Abstract
Objectives
Although endorsed by international guidelines, complete revascularization (CR) with Coronary Artery Bypass Grafting (CABG) remains underused. In higher-risk patients such as those with pre-operative atrial fibrillation (AF), the effects of CR are not well studied.
Methods
We analyzed patients’ data from the HEIST (HEart surgery In AF and Supraventricular Tachycardia) registry. Between 2012 and 2020 we identified 4770 patients with pre-operative AF and multivessel coronary artery disease who underwent isolated CABG. We divided the cohort according to the completeness of the revascularization and used propensity score matching (PSM) to minimize differences between baseline characteristics. The primary endpoint was all-cause mortality.
Results
Median follow-up was 4.7 years [interquartile range (IQR) 2.3–6.9]. PSM resulted in 1,009 pairs of complete and incomplete revascularization. Number of distal anastomoses varied, accounting for 3.0 + –0.6 vs. 1.7 + –0.6, respectively. Although early (< 24 h) and 30-day post-operative mortalities were not statistically different between non-CR and CR patients [Odds Ratio (OR) and 95% Confidence Intervals (CIs): 1.34 (0.46–3.86); P = 0.593, Hazard Ratio (HR) and 95% CIs: 0.88 (0.59–1.32); P = 0.542, respectively] the long term mortality was nearly 20% lower in the CR cohort [HR (95% CIs) 0.83 (0.71–0.96); P = 0.011]. This benefit was sustained throughout subgroup analyses, yet most accentuated in low-risk patients (younger i.e., < 70 year old, with a EuroSCORE II < 2%, non-diabetic) and when off-pump CABG was performed.
Conclusion
Complete revascularization in patients with pre-operative AF is safe and associated with improved survival. Particular survival benefit with CR was observed in low-risk patients undergoing off-pump CABG.
Keywords: atrial fibrillation, CABG, complete revascularization, survival, long-term
Introduction
Although never compared directly in a randomized controlled trial (RCT), complete revascularization (CR) during coronary artery bypass grafting (CABG) is considered to be superior to incomplete revascularization (ICR) in multi-vessel coronary artery disease (MV-CAD). The benefit is thought to originate from reduced risk of future cardiovascular events, namely periprocedural myocardial infarction (MI) and repeat revascularization (RR). Many observational studies, as well as insights from subgroup analysis of RCTs reinforced this notion (1–3). Several RCTs which investigated CR in the context of percutaneous intervention (PCI) for ST-elevation myocardial infarction (STEMI) have shown the benefit of complete, compared to ICR for MV-CAD (4–6). However, surgical revascularization is still the first-choice procedure in high-risk non-acute MI patients, specifically those with diabetes (7) and intermediate-to-high anatomical complexity coronary disease (8).
The 2018 ESC/EACTS guidelines on myocardial revascularization emphasized that the expected highest completeness of revascularization should guide the choice of treatment strategy (9). The question arises if surgeons should attempt CR at all costs and, if not, what type of risk factors may discourage them from pursuing one. Atrial fibrillation (AF) is an independent predictor of mortality and morbidity after CABG (10, 11). Apart from an increased risk of stroke, AF is also associated with an over a fourfold increased risk of developing heart failure (12). Moreover, an impaired graft flow in AF CABG patients was observed (13). Most reports estimate the pre-op AF prevalence in CABG patients at 6–10%, but in some reports, it was as high as 20% (14). Because of aging of the society, the prevalence of AF is likely to rise.
The disparity in reported results of CR in CABG, and the shortage of evidence in high-risk patients, requires further investigation (15, 16). The current study aimed to address whether there exists a survival benefit with CR in MV-CAD and underlying AF.
Patients and Methods
Study Population and Clinical Variables
Because of the retrospective nature of the study, the ethics committee approval was waived. Our investigation was a part of the HEIST (Heart Surgery In atrial fibrillation and Supraventricular tachycardia) Registry (NCT04860882). We included consecutive AF patients, over 18 year old, admitted to 8 tertiary centers in Poland, Netherlands, and Italy between January 2012 and December 2020 who had isolated CABG for MV-CAD performed (Supplementary Figure 1). Patients who (1) had no diagnosis of AF; (2) had CABG with concomitant valvular or aortic procedures, were not included in the study. Similarly, (3) patients with single-vessel CAD or (4) patients in whom the number of distal anastomoses and/or type of graft material used could not be determined were excluded from the analyses. (5) Patients undergoing hybrid revascularization by intention-to-treat protocol, or who were admitted for (6) staged revascularization strategy or (7) re-do surgery were not included.
Endpoints and Definitions
The primary endpoint was all-cause mortality following complete vs. incomplete surgical revascularization for MV-CAD. We defined CR as grafting two significantly stenotic lesions in two-vessel disease and three lesions in three-vessel disease of different territories: right coronary artery (RCA), left anterior descending- (LAD), and circumflex- (Cx) artery. Additional grafts to the diseased systems were encouraged and when the number of grafts was greater than the number required for CR, the approach was considered as “supracomplete” revascularization (SCR). Only the coronary vessels with significant stenosis were bypassed. We defined ICR as failure to graft two significantly stenotic lesions in two-vessel disease and three lesions in three-vessel disease of different territories for whatever reason (15). Each distal anastomosis was counted as a separate graft, e.g., sequential conduit was counted as more than one graft. Whenever the territory that sequential graft supplied couldn’t be determined from the registry or this data was missing, it was not taken into consideration when assessing the completeness of revascularization. We report data on early post-operative (< 24 h) mortality rates, in-hospital complications, lengths of stay in the intensive care unit (ICU) and in the hospital (HLoS).
Statistical Analyses
Continuous variables were summarized as mean with standard deviation if normally distributed; non-normal distributions were summarized as median with IQR and compared with the Mann–Whitney U test or standard t-test, as appropriate. Categorical variables [number (%)] were compared with the Fisher exact test. Propensity matching was generated for each patient from a non-parsimonious multivariable logistic regression model that was based on baseline characteristics (age, number of vessels diseased, comorbidities, EuroSCORE II, LVEF, CCS, NYHA, and others listed in Table 1) and procedural [concomitant ablation, type of surgery (Off-Pump, On-Pump), procedure urgency] covariates as independent variables with treatment type (CR vs. non-CR) as a dependent variable. We used and opt-match and matchIt packages, 1-to-1 pairing, without replacement within a specific caliper width of 0.2 standard deviation of the propensity score. We computed standardized mean differences (SMDs) to verify the balance between CR versus non-CR groups after matching (Supplementary Figure 2). Risk Ratios (RRs) were used for in-hospital outcomes, whereas Cox proportional-hazards models were used to determine factors related to the event-free survival at long-term follow-up. We calculated Hazard Ratios (HRs) point estimate and 95% confidence intervals (95% CIs) with ensuing statistical models. Mortality was assessed with Kaplan–Meier survival curves fitted after PS matching. As a further sensitivity analysis, defined subgroup analyses were performed to assess the mortality in different scenarios. STATA MP v13.0 software (StataCorp, College Station, TX, United States) and R (with Rcmdr package and EZR software) were used for computations.
TABLE 1.
Pre-operative characteristics after PS-matching.
| Total (2018) | Non-CR (1009) | CR (1009) | P-value | |
| Baseline characteristics | ||||
| Age years [median (IQR)] | 70 (64–76) | 70 (64–76) | 70 (64–76) | 0.792 |
| Male gender | 1589 (78.7) | 795 (78.8) | 794 (78.7) | 0.99 |
| EuroSCORE II | 1.91 (1.21,3.19) | 1.92 (1.22, 3.19) | 1.90 (1.18, 3.19) | 0.760 |
| Diabetes | 899 (44.5) | 457 (45.3) | 442 (43.8) | 0.531 |
| Insulin ± oral hypoglycemic drugs | 372 (18.4) | 186 (18.4) | 186 (18.4) | 0.99 |
| Smoking | 1247 (61.8) | 628 (62.2) | 619 (61.3) | 0.714 |
| Hypertension | 1829 (90.6) | 917 (90.9) | 912 (90.4) | 0.760 |
| Hyperlipidemia | 1293 (64.1) | 656 (65.0) | 637 (63.1) | 0.404 |
| BMI [median (IQR)] | 28.69 (25.78–31.70) | 28.70 (25.65, 31.71) | 28.69 (25.95, 31.67) | 0.815 |
| Pulmonary hypertensiona | 154 (7.6) | 86 (8.5) | 68 (6.7) | 0.154 |
| Renal impairment | 1128 (55.9) | 570 (56.5) | 558 (55.3) | 0.622 |
| Dialysis (regardless of CC) | 26 (1.3) | 13 (1.3) | 13 (1.3) | 0.99 |
| Peripheral artery disease | 515 (25.5) | 263 (26.1) | 252 (25.0) | 0.610 |
| Cerebrovascular disease | 162 (8) | 75 (7.4) | 87 (8.6) | 0.368 |
| History of stroke | 73 (3.6) | 34 (3.4) | 39 (3.9) | 0.634 |
| History of TIA/RIND | 72 (3.6) | 28 (2.8) | 44 (4.4) | 0.071 |
| chronic lung disease | 111 (5.5) | 56 (5.6) | 55 (5.5) | 0.99 |
| LVEF (%) [median (IQR)]a | 50 (40–55) | 50 (40–55) | 49 (40–55) | 0.527 |
| 3 vessel CAD | 1609 (79.7) | 813 (80.6) | 796 (78.9) | 0.376 |
| Previous MI | 1076 (53.3) | 517 (51.2) | 559 (55.4) | 0.067 |
| Previous PCI | 304 (15.1) | 152 (15.1) | 152 (15.1) | 0.99 |
| NYHA IV | 31 (1.5) | 14 (1.4) | 17 (1.7) | 0.718 |
| CCS 4 | 194 (9.6) | 93 (9.2) | 101 (10.0) | 0.597 |
| ACS | 95 (4.7) | 47 (4.7) | 48 (4.8) | 0.99 |
aMissing data.
PS, propensity score; IQR, interquartile range; BMI, body mass index; PA, pulmonary artery; CC, creatinine clearance; TIA, transient ischemic attack; LVEF, left ventricle ejection fraction; CAD, coronary artery disease; VD, vessel disease; MI, myocardial infarction; PCI, percutaneous coronary intervention; NYHA, New York Heart Association; CCS, Canadian Cardiovascular Society; ACS, Acute Coronary Syndrome.
Results
We identified 4,770 patients with pre-operative AF undergoing CABG; of those, in 3,193 (66.9%) patients, CR according to predefined criteria was achieved. During the 9-year follow-up, there were no marked differences in the proportion of complete vs. ICR, nor there were any differences in the adoption of multi-arterial grafting (MAG) (Figure 1). Using the propensity score matching (PSM) model, two groups of 1,009 patients each were determined, by pairing non-CR patients with CR controls to achieve similar baseline (Table 1) and surgical (Table 2) characteristics. We report details on matching quality in Supplementary Figure 2.
FIGURE 1.
Temporal trends in completeness of revascularization and utilization of arterial grafting. Complete revascularization (dark blue), non-complete revascularization (light blue), multiple arterial grafting (orange), single arterial grafting (yellow), total arterial revascularization (red).
TABLE 2.
Operative characteristics after PS-matching.
| Total (2018) | Non-CR (1009) | CR (1009) | P-value | |
| Procedural characteristics | ||||
| Critical pre-operative state | 43 (2.1) | 22 (2.2) | 21 (2.1) | 0.99 |
| IABP | 8 (0.4) | 4 (0.4) | 4 (0.4) | 0.99 |
| iv. inotropes | 35 (1.7) | 19 (1.9) | 16 (1.6) | 0.734 |
| Mechanical vent | 8 (0.4) | 5 (0.5) | 3 (0.3) | 0.726 |
| Emergency surgery | 74 (3.7) | 37 (3.7) | 37 (3.7) | 0.99 |
| OPCAB | 1031 (51.1) | 531 (52.6) | 500 (49.6) | 0.182 |
| CPB (min)a | 74 (55–95) | 60 (45–74) | 90 (71–109) | < 0.001 |
| X-clamp (min)a | 41 (29–54) | 32 (24–41) | 50 (40–61) | < 0.001 |
| Conversion to ONCAB | 17 (0.8%) | 11 (1.1) | 6 (0.6) | 0.330 |
| Concomitant ablation | 79 (3.9) | 41 (4.1) | 38 (3.8) | 0.819 |
aMissing data.
PS, propensity score; CPR, cardiopulmonary resuscitation; IABP, intra-aortic balloon pump; iv, intravenous; OPCAB, Off-Pump Coronary Artery Bypass; ONCAB, On-Pump Coronary Artery Bypass; CPB, cardiopulmonary bypass; LAAO, left atrial appendage occlusion; ±; SD, Standard Deviation.
Baseline and Surgical Characteristics
Baseline characteristics were balanced between groups, with similar EUROSCORE values [median (IQR): 1.92 (1.22–3.19) and 1.90 (1.18–3.19), respectively in patients with incomplete and CR]. The median age was identical in both groups – 70 years (64–76). Of included patients, 78.8% (non-CR) vs. 78.7% (CR) were men, 80.6% of non-CR patients in comparison with 78.9% in the CR group had a 3-vessel coronary artery disease (p = 0.376). Concomitant ablation was performed in 4.1% of non-CR and 3.8% of the CR group; 531 (52.6%) non-CR and 500 (49.6%) CR patients were operated on without the use of cardio-pulmonary bypass (p = 0.182). As expected, the number of distal anastomoses varied between groups (1.7 ± 0.6 non-CR vs. 3.0 ± 0.6 CR, P < 0.001). Table 3 lists information regarding grafts and anastomoses.
TABLE 3.
Grafts and anastomoses after PS-matching.
| Total (2018) | Non-CR (1009) | CR (1009) | |
| LIMA | 1860 (92.2) | 914 (90.5) | 950 (94.2) |
| RIMA | 83 (4.1) | 35 (3.5) | 48 (4.8) |
| BIMA | 77 (3.8) | 34 (3.4) | 43 (4.3) |
| Pedicled IMAa | 619 (30.7) | 295 (34.6) | 324 (38.0) |
| Skeletonized IMAa | 1085 (53.8) | 557 (65.4) | 528 (62.0) |
| Radial artery | 44 (2.2) | 25 (2.5) | 19 (1.9) |
| Multiple arterial grafts | 203 (10.1) | 82 (8.1) | 121 (12.0) |
| Total Arterial Revascularization | 42 (2.1) | 0 (0.0) | 42 (4.2) |
| Number of anastomoses (Mean + –SD) | 2 (1–2) | 3 (3–3) |
aMissing data.
LIMA/RIMA/BIMA, Left/Right/Bilateral Internal Mammary Artery; RA, Radial Artery. ±; SD, Standard Deviation.
Clinical Outcomes
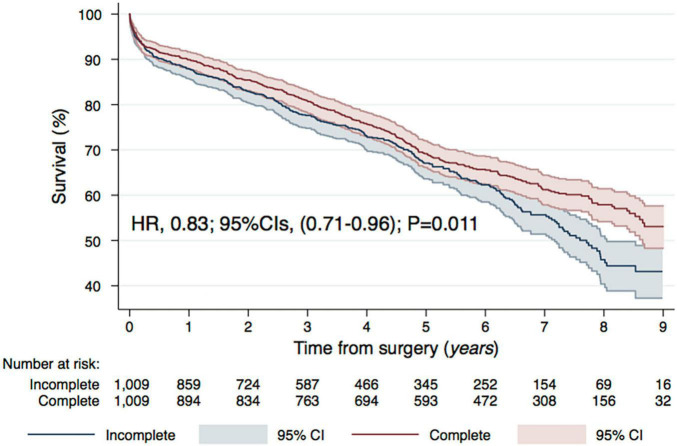
In-hospital outcomes and post-operative complications were consistent between groups (Table 4). Early mortality (24 h) and 30-day mortality were unaffected by CR [Odds Ratio (OR) and 95% Confidence Intervals (CIs): 1.34 (0.46–3.86), P = 0.593, Hazard Ratio (HR) and 95% CIs: 0.88 (0.59–1.32), P = 0.542, respectively]. Cardiac tamponade and/or re-thoracotomy for bleeding occurred in 3.1 vs. 5.1% and was statistically more frequent in the CR group [Risk Ratio (RR) and 95% CIs, 1.65 (1.06–2.55), P = 0.032]. Cardiopulmonary bypass (CBP) and aortic X-clamp times were significantly longer in the CR group: the median of CBP time was 65 vs. 79 min (P ≤ 0.001) in the CR and non-CR group and respectively 34 vs. 40 min (P ≤ 0.001) of aortic X-clamp time. In the long-term follow-up [As stated in the abstract median follow up was 4.7 years (2.3–6.9)], CR was associated with significantly lower mortality [HR (95% CIs) 0.83 (0.71–0.96), P = 0.011] (Figure 2).
TABLE 4.
In-hospital outcomes after PS-matching.
| Non-CR (1009) | CR (1009) | Risk ratio (95% CIs) | P-value | |
| Early post-operative mortality (< 24 h) | 7 (0.7) | 8 (0.8) | 1.14 (0.42–3.14) | 0.288 |
| Cardiac tamponade and/or rethoracotomy for bleeding | 31 (3.1) | 51 (5.1) | 1.65 (1.06–2.55) | 0.032 |
| Respiratory failure | 60 (5.9) | 74 (7.3) | 1.23 (0.89–1.71) | 0.245 |
| Neurologic complications | 25 (2.5) | 21 (2.1) | 0.84 (0.47–1.49) | 0.655 |
| Multiorgan failure | 21 (2.1) | 21 (2.1) | 1.00 (0.55–1.82) | 1.000 |
| Gastrointestinal complications | 13 (1.3) | 16 (1.6) | 1.23 (0.6–2.55) | 0.709 |
| Acute kidney failure and/or dialysis | 32 (3.2) | 34 (3.4) | 1.06 (0.66–1.71) | 0.901 |
| Superficial sternal wound infection | 19 (1.9) | 21 (2.1) | 1.11 (0.60–2.04) | 0.873 |
| Deep sternal wound infection | 18 (1.8) | 14 (1.4) | 0.78 (0.39–1.56) | 0.594 |
| Mediastinitis | 4 (0.4) | 6 (0.6) | 1.50 (0.42–5.3) | 0.753 |
| PPI | 4 (0.4) | 4 (0.4) | 1.00 (0.25–3.99) | 1.000 |
| ECMO | 1 (0%) | 1 (0%) | 1.00 (0.06–15.97) | 1.000 |
| IABP | 18 (1.8%) | 21 (2.1%) | 1.17 (0.63–2.18) | 0.628 |
PS, propensity score; CIs, confidence intervals; MI, myocardial infarction; ICU, intensive care unit; PPI, permanent pacemaker implantation; ECMO, extracorporeal membrane oxygenation; IABP, intra-aortic balloon pump.
FIGURE 2.
Long-term mortality, propensity matched Kaplan–Meier survival curves between the two groups: CR vs. non-CR CABG for the analysis of long-term survival. Hazard Ratios and respective 95% Confidence Intervals.
The CR group was further divided into patients who underwent complete- and “supracomplete” revascularization. The latter was associated with an even greater reduction in mortality HR (95% CIs) 0.76 (0.59–0.97), P = 0.023 (for SCR vs. ICR). Between ICR, CR, and SCR we observed a significant trend toward lower mortality (log rank P = 0.032, Figure 3).
FIGURE 3.
Long-term mortality, propensity matched Kaplan–Meier survival curves between three groups: CR, non-CR, SCR CABG. Logrank test for differences in survival between groups. CR, complete revascularization; SCR, “supracomplete” revascularization.
In subgroup analyses, the benefit of improved long-term survival was sustained across diverse patient populations. Especially beneficent were younger (< 70 years old.) patients [HR (95% CIs) 0.67 (0.53–0.85), P = 0.001 for < 70 year old Vs. HR (95% CIs) 0.94 (0.78–1.13), P = 0.497 for ≥ 70 year old; P interaction = 0.027]. The effect was also more pronounced in patients with lower EuroSCORE II, without diabetes and when off-pump CABG was performed. Further details on the subgroup analyses are shown in Figure 4.
FIGURE 4.
Subgroup Analysis, Hazard Ratios and 95% Confidence Intervals (Cis) for death from any cause in CR as compared to non-CR according to selected characteristics; CR, complete revascularization; VD, vessel disease; LVEF, left ventricle ejection fraction; MI, myocardial infarction.
Discussion
The current analysis is the first to focus on the long-term results of complete and incomplete surgical revascularization for MV-CAD in patients with pre-existing AF. Its main findings are as follows; (1) there was a high rate of ICR; (2) long-term benefit of CR; (3) even greater benefit with a higher number of additional grafts; (4) low prevalence of MAG and TAR in the population of AF patients, without significant temporal trends.
Complete revascularization, especially achieved through CABG, is characterized by improved long-term survival and a lower rate of reinterventions compared with ICR (1–3). It remains to be established whether this distinction is specifically because of ICR as a surgical method, deficiency, or anatomical obstacles during CABG or, whether the ICR is only a marker of more advanced and progressive coronary disease. The ICR usually indicates complex coronary pathology, with unfavorable outcomes originating from the patient’s baseline risk profile. In reality, even though ICR may contrarily influence long-term results (17), it may be the most appropriate treatment method in a specific subset of prohibitive-risk patients. When the risks of surgery must be minimized to reduce perioperative mortality and complications, target vessel revascularization represents possibly the best feasible course of action (6).
Patients presenting with AF are at markedly elevated, yet non-prohibitive, operative risk, nor is AF itself accounted for in prognostic scores (e.g., EuroSCORE II). Although, the prevalence of AF in patients undergoing CABG is much lower than in patients undergoing mitral valve surgery (18, 19), up to 20% of patients presenting for coronary surgical procedures may have preoperative AF (14, 20), which is often used as a marker for high-risk patients (10, 11, 21). This percentage rises with age and decreased left ventricular function, which is seen in an increasing number of patients referred for CABG surgery. Although no data exists on performing CR in this population, because of their high-risk nature, surgeons may be reluctant to aim for CR as it is associated with longer operative time. Until now, no single study has focused on a comparison of CR/ICR in the AF population undergoing CABG.
One subgroup analysis of the Atrial Fibrillation undergoing Coronary Artery Stenting (AFCAS) registry focusing on the impact of ICR, has shown that of 445 (46.8%) PCI subjects in whom physicians opted for ICR, at 1-year follow-up, had a higher rate of the composite endpoint of acute MI, stent thrombosis and RR, compared to patients with CR (13.9% vs. 9.4%, p = 0.003) (22). In an adjusted multivariable analysis, only creatinine clearance (inverse relationship) and ICR were independently associated with a higher risk of the composite endpoint [HR (95% CIs) 1.66, (1.10–2.50), p = 0.013] (22).
The latest reports and registries analyses present data on safety and efficacy of surgical CR in AF. In a study of 900 patients with end-stage renal disease, where 14.1% of all patients had pre-existing AF, emergency surgery, diabetes mellitus, the number of vein grafts and age were identified as risk factors for mortality (23). CR, the use of an internal thoracic artery and the sinus rhythm pre-op were recognized as beneficial factors for long-term survival (23). Although AF was not identified as an independent risk factor for perioperative mortality (p = 0.59), it was an independent predictor for late mortality (p < 0.001) (23). In an analysis of the KROK registry Off-Pump CABG offered a 30-day survival benefit to patients undergoing CABG surgery and presenting with underlying AF (24). On-Pump CABG, on the other hand, was associated with significantly improved long-term survival. CR was possible in 67.5% of patients and was significantly higher, by 10%, in patients undergoing On-Pump CABG (73.3 vs. 62.6%; P < 0.001) (24).
One finding of the preset report requires special attention; it was demonstrated that “supra-complete” revascularization, may further improve survival in AF patients undergoing CABG. A study by Schwann et al. investigated the effects of SCR in SAG and MAG and concluded that it conveyed a survival benefit in patients with 3-VD in a single arterial grafting group (which is the majority in our study) (25). Conversely, Chu et al. observed no survival benefit with multiple grafts to each myocardial territory (26). Supra-complete revascularization could be beneficial in several ways, by securing the vulnerable myocardium during the early phase post-op, particularly prone to arrhythmias and disturbances in blood flow, or protecting distal coronary arteries from MI in long-term when functionally non-significant and non-revascularized lesions become significant (27). However, aiming for SCR must increase operative times and since its benefit is not well established the surgeons face a difficult decision. Our results suggest that preoperative AF, although a poor prognostic factor in general, should not be deemed prohibitive while considering additional grafts to each coronary territory.
Several factors, beyond the completeness of the revascularization, can influence the outcome in the AF population. Analysis of the KROK registry (28) showed a significant survival benefit associated with concomitant surgical ablation (SA) in the setting of CABG. The same analysis showed that it remains severely underused, as it was performed only in 4.4%. Recent guidelines give a recommendation to concomitant SA during CABG surgery. Considering that both CR and SA prolong operative times, surgeons might decide to choose SA over the additional graft that would ensure completeness of the revascularization. Our results suggest that in non-prohibitive risk patients, both SA and CR should be aimed for. Another analysis of the KROK registry showed that patients undergoing multiple arterial grafting have survival benefits at long-term follow-up (13 years post-op) as compared to single arterial grafting (29). This benefit was further sustained in subgroup analyses, yet most appraised in low risk patients (< 70-year-old; EuroSCORE < 2; no diabetes) and when CR was achieved (P = 0.009). Some studies suggest that benefit with the CR may be conferred to SAG patients, wheares when multiple arterial grafts are used their superior patency could neutralize survival benefit associated with completeness of revascularization (30). Indeed, in our sensitivity analysis the benefit in MAG group was non-significant, although it has to be noted that the number of MAG patients was low.
Limitations
Our study has several limitations. First, only all-cause mortality assessment is possible; the information regarding the cause of death, reinterventions, MIs, heart failure hospitalizations, adherence to anticoagulation therapy, or angiographic patency follow-up is not recorded in the registry. Second, although we addressed a potential selection bias, with propensity score matching according to baseline clinical variables, several confounders could prevail, an important of which is the lack of coronary angiograms that would allow us to access the percentage of chronic total occlusions. Additionally, we did not include the grafting choice (arterial vs. venous grafting) and patient allocation in the propensity score model. Third, detailed anatomy of coronary vessels is not available and therefore the feasibility of CR in each case could not be assessed. Finally, our data regarding coronary revascularization concerns CABG surgery only. Perhaps, some patients, in whom CR during surgery was deemed infeasible, could benefit from a staged hybrid revascularization with PCI as a second stage. Unfortunately, the registry at that time did not gather data regarding subsequent interventions.
Conclusion
In this multicenter retrospective propensity-matched study of patients with preoperative AF, CR during CABG was associated with improved long-term survival. The particular benefit was observed in lower-risk patients. A significant trend was observed toward lower mortality with “supracomplete” revascularization.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics Statement
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author Contributions
MP, JS, JF, and MKow: conception and design. PS, RLo, MD, WWo, JR, MJ, MZ, and KB: administrative support. JS, RLi, GF, AK, WWa, AŁ, SS, DJ, FJ, MM, GM, and GR: provision of study materials or patients. NP-S, SM, TL, DR, and AS: collection and assembly of data. MP, MKow, MKoł, PGM, PM, and DF: data analysis and interpretation. MP, JS, JF, MKow, MKoł, and SM: manuscript writing. All authors approved the final manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2022.910811/full#supplementary-material
References
- 1.Garcia S, Sandoval Y, Roukoz H, Adabag S, Canoniero M, Yannopoulos D, et al. Outcomes after complete versus incomplete revascularization of patients with multivessel coronary artery disease: a meta-analysis of 89,883 patients enrolled in randomized clinical trials and observational studies. J Am Coll Cardiol. (2013) 62:1421–31. 10.1016/j.jacc.2013.05.033 [DOI] [PubMed] [Google Scholar]
- 2.Zimarino M, Ricci F, Romanello M, Di Nicola M, Corazzini A, De Caterina R. Complete myocardial revascularization confers a larger clinical benefit when performed with state-of-the-art techniques in high-risk patients with multivessel coronary artery disease: a meta-analysis of randomized and observational studies. Catheter Cardiovasc Interv. (2016) 87:3–12. 10.1002/ccd.25923 [DOI] [PubMed] [Google Scholar]
- 3.Takahashi K, Serruys PW, Gao C, Ono M, Wang R, Thuijs DJFM, et al. Ten-year all-cause death according to completeness of revascularization in patients with three-vessel disease or left main coronary artery disease: insights from the SYNTAX extended survival study. Circulation. (2021) 144:96–109. 10.1161/CIRCULATIONAHA.120.046289 [DOI] [PubMed] [Google Scholar]
- 4.Mehta SR, Wood DA, Storey RF, Mehran R, Bainey KR, Nguyen H, et al. Complete Revascularization with multivessel PCI for myocardial infarction. N Engl J Med. (2019) 381:1411–21. [DOI] [PubMed] [Google Scholar]
- 5.Engstrøm T, Kelbæk H, Helqvist S, Høfsten DE, Kløvgaard L, Holmvang L, et al. Complete revascularisation versus treatment of the culprit lesion only in patients with ST-segment elevation myocardial infarction and multivessel disease (DANAMI-3—PRIMULTI): an open-label, randomised controlled trial. Lancet. (2015) 386:665–71. 10.1016/s0140-6736(15)60648-1 [DOI] [PubMed] [Google Scholar]
- 6.Kowalewski M, Schulze V, Berti S, Waksman R, Kubica J, Kołodziejczak M, et al. Complete revascularisation in ST-elevation myocardial infarction and multivessel disease: meta-analysis of randomised controlled trials. Heart. (2015) 101:1309–17. 10.1136/heartjnl-2014-307293 [DOI] [PubMed] [Google Scholar]
- 7.Farkouh ME, Domanski M, Sleeper LA, Siami FS, Dangas G, Mack M, et al. Strategies for multivessel revascularization in patients with diabetes. N Engl J Med. (2012) 367:2375–84. [DOI] [PubMed] [Google Scholar]
- 8.Serruys PW, Morice MC, Kappetein AP, Colombo A, Holmes DR, Mack MJ, et al. Percutaneous coronary intervention versus coronary-artery bypass grafting for severe coronary artery disease. N Engl J Med. (2009) 360:961–72. [DOI] [PubMed] [Google Scholar]
- 9.Neumann FJ, Sousa-Uva M, Ahlsson A, Alfonso F, Banning AP, Benedetto U, et al. 2018 ESC/EACTS guidelines on myocardial revascularization. Eur Heart J. (2019) 40:87–165.30165437 [Google Scholar]
- 10.Batra G, Ahlsson A, Lindahl B, Lindhagen L, Wickbom A, Oldgren J. Atrial fibrillation in patients undergoing coronary artery surgery is associated with adverse outcome. Ups J Med Sci. (2019) 124:70–7. 10.1080/03009734.2018.1504148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Böning A, Diegeler A, Hilker M, Zacher M, Reents W, Faerber G, et al. Preoperative atrial fibrillation and outcome in patients undergoing on-pump or off-pump coronary bypass surgery: lessons learned from the GOPCABE trial. Interact Cardiovasc Thorac Surg. (2015) 20:74–8. 10.1093/icvts/ivu331 [DOI] [PubMed] [Google Scholar]
- 12.Ruddox V, Sandven I, Munkhaugen J, Skattebu J, Edvardsen T, Otterstad JE. Atrial fibrillation and the risk for myocardial infarction, all-cause mortality and heart failure: a systematic review and meta-analysis. Eur J Prev Cardiol. (2017) 24:1555–66. 10.1177/2047487317715769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shin H, Hashizume K, Iino Y, Koizumi K, Matayoshi T, Yozu R. Effects of atrial fibrillation on coronary artery bypass graft flow. Eur J Cardiothorac Surg. (2003) 23:175–8. 10.1016/s1010-7940(02)00730-3 [DOI] [PubMed] [Google Scholar]
- 14.McCarthy PM, Davidson CJ, Kruse J, Lerner DJ, Braid-Forbes MJ, McCrea MM, et al. Prevalence of atrial fibrillation before cardiac surgery and factors associated with concomitant ablation. J Thorac Cardiovasc Surg. (2020) 159:2245–2253.e15. 10.1016/j.jtcvs.2019.06.062 [DOI] [PubMed] [Google Scholar]
- 15.Schaefer A, Conradi L, Schneeberger Y, Reichenspurner H, Sandner S, Tebbe U, et al. Clinical outcomes of complete versus incomplete revascularization in patients treated with coronary artery bypass grafting: insights from the TiCAB trial. Eur J Cardiothorac Surg. (2020) 59:417–25. 10.1093/ejcts/ezaa330 [DOI] [PubMed] [Google Scholar]
- 16.Vander Salm TJ, Kip KE, Jones RH, Schaff HV, Shemin RJ, Aldea GS, et al. What constitutes optimal surgical revascularization? Answers from the bypass angioplasty revascularization investigation (BARI). J Am Coll Cardiol. (2002) 39:565–72. 10.1016/s0735-1097(01)01806-x [DOI] [PubMed] [Google Scholar]
- 17.Mocanu V, Buth KJ, Kelly R, Légaré JF. Incomplete revascularization after coronary artery bypass graft operations is independently associated with worse long-term survival. Ann Thorac Surg. (2014) 98:549–55. 10.1016/j.athoracsur.2014.02.090 [DOI] [PubMed] [Google Scholar]
- 18.Suwalski P, Kowalewski M, Jasiński M, Staromłyński J, Zembala M, Widenka K, et al. Survival after surgical ablation for atrial fibrillation in mitral valve surgery: analysis from the polish national registry of cardiac surgery procedures (KROK). J Thorac Cardiovasc Surg. (2019) 157:1007–1018.e4. 10.1016/j.jtcvs.2018.07.099 [DOI] [PubMed] [Google Scholar]
- 19.Rankin JS, He X, O’Brien SM, Jacobs JP, Welke KF, Filardo G, et al. The Society of Thoracic Surgeons risk model for operative mortality after multiple valve surgery. Ann Thorac Surg. (2013) 95:1484–90. 10.1016/j.athoracsur.2012.11.077 [DOI] [PubMed] [Google Scholar]
- 20.Malaisrie SC, McCarthy PM, Kruse J, Matsouaka RA, Churyla A, Grau-Sepulveda MV, et al. Ablation of atrial fibrillation during coronary artery bypass grafting: late outcomes in a Medicare population. J Thorac Cardiovasc Surg. (2021) 161:1251–61. 10.1016/j.jtcvs.2019.10.159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ad N, Barnett SD, Haan CK, O’Brien SM, Milford-Beland S, Speir AM. Does preoperative atrial fibrillation increase the risk for mortality and morbidity after coronary artery bypass grafting? J Thorac Cardiovasc Surg. (2009) 137:901–6. 10.1016/j.jtcvs.2008.09.050 [DOI] [PubMed] [Google Scholar]
- 22.Proietti M, Airaksinen KEJ, Rubboli A, Schlitt A, Kiviniemi T, Karjalainen PP, et al. P1177 Impact of incomplete revascularization in atrial fibrillation patients undergoing percutaneous coronary intervention: the afcas registry. EP Europace. (2018) 20:i227. 10.1093/europace/euy015.662 [DOI] [Google Scholar]
- 23.Schönburg M, Ziegelhoeffer T, Weinbrenner F, Bechtel M, Detter C, Krabatsch T, et al. Preexisting atrial fibrillation as predictor for late-time mortality in patients with end-stage renal disease undergoing cardiac surgery–a multicenter study. Thorac Cardiovasc Surg. (2008) 56:128–32. 10.1055/s-2007-989432 [DOI] [PubMed] [Google Scholar]
- 24.Kowalewski M, Jasiński M, Staromłyński J, Zembala M, Widenka K, Brykczyński M, et al. On-Pump vs Off-Pump coronary artery bypass surgery in atrial fibrillation. Analysis from the polish national registry of cardiac surgery procedures (KROK). PLoS One. (2020) 15:e0231950. 10.1371/journal.pone.0231950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schwann TA, Yammine MB, El-Hage-Sleiman AM, Engoren MC, Bonnell MR, Habib RH. The effect of completeness of revascularization during CABG with single versus multiple arterial grafts. J Card Surg. (2018) 33:620–8. 10.1111/jocs.13810 [DOI] [PubMed] [Google Scholar]
- 26.Chu D, Bakaeen FG, Wang XL, Coselli JS, LeMaire SA, Huh J. The impact of placing multiple grafts to each myocardial territory on longterm survival after coronary artery bypass grafting. J Thorac Cardiovasc Surg. (2009) 137:60–4. 10.1016/j.jtcvs.2008.09.018 [DOI] [PubMed] [Google Scholar]
- 27.Thuesen AL, Riber LP, Veien KT, Christiansen EH, Jensen SE, Modrau I, et al. Fractional flow reserve versus angiographically-guided coronary artery bypass grafting. J Am Coll Cardiol. (2018) 72:2732–43. 10.1016/j.jacc.2018.09.043 [DOI] [PubMed] [Google Scholar]
- 28.Suwalski P, Kowalewski M, Jasiński M, Staromłyński J, Zembala M, Widenka K, et al. Surgical ablation for atrial fibrillation during isolated coronary artery bypass surgery. Eur J Cardiothorac Surg. (2020) 57:691–7. [DOI] [PubMed] [Google Scholar]
- 29.Kowalewski M, Pasierski M, Litwinowicz R, Zembala M, Piekuś-Słomka N, Tobota Z, et al. Multiple versus single arterial coronary arterial bypass grafting surgery for multivessel disease in atrial fibrillation. Semin Thorac Cardiovasc Surg. (2021) 33:974–83. 10.1053/j.semtcvs.2020.11.015 [DOI] [PubMed] [Google Scholar]
- 30.Rosenblum JM, Binongo J, Wei J, Liu Y, Leshnower BG, Chen EP, et al. Priorities in coronary artery bypass grafting: is midterm survival more dependent on completeness of revascularization or multiple arterial grafts? J Thorac Cardiovasc Surg. (2021) 161:2070–8. 10.1016/j.jtcvs.2019.11.125 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.