Abstract
Introduction
Many patients with type 2 diabetes mellitus (T2DM) suffer from complications that impose substantial burdens on prognosis and medical costs. Accumulating evidence has demonstrated the clinical benefit of sodium–glucose cotransporter 2 inhibitors (SGLT2i) on cardiovascular and renal complications. However, the health economic impact of SGLT2i remains unclear. The aim of this study was to evaluate the cost-effectiveness of initiating antidiabetic therapy with an SGLT2i using Japanese real-world data.
Methods
We constructed a natural history model incorporating heart failure (HF), myocardial infarction, stroke, chronic kidney disease, and end-stage renal disease (ESRD) as complications. The target population comprised patients with T2DM who newly initiated their first oral glucose-lowering drugs. By using a population-based microsimulation, we estimated the 10-year medical costs in Japanese yen (JPY) and outcomes (hospitalization for/development of complications and quality-adjusted life years [QALY]) for patients who initiated antidiabetic therapy with an SGLT2i or conventional therapy. Sensitivity analyses included a probabilistic sensitivity analysis (PSA) with 1,000,000 iterations.
Results
In the base-case analysis, the total medical cost per person was JPY 1,638,806 versus JPY 1,825,033 and the QALYs were 8.732 versus 8.513 for the SGLT2i strategy versus the conventional strategy, respectively. Thus, initiating treatment with an SGLT2i was dominant, more effective (QALY gain), and lower cost. When treating 10,000 patients, the SGLT2i strategy would reduce all-cause deaths by 410 (552 vs 962), HF events by 201 (897 vs 1098), and ESRD events by 16 (16 vs 32) versus the conventional strategy. The PSA revealed that the probability of dominance for initiating SGLT2i therapy was 90.5%, demonstrating the robustness of the results.
Conclusion
Our results suggest that initiating T2DM treatment with SGLT2i, aimed at managing cardiovascular and renal complications from the early stages of diabetes, can improve the clinical outcome and reduce cost burden of T2DM.
Keywords: Type 2 diabetes mellitus, SGLT2 inhibitor, Cost-effectiveness, Diabetic complications
Key Summary Points
| Why carry out this study? |
| Heart failure and chronic kidney disease, which are the most frequent initial manifestations of patients in the early stages of diabetes, impose substantial clinical and economic burdens. |
| Despite the benefit of sodium–glucose cotransporter 2 inhibitors (SGLT2i) on primary and secondary prevention of these complications, the health economic impact of SGLT2i remains unclear. |
| What was learned from the study? |
| Initiating diabetes treatment with an SGLT2i decreased medical costs and increased the earned-QALY compared with conventional treatment. |
| The results demonstrate the economic benefit of initiating therapy with an SGLT2i for patients with type 2 diabetes. |
Introduction
More than 400 million people live with diabetes worldwide, and each year 4 million deaths are caused by diabetes [1, 2]. The prevalence of diabetes is expected to increase to 578 million by 2030 and 700 million by 2045 as the global population ages [1]. The burden of diabetes is mainly caused by its complications. In a US registry, only 6% of patients had isolated type 2 diabetes mellitus (T2DM), the rest had one or multiple complications [3, 4]. The development of complications, including cardiovascular and renal diseases, exacerbates the prognosis of patients with T2DM [5, 6].
Diabetes also imposes a significant financial burden. The global health expenditure due to diabetes in 2015 was estimated to be 673 billion USD [1], equivalent to approximately 12% of global health expenditure, and it is predicted to reach 802 billion USD by 2045. It is noteworthy that a substantial proportion of the total expenditure for diabetes was due to the treatment of complications [1]. Given the clinical and economic burden of diabetes, a treatment strategy that can prevent or slow the progression of complications from the early stage of diabetes is of particular importance.
Several cardiovascular outcome trials of sodium–glucose cotransporter 2 inhibitors (SGLT2i) have demonstrated that they reduce cardiovascular and renal events, including hospitalization for heart failure (HF), in patients with T2DM [7–9]. A recent multinational observational study confirmed the importance of prevention and management of HF and chronic kidney disease (CKD) from the early stage of diabetes by showing that HF and CKD are the most frequent initial complications among patients without a history of cardiovascular and renal diseases [5]. Furthermore, several large clinical trials have shown protective effects of SGLT2i on HF and renal events [10–12]. Based on these findings, guidelines on diabetes developed by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) recommend the use of SGLT2i in patients with diabetes at high risk of atherosclerotic cardiovascular disease, HF, or CKD [13–16].
Japanese real-world data (RWD) have further provided evidence of the frequent manifestation of HF and CKD in the early stages of T2DM [5, 17, 18] and clinical benefits of SGLT2i in these patients [19, 20]. Unlike the ADA and EASD guidelines, the Japanese clinical practice guidelines do not recommend specific pharmacotherapies for T2DM, and instead endorse the selection of drugs on the basis of the patient’s disease condition. Although the number of patients initiating antidiabetic therapy with an SGLT2i is increasing, the proportion is still below 10% [17, 21]. SGLT2i are also associated with lower total healthcare cost compared with other glucose-lowering drugs (GLDs) in real-world settings [22], although the cost-effectiveness of SGLT2i in Japanese clinical settings remains unclear.
In the present study, we constructed a health economic model that considered T2DM-related complications to assess the economic effectiveness of initiating antidiabetic therapy with an SGLT2i in Japanese clinical settings. The results are expected to contribute to better treatment strategies for T2DM.
Methods
Ethical Statement
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Target Population
The model population consisted of patients with T2DM who had not previously been treated with oral GLDs.
Intervention and Comparators
Two treatment strategies were assessed: (1) SGLT2i strategy and (2) conventional treatment strategy. For the SGLT2i strategy, antidiabetic therapy was initiated as an SGLT2i. In the conventional strategy, oral GLDs were prescribed as initial therapy on the basis of the results of a previous study that examined the real-world prescription patterns in Japanese patients with T2DM [17], in which antidiabetic therapy was initiated as a dipeptidyl peptidase 4 inhibitors (DPP4i) in 63.5% of patients, biguanide in 9.3%, α-glucosidase inhibitor in 5.1%, sulfonylurea in 4.9%, SGLT2i in 2.6%, and thiazolidinedione in 1.8% [17].
Model Framework
The state transition model was constructed using TreeAge Pro 2020 (TreeAge Software, Inc.), which predicts the costs and outcomes associated with hospitalization for HF, myocardial infarction (MI), stroke, and end-stage renal disease (ESRD) in patients in the early stages of T2DM. A population-based Monte Carlo simulation was conducted to incorporate different patterns of clinical characteristics, including age, sex, and medical history.
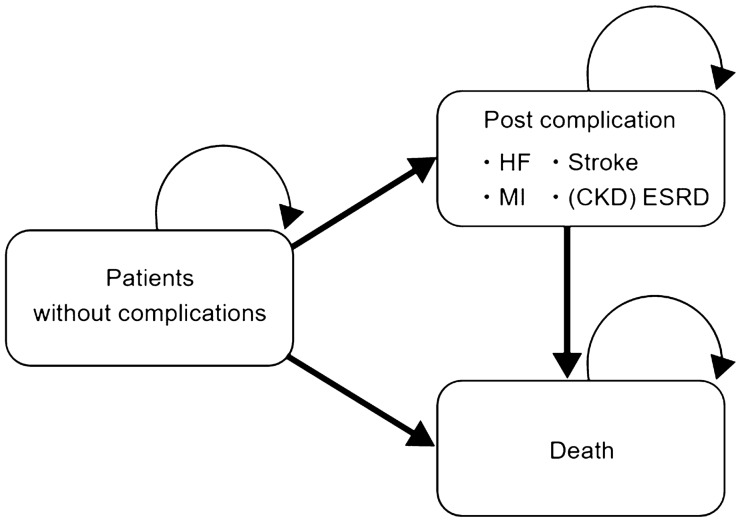
The model consisted of the following three states (Fig. 1): stable (without complications); occurrence of a complication (post complication); and death. Four complications (HF, MI, stroke, and ESRD) were separately incorporated into the model. Once a patient develops a complication, the patient is moved to the “post complication” node and does not develop another complication. This is a conservative assumption for the SGLT2i strategy because it protected against cardiovascular and renal events in previous studies. The effect of CKD was also included in the model. The development of CKD did not affect the incidence rate of complications, except for ESRD, and the incidence rate of ESRD would not be considered before the occurrence of CKD. The cycle length was set to 1 month. In the base-case analysis, the time horizon was set to 10 years.
Fig. 1.
Model structure. CKD chronic kidney disease, ESRD end-stage renal disease, HF heart failure, MI myocardial infarction
The analyses were conducted from the healthcare payer perspective. Medical costs covered by the public health insurance system were included in the analysis. Healthcare costs and outcome were discounted at a rate of 2%, as recommended in Japanese guidelines for cost-effectiveness analysis [23].
The primary outcome of this study was quality-adjusted life years (QALY). The incremental cost-effectiveness ratio (ICER) was calculated if applicable. We also captured the numbers of death, hospitalization for complications, and the development of complications to estimate the overall impact in the whole population.
Data Sources
Cost of Oral Glucose-Lowering Drugs
For the SGLT2i strategy, the average daily cost of six SGLT2i available in Japan was used (Japanese yen [JPY] 189.6/day, Table 1). For the conventional strategy, the daily cost was estimated by calculating the weighted average of oral GLDs classified by drug categories, based on the reported distribution of initial oral GLDs in Japan [17]. The resultant daily cost for conventional therapy was JPY 125.9/day (Table 1).
Table 1.
Daily cost of glucose-lowering drugs (JPY)
| Drug | Cost/day range | Average cost/day |
|---|---|---|
| SGLT2i | 180.0–195.2 | 189.6 |
| Other GLD | 125.9 | |
| DPP4i | 115.0–170.1 | 137.6 |
| Sulfonylurea | 5.7–27.4 | 14.1 |
| Thiazolidinedione | 19.9–58.4 | 39.2 |
| Biguanide | 14.4–19.6 | 17.0 |
| α-Glucosidase inhibitor | 31.8–104.7 | 67.5 |
As of March 2021
DPP4i dipeptidyl peptidase 4 inhibitor, GLD glucose-lowering drug, JPY Japanese yen, SGLT2i, sodium–glucose cotransporter 2 inhibitor
Treatment Cost
All cost data were derived from Japanese studies. The treatment costs of each HF, MI, and stroke event were derived from published studies identified via a systematic literature review (Table 2). For cardiovascular diseases, the initial costs (up to 1 year from disease onset) and chronic costs (subsequent years) were adopted separately. Among eight articles included in our analysis, four [24–27] used claim data for cost calculation and four [28–31] used hospital-based data. The initial costs of HF, MI, and stroke were JPY 770,428, JPY 2,156,290, and JPY 1,440,107, respectively, and the annual maintenance cost was JPY 1,207,920, JPY 900,432, and JPY 1,160,562, respectively. For CKD and ESRD, we used monthly costs of JPY 30,000 and JPY 350,000, respectively, based on prior studies [32–35].
Table 2.
Direct cost inputs (JPY) applied in the base-case and sensitivity analyses
| Variable | Mean | SEa | Source |
|---|---|---|---|
| Hospitalization for HF | |||
| Base case | 770,428 | 154,086 | [24] |
| Lower | 770,428 | – | [24] |
| Upper | 991,240 | – | [25] |
| HF maintenance | |||
| Base case | 1,207,920 | 845,544 | [29] |
| Lower | 160,000 | – | [29] |
| Upper | 5,400,000 | – | [29] |
| MI event | |||
| Base case | 2,156,290 | 431,258 | [26] |
| Lower | 991,078 | – | [26] |
| Upper | 2,785,000 | – | [27] |
| MI maintenance | |||
| Base case | 900,432 | 225,108 | [26] |
| Lower | 365,760 | – | [27] |
| Upper | 1,115,750 | – | [30] |
| Stroke event | |||
| Base case | 1,440,107 | 288,021 | [26] |
| Lower | 298,855 | – | [27] |
| Upper | 1,596,639 | – | [28] |
| Stroke maintenance (annually) | |||
| Base case | 1,160,562 | 290,141 | [26] |
| Lower | − 25% | – | [31] |
| Upper | + 25% | – | |
| ESRD maintenance (monthly) | |||
| Base case | 350,000 | Assumed from [34, 35] | |
| Lower | − 25% | ||
| Upper | + 25% | ||
| CKD maintenance (monthly) | |||
| Base case | 30,000 | Assumed from [32, 33] | |
| Lower | − 25% | ||
| Upper | + 25% | ||
aGamma distribution was applied for the probabilistic sensitivity analysis
CKD chronic kidney disease, ESRD end-stage renal disease, HF heart failure, JPY Japanese yen, MI myocardial infarction, SE standard error
Clinical Outcome Data
Incidence rates of hospitalization for HF, MI, and stroke were derived from a study using an administrative claims database of enrollees in the municipal government health insurance program in Shizuoka prefecture [17]. Data were extracted from 23,340 patients (mean follow-up period of 2.8 years). The incidence rates were 10.4/1000 person-years for HF hospitalization, 4.1/1000 person-years for MI hospitalization, and 12.9/1000 person-years for stroke hospitalization. The risk ratio for the SGLT2i strategy was obtained from the Japanese subset of the CVD-REAL 2 study by comparing the two-parameterized risk of SGLT2i use and other GLD use (Table 3) [19]. The CVD-REAL 2 study was a large-scale real-world study of SGLT2i that examined the cardiovascular outcomes of patients with T2DM initiating an SGLT2i or other oral GLD, including DPP4i. The risk ratio of the SGLT2i strategy versus other oral GLD was 0.75 for HF hospitalization, 0.73 for MI hospitalization, 0.66 for stroke hospitalization, and 0.56 for all-cause death. The incidence of events over the modeled horizon was estimated by applying exponential survival distributions parameterized utilizing the Japanese-specific event rates reported in CVD-REAL 2 [19]. For CKD, Komuro et al. [20] reported an incidence rate of CKD of 3.1/1000 person-years among patients prescribed an SGLT2i and 7.0/1000 person-years among patients prescribed other oral GLDs. Fukuma et al. [36] reported that the incidence of progressing from CKD to ESRD was 13.3/1000 person-years among patients with diabetes. Komuro et al. [20] reported that the hazard ratio for the onset of CKD between SGLT2i and oral GLD was 0.45. These parameters were adopted in our model. Additionally, information on patient-associated risk factors and their impact on hospitalizations for HF, MI, and stroke were derived from Japanese RWD [17], and incorporated into the Monte Carlo simulation (Table 4).
Table 3.
Effect of the SGLT2i strategy versus the conventional GLD strategy on all-cause death, cardiovascular outcomes, and renal outcomes
| Outcome | Risk ratio | Rate for SGLT2ia | Rate for other oral GLDsa | Source |
|---|---|---|---|---|
| All-cause death | 0.56 | 1.56 × 10−5 | 2.79 × 10−5 | [19] |
| HF | 0.75 | 1.92 × 10−5 | 2.55 × 10−5 | |
| MI | 0.73 | 3.01 × 10−6 | 4.11 × 10−6 | |
| Stroke | 0.66 | 8.49 × 10−6 | 1.29 × 10−5 |
| Outcome | HR | Event rate for SGLT2i (/1000 person-years) | Event rate for other oral GLD (/1000 person-years) | Source |
|---|---|---|---|---|
| CKD | 0.45 | 3.1 | 7.0 | [20] |
CKD chronic kidney disease, GLD glucose-lowering drug, HF heart failure, HR hazard ratio, MI myocardial infarction, SGLT2i sodium–glucose cotransporter 2 inhibitor
aThe incidence of cardiovascular events over the modeled horizon was estimated by applying an exponential survival distribution parameterized utilizing Japanese-specific event rates [19]
Table 4.
Hazard ratios for hospitalization for HF, MI or stroke according to patient characteristics
| Characteristic | HF | MI | Stroke | |||
|---|---|---|---|---|---|---|
| sHR | 95% CI | sHR | 95% CI | sHR | 95% CI | |
| Age group | ||||||
| 40–65 years | 1 | Reference | 1 | Reference | 1 | Reference |
| 66–74 years | 1.40 | 1.11–1.75 | 1.04 | 0.75–1.45 | 1.23 | 1.01–1.50 |
| 74–85 years | 2.84 | 2.23–3.6 | 2.17 | 1.51–3.11 | 2.49 | 2.01–3.08 |
| ≥ 85 years | 5.68 | 4.4–7.33 | 3.88 | 2.60–5.78 | 4.45 | 3.53–5.61 |
| Sex | ||||||
| Male | 1 | Reference | 1 | Reference | 1 | Reference |
| Female | 0.69 | 0.59–0.81 | 0.54 | 0.42–0.70 | 0.68 | 0.59–0.79 |
| Baseline comorbidities | ||||||
| Hypertension | 1.31 | 1.1–1.56 | 1.04 | 0.80–1.37 | 1.39 | 1.17–1.63 |
| Cardiovascular disease | 1.31 | 1.11–1.54 | 1.60 | 1.24–2.07 | 1.88 | 1.63–2.18 |
| Hyperlipidemia | 0.72 | 0.62–0.84 | 0.76 | 0.59–0.97 | 0.84 | 0.73–0.96 |
| Initiated on combination therapy | 1.55 | 1.25–1.93 | 1.54 | 1.10–2.16 | 1.41 | 1.16–1.73 |
All data were derived from [17]
CI confidence interval, HF heart failure, MI myocardial infarction, sHR subdistribution hazard ratio
Quality of Life (Utility) Scores
The weighted average of the source studies identified through the systematic review was applied as a baseline utility score (Table 5). The baseline utility value was set to 0.917 and the magnitudes of utility decrements for HF, MI, and stroke were 0.101, 0.055, and 0.164, respectively.
Table 5.
Utility
| Variable | Mean | SE | Source |
|---|---|---|---|
| Utility: T2DM without complications | 0.917 | 0.183a | [57–59] |
| Disutility of HFb | 0.101 | 0.031 | [38] |
| Disutility of MI | 0.055 | 0.006 | [38] |
| Disutility of stroke | 0.164 | 0.030 | [38] |
HF heart failure, MI myocardial infarction, SE standard error, T2DM type 2 diabetes mellitus
aThe SE was assumed to be 20% of mean for the probabilistic sensitivity analysis using a normal distribution
bThe published value was decreased by 6.75% to account for history of HF at baseline in CVD-REAL 2
Sensitivity Analyses
A one-way sensitivity analysis was carried out to investigate the effect of each parameter included in the analysis. The net monetary benefit (NMB) was used to represent the results, in which the incremental QALY was converted into an incremental monetary benefit using the willingness-to-pay value of JPY 5,000,000/QALY gained. In order to explore the impact of joint uncertainty in all model parameters, a probabilistic sensitivity analysis (PSA) with 1,000,000 iterations was performed using the Monte Carlo method [37]. Appropriate statistical distributions were assigned to each model input. For each input value, the chosen distribution was parameterized on the basis of the available information (e.g., standard deviation and confidence intervals). In addition, we applied four scenario analyses with conservative approaches under the following conditions: (1) SGLT2i prevents death only after admission for a complication (HF, MI, stroke, and ESRD); (2) there is no treatment cost for HF, MI, and stroke in the chronic phase (later than 1 year after admission); (3) all patients were in the lowest risk category with no risk factors; and (4) all three scenarios combined.
Results
Base-Case Results
The expected total costs were JPY 1,638,806 for the SGLT2i strategy and JPY 1,825,033 for the conventional strategy. Thus, initiating an SGLT2i saved JPY 186,277. The expected QALYs were 8.732 and 8.513 for the SGLT2i and conventional strategies, respectively. These results indicate that the SGLT2i strategy was dominant, being associated with less cost and greater effectiveness, in the base-case scenario (Table 6). The ICER value was not calculated because the SGLT2i strategy was thought to be dominant. According to the breakdown of costs, although the antidiabetic medication costs were greater in the SGLT2i strategy by JPY 206,130 compared with the conventional strategy (SGLT2i strategy vs conventional strategy: JPY 548,767 vs JPY 342,637, respectively), the complication-related costs were lower with the SGLT2i strategy by JPY 392,357 (JPY 1,090,039 vs JPY 1,482,396, respectively) (Fig. 2). All of the complication-related costs examined were lower with the SGLT2i strategy than with the conventional strategy.
Table 6.
Results of the base-case analysis: cost–utility analysis, discounted and undiscounted, across a 10-year horizon
| SGLT2i strategy | Conventional strategy | Differencea | |
|---|---|---|---|
| Discounted (2%) | |||
| Cost (JPY) | 1,638,806 | 1,825,033 | − 186,227 |
| QALY | 8.732 | 8.513 | 0.219 |
| LY | 8.836 | 8.652 | 0.184 |
| Undiscounted | |||
| Cost (JPY) | 1,844,213 | 2,055,413 | − 211,200 |
| QALY | 9.602 | 9.349 | 0.253 |
| LY | 9.724 | 9.511 | 0.212 |
LY life years, QALY quality-adjusted life years, SGLT2i sodium–glucose cotransporter 2
aSGLT2i strategy − conventional strategy
Fig. 2.
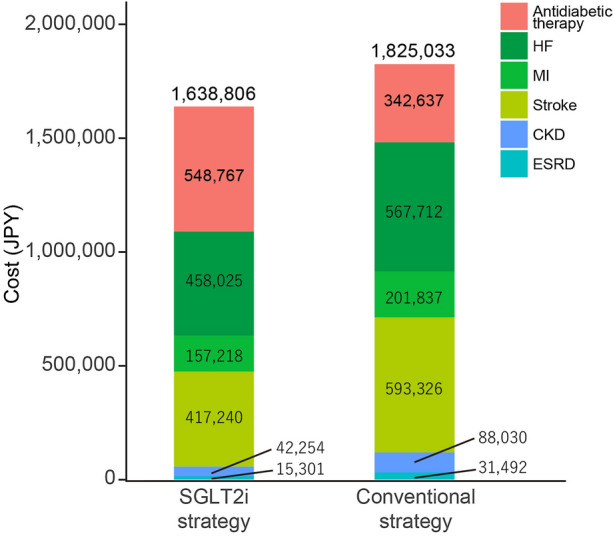
Breakdown of costs. CKD chronic kidney disease, ESRD end-stage renal disease, HF heart failure, JPY Japanese yen, MI myocardial infarction, SGLT2i sodium–glucose cotransporter 2 inhibitor
The SGLT2i strategy saved 410 deaths per 10,000 patients over the 10-year horizon (SGLT2i strategy vs conventional strategy: 552 vs 962 deaths) (Table 7). SGLT2i also reduced the development of all complications, as follows: 201 HF events (897 vs 1098, respectively), 83 MIs (305 vs 389, respectively), 306 strokes (759 vs 1065, respectively), and 16 ESRDs (16 vs 32, respectively) per 10,000 patients. The total cost saving associated with the SGLT2i strategy was JPY 1.86 billion (JPY 16.39 billion vs JPY 18.25 billion) per 10,000 patients versus the conventional strategy.
Table 7.
Results of the base-case analysis: incidence of events/10,000 person over the 10-year horizon
| Outcome | SGLT2i strategy | Conventional strategy | Differencea |
|---|---|---|---|
| All-cause deaths | 552 | 962 | − 410 |
| HF | 897 | 1098 | − 201 |
| MI | 305 | 389 | − 83 |
| Stroke | 759 | 1065 | − 306 |
| ESRD | 16 | 32 | − 16 |
| Total cost (billion JPY) | |||
| Discounted (2%) | 16.388 | 18.250 | − 1.862 |
| Undiscounted | 18.442 | 20.554 | − 2.112 |
ESRD end-stage renal disease, HF heart failure, JPY Japanese yen, MI myocardial infarction, SGLT2i sodium–glucose cotransporter 2
aSGLT2i strategy − conventional strategy
Sensitivity Analyses
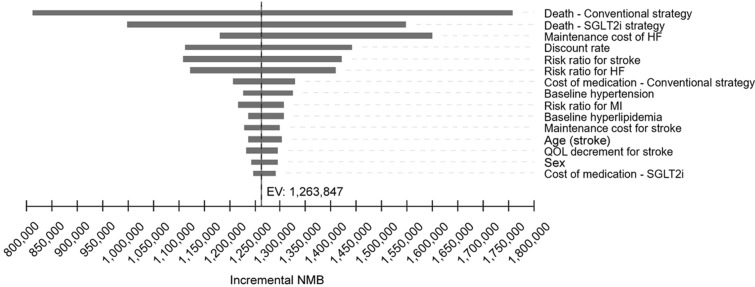
A tornado plot of the one-way sensitivity analysis is shown in Fig. 3. In all analyses, the NMB values were positive, which indicates that the SGLT2i strategy is the cost-effective option. The incidence rate of all-cause death in both strategies, followed by the cost of HF in the chronic phase, had the greatest impact on the results. Broad range of HF maintenance cost (JPY 160,000 to JPY 5,400,000), largely due to small sample size of the original study [29], may account for its large impact on the results.
Fig. 3.
One-way sensitivity analysis. The tornado plot shows the incremental cost-effectiveness ratio for each model input parameter. EV equivalent variation, HF heart failure, MI myocardial infarction, NMB net monetary benefit, QOL quality of life, SGLT2i sodium–glucose cotransporter 2 inhibitor
The results of the four scenario analyses are shown in Table 8. The SGLT2i strategy was dominant in scenarios 1 and 3. Although the SGLT2i strategy was more costly but more effective in scenarios 2 and 4, their ICERs in both scenarios (scenario 2: JPY 350,000; scenario 4: JPY 2,300,000) were well below the threshold value of JPY 5,000,000/QALY.
Table 8.
Scenario analyses
| Scenario | Cost (JPY) | QALY | |||||
|---|---|---|---|---|---|---|---|
| SGLT2i strategy | Conventional strategy | Differencea | SGLT2i strategy | Conventional strategy | Differencea | ICER | |
| 1 | 1,618,043 | 1,827,426 | − 209,383 | 8.554 | 8.503 | 0.052 | Dominant |
| 2 | 821,748 | 741,448 | 80,300 | 8.726 | 8.497 | 0.228 | 351,999 |
| 3 | 1,286,199 | 1,372,707 | − 86,508 | 8.765 | 8.554 | 0.211 | Dominant |
| 4 | 761,595 | 685,633 | 75,963 | 8.599 | 8.566 | 0.033 | 2,288,035 |
ICER incremental cost-effectiveness ratio, QALY quality-adjusted life years, SGLT2i sodium–glucose cotransporter 2
aSGLT2i strategy − conventional strategy
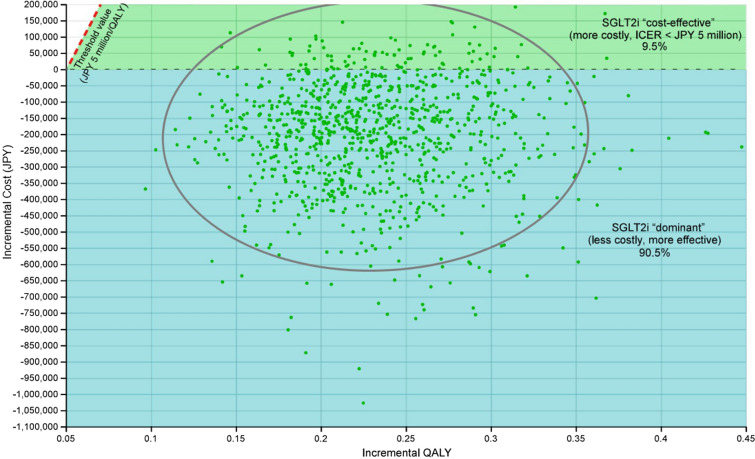
The PSA revealed that the probability of dominance was 90.5% for the SGLT2i strategy (Fig. 4). Under all remaining cases, or 9.5% of iterations, the SGLT2i strategy was cost-effective, with ICER values below JPY 5,000,000/QALY.
Fig. 4.
Probabilistic sensitivity analysis. The scatterplot shows results of 1,000,000 Monte Carlo simulations in the cost-effectiveness plane. ICER incremental cost-effectiveness ratio, JPY Japanese yen, QALY quality-adjusted life years, SGLT2i sodium–glucose cotransporter 2 inhibitor
Discussion
In the present study, we have demonstrated an economic benefit of initiating antidiabetic therapy with an SGLT2i by using a model of GLD-naive patients with T2DM. In the base-case scenario, initiating glucose-lowering therapy with an SGLT2i decreased the total costs and increased the earned-QALYs compared with the conventional GLD strategy. Although the medication cost of the SGLT2i strategy was higher, the total cost was decreased owing to lower costs of managing cardiovascular and renal complications. We performed several sensitivity analyses, including scenario analyses with conservative assumptions, and the results were consistent with the base-case analysis. Furthermore, the PSA demonstrated that the probability of dominance was 90.5%. The most common scenario in cost-effectiveness analyses is that a new treatment strategy improves the clinical outcome at increased cost, and the estimated value is derived using the ICER. In the present study, however, the SGLT2i strategy was dominant, with improved clinical outcomes and decreased cost, which indicates that initiating antidiabetic therapy with an SGLT2i offers extreme value from both clinical and economic perspectives.
Several simulation models for health economic assessments of diabetes have been reported; however, they were mainly conducted in Western countries [38–43]. The healthcare systems differ between Japan and other countries, and the clinical characteristics differ between Japanese and Caucasian patients with T2DM [44–46]. In addition, biguanides are predominantly used as the first-line GLD in Western countries, whereas DPP4i are the most commonly prescribed initial GLD in Japan [47, 48]. Considering these differences, the health economic impact of antidiabetic drugs in Japanese patients should be assessed using Japanese data. To our knowledge, this is the first study to report the health economics of initiating an SGLT2i in patients with T2DM using Japanese RWD taking into account cardiovascular and renal complications. Furthermore, RWD are potentially more suitable than data from a randomized controlled trial because the latter may not fully capture patient outcomes in daily clinical practice. In the present study, we developed a patient-level microsimulation model using Japanese RWD to assess T2DM-related cost in Japanese clinical practice. The microsimulation model provides estimates based on the demographics of the target patient population, including sex and age distribution, which enabled us to consider the characteristics of the target patient population. We used data from Shizuoka prefecture because its demographic distribution is similar to that of Japan. Including data from other regions will permit region-specific assessment of the clinical and economic impact, which could be implemented in healthcare policy planning by local government. The present model did not consider labor loss or cost of caregivers because this information is limited in Japan. The inclusion of this information in future studies will enable researchers to evaluate the effectiveness of SGLT2i in the context of value-based healthcare more comprehensively.
In the current study, we set CKD as a risk factor of ESRD and its effect on other clinical events was not evaluated, even though it is a strong risk factor for development of HF and other cardiovascular diseases [5, 17, 49]. Therefore, the current model probably underestimates the clinical and economic impact of CKD. Consequently, we probably underestimated the benefits of the SGLT2i strategy because SGLT2i has been shown to reduce the risk of cardiovascular death and hospitalization for HF hospitalization, as well as renal events, in patients with T2DM [7–12, 50].
The rate of SGLT2i use as the initial GLD was low in a study using an administrative claims database of a municipal government health insurance program [17]. A notable finding of that study was that only 2.2% of patients with T2DM were prescribed SGLT2i as an initial GLD even though they met the inclusion criteria of the EMPA-REG outcome or DECLARE-TIMI 58 studies [7, 9]. Those data may indicate that the burden of cardiovascular and renal diseases is not fully recognized. Although multifactorial interventions successfully reduce the risk of atherosclerotic cardiovascular diseases in patients with T2DM [51, 52], the incidence of HF remains high even after controlling for risk factors, including blood pressure and lipids within their target ranges [53]. Furthermore, CKD and HF develop from the early stages of T2DM and place a substantial burden on the prognosis of T2DM [5, 18], underscoring the need for early detection and early intervention. Recent real-world studies have demonstrated that the use of SGLT2i is associated with a slower decline in renal function [54] and lower incidence of cardiovascular events [17]. Another real-world study demonstrated an association between SGLT2i use and reduced risk of developing CKD and HF in patients with T2DM without cardiovascular or renal complications [20]. Considering the beneficial effects of SGLT2i on risk of CKD and HF, prescribing an SGLT2i from the early stage of diabetes may improve the prognosis and quality of life of patients with T2DM. The results of our study support this notion from both clinical and health economic perspectives.
The National Institute for Health and Care Excellence guidelines [55] and the ADA [56] guidance for managing diabetes refer to health economic evaluations, and include the medication cost as a factor to be considered when selecting the pharmacological treatment. In Japan, however, cost-effectiveness analyses are rarely used in treatment policies. In 2019, the Japanese Ministry of Health, Labor and Welfare introduced cost-effectiveness evaluation into drug price adjustment, and innovative drugs with higher price or large total sales are subject to this assessment. Because of the increase in medical expenses due to advances in medical technologies and societal aging, there is growing importance of health economic analyses. Further discussion on how to incorporate health economic analyses into healthcare policy and clinical practice in Japan is needed in order to provide treatment options with established therapeutic benefits and cost-effectiveness to patients and improve their overall outcomes.
The present study has some limitations. First, the results were derived from a model simulation rather than actual data. Nonetheless, we used RWD to develop the model to closely reflect Japanese clinical settings. Second, there were limited data on the maintenance cost of each complication, a topic that warrants further investigation. Third, the present study only targeted patients with T2DM. Considering the clinical benefit of SGLT2i in patients with HF or CKD, regardless of diabetes [10, 12], a model that includes patients with or without diabetes would allow for a more comprehensive assessment of the health economic effect of SGLT2i.
Conclusions
We performed health economic assessments of initiating an oral GLD in Japanese patients with T2DM using a microsimulation model that considering diabetes-related complications. The results demonstrate that initiating SGLT2i in patients with T2DM results in reduced clinical events and improved costs from a Japanese healthcare perspectives.
Acknowledgements
Funding
This study, including the journal’s Rapid Service Fee, was supported by the Japan Science and Technology Agency Program on Open Innovation Platform with Enterprises, Research Institute and Academia (JST-OPERA) (grant number, JPMJOP1842) and by AstraZeneca K.K., Osaka, Japan.
Authorship
All named authors meet the International Committee of Medical Journal Editors criteria for authorship, take responsibility for the integrity of the work as a whole, and approved the version of the manuscript to be published.
Author Contributions
All authors contributed to the study concept and design. AI developed the simulation model. AI and HA drafted the manuscript and all authors critically revised the manuscript for important intellectual content. The final version of the manuscript was approved by all authors.
Medical Writing Assistance
Nicholas D. Smith (EMC K.K.) provided English language editing, which was funded by AstraZeneca K.K.
Disclosures
Hiroki Akiyama and Toshitaka Yajima are full-time employees of AstraZeneca K.K. Ataru Igarashi has received lecture/other fees from AstraZeneca K.K., Novartis Pharma K.K., Novo Nordisk Pharma Ltd., Ono Pharmaceutical Co., Ltd., and Sanofi K.K.; and received donation from Takeda Pharmaceutical Company Limited for the endowed laboratory which AI belonged to. Shun Kohsaka has received grants from Daiichi Sankyo Company Limited. And Novartis Pharma K.K.; and lecture/other fees from AstraZeneca K.K., Bayer Yakuhin, Ltd., and Bristol Myers Squibb K.K. Hiroaki Miyata has received grants from Johnson & Johnson, Nipro Corporation, Takeda Pharmaceutical Company Limited, Mitsubishi Tanabe Pharma Corporation; and lecture/other fees from Boehringer Ingelheim GmbH, GE Healthcare Pharma, Astellas Pharma Inc., AstraZeneca K.K., Johnson & Johnson, Chugai Pharmaceutical Co., Ltd., and ROHTO Pharmaceutical Co., Ltd.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Data Availability
All data and parameters used in the present study are available in this article or in the cited articles.
References
- 1.Ogurtsova K, da Rocha Fernandes JD, Huang Y, et al. IDF Diabetes Atlas: global estimates for the prevalence of diabetes for 2015 and 2040. Diabetes Res Clin Pract. 2017;128:40–50. doi: 10.1016/j.diabres.2017.03.024. [DOI] [PubMed] [Google Scholar]
- 2.Saeedi P, Petersohn I, Salpea P, et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: results from the International Diabetes Federation Diabetes Atlas, 9(th) edition. Diabetes Res Clin Pract. 2019;157:107843. doi: 10.1016/j.diabres.2019.107843. [DOI] [PubMed] [Google Scholar]
- 3.Arnold SV, Inzucchi SE, McGuire DK, et al. Evaluating the quality of comprehensive cardiometabolic care for patients with type 2 diabetes in the U.S.: The Diabetes Collaborative Registry. Diabetes Care. 2016;39:e99–e101. doi: 10.2337/dc16-0585. [DOI] [PubMed] [Google Scholar]
- 4.Arnold SV, Kosiborod M, Wang J, Fenici P, Gannedahl G, LoCasale RJ. Burden of cardio-renal-metabolic conditions in adults with type 2 diabetes within the Diabetes Collaborative Registry. Diabetes Obes Metab. 2018;20:2000–2003. doi: 10.1111/dom.13303. [DOI] [PubMed] [Google Scholar]
- 5.Birkeland KI, Bodegard J, Eriksson JW, et al. Heart failure and chronic kidney disease manifestation and mortality risk associations in type 2 diabetes: a large multinational cohort study. Diabetes Obes Metab. 2020;22:1607–1618. doi: 10.1111/dom.14074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wen CP, Chang CH, Tsai MK, et al. Diabetes with early kidney involvement may shorten life expectancy by 16 years. Kidney Int. 2017;92:388–396. doi: 10.1016/j.kint.2017.01.030. [DOI] [PubMed] [Google Scholar]
- 7.Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117–2128. doi: 10.1056/NEJMoa1504720. [DOI] [PubMed] [Google Scholar]
- 8.Neal B, Perkovic V, Mahaffey KW, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377:644–657. doi: 10.1056/NEJMoa1611925. [DOI] [PubMed] [Google Scholar]
- 9.Wiviott SD, Raz I, Bonaca MP, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019;380:347–357. doi: 10.1056/NEJMoa1812389. [DOI] [PubMed] [Google Scholar]
- 10.McMurray JJV, Docherty KF, Jhund PS. Dapagliflozin in patients with heart failure and reduced ejection fraction. Reply N Engl J Med. 2020;382:973. doi: 10.1056/NEJMc1917241. [DOI] [PubMed] [Google Scholar]
- 11.Packer M, Anker SD, Butler J, et al. Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med. 2020;383:1413–1424. doi: 10.1056/NEJMoa2022190. [DOI] [PubMed] [Google Scholar]
- 12.Heerspink HJL, Stefansson BV, Correa-Rotter R, et al. Dapagliflozin in patients with chronic kidney disease. N Engl J Med. 2020;383:1436–1446. doi: 10.1056/NEJMoa2024816. [DOI] [PubMed] [Google Scholar]
- 13.American Diabetes Association. 9. Pharmacologic approaches to glycemic treatment: standards of medical care in diabetes-2021. Diabetes Care. 2021;44(Suppl 1):S111–24. [DOI] [PubMed]
- 14.American Diabetes Association. 10. Cardiovascular disease and risk management: standards of medical care in diabetes-2021. Diabetes Care. 2021;44(Suppl 1):S125–50.
- 15.Cosentino F, Grant PJ, Aboyans V, et al. 2019 ESC guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur Heart J. 2020;41:255–323. doi: 10.1093/eurheartj/ehz486. [DOI] [PubMed] [Google Scholar]
- 16.Visseren FLJ, Mach F, Smulders YM, et al. 2021 ESC guidelines on cardiovascular disease prevention in clinical practice. Eur Heart J. 2021;42:3227–3337. doi: 10.1093/eurheartj/ehab484. [DOI] [PubMed] [Google Scholar]
- 17.Kohsaka S, Kumamaru H, Nishimura S, et al. Incidence of adverse cardiovascular events in type 2 diabetes mellitus patients after initiation of glucose-lowering agents: a population-based community study from the Shizuoka Kokuho database. J Diabetes Investig. 2021;12:1452–1461. doi: 10.1111/jdi.13485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kadowaki T, Komuro I, Morita N, Akiyama H, Kidani Y, Yajima T. Manifestation of heart failure and chronic kidney disease are associated with increased mortality risk in early stages of type 2 diabetes mellitus: analysis of a Japanese real-world hospital claims database. Diabetes Ther. 2022;13:275–286. doi: 10.1007/s13300-021-01191-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kohsaka S, Takeda M, Bodegard J, et al. Sodium–glucose cotransporter 2 inhibitors compared with other glucose-lowering drugs in Japan: subanalyses of the CVD-REAL 2 Study. J Diabetes Investig. 2021;12:67–73. doi: 10.1111/jdi.13321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Komuro I, Kadowaki T, Bodegard J, Thuresson M, Okami S, Yajima T. Lower heart failure and chronic kidney disease risks associated with sodium-glucose cotransporter-2 inhibitor use in Japanese type 2 diabetes patients without established cardiovascular and renal diseases. Diabetes Obes Metab. 2021;23(Suppl 2):19–27. doi: 10.1111/dom.14119. [DOI] [PubMed] [Google Scholar]
- 21.Nishimura R, Kato H, Kisanuki K, et al. Treatment patterns, persistence and adherence rates in patients with type 2 diabetes mellitus in Japan: a claims-based cohort study. BMJ Open. 2019;9:e025806. doi: 10.1136/bmjopen-2018-025806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kohsaka S, Takeda M, Kidani Y, Yajima T. Healthcare resource utilization after initiation of sodium-glucose co-transporter-2 inhibitors versus dipeptidyl peptidase-4 inhibitors or other glucose-lowering drugs in Japanese patients with type 2 diabetes. Diabetes Obes Metab. 2021;23(Suppl 2):28–39. doi: 10.1111/dom.14289. [DOI] [PubMed] [Google Scholar]
- 23.Center for Outcomes Research and Economic Evaluation for Health. Guideline for cost-effectiveness analysis (3rd ed). 2022. https://c2h.niph.go.jp/tools/guideline/guideline_ja.pdf. Accessed 1 Apr 2022.
- 24.Mizuno A, Iguchi H, Sawada Y, et al. The impact of carperitide usage on the cost of hospitalization and outcome in patients with acute heart failure: high value care vs. low value care campaign in Japan. Int J Cardiol. 2017;241:243–248. doi: 10.1016/j.ijcard.2017.04.078. [DOI] [PubMed] [Google Scholar]
- 25.Sasaki N, Kunisawa S, Ikai H, Imanaka Y. Differences between determinants of in-hospital mortality and hospitalisation costs for patients with acute heart failure: a nationwide observational study from Japan. BMJ Open. 2017;7:e013753. doi: 10.1136/bmjopen-2016-013753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kamae I, Hashimoto Y, Koretsune Y, et al. Cost-effectiveness analysis of apixaban against warfarin for stroke prevention in patients with nonvalvular atrial fibrillation in Japan. Clin Ther. 2015;37:2837–2851. doi: 10.1016/j.clinthera.2015.10.007. [DOI] [PubMed] [Google Scholar]
- 27.Saito I, Kobayashi M, Matsushita Y, Mori A, Kawasugi K, Saruta T. Cost-utility analysis of antihypertensive combination therapy in Japan by a Monte Carlo simulation model. Hypertens Res. 2008;31:1373–1383. doi: 10.1291/hypres.31.1373. [DOI] [PubMed] [Google Scholar]
- 28.Toyonaga T. Factors affecting the sot for medical care and rehabilitation in stroke patients. Jpn Soc Occup Med Traumatol. 2006;54:175–182. [Google Scholar]
- 29.Nishi K, Sato Y, Miyamoto T, et al. Infusion therapy at outpatient clinic in chronic end-stage heart failure. J Cardiol. 2007;49:251–258. [PubMed] [Google Scholar]
- 30.Tanihata S, Nishigaki K, Kawasaki M, Takemura G, Minatoguchi S, Fujiwara H. Outcomes of patients with stable low-risk coronary artery disease receiving medical- and PCI-preceding therapies in Japan: J-SAP study 1–1. Circ J. 2006;70:365–369. doi: 10.1253/circj.70.365. [DOI] [PubMed] [Google Scholar]
- 31.Hattori N, Hirayama T, Katayama Y. Medical care for chronic-phase stroke in Japan. Neurol Med Chir (Tokyo) 2012;52:175–180. doi: 10.2176/nmc.52.175. [DOI] [PubMed] [Google Scholar]
- 32.Higashiyama A, Okamura T, Watanabe M, et al. Effect of chronic kidney disease on individual and population medical expenditures in the Japanese population. Hypertens Res. 2009;32:450–454. doi: 10.1038/hr.2009.51. [DOI] [PubMed] [Google Scholar]
- 33.Kondo M, Yamagata K, Hoshi SL, et al. Cost-effectiveness of chronic kidney disease mass screening test in Japan. Clin Exp Nephrol. 2012;16:279–291. doi: 10.1007/s10157-011-0567-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tsuji S, Ishikawa T, Morii Y, et al. Cost-effectiveness of a continuous glucose monitoring mobile app for patients with type 2 diabetes mellitus: analysis simulation. J Med Internet Res. 2020;22:e16053. doi: 10.2196/16053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hanafusa N, Fukagawa M. Global dialysis perspective: Japan. Kidney 360. 2020;1:416–419. doi: 10.34067/KID.0000162020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fukuma S, Ikenoue T, Shimizu S, et al. Quality of care in chronic kidney disease and incidence of end-stage renal disease in older patients: a cohort study. Med Care. 2020;58:625–631. doi: 10.1097/MLR.0000000000001301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Briggs A, Claxton K, Sculpher M. Decision modelling for health economic evaluation. Oxford: Oxford University Press; 2006. [Google Scholar]
- 38.Clarke P, Gray A, Holman R. Estimating utility values for health states of type 2 diabetic patients using the EQ-5D (UKPDS 62) Med Decis Mak. 2002;22:340–349. doi: 10.1177/0272989X0202200412. [DOI] [PubMed] [Google Scholar]
- 39.Clarke PM, Gray AM, Briggs A, et al. A model to estimate the lifetime health outcomes of patients with type 2 diabetes: the United Kingdom Prospective Diabetes Study (UKPDS) Outcomes Model (UKPDS no. 68) Diabetologia. 2004;47:1747–1759. doi: 10.1007/s00125-004-1527-z. [DOI] [PubMed] [Google Scholar]
- 40.Clarke PM, Glasziou P, Patel A, et al. Event rates, hospital utilization, and costs associated with major complications of diabetes: a multicountry comparative analysis. PLoS Med. 2010;7:e1000236. doi: 10.1371/journal.pmed.1000236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gray A, Raikou M, McGuire A, et al. Cost effectiveness of an intensive blood glucose control policy in patients with type 2 diabetes: economic analysis alongside randomised controlled trial (UKPDS 41). United Kingdom Prospective Diabetes Study Group. BMJ. 2000;320:1373–1378. doi: 10.1136/bmj.320.7246.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McEwan P, Foos V, Palmer JL, Lamotte M, Lloyd A, Grant D. Validation of the IMS CORE diabetes model. Value Health. 2014;17:714–724. doi: 10.1016/j.jval.2014.07.007. [DOI] [PubMed] [Google Scholar]
- 43.McEwan P, Ward T, Bennett H, Bergenheim K. Validation of the UKPDS 82 risk equations within the Cardiff Diabetes Model. Cost Eff Resour Alloc. 2015;13:12. doi: 10.1186/s12962-015-0038-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yoon KH, Lee JH, Kim JW, et al. Epidemic obesity and type 2 diabetes in Asia. Lancet. 2006;368:1681–1688. doi: 10.1016/S0140-6736(06)69703-1. [DOI] [PubMed] [Google Scholar]
- 45.Ma RC, Chan JC. Type 2 diabetes in East Asians: similarities and differences with populations in Europe and the United States. Ann N Y Acad Sci. 2013;1281:64–91. doi: 10.1111/nyas.12098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang J, Thornton JC, Russell M, Burastero S, Heymsfield S, Pierson RN., Jr Asians have lower body mass index (BMI) but higher percent body fat than do whites: comparisons of anthropometric measurements. Am J Clin Nutr. 1994;60:23–28. doi: 10.1093/ajcn/60.1.23. [DOI] [PubMed] [Google Scholar]
- 47.Katakami N, Mita T, Takahara M, et al. Baseline characteristics of patients with type 2 diabetes initiating second-line treatment in Japan: findings from the J-DISCOVER study. Diabetes Ther. 2020;11:1563–1578. doi: 10.1007/s13300-020-00846-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Khunti K, Heerspink HJL, Lam CSP, et al. Design and rationale of DISCOVER global registry in type 2 diabetes: real-world insights of treatment patterns and its relationship with cardiovascular, renal, and metabolic multimorbidities. J Diabetes Complic. 2021;35:108077. doi: 10.1016/j.jdiacomp.2021.108077. [DOI] [PubMed] [Google Scholar]
- 49.Parikh NI, Hwang SJ, Larson MG, Levy D, Fox CS. Chronic kidney disease as a predictor of cardiovascular disease (from the Framingham Heart Study) Am J Cardiol. 2008;102:47–53. doi: 10.1016/j.amjcard.2008.02.095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Perkovic V, Jardine MJ, Neal B, et al. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med. 2019;380:2295–2306. doi: 10.1056/NEJMoa1811744. [DOI] [PubMed] [Google Scholar]
- 51.Gaede P, Lund-Andersen H, Parving HH, Pedersen O. Effect of a multifactorial intervention on mortality in type 2 diabetes. N Engl J Med. 2008;358:580–591. doi: 10.1056/NEJMoa0706245. [DOI] [PubMed] [Google Scholar]
- 52.Ueki K, Sasako T, Okazaki Y, et al. Effect of an intensified multifactorial intervention on cardiovascular outcomes and mortality in type 2 diabetes (J-DOIT3): an open-label, randomised controlled trial. Lancet Diabetes Endocrinol. 2017;5:951–964. doi: 10.1016/S2213-8587(17)30327-3. [DOI] [PubMed] [Google Scholar]
- 53.Rawshani A, Rawshani A, Franzen S, et al. Risk factors, mortality, and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2018;379:633–644. doi: 10.1056/NEJMoa1800256. [DOI] [PubMed] [Google Scholar]
- 54.Heerspink HJL, Karasik A, Thuresson M, et al. Kidney outcomes associated with use of SGLT2 inhibitors in real-world clinical practice (CVD-REAL 3): a multinational observational cohort study. Lancet Diabetes Endocrinol. 2020;8:27–35. doi: 10.1016/S2213-8587(19)30384-5. [DOI] [PubMed] [Google Scholar]
- 55.NICE guideline [NG28]. Type 2 diabetes in adults: management. 2022. https://www.nice.org.uk/guidance/ng28. Accessed 1 Apr 2022.
- 56.American Diabetes Association Introduction: standards of medical care in diabetes-2021. Diabetes Care. 2021;44(Suppl 1):S1–S2. doi: 10.2337/dc21-Sint. [DOI] [PubMed] [Google Scholar]
- 57.Asakura R, Miyatake N, Mochimasu KD, Kurato R, Kuwana S. Comparison of health-related quality of life between type 2 diabetic patients with and without locomotive syndrome. Environ Health Prev Med. 2016;21:356–360. doi: 10.1007/s12199-016-0537-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sakamaki H, Ikeda S, Ikegami N, et al. Measurement of HRQL using EQ-5D in patients with type 2 diabetes mellitus in Japan. Value Health. 2006;9:47–53. doi: 10.1111/j.1524-4733.2006.00080.x. [DOI] [PubMed] [Google Scholar]
- 59.Takahara M, Katakami N, Shiraiwa T, et al. Evaluation of health utility values for diabetic complications, treatment regimens, glycemic control and other subjective symptoms in diabetic patients using the EQ-5D-5L. Acta Diabetol. 2019;56:309–319. doi: 10.1007/s00592-018-1244-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data and parameters used in the present study are available in this article or in the cited articles.