | 0.58 (0.51–0.66) | 0.59 (0.52–0.67) | 0.697 | 2.18 (1.89–2.46) | 2.00 (1.79–2.22) | 0.290 |
| Total cholesterol (mmol/L) | 4.39 (4.19–4.59) | 4.43 (4.26–4.60) | 0.871 | 4.79 (4.58–5.00) | 4.80 (4.63–4.97) | 0.978 |
| ApoAI (g/L) | 1.66 (1.58–1.74) | 1.62 (1.55–1.69) | 0.317 | 1.30 (1.27–1.33) | 1.27 (1.25–1.30) | 0.109 |
| ApoB (g/L) | 0.83 (0.77–0.88) | 0.87 (0.83–0.91) | 0.257 | 1.08 (1.03–1.14) | 1.12 (1.07–1.17) | 0.242 |
| ApoB/ApoAI ratio | 0.51 (0.47–0.55) | 0.55 (0.51–0.58) | 0.094 | 0.84 (0.80–0.88) | 0.89 (0.85–0.93) | 0.097 |
Threshold of significance after Bonferroni correction: p < 0.006.
95% CI, 95% confidence interval; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein; TG, triglyceride; ApoAI, Apolipoprotein A1; ApoB, Apolipoprotein B.
Comparative analysis of the HDL-C subfraction profiles of the study populations by HDL-C status
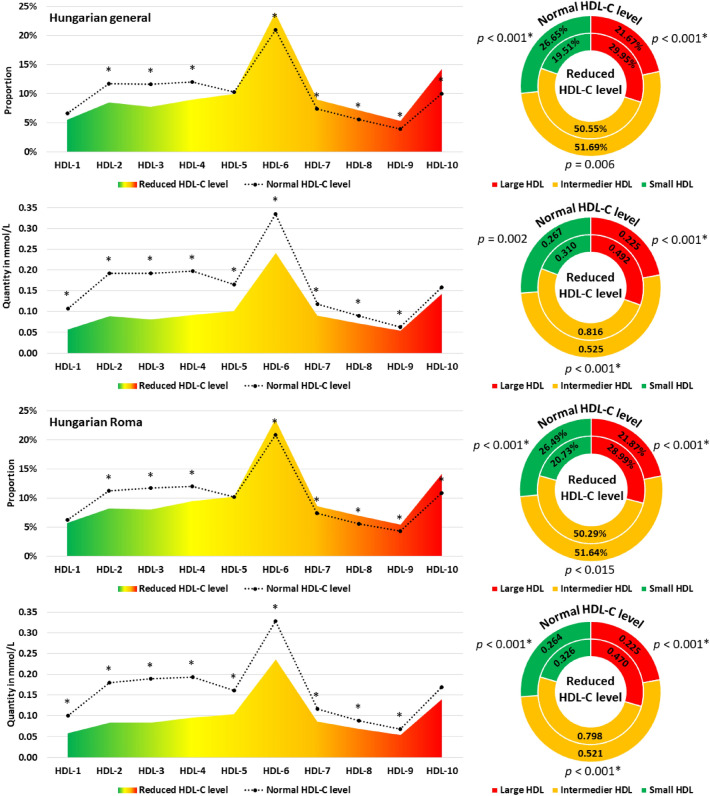
We compared the HDL subfraction profiles (quantity in mmol/L and proportion in %) of individuals with reduced and normal HDL-C levels. For both populations, individuals with reduced HDL-C levels showed a significant reduction in the proportion of HDL-2 to 4 and an increase in the proportion of HDL-6 to 10. The proportion of HDL-5 remained unchanged. The proportion of HDL-L showed a significant increase while HDL-S showed a decrease in those with reduced HDL-C levels, regardless of the population studied. The proportion of HDL-L remained unchanged. The concentration of HDL-1 to 9 subfractions was significantly lower in participants with reduced HDL-C status than in those with normal HDL-C status in both populations. See Fig. 2 for more details.
Figure 2.
Composition of the HDL subfraction profile (in mmol/L and proportion in %) by HDL-C status (normal and reduced) in the Hungarian general and Roma populations. Large HDL: from HDL-1 to 3; intermediate HDL: from HDL-4 to 7; small HDL: from HDL-8 to 10. *Significant results after test correction (p < 0.002).
There is no difference in the HDL subfraction profiles (neither mmol/L nor proportion in %) between those with normal or reduced HDL-C levels in the two study populations. In both cases, HDL-6 is the most predominant while HDL-9 is the least predominant subfraction. Regardless of the examined population and the HDL-C status, the HDL-I subclass was the most prevalent one. See Supplementary Fig. 1 for more details.
The effect of Roma ethnicity on HDL subfraction levels (in mmol/L) and proportions (in %) compared to the Hungarian general one
Linear regression analyses were applied to investigate the HDL subfraction profile (as a dependent variable in mmol/L and as a proportion in %) between the HG and the Roma populations. All analyses were adjusted for the independent variable as ethnicity (HG used as reference), sex, age (in years), LDL, and TG levels (in mmol/L).
The results of statistical analyses showed that the Roma population had significantly lower HDL-6 and -7 subfractions in mmol/L compared to the Hungarian general one. In addition, Roma ethnicity was associated with a decrease in all HDL subfractions (although not in all cases significantly). There was no significant effect of Roma ethnicity on the proportion of HDL subfractions. See Table 2 for more details.
Table 2.
Effect of Roma ethnicity on HDL subfractions (A—in mmol/L and B—in %) compared to the Hungarian general one.
| β | 95% CI | p value | |
|---|---|---|---|
| A | |||
| HDL-1 | − 0.003 | − 0.008 to 0.003 | 0.342 |
| HDL-2 | − 0.012 | − 0.021 to − 0.003 | 0.009 |
| HDL-3 | − 0.005 | − 0.015 to 0.005 | 0.324 |
| HDL-4 | − 0.003 | − 0.012 to − 0.006 | 0.550 |
| HDL-5 | − 0.003 | − 0.009 to 0.003 | 0.296 |
| HDL-6 | − 0.016 | − 0.027 to − 0.006 | 0.003* |
| HDL-7 | − 0.008 | − 0.013 to − 0.003 | 0.002* |
| HDL-8 | − 0.006 | − 0.010 to − 0.002 | 0.007 |
| HDL-9 | < 0.001 | − 0.003 to 0.003 | 0.884 |
| HDL-10 | − 0.003 | − 0.013 to 0.006 | 0.502 |
| HDL-L | − 0.020 | − 0.041 to 0.001 | 0.066 |
| HDL-I | − 0.032 | − 0.059 to − 0.005 | 0.022 |
| HDL-S | − 0.008 | − 0.023 to 0.007 | 0.290 |
| B | |||
| HDL-1 | 0.027 | − 0.384 to 0.439 | 0.896 |
| HDL-2 | − 0.513 | − 0.957 to − 0.069 | 0.024 |
| HDL-3 | 0.131 | − 0.344 to 0.606 | 0.589 |
| HDL-4 | 0.190 | − 0.214 to 0.594 | 0.355 |
| HDL-5 | 0.200 | − 0.044 to 0.443 | 0.108 |
| HDL-6 | − 0.339 | − 0.788 to 0.110 | 0.139 |
| HDL-7 | − 0.204 | − 0.466 to 0.058 | 0.127 |
| HDL-8 | − 0.149 | − 0.386 to 0.088 | 0.218 |
| HDL-9 | 0.193 | − 0.010 to 0.397 | 0.063 |
| HDL-10 | 0.333 | − 0.394 to 1.060 | 0.368 |
| HDL-L | − 0.416 | − 1.419 to 0.587 | 0.415 |
| HDL-I | − 0.047 | − 0.682 to 0.588 | 0.884 |
| HDL-S | 0.418 | − 0.594 to 1.430 | 0.417 |
HDL-L, large HDL (from HDL-1 to 3); HDL-I, intermediate HDL (from HDL-4 to 7); HDL-S, small HDL (from HDL-8 to 10).
*Significant results after test correction (p < 0.004).
Association of HDL subfractions with the 10-year risk of CVD events estimated by the FRS and the SCORE algorithms
Linear regression analyses (adjusted for ethnicity) were applied to examine the association between HDL subfractions and the estimated 10-year cardiovascular risk by SCORE and FRS algorithms.
Out of the 10 HDL subfractions examined, increasing levels (in mmol/L) of HDL-1 to 3 subfractions and HDL-L showed significant association with reduced cardiovascular risk estimated by SCORE. The HDL-1 to 6 subfractions as well as HDL-L and HDL-I levels were significantly associated with reduced cardiovascular risk estimated by FRSCHD and FRSCVD.
The proportion of HDL subfractions showed no significant correlation with the cardiovascular risk estimated by SCORE. The proportion of HDL-1 to 3 subfractions showed a significant association with reduced cardiovascular risk estimated by the FRS, whereas HDL-6 to 10 subfractions showed an association with an increased cardiovascular risk. The percentage of HDL-4 was only significantly associated with reduced risk estimated by FRSCHD. A higher proportion of HDL-L subfractions was associated with a significantly decreased cardiovascular risk, whereas HDL-S was associated with an increased cardiovascular risk estimated by FRS. See Table 3. for more details.
Table 3.
Effect of HDL subfractions (A—in mmol/L; B—in %) on the estimated cardiovascular risk by Systematic Coronary Risk Evaluation and Framingham Risk Scores.
| Systematic Coronary Risk Evaluation | Framingham Risk Scores | ||
|---|---|---|---|
| High-risk algorithm | CHD | CVD in general | |
| A | |||
| HDL-1 | − 11.26* | − 28.37* | − 53.98* |
| HDL-2 | − 6.27* | − 13.43* | − 24.99* |
| HDL-3 | − 5.95* | − 12.99* | − 23.85* |
| HDL-4 | − 4.84 | − 12.76* | − 23.23* |
| HDL-5 | − 8.50 | − 20.06* | − 39.80* |
| HDL-6 | − 5.10 | − 9.93* | − 19.9* |
| HDL-7 | − 8.85 | − 12.46 | − 25.98 |
| HDL-8 | − 8.61 | − 4.43 | − 14.83 |
| HDL-9 | − 6.17 | 11.85 | 10.57 |
| HDL-10 | 0.52 | 7.31 | 7.93 |
| HDL-L | − 2.65* | − 5.94* | − 10.94* |
| HDL-I | − 1.88 | − 4.32* | − 8.32* |
| HDL-S | − 0.81 | 2.48 | 1.84 |
| B | |||
| HDL-1 | − 0.13 | − 0.40* | − 0.71* |
| HDL-2 | − 0.08 | − 0.24* | − 0.41* |
| HDL-3 | − 0.10 | − 0.26* | − 0.42* |
| HDL-4 | − 0.10 | − 0.29* | − 0.45 |
| HDL-5 | 0.03 | − 0.18 | − 0.39 |
| HDL-6 | 0.04 | 0.18 | 0.33 |
| HDL-7 | 0.09 | 0.37 | 0.68 |
| HDL-8 | 0.13 | 0.54* | 0.92* |
| HDL-9 | 0.17 | 0.74* | 1.20* |
| HDL-10 | 0.07 | 0.18* | 0.29* |
| HDL-L | − 0.05 | − 0.12* | − 0.20* |
| HDL-I | 0.01 | 0.02 | 0.06 |
| HDL-S | 0.04 | 0.13* | 0.20* |
HDL-L, large HDL (from HDL-1 to 3); HDL-I, intermediate HDL (from HDL-4 to 7); HDL-S, small HDL (from HDL-8 to 10); CVD, cardiovascular diseases; CHD: coronary heart disease.
*Significant results after test correction (p < 0.004).
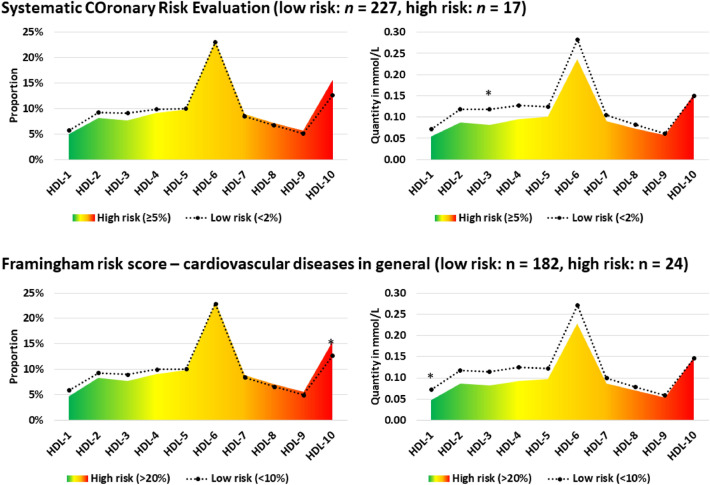
When comparing groups with low (< 2%) and high (≥ 5%) cardiovascular risk according to SCORE, no significant difference in the proportion of HDL subfractions was observed. The HDL-1 to 7 subfractions in mmol/L were significantly lower in the high-risk group compared with the low-risk group. The proportion of HDL subfractions did not differ significantly between the low (< 10%) and high (> 20%) cardiovascular risk groups based on FRSCVD. HDL-1, -4, and -5 subfractions in mmol/L were significantly lower in the high-risk group compared with the low-risk group. For FRSCHD, due to the low number of high-risk samples (n = 3), the analysis cannot be performed. See Fig. 3. for more details.
Figure 3.
Composition of the HDL subfraction profile (proportion in % and in mmol/L) by low- and high-risk status according to Systematic Coronary Risk Evaluation and Framingham risk score—cardiovascular diseases in general. *Significant results after test correction (p < 0.0013).
Trend analysis showed no significant association between HDL subfractions in mmol/L or % and cardiovascular categories in SCORE. A significant negative trend was measured for HDL-1 to 4, HDL-L, and -I in mmol/L among FRSCHD risk groups. Based on the analysis of the % proportions, HDL-1 to 3 and HDL-L showed a significantly negative trend, while HDL-9, -10, and HDL-S showed a significantly positive trend with the cardiovascular risk estimated by FRSCHD. Decreases in HDL-1, -3, -4, and HDL-L and -I subfractions were significantly associated with the increasing trend in cardiovascular risk estimated by FRSCVD. HDL-1 in % was the only one that showed a significant association with the risk estimated by FRSCVD. See Supplementary Tables 2 and 3 for more details.
Discussion
Recent guidelines for the treatment of dyslipidemia and CVDs focus mainly on LDL and do not include recommendations to increase HDL-C levels45. However, both the European SCORE risk calculator and the Framingham Risk Score calculator take HDL-C levels into account when assessing CVD risk and consistently show that increasing HDL-C levels (regardless of the subfraction profile) reduces the risk of developing CVDs in the future.
The present study is the first to investigate and compare the HDL subfraction profiles of the Hungarian general and Roma populations and the association of the HDL profile (based on ten subfractions) with the 10-year risk of CVD events estimated by the FRS and the SCORE algorithms. Although the sample size seems small, it is not significantly different from previous studies using the same methodology37,46–49. Identification and quantification of HDL subfractions need an expensive and time-consuming method, so careful identification of subjects who are included in the sample(s) is a crucial requirement in general. In our study, both the control samples and cases were selected from a sample of a complex survey with 832 subjects after careful evaluation of the results published previously43.
The HDL subfraction profiles of Roma and Hungarian general populations with the same HDL-C status (normal vs. reduced) were not significantly different suggesting that the reduced HDL-C status is associated with similar subfraction changes in both groups. The results of multi-adjusted linear regression analyses confirmed that significantly reduced levels of HDL-6 and -7 were associated with Roma ethnicity and all other subfractions were also lower than in the Hungarian general population, although not significantly. These results are not in full harmony with the findings of Hubková et al.41, who described reduced levels of HDL subfractions 8 to 10 (HDL-S) in Roma compared to the majority of the Slovak population. However, their analyses were based on the result of descriptive statistical analyses and did not adjust for other factors (such as age, sex, and other lipid parameters).
Continuing the analysis, we investigated how the cardiovascular risk estimated by the two most widely used risk algorithms in clinical practice (SCORE and FRS) are associated with changes in the HDL subfraction profile. HDL-1 to -3 and HDL-L in mmol/L showed an inverse significant association with SCORE-estimated cardiovascular risk. Elevated levels of HDL-1 to 6 and HDL-L and HDL-I subfractions expressed in mmol/L showed an inverse association with the FRS estimated 10-year risk of cardiovascular events.
HDL subfractions expressed in % showed no significant association with SCORE-estimated risk. HDL-1 to 3 subfractions and HDL-L expressed as a percentage had an FRS-estimated cardiovascular risk-reducing effect, whereas HDL-8 to 10 and HDL-S had an FRS-estimated cardiovascular risk-increasing effect.
Several methods are available for HDL separation, which are used with varying frequency in studies (gel electrophoresis49, nuclear magnetic resonance50, and ion mobility51). They are based on different physicochemical characteristics of the HDL molecules and separate the particles according to size, charge, density, etc. "Same" HDL subfractions isolated by different separation methods may contain HDL particles that differ greatly in their properties52. This phenomenon makes it considerably more difficult or even impossible to compare studies using different separation methods. Therefore, we aim to compare our present results with published results that have used the Lipoprint HDL® platform.
Currently, there is only one article49 known to have described the association between HDL subfractions and cardiovascular risk estimated by FRS. This study showed, similar to the present one, that a decrease in the representation of HDL-L is associated with a higher cardiovascular risk, but the association was not statistically significant. There are no known studies that have examined the association between SCORE-estimated cardiovascular risk and HDL subfraction profile. However, several studies have examined the association between HDL subfractions and cardiovascular events.
Goliasch and colleagues found that HDL-L showed an inverse association (OR = 0.48, 95% CI 0.31–0.74, p = 0.001), whereas HDL-I was a risk factor (OR = 1.81, 95% CI 1.26–2.60, p = 0.001) for myocardial infarction in people aged 40 years and younger46. Zhang and colleagues found a significant negative association between the prevalence and incidence of hypertension and the level and proportion of HDL-L, and a positive association between the level and proportion of HDL-S47. Xu and colleagues showed that the HDL-L levels and the percentage were significantly lower, while the HDL-S levels and percentage were significantly higher in the coronary artery disease (CAD) group compared with those in the non-CAD group48. Li et al. demonstrated that high HDL-L levels were significantly (p < 0.05) negatively associated with coronary artery severity as assessed by SYNTAX and Gensini scores. Furthermore, using a log-rank test, they demonstrated that there was a significant (p = 0.013) difference between high and low HDL-L subfraction groups in the analysis of event-free survival, but no significant difference between total HDL-C groups and medium or small HDL subfraction groups. In particular, the multivariate Cox proportional hazards model showed that high HDL-L levels were associated with a lower risk of major adverse cardiovascular events (HR = 0.531, 95% CI 0.295–0.959), independent of potential confounders37.
The HDL-I subfractions (HDL-4 to 7) accounted for more than half of the total HDL-C level, with HDL-6 being the most predominant. Decreased levels of these subfractions have been associated with several cardiovascular events and risk factors based on literature data. Munchova and colleagues published a paper53 in which they examined the distribution of HDL subfractions in 27 mildly hypercholesterolemic and 21 healthy controls and found that HDL-6 (and HDL-7) concentrations were significantly lower in the patient group. Ezhov and colleagues found similar results54 when they studied the association between HDL subfractions and coronary atherosclerosis in 120 men. Their results showed significantly decreased levels of HDL-6 and -7 subfractions in patients with coronary lesions and found that the concentration of intermediate HDL subfractions (from HDL-4 to HDL-7) was inversely related to the presence and number of affected coronary arteries in middle-aged men. Femlak et al.55 have shown that HDL-6 and intermediate HDL subfractions are significantly lower in patients with advanced T2DM than in healthy or newly diagnosed patients.
Results of the present study that HDL-L levels show an inverse association with CVD risk furthermore, a decrease in HDL-L and a consequent increase in HDL-S is associated with elevated cardiovascular risk are consistent with the literature37,42,56.
Our results suggest that a decrease in HDL-6 and -7 subfractions may play a role in the high CVD risk among the Roma in general40. The question of whether this is due to the genetic predisposition identified in previous studies among the Roma31,32,34 or to other environmental and lifestyle factors associated with the Roma ethnic group10–14 requires further research.
Our current study has its strengths and limitations. On the one hand, the accurate identification of ethnicity is a common challenge for studies like ours. Due to the criteria of sample selection used in our present study (individuals were excluded by the investigation protocol), the sample populations cannot be interpreted as representative ones for the Hungarian general or Roma population. Given that the Hungarian general population may also include Roma individuals, the impact of ethnic differences estimated in the study may be underestimated. The cardiovascular risk assessment models used in the present study include only a limited number of traditional cardiovascular risk factors (age, gender, smoking, diabetic status, blood pressure, and cholesterol levels) and do not consider all known ones. This represents a major limitation when applying these equations to genetically susceptible people such as the Roma. Since the calculation formula of the risk estimation models includes HDL-C levels (as a protective/risk-reducing factor), our results slightly overestimate the risk-reducing and underestimate the risk-increasing effect of the identified subfractions. One of the major limitations of the present study is the small sample size which may result in limited statistical power. Although our results showing the association between CVD risk identified by different scoring calculations and HDL subfractions profile are statistically significant even after Bonferroni correction further analyses on larger sample populations of different ethnicities would be useful to confirm our conclusions.
On the other hand, the present study has several strengths. It is the first which compares the HDL subfraction profile of the Hungarian general and Roma populations and investigates whether there are differences between the two populations with the same HDL status. It also examines the association of HDL subfraction profile (in mmol/l and %) with cardiovascular risk factors. The present study is the first one to investigate the association between SCORE-estimated cardiovascular risk and HDL subfractions and to confirm the existence of a strong relationship between the SCORE- and FRS-estimated CVD risk and the HDL subfractions profile.
In conclusion, we have identified the significantly reduced levels of HDL subfractions 6 and 7 among the Roma. Furthermore, the present study is the first one to demonstrate a statistically significant association between HDL subfraction profile and cardiovascular risk estimated by SCORE and FRS. The results of the present research, which show that certain HDL subfractions are significantly reduced in individuals of Roma origin and that reduced levels of these subfractions are associated with increased cardiovascular risk, suggest that the distribution of HDL subfractions also contributes to the overall unfavorable cardiovascular risk among the Roma. Since genetic and lifestyle/environmental factors contribute to HDL-C levels approximately equally, one can raise the question of whether differences in the HDL subfraction profile of the Roma are influenced by their genetic inheritance or environmental and lifestyle factors, which requires further research.
Supplementary Information
Acknowledgements
The authors thank Zsuzsa Peter for English proofreading.
Author contributions
Conceptualization, R.A.; methodology, R.A., and P.P.; data curation, formal analysis, and visualization, P.P.; investigation, Zs.K., J.S., I.S. and Gy.P.; writing—original draft preparation, P.P.; writing—review and editing, R.A.; supervision, R.A.; funding acquisition, R.A. All authors have read and agreed to the published version of the manuscript.
Funding
Open access funding provided by University of Debrecen. This project was co-financed by the European Regional Development Fund (GINOP-2.3.2-15-2016-00005), as well as by the Hungarian Academy of Sciences (TK2016-78). Project no. 135784 has also been implemented with the support provided by the National Research, Development, and Innovation Fund of Hungary, financed under the K_20 funding scheme. P.P. is a recipient of a fellowship from the ÚNKP-21-4 New National Excellence Program of the Ministry for Innovation and Technology from the source of the National Research, Development and Innovation Fund.
Data availability
The datasets generated and/or analyzed during the current study are not publicly available due to privacy/ethical restrictions but are available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-022-15192-9.
References
- 1.Cardiovascular diseases (CVDs) https://www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases-%28cvds%29 (2021).
- 2.Knowles JW, Ashley EA. Cardiovascular disease: The rise of the genetic risk score. PLoS Med. 2018;15:e1002546. doi: 10.1371/journal.pmed.1002546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Damen JA, et al. Prediction models for cardiovascular disease risk in the general population: systematic review. BMJ. 2016;353:i2416. doi: 10.1136/bmj.i2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.D'Agostino RB, Sr, et al. General cardiovascular risk profile for use in primary care: The Framingham Heart Study. Circulation. 2008;117:743–753. doi: 10.1161/CIRCULATIONAHA.107.699579. [DOI] [PubMed] [Google Scholar]
- 5.Conroy RM, et al. Estimation of ten-year risk of fatal cardiovascular disease in Europe: The SCORE project. Eur. Heart J. 2003;24:987–1003. doi: 10.1016/s0195-668x(03)00114-3. [DOI] [PubMed] [Google Scholar]
- 6.Dayimu A, et al. Trajectories of lipids profile and incident cardiovascular disease risk: A longitudinal cohort study. J. Am. Heart Assoc. 2019;8:e013479. doi: 10.1161/JAHA.119.013479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen Y, et al. Association of cardiovascular disease with premature mortality in the United States. JAMA Cardiol. 2019;4:1230–1238. doi: 10.1001/jamacardio.2019.3891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gheorghe A, et al. The economic burden of cardiovascular disease and hypertension in low- and middle-income countries: A systematic review. BMC Public Health. 2018;18:975. doi: 10.1186/s12889-018-5806-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Leal J, Luengo-Fernandez R, Gray A, Petersen S, Rayner M. Economic burden of cardiovascular diseases in the enlarged European Union. Eur. Heart J. 2006;27:1610–1619. doi: 10.1093/eurheartj/ehi733. [DOI] [PubMed] [Google Scholar]
- 10.Skodova Z, et al. Psychosocial factors of coronary heart disease and quality of life among Roma coronary patients: A study matched by socioeconomic position. Int. J. Public Health. 2010;55:373–380. doi: 10.1007/s00038-010-0153-4. [DOI] [PubMed] [Google Scholar]
- 11.Janevic T, Jankovic J, Bradley E. Socioeconomic position, gender, and inequalities in self-rated health between Roma and non-Roma in Serbia. Int. J. Public Health. 2012;57:49–55. doi: 10.1007/s00038-011-0277-1. [DOI] [PubMed] [Google Scholar]
- 12.Sedlakova D. Low socioeconomic status and unhealthy lifestyle lead to high morbidity in young Roma of East Slovakia. Cent. Eur. J. Public Health. 2014;22(Suppl):S3–5. doi: 10.21101/cejph.a4008. [DOI] [PubMed] [Google Scholar]
- 13.Voko Z, et al. Does socioeconomic status fully mediate the effect of ethnicity on the health of Roma people in Hungary? J Epidemiol Community Health. 2009;63:455–460. doi: 10.1136/jech.2008.079715. [DOI] [PubMed] [Google Scholar]
- 14.Sarvary A, et al. Socioeconomic status, health related behaviour, and self-rated health of children living in Roma settlements in Hungary. Cent. Eur. J. Public Health. 2019;27:24–31. doi: 10.21101/cejph.a4726. [DOI] [PubMed] [Google Scholar]
- 15.Health status of the Roma population Data collection in the Member States of the European Union (European Union, 2014).
- 16.Babinska I, et al. Is the cardiovascular risk profile of people living in Roma settlements worse in comparison with the majority population in Slovakia? Int. J. Public Health. 2013;58:417–425. doi: 10.1007/s00038-013-0463-4. [DOI] [PubMed] [Google Scholar]
- 17.Dobranici M, Buzea A, Popescu R. The cardiovascular risk factors of the Roma (gypsies) people in Central-Eastern Europe: A review of the published literature. J. Med Life. 2012;5:382–389. [PMC free article] [PubMed] [Google Scholar]
- 18.Weiss E, Japie C, Balahura AM, Bartos D, Badila E. Cardiovascular risk factors in a Roma sample population from Romania. Rom. J. Intern. Med. 2018;56:193–202. doi: 10.2478/rjim-2018-0010. [DOI] [PubMed] [Google Scholar]
- 19.Papon C, Delarche N, Le Borgne C, Bauduer F. Assessment of cardiovascular risk factors in a Roma community from Southwestern France. Am. J. Hum. Biol. 2017;29:e22895. doi: 10.1002/ajhb.22895. [DOI] [PubMed] [Google Scholar]
- 20.Zeljko HM, et al. Age trends in prevalence of cardiovascular risk factors in Roma minority population of Croatia. Econ. Hum. Biol. 2013;11:326–336. doi: 10.1016/j.ehb.2012.02.007. [DOI] [PubMed] [Google Scholar]
- 21.Piko P, Kosa Z, Sandor J, Adany R. Comparative risk assessment for the development of cardiovascular diseases in the Hungarian general and Roma population. Sci. Rep. 2021;11:3085. doi: 10.1038/s41598-021-82689-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kosa Z, et al. Prevalence of metabolic syndrome among Roma: a comparative health examination survey in Hungary. Eur. J. Public Health. 2015;25:299–304. doi: 10.1093/eurpub/cku157. [DOI] [PubMed] [Google Scholar]
- 23.Piko P, Dioszegi J, Sandor J, Adany R. Changes in the prevalence of metabolic syndrome and its components as well as in relevant preventive medication between 2006 and 2018 in the Northeast Hungarian population. J. Pers. Med. 2021;11:52. doi: 10.3390/jpm11010052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Macejova Z, et al. The Roma population living in segregated settlements in Eastern Slovakia has a higher prevalence of metabolic syndrome, kidney disease, viral hepatitis B and E, and some parasitic diseases compared to the majority population. Int. J. Environ. Res. Public Health. 2020;17:3112. doi: 10.3390/ijerph17093112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hubkova B, et al. Assessment of clinical biochemical parameters in Roma minority residing in eastern Slovakia compared with the majority population. Cent. Eur. J. Public Health. 2014;22(Suppl):S12–17. doi: 10.21101/cejph.a3895. [DOI] [PubMed] [Google Scholar]
- 26.Allard-Ratick MP, et al. HDL: Fact, fiction, or function? HDL cholesterol and cardiovascular risk. Eur. J. Prev. Cardiol. 2021;28:166–173. doi: 10.1177/2047487319848214. [DOI] [PubMed] [Google Scholar]
- 27.Mineo C, Deguchi H, Griffin JH, Shaul PW. Endothelial and antithrombotic actions of HDL. Circ. Res. 2006;98:1352–1364. doi: 10.1161/01.RES.0000225982.01988.93. [DOI] [PubMed] [Google Scholar]
- 28.Navab M, Anantharamaiah GM, Fogelman AM. The role of high-density lipoprotein in inflammation. Trends Cardiovasc. Med. 2005;15:158–161. doi: 10.1016/j.tcm.2005.05.008. [DOI] [PubMed] [Google Scholar]
- 29.Brites F, Martin M, Guillas I, Kontush A. Antioxidative activity of high-density lipoprotein (HDL): Mechanistic insights into potential clinical benefit. BBA Clin. 2017;8:66–77. doi: 10.1016/j.bbacli.2017.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kuvin JT, et al. A novel mechanism for the beneficial vascular effects of high-density lipoprotein cholesterol: Enhanced vasorelaxation and increased endothelial nitric oxide synthase expression. Am. Heart J. 2002;144:165–172. doi: 10.1067/mhj.2002.123145. [DOI] [PubMed] [Google Scholar]
- 31.Piko P, Fiatal S, Kosa Z, Sandor J, Adany R. Genetic factors exist behind the high prevalence of reduced high-density lipoprotein cholesterol levels in the Roma population. Atherosclerosis. 2017;263:119–126. doi: 10.1016/j.atherosclerosis.2017.05.028. [DOI] [PubMed] [Google Scholar]
- 32.Piko P, Fiatal S, Kosa Z, Sandor J, Adany R. Data to genetic risk assessment on high-density cholesterol level associated polymorphisms in Hungarian general and Roma populations. Data Brief. 2017;14:354–359. doi: 10.1016/j.dib.2017.07.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Piko P, Fiatal S, Kosa Z, Sandor J, Adany R. Generalizability and applicability of results obtained from populations of European descent regarding the effect direction and size of HDL-C level-associated genetic variants to the Hungarian general and Roma populations. Gene. 2019;686:187–193. doi: 10.1016/j.gene.2018.11.067. [DOI] [PubMed] [Google Scholar]
- 34.Piko P, et al. The effect of haplotypes in the CETP and LIPC genes on the triglycerides to HDL-C ratio and its components in the Roma and Hungarian general populations. Genes (Basel) 2020;11:56. doi: 10.3390/genes11010056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Barbagallo CM, et al. Lipoprotein profile and high-density lipoproteins: Subfractions distribution in centenarians. Gerontology. 1998;44:106–110. doi: 10.1159/000021992. [DOI] [PubMed] [Google Scholar]
- 36.Generoso G, et al. High-density lipoprotein-cholesterol subfractions and coronary artery calcium: The ELSA-Brasil study. Arch. Med. Res. 2019;50:362–367. doi: 10.1016/j.arcmed.2019.10.006. [DOI] [PubMed] [Google Scholar]
- 37.Li JJ, et al. Large HDL subfraction but not HDL-C is closely linked with risk factors, coronary severity and outcomes in a cohort of nontreated patients with stable coronary artery disease: A prospective observational study. Medicine (Baltimore) 2016;95:e2600. doi: 10.1097/MD.0000000000002600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Maeda S, et al. Associations between small dense LDL, HDL subfractions (HDL2, HDL3) and risk of atherosclerosis in Japanese-Americans. J. Atheroscler. Thromb. 2012;19:444–452. doi: 10.5551/jat.11445. [DOI] [PubMed] [Google Scholar]
- 39.Oravec S, et al. HDL subfractions analysis: A new laboratory diagnostic assay for patients with cardiovascular diseases and dyslipoproteinemia. Neuro Endocrinol Lett. 2011;32:502–509. [PubMed] [Google Scholar]
- 40.Arsenault BJ, et al. HDL particle size and the risk of coronary heart disease in apparently healthy men and women: The EPIC-Norfolk prospective population study. Atherosclerosis. 2009;206:276–281. doi: 10.1016/j.atherosclerosis.2009.01.044. [DOI] [PubMed] [Google Scholar]
- 41.Hubkova B, et al. Lipoprotein-cholesterol fractions in marginalized roma versus majority population. Int. J. Environ. Res. Public Health. 2018;15:81. doi: 10.3390/ijerph15010081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xu RX, et al. High-density lipoprotein subfractions in relation with the severity of coronary artery disease: A Gensini score assessment. J. Clin. Lipidol. 2015;9:26–34. doi: 10.1016/j.jacl.2014.11.003. [DOI] [PubMed] [Google Scholar]
- 43.Adany R, et al. Prevalence of insulin resistance in the Hungarian general and Roma populations as defined by using data generated in a complex health (interview and examination) survey. Int. J. Environ. Res. Public Health. 2020;17:4833. doi: 10.3390/ijerph17134833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) Third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III) final report. Circulation. 2002;106:3143–3421. [PubMed] [Google Scholar]
- 45.Mach F, et al. 2019 ESC/EAS guidelines for the management of dyslipidaemias: Lipid modification to reduce cardiovascular risk. Eur. Heart J. 2020;41:111–188. doi: 10.1093/eurheartj/ehz455. [DOI] [PubMed] [Google Scholar]
- 46.Goliasch G, et al. Relative importance of different lipid risk factors for the development of myocardial infarction at a very young age (<= 40 years of age) Eur. J. Clin. Invest. 2012;42:631–636. doi: 10.1111/j.1365-2362.2011.02629.x. [DOI] [PubMed] [Google Scholar]
- 47.Zhang Y, et al. Distribution of high-density lipoprotein subfractions and hypertensive status a cross-sectional study. Medicine. 2015;94:e1912. doi: 10.1097/MD.0000000000001912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xu RX, et al. Analysis of lipoprotein subfractions in Chinese Han patients with stable coronary artery disease. Heart Lung Circ. 2015;24:1203–1210. doi: 10.1016/j.hlc.2015.05.002. [DOI] [PubMed] [Google Scholar]
- 49.Vekic J, Topic A, Zeljkovic A, Jelic-Ivanovic Z, Spasojevic-Kalimanovska V. LDL and HDL subclasses and their relationship with Framingham risk score in middle-aged Serbian population. Clin. Biochem. 2007;40:310–316. doi: 10.1016/j.clinbiochem.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 50.Teis A, et al. Particle size and cholesterol content of circulating HDL correlate with cardiovascular death in chronic heart failure. Sci. Rep. 2021;11:3141. doi: 10.1038/s41598-021-82861-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Musunuru K, et al. Ion mobility analysis of lipoprotein subfractions identifies three independent axes of cardiovascular risk. Arterioscler. Thromb. Vasc. Biol. 2009;29:1975–1980. doi: 10.1161/ATVBAHA.109.190405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yamashita S, et al. Distinct differences in lipoprotein particle number evaluation between GP-HPLC and NMR: Analysis in dyslipidemic patients administered a selective PPAR alpha modulator, pemafibrate. J. Atheroscler. Thromb. 2021;28:974–996. doi: 10.5551/jat.60764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Muchova J, et al. High density lipoprotein subfractions and paraoxonase 1 in children. Acta Biochim. Pol. 2016;63:555–563. doi: 10.18388/abp.2015_1230. [DOI] [PubMed] [Google Scholar]
- 54.Ezhov M, et al. Hdl and Ldl subfractions and coronary atherosclerosis in adult males. Atherosclerosis. 2015;241:E108–E108. doi: 10.1016/j.atherosclerosis.2015.04.378. [DOI] [Google Scholar]
- 55.Femlak M, Gluba-Brzozka A, Franczyk B, Rysz J. Diabetes-induced alterations in HDL subfractions distribution. Curr. Pharm. Des. 2020;26:3341–3348. doi: 10.2174/1381612825666190227224246. [DOI] [PubMed] [Google Scholar]
- 56.Zhang Y, et al. HDL subfractions and very early CAD: novel findings from untreated patients in a Chinese cohort. Sci. Rep. 2016;6:30741. doi: 10.1038/srep30741. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated and/or analyzed during the current study are not publicly available due to privacy/ethical restrictions but are available from the corresponding author on reasonable request.