Abstract
About half of the brain tumours are primary and the rest are metastatic. The impact of each of these treatments alone or together on the prognosis of patients with astrocytoma tumours, especially low-grade astrocytoma, is unclear which may pose many challenges in the decision-making of surgeons and patients. Considering the importance of patient’s outcomes with astrocytoma and lack of general statistics, this study aimed to determine the survival of patients with high-grade astrocytoma and low-grade astrocytoma after treatments. This study follows a systematic review and a meta-analysis approach. Following a systematic review and meta-analysis method, articles dated from 1982 to March 2020 were extracted from Embase, ScienceDirect, Scopus, PubMed and Web of Science (WoS) international databases. Random effects model was used for analysis, and heterogeneity of studies was investigated considering the I2 index. Data were analysed using the Comprehensive Meta-Analysis software (version 2). According to a meta-analysis of studies, the mean overall survival in patients with high-grade astrocytoma was 31.9 ± 2.7 months, for 2-year survival, 38.1% (95% CI: 27.5–50.1%) and for 5-year survival was 28.6% (95% CI: 24.1–33.4%). Mean overall survival in patients with low-grade astrocytoma was 64.8 ± 7.4 months, for 2-year survival was 74.3% (95% CI: 32.6–94.5%) and for 5-year survival was 74.4% (95% CI: 57.9–86%). The highest mean for survival in patients with high-grade astrocytoma and in chemotherapy and radiation therapy treatments was 45.2 ± 5.2 months, and also the highest mean for survival in patients with low-grade astrocytoma in surgical treatment was 71.4 ± 8.8 months. The results of this study show that the average survival in patients with low-grade astrocytoma is high following the treatment, and in high-grade astrocytoma, there will be the highest survival rate, if the surgical treatment is combined with chemotherapy and radiation therapy. This study summarizes retrospective studies up to 2020 to evaluate the prognosis and survival of patients with brain astrocytoma tumours, and the results of this meta-analysis can be of interest to surgeons and specialists in this field.
Keywords: Astrocytoma, Primary brain tumours, High grade, Low grade, Meta-analysis
Introduction
By controlling infectious diseases and increasing life expectancy in the world, non-contagious and chronic diseases such as cancer are one of the most important causes of mortality [1]. About half of the brain tumours are primary, and the rest are metastatic [2]. CNS tumours have unique properties that distinguish them from neoplasms of other organs of the body. The distinction between benign and malignant lesions in the central nervous system (CNS) is less obvious than in other organs. Some glial tumours with benign microscopic features such as low mitosis, single nucleus formation and slow growth may infiltrate large areas of the brain, leading to severe clinical defects and poor prognosis. The operation to remove glial infiltrating neoplasms without damaging nerve function is very challenging. Additionally, the anatomical location of the neoplasm may have deadly consequences irrespective of its microscopic classification. The pattern of early CNS neoplasms differs from other tumours. Even the most malignant gliomas rarely metastasize outside the CNS. The subarachnoid space provides a pathway for expansion, such that implantation across the brain and spinal cord may occur in brain neoplasms, either severely anaplastic or well-differentiated, that have connections with the cerebrospinal fluid [2].
Astrocytoma is classified into four degrees according to the severity of the invasion; astrocytoma grade I usually does not penetrate or combine with surrounding tissues. Although the growth of tumours is slow at this rate, they may be relatively large. The most common type of astrocytoma is the pilocytic astrocytoma, which is more common in children, adolescents and young adults. Astrocytoma grade II, known as diffuse astrocytes, can penetrate and affect other adjacent structures. Astrocytoma grades I and II are called low-grade astrocytoma. Astrocytoma grade III, known as anaplastic or malignant astrocytoma, grows rapidly and is difficult to treat. These types of tumours are most common in people over 30 years old. Grade IV astrocytoma is the most aggressive type of tumour that grows rapidly and invades surrounding tissues. This tumour, known as glioblastoma or glioblastoma multiforme, occurs most commonly in men over 50 years of age. Astrocytoma grades III and IV are called high-grade astrocytoma [3, 4].
Economic growth appears to be related to astrocytoma in populations. The highest rates are in North America, Australia and Western Europe, and the lowest are in Asia, Central and South America. According to an estimate released by GLOBOCAN in 2008, it was predicted that Northern Europe in 2010 will have the highest diagnosis of malignant central nervous system tumours, and East African regions will have the lowest [5]. According to Bauchet et al. [6], the rate of primary brain tumours in France was 15.8%, from which 39.6% were benign, 56.3% malignant and 4.1% unknown categories [6]. The rate of primary malignant brain tumours worldwide is 3.7 per 100,000 for men and 2.6 per 100,000 for women. This is higher in developed countries i.e. 5.8 for men and 4.1 for women per 100,000 versus 3 and 1/2 for men and women in less developed countries respectively [5]. Jazayeri et al. [7], in a systematic literature review study in Iran, showed that primary malignant tumours of the nervous system during 2000–2009 accounted for 2.3% of all registered tumours. Among these, astrocytoma (32.3%) and glioblastoma (28.9%) were the most common brain tumours, and 51.9% of primary tumours were benign [7].
Chemotherapy, radiotherapy and surgery are the most common treatment options. In benign brain tumour cases, surgery may be successful; There are a large number of people who were treated postoperatively and were able to return to their normal lives [8].
Various studies have reported different survival rates in patients with high-grade and low-grade astrocytoma after treatment. However, there is lack in a comprehensive and holistic research that analyzes the results of these studies. Therefore, due to the importance of survival of patients with astrocytoma, and lack of general statistics about this globally, this study aims to determine the survival of patients with high-grade and low-grade astrocytoma after treatment; the study was performed following a systematic literature review and meta-analysis (Fig. 1).
Fig. 1.
Grading astrocytoma. a Grade I. b Grade II. c Grade III. d Grade IV
Methods
In this systematic review and meta-analysis, the survival of patients with high-grade and low-grade astrocytoma after treatment was evaluated based on studies performed between 1982 and March 2020. For this purpose, articles published in international databases Embase, ScienceDirect, Scopus, PubMed and Web of Science (WoS) were searched using the keywords Central Nervous System, CNS, High Grade, Low Grade, Astrocytoma and Survival.
The selection criteria were based on the availability of full-text clinical trials that examined the survival of patients with high-grade and low-grade astrocytoma after treatment. For more information, the sources of the reviewed articles were also reviewed for access to other potential suitable articles.
Article Selection
All articles referring to survival of patients with high-grade and low-grade astrocytoma after treatment were collected by researchers based on the inclusion and exclusion criteria. Exclusion criteria included unrelated cases, case reports, interventional studies, duplication of studies, unclear methodology and inaccessibility of the full text of the study. In order to reduce bias, the articles were searched independently by two reviewers, and if there were disagreements about the selection of article, the article was then reviewed by the lead reviewer. A total of 47 studies entered the third phase to assess the quality of the selected articles.
Assessing Articles’ Quality
The quality of articles was evaluated on the basis of the CONSORT checklist items that include the following criteria: study design, background and literature review, place and time of study, outcome, inclusion criteria, sample size and statistical analysis. Articles that fulfilled 6 to 7 of the criteria were considered high-quality articles, and those that did not fulfil 2 and more than 2 criteria from the 7 were considered medium and low-quality articles respectively [9]. In this study, 38 articles that were assessed as high-quality and medium-quality studies were systematically reviewed and meta-analysed; 9 other articles were of poor quality and were excluded.
Data Extraction
All final articles entered the meta-analysis stage and by using a checklist. Checklist included article title, first author’s name, year of publication, study location, sample size, mean survival time (months), survival rate of 2 and 5 years, type of astrocytoma and type of treatment.
Statistical Analysis
Since the review criteria were the survival of patients with high-grade and low-grade astrocytoma after treatment, frequency, rate and standardized mean difference were used as the measures to amalgamate and compare the results from different studies. I2 index was used to evaluate homogeneity between studies, and where there were heterogeneities in studies, random effects model was used to combine studies and conduct the meta-analysis. When the I2 index was less than 25%, it was considered low heterogeneity, between 25 and 75% moderate inhomogeneity and more than 75% as high heterogeneity. Moreover, P value less than 0.05 was considered significant. The funnel diagrams and Egger’s test were also used to evaluate the propagation bias.
Results
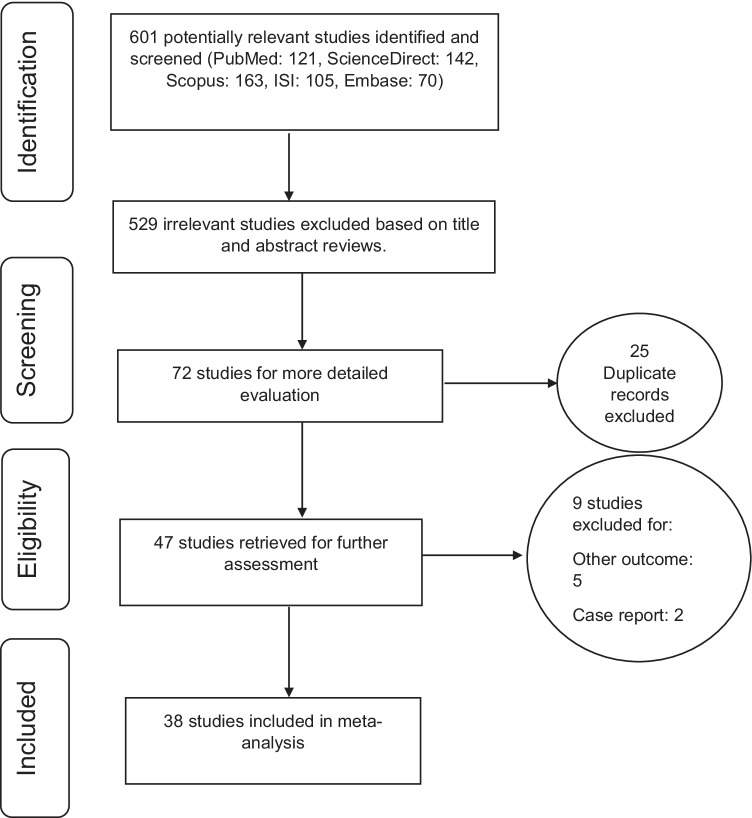
In this piece of research, all the studies regarding the survival of patients with high-grade and low-grade astrocytoma after unrestricted treatment were systematically reviewed according to PRISMA guidelines. In the initial search, 601 articles were identified, which eventually resulted in 38 studies selected for the final analysis; these were published between 1982 and March 2020 ( Fig. 2).
Fig. 2.
PRISMA flow diagram for the study selection
Mean survival rates in patients with high-grade astrocytoma in 18 articles with a total sample size of 4662 are reported in Table 1, 2-year survival of patients with high-grade astrocytoma in 10 articles with a total sample size of 3960, and also 5-year survival in 10 articles with a sample of 8748 individuals was included in the meta-analysis and presented in Table 2. Mean overall survival in patients with low-grade astrocytoma in 10 articles with a total sample size of 3956 is presented in Table 3, 2-year survival of patients with low-grade astrocytoma in 3 articles with a total sample size of 138, and also 5-year survival in 10 articles with a total sample size of 4875 individuals was included in the meta-analysis and reported in Table 4. The characteristics of the clinical trial studies compiled systematically and meta-analytically are also shown in Tables 1, 2, 3, and 4.
Table 1.
Characteristics of studies entered into the meta-analysis in terms of mean survival in the high-grade astrocytoma group
| Author, year, reference | Country | Sample size total | Average survival (months) | Type of treatment | Quality |
|---|---|---|---|---|---|
| Nikpour, 2009, [10] | Iran | 13 | 51.3 | Radiation therapy | Medium |
| Lin, 2003, [11] | Taiwan | 45 | 30.0 | Radiation therapy | High |
| Yamada, 2002, [12] | Japan | 41 | 22.3 | Radiation therapy | Medium |
| Beiko, 2014, [13] | USA | 128 | 19.6 | Surgery | High |
| Madajewicz, 2000, [14] | USA | 20 | 20.0 | Radiation therapy | High |
| Fukushima, 1998, [15] | Japan | 17 | 55.5 | Chemotherapy | High |
| Huddart, 1993, [16] | UK | 7 | 78.0 | Conservative surgery and radiotherapy | Medium |
| North, 1990, [17] | Australia | 285 | 6.0 | Radiation therapy | Medium |
| Deshpande, 2019, [18] | India | 479 | 34.0 | Surgery | High |
| Xie, 2018, [19] | China | 47 | 17.0 | Surgery | High |
| Grau, 2017, [20] | Germany | 56 | 33.0 | Surgical resection and adjuvant treatment | Medium |
| Dong-2, 2016, [21] | USA | 2755 | 21.0 | Radiation therapy | High |
| Strowd-1, 2004, [22] | USA | 74 | 27.0 | Radiation therapy | High |
| Strowd-2, 2004, [22] | USA | 122 | 37.0 | Radiation therapy | High |
| Juratli, 2015, [23] | Germany | 109 | 40.0 | Chemotherapy and radiation therapy | High |
| Barker, 2014, [24] | USA | 126 | 31.0 | Radiation therapy | High |
| Minniti, 2014, [25] | Italy | 97 | 50.5 | Chemotherapy and radiation therapy | Medium |
| Dey, 2014, [26] | USA | 241 | 7.0 | Surgical | High |
Table 2.
Characteristics of studies entered into the analysis in the 2 and 5-year survival rates in the high-grade astrocytoma group
| Author, year, reference | Country | Sample size total | 2-year survival % | 5-year survival % | Type of treatment | Quality |
|---|---|---|---|---|---|---|
| Lin, 2003, [11] | Taiwan | 45 | 73.3 | 64.4 | Radiation therapy | High |
| Fukushima, 1998, [15] | Japan | 17 | 29.4 | - | Chemotherapy | High |
| Nikpour, 2009, [10] | Iran | 13 | 69.2 | 38.5 | Radiation therapy | Medium |
| Davis, 1999, [27] | USA | 3128 | 36.2 | 27.6 | Surgical | High |
| North, 1990, [28] | Australia | 285 | 15.1 | - | Radiation therapy | High |
| Huddart, 1993, [16] | UK | 7 | - | 57.1 | Conservative surgery and radiotherapy | Medium |
| Salcman, 1982, [29] | USA | 74 | 25.7 | 20.3 | Chemotherapy and radiation therapy | High |
| Shahzadi-2, 1992, [8] | Iran | 8 | 62.5 | - | Radiation therapy | High |
| Aghajan, 2019, [30] | USA | 24 | 29.2 | - | Surgical | High |
| Barker, 2014, [24] | USA | 126 | 57.9 | - | Radiation therapy | High |
| Duffner-2, 1986, [31] | USA | 215 | - | 34.9 | Surgical | Medium |
| Okamoto-2, 2004, [32] | Switzerland | 35 | - | 16.4 | Radiation therapy | High |
| Shin, 2016, [33] | USA | 4807 | - | 29.8 | Chemotherapy and radiation therapy | High |
| Minniti, 2014, [25] | Italy | 97 | - | 38.1 | Chemotherapy and radiation therapy | Medium |
| Dey, 2014, [26] | USA | 241 | 19.6 | 10.0 | Surgical | High |
Table 3.
Characteristics of studies entered into the meta-analysis in terms of mean survival in the low-grade astrocytoma group
| Author, year, reference | Country | Sample size total | Average survival (months) | Type of treatment | Quality |
|---|---|---|---|---|---|
| McCormack, 1992, [34] | USA | 53 | 16.8 | Radiation therapy | High |
| Gary, 1985, [35] | USA | 30 | 70.9 | Surgery | High |
| Johannesen, 2003, [36] | USA | 993 | 76.8 | Radiation therapy | Medium |
| Abdulrauf, 1998, [37] | Spain | 74 | 68.4 | Radiation therapy | High |
| Phuphanich, 1993, [38] | USA | 14 | 42.0 | Radiation therapy | Medium |
| Jungk, 2019, [39] | Germany | 58 | 136.0 | O-6-methylguanine-DNA methyltransferase | High |
| Dong, 2016, [40] | USA | 2497 | 50.0 | Radiation therapy | High |
| Gimenez, 2015, [22] | Brazil | 4 | 43.0 | Radiation therapy | High |
| Jakola, 2013, [41] | Norway | 51 | 92.4 | Surgical | High |
| Sahgal, 2013, [42] | Canada | 182 | 49.2 | Surgical | High |
Table 4.
Characteristics of studies entered into the analysis in the 2 and 5-year survival rates in the low-grade astrocytoma group
| Author, year, reference | Country | Sample size total | 2-year survival % | 5-year survival % | Type of treatment | Quality |
|---|---|---|---|---|---|---|
| McCormack, 1992, [34] | USA | 53 | - | 64.2 | Radiation therapy | High |
| Abdulrauf, 1998, [37] | Spain | 74 | - | 64.9 | Radiation therapy | High |
| Hwang, 2000, [43] | Taiwan | 112 | 89.3 | 74.1 | Radiation therapy | High |
| Phuphanich, 1993, [38] | USA | 14 | 35.7 | - | Radiation therapy | Medium |
| Shahzadi-1, 1992, [8] | Iran | 12 | 83.3 | - | Radiation therapy | High |
| Duffner-1, 1986, [31] | USA | 106 | - | 70.8 | Radiation therapy | Medium |
| Ostertag-1, 1992, [44] | USA | 987 | - | 76.4 | Radiation therapy | High |
| Ostertag-2, 1992, [44] | USA | 122 | - | 64.9 | Radiation therapy | High |
| Ostertag-3, 1992, [44] | USA | 81 | - | 79.5 | Radiation therapy | High |
| Okamoto-1, 2004, [32] | Switzerland | 163 | - | 64.9 | Radiation therapy | High |
| Jungk, 2019, [39] | Germany | 58 | - | 67.2 | O-6-methylguanine-DNA methyltransferase | High |
| Tabash, 2019, [45] | USA | 3084 | - | 95.3 | Radiation therapy | High |
Evaluation of Heterogeneity and Propagation Bias of Mean Survival, 2 and 5-Year Survivals in High-Grade Astrocytoma Group
The heterogeneity of the studies was evaluated using I2 test and based on this test, the mean survival, 2-year survival and 5-year survival in patients with a high-grade astrocytoma were 100%, 93.2% and 89.5% respectively. These I2 values indicated a high heterogeneity in the included studies; therefore, the random effects model was used to amalgamate and compare the results from the selected papers.
The bias in the mean survival (months) (P = 0.110), 2-year survival rates (P = 0.960) and 5-year (P = 0.979) in the high-grade astrocytoma results are presented by the funnel charts and using the Egger’s test, and with the significant level of 0.05, demonstrate the non-existence of bias in our analysis.
Evaluation of Heterogeneity and Propagation Bias of Mean Survival, 2 and 5-Year Survivals in Low-Grade Astrocytoma Group
Similarly, the heterogeneities of the studies were analysed for results on low-grade astrocytoma; the heterogeneity of the studies was evaluated using I2 test and based on this test, the mean survival, 2-year survival and 5-year survival in patients with a low-grade astrocytoma were 100%, 88.9% and 93.8% respectively. These I2 values indicated a high heterogeneity in the included studies; therefore, the random effects model was used to amalgamate and compare the results from the selected papers.
The bias in the mean survival (months) (P = 0.428), 2-year survival rates (P = 0.594) and in 5-year survival (P = 0.668) in the low-grade astrocytoma results are presented by the funnel charts and using the Egger’s test, and with the significant level of 0.05, demonstrate the non-existence of bias in our analysis.
Meta-analysis of Results on Mean Survival, and 2 and 5-Year Survival of Patients with High-Grade Astrocytoma
Considering the meta-analysis of the studies, the mean overall survival in patients with high-grade astrocytoma in 18 articles with the overall sample size of 4662 was 31.9 ± 2.7 months. Two-year survival of patients with high-grade astrocytoma in 10 papers with the total sample size of 3960 was 38.1% (95% CI: 27.5–50%) and 5-year survival of patients with high-grade astrocytoma in 10 articles with the total sample size of 8748 was 28.6% (95% CI: 24.1–33.4%) (Figs. 3, 4, and 5).
Fig. 3.
Mean survival in patients with a high-grade astrocytoma (95% confidence interval). The middle point of each small rectangle represents the mean survival in each study, and the diamond represents the overall mean survival in high-grade astrocytoma patients considering all studies
Fig. 4.
Two-year survival in patients with a high-grade astrocytoma (95% confidence interval). The middle point of each small rectangle represents the 2-year survival in each study, and the diamond represents the overall 2-year survival in high-grade astrocytoma patients considering all studies
Fig. 5.
Five-year survival in patients with a high-grade astrocytoma (95% confidence interval). The middle point of each small rectangle represents the 5-year survival in each study, and the diamond represents the overall 5-year survival in high-grade astrocytoma patients considering all studies
Meta-analysis of Results on Mean Survival, and 2 and 5-Year Survival of Patients with Low-Grade Astrocytoma
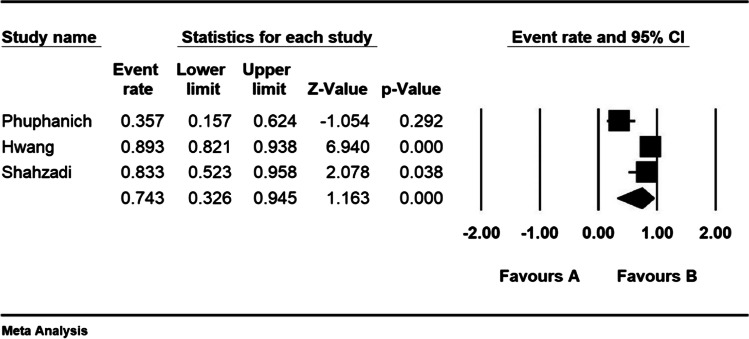
Considering the meta-analysis of the studies, the mean overall survival in patients with low-grade astrocytoma in 10 articles with the overall sample size of 3956 was 64.8 ± 7.4 months. Two-year survival of patients with low-grade astrocytoma in 3 papers with the total sample size of 138 was 74.3% (95% CI: 32.6–94.5%) and 5-year survival of patients with low-grade astrocytoma in 10 articles with the total sample size of 4875 was 74.4% (95% CI: 57.9–86%) (Figs. 6, 7, and 8).
Fig. 6.
Mean survival in patients with a low-grade astrocytoma (95% confidence interval). The middle point of each small rectangle represents the mean survival in each study, and the diamond represents the overall mean survival in low-grade astrocytoma patients considering all studies
Fig. 7.
Two-year survival in patients with a low-grade astrocytoma (95% confidence interval). The middle point of each small rectangle represents the 2-year survival in each study, and the diamond represents the overall 2-year survival in low-grade astrocytoma patients considering all studies
Fig. 8.
Five-year survival in patients with a low-grade astrocytoma (95% confidence interval). The middle point of each small rectangle represents the 5-year survival in each study, and the diamond represents the overall 5-year survival in low-grade astrocytoma patients considering all studies
Analysis of Sub-Categories Based on Treatments
Mean and rate of survival by type of treatment in patients with high and low-grade astrocytoma are presented in Tables 5 and 6. Considering Table 3, the highest mean survival in patients with a high-grade astrocytoma who had chemotherapy and radiation therapy was 45.3 ± 5.2 months. Moreover, the highest mean survival was reported in patients with a low-grade astrocytoma who had a surgical treatment with 71.4 ± 8.8 months (Table 5). Considering the results in Table 4, the highest 2-year survival rate in patients with high-grade astrocytoma who were treated with radiation therapy was 53.6% (95% CI: 24.4–80.5%), and the highest 5-year survival rate in patients with high-grade astrocytoma who were treated with radiation therapy was reported as 37.5% (95% CI: 1/11.24–74%). Furthermore, the highest 2-year survival rate in patients with a low-grade astrocytoma who had a radiation therapy treatment was 74.3% (95% CI: 32.6–94.5%), and the highest 5-year survival rate in patients with a low-grade astrocytoma who had a radiation therapy treatment was 75.1% (95% CI: 57.5–87.1%) (Table 6).
Table 5.
Mean survival rate for patients with a low or high-grade astrocytoma, based on treatment
| Grade | Type of treatment | N (articles) | N (sample size) | I2 | Egger’s test | Mean ± SD (months) |
|---|---|---|---|---|---|---|
| High grade | Radiation therapy | 10 | 3498 | 100 | 0.224 | 29.6 ± 3.3 |
| Surgical | 4 | 894 | 100 | 0.943 | 19.4 ± 7.4 | |
| Chemotherapy and radiation therapy | 2 | 206 | 100 | - | 45.2 ± 5.2 | |
| Low grade | Radiation therapy | 6 | 3635 | 100 | 0.995 | 49.6 ± 7.9 |
| Surgical | 3 | 263 | 100 | 0.880 | 71.4 ± 8.8 |
Table 6.
The 1 and 5-year survival rates for patients with a low or high-grade astrocytoma, based on treatment
| Grade | Survival (years) | Type of treatment | N (articles) | N (sample size) | I2 | Egger’s test | Percent (95% CI) |
|---|---|---|---|---|---|---|---|
| High-grade astrocytoma | 2 | Radiation therapy | 5 | 447 | 96 | 0.402 | 53.6 (24.4–80.5) |
| Surgical | 3 | 3392 | 93.3 | 0.479 | 27.8 (16.4–43.1) | ||
| Chemotherapy and radiation therapy | 2 | 91 | 0 | - | 26.4 (18.4–36.4) | ||
| 5 | Radiation therapy | 3 | 180 | 93.7 | 0.773 | 37.5 (11.2–74.1) | |
| Surgical | 3 | 3583 | 94.7 | 0.709 | 22.6 (13.6–35.2) | ||
| Chemotherapy and radiation therapy | 3 | 4978 | 68.4 | 0.984 | 29.8 (23–37.6) | ||
| Low-grade astrocytoma | 2 | Radiation therapy | 3 | 138 | 88.9 | 0.594 | 74.3 (32.6–94.5) |
| 5 | Radiation therapy | 9 | 4817 | 98.5 | 0.773 | 75.1 (57.5–87.1) |
Discussion
In this systematic review and meta-analysis study, the mean overall survival in patients with a low-grade astrocytoma was 64.8 ± 7.4 months. Low-grade astrocytoma is a less aggressive tumour and its most effective treatment is surgery [46, 47]. It is aggressive in the elderly who require adjuvant treatment and subsequently adjuvant therapy [48]. Pilocytic astrocytoma occurs in young people and children, and has a long-term prognosis and survival rate, and the best treatment is surgery. Nevertheless, rapid radiotherapy after surgery has no advantage in delaying tumour progression [49, 50]. An oligodendroglioma is a tumour with unpredictable biological behaviour and its primary treatment is also surgical. Radiation therapy and chemotherapy for this tumour may result in complications and are only performed where necessary. Long-term survival and surgery outcome depend on age and gender [51]. Ependymoma occurs at a young age and is treated with surgery and radiation. Diffuse astrocytoma most commonly occurs in people under the age of 50, and the most effective treatment is surgery followed by radiation therapy [51]. In one study, the mean survival of patients with oligodendroglioma in grades II and III was reported 11.6 and 6.5 years respectively; in astrocytoma tumours with grade II (diffuse astrocytoma), this was 5.6 years, and in 10-year pilocytic astrocytoma 96% [52].
The 2-year survival of patients with low-grade astrocytoma was 74.3% and the 5-year survival rate was 74.4%. The findings of this study indicate that survival rate in patients with low-grade astrocytoma is high, and this may be due to the quality of treatment and diagnostic methods. Our study found that the survival rates of 2 and 5 years were similar in these patients, indicating that these patients had a nearly normal survival after treatment.
According to Tables 5 and 6, the highest mean survival was found in patients with a low-grade astrocytoma in surgical treatment with 71.4 ± 8.8 months, and the highest survival of 2 and 5 years in patients with a low-grade astrocytoma is reported when the patients are treated by radiation therapy.
If a brain tumour is accessible, surgery is a good option to remove the tumour. In some cases, the tumour is small and detachable from the tissue around the brain and can be completely removed by surgery. In other cases, the tumour cannot be removed from the surrounding tissue, or the tumour is located in a sensitive area of the brain (eloquence areas), which makes the surgery a dangerous option. In this case, the doctor will continue the surgery until the removal process does not endanger the patient’s life, and does not lead to major neurological deficits. Even removing part of a brain tumour can help reduce a person’s symptoms. Moreover, a surgery may be combined with radiation therapy and chemotherapy [46, 47].
Brain tumour surgery has risks such as sensorimotor complications, seizures, infection and bleeding. The risks depend on the location of the tumour. For instance, tumour surgery near the optic nerve may present a risk of vision loss. Other side effects of surgery include increased existing symptoms, damage to normal brain tissue, swelling of brain tissue and seizures. The effects of altered brain function such as muscle weakness, mental changes and any decrease in brain function can be controlled. The side effects of surgery often decrease over time; however, sometimes this may not be the case [47].
Reducing the complications of neurosurgery, especially brain surgery, has been one of the main goals in the development of new surgery techniques in recent decades. Not long ago, brain surgeries were associated with irreversible complications such as limb paralysis or loss of speech. These complications were typically due to the surgical team’s lack of knowledge about brain eloquence areas, such as the speech or brain motor centres, tumour-induced displacement and/or the lack of precise brain mapping equipment. Our awareness has increased over the past few decades in rectifying these issues, especially with the use of imaging techniques. Although techniques such as functional MRI allow us to identify sensitive and high-risk brain areas, such as the centres for speech or movement of the limbs and face, the accuracy of these techniques is insufficient to fully and confidently remove brain tumours from all patients [53, 54].
Considering the current systematic review and meta-analysis undertaken in our study, the mean overall survival in patients with a high-grade astrocytoma was 31.9 ± 2.7 months. The World Health Organization (WHO) classified grades III and IV astrocytic tumours, oligo astrocytoma (grade III) and oligodendrogliomas (grade III) as a class of malignant gliomas of the central nervous system [55]. Malignant astrocytoma is diagnosed by histopathologic tests. The WHO considers the St. Anne-mayo system for diagnosis and histopathologic confirmation of malignant astrocytoma tumours (grade III) when 2 out of the 4 properties of (1) nuclear alteration, (2) mitosis, (3) endothelial proliferation and (4) necrosis are present [56]. Grade IV is identified by having at least 3 of the 4 properties mentioned above. Since the risk of systemic diffusion is low, the classification performed is solely based on pathological findings.
The Central Brain Tumor Registry of the United States (CBTRUS) reported 986,000 and 295,000 primary CNS tumours in 2004 and 2008 respectively. A total of 6.3% of them were polymorphic glioblastoma, which is the most common type of CNS tumour. Grade IV diagnoses were reported more in males than in females with a ratio of 1.5 to 1, and in such diagnoses in the white ethic group was reported to be more than the black ethnic group with a ratio of 2 to 1. The results of studies on the age of patients with this tumour suggest that with increasing life expectancy, this type of tumour is more likely to develop. The diagnosis of this tumour is higher in the age groups of 75–84 years and the mean age of the patients is 64 years [57].
According to the findings of this study, 2-year survival of patients with a high-grade astrocytoma was 38.1% and 5-year survival in these patients was 28.6%. The prognosis of patients with a high-grade astrocytoma is poor; therefore, the probability of recovery and death are relatively high [57]. From 1995 to 2008, 1-year survival rates of patients with grade IV were 35%, while their 5-year survival rate was only 5% [57]. The higher 5-year survival of patients found in this study may be due to the enhancements in the quality of treatment and surgical procedures in recent years.
Considering Tables 5 and 6, the highest mean survival in patients with a high-grade astrocytoma was when the patients were treated with chemotherapy and radiation therapy, whereas the highest 2 and 5-year survivals in patients with a high-grade astrocytoma were when the treatment was radiation therapy.
The first step in treating patients with high-grade astrocytoma is to have the tumour removed by a surgeon. One of the common limitations in tumour removal surgery is the exact location of the tumour in or near the eloquence areas. Recently, it has been shown that removal of at least the sub-total of a tumour increases patients’ life expectancy and success in surgery. This is dependent on the patient’s age, Karnofsky’s performance status, tumour volume and the ratio removed after surgery [58]. The mean survival after surgery and the removal of the minimum sub-total tumour volume was 12.5 months; in contrast, survival will be higher to 16 months after the chemotherapy treatment [58].
Astrocytoma patients who require radiotherapy make up a significant percentage of those who visit radiotherapy centres. In the treatment of astrocytoma, surgery is often only used for biopsy, to diagnose or reduce tumour size as much as possible [59], and the primary treatment of these patients is radiotherapy [59, 60]. Due to the surgical challenges for tumours in the eloquence area or in the deep tissues of the brain, tumours adjacent to vital organs such as brainstem, medulla oblongata and cranial nerve e.g. optic nerve and chiasma, and the nature of some tumours such as glial cell tumour that route in the adjacent tissues [61], a complete surgery with an acceptable margin from normal tissues is virtually impossible in most cases. Radical surgeries are often associated with serious complications that can severely affect quality of life of the patient. On the other hand, since chemotherapy is also a less successful treatment for these patients, as the blood–brain barrier prevents the chemotherapy drugs to enter the targeted brain tissue [60], the role of radiotherapy in these treatments gains more importance. Significant developments have also been made, in recent years, in the field of radiotherapy such as proton, neutron and gamma-ray and stereo-tactical surgery (one of radiotherapy techniques) [59, 61], and with the invention of sophisticated radiotherapy techniques such as IMRT or 3DRT. In addition, the use of linear accelerators with different energies and the use of treatment planning and planning systems to accurately determine the dose distribution of radiation and fixators to facilitate repeatability of radiotherapy sessions and reduction of radiotherapy errors all lead to increased radiation absorption to the tumour and preservation of adjacent normal tissues. Radiation and overall success radiation therapy has increased the 6-year survival rate of patients with a low-grade astrocytoma to more than 60–70% [60]. The average dose of these patients is usually between 5000 and 6000 cGy, and at times such as for malignant grade IV tumours could be up to 7000 cGy, that is administered as a single dose between 180 and 200 cGy daily [59–61].
In general, brain radiotherapy complications are divided into three distinct groups:
Acute complications due to radiation therapy: It is often due to swelling of the brain tissue and increased intra-brain pressure during treatment, and manifests itself mainly as headache, nausea, anorexia and fatigue [59].
Subacute complications: It begins 4–6 weeks after radiotherapy and can last up to 6–12 weeks, due to damage to the oligodendrial brain cells, which is a type of temporary demyelination [61].
Late complications: Starting after 3–6 months of radiation therapy and can manifest itself for years to come, such as brain tissue necrosis or damage to the optic nerve and chiasma, can usually be seen after doses above 5400–6000 cGy [59, 61].
Limitations
One of the limitations of this study is that some samples were not randomly selected. Moreover, one limitation was due to some deficiencies in some of the searched studies; examples of such deficiencies include lack of uniform reporting, inadequate implementation, lack of consistency and non-existence of the full text of the papers presented at the conference mentioned.
Conclusion
The results of this study show that the average survival in patients with low-grade astrocytoma is high, and surgical treatment with radiation therapy offers the highest survival; the results also demonstrate that the average survival in patients with high-grade astrocytoma in recent years has increased, due to the advancement of diagnostic and therapeutic techniques. Moreover, such patients are more likely to survive if surgical treatment is combined with chemotherapy and radiation therapy. This study is helpful in deciding which treatments to choose considering the type of tumour. The study also assists us to better understand types of treatments, and the potentials with patients’ survival, and can be certainly a very useful source for surgeons when selecting treatment of cerebral astrocytoma tumours.
Acknowledgements
The authors thank the Student Research Committee, Kermanshah University of Medical Sciences, Kermanshah, Iran.
Abbreviations
- CNS
Central nervous system
- CONSORT
Consolidated Standards of Reporting Trials
- PRISMA
Preferred Reporting Items for Systematic Reviews and Meta-Analysis
- WoS
Web of Science
Author Contribution
RF, NS and MK contributed to the design, and MM participated in statistical analysis and most of the study steps. MM, AHF and SHSH prepared the manuscript. MM, MK and NS assisted in designing the study, and helped in the interpretation of the study. All authors have read and approved the content of the manuscript.
Funding
The research funding was supported by the student research committee of Kermanshah University of Medical Sciences, grant no. 990652.
Data availability
Datasets are available through the corresponding author upon reasonable request.
Declarations
Ethics Approval and Consent to Participate
Ethics approval was received from the ethics committee of deputy of research and technology, Kermanshah University of Medical Sciences (IR.KUMS.REC.1399.652).
Consent for Publication
Not applicable.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Nader Salari, Email: n_s_514@yahoo.com.
Reza Fatahian, Email: r_fatahian@kums.ac.ir.
Mohsen Kazeminia, Email: mohsenkaz221@gmail.com.
Amin Hosseinian-Far, Email: amin.hosseinian-far@northampton.ac.uk.
Shamarina Shohaimi, Email: shamarina@upm.edu.my.
Masoud Mohammadi, Email: Masoud.mohammadi1989@yahoo.com.
References
- 1.WHO. The top 10 causes of death. [Webpage]: WHO; 2011; Available from: http://www.who.int/mediacentre/ Factsheets/fs310/en/index4.html
- 2.Shelly M, MacMahon P, Eustace S (2008) Radiology of soft tissue tumors including melanoma. Imaging in Oncology: Springer. pp 423–52 [DOI] [PubMed]
- 3.Xu J, Ma X, Tian Z, Chen C, Fan Y, Ou X, et al. Glioblastoma multiforme and anaplastic astrocytoma: differentiation using MRI texture analysis. Front Oncol. 2019;9:876. doi: 10.3389/fonc.2019.00876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wei J, Yang G, Hao X, Gu D, Tan Y, Wang X, et al. A multi-sequence and habitat-based MRI radiomics signature for preoperative prediction of MGMT promoter methylation in astrocytomas with prognostic implication. Eur Radiol. 2019;29(2):877–888. doi: 10.1007/s00330-018-5575-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bondy ML, Scheurer ME, Malmer B, Barnholtz-Sloan JS, Davis FG, D, Il’Yasova, et al. Brain tumor epidemiology: consensus from the Brain Tumor Epidemiology Consortium. Cancer. 2008;113(S7):1953–68. doi: 10.1002/cncr.23741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bauchet L, Rigau V, Mathieu-Daudé H, Figarella-Branger D, Hugues D, Palusseau L, et al. French brain tumor data bank: methodology and first results on 10,000 cases. J Neurooncol. 2007;84(2):189–199. doi: 10.1007/s11060-007-9356-9. [DOI] [PubMed] [Google Scholar]
- 7.Jazayeri RB, Shokraneh F, Ramezani R, Marjan A, Saadat S, Rahimi-Movaghar V. Epidemiology of primary brain and spinal cord tumors in Iran: a systematic review. Khatam Healing Magazine, Year. 2013;1(2):13–20. [Google Scholar]
- 8.Shahzadi S, Abbasanejad E (1992) Interstitial radiotherapy of brain tumor. J Ozavin Univ Med Sci. No. 12, Winter 2000. [In Persian]
- 9.Schulz KF, Altman DG, Moher D. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. BMC Med. 2010;8(1):18. doi: 10.1186/1741-7015-8-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nikpor F (2009). Epidemiologic characteristics and survival rate of malignant nervous system tumors in children referred to Ali Asghar Hospital during years 1999–2009. Master thesis, Iran Univ Med Sci [In Persian]
- 11.Lin C, Lieu A, Lee K, Yang Y, Kuo T, Hung M, et al. The conditional probabilities of survival in patients with anaplastic astrocytoma or glioblastoma multiforme. Surg Neurol. 2003;60(5):402–406. doi: 10.1016/s0090-3019(03)00322-7. [DOI] [PubMed] [Google Scholar]
- 12.Yamada SM, Yamada S, Hayashi Y, Takahashi H, Teramoto A, Matsumoto K. Fibroblast growth factor receptor (FGFR) 4 correlated with the malignancy of human astrocytomas. Neurol Res. 2002;24(3):244–248. doi: 10.1179/016164102101199864. [DOI] [PubMed] [Google Scholar]
- 13.Beiko J, Suki D, Hess KR, Fox BD, Cheung V, Cabral M, et al. IDH1 mutant malignant astrocytomas are more amenable to surgical resection and have a survival benefit associated with maximal surgical resection. Neuro Oncol. 2014;16(1):81–91. doi: 10.1093/neuonc/not159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Madajewicz S, Chowhan N, Tfayli A, Roque C, Meek A, Davis R, et al. Therapy for patients with high grade astrocytoma using intraarterial chemotherapy and radiation therapy. Cancer. 2000;88(10):2350–2356. doi: 10.1002/(sici)1097-0142(20000515)88:10<2350::aid-cncr20>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 15.Fukushima T, Yamamoto M, Ikeda K, Tsugu H, Kimura H, Soma G, et al. Treatment of malignant astrocytomas with recombinant mutant human tumor necrosis factor-alpha (TNF-SAM2) Anticancer Res. 1998;18(5D):3965–3970. [PubMed] [Google Scholar]
- 16.Huddart R, Traish D, Ashley S, Moore A, Brada M. Management of spinal astrocytoma with conservative surgery and radiotherapy. Br J Neurosurg. 1993;7(5):473–481. doi: 10.3109/02688699308995069. [DOI] [PubMed] [Google Scholar]
- 17.North B, Reilly P, Blumbergs P, Roder D, Esterman A. Malignant astrocytoma in South Australia: treatment and case survival. Med J Aust. 1990;153(5):250–254. doi: 10.5694/j.1326-5377.1990.tb136894.x. [DOI] [PubMed] [Google Scholar]
- 18.Deshpande RP, Chandrasekhar Y, Panigrahi M, Babu PP. Prognostic significance of anatomic origin and evaluation of survival statistics of astrocytoma patients—a tertiary experience. Indian J Surg Oncol. 2019;10(1):55–60. doi: 10.1007/s13193-018-0820-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xie JC, Yang S, Liu XY, Zhao YX. Marital status is associated with survival of patients with astrocytoma. J Clin Neurosci. 2018;56:79–87. doi: 10.1016/j.jocn.2018.07.005. [DOI] [PubMed] [Google Scholar]
- 20.Grau SJ, Hampl JA, Kohl A-C, Timmer M, Duval IV, Blau T, et al. Impact of resection on survival of isocitrate dehydrogenase 1–mutated World Health Organization grade ii astrocytoma after malignant progression. World Neurosurg. 2017;103:180–185. doi: 10.1016/j.wneu.2017.03.123. [DOI] [PubMed] [Google Scholar]
- 21.Strowd RE, Abuali I, Ye X, Lu Y, Grossman SA. The role of temozolomide in the management of patients with newly diagnosed anaplastic astrocytoma: a comparison of survival in the era prior to and following the availability of temozolomide. J Neurooncol. 2016;127(1):165–171. doi: 10.1007/s11060-015-2028-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gimenez M, Marie SKN, Oba-Shinjo S, Uno M, Izumi C, Oliveira JB, et al. Quantitative proteomic analysis shows differentially expressed HSPB1 in glioblastoma as a discriminating short from long survival factor and NOVA1 as a differentiation factor between low-grade astrocytoma and oligodendroglioma. BMC Cancer. 2015;15(1):481. doi: 10.1186/s12885-015-1473-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Juratli TA, Lautenschläger T, Geiger KD, Pinzer T, Krause M, Schackert G, et al. Radio-chemotherapy improves survival in IDH-mutant, 1p/19q non-codeleted secondary high-grade astrocytoma patients. J Neurooncol. 2015;124(2):197–205. doi: 10.1007/s11060-015-1822-1. [DOI] [PubMed] [Google Scholar]
- 24.Barker CA, Chang M, Beal K, Chan TA. Survival of patients treated with radiation therapy for anaplastic astrocytoma. Radiol Oncol. 2014;48(4):381–386. doi: 10.2478/raon-2014-0019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Minniti G, Scaringi C, Arcella A, Lanzetta G, Di Stefano D, Scarpino S, et al. IDH1 mutation and MGMT methylation status predict survival in patients with anaplastic astrocytoma treated with temozolomide-based chemoradiotherapy. J Neurooncol. 2014;118(2):377–383. doi: 10.1007/s11060-014-1443-0. [DOI] [PubMed] [Google Scholar]
- 26.Dey M, Lin Y, Melkonian S, Lam S. Prognostic factors and survival in primary adult high grade brainstem astrocytoma: a population based study from 1973–2008. J Clin Neurosci. 2014;21(8):1298–1303. doi: 10.1016/j.jocn.2013.12.011. [DOI] [PubMed] [Google Scholar]
- 27.Davis FG, McCarthy BJ, Freels S, Kupelian V, Bondy ML. The conditional probability of survival of patients with primary malignant brain tumors: surveillance epidemiology and end results (SEER) data. Cancer: Interdiscip Int J Am Cancer Soc. 1999;85(2):485–91. [PubMed] [Google Scholar]
- 28.North CA, North RB, Epstein JA, Piantadosi S, Wharam MD. Low-grade cerebral astrocytomas: survival and quality of life after radiation therapy. Cancer. 1990;66(1):6–14. doi: 10.1002/1097-0142(19900701)66:1<6::aid-cncr2820660103>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 29.Salcman M, Scholtz H, Kaplan RS, Kulik S. Long-term survival in patients with malignant astrocytoma. Neurosurg. 1994;34(2):213–220. doi: 10.1227/00006123-199402000-00002. [DOI] [PubMed] [Google Scholar]
- 30.Aghajan Y, Malicki DM, Levy ML, Crawford JR. Atypical anaplastic astrocytoma with unique molecular features and diffuse leptomeningeal spread in a child with long-term survival. BMJ Case Rep CP. 2019;12(2):e228153. doi: 10.1136/bcr-2018-228153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Duffner PK, Cohen ME, Myers MH, Heise HW. Survival of children with brain tumors: SEER program, 1973–1980. Neurol. 1986;36(5):597. doi: 10.1212/wnl.36.5.597. [DOI] [PubMed] [Google Scholar]
- 32.Okamoto Y, Di Patre P-L, Burkhard C, Horstmann S, Jourde B, Fahey M, et al. Population-based study on incidence, survival rates, and genetic alterations of low-grade diffuse astrocytomas and oligodendrogliomas. Acta Neuropathol. 2004;108(1):49–56. doi: 10.1007/s00401-004-0861-z. [DOI] [PubMed] [Google Scholar]
- 33.Shin JY, Diaz AZ. Anaplastic astrocytoma: prognostic factors and survival in 4807 patients with emphasis on receipt and impact of adjuvant therapy. J Neurooncol. 2016;129(3):557–565. doi: 10.1007/s11060-016-2210-1. [DOI] [PubMed] [Google Scholar]
- 34.McCormack BM, Miller DC, Budzilovich GN, Voorhees GJ, Ransohoff J. Treatment and survival of low-grade astrocytoma in adults–1977–1988. Neurosurg. 1992;31(4):636–642. doi: 10.1227/00006123-199210000-00004. [DOI] [PubMed] [Google Scholar]
- 35.Clark GB, Henry JM, McKeever PE. Cerebral pilocytic astrocytoma. Cancer. 1985;56(5):1128–1133. doi: 10.1002/1097-0142(19850901)56:5<1128::aid-cncr2820560529>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 36.Johannesen TB, Langmark F, Lote K. Progress in long-term survival in adult patients with supratentorial low-grade gliomas: a population-based study of 993 patients in whom tumors were diagnosed between 1970 and 1993. J Neurosurg. 2003;99(5):854–862. doi: 10.3171/jns.2003.99.5.0854. [DOI] [PubMed] [Google Scholar]
- 37.Abdulrauf SI, Edvardsen K, Ho KL, Yang XY, Rock JP, Rosenblum ML. Vascular endothelial growth factor expression and vascular density as prognostic markers of survival in patients with low-grade astrocytoma. J Neurosurg. 1998;88(3):513–520. doi: 10.3171/jns.1998.88.3.0513. [DOI] [PubMed] [Google Scholar]
- 38.Phuphanich S, Ferrall S, Greenberg H. Long-term survival in malignant glioma. Prognostic factors. J Fla Med Assoc. 1993;80(3):181–4. [PubMed] [Google Scholar]
- 39.Jungk C, Reinhardt A, Warta R, Capper D, von Deimling A, Herold-Mende C, et al. Extent of resection, MGMT promoter methylation status and tumor location independently predict progression-free survival in adult sporadic pilocytic astrocytoma. Cancers. 2019;11(8):1072. doi: 10.3390/cancers11081072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dong X, Noorbakhsh A, Hirshman BR, Zhou T, Tang JA, Chang DC, et al. Survival trends of grade I, II, and III astrocytoma patients and associated clinical practice patterns between 1999 and 2010: a SEER-based analysis. Neuro-Oncol Pract. 2016;3(1):29–38. doi: 10.1093/nop/npv016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jakola AS, Unsgård G, Myrmel KS, Kloster R, Torp SH, Losvik OK, et al. Surgical strategy in grade II astrocytoma: a population-based analysis of survival and morbidity with a strategy of early resection as compared to watchful waiting. Acta Neurochir. 2013;155(12):2227–2235. doi: 10.1007/s00701-013-1869-8. [DOI] [PubMed] [Google Scholar]
- 42.Sahgal A, Ironside S, Perry J, Mainprize T, Keith J, Laperriere N, et al. Factors influencing overall survival specific to adult low-grade astrocytoma: a population-based study. Clin Oncol. 2013;25(7):394–399. doi: 10.1016/j.clon.2013.04.005. [DOI] [PubMed] [Google Scholar]
- 43.Hwang S-L, Yang Y-HC, Lieu A-S, Chuang M-C, Chang S-J, Chang Y-Y, et al. The conditional survival statistics for survivors with primary supratentorial astrocytic tumors. J Neuro-Oncol. 2000;50(3):257–64. doi: 10.1023/a:1006484220764. [DOI] [PubMed] [Google Scholar]
- 44.Ostertag CB, Kreth F. Iodine-125 interstitial irradiation for cerebral gliomas. Acta Neurochir. 1992;119(1–4):53–61. doi: 10.1007/BF01541782. [DOI] [PubMed] [Google Scholar]
- 45.Tabash MA. Characteristics, survival and incidence rates and trends of pilocytic astrocytoma in children in the United States; SEER-based analysis. J Neurol Sci. 2019;400:148–152. doi: 10.1016/j.jns.2019.03.028. [DOI] [PubMed] [Google Scholar]
- 46.Mamelak AN, Jacoby DB. Targeted delivery of antitumoral therapy to glioma and other malignancies with synthetic chlorotoxin (TM-601) Expert Opin Drug Deliv. 2007;4(2):175–186. doi: 10.1517/17425247.4.2.175. [DOI] [PubMed] [Google Scholar]
- 47.Goodenberger ML, Jenkins RB. Genetics of adult glioma. Cancer Genet. 2012;205(12):613–621. doi: 10.1016/j.cancergen.2012.10.009. [DOI] [PubMed] [Google Scholar]
- 48.Bauman G, Fisher B, Watling C, Cairncross JG, Macdonald D. Adult supratentorial low-grade glioma: long-term experience at a single institution. Int J Radiat Oncol Biol Phys. 2009;75(5):1401–1407. doi: 10.1016/j.ijrobp.2009.01.010. [DOI] [PubMed] [Google Scholar]
- 49.Ahmadi R, Dictus C, Hartmann C, Zurn O, Edler L, Hartmann M, et al. Long-term outcome and survival of surgically treated supratentorial low-grade glioma in adult patients. Acta Neurochir (Wien) 2009;151(11):1359–1365. doi: 10.1007/s00701-009-0435-x. [DOI] [PubMed] [Google Scholar]
- 50.Schomas DA, Laack NN, Brown PD. Low-grade gliomas in older patients: long-term follow up from Mayo Clinic. Cancer. 2009;115(17):3969–3978. doi: 10.1002/cncr.24444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fisher PG, Tihan T, Goldthwaite PT, Wharam MD, Carson BS, Weingart JD, et al. Outcome analysis of childhood low-grade astrocytomas. Pediatr Blood Cancer. 2008;51(2):245–250. doi: 10.1002/pbc.21563. [DOI] [PubMed] [Google Scholar]
- 52.Andrychowski J, Taraszewska A, Czernicki Z, Jurkiewicz J, Netczuk T, Dabrowski P. Ten years observation and treatment of multifocal pilocytic astrocytoma. Folia Neuropathol. 2009;47(4):362–370. [PubMed] [Google Scholar]
- 53.Maiuri F, Del Basso De Caro ML, Iaconetta G, Peca C, Esposito M, de Divitiis E. Prognostic and survival-related factors in patients with well differentiated oligodendrogliomas. Zentralbl Neurochir 2006; 67(4): 204-9 [55] Ohgaki H, Kleihues P. Population-based studies on incidence, survival rates, and genetic alterations in astrocytic and oligodendroglial gliomas. J Neuropathol Exp Neurol. 2005;64(6):479–89. [Google Scholar]
- 54.Ohgaki H, Kleihues P. Population-based studies on incidence, survival rates, and genetic alterations in astrocytic and oligodendroglial gliomas. J Neuropathol Exp Neurol. 2005;64(6):479–489. doi: 10.1093/jnen/64.6.479. [DOI] [PubMed] [Google Scholar]
- 55.Kunieda T, Kikuchi T, Miyamoto S. Epilepsy surgery: surgical aspects. Curr Opin Anaesthesiol. 2012;25(5):533–539. doi: 10.1097/ACO.0b013e32835774d4. [DOI] [PubMed] [Google Scholar]
- 56.Conte V, Baratta P, Tomaselli P, Songa V, Magni L, Stocchetti N. Awake neurosurgery: an update. Minerva Anestesiol. 2008;74(6):289–292. [PubMed] [Google Scholar]
- 57.Perez CA, Burdy LW (2004) Principle and practice of radiation oncology. Philadelphia:Lippincott, Williams & Wilkins. pp.791–839;855–856
- 58.Devita T (2005) Cancer principle & practice of oncology. Philadelphia: Lippencott, Williams& Wilkins 5. pp.2108–2138.
- 59.Cox JD (1991) Radiation oncology JAMA 265(23): 3165-7 [PubMed]
- 60.Clifford KS, Preze CA (2004) Radiation oncology management decisions. Philadelphia: Lippencott –Reven. pp.103–111
- 61.Hall EJ (2000) Radiobiology for the radiologist. 4 Th ed. Philadelphia: JB Lippincott Co. pp.339–361;112–124
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Datasets are available through the corresponding author upon reasonable request.