Abstract
Ewing’s sarcoma (ES) is a tumor that often occurs in the long bones and rarely arises from visceral organs primarily. Here, we report a case of primary hepatic ES, discuss its computed tomography (CT) and gadobenate dimeglumine-enhanced magnetic resonance (MRI) features. This is the first Chinese and fifth primary hepatic ES case reported, based on a literature review. Imaging examinations showed that the tumor was solid, with necrosis and hemorrhage. Contrast-enhanced images showed that the tumor was hypervascular and especially had heterogeneous signal intensity on hepatobiliary phase MRI images. Intratumoral vessels and vascular invasion were also present.
Keywords: Ewing’s sarcoma, Primitive neuroectodermal tumor, CT, MRI, Literature review
Introduction
Ewing’s sarcoma (ES) is also known as primitive neuroectodermal tumor (PNET) because of the overlap of their genetic abnormalities. ES/PNET, together with skin tumors and atypical ES, are members of the ES tumor family.1,2 ES usually occurs in the long bones of the extremities and in the pelvic bones, and extraosseous ES can occur in the deep soft tissue around the extremities, chest wall, retroperitoneum, and solid organs, including the pancreas, kidney, uterus, ovary, gastrointestinal tract, and other visceral organs.3–8 Primary hepatic ES is uncommon, and only four cases of this disease have been reported.6,7,9,10 Herein, we present a case of primary hepatic ES/PNET and describe the computed tomography (CT) and gadobenate dimeglumine-enhanced magnetic resonance imaging (MRI) features of the tumor, with an accompanying review of the literature.
Case report
Patient information
A 27-year-old woman presented with severe epigastric pain for 20 days. The patient had an unremarkable medical and family history, and the physical examination was negative. After admission, routine laboratory examinations, including serum glutamic oxaloacetic transaminase (AST), glutamic pyruvic transaminase (ALT), and bilirubin index, were normal. However, serum lactate dehydrogenase was 320 U/L (normal range: 120–250 U/L). Viral serology tests for hepatitis B virus (HBV)/ hepatitis C virus (HCV) were negative. The serum tumor biomarker test revealed that the cancer antigen 125 (CA125) level was 44.5 U/mL (normal range: 0–35 U/mL), while alpha-fetoprotein (AFP) and cancer antigen 19-9 (CA19-9) levels were normal. Further laboratory investigations showed slightly increased monocytes (0.650 109/L; normal range: 0.10–0.60 109/L), a slightly decreased lymphocyte rate (18.1%; normal range: 20–50%), and decreased albumin (3.67 g/dL; normal range: 4–5.5 g/dL) and prealbumin (14.9 mg/dL; normal range: 16–45 mg/dL).
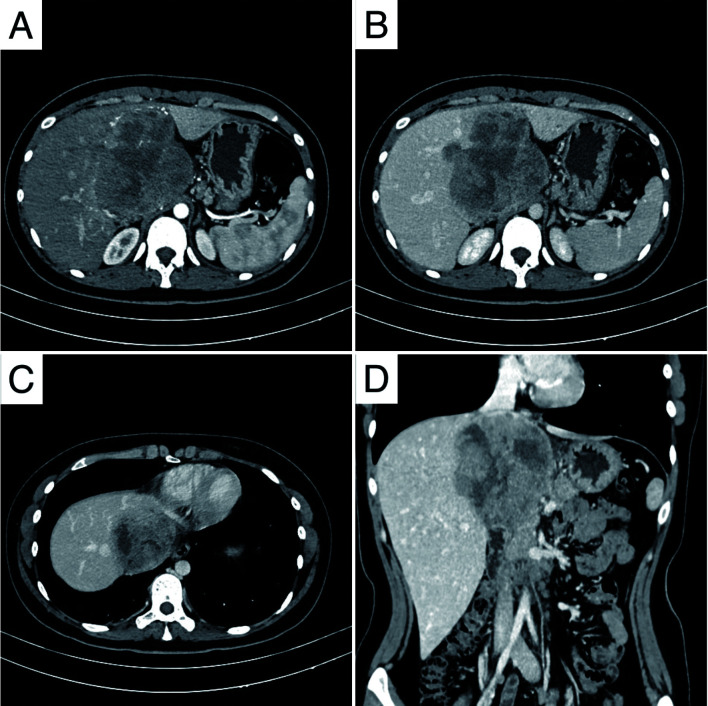
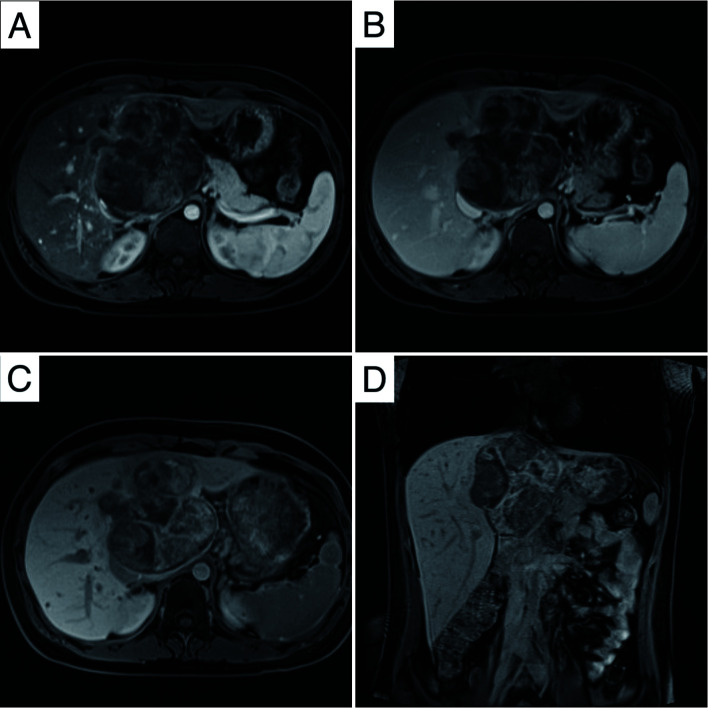
The imaging examination, including contrast-enhanced CT, indicated a heterogeneous, solid mass with areas of necrosis measuring 9.6×9.1×10 cm in the hepatic caudate lobe. The mass showed obvious heterogeneous enhancement with multiple tortuous vessels in the arterial phase and persistent enhancement with mild dilation of distal hepatic ducts in the portal venous phase (Fig. 1). The mass extended to the portacaval space and upward to invade the second porta hepatis, including the inferior vena cava (IVC) and the roots of the right hepatic vein (RHV), the middle hepatic vein (MHV), and the left hepatic vein (LHV). The left branch of the portal vein (PV) was also possibly invaded. On MRI, the tumor was hypointense on the T1 weighted image (T1WI) and heterogeneously hyperintense on the T2 weighted image (T2WI), showing areas of hemorrhage and necrosis. Diffusion-weighted imaging (DWI) also showed hyperintensity. After injection of gadobenate dimeglumine, the mass showed obvious heterogeneous enhancement in the arterial phase and persistent enhancement in the portal venous and delayed phases. In the hepatobiliary phase (HBP), the tumor showed heterogeneous signal intensity (SI) (Fig. 2). Both CT and MRI prompted suspicion of mesenchymal neoplasm, such as sarcoma, in the caudate lobe of the liver. A search for any other sites of involvement of the tumor using whole-body bone scan and positron emission tomography (PET) showed no abnormality.
Fig. 1. Contrast-enhanced CT scans of the hepatic ES.
(A–B) Obvious heterogeneous enhancement with multiple serpentine neovascular (axial arterial phase). (C) Persistent enhancement with mild dilation of distal hepatic ducts (portal venous phase). (D) IVC involvement (coronal portal venous phase image). IVC, inferior vena cava.
Fig. 2. Gadobenate dimeglumine-enhanced MRI of the hepatic ES.
(A) Hypointense (axial T1WI) image. (B) Heterogeneous hyperintense (axial T2WI) image. (C) Diffusion restriction in DWI. (D) IVC involvement (coronal image). T1WI, T1 weighted image; T2WI, T2 weighted image; DWI, diffusion-weighted imaging; IVC, inferior vena cava.
Surgical procedure
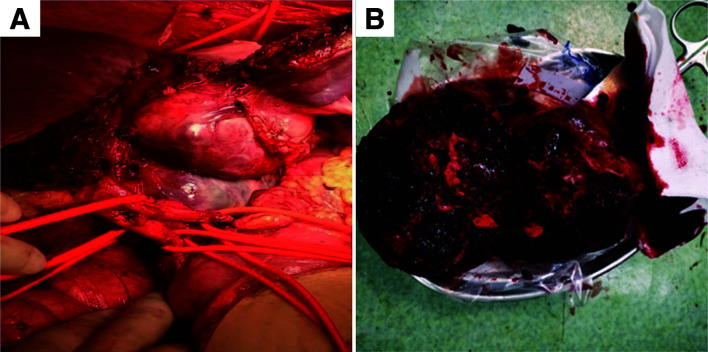
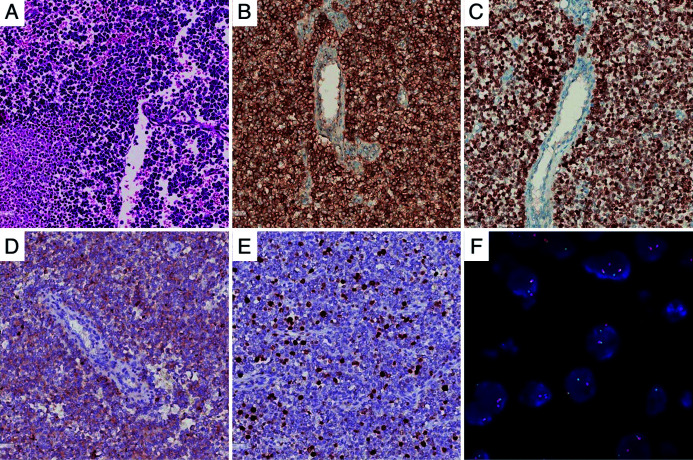
A laparoscopic exploration was performed and showed the mass located in the caudate lobe, 10×11 cm in size and involving segments 2/3/5/8 of the liver, the diaphragm, second porta hepatis, RHV, MHV, and IVC (Fig. 3). On the cut surface, the tumor was grayish red and hard, with necrosis. On microscopic examination, the tumor was composed of small blue tumor cells with necrosis. Immunohistochemical examination revealed positive expression for CD99 and NKX2.2 and weak positivity for synuclein (Syn) and Ki-67 (the positive rate was approximately 20%) (Fig. 4). Dual-color, break-apart probe fluorescence in situ hybridization (FISH) examination revealed that more than 30% of the cells (200 counted cells per slide) exhibited one yellow and one red signal, which indicated a break of the Ewing sarcoma breakpoint region 1 (EWSR1) locus (Fig. 4). These findings supported a diagnosis of localized ES arising from the liver. However, the patient refused standard postoperative chemotherapy and/or radiotherapy as adjuvant treatments. She is currently alive (3 months postoperatively) without any signs of recurrence.
Fig. 3. Intraoperative findings.
(A) The tumor originated from the hepatic caudate lobe. (B) The tumor was greyish red and hard, with necrosis.
Fig. 4. Pathological and immunohistochemical staining.
(A) The tumor was composed of hypercellular small, blue-colored, round cells microscopically (HE, 200×). (B) Strong positive staining for CD 99 (IHC, 200×). (C) Strong positive staining for NKX2.2 (IHC, 200×). (D) Weak positive staining for Syn (IHC, 200×). (E) Weak positive staining for Ki-67 (IHC, 200×). (F) Dual-color, break-apart probe FISH examination, showed one yellow and one red signal, which indicated a break of the EWSR1 locus. HE, hematoxylin and eosin; CD 99, Cluster of Differentiation 99; NKX2.2, NK2 homeobox 2; IHC, immunohistochemistry; FISH, fluorescence in situ hybridization; EWSR1, Ewing sarcoma breakpoint region 1.
Literature review
A literature search was initiated to review cases of primary hepatic ES. Based on the literature review, only four prior cases of primary hepatic ES have been reported. Following the previous reports and our case presented herein, 80% of patients with ES were younger than 20 years, and all patients with hepatic ES were younger than 30 years. The clinical symptoms of ES in the liver are nonspecific, with abdominal pain being the most common. Uncommon symptoms include abdominal distention, nausea, emesis, and diarrhea (Table 1).
Table 1. Clinical and pathological features of ES/PNET of the liver.
| Case | Age | Sex | Size | Site | Necrosis | Hemorrhage | Initial diagnosis | Treatment | Pathology by IHC | Follow-up duration | Recurrence | Metastasis | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 18 | Male | 21 cm | Right lobe | Yes | No | N/A | Right hepatic artery embolization and hepatectomy | CD99(+) | 1 month | No | Lung | Death |
| 2 | 20 | Female | 28 cm | Whole liver | No | No | Hepatomegaly | Chemotherapy | Mic2(+), FLI-1(+), CD99(+) | 1 year | No | No | Survival |
| 3 | 18 | Male | 13×8×7 cm | Segments 5 and 6 | Yes | No | Cystadenocarcinoma or hepatocellular carcinoma | Tumor resection and chemotherapy | PAS(+), NSE(+) | N/A | No | No | N/A |
| 4 | 27 | Female | 8.2×6.6 cm | Segment 8 | N/A | N/A | Mucinous cystadenoma | Hepatectomy and chemotherapy | PAS(+), CD99(+), NKX2.2(+), CD56(+), Syn(+) | 15 months | No | No | Survival |
| Present case | 27 | Female | 10×11 cm | Caudate lobe | Yes | Yes | Sarcoma | Hepatectomy | CD99(+), NKX2.2(+) | 2 months | No | No | Survival |
IHC, immunohistochemistry; D99, Cluster of Differentiation 99; CD56, Cluster of Differentiation 56; FLI-1, friend leukemia virus integration 1; PAS, Periodic Acid-Schiff; NKX2.2, NK2 homeobox 2; Syn, synaptophysin.
Discussion and conclusions
Due to the rarity of the tumor, little information on the imaging features of hepatic ES is available. Relying on CT and ultrasound findings, two previous reports only described the tumor as solid, with one report using CT to describe the tumor as a multilocular cystic mass with enhanced septa, and the other to describe an enlarged liver without a mass lesion.9–12 To the best of our knowledge, this is the first case of both CT and gadobenate dimenglumine-enhanced MRI features of ES in the liver.
In the present case, the tumor was solid, with necrosis and hemorrhage, as shown by CT and MRI imaging. The tumor also showed diffusion restriction in DWI. After enhancement, the tumor was hyper vascular, with prominent intratumoral vessels in the arterial phase and persistent enhancement to the portal venous phase and delayed phase from dynamic MRI scans. The tumor was aggressive, based on vascular invasion demonstrated by CT and MRI. On HBP, the tumor showed heterogeneous SI, which was only partly due to intratumoral hyperintensity representing intralesional hemorrhage. These findings suggested the tumor to be non-HCC or –ICC but raised a suspicion of sarcoma.
With similar clinical, immunohistochemical and cytogenetic profiles, ES and PNET are regarded as two extremes of a morphologic spectrum of the same tumor entity. ES/PNETs are divided into two main categories, according to the cell origin and location. Central PNETs are derived from the neural tube, mainly involving the brain and spinal cord. Peripheral PNETs are derived from the neural crest and occur outside the central nervous system, often involving the sympathetic nervous system or soft tissue and bones.4,13–16
In children, approximately 80% of ES are found in bones and <20% in soft tissues, while in adults, >50% of ES occur in soft tissues17 but ES rarely affects visceral organs. When visceral involvement does occur, the most common affected organ is the kidney.18–20 The liver is a rather rare organ of involvement.
The gross appearance of the tumor is usually multilobulated, soft, and friable, and it usually exceeds 10 cm in its largest dimension in the liver. Among the previous reports, only one presented a tumor <10 cm in its largest dimension.11 The tumors could be solid, cystic, or diffusely enlarged to involve the entire liver, but most of the tumors reported have been solid, with or without areas of necrosis and hemorrhage. Histologically, ES is composed of poorly differentiated small round cells containing dark staining and round or oval nuclei.9 Special stains, such as periodic acid-Schiff (PAS), usually show positivity for cytoplasmic glycogen,10 and immunostains usually show positivity for CD99, vimentin and NKX2.2.21 However, these findings are not specific to ES, so molecular biological examination is recommended to confirm the disease. FISH or real-time polymerase chain reaction (PCR) tests have demonstrated that most ES’s harbor the EWSR1-ETS fusion protein, which results in a chromosomal translocation t (11:22) between the EWS (22q12) and friend leukemia virus integration 1 (FLI-1) (11q24) genes.3–5
The major differential diagnosis for hepatic ES includes carcinosarcoma, angiosarcoma, leiomyosarcoma, and undifferentiated embryonal sarcoma. Carcinosarcoma is a rare malignant tumor containing both carcinomatous and sarcomatous components. It is more common in elderly males, and most patients have elevated tumor markers, including CEA, AFP, and CA19-9. Tumors usually show heterogeneous density/intensity accompanied by vast cystic changes and necrosis with moderate to clearly-irregular marginal enhancement.22,23 Angiosarcoma is the most common malignant mesenchymal tumor of the liver, accounting for <2% of primary hepatic tumors.24,25 It typically occurs in males in the fifth to seventh decade of life. Tumors are multifocal and have heterogeneous signals on T2WI and HBP images with intralesional hemorrhage. On dynamic scans, angiosarcoma demonstrates at least some extent of progressive enhancement with enhancing foci of irregular or rim-like nodular/linear shape, or bizarre shapes.26,27 Hepatic leiomyosarcoma derives from smooth muscle cells in hepatic vessels, bile ducts, or ligaments.28 The patient ages at the time of tumor detection ranged from 5 months to 86 years, with no obvious sex difference.29–32 On CT, the tumors are hypodense, with necrosis or bleeding, and heterogeneous enhancement. On MRI, the tumors are hypointense on T1WI and heterogeneous hyperintense on T2WI with heterogeneous enhancement.29,31,33,34 Undifferentiated embryonal sarcoma of the liver occurs mostly in children aged 6 to 10 years and rarely in adults.35,36 On CT scan, the tumor appears as a solitary, well-defined cystic mass with solid nodules and septations, showing progressive enhancement. Tortuous vessels within the tumor may be observed.35,37,38
The standard treatment plan for extraosseous ES has not been established and should correspond to the treatment method for all sarcomas in the Ewing family.39 ES/PNET is highly sensitive to chemotherapy and radiotherapy; however, surgical resection should also be considered for patients with localized extraosseous ES. In line with this, systemic multiagent chemotherapy combined with surgery and/or radiotherapy is recommended.40 Previous studies have shown a 5-year survival of 58–61%, with a median survival of 120 months for patients with PNETs.41,42 Finally, age and surgical treatment are recognized as important prognostic variables in the treatment of extraosseous ES, in particular.10
Abbreviations
- AFP
alpha-fetoprotein
- AST
glutamic oxaloacetic transaminase
- ALT
glutamic pyruvic transaminase
- CA125
cancer antigen 125
- CA19-9
cancer antigen 19-9
- CEA
carcinoembryonic antigen
- CT
computed tomography
- DWI
diffusion-weighted imaging
- ES
Ewing’s sarcoma
- EWSR1
Ewing sarcoma breakpoint region 1
- FISH
fluorescence in situ hybridization
- FLI-1
friend leukemia virus integration 1
- HBP
hepatobiliary phase
- HBV
hepatitis B virus
- HCC
hepatocellular carcinoma
- HCV
hepatitis C virus
- HE
hematoxylin and eosin
- ICC
intrahepatic cholangiocellular carcinoma
- IVC
inferior vena cava
- LHV
left hepatic vein
- MHV
middle hepatic vein
- MRI
magnetic resonance imagining
- PAS
periodic acid-Schiff
- PCR
polymerase chain reaction
- PECT
positron emission computerized tomography
- PET
positron emission tomography
- PNET
primitive neuroectodermal tumor
- PV
portal vein
- RHV
right hepatic vein
- SI
signal intensity
- Syn
synuclein
- T1WI
T1 weighted image
- T2WI
T2 weighted image
Ethical approval and consent for publication
The hospital board committee provided approval for the procedural application in clinic and case report publication. Written informed consent for publication was obtained from the patient.
Data sharing statement
All data generated or analyzed during this study are included in this published article.
References
- 1.Dehner LP. Primitive neuroectodermal tumor and Ewings-sarcoma. Am J Surg Pathol. 1993;17(1):1–13. doi: 10.1097/00000478-199301000-00001. [DOI] [PubMed] [Google Scholar]
- 2.Dehner LP. The evolution of the diagnosis and understanding of primitive and embryonic neoplasms in children: living through an epoch. Mod Pathol. 1998;11(7):669–685. [PubMed] [Google Scholar]
- 3.Li T, Zhang F, Cao Y, Ning S, Bi Y, Xue W, et al. Primary Ewing’s sarcoma/primitive neuroectodermal tumor of the ileum: case report of a 16-year-old Chinese female and literature review. Diagn Pathol. 2017;12(1):37. doi: 10.1186/s13000-017-0626-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Park JY, Lee S, Kang HJ, Kim HS, Park SY. Primary Ewing’s sarcoma-primitive neuroectodermal tumor of the uterus: a case report and literature review. Gynecol Oncol. 2007;106(2):427–432. doi: 10.1097/00005392-200009010-00036. [DOI] [PubMed] [Google Scholar]
- 5.Cheng L, Xu Y, Song H, Huang H, Zhuo D. A rare entity of primary Ewing sarcoma in kidney. BMC Surg. 2020;20(1):280. doi: 10.1186/s12893-020-00948-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Movahedi-Lankarani S, Hruban RH, Westra WH, Klimstra DS. Primitive neuroectodermal tumors of the pancreas - a report of seven cases of a rare neoplasm. Am J Surg Pathol. 2002;26(8):1040–1047. doi: 10.1097/00000478-200208000-00009. [DOI] [PubMed] [Google Scholar]
- 7.Shek TWH, Chan GCF, Khong PL, Chung LP, Cheung ANY. Ewing sarcoma of the small intestine. Pediatr Hematol Oncol. 2001;23(8):530–532. doi: 10.1097/00043426-200111000-00013. [DOI] [PubMed] [Google Scholar]
- 8.Kleinman GM, Young RH, Scully RE. Primary neuroectodermal tumors of the ovary - a report of 25 cases. Am J Surg Pathol. 1993;17(8):764–778. doi: 10.1097/00000478-199308000-00002. [DOI] [PubMed] [Google Scholar]
- 9.Cambruzzi E, Guerra EE, Hilgert HC, Schmitz HJ, Silva VL, Milani DM, et al. Primitive neuroectodermal tumor of the liver: a case report. Case Rep Med. 2011;2011:748194. doi: 10.1155/2011/748194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang SF, Chiang JH, Jan HC, Chou SJ, Chen TK, Chen TH. Intra-abdomen Ewing’s sarcoma. ANZ J Surg. 2011;81(5):377–378. doi: 10.1111/j.1445-2197.2011.05696.x. [DOI] [PubMed] [Google Scholar]
- 11.Ozaki Y, Miura Y, Koganemaru S, Suyama K, Inoshita N, Fujii T, et al. Ewing sarcoma of the liver with multilocular cystic mass formation: a case report. BMC Cancer. 2015;15:16. doi: 10.1186/s12885-015-1017-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mani S, Dutta D, De BK. Primitive neuroectodermal tumor of the liver: a case report. Jpn J Clin Oncol. 2010;40(3):258–262. doi: 10.1093/jjco/hyp158. [DOI] [PubMed] [Google Scholar]
- 13.Colovic RB, Grubor NM, Micey MT, Matic SV, Atkinson HDE, Latincic SM. Perigastric extraskeletal Ewing’s sarcoma: a case report. World J Gastroenterol. 2009;15(2):245–247. doi: 10.3748/wjg.15.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Welsch T, Mechtersheimer G, Aulmann S, Mueller SA, Buechler MW, Schmidt J, et al. Huge primitive neuroectodermal tumor of the pancreas: report of a case and review of the literature. World J Gastroenterol. 2006;12(37):6070–6073. doi: 10.3748/wjg.v12.i37.6070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Doi H, Ichikawa S, Hiraoka A, Ichiryu M, Nakahara H, Ochi H, et al. Primitive neuroectodermal tumor of the pancreas. Intern Med. 2009;48(5):329–333. doi: 10.2169/internalmedicine.48.1484. [DOI] [PubMed] [Google Scholar]
- 16.O’Sullivan MJ, Perlman EJ, Furman J, Humphrey PA, Dehner LP, Pfeifer JD. Visceral primitive peripheral neuroectodermal tumors: a clinicopathologic and molecular study. Hum Pathol. 2001;32(10):1109–1115. doi: 10.1053/hupa.2001.28247. [DOI] [PubMed] [Google Scholar]
- 17.Murray FB, Cristina RA, Robert GM. Management of soft tissue sarcoma. New York: Springer; 2013. p. 222. [Google Scholar]
- 18.Karnes RJ, Gettman MT, Anderson PM, Lager DJ, Blute ML. Primitive neuroectodermal tumor (extraskeletal Ewing’s sarcoma) of the kidney with vena caval tumor thrombus. J Urol. 2000;164(3 Pt 1):772. doi: 10.1097/00005392-200009010-00036. [DOI] [PubMed] [Google Scholar]
- 19.Marley EF, Liapis H, Humphrey PA, Nadler RB, Siegel CL, Zhu XP, et al. Primitive neuroectodermal tumor of the kidney - another enigma: a pathologic, immunohistochemical, and molecular diagnostic study. Am J Surg Pathol. 1997;21(3):354–359. doi: 10.1097/00000478-199703000-00013. [DOI] [PubMed] [Google Scholar]
- 20.Sheaff M, McManus A, Scheimberg I, Paris A, Shipley J, Baithun S. Primitive neuroectodermal tumor of the kidney confirmed by fluorescence in situ hybridization. Am J Surg Pathol. 1997;21(4):461–468. doi: 10.1097/00000478-199704000-00013. [DOI] [PubMed] [Google Scholar]
- 21.Yoshida A, Sekine S, Tsuta K, Fukayama M, Furuta K, Tsuda H. NKX2.2 is a useful immunohistochemical marker for Ewing sarcoma. Am J Surg Pathol. 2012;36(7):993–999. doi: 10.1097/PAS.0b013e31824ee43c. [DOI] [PubMed] [Google Scholar]
- 22.Bin F, Chen Z, Liu P, Liu J, Mao Z. The clinicopathological and imaging characteristics of primary hepatic carcinosarcoma and a review of the literature. J Hepatocell Carcinoma. 2020;7:169–180. doi: 10.2147/jhc.S272768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li J, Liang P, Zhang D, Liu J, Zhang H, Qu J, et al. Primary carcinosarcoma of the liver: imaging features and clinical findings in six cases and a review of the literature. Cancer Imaging. 2018;18(1):7. doi: 10.1186/s40644-018-0141-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Buetow PC, Buck JL, Ros PR, Goodman ZD. Malignant vascular tomors of the liver - radiologic-pathlolgicalcorrelation. Radiographics. 1994;14(1):153–166. doi: 10.1148/radiographics.14.1.8128048. [DOI] [PubMed] [Google Scholar]
- 25.Alrenga DP. Primary angiosarcoma of the liver. Review article. Int Surg. 1975;60(4):198–203. [PubMed] [Google Scholar]
- 26.Kim B, Byun JH, Lee JH, Park BJ, Kwon HJ, Lee JH, et al. Imaging findings of primary hepatic angiosarcoma on gadoxetate disodium-enhanced liver MRI: comparison with hepatic haemangiomas of similar size. Clin Radiol. 2018;73(3):244–253. doi: 10.1016/j.crad.2017.09.015. [DOI] [PubMed] [Google Scholar]
- 27.Ehman EC, Torbenson MS, Wells ML, Welch BT, Thompson SM, Garg I, et al. Hepatic tumors of vascular origin: imaging appearances. Abdom Radiol (NY) 2018;43(8):1978–1990. doi: 10.1007/s00261-017-1401-3. [DOI] [PubMed] [Google Scholar]
- 28.Gohrbandt AE, Hansen T, Ell C, Heinrich SS, Lang H. Portal vein leiomyosarcoma: a case report and review of the literature. BMC Surg. 2016;16(1):60. doi: 10.1186/s12893-016-0174-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chi M, Dudek AZ, Wind KP. Primary hepatic leiomyosarcoma in adults: analysis of prognostic factors. Onkologie. 2012;35(4):210–214. doi: 10.1159/000337416. [DOI] [PubMed] [Google Scholar]
- 30.Tsai PS, Yeh TC, Shih SL. Primary hepatic leiomyosarcoma in a 5-month-old female infant. Acta Radiol Short Rep. 2013;2(7):2047981613498722. doi: 10.1177/2047981613498722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shivathirthan N, Kita J, Iso Y, Hachiya H, KyungHwa P, Sawada T, et al. Primary hepatic leiomyosarcoma: case report and literature review. World J Gastrointest Oncol. 2011;3(10):148–152. doi: 10.4251/wjgo.v3.i10.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu W, Liang W. Primary hepatic leiomyosarcoma presenting as a thick-walled cystic mass resembling a liver abscess a case report. Medicine. 2018;97(51):e13861. doi: 10.1097/md.0000000000013861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Iida T, Maeda T, Amari Y, Yurugi T, Tsukamoto Y, Nakajima F. Primary hepatic leiomyosarcoma in a patient with autosomal dominant polycystic kidney disease. CEN Case Rep. 2017;6(1):74–78. doi: 10.1007/s13730-017-0247-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Metta H, Corti M, Trione N, Masini D, Monestes J, Rizzolo M, et al. Primary hepatic leiomyosarcoma-a rare neoplasm in an adult patient with AIDS: second case report and literature review. J Gastrointest Cancer. 2014;45(Suppl 1):36–39. doi: 10.1007/s12029-013-9525-3. [DOI] [PubMed] [Google Scholar]
- 35.Gabor F, Franchi-Abella S, Merli L, Adamsbaum C, Pariente D. Imaging features of undifferentiated embryonal sarcoma of the liver: a series of 15 children. Pediatr Radiol. 2016;46(12):1694–1704. doi: 10.1007/s00247-016-3670-3. [DOI] [PubMed] [Google Scholar]
- 36.Legou F, Ayav A, Cahn V, Elrifai R, Bruot O, Regent D, et al. Radiologic-pathologic comparison of undifferentiated embryonal sarcoma of the liver in a 61-year-old woman. Diagn Interv Imaging. 2012;93(3):e208–211. doi: 10.1016/j.diii.2012.01.003. [DOI] [PubMed] [Google Scholar]
- 37.Chung EM, Lattin GE, Jr, Cube R, Lewis RB, Marichal-Hernandez C, Shawhan R, et al. From the archives of the AFIP pediatric liver masses: radiologic-pathologic correlation part 2. malignant tumors. Radiographics. 2011;31(2):483–507. doi: 10.1148/rg.312105201. [DOI] [PubMed] [Google Scholar]
- 38.Qiu LL, Yu RS, Chen Y, Zhang Q. Sarcomas of abdominal organs: computed tomography and magnetic resonance imaging findings. Semin Ultrasound CT MRI. 2011;32(5):405–421. doi: 10.1053/j.sult.2011.04.003. [DOI] [PubMed] [Google Scholar]
- 39.Carvajal R, Meyers P. Ewing’s sarcoma and primitive neuroectodermal family of tumors. Hematol Oncol Clin North Am. 2005;19(3):501–25. doi: 10.1016/j.hoc.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 40.Biermann JS, Chow W, Reed DR, Lucas D, Adkins DR, Agulnik M, et al. NCCN guidelines (R) insights bone cancer, version 2.2017 featured updates to the NCCN guidelines. J Natl Compr Canc Netw. 2017;15(2):155–167. doi: 10.6004/jnccn.2017.0017. [DOI] [PubMed] [Google Scholar]
- 41.Jurgens H, Bier V, Harms D, Beck J, Brandeis W, Etspuler G, et al. Malignant peripheral neuroectodermal tumors. a retrospective analysis of 42 patients. Cancer. 1988;61(2):349–357. doi: 10.1002/1097-0142(19880115)61:2<349::Aid-cncr2820610226>3.0.Co;2-0. [DOI] [PubMed] [Google Scholar]
- 42.Smorenburg CH, van Groeningen CJ, Meijer OWM, Visser M, Boven E. Ewing’s sarcoma and primitive neuroectodermal tumours in adults: single-centre experience in the Netherlands. Neth J Med. 2007;65(4):132–136. [PubMed] [Google Scholar]