Abstract
Purpose
The purpose of this study is to analyze clinical information and combine significant parameters to generate a predictive model and achieve a better prognosis prediction of dermatomyositis-associated interstitial lung disease with positive melanoma differentiation-associated gene 5 antibody (MDA5+ DM-ILD) and stratify patients according to prognostic risk factors appropriately.
Methods
We retrospectively reviewed 63 patients MDA5+ DM-ILD who were treated in our hospital from January 2018 to January 2021. Our study incorporated most clinical characteristics in clinical practice to explore the associations and predictive functions of clinical characteristics and prognosis. Student's t-test, Mann-Whitney U-test, chi-squared test, Pearson correlation analysis, Cox regression analysis, R, receiver operating characteristic curves (ROC curves), and Kaplan-Meier survival curves were performed to identify independent predictors for the prognosis of MDA5+DM-ILD.
Results
In all the 63 patients with MDA5+DM-ILD, 44 improved but 19 did not. Poor prognosis was found more frequently in patients who were older, clinically amyopathic variant of dermatomyositis (CADM), and/or with short duration, short interval of DM and ILD, long length of stay, fever, dyspnea, non-arthralgia, pulmonary infection, pleural effusion (PE), high total computed tomography scores (TCTs), ground-glass opacity (GGO), consolidation score, reticular score and fibrosis score, decreased forced vital capacity (FVC), forced expiratory volume in 1s (FEV1), albumin, A/G, glomerular filtration rate (GFR) and tumor necrosis factor α (TNFα), high titer of anti-MDA5, proteinuria, high levels of monocyte, lactate dehydrogenase (LDH), ferritin (FER), neuron specific enolase (NSE) and glucocorticoid, antibiotic, antiviral, and non-invasive positive pressure ventilation (NPPV). The multivariate Cox regression analysis demonstrated that duration, fever, PE, TCTs and aspartate transaminase (AST) were independent predictors of poor prognosis in patients with MDA5+DM-ILD. The nomogram model quantified the risk of 400-day death as: duration ≤ 4 months (5 points), fever (88 points), PE (21 points), TCTs ≥10 points (22 points), and AST ≥200 U/L (100 points) with high predictive accuracy and convenience. The ROC curves possessed good discriminative ability for combination of fever, PE, TCTs, and AST, as reflected by the area under curve (AUC) being.954, 95% CI 0.902–1.000, and sensitivity and specificity being 84.2 and 94.6%, respectively.
Conclusion
We demonstrated that duration, fever, PE, TCTs, and AST could be integrated together to be independent predictors of poor prognosis in MDA5+ DM-ILD with highly predictive accuracy.
Keywords: interstitial lung disease, anti-MDA5, dermatomyositis, prognosis, nomogram
Introduction
Dermatomyositis (DM), a multisystem autoimmune disease and a common subtype of idiopathic inflammatory myopathy (IIM), attracts attention from the medical field. In addition to typical skin and muscle involvement, respiratory, digestive, and circulatory system damage, and even malignant tumor can complicate DM. CADM accounts for ~20% of all DM cases. Approximately 87% of MDA5+ DM-ILD cases fulfilled Sontheimer's CADM criteria in a Chinese multi-centered cohort (1). ILD, with an incidence of 5–80% and a high risk in positive ARS antibodies and Black ethnicity, is one of the important respiratory lesions in patients with DM (2). Overall, the prognosis of ILD in IIM is good: 50–66% may be expected to have a stable disease course over a substantial period of time. Frustratingly, the remaining proportion will show signs of worsening lung disease within 12 months.
MDA5, a cytoplasmic RNA helicase belonging to the retinoic acid-inducible gene-I (RIG-I) family, which can recognize ds-RNAof viruses and plays an important role in the innate immune system during RNA viral infections, has been identified as a DM-specific autoantigen (3, 4). Anti-MDA5, a 140-kDa polypeptide and one of the myositis-specific autoantibodies named after its autoantigen, was first found in 2005 by immunoprecipitation in Japanese patients (5). The incidence of MDA5+ DM ranges from 10 to 20% in Japan, 17.6–22.6% in China, and 7–13% in the United States (6–9). The cumulative 100-month survival rate for the entire patients with MDA5+ DM is 66%, and fatal outcomes occur remarkably often within the first 6 months of the diagnosis (10). Patients who responded to therapy and survived had a significantly lower mean titer of anti-MDA5, which was significantly decreased down to below the cutoff level after treatment, while those who did not respond and died had a high level of anti-MDA5 (7, 11), indicating from the side that anti-MDA5 titer is also useful for evaluation of treatment response.
Patients with DM with anti-MDA5 are prone to develop ILD, with a probability of 50–100% (8, 12, 13). Current views regard anti-MDA5 level as a novel parameter for monitoring disease activity and a good predictor of rapidly progressing ILD (RP-ILD) and decreased survival in patients with DM or CADM (11, 14). Early cohort studies reported a high 6-month mortality varying from 33 to 66% in MDA5+DM-ILD (10, 15, 16). A multivariate logistic analysis reported by Chen et al. (9) showed that anti-MDA5 is an independent risk factor for death in DM-ILD. Previous studies on the predictive role of clinical characteristics for MDA5+DM-ILD are relatively limited. For instance, the relationship between serum ferritin level and abnormality of T cell counts and the disease activity of RP-ILD was reported (17). As the increase of both the morbidity and mortality in MDA5+ DM-ILD and the etiology and pathogenesis remaining unknown, early recognition of risk factors for death is particularly important. The aim of this research project is, therefore, to try and establish a meritorious predictive model of prognosis in MDA5+ DM-ILD.
Materials and Methods
Patients and Inclusion Criteria
We retrospectively reviewed all patients with MDA5+DM-ILD from the Department of Rheumatology and the Department of Respiratory and Critical Care Medicine between January 2018 and January 2021 who fulfilled the Bohan and Peter (18, 19) myositis criteria for DM or the Sontheimer (20) criteria for CADM and ILD imaging features. A total of 63 patients were identified. Clinical characteristics consisted of basic information, prognosis, clinical symptoms and signs, complications, treatment means, imaging information, pulmonary functions, and laboratory examinations. We followed all the enrolled patients, and the primary outcome of interest was mortality during the 400-day follow-up.
Acquisition and Analysis of Computed Tomography Imaging
All CT scans were obtained in the supine position using one of the following scanners: SOMATOM Perspective, SOMATOM Spirit, or SOMATOM Definition AS+ (Siemens Healthineers, Forchheim, Germany). Scans were conducted from the level of the upper thoracic inlet to the inferior level of the costophrenic angle, and images were reconstructed with a slice thickness of 1 or 1.5 mm.
For each patient, predominant CT patterns such as GGO, consolidation, reticulation, emphysema, thickening of the adjacent pleura, pleural effusion, presence of nodules or masses, honeycombing, bronchiectasis, and interlobar pleural traction were independently reviewed by two experienced observers according to the Fleischner Society glossary (21). CT evidence of fibrotic-like changes was defined as the presence of traction bronchiectasis, parenchymal bands (22), and/or honeycombing (21, 23, 24). To quantify the extent of pulmonary abnormalities (GGO, consolidation, reticulation, and fibrotic-like changes), a semiquantitative CT score (25) was assigned on the basis of the area involved in each of the five lung lobes (right upper, middle, and lower, and left upper and lower lobes): 0, no involvement; 1, <5%; 2, 5–25%; 3, 26–49%; 4, 50–75%, and 5, >75%. Total CT score was calculated by summing the individual lobar scores (possible scores range from 0 to 25).
Pulmonary Function Test
The patients underwent standard pulmonary function testing (PFT) including ventilatory function and diffusion function using Pulmonary Function Testing System (MasterScreen, CareFusion Germany 234 GmbH or Vyaire Medical GmbH) with indoor temperature 24°C, relative humidity 50–70%, and standard atmospheric pressure 760 mmHg. Among all tested indexes, we had principally concentrated on FVC, FEV1, FEV1/FVC, and diffusion capacity of the lung for carbon monoxide (DLCO). The results were normalized with age-, sex-, height-and weight-matched control subjects.
Anti-MDA5 Examination
Serum samples were routinely collected from the patients at initial hospitalization. Anti-MDA5 was detected using commercially available kits (EUROIMMUN, Lübeck, Germany) by Guangzhou Oumeng Medical Laboratory, with a positive control provided in the kit and a negative control provided in the buffer. The criteria for interpretation of results were based on the staining degree of antigen band recognized automatically with EUROBlotOne (EuroImmun, Lübeck, Germany): negative (–) for colorless, doubtful [(+)] for very weakly colored, weakly positive (+) for weakly colored, positive (++) for strongly colored, and strongly positive (+++) for the same intensity with the quality control blot.
Statistical Analysis
Continuous variables were presented as the mean with standard deviation and categorical variables were expressed as frequency with percentage, and differences between clinical characteristics and prognosis were compared by Student's t-test or Mann-Whitney U-test and chi-squared test. Significant variables were selected for Pearson correlation analysis and univariate Cox regression analysis. Significant (P <0.05) and clinically focused variables in the univariate Cox regression analysis were selected for further multivariate Cox regression analysis. Regression coefficients were regarded as weights for the variables in ROC curves. The nomogram applied to create the scoring system was developed with independent risk factors based on multivariate Cox regression analysis using the “rms” package in R. Survival rates were calculated using the Kaplan-Meier method. A two-sided P-Value <0.05 was defined as statistically significant. All the analyses were performed using SPSS 25.0 and GraphPad Prism 8.0.2.
Results
Patients and Clinical Characteristics
Among the 63 patients with MDA5+DM-ILD admitted in our hospital between January 2018 and January 2021, 44 survived and improved, but 19 lost their lives during the 400-day follow-up. The 400-day mortality in our data of all the 63 patients with MDA5+DM-ILD was 30.16%. The clinical characteristics are summarized in Tables 1–4, Supplementary Table 1 based on prognosis. Of the 19 patients who died during follow-up, 11 (57.9%) and 8 (42.1%) were confirmed to be anti-MDA5-positive and strongly positive, respectively. The group included nine women (47.4%) and 10 men (52.6%) with a median age of 56.95 years (range 40–68) and mean TCTs of 23.21 (range 6–54), and 2 (10.5%) being smoker, and 11 (57.9%) being CADM. Of the 44 patients who improved, 7 (15.9%), 14 (31.8%), and 23 (52.3%) were confirmed to be anti-MDA5 weakly positive, positive, and strongly positive, respectively. This group included 28 women (63.6%) and 16 men (36.4%) with a median age of 45.8 years (range 19–72) and mean TCTs of 10.02 (range, 0–39), and 8 (18.2%) being smoker and 11 (25%) being CADM.
Table 1.
Basic information of MDA5+DM-ILD.
| Characteristics | Total (N = 63) | Not improved (n = 19) | Improved (n = 44) | P-value |
|---|---|---|---|---|
| Age, years | 49.16 ± 12.16 | 56.95 ± 7.81 | 45.80 ± 12.22 | 0.000 |
| Sex, male/female | 26/37 | 10/9 | 16/28 | 0.229 |
| Ever smoker, n (%) | 10 (15.9) | 2 (10.5) | 8 (18.2) | 0.698 |
| CADM, n (%) | 22 (34.9) | 11 (57.9) | 11 (25.0) | 0.012 |
| Duration, m | 7.83 ± 14.53 | 2.87 ± 3.50 | 9.97 ± 16.85 | 0.010 |
| Interval of DM and ILD, m | 5.47 ± 13.63 | 1.16 ± 3.40 | 7.33 ± 15.85 | 0.017 |
| Length of stay, days | 15.11 ± 9.89 | 21.21 ± 15.29 | 12.48 ± 4.44 | 0.024 |
| Fever, n (%) | 34 (54.0) | 18 (94.7) | 16 (36.4) | 0.000 |
| Cough, n (%) | 39 (61.9) | 15 (78.9) | 24 (54.5) | 0.067 |
| Dyspnea, n (%) | 40 (63.5) | 18 (94.7) | 22 (50.0) | 0.001 |
| Arthralgia, n (%) | 46 (73.0) | 10 (52.6) | 36 (81.8) | 0.017 |
| Myalgia or myasthenia, n (%) | 49 (77.8) | 14 (73.7) | 35 (79.5) | 0.854 |
| Skin ulcer, n (%) | 23 (36.5) | 4 (21.1) | 19 (43.2) | 0.094 |
| Gottron sign, n (%) | 23 (36.5) | 7 (36.8) | 16 (36.4) | 0.971 |
| Helicotrop rash, n (%) | 43 (68.3) | 10 (52.6) | 33 (75.0) | 0.080 |
| Raynaud phenomenon, n (%) | 9 (14.3) | 2 (10.5) | 7 (15.9) | 0.867 |
| Pulmonary infection, n (%) | 35 (55.6) | 19 (100.0) | 16 (36.4) | 0.000 |
| Pleural effusion, n (%) | 23 (36.5) | 14 (73.7) | 9 (20.5) | 0.000 |
| Subcutaneous emphysema, n (%) | 1 (1.6) | 1 (5.3) | 0 (0.0) | 0.302 |
| Mediastinal emphysema, n (%) | 20 (3.2) | 1 (5.3) | 1 (2.3) | 0.516 |
| Pleural thickness, n (%) | 17 (27.4) | 4 (21.1) | 13 (30.2) | 0.486 |
| Internal malignancy, n (%) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Glucocorticoid, n (%) | 63 (100.0) | 19 (100.0) | 44 (100.0) | |
| Glucocorticoid, mg | 159.02 ± 208.45 | 326.32 ± 284.65 | 86.77 ± 105.02 | 0.002 |
| Immunosuppressor, n (%) | 40 (63.5) | 11 (57.9) | 29 (65.9) | 0.544 |
| Antibiotic, n (%) | 48 (76.2) | 19 (100.0) | 29 (65.9) | 0.010 |
| Antiviral, n (%) | 17 (27.0) | 11 (57.9) | 6 (13.6) | 0.000 |
| Anti-fibrosis, n (%) | 21 (33.3) | 8 (42.1) | 13 (29.5) | 0.332 |
| NPPV, n (%) | 12 (19.0) | 12 (63.2) | 0 (0.0) | 0.000 |
| Survival time, days | 298.89 ± 152.45 | 102.00 ± 95.27 | 400.00 ± 0.00 | 0.000 |
The dosage of glucocorticoid was changed to that of methylprednisolone. Immunosuppressors included ciclosporin, cyclophosphamide, tripterygium wilfordii glycosides, mycophenolate mofetil, thalidomide, leflunomide, tacrolimus, and methotrexate. Antifibrotic drugs referred to nintedanib and pirfenidone. CADM, clinically amyopathic variant of dermatomyositis; DM, dermatomyositis; ILD, interstitial lung disease; NPPV, non-invasive positive pressure ventilation.
Table 4.
Immunologic tests for MDA5+DM-ILD.
| Characteristics | Total (N = 63) | Not improved (n = 19) | Improved (n = 44) | P-value |
|---|---|---|---|---|
| Anti-MDA5 | 0.024 | |||
| Weakly positive, n (%) | 7 (11.1) | 0 (0.0) | 7 (15.9) | |
| Positive, n (%) | 25 (39.7) | 11 (57.9) | 14 (31.8) | |
| Strongly positive, n (%) | 31 (49.2) | 8 (42.1) | 23 (52.3) | |
| CD3+ T, % | 72.46 ± 12.13 | 70.15 ± 13.84 | 73.46 ± 11.37 | 0.367 |
| CD4+ T, % | 46.70 ± 13.19 | 48.04 ± 15.87 | 46.12 ± 12.05 | 0.630 |
| CD8+ T, % | 23.14 ± 11.19 | 19.41 ± 10.15 | 24.75 ± 11.36 | 0.112 |
| B lymphocyte, % | 17.54 ± 9.71 | 17.26 ± 8.54 | 17.67 ± 10.33 | 0.891 |
| NK lymphocyte, % | 7.95 ± 7.72 | 9.88 ± 11.59 | 7.04 ± 4.98 | 0.230 |
| IL-2, pg/ml | 2.62 ± 1.26 | 2.74 ± 1.01 | 2.57 ± 1.36 | 0.657 |
| IL-4, pg/ml | 2.59 ± 1.11 | 2.77 ± 0.98 | 2.52 ± 1.16 | 0.458 |
| IL-6, pg/ml | 39.76 ± 92.17 | 30.61 ± 54.40 | 43.71 ± 104.81 | 0.639 |
| IL-10, pg/ml | 5.77 ± 3.01 | 6.24 ± 3.51 | 5.57 ± 2.82 | 0.475 |
| TNFα, pg/ml | 10.56 ± 26.26 | 3.08 ± 1.77 | 13.67 ± 30.82 | 0.047 |
| IFNγ, pg/ml | 2.61 ± 1.39 | 2.54 ± 1.48 | 2.64 ± 1.38 | 0.809 |
IL, interleukin; TNF, tumor necrosis factor; IFN, interferon.
Table 2.
Pulmonary examinations for MDA5+DM-ILD.
| Characteristics | Total (N = 63) | Not improved (n = 19) | Improved (n = 44) | P-value |
|---|---|---|---|---|
| TCTs | 14.06 ± 12.49 | 23.21 ± 14.14 | 10.02 ± 9.28 | 0.001 |
| GGO score | 5.05 ± 4.51 | 7.32 ± 5.19 | 4.05 ± 3.83 | 0.007 |
| Consolidation score | 3.05 ± 4.25 | 6.63 ± 5.27 | 1.47 ± 2.43 | 0.001 |
| Reticular score | 2.76 ± 3.42 | 4.26 ± 3.77 | 2.09 ± 3.06 | 0.020 |
| Fibrosis score | 3.23 ± 4.20 | 5.05 ± 5.02 | 2.42 ± 3.56 | 0.022 |
| FVC (L) | 2.60 ± 0.88 | 2.12 ± 0.52 | 2.75 ± 0.92 | 0.022 |
| FEV1 (L) | 2.09 ± 0.68 | 1.75 ± 0.33 | 2.19 ± 0.72 | 0.022 |
| FEV1/FVC | 81.18 ± 9.40 | 83.91 ± 12.14 | 80.37 ± 8.53 | 0.357 |
| DLCO (mmol/min/kPa) | 4.92 ± 2.25 | 4.06 ± 2.26 | 5.18 ± 2.23 | 0.253 |
TCTs, total CT score; GGO, ground-glass opacity; FVC, forced vital capacity; FEV1, forced expiratory volume in 1s; DLCO, diffusion capacity of the lungs for carbon monoxide.
Table 3.
General laboratory tests for MDA5+DM-ILD.
| Characteristics | Total (N = 63) | Not improved (n = 19) | Improved (n = 44) | P-value |
|---|---|---|---|---|
| Leukocyte, G/L | 5.56 ± 2.83 | 6.61 ± 4.28 | 5.10 ± 1.79 | 0.052 |
| Monocyte, G/L | 0.41 ± 0.20 | 0.53 ± 0.20 | 0.35 ± 0.17 | 0.001 |
| Monocyte, % | 7.91 ± 3.64 | 8.95 ± 3.02 | 7.47 ± 3.82 | 0.140 |
| Neutrophil, G/L | 4.24 ± 2.67 | 5.20 ± 4.01 | 3.82 ± 1.72 | 0.059 |
| Neutrophil, % | 73.75 ± 11.24 | 76.11 ± 8.02 | 72.73 ± 12.32 | 0.202 |
| Lymphocyte, G/L | 0.83 ± 0.42 | 0.78 ± 0.32 | 0.85 ± 0.45 | 0.581 |
| Lymphocyte, % | 16.78 ± 9.11 | 13.70 ± 7.18 | 18.11 ± 9.60 | 0.078 |
| Blood urine | ||||
| Negative, n (%) | 44 (75.9) | 9 (56.3) | 35 (83.3) | 0.070 |
| Positive, n (%) | 14 (24.1) | 7 (43.8) | 7 (16.7) | |
| Proteinuria | ||||
| Negative, n (%) | 36 (62.1) | 6 (37.5) | 30 (71.4) | 0.017 |
| Positive, n (%) | 22 (37.9) | 10 (62.5) | 12 (28.6) | |
| AST, U/L | 94.48 ± 121.17 | 150.63 ± 161.88 | 70.23 ± 90.58 | 0.054 |
| ALT, U/L | 70.63 ± 103.17 | 94.63 ± 84.83 | 60.02 ± 109.54 | 0.226 |
| LDH, U/L | 371.81 ± 145.23 | 458.05 ± 142.07 | 333.71 ± 130.89 | 0.001 |
| Alb, g/L | 32.68 ± 4.74 | 29.31 ± 3.77 | 34.14 ± 4.39 | 0.000 |
| Glb, g/L | 27.68 ± 5.05 | 27.27 ± 3.25 | 27.85 ± 5.68 | 0.615 |
| A/G | 1.22 ± 0.29 | 1.08 ± 0.21 | 1.28 ± 0.30 | 0.006 |
| CK, U/L | 201.30 ± 300.15 | 236.26 ± 374.21 | 185.48 ± 263.69 | 0.545 |
| GFR, ml/(min/1.73 m2) | 111.65 ± 21.96 | 100.95 ± 28.74 | 115.76 ± 17.49 | 0.025 |
| Bun, mmol/L | 4.47 ± 3.21 | 5.81 ± 5.32 | 3.89 ± 1.37 | 0.137 |
| Cr, μmol /L | 62.40 ± 80.92 | 84.87 ± 146.37 | 52.69 ± 12.36 | 0.351 |
| ESR, mm/h | 34.67 ± 23.69 | 38.56 ± 24.57 | 33.05 ± 23.42 | 0.412 |
| CRP, mg/L | 16.80 ± 27.58 | 31.72 ± 44.49 | 10.26 ± 10.85 | 0.059 |
| FER, μg /L | 1,082.04 ± 870.39 | 1,512.62 ± 1,125.17 | 866.75 ± 623.90 | 0.033 |
| CEA, μg /L | 7.05 ± 5.39 | 8.74 ± 7.13 | 6.26 ± 4.25 | 0.142 |
| CYFRA, ng/ml | 5.70 ± 8.19 | 9.54 ± 12.23 | 3.40 ± 2.63 | 0.075 |
| SCCA, ng/ml | 2.51 ± 10.96 | 5.48 ± 17.86 | 0.72 ± 0.44 | 0.320 |
| NSE, μg /L | 20.10 ± 8.59 | 25.11 ± 10.16 | 17.10 ± 5.89 | 0.003 |
AST, aspartate transaminase; ALT, alanine transaminase; LDH, lactate dehydrogenase; Alb, albumin; Glb, globulin; CK, creatine kinase; GFR, glomerular filtration rate; BUN, blood urea nitrogen; Cr, creatinine; ESR, erythrocyte sedimentation rate; CRP, C-reactive protein; FER, ferritin; CEA, carcino-embryonic antigen; CYFRA, cytokeratin 19 fragment; SCCA, squamous cell carcinoma antigen; NSE, neuron-specific enolase.
Association Between Clinical Characteristics and Prognosis
The clinical characteristics of the patients with MDA5+ DM-ILD are summarized in Tables 1–4, Supplementary Table 1 according to basic information, pulmonary examinations, general laboratory tests, and immunologic tests based on their prognosis in the 400-day follow-up. CT scores of each lobe assessed by fibrotic-liking changes including GGO score, consolidation score, reticular score, and fibrosis score are shown in Supplementary Table 2.
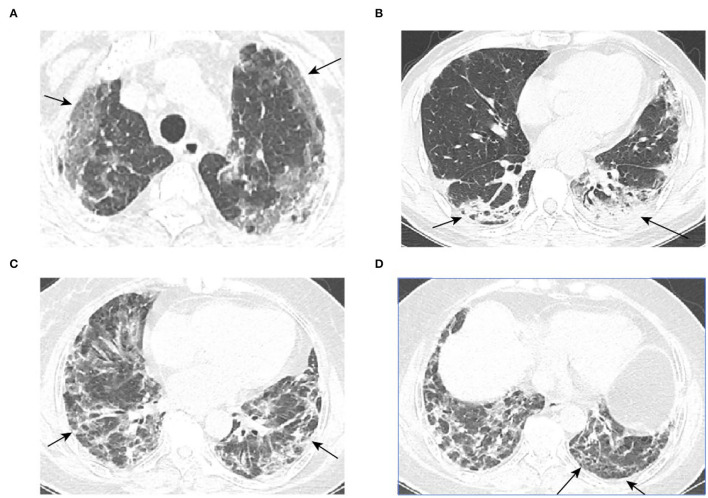
Previous research determined that prognosis was poor in elderly patients with MDA5+ DM-ILD (12). In our study, poor prognosis was found more frequently in acute-onset patients (2.87 ± 3.5 vs. 9.97 ± 16.85, P = 0.01). Abnormal symptoms such as fever (94.7% vs.36.4%, P < 0.001) and some complications such as pulmonary infection (100.0% vs. 36.4%, P < 0.001) and pleural effusion (73.7% vs. 20.5%, P < 0.001) were significantly associated with high mortality. The Gottron sign, skin ulceration, and heliotrope rash are characteristic cutaneous phenotypes in patients with MDA5+ DM and are significantly associated with increased risk of subacute ILD or RP-ILD (6, 26, 27). However, our results did not find that the signs above had an apparent link with prognosis of patients with MDA5+ DM-ILD. All the 63 patients here were not diagnosed with any internal malignancy. Despite many scholars suggesting MDA5+ DM is likely to complicate malignancy, malignancy is uncommon in MDA5+ DM-ILD, with an incidence of <5% (15, 28). As expected, TCTs, GGO score, consolidation score, reticular score, and fibrosis score were higher in patients with poor prognosis. Representative CT images of GGO, consolidation, and reticular and fibrotic changes are shown in Figure 1. In contrast, the value of FVC and FEV1 was lower in poor prognosis. It had been noted the severely affected pulmonary function especially the baseline FVC% was validated to be the most significant prognostic factor to predict the 6-month all-cause mortality based on a multi-center MDA5+ DM-ILD data with a cutoff value of 50%, which means mortality being 15% while FVC% >50% and mortality being 70% while FVC% <50% (29, 30).
Figure 1.
Representative CT images. (A–D) are typical GGO, consolidation, reticular, and honeycomb images, respectively (red arrows).
A new AI algorithm-based analysis suggested that “MDA5 score” may serve as an applicable prognostic predictor for MDA5+ DM-ILD (31). Regarding the laboratory examination indicators in our research, we found that poor prognosis patients had more positive and strongly positive anti-MDA5 results (P = 0.024) than the survivors. Research studies have mentioned that predictive cytokines and chemokines including IL-6, IL-8, IL-10, IL-15, IL-18, TNFα, IFN-α, IP-10, and CX3CL1 had high levels in MDA5+ DM-ILD (32–35), especially CX3CL1, which was identified as involved in the pathogenesis of MDA5+ DM-ILD with a strong correlation of r = 0.89 between anti-MDA5 titer and CX3CL1.
Early and intensive immunomodulatory therapy has some effects on clinical parameters such as cytokines, antibodies, and hyperferritinemia and may lead to better prognosis of concomitant ILD (29). Nakashima et al. (36) reported that combined immunosuppressive therapy markedly improved the prognosis from 28.6 to 75%. An existing report revealed that the application of non-invasive positive pressure ventilation was an independent risk factor for survival (37). Based on this study, we were surprised to find that patients who received a larger dose of glucocorticoid (326.32 ± 284.65 vs. 86.77 ± 105.02, P = 0.002), antibiotic therapy (100% vs. 65.9%, P = 0.01), antiviral therapy (57.9% vs. 13.6%, P < 0.001), and NPPV (63.2% vs. 0%, P < 0.001) were more inclined to suffer a bad end. We had to owe poor prognosis after receiving intensive therapies to their complex and severe status liking secondary multiple infections. Although a previous clinical trial suggested that pirfenidone, in addition to conventional immunosuppressive treatment, did not result in improvement in terms of survival (38). We wanted to see if there is anti-fibrosis benefit. However, contrary to our expectations, the results showed that anti-fibrosis therapy did not improve the outcomes, maybe because the population incorporated was small and the follow-up was short.
Besides, we specially analyzed the correlation between the above clinical characteristics showing significant differences with prognosis and the survival time in the 400-day follow-up through Pearson correlation coefficient (Table 5). Majority of the results were consistent with those aforementioned.
Table 5.
Correlation analysis of clinical characteristics and prognosis.
| Characteristics | Prognosis | Survival time | ||
|---|---|---|---|---|
| Pearson | P-value | Pearson | P-value | |
| Age | 0.424 | 0.001 | −0.365 | 0.006 |
| CADM | 0.317 | 0.011 | −0.293 | 0.028 |
| Duration | −0.226 | 0.075 | 0.294 | 0.028 |
| Interval of DM and ILD | −0.210 | 0.099 | 0.258 | 0.054 |
| Length of stay | 0.408 | 0.001 | −0.414 | 0.002 |
| Fever | 0.537 | 0.000 | −0.509 | 0.000 |
| Dyspnea | 0.426 | 0.000 | −0.379 | 0.004 |
| Arthralgia | −0.302 | 0.016 | 0.272 | 0.043 |
| Pulmonary infection | 0.588 | 0.000 | −0.538 | 0.000 |
| Pleural effusion | 0.507 | 0.000 | −0.526 | 0.000 |
| TCTs | 0.491 | 0.000 | −0.385 | 0.003 |
| GGO score | 0.337 | 0.007 | −0.223 | 0.099 |
| Consolidation score | 0.565 | 0.000 | −0.479 | 0.000 |
| Reticular score | 0.295 | 0.020 | −0.297 | 0.026 |
| Fibrosis score | 0.291 | 0.022 | −0.170 | 0.211 |
| FVC | −0.305 | 0.075 | 0.276 | 0.126 |
| FEV1 | −0.280 | 0.103 | 0.262 | 0.148 |
| Anti-MDA5 | 0.039 | 0.762 | −0.051 | 0.709 |
| Leukocyte | 0.246 | 0.052 | −0.279 | 0.038 |
| Monocyte | 0.426 | 0.001 | −0.482 | 0.000 |
| Neutrophil | 0.239 | 0.059 | −0.279 | 0.038 |
| Proteinuria | 0.313 | 0.017 | −0.293 | 0.035 |
| AST | 0.307 | 0.014 | −0.407 | 0.002 |
| LDH | 0.398 | 0.001 | −0.378 | 0.004 |
| Alb | −0.472 | 0.000 | 0.482 | 0.000 |
| A/G | −0.307 | 0.014 | 0.273 | 0.042 |
| GFR | −0.305 | 0.025 | 0.227 | 0.124 |
| CRP | 0.361 | 0.005 | −0.353 | 0.010 |
| FER | 0.353 | 0.009 | −0.254 | 0.072 |
| TNFα | −0.186 | 0.192 | 0.181 | 0.224 |
| CEA | 0.217 | 0.142 | −0.221 | 0.141 |
| CYFRA | 0.367 | 0.020 | −0.320 | 0.047 |
| NSE | 0.217 | 0.142 | −0.459 | 0.003 |
| Glucocorticoid | 0.532 | 0.000 | −0.410 | 0.002 |
| Antibiotic | 0.367 | 0.003 | −0.386 | 0.003 |
| Antiviral | 0.458 | 0.000 | −0.366 | 0.006 |
| NPPV | 0.738 | 0.000 | −0.649 | 0.000 |
Prediction of the Prognosis of MDA5+DM-ILD
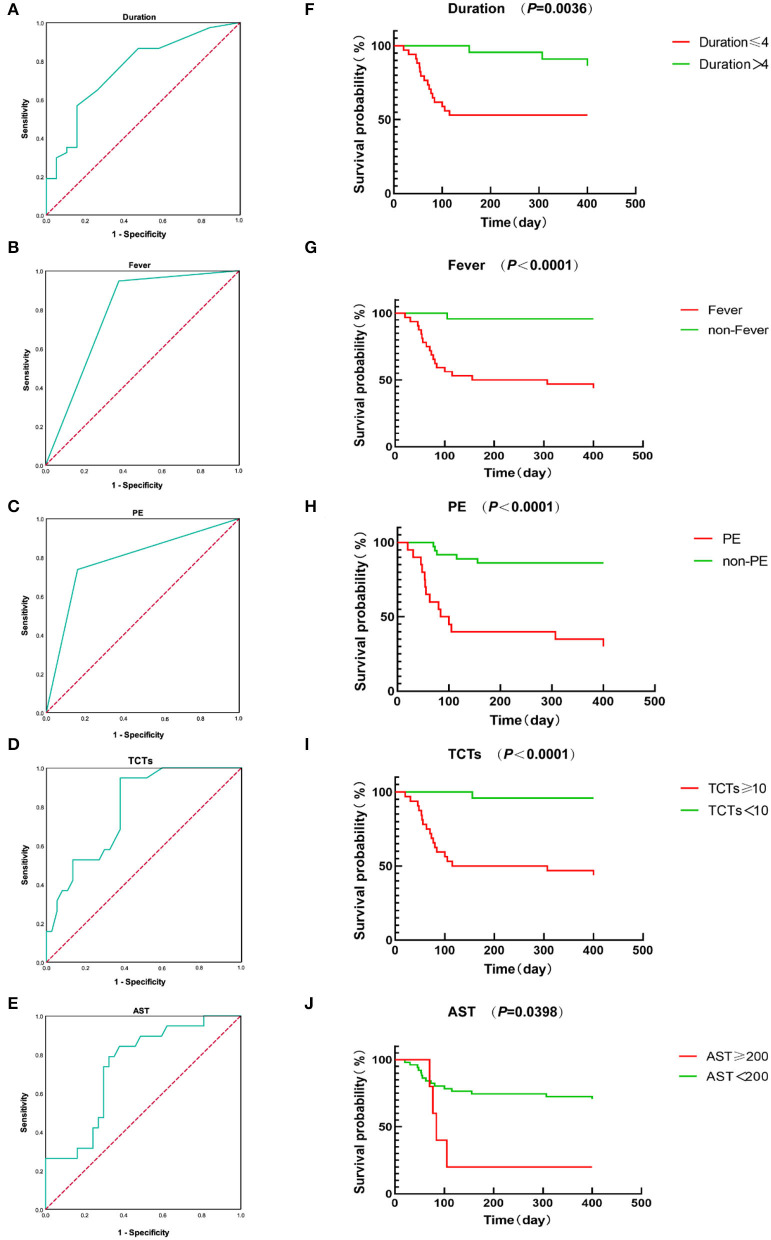
The above studies have revealed some significant differences and associations between clinical characteristics and prognosis. Based on them, we next performed a univariate Cox regression analysis. Although there were many significant indicators included in our research, we selected only seven of them for the univariate Cox regression analysis following the rules of statistics (one indicator for 10 observations). As seen in Table 6, the univariate Cox regression analysis showed that fever, pulmonary infection, pleural effusion, TCTs, AST, and FER were significantly correlated with the prognosis of MDA5+DM-ILD. Then, inclusion of these factors and duration together in the multivariate Cox regression analysis revealed that duration, fever, PE, TCTs, and AST remained independent variables for predicting the prognosis of MDA5+DM-ILD. That is to say, acute onset (HR 0.827, P = 0.011), fever (HR 17.486, P = 0.012), pleural effusion (HR 0.174, P = 0.001), high TCTs (HR 1.048, P = 0.011), and high AST (HR 1.005, P = 0.004) were significant predictors of poor prognosis for MDA5+DM-ILD. Additionally, an ROC curve analysis was conducted to evaluate the predictive value of these factors (Table 7 and Figures 2A–E).
Table 6.
Cox regression analysis of various predictive factors for the prognosis of MDA5+ DM-ILD.
| Characteristics | Univariate HR (95% CI) | P-value | Multivariate HR (95% CI) | P-value |
|---|---|---|---|---|
| Duration | 0.056 | 0.827 (0.704–0.972) | 0.011 | |
| Fever | 0.052 (0.007–0.388) | 0.004 | 17.486 (1.861–164.255) | 0.012 |
| Pulmonary infection | 61.142 (1.494–2,501.471) | 0.030 | ||
| Pleural effusion | 8.061 (2.884–22.532) | 0.000 | 0.174 (0.059–0.511) | 0.001 |
| TCTs | 1.049 (1.020–1.078) | 0.001 | 1.048 (1.011–1.086) | 0.011 |
| AST | 1.005 (1.002–1.008) | 0.001 | 1.005 (1.002–1.009) | 0.004 |
| FER | 1.000 (1.000–1.001) | 0.032 |
Table 7.
ROC analysis of duration, fever, PE, TCTs, and AST.
| Characteristics | AUC | Youden index | 95% CI | Sensitivity | Specificity | P-value |
|---|---|---|---|---|---|---|
| Duration | 0.757 | 0.410 | 0.623–0.890 | 56.8% | 84.2% | 0.002 |
| Fever | 0.784 | 0.569 | 0.664–0.905 | 94.7% | 62.2% | 0.001 |
| PE | 0.787 | 0.575 | 0.652–0.923 | 73.7% | 83.8% | 0.000 |
| TCTs | 0.788 | 0.569 | 0.671–0.905 | 94.7% | 62.2% | 0.000 |
| AST | 0.738 | 0.465 | 0.604–0.871 | 78.9% | 67.6% | 0.004 |
| Combination | 0.954 | 0.788 | 0.902–1.000 | 84.2% | 94.6% | 0.000 |
Combination: the combination of fever, PE, TCTs, and AST.
Figure 2.
(A–E) ROC curves of duration, fever, PE, TCTs, and AST. (F–J) Kaplan-Meier survival curves of duration, fever, PE, TCTs, and AST.
Development of Prognostic Nomogram Models of MDA5+DM-ILD
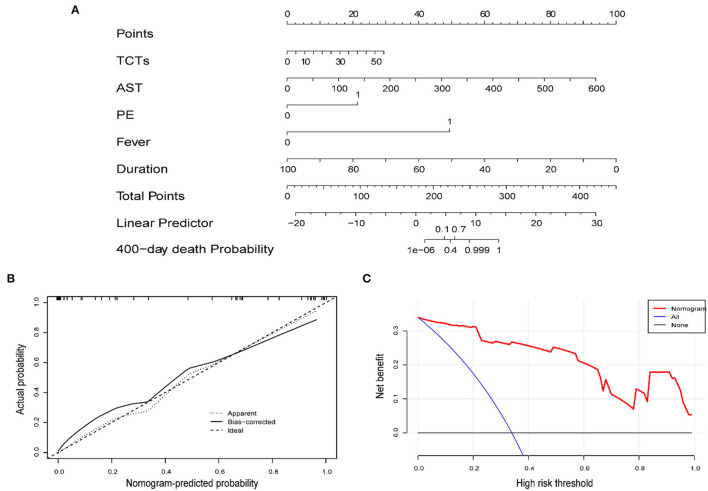
A nomogram to predict the mortality of MDA5+DM-ILD was preliminarily constructed on the basis of multivariate Cox regression results (Figure 3A). Particularly, the nomogram was generated by assigning a weighed score on the point scale to each independent predictor. A higher score calculated from the sum of the assigned number of points for each prognostic parameter in the nomogram corresponds to a higher likelihood of death. The calibration curve showed that this predictive nomogram exhibited good calibration (Figure 3B). Moreover, a decision curve analysis (DCA) was conducted to assess the clinical utility of the predictive nomogram in Figure 3C.
Figure 3.
(A) Nomogram predicting the prognosis of MDA5+DM-ILD. (B) Calibration curve of the nomogram. (C) DCA of the nomogram.
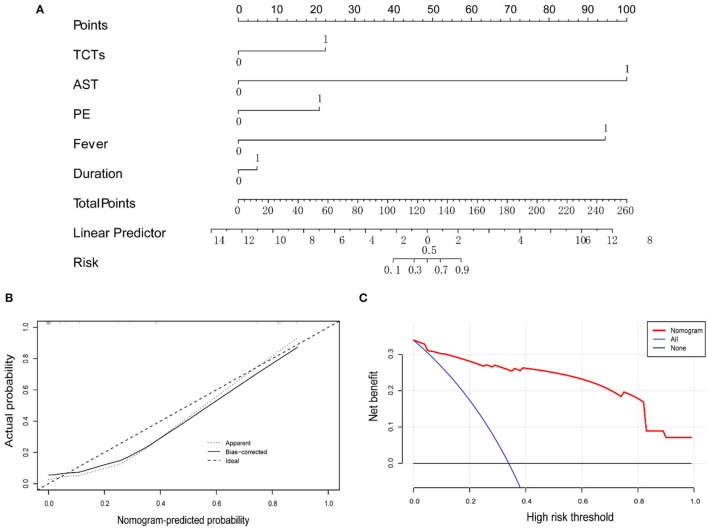
To make this predictive model more convenient for physicians to use in clinical practice, we modified three predictors (duration, TCTs, and AST) into binary variables. Then three transformed binary variables together with fever and PE were used to conduct another nomogram model, in which all five predictors were evaluated with specific integer points: duration ≤ 4 m (5 points), fever (88 points), PE (21 points), TCTs ≥10 points (22 points), and AST ≥200 U/L (100 points) (Figures 4A–C). Then, we obtained Kaplan-Meier survival curves subdivided by duration ≤ 4 mouths, fever, PE, TCTs ≥10 points, and AST ≥200 U/L (Figures 2F–J). In the end, we created a new indicator by combining fever, PE, TCTs, and AST, which possessed good predictive ability, as reflected by an AUC of.954 (Table 7 and Figure 5).
Figure 4.
(A) Nomogram predicting the prognosis of MDA5+DM-ILD, while all predictors are binary variables. (B) Calibration curve of the nomogram. (C) DCA of the nomogram.
Figure 5.
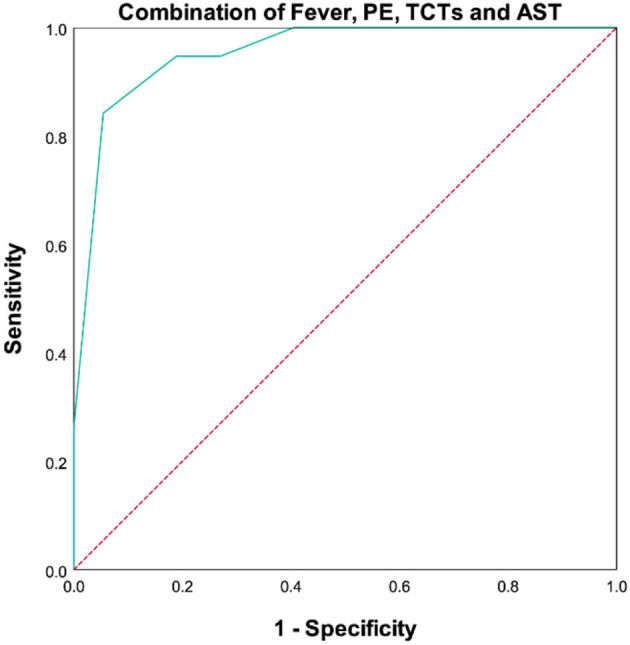
ROC curves of combination of fever, PE, TCTs, and AST.
Discussion
The presence of MDA5+ DM-ILD can seriously impair the quality of life and shorten the survival of patients. The 6-month mortality of patients with MDA5+ DM-ILD ranges from 33 to 66% (10, 15, 16). A multicenter observational study (39) from 37 medical centers including 121 patients showed that MDA5+ RP-ILD had a noteworthy high mortality rate. Early and intensive immunomodulatory therapy has some effects on clinical parameters such as cytokines, antibodies, and hyperferritinemia, and may lead to better prognosis of concomitant ILD (29). Nakashima et al. (36) reported that combined immunosuppressive therapy markedly improved the prognosis from 28.6 to 75%. An existing report revealed that application of NPPV was an independent risk factor for survival (37). Previous studies on the predictive role of clinical characteristics for patients with MDA5+DM-ILD were relatively limited. Our study aimed to design a novel quantitative tool so clinicians can predict the probability of death. Thus, we integrated a total of 122clinical characteristics, 46 of which are shown in Supplementary Table 1. Although numerous clinical features were associated with prognosis, the clinical significance of a single index in the prediction of prognosis was quite limited because of one-sidedness. As a result, we selected the independent variables duration, fever, PE, TCTs, and AST, all being routine clinical practice, based on the multivariate Cox regression analysis to construct a predictive model.
Nakashima et al. (40) determined that the prognosis was poor in MDA5+DM-ILD patients who went through a long interval from appearance of skin lesions to diagnosis of ILD. Our data indicated the average course of disease and interval of DM and ILD in poor prognosis patients was 2.87 and 1.16 months, respectively, meaning acute onset of DM and ILD and serious, fractious conditions. Tanizawa et al. (14) also indicated that high fever was associated with poor prognosis of DM-ILD. Pleural effusion (73.7% vs. 20.5%, P < 0.001) was significantly associated with high mortality in this research. We systematically evaluated every patient's CT imaging and made points according to standard as mentioned above, finding the poor prognosis population getting visibly higher points not only on TCTs but also on GGO score, consolidation score, reticular score, and fibrosis score. It was reported that consolidation, GGO, and reticular opacities were distinctive findings in high-resolution computed tomography (HRCT) (14, 41) and that an initial right middle lobe GGO score of ≥2 (GGO ≥5% of the lobe) was a poor prognostic factor (42) for patients with MDA5+DM-ILD. Besides, a semi-quantitative HRCT scoring method including GGO, consolidation, and fibrosis was applied for the assessment of MDA5+ DM-ILD and confirmed an independent risk factor for 1-year mortality (43). However, the fibrosis components were heavily weighted in this scoring method. Recent research studies including an AI algorithm-based analysis named “AI score” revealed that lower zone GGO and consolidation demonstrated to be correlated with RP-ILD and were applicable prognostic predictors for MDA5+ DM-ILD (31, 44). Besides, the scores of microhemorrhage, capillary disorganization, spontaneous pneumomediastinum, and neoangiogenesis were significantly correlated with known poor prognosis factors of DM-ILD and total fibrosis scores of chest HRCT (37, 45, 46). Some research studies (9, 29, 37, 40, 47) have reported that anti-MDA5-positive and non-survivors presented higher serum AST level.
We can believe that each enrolled index in our model has a definite guiding function and an undoubted effect on clinic work. However, this model was generated in a specific patient population and specific clinical characteristics. Inevitably, this model may not be the standard model that represents all patients with MDA5+ DM-ILD and covers all possible clinical indicators. What we can do is to build a model that is as comprehensive and reliable as possible under existing conditions. Therefore, we suggest that one flaw of our model is that hyperferritinemia was not included. In fact, hyperferritinemia has been indicated as a key risk factor for patients with MDA5+ DM and RP-ILD (1, 10, 48–51). It is just that our model dropped it in the fitting process for some reason. Nevertheless, non-hyperferritinemia in the model does not mean that hyperferritinemia is not important, and it absolutely can be an independent prognostic factor.
Different predictive models have been reported in the past 10 years. “FLAIR score,” including ferritin, LDH, semi-quantitative anti-MDA5 grade, HRCT imaging score, and RPILD/non-RPILD based on a large-scale Chinese single-center cohort (n = 207), was proposed to predict mortality in CADM-ILD (1). Other reports also stated that ferritin, LDH, and KL-6 were independent high-risk factors for poor outcomes (1, 52, 53). A multivariate logistic regression analysis (27) previously indicated that positive anti-MDA5, elevated CRP, and decreased counts of lymphocyte can provide a precise prediction for RP-ILD in patients with CADM. The evidence-based risk prediction model using CRP and KL-6 combined with anti-MDA5 might also be useful for predicting prognosis in patients with DM-ILD; it is called the MCK (MDA5, CRP, and KL-6) model, identifying patients at low (<15%), moderate (15–49%), or high risk (≥50%) of mortality based on the number of risk factors. Respiratory physiological parameters such as lower arterial partial pressure of oxygen (PaO2) and higher alveolar-arterial oxygen difference (AaDO2) have been associated with the development of RPILD and poor prognosis in several small-sample MDA5+ DM/CADM studies (10, 42). Unfortunately, the heterogeneity of these cohorts was obvious, and the pulmonary function and structure evaluation were suboptimal.
This is the first time that duration, fever, PE, TCTs, and AST are recommended together as a predictor for the prognosis of MDA5+ DM-ILD. This nomogram has high predictive accuracy and can be applied in most hospitals because of convenience. With the aim of establishing a novel scoring system, we converted the nomogram into a scoring system. If the total score is over 116 points, a high probability (≥30%) of mortality exists. Meanwhile, when we combined fever, PE, TCTs, and AST together, a nice predictive function can be seen: AUC being.954, sensitivity being 84.2%, and specificity being 94.6% on the ROC curve. Hence, this method is not only feasible and simple but could also accurately recognize poor prognosis with high calibration.
This study is not exempt from limitations. First, this study was based on retrospective data, and the validity of the retrospective data was limited. Moreover, the size of the sample included in this study was small. Next, the nomogram model was not validated in the external validation set from other medical centers. Finally, our follow-up time was relative short, lacking assessment of long-term survival conditions. Therefore, multicenter validation of the scoring system with a large study population is urgently needed to obtain high-level evidence for its clinical application in the future.
In conclusion, the predictive model for the prognosis of MDA5+ DM-ILD assists in identifying cases accurately, intensifying treatment early, and saving as many patient lives as possible in clinical practice. This study is based on a unicentric and small sample of participants suggesting a favorable predictive performance and should be further validated in multicenter prospective studies in the near future.
Data Availability Statement
The datasets presented in this article are not readily available because the data are being expanded for use in another study. Requests to access the datasets should be directed to 476839887@qq.com.
Ethics Statement
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author Contributions
QN and L-qZ conceived the study, collected the data, and performed the analysis. QN wrote the manuscript. W-lM, LX, X-rW, X-lH, and FY made suggestions on the revision of manuscript. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2022.908365/full#supplementary-material
References
- 1.Lian X, Zou J, Guo Q, Chen S, Lu L, Wang R, et al. Mortality risk prediction in amyopathic dermatomyositis associated with interstitial lung disease: the FLAIR model. Chest. (2020) 158:1535–45. 10.1016/j.chest.2020.04.057 [DOI] [PubMed] [Google Scholar]
- 2.Kiely PD, Chua F. Interstitial lung disease in inflammatory myopathies: clinical phenotypes and prognosis. Curr Rheumatol Rep. (2013) 15:359. 10.1007/s11926-013-0359-6 [DOI] [PubMed] [Google Scholar]
- 3.Takeuchi O, Akira S. MDA5/RIG-I and virus recognition. Curr Opin Immunol. (2008) 20:17–22. 10.1016/j.coi.2008.01.002 [DOI] [PubMed] [Google Scholar]
- 4.Kato H, Takeuchi O, Mikamo-Satoh E, Hirai R, Kawai T, Matsushita K, et al. Length-dependent recognition of double-stranded ribonucleic acids by retinoic acid-inducible gene-I and melanoma differentiation-associated gene 5. J Exp Med. (2008) 205:1601–10. 10.1084/jem.20080091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sato S, Hirakata M, Kuwana M, Suwa A, Inada S, Mimori T, et al. Autoantibodies to a 140-kd polypeptide, CADM-140, in Japanese patients with clinically amyopathic dermatomyositis. Arthritis Rheum. (2005) 52:1571–6. 10.1002/art.21023 [DOI] [PubMed] [Google Scholar]
- 6.Fiorentino D, Chung L, Zwerner J, Rosen A, Casciola-Rosen L. The mucocutaneous and systemic phenotype of dermatomyositis patients with antibodies to MDA5 (CADM-140): a retrospective study. J Am Acad Dermatol. (2011) 65:25–34. 10.1016/j.jaad.2010.09.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sato S, Kuwana M, Fujita T, Suzuki Y. Anti-CADM-140/MDA5 autoantibody titer correlates with disease activity and predicts disease outcome in patients with dermatomyositis and rapidly progressive interstitial lung disease. Mod Rheumatol. (2012) 23:496–502. 10.1007/s10165-012-0663-4 [DOI] [PubMed] [Google Scholar]
- 8.Hall JC, Casciola-Rosen L, Samedy LA, Werner J, Owoyemi K, Danoff SK, et al. Anti-melanoma differentiation-associated protein 5-associated dermatomyositis: expanding the clinical spectrum. Arthritis Care Res. (2013) 65:1307–15. 10.1002/acr.21992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen F, Wang D, Shu X, Nakashima R, Wang G. Anti-MDA5 antibody is associated with A/SIP and decreased T cells in peripheral blood and predicts poor prognosis of ILD in Chinese patients with dermatomyositis. Rheumatol Int. (2012) 32:3909–15. 10.1007/s00296-011-2323-y [DOI] [PubMed] [Google Scholar]
- 10.Gono T, Sato S, Kawaguchi Y, Kuwana M, Hanaoka M, Katsumata Y, et al. Anti-MDA5 antibody, ferritin and IL-18 are useful for the evaluation of response to treatment in interstitial lung disease with anti-MDA5 antibody-positive dermatomyositis. Rheumatology. (2012) 51:1563–70. 10.1093/rheumatology/kes102 [DOI] [PubMed] [Google Scholar]
- 11.Matsushita T, Mizumaki K, Kano M, Yagi N, Tennichi M, Takeuchi A, et al. Antimelanoma differentiation-associated protein 5 antibody level is a novel tool for monitoring disease activity in rapidly progressive interstitial lung disease with dermatomyositis. Br J Dermatol. (2017) 176:395–402. 10.1111/bjd.14882 [DOI] [PubMed] [Google Scholar]
- 12.Li S, Ge Y, Yang M. Correlation analysis of myositis specific antibody spectrum and clinical features in 427 patients with dermatomyositis. Chin J Rheumatol. (2017) 21:585–94. 10.3760/cma.j.issn.1007-7480.2017.09.003 [DOI] [Google Scholar]
- 13.Motegi SI, Sekiguchi A, Toki S, Kishi C, Endo Y, Yasuda M, et al. Clinical features and poor prognostic factors of anti-melanoma differentiation-associated gene 5 antibody-positive dermatomyositis with rapid progressive interstitial lung disease. Eur J Dermatol. (2019) 29:511–7. 10.1684/ejd.2019.3634 [DOI] [PubMed] [Google Scholar]
- 14.Tanizawa K, Handa T, Nakashima R, Kubo T, Hosono Y, Watanabe K, et al. HRCT features of interstitial lung disease in dermatomyositis with anti-CADM-140 antibody. Respir Med. (2011) 105:1380–7. 10.1016/j.rmed.2011.05.006 [DOI] [PubMed] [Google Scholar]
- 15.Koga T, Fujikawa K, Horai Y, Okada A, Kawashiri SY, Iwamoto N, et al. The diagnostic utility of anti-melanoma differentiation-associated gene 5 antibody testing for predicting the prognosis of Japanese patients with DM. Rheumatology. (2012) 51:1278–84. 10.1093/rheumatology/ker518 [DOI] [PubMed] [Google Scholar]
- 16.Tsuji H, Nakashima R, Hosono Y, Imura Y, Yagita M, Yoshifuji H, et al. Multicenter prospective study of the efficacy and safety of combined immunosuppressive therapy with high-dose glucocorticoid, tacrolimus, and cyclophosphamide in interstitial lung diseases accompanied by anti-melanoma differentiation-associated gene 5-positive dermatomyositis. Arthritis Rheumatol. (2020) 72:488–98. 10.1002/art.41105 [DOI] [PubMed] [Google Scholar]
- 17.Gono T, Kawaguchi Y, Ozeki E, Ota Y, Satoh T, Kuwana M, et al. Serum ferritin correlates with activity of anti-MDA5 antibody-associated acute interstitial lung disease as a complication of dermatomyositis. Mod Rheumatol. (2011) 21:223–7. 10.3109/s10165-010-0371-x [DOI] [PubMed] [Google Scholar]
- 18.Bohan A, Peter JB. Polymyositis and dermatomyositis. N Engl J Med. (1975) 292:344–7. 10.1056/NEJM197502132920706 [DOI] [PubMed] [Google Scholar]
- 19.Bohan A, Peter JB. Polymyositis and dermatomyositis. N Engl J Med. (1975) 292:403–7. 10.1056/NEJM197502202920807 [DOI] [PubMed] [Google Scholar]
- 20.Sontheimer RD. Would a new name hasten the acceptance of amyopathic dermatomyositis (dermatomyositis sine myositis) as a distinctive subset within the idiopathic inflammatory dermatomyopathies spectrum of clinical illness? J Am Acad Dermatol. (2002) 46:626–36. 10.1067/mjd.2002.120621 [DOI] [PubMed] [Google Scholar]
- 21.Hansell DM, Bankier AA, MacMahon H, McLoud TC, Müller NL, Remy J. Fleischner society: glossary of terms for thoracic imaging. Radiology. (2008) 246:697–722. 10.1148/radiol.2462070712 [DOI] [PubMed] [Google Scholar]
- 22.Antonio GE, Wong KT, Hui DS, Wu A, Lee N, Yuen EH, et al. Thin-section CT in patients with severe acute respiratory syndrome following hospital discharge: preliminary experience. Radiology. (2003) 228:810–5. 10.1148/radiol.2283030726 [DOI] [PubMed] [Google Scholar]
- 23.Xu W, Wu W, Zheng Y, Chen Z, Tao X, Zhang D, et al. A computed tomography radiomics-based prediction model on interstitial lung disease in anti-MDA5-positive dermatomyositis. Front Med. (2021) 8:768052. 10.3389/fmed.2021.768052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ruaro B, Baratella E, Confalonieri P, Wade B, Marrocchio C, Geri P, et al. High-resolution computed tomography: lights and shadows in improving care for SSc-ILD patients. Diagnostics. (2021) 11:1960. 10.3390/diagnostics11111960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chang YC, Yu CJ, Chang SC, Galvin JR, Liu HM, Hsiao CH, et al. Pulmonary sequelae in convalescent patients after severe acute respiratory syndrome: evaluation with thin-section CT. Radiology. (2005) 236:1067–75. 10.1148/radiol.2363040958 [DOI] [PubMed] [Google Scholar]
- 26.Cao H, Xia Q, Pan M, Zhao X, Li X, Shi R, et al. Gottron papules and Gottron sign with ulceration: a distinctive cutaneous feature in a subset of patients with classic dermatomyositis and clinically amyopathic dermatomyositis. J Rheumatol. (2016) 43:1735–42. 10.3899/jrheum.160024 [DOI] [PubMed] [Google Scholar]
- 27.Xu Y, Yang CS, Li YJ, Liu XD, Wang JN, Zhao Q, et al. Predictive factors of rapidly progressive-interstitial lung disease in patients with clinically amyopathic dermatomyositis. Clin Rheumatol. (2016) 35:113–6. 10.1007/s10067-015-3139-z [DOI] [PubMed] [Google Scholar]
- 28.So H, Ip RW, Wong VT, Yip RM. Analysis of anti-melanoma differentiation-associated gene 5 antibody in Hong Kong Chinese patients with idiopathic inflammatory myopathies: diagnostic utility and clinical correlations. Int J Rheum Dis. (2018) 21:1076–81. 10.1111/1756-185X.13268 [DOI] [PubMed] [Google Scholar]
- 29.Gono T, Kawaguchi Y, Satoh T, Kuwana M, Katsumata Y, Takagi K, et al. Clinical manifestation and prognostic factor in anti-melanoma differentiation-associated gene 5 antibody-associated interstitial lung disease as a complication of dermatomyositis. Rheumatology. (2010) 49:1713–9. 10.1093/rheumatology/keq149 [DOI] [PubMed] [Google Scholar]
- 30.Wu W, Xu W, Sun W, Zhang D, Zhao J, Luo Q, et al. Forced vital capacity predicts the survival of interstitial lung disease in anti-MDA5 positive dermatomyositis: a multi-center cohort study. Rheumatology. (2021) 61:230–9. 10.1093/rheumatology/keab305 [DOI] [PubMed] [Google Scholar]
- 31.Xu W, Wu W, Zhang D, Chen Z, Tao X, Zhao J, et al. A novel CT scoring method predicts the prognosis of interstitial lung disease associated with anti-MDA5 positive dermatomyositis. Sci Rep. (2021) 11:17070. 10.1038/s41598-021-96292-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gono T, Kaneko H, Kawaguchi Y, Hanaoka M, Kataoka S, Kuwana M, et al. Cytokine profiles in polymyositis and dermatomyositis complicated by rapidly progressive or chronic interstitial lung disease. Rheumatology. (2014) 53:2196–203. 10.1093/rheumatology/keu258 [DOI] [PubMed] [Google Scholar]
- 33.Horai Y, Koga T, Fujikawa K, Takatani A, Nishino A, Nakashima Y, et al. Serum interferon-α is a useful biomarker in patients with anti-melanoma differentiation-associated gene 5 (MDA5) antibody-positive dermatomyositis. Mod Rheumatol. (2015) 25:85–9. 10.3109/14397595.2014.900843 [DOI] [PubMed] [Google Scholar]
- 34.Takada T, Ohashi K, Hayashi M, Asakawa K, Sakagami T, Kikuchi T, et al. Role of IL-15 in interstitial lung diseases in amyopathic dermatomyositis with anti-MDA-5 antibody. Respir Med. (2018) 141:7–13. 10.1016/j.rmed.2018.06.012 [DOI] [PubMed] [Google Scholar]
- 35.Chen M, Quan C, Diao L, Xue F, Xue K, Wang B, et al. Measurement of cytokines and chemokines and association with clinical severity of dermatomyositis and clinically amyopathic dermatomyositis. Br J Dermatol. (2018) 179:1334–41. 10.1111/bjd.17079 [DOI] [PubMed] [Google Scholar]
- 36.Nakashima R, Mimori T. Anti-MDA5 (melanoma differentiation-associated gene 5) antibody and dermatomyositis with rapidly progressive interstitial pneumonia. Nihon Rinsho Meneki Gakkai Kaishi. (2013) 36:711–6. 10.2177/jsci.36.71 [DOI] [PubMed] [Google Scholar]
- 37.Zhou M, Ye Y, Yan N, Lian X, Bao C, Guo Q. Non-invasive positive pressure ventilator deteriorates the outcome of pneumomediastinum in anti-MDA5 antibody-positive clinically amyopathic dermatomyositis. Clin Rheumatol. (2020) 39:1919–27. 10.1007/s10067-019-04918-2 [DOI] [PubMed] [Google Scholar]
- 38.Li T, Guo L, Chen Z, Gu L, Sun F, Tan X, et al. Pirfenidone in patients with rapidly progressive interstitial lung disease associated with clinically amyopathic dermatomyositis. Sci Rep. (2016) 6:33226. 10.1038/srep33226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Allenbach Y, Uzunhan Y, Toquet S, Leroux G, Gallay L, Marquet A, et al. Different phenotypes in dermatomyositis associated with anti-MDA5 antibody: study of 121 cases. Neurology. (2020) 95:e70–8. 10.1212/WNL.0000000000009727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nakashima R, Imura Y, Kobayashi S, Yukawa N, Yoshifuji H, Nojima T, et al. The RIG-I-like receptor IFIH1/MDA5 is a dermatomyositis-specific autoantigen identified by the anti-CADM-140 antibody. Rheumatology. (2009) 49:433–40. 10.1093/rheumatology/kep375 [DOI] [PubMed] [Google Scholar]
- 41.Hozumi H, Fujisawa T, Nakashima R, Johkoh T, Sumikawa H, Murakami A, et al. Comprehensive assessment of myositis-specific autoantibodies in polymyositis/dermatomyositis-associated interstitial lung disease. Respir Med. (2016) 121:91–9. 10.1016/j.rmed.2016.10.019 [DOI] [PubMed] [Google Scholar]
- 42.Fujiki Y, Kotani T, Isoda K, Ishida T, Shoda T, Yoshida S, et al. Evaluation of clinical prognostic factors for interstitial pneumonia in anti-MDA5 antibody-positive dermatomyositis patients. Mod Rheumatol. (2018) 28:133–40. 10.1080/14397595.2017.1318468 [DOI] [PubMed] [Google Scholar]
- 43.Zou J, Guo Q, Chi J, Wu H, Bao C. HRCT score and serum ferritin level are factors associated to the 1-year mortality of acute interstitial lung disease in clinically amyopathic dermatomyositis patients. Clin Rheumatol. (2015) 34:707–14. 10.1007/s10067-015-2866-5 [DOI] [PubMed] [Google Scholar]
- 44.Zuo Y, Ye L, Liu M, Li S, Liu W, Chen F, et al. Clinical significance of radiological patterns of HRCT and their association with macrophage activation in dermatomyositis. Rheumatology. (2020) 59:2829–37. 10.1093/rheumatology/keaa034 [DOI] [PubMed] [Google Scholar]
- 45.Wakura R, Matsuda S, Kotani T, Shoda T, Takeuchi T. The comparison of nailfold videocapillaroscopy findings between anti-melanoma differentiation-associated gene 5 antibody and anti-aminoacyl tRNA synthetase antibody in patients with dermatomyositis complicated by interstitial lung disease. Sci Rep. (2020) 10:15692. 10.1038/s41598-020-72752-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yamaguchi K, Yamaguchi A, Itai M, Kashiwagi C, Takehara K, Aoki S, et al. Clinical features of patients with anti-melanoma differentiation-associated gene-5 antibody-positive dermatomyositis complicated by spontaneous pneumomediastinum. Clin Rheumatol. (2019) 38:3443–50. 10.1007/s10067-019-04729-5 [DOI] [PubMed] [Google Scholar]
- 47.Hoshino K, Muro Y, Sugiura K, Tomita Y, Nakashima R, Mimori T. Anti-MDA5 and anti-TIF1-γ antibodies have clinical significance for patients with dermatomyositis. Rheumatology. (2010) 49:1726–33. 10.1093/rheumatology/keq153 [DOI] [PubMed] [Google Scholar]
- 48.Zuo Y, Ye L, Chen F, Shen Y, Lu X, Wang G, et al. Different multivariable risk factors for rapid progressive interstitial lung disease in anti-MDA5 positive dermatomyositis and anti-synthetase syndrome. Front Immunol. (2022) 13:845988. 10.3389/fimmu.2022.845988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shirakashi M, Nakashima R, Tsuji H, Tanizawa K, Handa T, Hosono Y, et al. Efficacy of plasma exchange in anti-MDA5-positive dermatomyositis with interstitial lung disease under combined immunosuppressive treatment. Rheumatology. (2020) 59:3284–92. 10.1093/rheumatology/keaa123 [DOI] [PubMed] [Google Scholar]
- 50.Wang LM, Yang QH, Zhang L, Liu SY, Zhang PP, Zhang X, et al. Intravenous immunoglobulin for interstitial lung diseases of anti-melanoma differentiation-associated gene 5-positive dermatomyositis. Rheumatology. (2021). 10.1093/rheumatology/keab928 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 51.Koguchi-Yoshioka H, Okiyama N, Iwamoto K, Matsumura Y, Ogawa T, Inoue S, et al. Intravenous immunoglobulin contributes to the control of antimelanoma differentiation-associated protein 5 antibody-associated dermatomyositis with palmar violaceous macules/papules. Br J Dermatol. (2017) 177:1442–6. 10.1111/bjd.15499 [DOI] [PubMed] [Google Scholar]
- 52.Kurasawa K, Arai S, Namiki Y, Tanaka A, Takamura Y, Owada T, et al. Tofacitinib for refractory interstitial lung diseases in anti-melanoma differentiation-associated 5 gene antibody-positive dermatomyositis. Rheumatology. (2018) 57:2114–9. 10.1093/rheumatology/key188 [DOI] [PubMed] [Google Scholar]
- 53.Bandoh S, Fujita J, Ohtsuki Y, Ueda Y, Hojo S, Tokuda M, et al. Sequential changes of KL-6 in sera of patients with interstitial pneumonia associated with polymyositis/dermatomyositis. Ann Rheum Dis. (2000) 59:257–62. 10.1136/ard.59.4.257 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets presented in this article are not readily available because the data are being expanded for use in another study. Requests to access the datasets should be directed to 476839887@qq.com.