Abstract
Since their introduction in 1987, hydroxymethyl glutaryl coenzyme A reductase (HMG-CoA) inhibitors, more commonly known as statins, have become some of the most widely prescribed medications in the world. Though generally considered to be safe and well tolerated, statins have been associated with several side effects including mild liver dysfunction manifested by increases in aminotransferases. Rarely, statins have been noted to induce more serious hepatic injury, including liver injury with autoimmune features. Current literature supports statin induced liver injury presenting in either hepatocellular or cholestatic patterns, though with the former being the prevailing pattern of injury. Fortunately, severe liver injury is uncommon with statin use and is generally reversible without any intervention other than offending statin cessation. When evaluating cases of suspected statin-induced liver injury, a complete medical history, laboratory tests including a complete metabolic panel, autoimmune markers, and viral panel, as well as hepatic imaging, are crucial for a complete causality analysis with validated tools such as Roussel Uclaf Causality Assessment Method. The aim of this review is to review the current evidence for statin-induced liver injury and cholestasis.
Keywords: Drug-induced liver injury, Statin, Cholestatic liver injury, Hepatocellular liver injury, Cholestasis, Autoimmune hepatitis
Introduction
Hydroxymethyl glutaryl coenzyme A reductase (HMG-CoA) inhibitors, commonly known as statins, work by competitively inhibiting HMG-CoA reductase, the rate limiting enzyme of the cholesterol synthetic pathway. Statins have enjoyed widespread acceptance and use because of their lipid lowering activity which in turn helps prevent the development of atherosclerosis.1 At present, statins are most commonly used in the treatment of hypercholesterolemia and dyslipidemia for primary reduction cardiovascular disease and secondary risk reduction in patients with pre-existing coronary artery disease-related events.1 Lovastatin was the first statin approved for cholesterol lowering in the US. Since then, seven other statins (atorvastatin, fluvastatin, mevastatin, pitavastatin, pravastatin, rosuvastatin, and simvastatin) have received Federal Drug Administration approval. Cerivastatin was withdrawn in 2001 because of a high risk of rhabdomyolysis.
Statins have been associated with several dose-independent symptomatic side effects including headache, nausea, rash, and decreased libido with an incidence of roughly 10%. Dose-related side effects include myopathy, rhabdomyolysis, and increased aminotransferases with incidences of 0.1%, 0.002%, and 0.2–2.4%, respectively.2 While generally noted in the first 3 months of statin therapy initiation, elevated aminotransferases have been observed to return to baseline in approximately 70% of patients with continued statin therapy, and in other cases, return to baseline with medication cessation.3 Only about 3% of patients with aminotransferase elevation experienced persistent elevation of over three times the upper limit of normal (ULN).3 It has been surmised that the etiology of asymptomatic and transient abnormalities in the aminotransferases of affected patients may be the result of changes in hepatocyte lipid membranes leading to an increase in its permeability and leakage of liver enzymes.4 The phenomenon has been observed with all lipid-lowering agents and may not be specific to statins.5 As previous investigations have found no significant histopathological changes associated with minor aminotransferase elevations in patients started on statins, the changes have recently been termed hepatic “adaptations” rather than injury.5 The actual incidence of liver injury by statins is considered to be much lower than general aminotransferase abnormalities, around 1%.6 In this review, we evaluated available cases of liver injury attributed to statin therapy and categorized them by injury pattern.
Statin-induced hepatocellular patterns of liver injury
Definition and epidemiology
Statin-induced liver injury has been associated with both hepatocellular and cholestatic patterns of injury. Hepatocellular pattern liver injury is defined by a predominant rise in aminotransferases, more specifically, alanine aminotransferase (ALT); a cholestatic pattern is associated with a principal rise in alkaline phosphatase (ALP) and bilirubin.7,8 Statin-associated hepatocellular injury frequently occurs 5 to 90 days after the initiation of therapy.7,8 Bilirubin levels more than twice the ULN imply severe hepatocellular liver injury with a mortality of 10% and an incidence of 0.7–1.3 per 100,000 cases of drug-induced liver injury (DILI).8 Unfortunately, clear incidence rates of statin-induced liver injury and pretreatment risks are difficult to obtain.9 Most retrospective epidemiological studies tend to underreport and underestimate the true incidence of statin-induced liver injury.9 Recent population-based studies reported an incidence of statin-induced liver injury of 19 cases per 100,000 per year.9 While there is an established association between statin therapy and liver injury, it is important to rule out other causes of liver injury.
Reported cases
Using the Scopus, PubMed, and Google Scholar databases, we identified 531 manuscripts using the key words “statin” and “liver injury”. Of the 15 manuscripts evaluated, there were nine case reports,10-18 two of which discussed two patients.11,17 The case reports are summarized in Table 1. Only one of the six case patients was female.12 While there were no data to explain the difference in adverse reactions associated with statins in men and women, the disproportion was also noted in cholestatic DILI, and is discussed below. The finding raises the question of whether the risk of hepatotoxicity from statins is higher for men or if it more reflective of sex differences in statin prescription rates, being higher in men than in women, as has been previously identified in studies evaluating patients with conditions ranging from heart failure to HCV and HIV.19,20 Again, given the small number of cases reviewed, no conclusions can be drawn, but additional research may further investigate this observation.
Table 1. Summary of reported statin-induced hepatitis cases.
| Case report | Age/Sex | Statin | Symptoms | Labs (peak) | Timeframe | Liver biopsy | Rechallenge | Reference ranges |
|---|---|---|---|---|---|---|---|---|
| Mohamed 201910 | 67 M | Atorvastatin 80 mg | Generalized body aches, muscle weakness, jaundice, dark urine, decreased urine output | Bili: 211; AST/ALT: 1,612/1,325; ALP: 278 | 4 months | No | No | Bili >20.5 µmol/L, AST/ALT >34/55 UI/L, ALP >150 UI/L |
| Kawasaki 202011 | 46 M | Atorvastatin 10 mg | Generalized weakness, abdominal pain | Bili: 5.1; AST/ALT: 860/1,632; ALP: NA; ANA (+) | 6 months | Yes | No | Not provided |
| 54 M | Rosuvastatin 2.5 mg | Generalized weakness, abdominal pain | Bili: N/a; AST/ALT: 606/709; ALP 2,055; IgG: 1,857 | 8 months | Yes | No | ||
| Khan 202012 | 57 F | Atorvastatin 40 mg | Generalized weakness, abdominal pain | Bili: 122.8; AST/ALT:; 2,999/3,195; ALP: 435; ANA (+); ASMA (+) | 3 months | No | Yes, no recurrence | Bili >20.5 µmol/L, AST/ALT >34/55 UI/L, ALP >150 UI/L |
| Sanchez 201813 | 47 M | Rosuvastatin 5 mg | Generalized weakness, abdominal pain | Bili: NA; AST/ALT: 112/201; ALP: N/a; ANA (+); ASMA (+) | 4 months | No | No | Bili >1.1 mg/dl, AST/ALT >37/41 UI/L, ALP >129 UI/L |
| Saha 202114 | 71 M | Atorvastatin 80 mg | No symptoms | Bili: 1.5; AST/ALT: 1,093/1,385; ALP: 265; ASMA (+) | 1 month | No | Yes, simvastatin 20mg rechallenge after 3 months, no recurrence | Bili >1.2 mg/dL, AST/ALT >41/58 UI/L, ALP >129 UI/L |
| Shah 201915 | 47 M | Rosuvastatin 5 mg | Jaundice, pruritis, fatigue | Bili: 3.53; AST/ALT:; 1,142/2,260; ALP: 277 | 1.5 months | Yes | No | Bili >1.2 mg/dL; AST/ALT >40/40 UI/L, ALP >115 UI/L |
| Vishwakarma 201416 | 63 M | Atorvastatin 20 mg | Jaundice, Nausea, fatigue, appetite loss, abdominal pain | Bili: 5.2; AST/ALT: 1,124/1,049; ALP: 214 | 2 months | No, patient refused | Yes, rosuvastatin 10mg rechallenge upon noted elevation with enzyme normalization and no recurrence | Not provided |
| Liu 201017 | 58 M | Atorvastatin 20 mg | Fatigue, anorexia | Bili: 9.2; AST/ALT: 23/120; ALP: 62 | 10 hours | No | Yes, Pravastatin (dose unknown) with no recurrence | Bili >22 µmol/L, AST/ALT >40/40 UI/L, ALP >120 UI/L |
| 53 M | Atorvastatin 10 mg | None symptoms | Bili: 9.3; AST/ALT: 119/124; ALP: 93 | Immediate | No | Yes, Pravastatin 20mg after 14 days with no recurrence | ||
| Famularo 200718 | 64 M | Rosuvastatin 10 mg | Fatigue, anorexia, malaise, abdominal pain, jaundice | Bili: 2.6; AST/ALT: 880/775; ALP: “normal” | 4 months | No | No | Bili >2.0 mg/dL, AST/ALT >36/36 UI/L, ALP not given |
ALP, alkaline phosphatase, ANA, antinuclear antibody; ALT, alanine aminotransferase; ASMA, anti-smooth antibody AST, aspartate aminotransferase; Bili, bilirubin; IgG, immunoglobulin G.
Of the ten patient cases reviewed, only three underwent liver biopsies (Table 1). The other seven patients were followed by serial aminotransferase and imaging studies.10–18 Eight of the patients had reported normal baseline aminotransferases prior to initiation of statin therapy, and nine had resolution of aminotransferase elevations following discontinuation of statin therapy, although three had also received steroid maintenance therapy.10–18 None of the case reports took into consideration the possible presence of nonalcoholic fatty liver disease or elevated body mass index.
Autoimmune modulation in statin-induced liver injury
Seven patients experienced hepatocellular pattern liver injury after being started on atorvastatin, and three experienced it after starting rosuvastatin (Table 1). In five cases, statin rechallenge with pravastatin, rosuvastatin, or simvastatin was used instead of resumption of the original atorvastatin without subsequent recurrence of hepatitis (Table 1). Of the ten cases reviewed, four patients experienced liver injury with autoimmune features.11–13 One had a positive 1:80 antinuclear antibody (ANA) titer, a liver biopsy significant for piecemeal necrosis (interface hepatitis) with lymphocyte and plasma cell infiltration, and a revised International Autoimmune Hepatitis Group (IAHG) score of 18 points, indicating definite autoimmune hepatitis (AIH).11 A second patient had an elevated immunoglobulin G level (1,857 mg/dL) along with a liver biopsy significant for lobular, portal and interface hepatitis with lymphocyte and plasma cell infiltration and a IAHG score of 13, suggestive of probable AIH.11 A third patient with presumed AIH did not have an IAHG score, but was weakly ANA positive and had a positive anti-smooth muscle antibody (ASMA) titer (1:640).12 The fourth patient initially was only positive for ANA (1:160) 2 weeks after discontinuation of rosuvastatin, but subsequently became ASMA-positive (1:80) on repeat evaluation 1 month after medication discontinuation. The patient experienced a complete normalization of aminotransferases and negative auto-antibody titers within 3 months of statin discontinuation without any further intervention. The authors noted an IAHG score of 11 indicating probable AIH and a Roussel Uclaf Causality Assessment Method (RUCAM) score of 5, indicating possible drug-induced liver injury (DILI).13 RUCAM is a seven part investigative method for predicting the likelihood of causation between drug use and liver injury using a point system based on the relation to time of liver injury onset after drug use, the course of liver enzyme changes, risk factors, concomitant drug use, evaluation of alternative causes for liver injury, previously known drug hepatotoxicity, and response to unintentional re-exposure. A score of <1 is considered exclusionary, 1–3 is unlikely, 3–5 is possible, 6–8 is probable, and >8 is highly probable DILI.21
Of the five cases with positive autoimmune markers/features, all had normal baseline aminotransferase levels prior to the initiation of statin therapy which decreases the chance of confounding variables. Only two statins were administered, which makes the results less applicable to other statin formulations. Only two cases involved a statin rechallenge, neither with the same statin, making assignment of causality more difficult. The majority of cases had detailed descriptions of their serology data, and not all were similar. Only three patients had IAHG scores, which makes the comparison of autoimmune hepatitis between the cases more challenging.
There has been speculation and considerable debate as to whether all cases of reported drug-induced AIH are truly cases of AIH rather than immune-mediated DILI with overlapping features. A framework that differentiates the two conditions based on patient clinical course has been proposed. With true AIH, offending drug cessation should not result in resolution of AIH symptoms and laboratory findings, while in immune-mediated DILI, complete resolution of hepatitis should be seen with drug cessation or after a brief course of steroids.22 This association was investigated by Björnsson et al.23 in a retrospective study that reported hepatitis resolution following drug discontinuation without steroid use was indeed more supportive of autoimmune DILI than AIH was. In the two cases reported by Kawasaki et al.,10,11 patients were started on long-term steroid maintenance once diagnosed with features of AIH. Given the use of maintenance steroid administration, it is not possible to determine whether the persistent aminotransferase normalization after statin cessation was immune-mediated DILI or AIH controlled by steroid therapy. In the case described by Khan et al.,12 however, statin cessation led to aminotransferase normalization without any further intervention, which is consistent with immune-mediated DILI secondary to statin administration. Interestingly, in that case, the patient was rechallenged with pravastatin rather than the previously administered agent, atorvastatin, after aminotransferase normalization. While recurrence in hepatitis features was not observed, trial of pravastatin cannot be considered a true rechallenge, as structural differences between the statins may have accounted for the lack of recurrence.
It is important to note that two of four patients who developed liver injury with autoimmune features had type 1 diabetes mellitus,11 which is often associated with other autoimmune conditions, but there is literature to support its coexistence with autoimmune hepatitis.11 The exact mechanism of statin-induced autoimmune hepatitis/immune-mediated DILI is not clear. Some studies have suggested that statins may upregulate toll-like receptors on activated dendritic cells, enhance the secretion of proinflammatory cytokines, and could be related to the development of autoimmune hepatitis.24–26
Treatment and conclusions
Currently available evidence suggests that hepatocellular pattern liver injury is a rare complication of statin administration. The most commonly reported symptoms are jaundice, generalized weakness, and abdominal pain, but some patients are completely asymptomatic when abnormal laboratory values appear. Changes in aminotransferases suggestive of hepatocellular pattern liver injury have been observed as early as a few hours after initial statin exposure to as late as 8 months after initiation.10–18 While statin-induced hepatocellular liver injury is predominantly self-limiting and generally resolves within 6 months of offending agent cessation, some cases had autoimmune features and were treated with a brief steroid course to accelerate resolution.10,11 A few reports described attempts at statin rechallenge, although with alternate formulations that did not induce liver injury. While statin rechallenge with congeners further helps prove causality, the data obtained is inferior to rechallenge with the same statin. It is understandable, however, that few clinicians would choose to restart a medication highly suspected of DILI so as not to expose their patients to further harm.
Statin-induced cholestatic pattern liver injury
Definition and epidemiology
Cholestatic pattern liver injury represents a course of hepatic illness and inflammation that is characterized by elevations of aminotransferases, cholestatic markers, ALP, and bilirubin. While there is no incidence data specific to cholestatic DILI, a figure of 1.47 per 300,000 can be extrapolated from data reported by Björnsson et al.7 Unfortunately, no data are available on genetic predisposition specific for statin-induced cholestatic liver injury. Differentiating cholestasis from cholestatic liver injury often requires examining liver biopsies in addition to biochemical features.27 Symptoms of cholestatic liver injury include right upper quadrant pain, jaundice, anorexia, nausea, and vomiting. Laboratory values usually reveal ALP levels greater than three times the ULN along with hyperbilirubinemia and an AST and ALT two to 10 times the ULN. Liver pathology is also consistent with hepatic portal inflammation with or without hepatic necrosis and eosinophils.27
In reviewing the data on cholestatic pattern liver injury, we used the definition of drug-induced cholestatic hepatitis as defined by the National Institute of Diabetes and Digestive and Kidney Disease, which includes six criteria.28 (1) In this review, R values ≤5 were used to identify cases of cholestatic hepatic injury. With that criterion, cases of “mixed hepatitis” described in the literature were included in the cholestatic hepatitis cohort. R is calculated as (ALT × ULN ALP)/(ALP × ULN ALT). (2) The cases had a latency of 2–24 weeks. (3) If symptoms were present, they included dark urine or pruritis early during the disease course. (4) The bilirubin concentration was >2.5 mg/dL. (5) For patients with liver biopsies, the histology should show changes of intrahepatic cholestasis with inflammatory cells and mild to moderate focal hepatocellular necrosis. (6) If the etiology of cholestatic hepatitis is suspected to be in the setting of medication use, there should be exposure to a known cholestatic agent.
Case reports
A total of 531 manuscripts were retrieved from Scopus, PubMed, and Google Scholar using the key words “statin” and “liver injury”. Of those, 23 were reviewed. Six were not included here because they reviewed the available literature without reporting cases or had confounding variables of patient evaluation such as pre-existing liver disease. Eleven case reports on statin-induced cholestatic hepatitis and three database studies are included in this review.7,29–41 The case reports are summarized in Table 2.29–39 One case report included a patient younger that 40 years of age,29 which makes it less likely to represent potential age-related toxicity than the patient population most likely to be prescribed statin therapy. Only three case reports included women.29,36,39 No of the studies evaluated sex differences in susceptibility to cholestatic DILI, but some have reported conflicting results on which sex is more frequently affected by statin-induced DILI.7,38 As noted previously, the differences may also involve lower statin prescription rates for women than for men. The small sample size of eleven cases reports included in this review makes conclusions on sex differences in development of statin-induced cholestatic hepatitis unreliable.
Table 2. Summary of reported statin-induced cholestatic hepatitis cases.
| Case report | Age/Sex | Statin | Symptoms | Labs (peak) | Timeframe | Liver biopsy | Rechallenge | Reference ranges |
|---|---|---|---|---|---|---|---|---|
| Jimenez 199929 | 20, F | Atorvastatin 10 mg (3 weeks) then 20 mg (5.5 weeks) | Fatigue, anorexia, jaundice | Bili: 7.0; AST/ALT: 869/783; ALP: 669 | 8.5 weeks | No | No | Not provided |
| Hartleb 199930 | 57, M | Pravastatin 20 mg | Jaundice | Bili: 13.6; AST/ALT: 297/421; ALP: 482 | 7 weeks | Yes | No | Not provided |
| Ridruejo 200231 | 69, M | Atorvastatin 10 mg | asthenia, nausea, pruritus and dark urine | Bili: 2.6; AST/ALT: 669/727; ALP: 3,767 | 6 months* | No | No | Bili reference range not provided; AST/ALT <40/35 UI/L, ALP <270 UI/L |
| Perger 200332 | 83, M | Atorvastatin 20 mg | Fatigue, anorexia, jaundice | Bili: 24.85; AST/ALT: 1,312/1,401; ALP: 393 | 2 weeks | Yes | No | Bili <17 µmol/L, AST/ALT <40/40 UI/L, ALP <115 UI/L |
| De Castro 200633 | 72, M | Atorvastatin, 40 mg | Jaundice, dark urine | Bili: 7.4; AST/ALT: 679; ALP 1,259 | 1 week | Yes | Yes, recurrence | Not provided |
| Vergura 200734 | 77, M | Atorvastatin, 20 mg | Asymptomatic, incidental | Bili: N/A; AST/ALT: 564/738; ALP: 519 | 1 month | Yes | No | Bili reference range not provided, AST/ALT <45/50 UI/L, ALP <129 UI/L |
| Kleiner 201135 | 82, M | Simvastatin (N/A) | Jaundice | Bili: 5.2; AST/ALT: 1,919/1,737; ALP: 260 | 4 months | Yes | No | Not provided |
| Krezner 201336 | 79, F | Atorvastatin (N/A) | Pruritis, Jaundice, Scleral icterus | Bili: 2.5; AST/ALT: 124/307; ALP: 953 | 1 month | No | Yes, recurrence | Not provided |
| Alanazi 202137 | 69, M | Fluvastatin 40 mg | Fatigue, abdominal pain, vomiting, pruritis, weakness | Bili: 318; AST/ALT: 202/108; ALP: 1,200 | 7 weeks | No | Not mentioned | Bili less than 21 µmol/L, AST/ALT >45/45 UI/L, ALP <100 UI/L |
| Xu 202038 | 47, M | Atorvastatin 10 mg | Not described | Bili: “normal”; AST/ALT: “normal”/51; ALP: 178 | 2 months | No | Not mentioned | ALT <41 UI/L, ALP <129 UI/L |
| Kuniyoshi 201939 | 44, F | Atorvastatin 5 mg | Fatigue | Bili:.7; AST/ALT: 66/84; ALP: 1,557 | 10 months | Yes | Not mentioned | Not provided |
*Patient paused therapy for 1 week because of partial colonic resection with re-initiation 1 week prior to symptom onset. ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; Bili, bilirubin.
Of the evaluated statins, atorvastatin was the most frequently implicated in the development of cholestatic DILI (i.e., in eight of the eleven cases),29,31–34,37–39 which is consistent with the findings of a retrospective study by Björnsson et al.7 in which atorvastatin was the statin found to be the most likely to induce cholestatic DILI. However, prescriber habits need to be considered. While we did not find European data on statin prescription habits, according to the United States Center for Disease Control and Prevention, simvastatin accounted for 42% of statin prescriptions followed by atorvastatin at 20.2% between 2011 and 2012.42 Interestingly, four of the reviewed cases described prior statin use in affected patients without notable side effects.29,33,37,38 Ridruejo et al.31 described a patient who had previously been asymptomatic on a lower dose of atorvastatin (20 mg) though with intermittent compliance. De Castro et al.33 described a patient who had previously tolerated other statins (pravastatin and simvastatin) without notable side effects until developing DILI on the current medication regimen. Alanazi et al.37 and Xu et al.38 described patients previously on simvastatin and pitavastatin, respectively, without any notable complications. Those cases raise the possibility that statin-induced hepatotoxicity may depend both on both formulation and dose. Overall, patients appear to have most often presented with jaundice or were incidentally found to have abnormal aminotransferase levels. No evidence of liver failure was described in the reviewed cases, and statin cessation led to complete symptom and laboratory marker resolution without further pharmacologic intervention. However, none of the case reports included long-term follow-up, making it difficult to assess any delayed complications.
The strengths of the reviewed case reports include available data on liver enzymes before and after starting statin therapy as well as follow-up laboratory findings after cessation of statin use in all but two cases. Ridruejo et al.31 and Alanazi et al.37 did not report baseline aminotransferase levels. Six of the evaluated cases included data from liver biopsies (Table 2). However, differences in reporting and patient evaluation potentially allow for confounding variables that affecting the presumed correlation between statin use and the development of cholestatic hepatitis. All the reviewed cases reported elevated aminotransferases, imaging studies, and viral/infectious etiology workups, and three of the 11 case reports did not investigate autoimmune markers of hepatic injury.26,27,31 None of the cases took into consideration the possible presence of nonalcoholic fatty liver disease or elevated body mass index.
A female patient was reported to have cholestatic hepatitis in the setting of atorvastatin administration, but had pre-existing lupus erythematosus complicated by lupus nephritis.29 At the time of the evaluation for hepatitis, her double-stranded (ds)DNA titer was 1:340, which was significantly lower than the levels she had 5 months previously (1:1,300), and it was believed that the patient was in remission at the time of her diagnosis of liver injury. It cannot be excluded that this patient was at a increased risk of autoimmune overlap, given her medical history. Unfortunately, no autoimmune workup was conducted, and no liver biopsy or medication rechallenge was attempted. Given the time frame of symptom onset of 8.5 weeks after initiation of statin therapy and normalization of aminotransferases after only medication cessation without additional therapy, it is conceivable that the cholestatic liver injury was secondary to atorvastatin use. It cannot, however, be excluded that her lupus contributed to the disease process.
In the case reported by Kuniyoshi et al.,39 the patient experienced transiently elevated antimitochondrial antibody (AMA) titers that normalized following cessation of atorvastatin. A liver biopsy performed while the AMA titers were elevated did not show any evidence of primary biliary cholangitis. The authors suspected that a transiently positive AMA was in line with a prior hypotheses that oxidative stress-induced liver damage may lead to AMA production, and that without additional factors, atorvastatin exposure alone does not result in the development of primary biliary cholangitis.39 Of note, the authors did treat the patient with ursodeoxycholic acid 600 mg/day for close to a year. The exact duration not reported, and the aminotransferases remained normal even after discontinuation of therapy.
Six of the eleven case reports reported laboratory values without reference ranges, and histories of alcohol use and alcohol-related liver disease were generally not clearly delineated. Furthermore, only three of the cases assessed the likelihood of correlation between statin use and ensuing DILI with an analytic model like RUCAM. One case described the use of a drug-induced lymphocyte test to prove causation.37–39 Medication rechallenge was attempted in only two cases with both describing recurrence of cholestatic hepatitis.30,33
Database literature review and analysis
Three investigations of statin use and hepatotoxicity included patient database records.7,40,41 The first, by the Swedish Adverse Reactions Advisory Committee, evaluated suspected statin-induced side effects.7 Using RUCAM to identify likely causation and DILI criteria including aminotransferases >5 times the ULN, and/or ALP >2 times the ULN, or bilirubin >2 times the ULN, 73 cases of possible or highly probable cases of statin-induced DILI between 1988 and 2010 were identified. Retrospective analysis found that simvastatin and atorvastatin were most frequently associated with DILI and that atorvastatin was the most likely to elicit a cholestatic/mixed hepatitis pattern, and associated with 57% of identified atorvastatin-induced cases of DILI.7 In total, 30 of 73 patients were reported to have experienced cholestatic/mixed picture liver injury. Of the 30 patients who experienced cholestatic pattern liver injury, only one underwent statin rechallenge, with simvastatin 20 mg. The patient subsequently had a recurrence of liver injury, but the authors did not report the pattern of injury on recurrence. One patient died from suspected cholestatic pattern DILI after atorvastatin use, but that case had the weakest causality association, with a RUCAM indicative of possible DILI. Unfortunately, no further information was provided about the case. The Swedish patient database study benefited from a large sample size of 73 patients and exclusion of patients with a confounding history of liver disease. The authors did report some patient information deficits, such as cases from 1988–1991 not being tested for hepatitis C as there was no commercially available test for the virus at the time. Additionally, baseline alcohol consumption was not available for review in any of the cases, although alcoholic liver disease was an excluded etiology for liver dysfunction at the time of proposed DILI. Even though the study was retrospective, there was enough medical history for a complete RUCAM to be run on all 73 included cases.
The second retrospective study evaluated DILI cases diagnosed between 1994 and August 2012 and retrieved from the Spanish Hepatotoxicity Registry (REH).40 Causality was assessed with RUCAM paired with two liver injury criteria, two times the ULN of ALT or conjugated bilirubin or, the combination of increases in aspartate aminotransferase (AST), ALP, and total bilirubin, with one of these greater than twice the ULN. Given the expert consensus in 2011, the definition of liver injury for cases reported in 2011 and 2012 was refined to raise the cutoff threshold for liver injury of ALT or AST when elevated in an isolated manner to ≥5 ULN. The second liver injury definition was an ALP of at least two times ULN or the combination of an increase in ALT greater three times the ULN and total bilirubin greater than two times the ULN. A total of 858 cases of DILI were identified, of which, 47 were identified in the setting of statin use, 16 were attributed to atorvastatin, 13 to simvastatin, 12 to fluvastatin, four to lovastatin, and two to pravastatin.40 Cholestatic DILI was noted in eight (50%) of the patients using atorvastatin, two (15%) using simvastatin, eight (67%) using fluvastatin, three (75%) using lovastatin, and two (100%) using pravastatin. In total, 23/47 (49%) of patients with presumed statin-induced liver injury experienced a cholestatic/mixed pattern of liver injury. Workup including liver biopsy and autoimmune markers did not identify instances of concern for autoimmune DILI in cases involving atorvastatin, simvastatin, and fluvastatin, there was no indication as to whether the cases involved predominantly hepatitic vs cholestatic liver injury. The strength of this study was its large sample size, with 23 patients experiencing a cholestatic liver injury. However, as with the Swedish database study, it was retrospective, which makes it difficult to completely evaluate of the clinical picture of each reported case. However, this study also had enough patient information in each case for RUCAM analysis. Other study limitations include the use of multiple aminotransferase cutoff definitions for hepatic injury, including one with a lower threshold for diagnosing liver injury that was used prior to an expert consensus change in 2011.
The third study, by Russo et al.,41 was a prospective evaluation of reported liver injuries in the setting of statin administration within the US Drug-Induced Liver Injury Network between 2004 and 2012. Among 1,188 cases of DILI, the authors identified 22 patients whose DILI was attributed to statin therapy. For those 22 patients, common causes of liver injury such as viral hepatitis, alcohol, pancreatic, biliary, and metabolic liver diseases were excluded. All patients were followed for at least 6 months to exclude other diagnoses. RUCAM analysis was used for causality as well as adjudication of each case by expert opinion as being definite, very likely, or probable likelihood of statin DILI. In cases where multiple medications were involved, injury was attributed to statin therapy if it had the highest causality score. The ratio of serum ALT to ALP, both expressed as multiples of the upper limit of normal (ULN) were used to define the type of hepatic injury, with ratios of <2 for cholestatic liver injury, from 2 to 5 for mixed pattern liver injury, and >5 for hepatocellular pattern liver injury. Of the 22 documented cases of statin DILI, seven had a purely cholestatic liver injury pattern. Three of the patients received atorvastatin, and one each received fluvastatin, rosuvastatin, lovastatin, and simvastatin. Two patients were men and five were women. Six cases of DILI resolved without interventions other than offending statin cessation. One patient, treated with rosuvastatin, was reported to subsequently experience chronic liver disease defined as having cholestatic pattern liver injury at the last follow-up evaluation at 6 months after statin cessation. None of the patients with cholestatic liver injury died or required liver transplantation. When compared with patients who experienced hepatocellular pattern DILI, those with cholestatic liver injury were found to be older (65 vs. 57 years of age), but the difference was not statistically significant. Interestingly, while three of the cases with cholestatic liver injury had liver biopsies, only two of the three had histological evidence of a cholestatic pattern of injury. Two other cases with hepatocellular-autoimmune DILI had histological evidence of cholestatic hepatitis (i.e., hepatocellular and canalicular cholestasis and bile duct injury combined with portal and lobular inflammation). The significance of this finding remains unclear. This study was prospective, enabling a more complete evaluation of the medical history as well as a scheduled follow-up for 6 months after hepatic insult, thereby allowing for classification each case as resulting in acute or chronic liver injury. As with the two other database studies, pre-existing etiologies of liver disease were excluded and as with the Swedish study, other medications patients were concomitantly prescribed were also evaluated as potential causal agents with a RUCAM score. This study used both RUCAM analysis as well as expert opinion for a combined evaluation of each patient case to determine the likelihood of causality. A limitation of this study was the inclusion of only seven cases of cholestatic liver injury, limiting the power for comparison of the statin-induced hepatocellular pattern liver injuries. The study also did not assess cross reactivity of statins, and statin rechallenge was only described in one case, the authors did not report whether the patient was in the hepatocellular or cholestatic liver injury cohort.
Treatment and conclusions
In reviewing the available literature, it appears that statins can induce cholestatic liver injury. However, hepatic failure and the development of chronic liver disease in this setting is exceedingly rare. Regarding presenting symptoms, jaundice and pruritis are most commonly seen, though many patients are asymptomatic and hepatic abnormalities are only incidentally found on bloodwork. Aside from statin cessation, no other treatment is generally required, as most cases of statin-associated cholestatic DILI resolved within 6 months. Latency for hepatic injury with statins appeared to vary, but abnormal aminotransferases developed from within 3 months to up to a year after starting statin therapy. Analytic tools that take into account the medical work up, such as RUCAM, should be used to further delineate the likelihood of causation. Ideally, if safe, statin rechallenge should be attempted to further prove causation. Liver biopsy, may be helpful in identifying liver disease pattern and ruling out of some disease pathologies, is unnecessary. It also appears that the development of cholestatic DILI with one statin does not preclude the use of other statins without liver injury recurrence. Atorvastatin appears to be the statin most commonly associated with a cholestatic pattern of liver injury.
Statin-induced cholestasis
Definition and epidemiology
Statin-induced cholestasis is a decrease in bile flow in the absence of, or with minimal hepatocellular damage. The spectrum of statin-induced cholestasis ranges from mild reversible cholestasis to chronic forms such as vanishing bile duct syndrome.43 Unfortunately, pure statin-induced cholestasis without overlap with hepatitis is very rare, and for that reason, no prevalence or epidemiological data are currently available.
Case reports
Fourteen publications were retrieved from Scopus, PubMed, and Google Scholar using the key words “statin” and “cholestasis,” eleven of which were not included in this review as they were either literature reviews themselves or had no reported cases. Of the remaining three manuscripts, we were able to obtain access to one case report.44 The limited number of cases describing statin-induced cholestasis can be explained by its overlap with cholestatic DILI.
Merli et al.44 described a case of statin-induced cholestasis in a 72-year-old man who had been treated with atorvastatin and developed nausea, jaundice, and scleral icterus with marked elevations of bilirubin (total bilirubin 22 mg/dL) and ALP (four times the ULN) and only marginal increases of aminotransferases. A biopsy revealed a histology consistent with cholestasis, including a lymphocytic and neutrophilic infiltrate without evidence of active hepatitis.44 Merli et al.44 used Maria and Victorino clinical scales as well as the Naranjo Adverse Drug Reaction Probability Scale to prove causality between atorvastatin use and cholestasis. The authors did not comment on the patient’s past medical history, making it unclear whether there were any confounding variables such as underlying biliary atresia/sclerosis or cholelithiasis, or autoimmune disease that could have presented as a transient cholestasis in the laboratory workup. There was no discussion of the imaging modalities used in the evaluation of this patient, which would have clarified any potential underlying biliary disease predisposing to cholestasis. Cholestasis in the setting of statin use is a complex condition and is considerably rarer than cholestatic and hepatocellular DILI discussed earlier. The infrequent clinical presentation has been associated with the use of pravastatin and atorvastatin.32,45
Pre-existing cholestatic disease does not appear to be a risk factor for developing cholestasis while on statin therapy. One of the rare risk factors for the development of cholestasis while on a statin is voriconazole treatment.44 This rare association was described in a case report in which the patient was simultaneously on voriconazole and simvastatin therapy. Voriconazole has a very narrow therapeutic index for development of cholestasis. Because voriconazole is metabolized by cytochrome P450 3A4 (CYP3A4) and statins inhibit that enzyme, co-administration puts patients at risk for the development of cholestasis. It is important to note that simvastatin effects could have been a confounding factor in this case. Because only one case has been reported, the findings may not be reliable.
Treatment and conclusions
The treatment of statin-induced cholestasis is generally supportive and starts with discontinuation of the offending statin.46 In the rare instances that medication cessation does not lead to cholestasis resolution, cholestyramine or ursodeoxycholic acid has been used, followed by rifampicin and opioid antagonists if the first-line agents fail. Nutritional support is critical in patients with prolonged cholestasis, especially because they are at risk for the development of biliary cirrhosis and liver failure.46 It is also important to refer patients with prolonged cholestasis for liver transplant assessment, as early referral has been shown to improve outcomes.46
Molecular mechanisms of statin-induced liver injury
In general, statin-induced liver injury is more likely to be seen in patients taking maximal statin doses together with other lipid lower medications, other medications that use the cytochrome P450 enzymatic pathway, and/or patients who are elderly or have severe hepatic or renal impairment.47
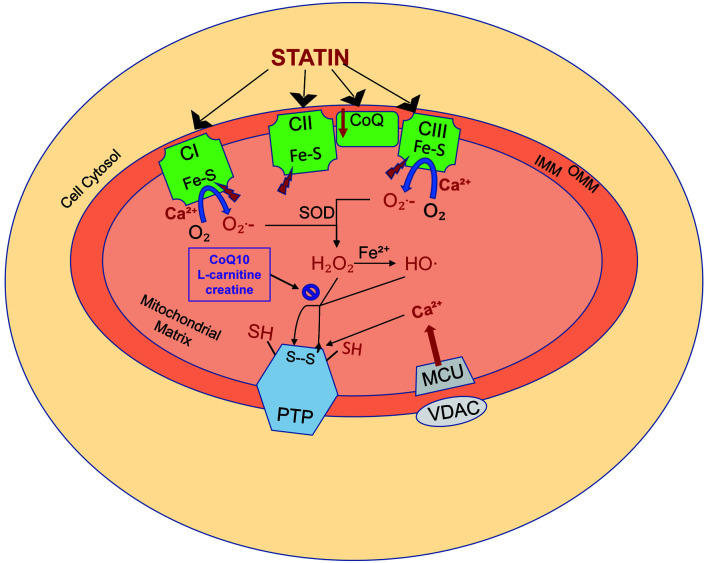
Several mechanisms of statin-induced liver injury have been proposed, but none have clearly delineated the mechanisms based on the ensuing liver injury pattern. Golomb et al.46 hypothesized that the underlying mechanism was mitochondrial damage. They reported that the risk of statin-induced liver injury increased with statin potency and its effect on the cytochrome P450 system.46 Cytochrome P450-dependant metabolism acts as a reactive oxygen species generator and participates in cell apoptosis. With the use of statins, that promotes the production of reactive oxygen species and lipid peroxidation, leading to a decrease in mitochondrial membrane potential and subsequent cytotoxicity.47 While the mechanism does apply to most statin medications, it should be noted that pravastatin and rosuvastatin are not metabolized by isoenzymes of the cytochrome P450 pathway. Other proposed mechanisms of mitochondrial damage that could result in statin-induced liver injury include inhibition of the respiratory chain (i.e., complexes I, II, and III) and the release of calcium (Fig. 1).48
Fig. 1. Statin-induced mitochondrial oxidative stress.
Statins induce both mitochondrial oxidative stress and calcium-dependent permeability transition, which may lead to cell injury and death. (1) Statins diminish respiratory capacity at the level of complexes I (CI), II (CII), and III (CIII) of the respiratory chain, which in turn results in the increased generation of superoxide (O2−) generation. The iron sulfur centers (Fe-S) within the respiratory complexes are in turn inhibited, diminishing their resistance to calcium (Ca2+)-dependent mitochondrial permeability transition. (2) The mitochondrial antioxidant defense system uses superoxide dismutase to convert superoxide to hydrogen peroxide. Hydrogen peroxide is normally then metabolized by the mitochondrial antioxidant system, including coenzyme Q10 (CoQ10), L-carnitine, and creatine. With statin toxicity, a large quantity of hydrogen peroxide production results in the depletion of the antioxidant system. Remaining hydrogen peroxide induces membrane protein sulfhydryl-disulfide transition (SH, S–S), promoting permeability transition pore (PTP) opening. (3) Statins also impair cellular Ca2+homeostasis by increasing Ca2+release from the endoplasmic reticulum thereby increasing cytosolic Ca2+levels. Cytosolic Ca2+is taken up by a voltage-dependent anion-selective channel and mitochondrial calcium uniporter channels, leading to accumulation of Ca2+ in the mitochondrial matrix. The accumulated Ca2+ binds to the membrane, exposing specific buried thiols to the oxidants and impairing mitochondrial respiration, resulting in increased superoxide formation. The combination of increasing reactive oxygen species and mitochondrial Ca2+ load leads to PTP opening and subsequent cell death. OMM, outer mitochondrial membrane; IMM, inner mitochondrial membrane. Adapted from Busanello et al., 2017.48
Genetic factors may affect patient susceptibility to liver injury in the setting of statin use.49 A recent genome-wide association study identified transporter genes, cytochrome P450, organic anion-transporting polypeptides, and ATP-binding cassette genes ABCB1 and ABCC1 as possibly predisposing patients to statin-induced liver injury.50 However, genetic and iatrogenic variations alone may not be to blame, as other comorbidities such as thyroid disease, which amplify mitochondrial or metabolic vulnerability, may increase the risk of statin-associated liver injury.46 Another proposed mechanism of statin-induced liver injury involves the triggering of autoimmune responses, with subsequent findings similar to those of autoimmune hepatitis.49 Unfortunately, the mechanism by which statins induce this state remains unclear. However, there is evidence of associations of autoimmune states with statin use. For example, statins have been implicated in directly causing immune-mediated necrotizing myopathy thought to be caused by upregulation of 3-hydroxy-3-methylglutaryl-CoA reductase (HMGCR), the molecular target of statins, resulting in presentation of highly immunogenic HMGCR peptides by human leukocyte antigens.39
For patients with chronic liver disease, the risk of statin use and subsequent liver injury remains controversial. While statins were previously thought to increase the risk of injury in those with chronic liver disease, Kim et al.51 found that statin use in patients with chronic liver disease was associated with a decreased risk of liver injury and subsequent cirrhosis. The study was a systematic literature review and meta-analysis limited to 13 studies, only 3 of which were randomized trials. The majority of the cases (84.5%) involved hepatitis C infection, which may have made conclusions less applicable to cases of chronic liver disease secondary to other etiologies. They did, however, use the Grading of Recommendations Assessment and Development and Evaluation criteria to assess the quality of evidence. The authors did not differentiate between statin-induced hepatocellular and cholestatic liver injury.
Conclusions
The literature on statin-induced hepatocellular pattern liver injury with and without autoimmune features as well as cholestatic pattern liver injury and cholestasis suggests that chronic liver disease does not occur unless patients develop true AIH.49 The use of steroids in statin DILI, and DILI in general, remains controversial. Most data demonstrating the benefit of steroid administration are in the form of case reports or observational studies. The subject is difficult to analyze given the considerable variability of therapeutic strategies among clinicians.52 Hu et al.52 reported some benefit of steroid administration in patients with severe DILI, did not report the presence of autoimmune markers. There is no standard therapy for patients who experience DILI with autoimmune features. Review of the available literature on DILI with autoimmune features attributed to other medication classes has shown complete liver injury resolution without relapse with offending drug cessation alone or with a short course of steroids. In cases where a true AIH develops secondary to DILI, standard AIH therapy involving long-term corticosteroid administration is warranted.50
Statins have a definite association with liver injury, most commonly manifesting a hepatocellular pattern. Statin-induced cholestasis may also occur but is very rare. In cases of suspected statin-induced liver injury, a complete medical history, laboratory tests including a complete metabolic panel, autoimmune markers, and a viral panel, as well as hepatic imaging, are crucial for a complete causality analysis with validated tools such as RUCAM. Liver biopsy may be required if noninvasive tests are inconclusive. Discontinuation of the offending statin is important for both preventing progression of liver injury as well as monitoring subsequent hepatic injury evolution. For the latter reason, it is included in the RUCAM scoring system, as the rate of liver injury resolution strengthens or weakens the case for causality. Statin rechallenge is helpful in determining causality, but may not be feasible in most cases. Fortunately, severe liver injury is uncommon with statin use and is generally reversible without any intervention other than drug cessation. Suspicion of stain-induced liver injury can lead to early recognition and appropriate intervention to prevent severe injury.
Acknowledgments
This work was made possible by the Herman Lopata Chair in Hepatitis Research.
Abbreviations
- AIH
autoimmune hepatitis
- ALP
alkaline phosphatase
- ALT
alanine aminotransferase
- AMA
antimitochondrial antibody
- ANA
antinuclear antibody
- ASMA
anti-smooth muscle antibody
- AST
aspartate aminotransferase
- Bili
bilirubin
- DILI
drug-induced liver injury
- HMG-CoA
hydroxymethyl glutaryl coenzyme A reductase
- HMGCR
3-hydroxy-3-methylglutaryl-CoA reductase
- IAHG
International Autoimmune Hepatitis Group
- RUCAM
Roussel Uclaf Causality Assessment Method
- ULN
upper limit of normal
References
- 1.Lee MY, Sattar N, McMurray JV, Packard CJ. Statins in the Prevention and Treatment of Heart Failure: a Review of the Evidence. Curr Atheroscler Rep. 2019;21(10):41. doi: 10.1007/s11883-019-0800-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Smiley III WH, Khan BV, Sperling LS. Management of the statin-intolerant patient. Curr Treat Options Cardiovasc Med. 2009;11(4):263–271. doi: 10.1007/s11936-009-0027-3. [DOI] [PubMed] [Google Scholar]
- 3.Bhardwaj SS, Chalasani N. Lipid-lowering agents that cause drug-induced hepatotoxicity. Clin Liver Dis. 2007;11(7):597–613. doi: 10.1016/j.cld.2007.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jose J. Statins and its hepatic effects: Newer data, implications, and changing recommendations. J Pharm Bioallied Sci. 2016;8(1):23–28. doi: 10.4103/0975-7406.171699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thapar M, Russo MW, Bonkovsky HL. Statins and liver injury. Gastroenterol Hepatol (N Y) 2013;9(9):605–606. [PMC free article] [PubMed] [Google Scholar]
- 6.Gillett RC, Norrell A. Considerations for safe use of statins: Liver enzyme abnormalities and muscle toxicity. Am Fam Physician. 2011;83:711–716. [PubMed] [Google Scholar]
- 7.Björnsson E, Jacobsen EI, Kalaitzakis E. Hepatotoxicity associated with statins: reports of idiosyncratic liver injury post-marketing. J Hepatol. 2012;56(2):374–380. doi: 10.1016/j.jhep.2011.07.023. [DOI] [PubMed] [Google Scholar]
- 8.Hussaini SH, Farrington EA. Idiosyncratic drug-induced liver injury: an overview. Expert Opin Drug Saf. 2007;6(6):673–684. doi: 10.1517/14740338.6.6.673. [DOI] [PubMed] [Google Scholar]
- 9.Björnsson ES. Epidemiology and risk factors for idiosyncratic drug-induced liver injury. Semin Liver Dis. 2014;34(2):115–122. doi: 10.1055/s-0034-1375953. [DOI] [PubMed] [Google Scholar]
- 10.Mohamed MH, Salameh OK, Saeed AM. Statin-Induced Rhabdomyolysis, Acute Kidney Injury, and Hepatitis Leading to Death. Am J Case Rep. 2019;20:709–712. doi: 10.12659/AJCR.914707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kawasaki E, Fukuyama T, Kuriyama E, Uchida A, Sagara Y, Tamai H, et al. Statin-induced autoimmune hepatitis in patients with type 1 diabetes: A report of two cases and literature review. J Diabetes Investig. 2020;11(6):1673–1676. doi: 10.1111/jdi.13271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Khan AA, Ahmed S, Mohammed A, Elzouki AY. Autoimmune-like Drug-induced Liver Injury Caused by Atorvastatin and Demonstration of the Safety Profile of Pravastatin: A Case Report and Literature Review. Cureus. 2020;12(3):e7299. doi: 10.7759/cureus.7299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sánchez M, Castiella A, Zapata E, Zubiaurre L, Pérez-Yeboles J, Mendibil L, et al. Autoimmune Hepatitis (Immune-Mediated Liver Injury) Induced By Rosuvastatin. Gastroenterol Hepatol. 2018;41(5):311–313. doi: 10.1016/j.gastrohep.2017.05.010. [DOI] [PubMed] [Google Scholar]
- 14.Saha A, Garg A. Severe Liver Injury Associated With High-Dose Atorvastatin Therapy. J Investig Med High Impact Case Rep. 2021;9:23247096211014050. doi: 10.1177/23247096211014050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shah J, Lingiah V, Pyrsopoulos N, Galan M. Acute Liver Injury in a Patient Treated With Rosuvastatin: A Rare Adverse Effect. Gastroenterology Res. 2019;12(5):263–266. doi: 10.14740/gr1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vishwakarma P, Nehra R, Kumar A. Acute hepatic injury with atorvastatin: an unusual occurrence. Indian J Pharmacol. 2014;46(3):343–344. doi: 10.4103/0253-7613.132197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu Y, Cheng Z, Ding L, Fang F, Cheng KA, Fang Q, et al. Atorvastatin-induced acute elevation of hepatic enzymes and the absence of cross-toxicity of pravastatin. Int J Clin Pharmacol Ther. 2010;48(12):798–802. doi: 10.5414/cpp48798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Famularo G, Miele L, Minisola G, Grieco A. Liver toxicity of rosuvastatin therapy. World J Gastroenterol. 2007;13(8):1286–1288. doi: 10.3748/wjg.v13.i8.1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Al-Kindi SG, Zidar DA, McComsey GA, Longenecker CT. Gender Differences in Statin Prescription Rate Among Patients Living With HIV and Hepatitis C Virus. Clin Infect Dis. 2016;63(7):993–994. doi: 10.1093/cid/ciw448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ballo P, Balzi D, Barchielli A, Turco L, Franconi F, Zuppiroli A. Gender differences in statin prescription rates, adequacy of dosing, and association of statin therapy with outcome after heart failure hospitalization: a retrospective analysis in a community setting. Eur J Clin Pharmacol. 2016;72(3):311–319. doi: 10.1007/s00228-015-1980-2. [DOI] [PubMed] [Google Scholar]
- 21. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-. Roussel Uclaf Causality Assessment Method (RUCAM) in Drug Induced Liver Injury. [Updated 2019 May 4]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK548272/ [PubMed]
- 22.Averbukh LD, Wu GY. Role of Biologics in the Development of Autoimmune Hepatitis: A Review. J Clin Transl Hepatol. 2018;6(4):402–409. doi: 10.14218/JCTH.2018.00039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Björnsson E, Talwalkar J, Treeprasertsuk S, Kamath PS, Takahashi N, Sanderson S, et al. Drug-induced autoimmune hepatitis: clinical characteristics and prognosis. Hepatology. 2010;51(6):2040–2048. doi: 10.1002/hep.23588. [DOI] [PubMed] [Google Scholar]
- 24.Yilmaz A, Reiss C, Weng A, Cicha I, Stumpf C, Steinkasserer A, et al. Differential effects of statins on relevant functions of human monocyte-derived dendritic cells. J Leukoc Biol. 2006;79(3):529–538. doi: 10.1189/jlb.0205064. [DOI] [PubMed] [Google Scholar]
- 25.Chi G, Feng XX, Ru YY, Xiong T, Gao Y, Wang H, et al. TLR2/4 ligand-amplified liver inflammation promotes initiation of autoimmune hepatitis due to sustained IL-6/IL-12/IL-4/IL-25 expression. Mol Immunol. 2018;99:171–181. doi: 10.1016/j.molimm.2018.05.005. [DOI] [PubMed] [Google Scholar]
- 26.Castiella A, Zapata E, Lucena MI, Andrade RJ. Drug-induced autoimmune liver disease: A diagnostic dilemma of an increasingly reported disease. World J Hepatol 2014;27. 6(4):160–168. doi: 10.4254/wjh.v6.i4.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Licata A, Giammanco A, Minissale MG, Pagano S, Petta S, Averna M. Liver and Statins: A Critical Appraisal of the Evidence. Curr Med Chem. 2018;25(42):5835–5846. doi: 10.2174/0929867325666180327095441. [DOI] [PubMed] [Google Scholar]
- 28.Kim RG, Loomba R, Prokop LJ, Singh S. Statin Use and Risk of Cirrhosis and Related Complications in Patients With Chronic Liver Diseases: A Systematic Review and Meta-analysis. Clin Gastroenterol Hepatol. 2017;15(10):1521–1530.e8. doi: 10.1016/j.cgh.2017.04.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jiménez-Alonso J, Osorio JM, Gutiérrez-Cabello F, López de la Osa A, León L, Mediavilla García JD. Atorvastatin-induced cholestatic hepatitis in a young woman with systemic lupus erythematosus. Grupo Lupus Virgen de las Nieves. Arch Intern Med. 1999;159(15):1811–1812. doi: 10.1001/archinte.159.15.1811-a. [DOI] [PubMed] [Google Scholar]
- 30.Hartleb M, Rymarczyk G, Januszewski K. Acute cholestatic hepatitis associated with pravastatin. Am J Gastroenterol. 1999;94(5):1388–1390. doi: 10.1111/j.1572-0241.1999.01091.x. [DOI] [PubMed] [Google Scholar]
- 31.Ridruejo E, Mandó OG. Acute cholestatic hepatitis after reinitiating treatment with atorvastatin. J Hepatol. 2002;37(1):165–166. doi: 10.1016/s0168-8278(02)00092-2. [DOI] [PubMed] [Google Scholar]
- 32.Perger L, Kohler M, Fattinger K, Flury R, Meier PJ, Pauli-Magnus C. Fatal liver failure with atorvastatin. J Hepatol. 2003;39(6):1095–1097. doi: 10.1016/s0168-8278(03)00464-1. [DOI] [PubMed] [Google Scholar]
- 33.de Castro ML, Hermo JA, Baz A, de Luaces C, Pérez R, Clofent J. Hepatitis colestásica aguda tras la reintroducción de atorvastatina [Acute cholestatic hepatitis after atorvastatin reintroduction] . Gastroenterol Hepatol. 2006;29(1):21–24. doi: 10.1157/13083248. Spanish. [DOI] [PubMed] [Google Scholar]
- 34.Vergura M, Tomaiuolo P. Cholestatic hepatitis induced by atorvastastin: a Case Report. Italian Journal of Medicine. 2007;1(1):29–31. doi: 10.4081/itjm.2007.1.29. [DOI] [Google Scholar]
- 35.Ferrell LD, Kakar S, editors. Liver Pathology. 1st ed. Demos Medical Publishing; 2011. [Google Scholar]
- 36.Kerzner S, Irabagon N, Berkelhammer C. Statin-induced cholestatic hepatitis: confirmed on rechallenge. Gastroenterol Hepatol (N Y) 2013;9(9):603–605. [PMC free article] [PubMed] [Google Scholar]
- 37.Alanazi NS, Alenazi TS, Alenzi KA. Hepatotoxicity Induced by Fluvastatin: A Reversible Acute Cholestatic Liver Injury. Am J Case Rep. 2021;12(22):e931418. doi: 10.12659/AJCR.931418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xu Y, Wu Y. Atorvastatin associated with gamma glutamyl transpeptidase elevation in a hyperlipidemia patient: A case report and literature review. Medicine (Baltimore) 2020;99(40):e22572. doi: 10.1097/MD.0000000000022572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kuniyoshi N, Miyakawa H, Matsumoto K, Tsunashima H, Sekine K, Tsujikawa T, et al. Detection of Anti-mitochondrial Antibodies Accompanied by Drug-induced Hepatic Injury due to Atorvastatin. Intern Med. 2019;58(18):2663–2667. doi: 10.2169/internalmedicine.2708-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Perdices EV, Medina-Cáliz I, Hernando S, Ortega A, Martín-Ocaña F, Navarro JM, et al. Hepatotoxicity associated with statin use: analysis of the cases included in the Spanish Hepatotoxicity Registry. Rev Esp Enferm Dig. 2014;106(4):246–254. [PubMed] [Google Scholar]
- 41.Russo MW, Hoofnagle JH, Gu J, Fontana RJ, Barnhart H, Kleiner DE, et al. Spectrum of statin hepatotoxicity: experience of the drug-induced liver injury network. Hepatology. 2014;60(2):679–686. doi: 10.1002/hep.27157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gu Q, Paulose-Ram R, Burt VL, Kit BK. Prescription cholesterol-lowering medication use in adults aged 40 and over: United States, 2003-2012. NCHS Data Brief. 2014;(177):1–8. [PubMed] [Google Scholar]
- 43.Chitturi S, Farrell GC. Drug-induced cholestasis. Semin Gastrointest Dis. 2001;12(2):113–124. [PubMed] [Google Scholar]
- 44.Merli M, Bragazzi MC, Giubilo F, Callea F, Attili AF, Alvaro D. Atorvastatin-induced prolonged cholestasis with bile duct damage. Clin Drug Investig. 2010;30(3):205–209. doi: 10.2165/11531660-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 45.Kuver R. Effects of statins on cholestasis: good, bad or indifferent? J Gastroenterol Hepatol. 2011;26(10):1467–1469. doi: 10.1111/j.1440-1746.2011.06836.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Golomb BA, Evans MA. Statin adverse effects: a review of the literature and evidence for a mitochondrial mechanism. Am J Cardiovasc Drugs. 2008;8(6):373–418. doi: 10.2165/0129784-200808060-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Karahalil B, Hare E, Koç G, Uslu İ, Şentürk K, Özkan Y. Hepatotoxicity associated with statins. Arh Hig Rada Toksikol. 2017;68(4):254–260. doi: 10.1515/aiht-2017-68-2994. [DOI] [PubMed] [Google Scholar]
- 48.Busanello ENB, Marques AC, Lorza-Gil E, Oliveira HCF, Vercesi AE. Mitochondrial Diseases. 2018. Mitochondrial oxidative stress and calcium-dependent permeability transition are key players in the mechanisms of statins-associated side effects. [DOI] [Google Scholar]
- 49.Alla V, Abraham J, Siddiqui J, Raina D, Wu GY, Chalasani NP, et al. Autoimmune hepatitis triggered by statins. J Clin Gastroenterol. 2006;40(8):757–761. doi: 10.1097/00004836-200609000-00018. [DOI] [PubMed] [Google Scholar]
- 50.Licata A, Giammanco A, Minissale MG, Pagano S, Petta S, Averna M. Liver and Statins: A Critical Appraisal of the Evidence. Curr Med Chem. 2018;25(42):5835–5846. doi: 10.2174/0929867325666180327095441. [DOI] [PubMed] [Google Scholar]
- 51.Kim RG, Loomba R, Prokop LJ, Singh S. Statin Use and Risk of Cirrhosis and Related Complications in Patients With Chronic Liver Diseases: A Systematic Review and Meta-analysis. Clin Gastroenterol Hepatol. 2017;15(10):1521–1530.e8. doi: 10.1016/j.cgh.2017.04.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hu PF, Xie WF. Corticosteroid therapy in drug-induced liver injury: Pros and cons. J Dig Dis. 2019;20(3):122–126. doi: 10.1111/1751-2980.12697. [DOI] [PubMed] [Google Scholar]