Abstract
Intrahepatic cholangiocarcinoma (ICC) is the second most common primary liver cancer and causes major economic and health burdens throughout the world. Although the incidence of ICC is relatively low, an upward trend has been seen over the past few decades. Owing to the lack of specific manifestations and tools for early diagnosis, most ICC patients have relatively advanced disease at diagnosis. Thus, neoadjuvant therapy is necessary to evaluate tumor biology and downstage these patients so that appropriate candidates can be selected for radical liver resection. However, even after radical resection, the recurrence rate is relatively high and is a main cause leading to death after surgery, which makes adjuvant therapy necessary. Because of its low incidence, studies in both neoadjuvant and adjuvant settings of ICC are lagging compared with other types of malignancy. While standard neoadjuvant and adjuvant regimens are not available in the current guidelines due to a lack of high-level evidence, some progress has been achieved in recent years. In this review, the available literature on advances in neoadjuvant and adjuvant strategies in ICC are evaluated, and possible challenges and opportunities for clinical and translational investigations in the near future are discussed.
Keywords: Intrahepatic cholangiocarcinoma, Neoadjuvant therapy, Adjuvant therapy, Recurrence, Liver resection, Liver transplant
Graphical abstract
Introduction
Intrahepatic cholangiocarcinoma (ICC), the second most common primary liver cancer, accounts for 6.4–12.0% of primary malignancies arising in liver itself.1,2 Although most ICC cases are sporadic, some risk factors have been identified, including liver fluke infection, bile tract conditions (e.g., primary sclerosing cholangitis, choledochal cysts, choledocholithiasis, cholelithiasis, and cholecystocholithiasis), hepatitis B and C virus infection, cirrhosis, alcohol consumption, smoking, metabolism-related factors (e.g., nonalcoholic fatty liver disease (NAFLD), nonalcoholic steatohepatitis (NASH), obesity, and diabetes mellitus), inflammatory bowel disease, thyrotoxicosis, hemochromatosis, gout, and environmental chemical exposure etc.3–8 The incidence of ICC is relatively low, but an upward trend has been noted in the last few decades, in contrast to a stable or decreasing incidence of extrahepatic cholangiocarcinoma (ECC).9,10 National Cancer Database (NCDB) records reveal that ICC cases rose from 1,194 in 2004 to 3,821 in 2015, with an average annual increase of 4.16%.11 It has been suggested that the increase is linked to the mounting incidence of type 2 diabetes mellitus, cirrhosis, alcoholic liver disease, and cholelithiasis.4 Significantly, a definitive association between cirrhosis and ICC occurrence has been confirmed by several studies and patients with cirrhosis,3,4 mainly secondary to hepatitis B and C virus infection, who are a population at high risk of ICC, which can be detected in a timely manner with an appropriate surveillance modality, such as magnetic resonance imaging (MRI) with hepatocyte-specific Gd-based contrast agents.12 The enlarging gap between ICC and ECC can be partially accounted for by the high incidence of metabolism-associated conditions, especially NAFLD. NAFLD affects approximately 24% of the global population and is now the leading cause of chronic liver disease. Meanwhile, increasing studies indicate a definitive association between NAFLD and ICC, but not in ECC.5,6,13,14 Owing to its highly aggressive biological behavior and the lack of specific symptoms and signs, most ICC patients present with relatively advanced disease at the initial diagnosis. An analysis of the Surveillance, Epidemiology, and End Results (SEER) database showed that 65.1–70.0% of ICC patients in the USA were classified as stage III or IV according to the American Joint Committee on Cancer (AJCC) tumor-node-metastasis (TNM) staging system.15 Accordingly, only 23.0–53.0% of patients have the opportunity to undergo surgical resection and then experience long-term survival.16,17 However, the high recurrence rate after curative treatment leads to a dismal prognosis. Even after radical resection, 57.9–73.4% of patients experience recurrence and 41.3–42.5% patients die of recurrence.18–20 Postoperative recurrence occurs not only in the liver remnant, but also in adjacent and distant organs. Hu et al.20 reported that intrahepatic-only recurrence was observed in 53.2% of patients, extrahepatic-only recurrence in 14.8% of patients, and both intrahepatic and extrahepatic recurrence in 32.0% of patients. Similar findings were observed in other studies.18,19 While more than half recurrent ICC patients have liver involvement, extrahepatic recurrence is not an uncommon event. The most common recurrence sites outside the liver are the lungs, lymph nodes, and peritoneum.18–20
Owing to the scarce experience in liver transplantation (LT) for ICC patients and the shortage of donors, liver resection remains the main modality for curing ICC patients. However, because of the relatively advanced stage at diagnosis and the high recurrence rate after resection, both neoadjuvant and adjuvant therapies are necessary in those situations. Neoadjuvant chemotherapy or radiotherapy enables initially unresectable patients to be downstaged and converted to surgical candidates, which is frequently undertaken in other malignancies.21 On the other hand, disseminated micrometastases in the liver remnant, lymph nodes, blood, or other organs can be eradicated by adjuvant chemotherapy or radiotherapy. The efficacy of neoadjuvant and adjuvant therapy has been validated in other types of cancer, and they are recommended as standard treatments in various guidelines.21 In contrast, the benefits of neoadjuvant and adjuvant therapy in ICC are poorly understood. No standard neoadjuvant and adjuvant regimens are included in the latest National Comprehensive Cancer Network (NCCN) guidelines.22 Thus, we endeavored to assess the available evidence on the use of neoadjuvant and adjuvant therapies in ICC patients undergoing resection or LT in this review.
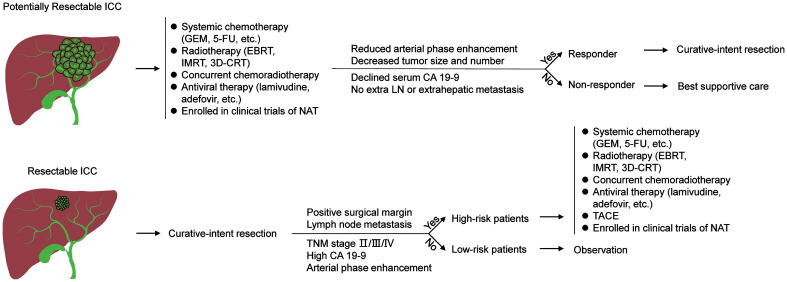
Neoadjuvant therapy (NAT) for ICC
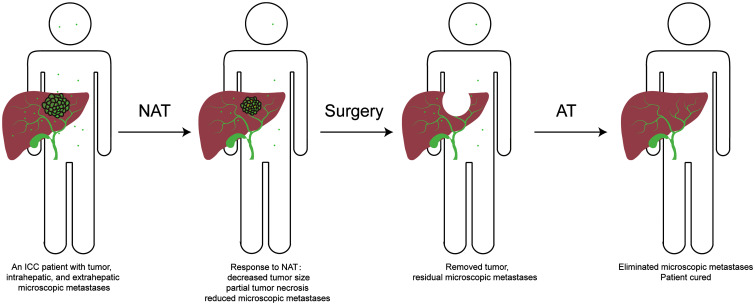
Neoadjuvant therapy is often used in other malignancies as an important modality to evaluate tumor response and biological nature, downstage initially borderline resectable or unresectable patients, and then select appropriate patients for resection. Nevertheless, NAT is not commonly used in ICC patients. The reported percentage of patients who received NAT is less than 10% in most studies.19,23–45 Indeed, no high-level evidence supports the use of pre-operative chemotherapy or radiotherapy in ICC, and current guidelines do not recommend it in ICC patients undergoing resection or LT. However, the unique clinical manifestations of ICC resulting from its aggressive biology, including relatively advanced disease at diagnosis, and rapid recurrence in some cases after surgery, imply that NAT might be necessary before surgery (Fig. 1).
Fig. 1. Rationale for neoadjuvant and adjuvant therapy in ICC.
AT, adjuvant therapy; ICC, intrahepatic cholangiocarcinoma; NAT, neoadjuvant therapy.
Chemotherapy and radiotherapy are frequently combined to obtain maximum neoadjuvant effectiveness in ICC. The first case of aggressive surgical resection following neoadjuvant chemoradiation therapy was reported by Kato et al.46 in 2009, in which intravenous gemcitabine and three-dimensional conformation radiotherapy were administered to a patient with locally advanced disease. Decreased enhancement of the tumor on CT scan and decreased serum CA19-9 levels demonstrated an active treatment response, which was validated by extensive fibrosis in the resected tumor and lymph nodes. A similar case was reported in 2015, in which a complete pathological response was achieved by gemcitabine-based chemotherapy. The patient remained alive with no evidence of recurrence 6 months after surgery.47 The first small-sample study was by Rayar et al.48 in 2015, in which 10 patients with potentially resectable disease were given gemcitabine-based systemic chemotherapy and yttrium-90 radioembolization. Eight patients accepted R0 resection, and the conversion rate was 80%. Six patients achieved long survival, with one patient remaining alive 40 months after initial treatment. Similarly, Sumiyoshi et al.49 reported a conversion rate of 71% (5/7) with S-1-based chemoradiotherapy and two patients having an overall survival of more than 40 months. The long survival of the patients in those studies is encouraging. Investigators have begun to explore its impact of NAT on long-term outcomes by comparing patients receiving NAT and surgery with those undergoing upfront surgery.
Buettner et al.40 identified 1,057 patients with curative-intent resection for ICC in an international multi-institutional cohort, among whom 62 patients had received preoperative chemotherapy. Both overall survival (OS) and disease-free survival (DFS) in patients with and without preoperative chemotherapy were comparable in propensity-score matched cohorts. Similar results were reported by others.19,24,27,29,34,43,50,51 However, the results should be interpreted with caution for several reasons. To begin with, the sample size was relatively small or the proportion of patients with NAT was low in these studies. Then, there might be selection bias regarding choosing candidates for NAT. It has been established that locally advanced patients or borderline resectable patients, who are deemed to have a dismal prognosis, are more likely to receive NAT. Finally, NAT regimens varied greatly among individuals in different studies and even in the same study in terms of the administration routine, dosage, and duration. Nevertheless, a recent study by Mason et al.52 reported a positive effect of neoadjuvant chemotherapy, with a 23% decrease in the risk of death compared with surgery alone (HR=0.77, p<0.05). Similarly, Utuama et al.53 observed that NAT prolonged survival (HR=0.58, p=0.02), but only in stage II–III disease. Both studies evaluated cases included in the NCDB database, and used propensity score matching to reduce the bias caused by the low proportion of patients with NAT. Another recent study from the USA confirmed the protective role of NAT in prolonging OS in ICC patients (HR=0.16, p=0.001).54
Traditionally, ICC is considered as a contradiction for LT because of unfavorable results. However, promising results were observed in a recent study, caused in part by NAT as the bridging treatment. Lunsford et al.55 performed LT in six of 12 (conversion rate: 50%) locally advanced ICC patients, all of whom had received NAT in the waiting period and had stable disease or tumor regression after 6 months or more on NAT. Most NAT regimens included gemcitabine-based systemic chemotherapy, fluoropyrimidines, and targeted drugs. The 5-year OS and DFS were 83.3% and 50% respectively, indicating favorable long-term outcomes. That was the first study focusing on LT in locally advanced ICC patients in the setting of NAT, which implies that the tumor response to NAT can be useful to measure tumor biology and to select candidates who might benefit from LT. While a satisfying conversion rate and improved long-term survival indicate the effectiveness of preoperative therapy, well-designed prospective studies are still necessary to confirm the role of NAT prior to liver resection or transplantation.
Adjuvant therapy (AT) for ICC
While NAT is usually employed to downstage and convert initially unresectable patients to surgical candidates, immediate resection remains the first choice in ICC patients with resectable tumors and sufficient future liver remnant volume and function. However, recurrence is a relatively common event in patients who receive upfront surgery, which is associated with residual micrometastasis from the primary tumor or de novo carcinogenesis from the underlying liver background. Extrahepatic recurrence or metastasis is also not uncommon. Therefore, effective adjuvant therapy must address all those issues (Fig. 1).
Transcatheter arterial chemoembolization (TACE)
While TACE is thought to be safe, feasible, and effective as a palliative treatment in unresectable ICC patients, the role of TACE in adjuvant settings is less well understood. Nearly all relevant studies are from China (Table 1), and report mixed results. Shen et al.56 published the first study in this field in 2011, in which patients receiving TACE after surgery had significantly better OS than those receiving surgery alone in an early recurrence subgroup, with a median OS of 12 vs. 5 months, p<0.001), but not in a late recurrence subgroup. The investigators concluded that TACE controlled early recurrence by eradicating recurrent foci in the remnant liver. Another study from the same center observed that TACE improved survival (3-year OS: 34% vs. 0%, p<0.001 and 3-year DFS: 27% vs. 0%, p=0.008) in patients with poor prognostic factors, while having no effect on the survival of patients without poor prognostic factors.57 The positive effect of TACE in ICC patients at high risk of recurrence or death was also confirmed by other studies that included patients with high-risk features such as being in the lowest tertile of a prognostic nomogram, having a preoperative GGT of >54 U/L, arterial phase enhancement on CT scans, relatively advanced TNM stages, elevated CA19-9, and without lymphadenectomy.58–63 Liu et al.64 observed no survival benefit with TACE, which instead promoted recurrence, similar to the findings of Li et al.59 in TNM stage I patients. The hypoxia caused by the blockage of liver blood flow during TACE may increase the malignant potential of residual tumor cells. Two meta-analyses also drew conflicting conclusions.65,66 Randomized controlled trials (RCTs) are not available, but the findings of the above studies indicate that adjuvant TACE might benefit patients at high risk of recurrence or death.
Table 1. Selected studies of adjuvant TACE in ICC.
| Reference | Study type | Arms and interventions | Patients, n intervention/observation | Main findings | Remarks |
|---|---|---|---|---|---|
| Shen et al. (2011)56 | Retrospective | TACE vs. observation | 53/72 | Patients with recurrence time ≤ 3 months: improved 1-, 3-, 5-year OS with TACE. | TACE can eradicate recurrent foci in remnant liver and control early recurrence. |
| Wu et al. (2012)57 | Retrospective | TACE vs. observation | 57/57 | Patients with poor prognostic factors: improved 1-, 3-, 5-year OS and DFS with TACE. | Poor prognostic factors: tumor size ≥ 5 cm, advanced TNM stage (stage III or IV). |
| Li et al. (2014)58 | Retrospective | TACE vs. observation | 68/143 | TNM stage II, III, and IV patients: improved OS with TACE. | TNM stage I patients: higher recurrence rate with TACE. |
| Li et al. (2015)59 | Retrospective | TACE vs. observation | 122/431 | Patients with nomogram scores ≥ 77: improved 1-, 3-, 5-year OS and recurrence rate with TACE. | ICC nomogram: CEA, CA19-9, tumor diameter, tumor number, vascular invasion, lymph node metastasis, direct invasion and local metastasis; study with the largest sample size. |
| Jeong et al. (2017)60 | Retrospective | TACE vs. observation | 9/33 | ICC with arterial phase enhancement on CT scans: improved 1-, 3-, 5-year OS with TACE. | HBV-associated ICC; preoperative CT scan manifestation can serve as a selection criterion for TACE candidates; limited by small sample. size |
| Lu et al. (2017)61 | Retrospective | TACE vs. observation | 89/183 | Patients with GGT levels > 54 U/L: improved OS with TACE. | PSM; preoperative serum GGT level can serve as a selection criterion for TACE candidates. |
| Wang et al. (2020)62 | Retrospective | TACE vs. observation | 39/296 | Patients with stage II, III or risk factors < 2: improved OS with TACE. | PSM; the incidence of patients having adjuvant TACE is relatively low (11.6%). |
| Cheng et al. (2021)63 | Retrospective | TACE vs. observation | 68/155 | Patients with elevated CA19-9 or no lymphadenectomy: improved OS with TACE. | PSM and IPTW; all patients have microvascular invasion. |
| Liu et al. (2021)64 | Retrospective | TACE vs. observation | 35/234 | TNM stage I patients: TACE cannot prolong OS; instead, TACE might increase the recurrence risk. | All patients have TNM stage I disease; relatively low proportions (13.0%) of patients receive adjuvant TACE. |
CEA, carcino-embryonic antigen; CA19-9, carbohydrate antigen 19–9; DFS, disease-free survival; CT, computed tomography; GGT, gamma-glutamyl transpeptidase; HBV, hepatitis B virus; ICC, intrahepatic cholangiocarcinoma; IPTW, inverse probability of treatment weighting; LR, liver resection; OS, overall survival; PSM, propensity score matching; TACE, transcatheter arterial chemoembolization; TNM, tumor-node-metastasis.
Systemic chemotherapy
The NCCN or other guidelines do not include a standard adjuvant regimen, it is not uncommon for ICC patients to receive adjuvant chemotherapy after surgery.22,67 The majority of relevant studies report that more than 30% patients receive systemic chemotherapy.18,20,26,28,33,40 Owing to a lack of RCT results, chemotherapy regimens vary among centers, and include gemcitabine, 5-fluorouracil, capecitabine, S-1, oxaliplatine, and cisplatine, etc.28,68–72 The most common regimens include gemcitabine and 5-fluorouracil. Detailed information on chemotherapy reagent dose, duration, and number of cycles is often not provided, and might partially explain inconsistent reports of treatment effectiveness.
Owing to the relatively low incidence of ICC, the role of adjuvant chemotherapy in resected biliary tract cancer including ICC has been evaluated in only two RCTs, neither of which achieved the primary endpoint of improving OS in the whole cohort as well as in the ICC subgroup. ICC only accounted for a minority (84/447,18.8%; and 86/194, 44.3%) of the entire study cohort in the two RCTs.73,74 Given the fact that ICC differs from other bile duct cancers at the clinicopathological and molecular levels, studies with large groups of ICC patients are needed.75 The first study focusing on AT in ICC patients was an analysis by Sur et al.76 of 638 ICC patients with surgical resection who were included in the NCDB database. Seventy-five had received adjuvant chemotherapy alone and 147 had received adjuvant chemoradiation. The patients with significant benefits from adjuvant chemotherapy or chemoradiation had positive surgical margins (chemotherapy HR=0.44, p=0.0016 and chemoradiation HR=0.57, p=0.0039) or lymph node metastasis (chemotherapy HR=0.54, p=0.0365 and chemoradiation HR=0.50, p=0.005). Three other studies that included patients in the NCDB or SEER databases who were treated at different time periods also reported that high-risk patients benefited from AT. The use of chemotherapy has increased from 33% of patients in 2000–2004, to 37% in 2005–2009 and 41% in 2010–2014 (p=0.027).45,77–78 The findings are echoed by similar results from the Taiwan Cancer Registry database and a multi-institutional cohort.79,80 Unlike the various high-risk characteristics of adjuvant TACE, those associated with adjuvant systemic chemotherapy are limited to positive margins, positive lymph nodes, or relatively advanced stage.79,80 A study by Schweitzer et al.81 could not evaluate high-risk subgroups because of a small sample size, but did report a survival advantage of adjuvant chemotherapy in a propensity-score matching analysis (median OS: 33.5 vs. 18.0 months, p=0.002). However, many studies did not find a significant positive or negative correlation between AT and patient survival.19,20,29,31,33–35,82 However, the studies mainly focused on other factors, such as albumin and bilirubin, and AT was only an incidental variable. As no subgroup analysis or propensity-score matched analysis was used to evaluate AT, and the role of AT was underestimated. In these circumstances, the view that selected ICC patients can benefit from AT seems more convincing. Selected studies are presented in Table 2.
Table 2. Selected studies of adjuvant chemotherapy/radiotherapy/chemoradiotherapy for intrahepatic cholangiocarcinoma and extrahepatic cholangiocarcinoma.
| Category | Reference | Regimen | Study type | Patients, n* intervention/observation | ICC | ECC |
|---|---|---|---|---|---|---|
| Adjuvant CT | (i) Sur et al. (2015)76; (ii) Miura et al. (2015)45; (iii) Reames et al. (2017)80; (iv) Schweitzer et al. (2017)81; (v) Lee et al. (2019)77; (vi) Altman et al. (2020)78 | (i) CT; (ii) CT; (iii) CIS/GEM/5-FU; (iv) GEM-based CT; (v) CT; (vi) CT | (i) Retrospective; (ii) Retrospective; (iii) Retrospective; (iv) Retrospective; (v) Retrospective; (vi) Retrospective | (i) 75/416; (ii) 985/1,766; (iii) 347/807; (iv) 39/171; (v) 1,189/1,624; (vi) 470/753 | (i) Positive LN subgroup OS: HR=0.54, p=0.037; positive margin subgroup: HR=0.44, p=0.002. (ii) T3/T4 subgroup mOS: 21.3 vs. 15.6 months, p<0.001; N1 subgroup mOS: 19.8 vs. 10.7 months, p<0.001; R1/R2 subgroup mOS: 19.5 vs. 11.6 months, p=0.006. (iii) T3/T4 subgroup OS: HR=0.44, p<0.01; N1 subgroup: HR=0.24, p<0.001. (iv) PSM cohort OS: HR=0.33, p=0.002. (v) high-risk subgroup OS: HR=0.66, p<0.001. (vi) N1 subgroup OS: HR=0.46, p=0.001 | (a) PCC; OS: HR=0.25, p=0.035. (b) ECC; positive LN subgroup OS: HR=0.85, p<0.05. (c) DCC; OS: HR=0.21, p=0.001; DFS: HR=0.34, p=0.002. (d) ECC; OS: HR=0.62, p=0.008; PFS, HR=0.62, p=0.007. (e) PCC; PSM cohort 5-year OS: 43.2% vs. 15.6%, p=0.001; 3-year RFS: 46.9% vs. 15.9, p=0.01. (f) ECC; OS: HR not reported, p=0.114; DFS: HR=1.64, p=0.035. (g) ECC; OS: HR=1.01, p=0.964; RFS: HR=0.9, p=0.693. (h) DCC; PSM cohort mOS: 26.3 vs. 43.3 months, p=0.340; mDFS: 15.5 vs. 14.7 months, p=0.790. (i) ECC; 5-year OS: 40.8% vs 44.6%, p=0.916; RFS, 30.9% vs. 38.4%, p=0.794. (j) PCC; early recurrence subgroup OS: HR=0.38, p=0.039. (k) PCC; OS: HR=0.47, p=0.001; DFS: HR=0.69, p=0.068 |
| (a) Murakami et al. (2009)97; (b) Hoehn et al. (2015)98; (c) Kim et al. (2016)92; (d) Im et al. (2016)95; (e) Mizuno et al. (2017)99; (f) Yin et al. (2018)100; (g) Ebata et al. (2018)101; (h) Bergeat et al. (2018)102; (i) Morino et al. (2019)103; (j) Zhao et al. (2020)104; (k) Im et al. (2021)94; | (a) GEM-based CT; (b) CT; (c) 5-FU/DOX/GEM; (d) 5-FU/CIS/GEM; (e) GEM monotherapy; (f) GEM/CIS/OXA/S-1/CAP; (g) GEM monotherapy; (h) GEM-based CT; (i) GEM/S-1; (j) GEM/S-1; (k) 5-FU/GEM-based CT | (a) Retrospective; (b) Retrospective; (c) Retrospective; (d) Retrospective; (e) Retrospective; (f) Retrospective; (g) RCT; (h) Retrospective; (i) Retrospective; (j) Retrospective; (k) Retrospective | (a) 18/20; (b) 444/5,739; (c) 27/102; (d) 90/168; (e) 67/113; (f) 40/40; (g) 117/108; (h) 56/122; (i) 57/49; (j) 25/310; (k) 67/90 | |||
| Adjuvant RT | (i) Shinohara et al. (2008)83; (ii) Jiang et al. (2010)84; (iii) Hammad et al. (2016)86; (iv) Zheng et al. (2018)85 | (i) RT; (ii) EBRT; (iii) RT; (iv) IMRT/VMAT | (i) Retrospective; (ii) Retrospective; (iii) Retrospective; (iv) Retrospective | (i) 286/948; (ii) 24/66; (iii) 525/2,372; (iv) 26/23 | (i) mOS: 11 vs. 6 months, p=0.014. (ii) OS: HR=0.48, p=0.013.(iii) PSM cohort OS: HR=1.01, p=0.923. (iv) OS: HR=0.27, p=0.011 | (a) ECC; OS: HR=0.89, p=0.074. (b) ECC; OS: HR=0.59, p=0.078; PFS, HR=0.57, p=0.045. (c) DCC; OS: HR=2.38, p=0.040; RFS: HR=1.42, p=0.361. (d) PCC; mOS: 22 vs. 23 months, p=0.978; mCSS, 17 vs 18 months, p=0.554. (e) ECC; OS: HR=0.49, p=0.085; LRFS, HR=0.31, p=0.043. (f) PCC; OS: HR=0.81, p=0.538; DFS: HR=1.32, p=0.368 |
| (a) Vern-Gross et al. (2011)91; (b) Im et al. (2016)95; (c) Kim et al. (2016)92; (d) Leng et al. (2017)93; (e) Kim et al. (2017)96; (f) Im et al. (2021)94 | (a) RT; (b) 3D-CRT; (c) EBRT; (d) RT; (e) RT; (f) EBRT/IMRT | (a) Retrospective; (b) Retrospective; (c) Retrospective; (d) Retrospective; (e) Retrospective; (f) Retrospective | (a) 473/1,018; (b) 29/168; (c) 9/102; (d) 762/1,155; (e) 23/36; (f) 18/90 | |||
| Adjuvant CRT | (i) Sur et al. (2015)76 | (i) CRT | (i) Retrospective | (i) 147/416 | (i) positive LN subgroup OS: HR=0.50, p=0.005; positive margin subgroup: HR=0.57, p=0.004 | (a) ECC; OS: HR=0.53, p=0.005; DFS: HR=0.55 p=0.005. (b) ECC; OS: HR=0.82, p<0.001. (c) DCC; OS: HR=0.25, p=0.024; DFS: HR=0.33, p=0.004. (d) PCC; OS: HR=0.46, p=0.003; PFS, HR=0.41, p=0.001. (e) PCC; OS: HR=0.26, p<0.001; DFS: HR=0.31, p<0.001 |
| (a) Kim et al. (2011)105; (b) Hoehn et al. (2015)98; (c) Kim et al. (2016)92; (d) Im et al. (2016)95; (e) Im et al. (2021)94 | (a) 5-FU-based CT+EBRT; (b) CRT; (c) 5-FU/CAP/GEM+EBRT; (d) 5-FU/CIS/GEM+3D-CRT; (e) 5-FU/GEM-based CT+EBRT/IMRT | (a) Retrospective; (b) Retrospective; (c) Retrospective; (d) Retrospective; (e) Retrospective | (a) 115/53; (b) 1,902/5,739; (c) 20/102; (d) 49/168; (e) 21/90 |
*Numbers of patients in the intervention/observation groups. i–vi are studies of adjuvant CT/RT/CRT in ICC; a–k are studies of adjuvant CT/RT/CRT in ECC. 3D-CRT, three-dimensional conformal radiotherapy; 5-FU, 5-Fluoropyrimidine; CAP, capecitabine; CIS, cisplatin; CRT, chemoradiotherapy; CSS, cancer-specific survival; CT, chemotherapy; DCC, distal cholangiocarcinoma; DFS, disease-free survival; DOX, doxorubicin; EBRT, external beam radiotherapy; ECC, extrahepatic cholangiocarcinoma; GEM, gemcitabine; HR, hazard ratio; ICC, intrahepatic cholangiocarcinoma; IMRT, intensity-modulated radiotherapy; LN, lymph nodes; LRFS, local recurrence-free survival; mOS, median OS; OS, overall survival; OXA, oxaliplatin; PCC, perihilar cholangiocarcinoma; PFS, progression-free survival; PSM, propensity score matching; RCT, randomized controlled trial; RFS, recurrence-free survival; RT, radiotherapy; VMAT, volumetric modulated arc therapy.
Radiotherapy
Radiotherapy is often combined with chemotherapy both in neoadjuvant and adjuvant settings of ICC patients. As chemotherapy has been discussed above, only radiotherapy is included in this section. The first study of adjuvant radiotherapy in ICC evaluated patients included in the SEER database. Those with surgery and adjuvant radiotherapy had significantly better OS then those with surgery alone (median: 11 vs. 6 months, p=0.014). However, information on the radiotherapy modality, dose, and duration and information on other variables such as adjuvant chemotherapy and lymph node metastasis was missing, which is an inherent drawback of the SEER database.83 Jiang et al.84 reported that adjuvant radiotherapy improved the prognosis of patients with resected ICC and concurrent macroscopic lymph node metastases, and Zheng et al.85 reported a similar role of radiotherapy in ICC patients with tumors adhering to major vessels. The survival of patients with narrow margins and adjuvant radiotherapy was comparable to that of patients with wide margins and no adjuvant radiology, and adjuvant radiotherapy improved the survival of patients who had narrow margins. The results indicated that adjuvant radiotherapy overcame the negative impact of narrow margins to some extent. However, an analysis of patients in the NCDB database did not find a survival benefit of adjuvant radiotherapy, even in patients with positive resection margins or node-negative disease.86 Even though RCTs of adjuvant radiotherapy in ICC are lacking, the findings of current studies support the use of adjuvant radiotherapy in high-risk patients.
Antiviral therapy (AVT)
The role of viral hepatitis is not as prominent in ICC as it is in HCC, and is involved in only 6.1–7.0% of all cases.42,43 To the best of our knowledge, Lei et al.87 published the only study on adjuvant antiviral therapy in ICC patients. Of 1,064 consecutive patients with liver resection for ICC and concurrent HBV infection, 198 received antiviral therapy. Eighty-seven of the 198 patients began AVT before surgery and all continued it after surgery. The remaining 111 patients initiated AVT after liver resection. That is to say, all 198 received AVT as AT and some also received AVT as NAT. AVT regimens included lamivudine, adefovir, telbivudine, and entecavir, and interferon alpha. The patients were required to receive an AVT regimen for at least 3 months. AVT reduced postoperative viral reactivation to 3.3%; viral reactivation occurred in 8.3% of patients who did not receive AVT. Compared with patients who had high HBV-DNA levels and no AVT, those with AVT had significantly better long-term outcomes (5-year OS: 43.0% vs. 20.5%, p<0.001), but the difference was not significant in patients with low HBV-DNA levels. In that study, neoadjuvant and adjuvant AVT decreased viral reactivation and improved long-term outcomes in ICC patients with a high viral burden.88 AVT should thus be considered in such ICC patients.
AT in ICC and ECC
Owing to the low incidence of biliary cancer, ICC and ECC are often reported together and are not evaluated separately in many studies even though they have distinct anatomical, clinical, and molecular characteristics. The outcomes of AT in ICC and ECC in selected studies are shown and compared in Table 2.73,74,88–111 Both ICC and ECC patients are more likely to receive adjuvant chemotherapy than radiotherapy, as there are more studies on adjuvant chemotherapy than radiotherapy in ICC as well as ECC. In both ICC and ECC, most AT regimens include gemcitabine- or fluorouracil, and the results are mixed in both diseases. Overall, there are far more differences than similarities between ICC and ECC studies. First, more studies have evaluated AT in ECC than in ICC, regardless of the treatment modalities (i.e., adjuvant chemotherapy, radiotherapy, or chemoradiotherapy). There is even one RCT of adjuvant chemotherapy for ECC. Secondly, it seems to be easier for ECC patients to benefit from AT. In studies reporting positive results, it was often the case that the benefit emerged in the analysis of the whole ECC cohort, while the benefit was only apparent in subgroup analyses of high-risk ICC patients. Five studies included both ICC and ECC patients, with subgroup analysis of each cancer type.73,74,88–90 In the two of three retrospective studies, ICC patients benefitted from AT but ECC patients did not. This difference was not observed in the two RCTs and in another retrospective study. In the RCTs, neither ICC nor ECC benefitted from AT. In the third retrospective study, only ECC patients with distal cholangiocarcinoma benefitted from AT. Finally, owing to the anatomical location of ICC (buried inside the liver) and a proportion of patients having a background of HBV infection, ICC patients can be given TACE and antiviral therapy as AT, but that is obviously not the case for ECC patients.
Discussion
Although substantial progress has been made in understanding the epidemiology, risk factors, and molecular characteristics of ICC in the past few decades, the management of ICC remains extremely challenging.3–10,75 Radical resection remains the main treatment for ICC patients to achieve long-term survival, but only a minority of patients are diagnosed at an early stage eligible for surgery because of a lack of specific symptoms and method suitable for an early diagnosis.16,17 Relatively advanced disease at diagnosis means that NAT should be used to downstage patients and select appropriate candidates with tumor biology allowing hepatectomy. On the other hand, the aggressive behavior of ICC leads to a high recurrence rate even after radical resection, which calls for the use of AT.18–20 Unfortunately, owing to the relatively low incidence and then the paucity of conclusive evidence in neoadjuvant as well as adjuvant settings, the latest guidelines do not recommend routine use of neoadjuvant or adjuvant regimens in managing ICC.22,67
NAT, as a means to downstage relatively advanced patients to resection or a bridge therapy before LT, has a relatively high objective response and conversion rates. Patients who respond to NAT also experience satisfying long-term outcomes similar to or superior to those with upfront surgery. The main challenge of promoting these conclusions lies in the fact that the sample size is relatively small, and the criteria for selecting candidates for NAT and NAT regimens vary greatly among different centers. Well-designed prospective trials are needed to identify those who will benefit from NAT, as well as the efficacy of various regimens. In comparison, there is evidence that supports adjuvant strategies in ICC, including TACE,55–66 systematic chemotherapy,76–81 radiotherapy,83–86 and antiviral therapy.87 Notably, most studies support the use of AT in ICC patients with high-risk features like positive margins or positive lymph nodes, indicating that not all patients can benefit from AT.55–66,76–81 Instead, AT might harm selected ICC patients for some unknown reasons.59,64 Future prospective studies on the role of AT are more likely to have positive results if they are designed to include candidates at high risk of recurrence or death. For HBV-infected ICC patients, especially these with high viral levels, active antiviral therapy before and after surgery can improve outcomes and should be implemented.87 It is worth noting that all evidence in support of NAT and AT was obtained in retrospective studies and needs to be further confirmed by carefully designed prospective trials. Because the study of NAT and AT in ICC lags behind that in ECC, and that ICC has clinical and molecular characteristics distinct from ECC, prospective trials including only ICC patients are especially anticipated.
During our review of existing evidence for NAT and AT in ICC, we noticed some limitations of the available therapeutic modalities. The disadvantages of systemic chemotherapy include relative insensitivity to currently available chemotherapy regimens, toxic side effects, and the development of drug resistance.73,74 Compared with systemic chemotherapy, TACE, as a locoregional therapy, causes fewer general side effects than systemic chemotherapy agents, but also increases the chance of liver-related complications and an inability to control disease outside the liver, such as metastasis in the lungs and lymph nodes.56,61 Radiotherapy, usually with an enlarged irradiation volume that includes surrounding organs like the kidneys and pancreas, can control micrometastasis with direct spread from the primary tumors, but also can harm those fields.84,85 Radiotherapy, however, fails to manage distant metastasis. Antiviral therapy, given either before or after resection, improves outcomes in ICC patients with a background HBV infection. Recurrence after surgery depends on different mechanisms that include intrahepatic metastasis from the primary tumor and de novo carcinogenesis from the underlying inflammation or cirrhosis caused by HBV infection. Antiviral therapy can control neocarcinogenesis, but is less effective in eradicating intrahepatic metastasis.87
Given the limited effectiveness and drawbacks of existing therapeutic modalities, more effective strategies based on immunotherapy and targeted agents should be pursued. Immunotherapy, including inhibitors of immune checkpoints such as programmed death 1 (PD-1), programmed death-ligand 1 (PD-L1), and cytotoxic T-lymphocyte antigen-4, (CTLA-4), cancer vaccines, and adoptive cell transfer, have received ongoing attention in recent years. No immunotherapy has been approved for treating ICC, but the evidence has been increasing. Job et al.112 confirmed the existence of an inflamed ICC subtype characterized by massive T lymphocyte infiltration and activation of inflammatory and immune checkpoint pathways and classification of the tumor microenvironment that showed a high response rate to immune checkpoint inhibitors. High expression of PD-1/PD-L1 in ICC has been observed in several studies, and was negatively correlated with unfavorable prognosis, which indicated a promising role of immunotherapy in ICC.112–115 Although no conclusive results yet been reported, clinical trials of NAT and AT of ICC with immunotherapy alone or in combination with other therapeutic reagents are now underway (Table 3). Considering the limited treatment options and efficacy of existing therapeutic modalities, the outcomes of ongoing clinical trials are eagerly anticipated. Two drugs, pemigatinib and infigratinib, have been approved by the FDA for the targeted treatment of advanced or metastatic cholangiocarcinoma patients with FGFR2 fusions or rearrangements. Relatively high objective response rates indicate promising anticancer activity of the two drugs in cholangiocarcinoma,116,117 but no clinical trials of either FGFR inhibitor for NAT and AT of ICC have been registered. Given that FGFR2 fusions or rearrangements almost exclusively occur in ICC, the use of pemigatinib and infigratinib for NAT and AT warrants exploration.
Table 3. Selected clinical trials of neoadjuvant and adjuvant therapy in ICC.
| Trial identifier | Regimen/intervention | Estimated enrollment | Study type | Primary outcome | Country | Setting |
|---|---|---|---|---|---|---|
| NCT04506281 | Toripalimab (PD-1 antibody) + GEMOX + lenvatinib vs. observation | 128 | Phase 2, RCT | EFS | China | Neoadjuvant |
| NCT04523402 | GEMOX vs. observation | 100 | Phase 2, RCT | EFS | China | Neoadjuvant |
| NCT04546828 | Gemcitabine + cisplatin + nab-paclitaxel | 34 | Phase 2, single arm | Increased rate of R0 resection | Korea | Neoadjuvant |
| NCT04669496 | Toripalimab (PD-1 antibody) + GEMOX + lenvatinib vs. observation | 178 | Phase 2–3, RCT | EFS | China | Neoadjuvant |
| NCT04989218 | Gemcitabine + cisplatin + durvalumab (PD-L1 antibody) + tremelimumab (CTLA4 antibody) | 20 | Phase 1–2, single arm | ORR | USA | Neoadjuvant |
| NCT03579771 | Gemcitabine + cisplatin + nab-paclitaxel | 34 | Phase 2, single arm | Completion of all therapy rate, AE | USA | Neoadjuvant |
| NCT04295317 | SHR-1210 (PD-1 antibody) + capecitabine | 65 | Phase 2, single arm | RFS | China | Adjuvant |
| NCT03820310 | Traditional therapy plus autologous Tcm cellular immunotherapy vs. traditional therapy alone | 20 | Phase 2, RCT | PFS, OS | China | Adjuvant |
| NCT04782804 | Tislelizumab (PD-1 antibody) + capecitabine vs. capecitabine alone | 30 | Phase 1–2, non-randomized | RFS | China | Adjuvant |
| NCT04077983 | Nab-paclitaxel + gemcitabine | 40 | Phase 2, single arm | DFS | China | Adjuvant |
AE, adverse event; CTLA4, cytotoxic T-lymphocyte associated protein 4; DFS, disease-free survival; EFS, event-free survival; GEMOX, gemcitabine + oxaliplatin; ORR, objective response rate; OS, overall survival; PD-1, programmed cell death protein 1; PD-L1, programmed death-ligand 1; PFS, progression-free survival; RCT, randomized controlled trial; RFS, recurrence-free survival.
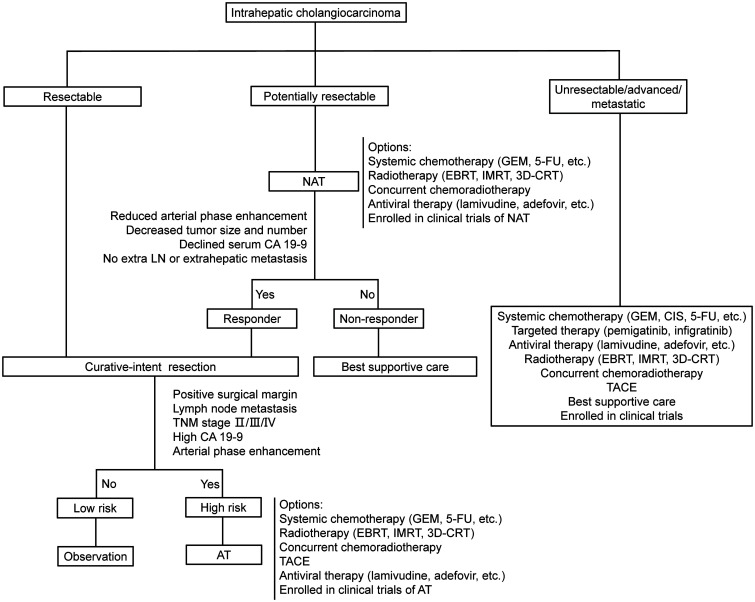
In conclusion, more effort should be addressed the improvement of multidisciplinary management of ICC, despite advances that have been made in recent years. The flow diagram in Figure 2 illustrates proposed ICC treatment based on the current evidence. Liver resection remains an important treatment with curative-intent, but additional neoadjuvant and adjuvant therapies might increase the number of surgical candidates, reduce recurrence rates after surgery, and improve the long-term outcomes. Progress has been achieved in the use of NAT and AT for ICC, future investigation is needed to identify the optimal therapeutic regimens, including chemotherapy, radiotherapy, TACE, immunotherapy, targeted therapy, and antiviral therapy, or an appropriate combination of those modalities.
Fig. 2. Proposed treatment flow for ICC.
3D-CRT, three-dimensional conformal radiotherapy; 5-FU, 5-Fluoropyrimidine; AT, adjuvant therapy; CIS, cisplatin; EBRT, external beam radiotherapy; GEM, gemcitabine; IMRT, intensity-modulated radiotherapy; NAT: neoadjuvant therapy; TACE, transcatheter arterial chemoembolization; TNM, tumor-node-metastasis.
Acknowledgments
We thank H. Nikki March, PhD, from Edanz (https://www.edanz.com/ac) for editing a draft of this manuscript.
Abbreviations
- 3D-CRT
three-dimensional conformal radiotherapy
- 5-FU
5-Fluoropyrimidine
- AE
adverse event
- AJCC
the American Joint Committee on Cancer
- AT
adjuvant therapy
- AVT
antiviral therapy
- CA19-9
carbohydrate antigen 19-9
- CAP
capecitabine
- CEA
carcino-embryonic antigen
- CIS
cisplatin
- CRT
chemoradiotherapy
- CSS
cancer-specific survival
- CT
chemotherapy
- CTLA4
cytotoxic T-lymphocyte associated protein 4
- DCC
distal cholangiocarcinoma
- DFS
disease-free survival
- DOX
doxorubicin
- EBRT
external beam radiotherapy
- ECC
extrahepatic cholangiocarcinoma
- EFS
event-free survival
- GEM
gemcitabine
- GEMOX
gemcitabine + oxaliplatin
- GGT
gamma-glutamyl transpeptidase
- HBV
hepatitis B virus
- HR
hazard ratio
- ICC
intrahepatic cholangiocarcinoma
- IMRT
intensity-modulated radiotherapy
- IPTW
inverse probability of treatment weighting
- LN
lymph nodes
- LR
liver resection
- LRFS
local recurrence-free survival
- LT
liver transplantation
- mOS
median OS
- MRI
magnetic resonance imaging
- NAFLD
nonalcoholic fatty liver disease
- NASH
nonalcoholic steatohepatitis
- NAT
neoadjuvant therapy
- NCCN
National Comprehensive Cancer Network
- NCDB
National Cancer Database
- ORR
objective response rate
- OS
overall survival
- OXA
oxaliplatin
- PCC
perihilar cholangiocarcinoma
- PD-1
programmed cell death protein 1
- PD-L1
programmed death-ligand 1
- PFS
progression-free survival
- PSM
propensity score matching
- RCTs
randomized controlled trials
- RFS
recurrence-free survival
- RT
radiotherapy
- SEER
Surveillance, Epidemiology, and End Results
- TACE
transcatheter arterial chemoembolization
- TNM
tumor-node-metastasis
- VMAT
volumetric modulated arc therapy
Data sharing statement
The clinical data used to support the findings of this study are included within the cited articles.
References
- 1.Kudo M, Izumi N, Kokudo N, Sakamoto M, Shiina S, Takayama T, et al. Report of the 22nd nationwide follow-up survey of primary liver cancer in Japan (2012-2013) Hepatol Res. 2021 doi: 10.1111/hepr.13675. [DOI] [PubMed] [Google Scholar]
- 2.Petrick JL, Campbell PT, Koshiol J, Thistle JE, Andreotti G, Beane-Freeman LE, et al. Tobacco, alcohol use and risk of hepatocellular carcinoma and intrahepatic cholangiocarcinoma: The Liver Cancer Pooling Project. Brit J Cancer. 2018;118(7):005–1012. doi: 10.1038/s41416-018-0007-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Palmer WC, Patel T. Are common factors involved in the pathogenesis of primary liver cancers? A meta-analysis of risk factors for intrahepatic cholangiocarcinoma. J Hepatol. 2012;57(1):69–76. doi: 10.1016/j.jhep.2012.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clements O, Eliahoo J, Kim JU, Taylor-Robinson SD, Khan SA. Risk factors for intrahepatic and extrahepatic cholangiocarcinoma: A systematic review and meta-analysis. J Hepatol. 2020;72(1):95–103. doi: 10.1016/j.jhep.2019.09.007. [DOI] [PubMed] [Google Scholar]
- 5.Corrao S, Natoli G, Argano C. Nonalcoholic fatty liver disease is associated with intrahepatic cholangiocarcinoma and not with extrahepatic form: definitive evidence from meta-analysis and trial sequential analysis. Eur J Gastroenterol Hepatol. 2021;33(1):62–68. doi: 10.1097/MEG.0000000000001684. [DOI] [PubMed] [Google Scholar]
- 6.De Lorenzo S, Tovoli F, Mazzotta A, Vasuri F, Edeline J, Malvi D, et al. Non-alcoholic steatohepatitis as a risk factor for intrahepatic cholangiocarcinoma and its prognostic role. Cancers (Basel) 2020;12(11):3182. doi: 10.3390/cancers12113182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Petrick JL, Thistle JE, Zeleniuch-Jacquotte A, Zhang X, Wactawski-Wende J, Van Dyke AL, et al. Body mass index, diabetes and intrahepatic cholangiocarcinoma risk: the liver cancer pooling project and meta-analysis. Am J Gastroenterol. 2018;113(10):1494–1505. doi: 10.1038/s41395-018-0207-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Makiuchi T, Sobue T, Kitamura T, Sawada N, Iwasaki M, Yamaji T, et al. Smoking, alcohol consumption, and risks for biliary tract cancer and intrahepatic bile duct cancer. J Epidemiol. 2019;29(5):180–186. doi: 10.2188/jea.JE20180011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shaib YH, Davila JA, McGlynn K, El-Serag HB. Rising incidence of intrahepatic cholangiocarcinoma in the United States: a true increase? J Hepatol. 2004;40(3):472–477. doi: 10.1016/j.jhep.2003.11.030. [DOI] [PubMed] [Google Scholar]
- 10.Witjes CDM, Karim Kos HE, Visser O, de Vries E, IJzermans JNM, de Man RA, et al. Intrahepatic cholangiocarcinoma in a low endemic area: rising incidence and improved survival. Hpb. 2012;14(11):777–781. doi: 10.1111/j.1477-2574.2012.00536.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu L, Tsilimigras DI, Paredes AZ, Mehta R, Hyer JM, Merath K, et al. Trends in the incidence, treatment and outcomes of patients with intrahepatic cholangiocarcinoma in the usa: facility type is associated with margin status, use of lymphadenectomy and overall survival. World J Surg. 2019;43(7):1777–1787. doi: 10.1007/s00268-019-04966-4. [DOI] [PubMed] [Google Scholar]
- 12.Piscaglia F, Iavarone M, Galassi M, Vavassori S, Renzulli M, Forzenigo LV, et al. Cholangiocarcinoma in cirrhosis: value of hepatocyte specific magnetic resonance imaging. Dig Dis. 2015;33(6):735–744. doi: 10.1159/000439097. [DOI] [PubMed] [Google Scholar]
- 13.Younossi Z, Anstee QM, Marietti M, Hardy T, Henry L, Eslam M, et al. Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol. 2018;15(1):11–20. doi: 10.1038/nrgastro.2017.109. [DOI] [PubMed] [Google Scholar]
- 14.Wongjarupong N, Assavapongpaiboon B, Susantitaphong P, Cheungpasitporn W, Treeprasertsuk S, Rerknimitr R, et al. Non-alcoholic fatty liver disease as a risk factor for cholangiocarcinoma: a systematic review and meta-analysis. BMC Gastroenterol. 2017;17(1):149. doi: 10.1186/s12876-017-0696-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Meng Z, Pan W, Hong H, Chen J, Chen Y. Modified staging classification for intrahepatic cholangiocarcinoma based on the sixth and seventh editions of the AJCC/UICC TNM staging systems. Medicine. 2017;96(34):e7891. doi: 10.1097/MD.0000000000007891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sakamoto Y, Kokudo N, Matsuyama Y, Sakamoto M, Izumi N, Kadoya M, et al. Proposal of a new staging system for intrahepatic cholangiocarcinoma: analysis of surgical patients from a nationwide survey of the Liver Cancer Study Group of Japan. Cancer. 2016;122(1):61–70. doi: 10.1002/cncr.29686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dover LL, Jacob R, Wang TN, Richardson JH, Redden DT, Li P, et al. Improved postoperative survival for intraductal-growth subtype of intrahepatic cholangiocarcinoma. Am Surg. 2016;82(11):1133–1139. [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang XF, Beal EW, Bagante F, Chakedis J, Weiss M, Popescu I, et al. Early versus late recurrence of intrahepatic cholangiocarcinoma after resection with curative intent. Brit J Surg. 2018;105(7):848–856. doi: 10.1002/bjs.10676. [DOI] [PubMed] [Google Scholar]
- 19.Merath K, Mehta R, Hyer JM, Bagante F, Sahara K, Alexandrescu S, et al. Impact of body mass index on tumor recurrence among patients undergoing curative-intent resection of intrahepatic cholangiocarcinoma- a multi-institutional international analysis. Eur J Surg Oncol. 2019;45(6):1084–1091. doi: 10.1016/j.ejso.2019.03.004. [DOI] [PubMed] [Google Scholar]
- 20.Hu L, Zhang X, Weiss M, Popescu I, Marques HP, Aldrighetti L, et al. Recurrence patterns and timing courses following curative-intent resection for intrahepatic cholangiocarcinoma. Ann Surg Oncol. 2019;26(8):2549–2557. doi: 10.1245/s10434-019-07353-4. [DOI] [PubMed] [Google Scholar]
- 21.Burotto M, Wilkerson J, Stein WD, Bates SE, Fojo T. Adjuvant and neoadjuvant cancer therapies: a historical review and a rational approach to understand outcomes. Semin Oncol. 2019;46(1):83–99. doi: 10.1053/j.seminoncol.2019.01.002. [DOI] [PubMed] [Google Scholar]
- 22. National Comprehensive Cancer Network. Hepatobiliary Cancers (Version: 4.2021). Available from: https://www.nccn.org/professionals/physician_gls/pdf/hepatobiliary.pdf.
- 23.Merath K, Chen Q, Bagante F, Alexandrescu S, Marques HP, Aldrighetti L, et al. A multi-institutional international analysis of textbook outcomes among patients undergoing curative-intent resection of intrahepatic cholangiocarcinoma. JAMA Surg. 2019;154(6):e190571. doi: 10.1001/jamasurg.2019.0571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bagante F, Spolverato G, Merath K, Weiss M, Alexandrescu S, Marques HP, et al. Intrahepatic cholangiocarcinoma tumor burden: a classification and regression tree model to define prognostic groups after resection. Surgery. 2019;166(6):983–990. doi: 10.1016/j.surg.2019.06.005. [DOI] [PubMed] [Google Scholar]
- 25.Gil E, Joh J, Park HC, Yu JI, Jung SH, Kim JM. Predictors and patterns of recurrence after curative liver resection in intrahepatic cholangiocarcinoma, for application of postoperative radiotherapy: a retrospective study. World J Surg Oncol. 2015;13(1):227. doi: 10.1186/s12957-015-0637-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moro A, Paredes AZ, Farooq A, Sahara K, Tsilimigras DI, Mehta R, et al. Discordance in prediction of prognosis among patients with intrahepatic cholangiocarcinoma: A preoperative vs postoperative perspective. J Surg Oncol. 2019;120(6):946–955. doi: 10.1002/jso.25671. [DOI] [PubMed] [Google Scholar]
- 27.Sahara K, Tsilimigras DI, Mehta R, Bagante F, Guglielmi A, Aldrighetti L, et al. A novel online prognostic tool to predict long-term survival after liver resection for intrahepatic cholangiocarcinoma: the “metro-ticket” paradigm. J Surg Oncol. 2019;120(2):223–230. doi: 10.1002/jso.25480. [DOI] [PubMed] [Google Scholar]
- 28.Yoh T, Seo S, Morino K, Fuji H, Ikeno Y, Ishii T, et al. Reappraisal of prognostic impact of tumor SUVmax by 18F-FDG-PET/CT in intrahepatic cholangiocarcinoma. World J Surg. 2019;43(5):1323–1331. doi: 10.1007/s00268-019-04917-z. [DOI] [PubMed] [Google Scholar]
- 29.Hobeika C, Cauchy F, Poté N, Rautou P, Durand F, Farges O, et al. Short- and long-term outcomes of liver resection for intrahepatic cholangiocarcinoma associated with the metabolic syndrome. World J Surg. 2019;43(8):2048–2060. doi: 10.1007/s00268-019-04996-y. [DOI] [PubMed] [Google Scholar]
- 30.Buettner S, Ten Cate DWG, Bagante F, Alexandrescu S, Marques HP, Lamelas J, et al. Survival after resection of multiple tumor foci of intrahepatic cholangiocarcinoma. J Gastrointest Surg. 2019;23(11):2239–2246. doi: 10.1007/s11605-019-04184-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Addeo P, Jedidi I, Locicero A, Faitot F, Oncioiu C, Onea A, et al. Prognostic impact of tumor multinodularity in intrahepatic cholangiocarcinoma. J Gastrointest Surg. 2019;23(9):1801–1809. doi: 10.1007/s11605-018-4052-y. [DOI] [PubMed] [Google Scholar]
- 32.Kim SH, Han DH, Choi GH, Choi JS, Kim KS. Oncologic impact of lymph node dissection for intrahepatic cholangiocarcinoma: a propensity score-matched study. J Gastrointest Surg. 2019;23(3):538–544. doi: 10.1007/s11605-018-3899-2. [DOI] [PubMed] [Google Scholar]
- 33.Conci S, Ruzzenente A, Viganò L, Ercolani G, Fontana A, Bagante F, et al. Patterns of distribution of hepatic nodules (single, satellites or multifocal) in intrahepatic cholangiocarcinoma: prognostic impact after surgery. Ann Surg Oncol. 2018;25(12):3719–3727. doi: 10.1245/s10434-018-6669-1. [DOI] [PubMed] [Google Scholar]
- 34.Akgul O, Bagante F, Olsen G, Cloyd JM, Weiss M, Merath K, et al. Preoperative prognostic nutritional index predicts survival of patients with intrahepatic cholangiocarcinoma after curative resection. J Surg Oncol. 2018;118(3):422–430. doi: 10.1002/jso.25140. [DOI] [PubMed] [Google Scholar]
- 35.Bagante F, Weiss M, Alexandrescu S, Marques HP, Aldrighetti L, Maithel SK, et al. Long-term outcomes of patients with intraductal growth sub-type of intrahepatic cholangiocarcinoma. Hpb. 2018;20(12):1189–1197. doi: 10.1016/j.hpb.2018.05.017. [DOI] [PubMed] [Google Scholar]
- 36.Bagante F, Spolverato G, Weiss M, Alexandrescu S, Marques HP, Aldrighetti L, et al. Surgical management of intrahepatic cholangiocarcinoma in patients with cirrhosis: impact of lymphadenectomy on peri-operative outcomes. World J Surg. 2018;42(8):2551–2560. doi: 10.1007/s00268-017-4453-1. [DOI] [PubMed] [Google Scholar]
- 37.Miyata T, Yamashita Y, Higashi T, Taki K, Izumi D, Kosumi K, et al. The prognostic impact of controlling nutritional status (conut) in intrahepatic cholangiocarcinoma following curative hepatectomy: a retrospective single institution study. World J Surg. 2018;42(4):1085–1091. doi: 10.1007/s00268-017-4214-1. [DOI] [PubMed] [Google Scholar]
- 38.Bagante F, Spolverato G, Weiss M, Alexandrescu S, Marques HP, Aldrighetti L, et al. Assessment of the lymph node status in patients undergoing liver resection for intrahepatic cholangiocarcinoma: the new Eighth Edition AJCC Staging System. J Gastrointest Surg. 2018;22(1):52–59. doi: 10.1007/s11605-017-3426-x. [DOI] [PubMed] [Google Scholar]
- 39.Spolverato G, Bagante F, Weiss M, Alexandrescu S, Marques HP, Aldrighetti L, et al. Comparative performances of the 7th and the 8th editions of the American Joint Committee on Cancer staging systems for intrahepatic cholangiocarcinoma. J Surg Oncol. 2017;115(6):696–703. doi: 10.1002/jso.24569. [DOI] [PubMed] [Google Scholar]
- 40.Buettner S, Koerkamp BG, Ejaz A, Buisman FE, Kim Y, Margonis GA, et al. The effect of preoperative chemotherapy treatment in surgically treated intrahepatic cholangiocarcinoma patients - a multi-institutional analysis. J Surg Oncol. 2017;115(3):312–318. doi: 10.1002/jso.24524. [DOI] [PubMed] [Google Scholar]
- 41.Bagante F, Spolverato G, Weiss M, Alexandrescu S, Marques HP, Aldrighetti L, et al. Defining long-term survivors following resection of intrahepatic cholangiocarcinoma. J Gastrointest Surg. 2017;21(11):1888–1897. doi: 10.1007/s11605-017-3550-7. [DOI] [PubMed] [Google Scholar]
- 42.Doussot A, Lim C, Gomez-Gavara C, Fuks D, Farges O, Regimbeau JM, et al. Multicentre study of the impact of morbidity on long-term survival following hepatectomy for intrahepatic cholangiocarcinoma. Br J Surg. 2016;103(13):1887–1894. doi: 10.1002/bjs.10296. [DOI] [PubMed] [Google Scholar]
- 43.Doussot A, Gonen M, Wiggers JK, Groot-Koerkamp B, DeMatteo RP, Fuks D, et al. Recurrence patterns and disease-free survival after resection of intrahepatic cholangiocarcinoma: preoperative and postoperative prognostic models. J Am Coll Surgeons. 2016;223(3):493–505. doi: 10.1016/j.jamcollsurg.2016.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ratti F, Cipriani F, Ariotti R, Gagliano A, Paganelli M, Catena M, et al. Safety and feasibility of laparoscopic liver resection with associated lymphadenectomy for intrahepatic cholangiocarcinoma: a propensity score-based case-matched analysis from a single institution. Surg Endosc. 2016;30(5):1999–2010. doi: 10.1007/s00464-015-4430-4. [DOI] [PubMed] [Google Scholar]
- 45.Miura JT, Johnston FM, Tsai S, George B, Thomas J, Eastwood D, et al. Chemotherapy for surgically resected intrahepatic cholangiocarcinoma. Ann Surg Oncol. 2015;22(11):3716–3723. doi: 10.1245/s10434-015-4501-8. [DOI] [PubMed] [Google Scholar]
- 46.Kato H, Tabata M, Kobayashi M, Ohsawa I, Kishiwada M, Mizuno S, et al. Aggressive surgical resection after neoadjuvant chemoradiation therapy for locally advanced intrahepatic cholangiocarcinoma. Clin J Gastroenterol. 2009;2(5):351–354. doi: 10.1007/s12328-009-0102-z. [DOI] [PubMed] [Google Scholar]
- 47.Tran TB, Bal CK, Schaberg K, Longacre TA, Chatrath BS, Poultsides GA. Locally advanced intrahepatic cholangiocarcinoma: complete pathologic response to neoadjuvant chemotherapy followed by left hepatic trisectionectomy and caudate lobectomy. Dig Dis Sci. 2015;60(11):3226–3229. doi: 10.1007/s10620-015-3640-x. [DOI] [PubMed] [Google Scholar]
- 48.Rayar M, Sulpice L, Edeline J, Garin E, Levi Sandri GB, Meunier B, et al. Intra-arterial Yttrium-90 radioembolization combined with systemic chemotherapy is a promising method for downstaging unresectable huge intrahepatic cholangiocarcinoma to surgical treatment. Ann Surg Oncol. 2015;22(9):3102–3108. doi: 10.1245/s10434-014-4365-3. [DOI] [PubMed] [Google Scholar]
- 49.Sumiyoshi T, Shima Y, Okabayashi T, Negoro Y, Shimada Y, Iwata J, et al. Chemoradiotherapy for initially unresectable locally advanced cholangiocarcinoma. World J Surg. 2018;42(9):2910–2918. doi: 10.1007/s00268-018-4558-1. [DOI] [PubMed] [Google Scholar]
- 50.Bagante F, Spolverato G, Weiss M, Alexandrescu S, Marques HP, Aldrighetti L, et al. Impact of morphological status on long-term outcome among patients undergoing liver surgery for intrahepatic cholangiocarcinoma. Ann Surg Oncol. 2017;24(9):2491–2501. doi: 10.1245/s10434-017-5870-y. [DOI] [PubMed] [Google Scholar]
- 51.Le Roy B, Gelli M, Pittau G, Allard MA, Pereira B, Serji B, et al. Neoadjuvant chemotherapy for initially unresectable intrahepatic cholangiocarcinoma. Brit J Surg. 2018;105(7):839–847. doi: 10.1002/bjs.10641. [DOI] [PubMed] [Google Scholar]
- 52.Mason MC, Massarweh NN, Tzeng CD, Chiang YJ, Chun YS, Aloia TA, et al. Time to rethink upfront surgery for resectable intrahepatic cholangiocarcinoma? Implications from the neoadjuvant experience. Ann Surg Oncol. 2021;28(11):6725–6735. doi: 10.1245/s10434-020-09536-w. [DOI] [PubMed] [Google Scholar]
- 53.Utuama O, Permuth JB, Dagne G, Sanchez-Anguiano A, Alman A, Kumar A, et al. Neoadjuvant chemotherapy for intrahepatic cholangiocarcinoma: a propensity score survival analysis supporting use in patients with high-risk disease. Ann Surg Oncol. 2021;28(4):1939–1949. doi: 10.1245/s10434-020-09478-3. [DOI] [PubMed] [Google Scholar]
- 54.Sutton TL, Billingsley KG, Walker BS, Enestvedt CK, Dewey EN, Orloff SL, et al. Neoadjuvant chemotherapy is associated with improved survival in patients undergoing hepatic resection for intrahepatic cholangiocarcinoma. Am J Surg. 2021;221(6):1182–1187. doi: 10.1016/j.amjsurg.2021.02.029. [DOI] [PubMed] [Google Scholar]
- 55.Lunsford KE, Javle M, Heyne K, Shroff RT, Abdel-Wahab R, Gupta N, et al. Liver transplantation for locally advanced intrahepatic cholangiocarcinoma treated with neoadjuvant therapy: a prospective case-series. Lancet Gastroenterol Hepatol. 2018;3(5):337–348. doi: 10.1016/S2468-1253(18)30045-1. [DOI] [PubMed] [Google Scholar]
- 56.Shen WF, Zhong W, Liu Q, Sui CJ, Huang YQ, Yang JM. Adjuvant transcatheter arterial chemoembolization for intrahepatic cholangiocarcinoma after curative surgery: retrospective control study. World J Surg. 2011;35(9):2083–2091. doi: 10.1007/s00268-011-1171-y. [DOI] [PubMed] [Google Scholar]
- 57.Wu ZF, Zhang HB, Yang N, Zhao WC, Fu Y, Yang GS. Postoperative adjuvant transcatheter arterial chemoembolisation improves survival of intrahepatic cholangiocarcinoma patients with poor prognostic factors: Results of a large monocentric series. Eur J Surg Oncol. 2012;38(7):602–610. doi: 10.1016/j.ejso.2012.02.185. [DOI] [PubMed] [Google Scholar]
- 58.Li T, Qin LX, Zhou J, Sun HC, Qiu SJ, Ye QH, et al. Staging, prognostic factors and adjuvant therapy of intrahepatic cholangiocarcinoma after curative resection. Liv Int. 2014;34(6):953–960. doi: 10.1111/liv.12364. [DOI] [PubMed] [Google Scholar]
- 59.Li J, Wang Q, Lei Z, Wu D, Si A, Wang K, et al. Adjuvant transarterial chemoembolization following liver resection for intrahepatic cholangiocarcinoma based on survival risk stratification. Oncologist. 2015;20(6):640–647. doi: 10.1634/theoncologist.2014-0470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jeong S, Zheng B, Wang J, Chi J, Tong Y, Xia L, et al. Transarterial chemoembolization: a favorable postoperative management to improve prognosis of hepatitis b virus-associated intrahepatic cholangiocarcinoma after surgical resection. Int J Biol Sci. 2017;13(10):1234–1241. doi: 10.7150/ijbs.21149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lu Z, Liu S, Yi Y, Ni X, Wang J, Huang J, et al. Serum gamma-glutamyl transferase levels affect the prognosis of patients with intrahepatic cholangiocarcinoma who receive postoperative adjuvant transcatheter arterial chemoembolization: a propensity score matching study. Int J Surg. 2017;37:24–28. doi: 10.1016/j.ijsu.2016.10.015. [DOI] [PubMed] [Google Scholar]
- 62.Wang L, Lin ZG, Ke Q, Lou JY, Zheng SG, Bi XY, et al. Adjuvant transarterial chemoembolization following radical resection for intrahepatic cholangiocarcinoma: A multi-center retrospective study. J Cancer. 2020;11(14):4115–4122. doi: 10.7150/jca.40358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cheng Z, Lei Z, Jin X, Zhang Q, Si A, Yang P, et al. Postoperative adjuvant transarterial chemoembolization for intrahepatic cholangiocarcinoma patients with microvascular invasion: a propensity score analysis. J Gastrointest Oncol. 2021;12(2):819–830. doi: 10.21037/jgo-20-443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Liu G, Guo W, Wang H, Liu W, Lei L, Xie Q, et al. Influence of postoperative adjuvant transarterial chemoembolization on the prognosis of early-stage intrahepatic cholangiocarcinoma: a single center study. Ann Palliat Med. 2021;10(4):3673–3683. doi: 10.21037/apm-20-1337. [DOI] [PubMed] [Google Scholar]
- 65.Ma KW, Cheung TT, Leung B, She B, Chok K, Chan A, et al. Adjuvant chemotherapy improves oncological outcomes of resectable intrahepatic cholangiocarcinoma: a meta-analysis. Medicine (Baltimore) 2019;98(5):e14013. doi: 10.1097/MD.0000000000014013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Liu JB, Chu KJ, Ling CC, Wu TM, Wang HM, Shi Y, et al. Prognosis for intrahepatic cholangiocarcinoma patients treated with postoperative adjuvant transcatheter hepatic artery chemoembolization. Curr Probl Cancer. 2020;44(6):100612. doi: 10.1016/j.currproblcancer.2020.100612. [DOI] [PubMed] [Google Scholar]
- 67.Bridgewater J, Galle PR, Khan SA, Llovet JM, Park JW, Patel T, et al. Guidelines for the diagnosis and management of intrahepatic cholangiocarcinoma. J Hepatol. 2014;60(6):1268–1289. doi: 10.1016/j.jhep.2014.01.021. [DOI] [PubMed] [Google Scholar]
- 68.Doussot A, Groot-Koerkamp B, Wiggers JK, Chou J, Gonen M, DeMatteo RP, et al. Outcomes after resection of intrahepatic cholangiocarcinoma: external validation and comparison of prognostic models. J Am Coll Surg. 2015;221(2):452–461. doi: 10.1016/j.jamcollsurg.2015.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kim DH, Choi DW, Choi SH, Heo JS, Kow AW. Is there a role for systematic hepatic pedicle lymphadenectomy in intrahepatic cholangiocarcinoma? A review of 17 years of experience in a tertiary institution. Surgery. 2015;157(4):666–675. doi: 10.1016/j.surg.2014.11.006. [DOI] [PubMed] [Google Scholar]
- 70.Luo X, Yuan L, Wang Y, Ge R, Sun Y, Wei G. Survival outcomes and prognostic factors of surgical therapy for all potentially resectable intrahepatic cholangiocarcinoma: a large single-center cohort study. J Gastrointest Surg. 2014;18(3):562–572. doi: 10.1007/s11605-013-2447-3. [DOI] [PubMed] [Google Scholar]
- 71.Murakami Y, Uemura K, Sudo T, Hashimoto Y, Nakashima A, Sueda T. Intrahepatic cholangiocarcinoma: clinicopathological differences between peripheral type and hilar type. J Gastrointest Surg. 2012;16(3):540–548. doi: 10.1007/s11605-011-1730-4. [DOI] [PubMed] [Google Scholar]
- 72.Cho SY, Park S, Kim SH, Han S, Kim Y, Lee K, et al. Survival analysis of intrahepatic cholangiocarcinoma after resection. Ann Surg Oncol. 2010;17(7):1823–1830. doi: 10.1245/s10434-010-0938-y. [DOI] [PubMed] [Google Scholar]
- 73.Edeline J, Benabdelghani M, Bertaut A, Watelet J, Hammel P, Joly JP, et al. Gemcitabine and oxaliplatin chemotherapy or surveillance in resected biliary tract cancer (PRODIGE 12-ACCORD 18-UNICANCER GI): A Randomized Phase III Study. J Clin Oncol. 2019;37(8):658–667. doi: 10.1200/JCO.18.00050. [DOI] [PubMed] [Google Scholar]
- 74.Primrose JN, Fox RP, Palmer DH, Malik HZ, Prasad R, Mirza D, et al. Capecitabine compared with observation in resected biliary tract cancer (BILCAP): a randomised, controlled, multicentre, phase 3 study. Lancet Oncol. 2019;20(5):663–673. doi: 10.1016/S1470-2045(18)30915-X. [DOI] [PubMed] [Google Scholar]
- 75.Banales JM, Marin J, Lamarca A, Rodrigues PM, Khan SA, Roberts LR, et al. Cholangiocarcinoma 2020: the next horizon in mechanisms and management. Nat Rev Gastroenterol Hepatol. 2020;17(9):557–588. doi: 10.1038/s41575-020-0310-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sur MD, In H, Sharpe SM, Baker MS, Weichselbaum RR, Talamonti MS, et al. Defining the benefit of adjuvant therapy following resection for intrahepatic cholangiocarcinoma. Ann Surg Oncol. 2015;22(7):2209–2217. doi: 10.1245/s10434-014-4275-4. [DOI] [PubMed] [Google Scholar]
- 77.Lee GC, Ferrone CR, Tanabe KK, Lillemoe KD, Blaszkowsky LS, Zhu AX, et al. Predictors of adjuvant treatment and survival in patients with intrahepatic cholangiocarcinoma who undergo resection. Am J Surg. 2019;218(5):959–966. doi: 10.1016/j.amjsurg.2019.02.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Altman AM, Kizy S, Marmor S, Hui J, Tuttle TM, Jensen EH, et al. Adjuvant chemotherapy for intrahepatic cholangiocarcinoma: approaching clinical practice consensus? Hepatobiliary Surg Nutr. 2020;9(5):577–586. doi: 10.21037/hbsn.2019.06.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lin Y, Hsieh M, Wang W, Lin Y, Chang W, Chang C, et al. Outcomes of adjuvant treatments for resectable intrahepatic cholangiocarcinoma: Chemotherapy alone, sequential chemoradiotherapy, or concurrent chemoradiotherapy. Radiother Oncol. 2018;128(3):575–583. doi: 10.1016/j.radonc.2018.05.011. [DOI] [PubMed] [Google Scholar]
- 80.Reames BN, Bagante F, Ejaz A, Spolverato G, Ruzzenente A, Weiss M, et al. Impact of adjuvant chemotherapy on survival in patients with intrahepatic cholangiocarcinoma: a multi-institutional analysis. Hpb. 2017;19(10):901–909. doi: 10.1016/j.hpb.2017.06.008. [DOI] [PubMed] [Google Scholar]
- 81.Schweitzer N, Weber T, Kirstein MM, Fischer M, Kratzel A, Reineke-Plaaß T, et al. The effect of adjuvant chemotherapy in patients with intrahepatic cholangiocarcinoma: a matched pair analysis. J Cancer Res Clin. 2017;143(7):1347–1355. doi: 10.1007/s00432-017-2392-8. [DOI] [PubMed] [Google Scholar]
- 82.Tsilimigras DI, Hyer JM, Moris D, Sahara K, Bagante F, Guglielmi A, et al. Prognostic utility of albumin-bilirubin grade for short- and long-term outcomes following hepatic resection for intrahepatic cholangiocarcinoma: A multi-institutional analysis of 706 patients. J Surg Oncol. 2019;120(2):206–213. doi: 10.1002/jso.25486. [DOI] [PubMed] [Google Scholar]
- 83.Shinohara ET, Mitra N, Guo M, Metz JM. Radiation therapy is associated with improved survival in the adjuvant and definitive treatment of intrahepatic cholangiocarcinoma. Int J Radiat Oncol Biol Phys. 2008;72(5):1495–1501. doi: 10.1016/j.ijrobp.2008.03.018. [DOI] [PubMed] [Google Scholar]
- 84.Jiang W, Zeng ZC, Tang ZY, Fan J, Zhou J, Zeng MS, et al. Benefit of radiotherapy for 90 patients with resected intrahepatic cholangiocarcinoma and concurrent lymph node metastases. J Cancer Res Clin Oncol. 2010;136(9):1323–1331. doi: 10.1007/s00432-010-0783-1. [DOI] [PubMed] [Google Scholar]
- 85.Zheng X, Chen B, Wu J, Jia AY, Rong W, Wang L, et al. Benefit of adjuvant radiotherapy following narrow-margin hepatectomy in patients with intrahepatic cholangiocarcinoma that adhere to major vessels. Cancer Manag Res. 2018;10:3973–3981. doi: 10.2147/CMAR.S172940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hammad AY, Berger NG, Eastwood D, Tsai S, Turaga KK, Christian KK, et al. Is radiotherapy warranted following intrahepatic cholangiocarcinoma resection? The impact of surgical margins and lymph node status on survival. Ann Surg Oncol. 2016;23(S5):912–920. doi: 10.1245/s10434-016-5560-1. [DOI] [PubMed] [Google Scholar]
- 87.Lei Z, Xia Y, Si A, Wang K, Li J, Yan Z, et al. Antiviral therapy improves survival in patients with HBV infection and intrahepatic cholangiocarcinoma undergoing liver resection. J Hepatol. 2018;68(4):655–662. doi: 10.1016/j.jhep.2017.11.015. [DOI] [PubMed] [Google Scholar]
- 88.Yamanaka K, Hatano E, Kanai M, Tanaka S, Yamamoto K, Narita M, et al. A single-center analysis of the survival benefits of adjuvant gemcitabine chemotherapy for biliary tract cancer. Int J Clin Oncol. 2014;19(3):485–489. doi: 10.1007/s10147-013-0578-x. [DOI] [PubMed] [Google Scholar]
- 89.Wirasorn K, Ngamprasertchai T, Khuntikeo N, Pakkhem A, Ungarereevittaya P, Chindaprasirt J, et al. Adjuvant chemotherapy in resectable cholangiocarcinoma patients. J Gastroenterol Hepatol. 2013;28(12):1885–1891. doi: 10.1111/jgh.12321. [DOI] [PubMed] [Google Scholar]
- 90.Kim YS, Jeong CY, Song HN, Kim TH, Kim HJ, Lee YJ, et al. The efficacy of fluoropyrimidine-based adjuvant chemotherapy on biliary tract cancer after R0 resection. Chin J Cancer. 2017;36(1):9. doi: 10.1186/s40880-017-0182-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Vern-Gross TZ, Shivnani AT, Chen K, Lee CM, Tward JD, MacDonald OK, et al. Survival outcomes in resected extrahepatic cholangiocarcinoma: effect of adjuvant radiotherapy in a surveillance, epidemiology, and end results analysis. Int J Radiat Oncol Biol Phys. 2011;81(1):189–198. doi: 10.1016/j.ijrobp.2010.05.001. [DOI] [PubMed] [Google Scholar]
- 92.Kim YS, Hwang IG, Park SE, Go SI, Kang JH, Park I, et al. Role of adjuvant therapy after R0 resection for patients with distal cholangiocarcinoma. Cancer Chemother Pharmacol. 2016;77(5):979–985. doi: 10.1007/s00280-016-3014-x. [DOI] [PubMed] [Google Scholar]
- 93.Leng KM, Liu YP, Wang ZD, Zhong XY, Liao GQ, Kang PC, et al. Results of adjuvant radiation therapy for locoregional perihilar cholangiocarcinoma after curative intent resection. Onco Targets Ther. 2017;10:2257–2266. doi: 10.2147/OTT.S131873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Im JH, Choi GH, Lee WJ, Han DH, Park SW, Bang S, et al. Adjuvant radiotherapy and chemotherapy offer a recurrence and survival benefit in patients with resected perihilar cholangiocarcinoma. J Cancer Res Clin Oncol. 2021;147(8):2435–2445. doi: 10.1007/s00432-021-03524-7. [DOI] [PubMed] [Google Scholar]
- 95.Im JH, Seong J, Lee IJ, Park JS, Yoon DS, Kim KS, et al. Surgery alone versus surgery followed by chemotherapy and radiotherapy in resected extrahepatic bile duct cancer: treatment outcome analysis of 336 patients. Cancer Res Treat. 2016;48(2):583–595. doi: 10.4143/crt.2015.091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kim YJ, Kim K, Min SK, Nam EM. Role of adjuvant radiotherapy for localized extrahepatic bile duct cancer. Br J Radiol. 2017;90(1071):20160807. doi: 10.1259/bjr.20160807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Murakami Y, Uemura K, Sudo T, Hayashidani Y, Hashimoto Y, Nakamura H, et al. Gemcitabine-based adjuvant chemotherapy improves survival after aggressive surgery for hilar cholangiocarcinoma. J Gastrointest Surg. 2009;13(8):1470–1479. doi: 10.1007/s11605-009-0900-0. [DOI] [PubMed] [Google Scholar]
- 98.Hoehn RS, Wima K, Ertel AE, Meier A, Ahmad SA, Shah SA, et al. Adjuvant chemotherapy and radiation therapy is associated with improved survival for patients with extrahepatic cholangiocarcinoma. Ann Surg Oncol. 2015;22(Suppl 3):S1133–S1139. doi: 10.1245/s10434-015-4599-8. [DOI] [PubMed] [Google Scholar]
- 99.Mizuno T, Ebata T, Yokoyama Y, Igami T, Sugawara G, Yamaguchi J, et al. Adjuvant gemcitabine monotherapy for resectable perihilar cholangiocarcinoma with lymph node involvement: a propensity score matching analysis. Surg Today. 2017;47(2):182–192. doi: 10.1007/s00595-016-1354-0. [DOI] [PubMed] [Google Scholar]
- 100.Yin L, Xu Q, Li J, Wei Q, Ying J. The efficiency and regimen choice of adjuvant chemotherapy in biliary tract cancer: A STROBE-compliant retrospective cohort study. Medicine (Baltimore) 2018;97(50):e13570. doi: 10.1097/MD.0000000000013570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ebata T, Hirano S, Konishi M, Uesaka K, Tsuchiya Y, Ohtsuka M, et al. Randomized clinical trial of adjuvant gemcitabine chemotherapy versus observation in resected bile duct cancer. Br J Surg. 2018;105(3):192–202. doi: 10.1002/bjs.10776. [DOI] [PubMed] [Google Scholar]
- 102.Bergeat D, Turrini O, Courtin-Tanguy L, Truant S, Darnis B, Delpero JR, et al. Impact of adjuvant chemotherapy after pancreaticoduodenectomy for distal cholangiocarcinoma: a propensity score analysis from a French multicentric cohort. Langenbecks Arch Surg. 2018;403(6):701–709. doi: 10.1007/s00423-018-1702-1. [DOI] [PubMed] [Google Scholar]
- 103.Morino K, Seo S, Yoh T, Nishino H, Yamanaka K, Fukumitsu K, et al. The efficacy and limitations of postoperative adjuvant chemotherapy in patients with extrahepatic cholangiocarcinoma. Anticancer Res. 2019;39(4):2155–2161. doi: 10.21873/anticanres.13329. [DOI] [PubMed] [Google Scholar]
- 104.Zhao J, Zhang W, Zhang J, Chen YT, Ma WJ, Liu SY, et al. Independent risk factors of early recurrence after curative resection for perihilar cholangiocarcinoma: adjuvant chemotherapy may be beneficial in early recurrence subgroup. Cancer Manag Res. 2020;12:13111–13123. doi: 10.2147/CMAR.S289094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kim TH, Han SS, Park SJ, Lee WJ, Woo SM, Moon SH, et al. Role of adjuvant chemoradiotherapy for resected extrahepatic biliary tract cancer. Int J Radiat Oncol Biol Phys. 2011;81(5):e853–e859. doi: 10.1016/j.ijrobp.2010.12.019. [DOI] [PubMed] [Google Scholar]
- 106.Han IW, Jang JY, Lee KB, Kang MJ, Kwon W, Park JW, et al. Clinicopathological analysis and prognosis of extrahepatic bile duct cancer with a microscopic positive ductal margin. HPB (Oxford) 2014;16(6):575–581. doi: 10.1111/hpb.12193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Yang H, Zhou J, Wei X, Wang F, Zhao H, Li E. Survival outcomes and progonostic factors of extrahepatic cholangiocarcinoma patients following surgical resection: Adjuvant therapy is a favorable prognostic factor. Mol Clin Oncol. 2014;2(6):1069–1075. doi: 10.3892/mco.2014.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Hester C, Nassour I, Adams-Huet B, Augustine M, Choti MA, Minter RM, et al. Improved survival in surgically resected distal cholangiocarcinoma treated with adjuvant therapy: a propensity score matched analysis. J Gastrointest Surg. 2018;22(12):2080–2087. doi: 10.1007/s11605-018-3875-x. [DOI] [PubMed] [Google Scholar]
- 109.Nassour I, Mokdad AA, Porembka MR, Choti MA, Polanco PM, Mansour JC, et al. Adjuvant therapy is associated with improved survival in resected perihilar cholangiocarcinoma: a propensity matched study. Ann Surg Oncol. 2018;25(5):1193–1201. doi: 10.1245/s10434-018-6388-7. [DOI] [PubMed] [Google Scholar]
- 110.Ecker BL, Vining CC, Roses RE, Maggino L, Lee MK, Drebin JA, et al. Identification of patients for adjuvant therapy after resection of carcinoma of the extrahepatic bile ducts: a propensity score-matched analysis. Ann Surg Oncol. 2017;24(13):3926–3933. doi: 10.1245/s10434-017-6095-9. [DOI] [PubMed] [Google Scholar]
- 111.Krasnick BA, Jin LX, Davidson JT, Sanford DE, Ethun CG, Pawlik TM, et al. Adjuvant therapy is associated with improved survival after curative resection for hilar cholangiocarcinoma: a multi-institution analysis from the U.S. extrahepatic biliary malignancy consortium. J Surg Oncol. 2018;117(3):363–371. doi: 10.1002/jso.24836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Job S, Rapoud D, Dos SA, Gonzalez P, Desterke C, Pascal G, et al. Identification of four immune subtypes characterized by distinct composition and functions of tumor microenvironment in intrahepatic cholangiocarcinoma. Hepatology. 2020;72(3):965–981. doi: 10.1002/hep.31092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lu JC, Zeng HY, Sun QM, Meng QN, Huang XY, Zhang PF, et al. Distinct PD-L1/PD1 profiles and clinical implications in intrahepatic cholangiocarcinoma patients with different risk factors. Theranostics. 2019;9(16):4678–4687. doi: 10.7150/thno.36276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Tian L, Ma J, Ma L, Zheng B, Liu L, Song D, et al. PD-1/PD-L1 expression profiles within intrahepatic cholangiocarcinoma predict clinical outcome. World J Surg Oncol. 2020;18(1):303. doi: 10.1186/s12957-020-02082-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Gani F, Nagarajan N, Kim Y, Zhu Q, Luan L, Bhaijjee F, et al. Program death 1 immune checkpoint and tumor microenvironment: implications for patients with intrahepatic cholangiocarcinoma. Ann Surg Oncol. 2016;23(8):2610–2617. doi: 10.1245/s10434-016-5101-y. [DOI] [PubMed] [Google Scholar]
- 116.Abou-Alfa GK, Sahai V, Hollebecque A, Vaccaro G, Melisi D, Al-Rajabi R, et al. Pemigatinib for previously treated, locally advanced or metastatic cholangiocarcinoma: a multicentre, open-label, phase 2 study. Lancet Oncol. 2020;21(5):671–684. doi: 10.1016/S1470-2045(20)30109-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Javle M, Roychowdhury S, Kelley RK, Sadeghi S, Macarulla M, Waldschmidt DT, et al. Final results from a phase II study of infigratinib (BGJ398), an FGFR-selective tyrosine kinase inhibitor, in patients with previously treated advanced cholangiocarcinoma harboring an FGFR2 gene fusion or rearrangement. J Clin Oncol. 2021;39(suppl 3):265. doi: 10.1200/JCO.2021.39.3_suppl.265. [DOI] [Google Scholar]