Abstract
Novel biological agents including cytokines and recombinant fusion proteins are increasingly prescribed for cancer, rheumatologic, autoimmune, and inflammatory diseases, and are currently being evaluated in hepatocellular carcinoma (HCC). They are classified by their mechanism of action and include tumor necrosis factor-alpha (TNF-α) antagonists, T cell mediated antitumor inhibitors, interleukin receptor antagonists, and immune checkpoint inhibitors (ICIs). Some ICIs cause frequent hepatotoxicity with a variable clinical, biochemical, and serological presentation, especially in patients receiving another immunomodulatory agent. Half of the cases of liver damage induced by biological agents spontaneously regress after drug withdrawal, but the others require steroid therapy. Unfortunately, there are no widely accepted recommendation for the use of corticosteroids in these patients, even though international cancer societies have their own guidelines. Differentiating drug-induced autoimmune hepatitis (DIAIH) from classic AIH is challenging for pathologists, but liver biopsy is valuable, particularly in cases with unclear clinical presentation. Interesting, novel histological patterns have been described in liver damage induced by these agents (i.e., endothelitis, ring granuloma and secundary sclerosing cholangitis associated with lymphocytic infiltration of cytotoxic CD8+T cells). Here, we describe the clinical and biochemical characteristics of patients with hepatotoxicity induced by TNF-α antagonists and ICIs. Controversial issues involved in the administration of corticosteroid therapy, and hepatitis B virus (HBV) reactivation induced by immunosuppressive therapy are also discussed.
Keywords: Hepatotoxicity, Checkpoint inhibitors, Biologics, Autoimmune hepatitis, Drug-induced liver injury
Graphical abstract
Introduction
The term biologics derives from the notion that these agents target biological pathways that play a critical pathogenic role in a given disease. They are usually obtained from or produced by a living organism and are used to prevent, diagnose, or treat disease. Most of them are administered by subcutaneous or intramuscular injection or intravenous infusion. Their use has grown steadily over the past years to meet the need to improve the treatment of diverse diseases. Industry-sponsored clinical trials have led to new approvals and expanded indications. Monoclonal antibodies (mAbs) represent one-third of the approximately 300 Food and Drug Administration (FDA)-licensed biologic agents.1 These novel targeted therapies, which also include cytokines and recombinant fusion proteins, are increasingly prescribed for cancer, rheumatologic, autoimmune and inflammatory diseases.
These drugs are classified according their mechanism of action as tumor necrosis factor-alpha (TNF-α) antagonists, T cell mediated antitumor inhibitors, interleukin receptor antagonists, and immune checkpoint inhibitors (ICIs). mAbs are the most commonly used biologics, and are associated with the risk of adverse reactions caused by immunogenic responses. Reports of drug-induced liver injury (DILI) have followed recent approvals and widespread use of most mAbs, and several of them have been included among those with category A or B likelihood scores proposed by the Drug-Induced Liver Injury Network (DILIN, Table 1).2
Table 1. Mechanisms of hepatoxicity and patterns of liver damage associated with treatment by biological agents.
| Category (mechanism of action) | Mechanistic pathway | FDA approval | Indication | Pattern of liver damage |
|---|---|---|---|---|
| Anti-TNF-α | ||||
| Infliximab* | Chimeric monoclonal antibody to human tumor necrosis factor-alpha | 1998 | Rheumatoid arthritis, ankylosing spondylitis, psoriatic arthritis and severe psoriasis, Crohn’s disease and ulcerative colitis | Hepatocellular or cholestatic. Hepatocellular injury with markers and histological findings distinctive of autoimmune hepatitis. Reactivation of chronic hepatitis B. |
| Adalimumab* | Human monoclonal antibody to human tumor necrosis factor-alpha | 2002 | Rheumatoid arthritis, ankylosing spondylitis, juvenile idiopathic (rheumatoid) arthritis, severe psoriasis and psoriatic arthritis, Crohn’s disease and ulcerative colitis | Hepatocellular. Reactivation of chronic hepatitis B |
| Certolizumab** | Humanized monoclonal antibody to human tumor necrosis factor-alpha | 2007 | Crohn’s disease, rheumatoid and psoriatic arthritis and ankylosing spondylitis | Hepatocellular. Reactivation of chronic hepatitis B |
| Golimumab** | Human monoclonal antibody to human tumor necrosis factor-alpha | 2009 | Rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis and ulcerative colitis | Hepatocellular. Reactivation of chronic hepatitis B |
| Immune checkpoint inhibitor | ||||
| Nivolumab* | Human anti-PD-1 | 2014 | Malignant melanoma | Hepatocellular but also mixed, Autoantibodies are rare. Histological findings of immune-mediated hepatic injury. |
| Pembrolizumab** | Humanizedanti-PD-1 | 2014 | Malignant melanoma | Hepatocellular injury usually with no serological markers but with histology demonstrates an hepatitis-like pattern. |
| Ipilimumab* | Human anti-CTLA-4 | 2011 | Malignant melanoma | Most frequently hepatocellular, but also mixed. Histological liver pattern of immune-related hepatitis with pathognomic findings such as fibrin ring granulomas and central vein endothelitis. |
| Atezolizumab | Humanized anti-PDL-1 | 2015 | Non-small cell lung cancer, hepatocellular carcinoma | Hepatocellular injury |
*Score A (well known, described, and reported to cause liver injury with more than 50 cases described), or score B (highly likely to cause liver injury with between 12 and 50 cases described); ‡score D (possible cause of clinically apparent liver injury), and **score E (unproven but suspected cause of clinically apparent liver injury).2 Anti-TNF-α, Anti-tumor necrosis factor-α; Anti-PD-1, Anti-programmed deathprotein-1; Anti-PDL-1, Anti-programmed death-ligand-1; Anti-CTLA-4, Anti- T lymphocyte-associated antigen-4.
This review focuses on current advances in understanding the clinical aspects and mechanisms of DILI induced by anti-TNF-α agents and immune checkpoint inhibitors, which are the most used biologic agents. The contribution of liver biopsy to DILI diagnosis in this clinical setting, the spectrum and distinctive features of liver histology, and controversies associated with corticosteroid treatment are discussed.
DILI induced by TNF-α blocking agents
TNF-α was discovered in 1975. It is mainly released by macrophages and lymphocytes, and is a critical regulator of the inflammatory response to infection, and inappropriate or excess production can lead to autoimmune disease. Its extracellular domain can be cleaved and released in a soluble form that activates target cells (i.e., endothelial cells) by binding to two different receptors. Pro-inflammatory pathways are activated when the receptors stimulate signaling molecules.3 The central role of TNF-α in inflammation has been demonstrated by the ability of its blocking agents to treat a variety of inflammatory conditions, including rheumatoid arthritis, ankylosing spondylitis, inflammatory bowel disease, and psoriasis.
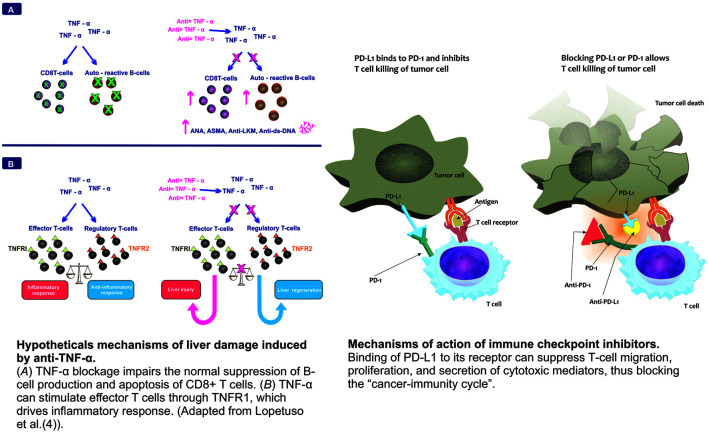
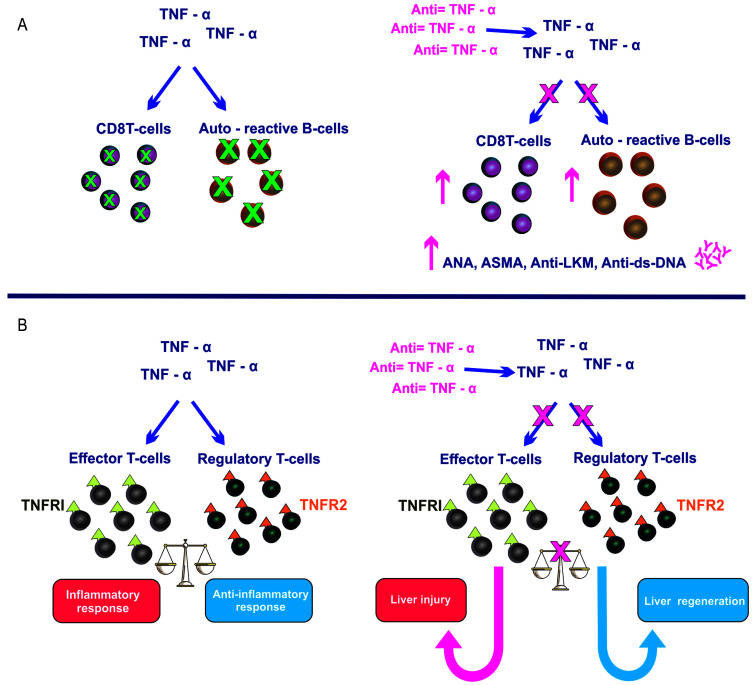
Although the pathogenesis of liver injury induced by anti-TNF-α is still unclear, TNF-blockade impairs the normal suppression of autoreactive B cell production and apoptosis of CD8+ T cells, favoring the production of autoantibodies. On the other hand, TNF-α stimulates effector T cells through TNF receptor 1, which drives an inflammatory response (Fig. 1) that is influenced by host genetic and immunological status.4 Anti-TNF-α drugs were developed in the 1990s, and their extensive use continues to benefit many patients. Initially, several systemic adverse events were reported to be associated with these agents, and liver injury was noted in post-marketing surveillance studies. The number of cases of hepatotoxicity has increased with time, and DILI triggered by anti-TNF agents has been well characterized both clinically and histologically.5
Fig. 1. Hypothetical mechanisms of liver damage induced by anti-TNF-α.
(A) TNF-α blockage impairs the normal suppression of B-cell production and apoptosis of CD8+ T cells. (B) TNF-α can stimulate effector T cells through TNFR1, which drives inflammatory response. (Adapted from Lopetuso et al.4). Anti-TNF-α, Anti-tumor necrosis factor-α.
The most commonly used drug, infliximab, can cause mild to severe DILI cases. Thus, the FDA issued a warning in 2004, based on 130 reported cases of suspected liver injury, which exceeds the rate of hepatotoxicity of similar agents.6 Hepatocellular injury is commonly mild and transient, and can be observed in patients suffering from inflammatory bowel disease (IBD) receiving infliximab monotherapy. Liver damage has been reported more frequently in patients with high body mass index, hepatic steatosis, and longer disease duration,7 but not all studies have confirmed those findings.8 Although Infliximab-induced DILI seems to be relatively rare in IBD patients, it is often reported in the rheumatology literature, probably mirroring the use of the drugs, as rheumatological patients are the most common type of patients treated. The reported serologic and pathologic characteristics are similar to idiopathic AIH, and liver injury resolves in most patients following infliximab withdrawal.8 In a prospective study, Bjornsson et al.9 identified 11 well-documented cases of DILI induced by anti-TNF agents among 1,176 treated patients. The mean age of the 11 patients was 46 years, eight were women, and nine were caused by infliximab. It seems that infliximab can trigger four forms of liver injury: (1) transient and asymptomatic ALT elevation, (2) hepatocellular injury associated with autoimmunity markers, (2) a cholestatic form that is usually self-limiting but can also be prolonged and can lead to liver failure and the need for liver transplantation,6 and (4) liver injury associated with chronic hepatitis B reactivation.10
Bjornsson et al.9 reported that one of 120 patients who received infliximab, one in 270 who received adalimumab, and one in 430 who received etanercept developed DILI. In most patients (n=6) infliximab-associated DILI developed after the fourth infusion of the drug. Four DILI patients presented with jaundice at diagnosis, and eight had hepatocellular liver injury. At DILI diagnosis, eight of the 11 patients had positive antinuclear antibody titers, and severe acute hepatitis was confirmed in three of the five liver biopsies that were performed. A short course of steroid therapy was successful in four of five patients in whom liver test abnormalities persisted despite drug withdrawal. In eight DILI patients, successful retreatment with a second TNF antagonist was achieved. The observation that nine of the patients who had been treated with a second TNF-α antagonist developed liver injury suggests that cross-reactivity is unlikely.9
Similar findings were reported by Ghabril et al.,11 who described six cases of DILI triggered by an anti-TNF agent registered in the DILIN and 26 additional cases identified in studies retrieved from PubMed. Infliximab triggered DILI in 26 patients whereas etanercept and adalimumab induced hepatitis in four patients each. The most common pattern at presentation was an autoimmune phenotype associated with severe hepatocellular injury, with both mixed non-autoimmune and predominant cholestatic patterns. In that analysis liver enzymes normalized in most patients after drug discontinuation, and a course of corticosteroids was received in several of them.
A recent study by Bjornsson H et al.,12 assessed 36 patients with infliximab-induced DILI. Approximately half of them required steroid treatment due to a slow improvement in ALT despite cessation of therapy. Treatment response was good with prompt resolution of liver test abnormalities. Relapse of liver injury was not observed after tapering of corticosteroids despite prolonged follow-up. No patient developed DILI when a second biologic was added.
The Austrian evidence-based consensus on the safe use of infliximab in IBD recommends that anti-TNF agents should be avoided in patients with baseline elevations of AST/ALT >3× the upper limit of normal (ULN).13 These guidelines recommend that liver tests should be monitored for adverse effects before initiation, after induction, and at least every 4 months while patients are on anti-TNF maintenance therapy. Even though it may not be wise to use drugs in patients with elevated liver enzymes, there is no data to support that there is a higher risk of DILI in patients with biochemical liver abnormalities.
Hepatotoxicity induced by ICIs
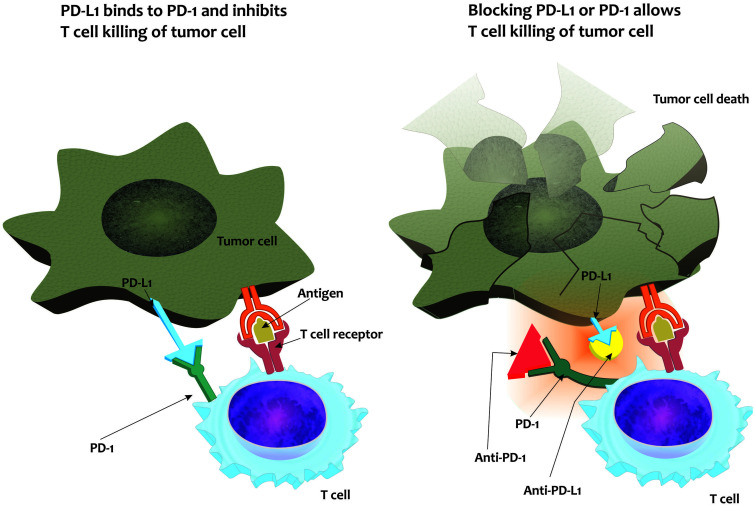
These agents belong to a large family of costimulatory molecules that play a crucial role in regulating the immune response. They modulate T cell receptor signaling via phosphorylation cascades. The most conspicuous immune checkpoints are cytotoxic T lymphocyte-associated antigen-4 (CTLA4), programmed cell death protein-1 (PD1), and programmed cell death-ligand-1 (PDL1). Altogether, they promote self-tolerance and help tumors to avoid an immune response (Fig. 2). When ICIs block these proteins, their inhibitory effects are disabled, which favors an immune response leading to both proliferation and T cell activation, which in turn ultimately lead to tumor-cell killing (Fig. 2). T cell inactivation by the PD1 and CTLA4 pathways is also involved in immune tolerance to self-antigens, and immune-mediated adverse events may affect almost all human organs and systems.14
Fig. 2. Mechanisms of action of immune checkpoint inhibitors.
Binding of PD-L1 to its receptor can suppress T cell migration, proliferation, and secretion of cytotoxic mediators, thus blocking the “cancer-immunity cycle”. PDL-1, Programmed death-ligand-1.
Emerging data indicate that ICIs benefit patients with a variety of advanced cancers, but also cause a broad spectrum of immune-related adverse events (irAEs). ICIs were initially approved to treat advanced melanoma, lung, and renal cancer, and more recently hepatocellular carcinoma (HCC).15 They have linked to both an improved and clinically significant survival rates.16 DILI occurs less frequently than other ICI-related irAEs, but fatal immune-related liver injury17 and a broad spectrum of ICI-associated liver damage have been reported.18 The incidence of treatment-associated hepatitis has been estimated at 3% to 9% for anti-CTLA4 agents, and 1% to 2% for anti-PD1 agents. Abnormal liver function tests are seen in up to 17% of patients receiving combined ICI schemes. Clinical patterns at presentation tend to be hepatocellular rather than cholestatic, and range from mild disease to acute liver failure. In most cases, liver autoantibodies are absent. The time to DILI onset after starting drug therapy usually ranges between 1 and 3 months. Following drug discontinuation and completing a short course of steroids, disease recurrence is uncommon.17,19 De Martin et al.20 reported the results of 16 patients treated with ICI and developing grade 3 hepatitis (i.e., cytolysis or cholestasis more than five times the ULN, and bilirubin >3). Anti-PD-1 agents caused nine cases, and anti-CTLA4 agents caused seven. The median DILI latency was 5 weeks, and autoantibodies were either negative or at low titers in most patients. Biopsies were performed in all 16 patients after a comprehensive workup to rule out other causes of liver disease. This interesting study helps to understand some histological features of this form of DILI. Inflammatory lymphocytic infiltrate, mainly involving CD8+ lymphocytes, was a characteristic pattern of portal cellularity in the absence of plasma cells, and lymphocytic cholangitis and ductal dystrophy were observed in more than half the biopsies. Anti-CTLA4-induced hepatitis included granulomas characterized by fibrin ring deposition, and was sometimes associated with central vein endotheliitis. The findings are consistent with a previous clinicopathological study that included liver biopsies from 11 patients with liver patterns of immune-related hepatitis induced by ipilimumab. All 11 had panlobular hepatitis and prominent histiocytic infiltration with no evidence of plasma cell infiltration.21 Johncilla et al.22 reported similar histological characteristics, with endotheliitis of central veins and perivenular hepatocyte necrosis. These findings might be helpful to support a diagnosis of ipilimumab-associated hepatitis, particularly in patients exposed to a combination of anti-PD1/PDL1 and anti-CTLA4 agents. A study from Japan that included 546 patients with advanced malignancies treated with ICI as monotherapy or combination therapy found immune-related liver injury in 29 patients (5.3%).23 The most common liver damage pattern was cholestatic/mixed injury (79%), serum IgG levels were within normal limits, and only two patients had positive ANA titers. Interestingly, the onset of fever within 24 h of drug administration was an independent risk factor for hepatotoxicity. The authors also described a unique type of ICI-induced cholangitis in four patients with a cholestatic/mixed pattern and severe bile duct dilatation. Liver biopsy was performed in only three patients, and included a compelling case with both liver test abnormalities and dilation of the intra- and extrahepatic bile ducts after the third treatment cycle. Immediate withdrawal of nivolumab and treatment with prednisolone was ineffective, and the patient died because of sepsis 15 weeks after the initial ICI dose.23
More than 90% of HCC develops on cirrhotic livers and usually are associated with hepatitis B virus, hepatitis C virus, and alcohol,24 and the association of HCC with nonalcoholic steatohepatitis (NASH) is increasingly described.25 Unfortunately, this widespread tumor is the fourth leading cause of cancer deaths worldwide. Although close screening of cirrhotic patients drastically decreases the occurrence of unresectable primary HCC, many patients still present with advanced tumor stages.26 Current medical treatment of advanced unresectable HCC is based on multikinase inhibitors sorafenib and lenvatinib, which can limit the quality of life because of adverse events.27 More recently, ICIs are being tested in intermediate stages, which were traditionally treated with chemoembolization. Although the use of ICIs in cirrhotic patients is concerning, those agents are being investigated in several ongoing trials, both as monotherapy and combination therapy.28 Results of a randomized phase 3 study of atezolizumab, a PD-L1 inhibitor together with bevacizumab, an inhibitor of angiogenesis via targeting of vascular endothelial growth factor, reported encouraging results, with better survival and recurrence-free disease compared with sorafenib.29 The incidence of grade 3 and 4 hepatic adverse effects was 1.2% and 3.6%, respectively. The study supports the use of this combined regimen in patients with underlying cirrhosis.
A recent meta-analysis by Fu et al.30 compared ICIs associated with DILI in HCC and other cancers. Anti-PD1 treatment was associated with a higher risk of hepatotoxicity than anti-PD-L1 treatment, and the presence of HCC was associated with a higher risk of all- and high-grade hepatotoxicity compared with other solid tumors. While awaiting the results of several ongoing trials, the use of ICIs in cirrhotic patients with underlying cirrhosis seems feasible, yet the specific contraindications for this type of drug in this population need to be determined.
Management of DILI induced by ICIs
DILI associated with cancer chemotherapy is graded by the Common Terminology Criteria for Adverse Events (CTCAE) of the National Cancer Institute.31 The grade of liver injury is based on peak abnormalities of alanine aminotransferase (ALT), aspartate aminotransferase (AST), gamma-glutamyl transpeptidase (GGT), and bilirubin. The FDA defines severe DILI as as ALT >3 times the ULN and total bilirubin >2 times ULN.32 Liver cell damage may be present with both normal serum bilirubin and other liver function tests. Although hepatotoxicity may manifest as early as 7 days, it usually occurs between 4 and 12 weeks after drug administration.32,33 Diagnosis of ICI-induced liver injury requires excluding other causes of DILI and underlying liver diseases that may occur with any type of hepatotoxicity.34 An asymptomatic increase of liver tests is a widespread event linked to these agents and usually represents an incidental finding. Many cases are diagnosed by asymptomatic elevated liver enzymes before a new ICI infusion.35 Underlying NASH or chronic hepatitis C virus (HCV) are confounding factors for a DILI diagnosis in patients with cirrhosis and HCC. The Roussel Uclaf Causality Assessment Method scale is a helpful for assessing DILI causality in clinical practice.36
The indication and timing of corticosteroid treatment in patients developing immune-related hepatitis is controversial. The EASL clinical practice guidelines recommend that the decision of steroid therapy in severely ill DILI patients should follow a multidisciplinary approach based on clinical and histological assessment.37 The American Cancer Association and the Consensus Recommendations of the Society for Immunotherapy of Cancer recommend oral prednisone and intravenous methylprednisone for grade 2–4 immune-related ICI hepatitis.38,39 However, a French study reported that almost half the patients with grade 3 or 4 DILI and individualized treatment spontaneously improved without corticosteroid therapy.20 A recent systematic review of immune-related ICI-induced hepatitis by Peeraphatdit et al.32 proposed an algorithm based on the severity of hepatotoxicity, using baseline liver tests and subsequent evaluation before each treatment to assess the presence if granulomatous hepatitis. The criteria were AST/ALT >3–5 the ULN, ALP/GGT >2.5–5 times the ULN, and total bilirubin >1.5–3 times the ULN. These authors also recommended liver biopsy in patients on anti-CTLA-4 therapy developing not only hepatitis grade 2 but also in patients with more severe hepatic compromise. They stated that the main limitation of liver biopsy in this study was that immune-mediated liver reactions do not have pathognomonic findings and did not always contribute to patient management. The authors also recommended temporary discontinuation of the ICI in patients with grade 2 hepatitis, resuming treatment after improvement or normalized liver tests, and switching from PD-1 or PD-L1 in patients receiving CTLA-4 inhibitors. They recommended oral prednisone in grade 3 hepatitis (AST/ALT/ALP/GGT >5–20 times the ULN and TBL >3–10 times the ULN) and in patients no improvement in the liver tests despite drug discontinuation. Intravenous methylprednisolone was recommended only for grade 4 hepatitis (AST/ALT/ALP/GGT >20 times the ULN and TBL >10 times the ULN).32 A cohort study by Dolladille et al.40 reported 29% recurrence of the same irAE after rechallenge with the ICI therapy that initially led to discontinuation. They recommend that resuming ICI therapy should be considered in selected patients, with appropriate monitoring and use of standard treatment algorithms to identify and treat toxic effects.
A recent retrospective cohort study including 40 patients who received anti-PD-1 or PD-L1 agents, reported 55% of patients with a second adverse event (three of the five patients had a recurrence of hepatitis) after experiencing an initial serious IrAE.41 Budesonide, a corticosteroid with 90% first-pass metabolism in the liver, was used successfully to treat autoimmune hepatitis induced by ICI in isolated case reports.42
The EASL statement on DILI induced by ICIs notes that those agents induce immune-related hepatotoxicity in a substantial proportion of patients. CTLA-4 inhibitors (e.g., ipilimumab) are more hepatotoxic than PD-L1 agents (e.g., nivolumab), and that combination treatments carry increased risk.37 They recommend that decisions regarding corticosteroid treatment of immune-mediated hepatitis associated with ICIs be made by a multidisciplinary team including hepatologists. Recently published DILI guidelines from the American College of Gastroenterology (ACG) recommend withholding or delaying ICI administration and to initiate immunosuppressive therapy for treating moderate to severe ICI hepatotoxicity.43 Corticosteroids are the primary immunosuppressants, and mycophenolate mofetil can be used in cases unresponsive to or with adverse events following corticosteroids.44 In those with HBV reactivation, appropriate therapy should be directed at the HBV infection.45 Tenofovir and entecavir should be used as the first-line drugs to treat HBV reactivation in immunosuppressed patients.45
In conclusion, ICIs may be associated with a high rate of DILI in up to 17 % of patients when combinations of two ICI drugs are prescribed. Although around 50% of hepatotoxicity induced by these agents spontaneously resolve when the drug is discontinued, a high percentage of patients will require corticosteroids. Whether patients with grade 2–3 liver toxicity should be treated continues to be controversial (Table 2).37,39,46,47 Liver histology is still a valuable tool for both a diagnosis and decision making.
Table 2. Proposed guidelines for the management of ICIs-induced liver toxicity.
| Society for Immunotherapy of Cancer (SITC) Toxicity Management Working Group46 |
Management of toxicities from immunotherapy: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up47 |
American Society of Clinical Oncology Clinical Practice Guideline39 |
EASL Clinical Practice Guidelines: Drug-induced liver injury37 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Definition | Management | Definition | Management | Definition | Management | Definition | Management | |||||
| Grade 1 | AST, ALT > ULN -3× ULN; TB > ULN -1.5× ULN | Corticoids are not indicated | Continue immunotherapy | AST, ALT > ULN -3× ULN | No treatment | Continue immunotherapy | AST, ALT > ULN-3× ULN; TB > ULN-1.5× ULN | No treatment | Continue ICIs treatment | AST, ALT ≤3× ULN; TB≤ 1.5× ULN; ALP≤2.5× ULN | No treatment | Continue ICIs treatment |
| Grade 2 | AST, ALT 3–5× ULN; TB 1.5–3× ULN | oral prednisone 0.5–1 mg/kg/day | Hold immunotherapy | AST, ALT > 3- ≤5× ULN | Prednisone 1 mg/kg/day | Hold immunotherapy | AST, ALT 3-5× ULN; TB1.5-3×ULN | 0.5–1 mg/kg/d prednisone | Hold ICIs treatment | AST, ALT 3-5× ULN; TB 1.5-3× ULN; ALP 2.5-5× ULN | Treat with corticosteroids only if biochemical abnormalities persist beyond 2 weeks | Skip dose immunotherapy |
| Grade 3 | AST, ALT >5× ULN; TB >3× ULN | prednisone 1–2 mg/kg/day | Discontinue immunotherapy | AST, ALT > 5×-≤20 ULN | Prednisone 1 mg/kg/day or Methylprednisolone 2 mg/k/day if raised TB/INR | Cease immunotherapy | AST, ALT >5- ≤20× ULN; TB>3- ≤10× ULN | 1–2 mg/kg methylprednisolone | Permanently discontinue ICIs teratment | AST, ALT 5-20× ULN; TB 3–10× ULN; ALP 5–20× ULN | 1 to 2 mg/kg methylprednisolone | Discontinue Immunotherapy |
| Grade 4 | AST, ALT > 20× ULN | Methylprednisolone 2 mg/k/day | Discontinue immunotherapy | AST, ALT >20× ULN; TB >10× ULN | 2 mg/kg/d methylprednisolone | Permanently discontinue ICIs treatment | AST, ALT >20× ULN; TB >10× ULN; ALP >20× ULN | 1 to 2 mg/kg methylprednisolone | Discontinue Immunotherapy | |||
EASL, European Association Study of the Liver; ALT, Alkaline aminoransferase; AST, Asparte aminotransferase; GGT, Gamma-glutamyl-transpeptidase; ALP, Alcaline phosphatase; TB, Total bilirubin; ULN, Upper limit of normal; ICIs, Immunological checkpoint inhibitors.
Controversies in histological assessment and the role of liver biopsy
Distinguishing between the induction of biochemical evidence of autoimmunity and clinically evident autoimmune disease represents a challenge in clinical practice.46 Differentiating DIAIH from a classical AIH is a complex issue for pathologists.47,48 A pioneering study published by Bjornsson et al. in 2010 compared 24 cases of DIAIH, 11 triggered by nitrofurantoin and minocycline, compared with classic AIH. Patients with presumed DIAIH rarely had cirrhosis-like changes and no recurrence of liver damage was observed after corticosteroid withdrawal.49
Infliximab is the best-studied biological drug, given its widespread use and relatively frequent hepatotoxicity. Ghabril et al.11 found that the most common histological patterns of infliximab-induced DILI were acute or chronic hepatitis with features of AIH, elevated titers of antinuclear antibodies and occasionally other serological findings. Other patterns included acute cholestasis and hepatocellular necrosis without any associated inflammatory infiltrate. Eight cases reported by Rodriguez et al.,50 seven induced by infliximab and one by adalimumab, had signs of AIH (i.e. chronic lymphoplasmocytic infiltrate and interface hepatitis). The International Diagnostic Criteria for AIH scores were all ≥ 19 after treatment, leading to an AIH diagnosis.51 It is noteworthy that out of 20 recently described patients 18 of them showed autoimmune like features while one case showed acute cholestasis and a mild reactive hepatitis was observed in the remaining patient.52
Regarding ICIs, Ipilimumab has been associated with autoimmune-like reactions, and a minority involved the liver.53,54 A series evaluated by Johncilla et al.22 described panlobular hepatitis with central vein endothelialiitis as a distinctive pattern of liver damage. About half the cases had a prominent plasma cell infiltrate. Of note, several patients had also received nivolumab combined with ipilimumab, another type of immune checkpoint inhibitor. None of the patients in the series had serological markers of AIH, in contrast to observations in idiopathic AIH and DILI associated with anti-TNF agents. Combination therapy has been linked to increased irAEs and liver injury.55 There has been only one report of the association of natalizumab, which blocks the migration of lymphocytes to areas of inflammation, with liver injury. The patient developed a lymphoplasmacytic infiltrate in portal areas with interface hepatitis and hepatocyte dropout in zone 3. Serology was positive for low titers of anti-smooth muscle antibody and anti-F-actin. Hepatitis resolved without corticosteroid treatment, as documented by a follow-up liver biopsy.56
A new ICI-induced cholestatic pattern presenting with clinical, biochemical and histological features similar to sclerosing cholangitis was recently described.57–59 This is a cholestatic disease showing diffuse biliary duct dilatation and thickening of the bile ducts.60–62 Dilatation of the bile ducts without biliary obstruction was also observed in almost 80% of cases, according to recently reported data.63 In addition, diffuse hypertrophy in the extrahepatic bile ducts was documented in most of them.63 Although the biliary involvement has already been clearly described, its long-term consequences are still unknown.
Histological study of the thickened bile ducts showed a characteristic lymphocytic infiltration associated with a frank predominance of cytotoxic CD8+T cells.57–59 These cellular changes indicate a probable alteration of the balance between effector and regulatory T cells capable of generating an immune-mediated hepatobiliary lesion.61,62
In conclusion, liver biopsy may be helpful in patients with clinical presentations that do not have typical features of idiopathic AIH. The presence of ring granulomas and endothelitis are suggestive of ICI-induced DILI.20 Histology can distinguish anti-PD1/PD-L1 and anti-CTLA4 mAb toxicity, and can establish the severity of liver injury. Histological findings may also help clinical decision making including the need for corticosteroid administration.20 The presence of cholestasis in patients with suspected DIAIH suggests drug toxcicity.48 A magnetic resonance cholangiopancreatography is often helpful when secondary sclerosing cholangitis is suspected.
Hepatitis B reactivation induced by immunosuppressive agents with emphasis in anti-TNF agents and checkpoint inhibitors
There are different virological scenarios, and a wide spectrum of associated drugs with specific and stratified risk for the development of HBV reactivation in immunosuppressed patients. Some agents trigger severe hepatocellular damage, including hepatitis, acute liver failure, and even death despite administration of effective antiviral agents.64 The potential consequences of HBV reactivation is of concern when patients are exposed to either immunosuppressive or biologic therapies for the management of cancer, rheumatologic, inflammatory bowel. and dermatologic diseases. Screening with serological hepatitis B virus markers and prophylactic or pre-emptive antiviral treatment with nucleos(t)ide analogues should be considered to diminish the risk of HBV reactivation in those patients.65 The risk of HBV reactivation should be graded according to the potency of immunosuppressive drugs used for the treatment of neoplastic and rheumatic diseases. The risk can be classified as (1) high in patients treated with anti-CD20 monoclonal antibodies including rituximab or systemic cancer chemotherapy agents such as doxorubicin, where the HBV reactivation rate is >10%; (2) moderate, with HBV reactivation in 1% to 10% with imatinib, ibrutinib, and other tyrosine kinase inhibitors or corticosteroids equivalent to prednisone >20 mg daily for more than 4 weeks; and (3) low in patients where the reactivation rate of HBV is <1% with azathioprine and methotrexate or corticosteroid use for <4 weeks.66 Anti-TNF agents with higher potency, including adalimumab, infliximab, golimumab, and certolizumab) are associated a high probability of HBV reactivation. HBsAg-positive patients using ICIs including anti-PD1 (nivolumab, pembrolizumab), anti-PD-L1 (atezolizumab), or anti-CTLA4 (ipilimumab) agents have a moderate to high risk of HBV reactivation.67 HBV reactivation without antiviral prophylaxis was reported as 29%, 27%, and 50%, respectively, of patients treated with IL12/23, IL17, and JAK inhibitors, which suggests that non-TNF-targeted biologics may have a higher risk of HBV reactivation than TNF-α inhibitors.68 A meta-analysis by Cantini et al.69 including 10 studies of anti-TNF agents reported a pooled estimate of HBV reactivation of 4.2% (95% CI: 1.4–8.2%). The pooled prevalence of reactivation was 3.0% (95% CI: 0.6–7.2) for patients with occult infection and 15.4% (95% CI: 1.2–41) for those with overt infection. The prevalence of reactivation was 3.9% (95% CI: 1.1%–8.4%) for treatment with etanercept and 4.6% (95% CI: 0.5%–12.5%) for adalimumab. For the subset of patients without any antiviral prophylaxis, pooled reactivation was 4.0% (95% CI: 1.2%–8.3%). The authors concluded that although HBV reactivation was low in patients treated with anti-TNF-α agents for rheumatic and dermatological conditions, antiviral prophylaxis should be recommended in patients with overt chronic HBV infection.68 Recent APASL guidelines note that the TNF-α inhibitors used to treat autoimmune diseases, such as rheumatic disorders and inflammatory bowel diseases, have HBV reactivation risks of between 14% and 63% in published reports of relatively small case series.67
The liver toxicity associated with ICI treatment is mostly immune-mediated hepatitis. The evidence is from a retrospective analysis of the Adverse Events Reporting System of the FDA that queried reported cases of hepatitis B reactivation involving the PD1/PD-L1 inhibitors pembrolizumab, atezolizumab, nivolumab, durvalumab, avelumab, and ipilimumab from their initial FDA approval to June 30, 2020.70 HBsAg-positive and HBsAg-negative patients who are anti-HBc positive and treated with drugs associated with a high risk of HBV reactivation and those at moderate risk (i.e., HBsAg positive and HBsAg negative but anti-HBc positive) who have advanced liver fibrosis or cirrhosis, would benefit from preemptive high-resistant barrier drug therapy (entecavir, tenofovir, or TAF).70 Few clinical studies have investigated the risk of HBV reactivation during ICI therapy and real-life data are currently based on five reports of HBV reactivation, one with a fatal outcome.71
Summary and future directions
Hepatotoxicity induced by biological agents is a novel emerging cause of DILI. The causative drugs induce liver injury via different mechanisms triggered by immune dysregulation. Hepatic adverse reactions are being increasingly reported in association with ICIs, and they represent a diagnostic and therapeutic challenge. As liver damage is not an uncommon event clinicians should be vigilant when using biological agents. A wide range of severity, from transient and mild forms to fulminant liver failure including prolonged immune-mediated hepatitis, have been observed. The indications and optimal timing, dosage, and duration of steroid treatment are a dilemma. Currently, there is no consensus on treatment guidelines and consistent expert opinion on which patients should receive corticosteroid therapy. Approximately half the patients who develop liver damage caused by anti-TNF agents and ICIs receive steroids, and many of them have a spontaneous resolution. Future consensus will shed light on this still controversial point and will establish when immunosuppression should be started and when mycophenolate mofetil should be used. The role of the pathologist can be very valuable in selected patients as the histological findings could suggest a causality. In particular, ICIs-induced liver damage may present distinctive histologic features characterized by ring granuloma, endothelitis and secondary sclerosing cholangitis. The clinician should consider testing of HBV markers prior to the use of biological agents. There is an increased risk of HBV reactivation in either current or past HBV-induced liver disease, which is of concern because it may be associated with fatal liver failure. Novel noninvasive biomarkers are needed to establish the diagnosis of biological agent-induced DILI and to monitor prognosis and therapeutic response. They might also be useful to identify patients who will experience complete biochemical remission after drug withdrawal and tolerate retreatment with immunotherapeutic drugs. The management of such patients should be personalized.
Acknowledgments
The authors wish to thank Professors Ramon Bataller and Einar Björnsson for reviewing this manuscript.
Abbreviations
- ALP
Alkaline phosphatase
- ALT
Alanine aminotransferase
- APASL
Asian Pacific Association for the study of the Liver
- AST
Asparte aminotransferase
- CTLA4
T lymphocyte-associated antigen-4
- DIAIH
Drug-induced autoinmmune hepatitis
- DILI
Drug-induced liver injury
- DILIN
Drug-induced liver injury network
- EASL
European Association for the Study of the Liver
- FDA
Food and Drug Administration
- GGT
Gamma-glutamyl-transpeptidase
- HBV
Hepatitis B virus
- HCC
Hepatocellular carcinoma
- IBD
Inflammatory bowel disease
- ICIs
Immunological checkpoint inhibitors
- irAEs
Immune-related adverse events
- mAbs
Monoclonal antibodies
- NASH
Nonalcoholic steatohepatitis
- PD1
Programmed death protein-1
- PDL-1
Programmed death-ligand-1
- TB
Total bilirrubin
- TNF-α
Tumor necrosis factor-α
- ULN
Upper Limit of Normal
References
- 1. Purple Book: Lists of Licensed Biological Products with Reference Product Exclusivity and Biosimilarity or Interchangeability Evaluations. Available from: https://www.fda.gov/Drugs/DevelopmentApprovalProcess/HowDrugsareDevelopedandApproved/ApprovalApplications/TherapeuticBiologicApplications/Biosimilars/ucm411418.htm.
- 2. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012. Available from: https://www.ncbi.nlm.nih.gov/books/NBK548335/ [PubMed]
- 3.Bradley JR. TNF-mediated inflammatory disease. J Pathol. 2008;214:149–160. doi: 10.1002/path.2287. [DOI] [PubMed] [Google Scholar]
- 4.Lopetuso LR, Mocci G, Marzo M, D’Aversa F, Rapaccini GL, Guidi L, et al. Harmful Effects and Potential Benefits of Anti-Tumor Necrosis Factor (TNF)-α on the Liver. Int J Mol Sci. 2018;19:2199. doi: 10.3390/ijms19082199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Granier C, De Guillebon E, Blanc C, Roussel H, Badoual C, Colin E, et al. Mechanisms of action and rationale for the use of checkpoint inhibitors in cancer. ESMO Open. 2017;2:e000213. doi: 10.1136/esmoopen-2017-000213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shah P, Sundaram V. Bjornsson E., Biologic and Checkpoint Inhibitor-Induced liver Injury: A Systematic Literature Review. Hepatol Commun. 2020;4(2):172–184. doi: 10.1002/hep4.1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Koller T, Galambosova M, Filakovska S, Kubincova M, Hlavaty T, Toth J, et al. Drug-induced liver injury in inflammatory bowel disease: 1-year prospective observational study. World J Gastroenterol. 2017;23(22):4102–4111. doi: 10.3748/wjg.v23.i22.4102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barnhill M, Steinberg JM, Jennings JJ, Lewis JH. Hepatotoxicty of Agents Used in the Management of Inflammatory Bowel Disease: a 2020 Update. Curr Gastroenterol Rep. 2020;22(9):47. doi: 10.1007/s11894-020-00781-3. [DOI] [PubMed] [Google Scholar]
- 9.Björnsson ES, Gunnarsson BI, Gröndal G, Jonasson JG, Einarsdottir R, Ludviksson BR, et al. Risk of Drug-Induced Liver Injury From Tumor Necrosis Factor Antagonists. Clin Gastroenterol Hepatol. 2015;13:602–608. doi: 10.1016/j.cgh.2014.07.062. [DOI] [PubMed] [Google Scholar]
- 10.LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet] Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012. [PubMed] [Google Scholar]
- 11.Ghabril M, Bonkovsky H, Kum C, Davern T, Hayashi P, Kleiner DE, et al. Liver Injury From Tumor Necrosis Factor-α Antagonists: Analysis of Thirty-four Cases. Clin Gastroenterol Hepatol. 2013;11:588–564. doi: 10.1016/j.cgh.2012.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Björnsson HK, Gudbjornsson B, Björnsson ES. Infliximab-induced liver injury: Clinical phenotypes, autoimmunity and the role of corticosteroid treatment. J Hepatol. 2022;76(1):86–92. doi: 10.1016/j.jhep.2021.08.024. [DOI] [PubMed] [Google Scholar]
- 13.Miehsler W, Novacek G, Wenzl H, Vogelsang H, Knoflach P Kaser A, et al. A decade of infliximab: the Austrian evidence based consensus on the safe use of infliximab in inflammatory bowel disease. J Crohns Colitis. 2010;4(3):221–256. doi: 10.1016/j.crohns.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 14. FDA approves atezolizumab plus bevacizumab for unresectable hepatocellular carcinoma. Available from: www.fda.gov/drugs/drug-approvals-and-databases/fda-approves-atezolizumab-plus-bevacizumab-unresectable-hepatocellular-carcinoma.
- 15.Wolchok JD, Chiarion-Sileni V, Gonzalez R, Rutkowski P, Grob JJ, Cowey CL, et al. Overall survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med. 2017;377:1345–1356. doi: 10.1056/NEJMoa1709684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Inamori O, Miyagawa-Hayashino A, Ueno A, Hongo F, Sonobe Y, Hojo T, et al. Fulminant hepatitis as an immune-related adverse event after nivolumab treatment. Pathol Int. 2019;69:434–436. doi: 10.1111/pin.12812. [DOI] [PubMed] [Google Scholar]
- 17.Xu F, Jin T, Zhu Y, Dai C. Immune checkpoint therapy in liver cancer. J Exp Clin Cancer Res. 2018;37(1):110. doi: 10.1186/s13046-018-0777-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Perdigoto AL, Kluger H, Herold KC. Adverse events induced by immune checkpoint inhibitors. Curr Opin Immunol. 2021;69:29–38. doi: 10.1016/j.coi.2021.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Suzman DL, Pelosof L, Rosenberg A, Avigan MI. Hepatotoxicity of immune checkpoint inhibitors: An evolving picture of risk associated with a vital class of immunotherapy agents. Liver Int. 2018;38:976–987. doi: 10.1111/liv.13746. [DOI] [PubMed] [Google Scholar]
- 20.De Martin E, Michot JM, Papouin B, Champiat S, Mateus C, Lambotte O, et al. Characterization of liver injury induced by cancer immunotherapy using immune checkpoint inhibitors. J Hepatol. 2018;68:1181–1190. doi: 10.1016/j.jhep.2018.01.033. [DOI] [PubMed] [Google Scholar]
- 21.Uetrecht J. Mechanistic Studies of Idiosyncratic DILI: Clinical Implications. Front Pharmacol. 2019;10:837. doi: 10.3389/fphar.2019.00837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Johncilla M, Misdraji J, Pratt DS, Agoston AT, Lauwers GY, Srivastava A, et al. Ipilimumab-associated hepatitis: clinicopathologic characterization in a series of 11 cases. Am J Surg Pathol. 2015;39:1075–1084. doi: 10.1097/PAS.0000000000000453. [DOI] [PubMed] [Google Scholar]
- 23.Kazuyuki M, Takanori I, Matsatoshi I, Yoji I, Teiji K, Takashi H, et al. Real world data of liver injury induced by immune checkpoint inhibitors in Japanese patients with advanced malignancies. J Gastroenterol. 2020;55(6):653–661. doi: 10.1007/s00535-020-01677-9. [DOI] [PubMed] [Google Scholar]
- 24.Desai A, Sandhu S, Lai JP, Sandhu DS. Hepatocellular carcinoma in non-cirrhotic liver: A comprehensive review. World J Hepatol. 2019;11(1):1–18. doi: 10.4254/wjh.v11.i1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Arab JP, Dirchwolf M, Álvares-da-Silva MR, Barrera F, Benítez C, Castellanos-Fernandez M, et al. Latin American Association for the study of the liver (ALEH) practice guidance for the diagnosis and treatment of non-alcoholic fatty liver disease. Ann Hepatol. 2020;19(6):674–690. doi: 10.1016/j.aohep.2020.09.006. [DOI] [PubMed] [Google Scholar]
- 26.Rao A, Rich NE, Marrero JA, Yopp AC, Singal AG. Diagnostic and Therapeutic Delays in Patients with Hepatocellular Carcinoma. J Natl Compr Canc Netw. 2021;28:1–14. doi: 10.6004/jnccn.2020.7689. [DOI] [PubMed] [Google Scholar]
- 27.Copur MS. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:2498. [PubMed] [Google Scholar]
- 28.Finn RS, Ryoo BY, Merle P, Kudo M, Bouattour M, Yeong Lim H, et al. Pembrolizumab As Second-Line Therapy in Patients with Advanced Hepatocellular Carcinoma in KEYNOTE-240: A Randomized, Double-Blind, Phase III Trial. J Clin Oncol. 2019;38:193–202. doi: 10.1200/JCO.19.01307. [DOI] [PubMed] [Google Scholar]
- 29.Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. 2020;382:1894–1905. doi: 10.1056/NEJMoa1915745. [DOI] [PubMed] [Google Scholar]
- 30.Fu J, Li WZ, McGrath NA, Lai CW, Brar G, Xiang YQ, et al. Immune Checkpoint Inhibitor Associated Hepatotoxicity in Primary Liver Cancer Versus Other Cancers: A Systematic Review and Meta-Analysis. Front Oncol. 2021;11:1279. doi: 10.3389/fonc.2021.650292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kluetz PG, Chingos DT, Basch EM, Mitchell SA. Patient-Reported Outcomes in Cancer Clinical Trials: Measuring Symptomatic Adverse Events With the National Cancer Institute’s Patient-Reported Outcomes Version of the Common Terminology Criteria for Adverse Events (PRO-CTCAE) Am Soc Clin Oncol Educ Book. 2016;35:67–73. doi: 10.1200/EDBK_159514. [DOI] [PubMed] [Google Scholar]
- 32.Peeraphatdit TB, Wang J, Odenwald MA, Hu S, Hart J, Charlton MR. Hepatotoxicity From Immune Checkpoint Inhibitors: A Systematic Review and Management Recommendation. Hepatology. 2020;72:315–329. doi: 10.1002/hep.31227. [DOI] [PubMed] [Google Scholar]
- 33.Cho YA, Han JM, Kang SY, Kim DC, Youn YJ, Choi KH, et al. Analysis of Risk Factors for Hepatotoxicity Induced by Immune Checkpoint Inhibitors. J Immunother. 2021;44(1):16–21. doi: 10.1097/CJI.0000000000000347. [DOI] [PubMed] [Google Scholar]
- 34.Bessone F, Robles-Diaz M, Hernandez N, Medina-Caliz I, Lucena MI, Andrade RJ. Assessment of serious acute and chronic idiosyncratic drug-induced liver injury in clinical practice. Semin Liver Dis. 2019;39:381–394. doi: 10.1055/s-0039-1685519. [DOI] [PubMed] [Google Scholar]
- 35.Malnick SDH, Abdullah A, Neuman MG. Checkpoint Inhibitors and Hepatotoxicity. Biomedicines. 2021;9(2):101. doi: 10.3390/biomedicines9020101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Danan G, Teschke R. Roussel UCLAF causality assessment method for drug-induced liver injury: Present and future. Front Pharmacol. 2019;10:853. doi: 10.3389/fphar.2019.00853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.European Association for the Study of the Liver EASL Clinical Practice Guidelines: Drug-induced liver injury. J Hepatol. 2019;70:1222–1261. doi: 10.1016/j.jhep.2019.02.014. [DOI] [PubMed] [Google Scholar]
- 38.Puzanov I, Diab A, Abdallah K, Bingham C, Brogdon C, Dadu R, et al. Managing toxicities associated with immune checkpoint inhibitors: consensus recommendations from the Society for Immunotherapy of Cancer (SITC) Toxicity Management Working Group. J Immunother Cancer. 2017;5(1):95. doi: 10.1186/s40425-017-0300-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brahmer JR, Lacchetti C, Schneider BJ, Atkins MB, Brassil KJ, Caterino JM, et al. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol. 2018;36:1714–1768. doi: 10.1200/JCO.2017.77.6385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dolladille C, Ederhy S, Sassier M, Cautela J, Thuny F, Cohen AA, et al. Immune Checkpoint Inhibitor Rechallenge After Immune-Related Adverse Events in Patients With Cancer. JAMA Oncol. 2020;6(6):865–871. doi: 10.1001/jamaoncol.2020.0726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Simonaggio A, Michot JM, Voisin AL, Pavec J, Collins M, Lallart A, et al. Evaluation of readministration of Immune Checkpoint Inhibitors After Immune-Related Adverse Events in Patients with Cancer. JAMA Oncol. 2019;5(9):1310–1317. doi: 10.1001/jamaoncol.2019.1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ziemer M, Koukoulioti E, Beyer S, Simon JC, Berg T. Managing immune checkpoint-inhibitor induced severe autoimmune-like hepatitis by liver-directed topical steroids. J Hepatol. 2017;66:657–659. doi: 10.1016/j.jhep.2016.11.015. [DOI] [PubMed] [Google Scholar]
- 43.Chalasani NP, Maddur H, Russo MW, Wong RJ, Reddy KR. ACG Clinical Guideline: Diagnosis and Management of Idiosyncratic Drug-Induced Liver Injury. Am J Gastroenterol. 2021;116(5):878–898. doi: 10.14309/ajg.0000000000001259. [DOI] [PubMed] [Google Scholar]
- 44.Nakano K, Nishizawa M, Fukuda N, Urasaki T, Wang XF, Mitani H, et al. Mycophenolate mofetil as a successful treatment of corticosteroid-resistant immune checkpoint inhibitor-induced hepatitis. Oxf Med Case Reports. 2020;2020(4):omaa027. doi: 10.1093/omcr/omaa027Oxf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bessone F, Dirchwolf M. Management of hepatitis B reactivation in immunosuppressed patients: An update on current recommendations. World J Hepatol. 2016;8(8):385–394. doi: 10.4254/wjh.v8.i8.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zen Y, Yeh MM. Checkpoint inhibitor-induced liver injury: A novel form of liver disease emerging in the era of cancer immunotherapy. Semin Diagn Pathol. 2019;36(6):434–440. doi: 10.1053/j.semdp.2019.07.009. [DOI] [PubMed] [Google Scholar]
- 47.Haanen JBAG, Carbonnel F, Robert C, Kerr KM, Peters S, Larkin J, et al. Management of toxicities from immunotherapy: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2017;28(suppl_4):iv119–iv142. doi: 10.1093/annonc/mdx225. [DOI] [PubMed] [Google Scholar]
- 48.Suzuki A, Brunt EM, Kleiner DE, Miquel R, Smyrk TC, Andrade RJ, et al. The use of liver biopsy evaluation in discrimination of idiopathic autoimmune hepatitis versus drug-induced liver injury. Hepatology. 2011;54(3):931–939. doi: 10.1002/hep.24481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bjornsson E, Talwalkar J, Treeprasertsuk S, Kamath PS, Takahashi N, Sanderson S, et al. Drug-induced autoimmune hepatitis: clinical characteristics and prognosis. Hepatology. 2010;51(6):2040–2048. doi: 10.1002/hep.23588. [DOI] [PubMed] [Google Scholar]
- 50.Rodrigues S, Lopes S, Magro F, Cardoso H, Horta e Vale AM, Marques M, et al. Autoimmune hepatitis and anti-tumor necrosis factor alpha therapy: A single center report of 8 cases. World J Gastroenterol. 2015;21(24):7584–7588. doi: 10.3748/wjg.v21.i24.7584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Manns MP, Czaja AJ, Gorham JD, Krawitt EL, Mieli-Vergani G, Vergani D, et al. Diagnosis and management of autoimmune hepatitis. Hepatology. 2010;51:2193–2213. doi: 10.1002/hep.23584. [DOI] [PubMed] [Google Scholar]
- 52.Kleiner DE. Recent Advances in the Histopathology of Drug-Induced Liver Injury. Surg Pathol Clin. 2018;11(2):297–311. doi: 10.1016/j.path.2018.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kleiner DE, Berman D. Pathologic changes in ipilimumab-related hepatitis in patients with metastatic melanoma. Dig Dis Sci. 2012;57(8):2233–2240. doi: 10.1007/s10620-012-2140-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Postow MA, Chesney J, Pavlick AC, Robert C, Grossmann K, McDermott D, et al. Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. N Engl J Med. 2015;372(21):2006–2017. doi: 10.1056/NEJMoa1414428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Beck KE, Blansfield JA, Tran KQ, Feldman AL, Hughes MS, Royal RE, et al. Enterocolitis in patients with cancer after antibody blockade of cytotoxic T-lymphocyte-associated antigen 4. J Clin Oncol. 2006;24(15):2283–2289. doi: 10.1200/JCO.2005.04.5716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Antezana A, Sigal S, Herbert J, Kister I. Natalizumab-induced hepatic injury: a case report and review of literature. Mult Scler Relat Disord. 2015;4(6):495–498. doi: 10.1016/j.msard.2015.08.008. [DOI] [PubMed] [Google Scholar]
- 57.Doherty GJ, Duckworth AM, Davies SE, Mells GF, Brais R, Harden SV, et al. Severe steroid-resistant anti-PD1 T-cell checkpoint inhibitor-induced hepatotoxicity driven by biliary injury. ESMO. 2017;2(4):e000268. doi: 10.1136/esmoopen-2017-000268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gelsomino F, Vitale G, D’Errico A, Bertuzzi C, Andreone P, Ardizzoni A. Nivolumab-induced cholangitic liver disease: a novel form of serious liver injury. Ann Oncol. 2017;28:671–672. doi: 10.1093/annonc/mdw649. [DOI] [PubMed] [Google Scholar]
- 59.Kawakami H, Tanizaki J, Tanaka K, Haratani K, Hayashi H, Takeda M, et al. Imaging and clinicopathological features of nivolumab-related cholangitis in patients with non-small cell lung cancer. Invest N Drugs. 2017;35:529–536. doi: 10.1007/s10637-017-0453-0. [DOI] [PubMed] [Google Scholar]
- 60.Gelsomino F, Vitale G, Ardizzoni A. A case of nivolumab-related cholangitis and literature review: how to look for the right tools for a correct diagnosis of this rare immune-related adverse event. Invest N Drugs. 2018;36:144–146. doi: 10.1007/s10637-017-0484-6. [DOI] [PubMed] [Google Scholar]
- 61.Cho JH, Sun JM, Lee SH, Ahn JS, Park K, Ahn MJ. Late-onset cholecystitis with cholangitis after avelumab treatment in non-small cell lung cancer. J Thorac Oncol. 2018;13:e34–e36. doi: 10.1016/j.jtho.2017.10.007. [DOI] [PubMed] [Google Scholar]
- 62.Hamoir C, de Vos M, Clinckart F, Nicaise G, Komuta M, Lanthier N. Hepatobiliary and pancreatic: nivolumab-related cholangiopathy. J Gastroenterol Hepatol. 2018;33:1695. doi: 10.1111/jgh.14136. [DOI] [PubMed] [Google Scholar]
- 63.Onoyama T, Takeda Y, Yamashita T, Hamamoto W, Sakamoto Y, Koda H, et al. Programmed cell death-1 inhibitor-related sclerosing cholangitis: a systematic review. World J Gastroenterol. 2020;26:353–365. doi: 10.3748/wjg.v26.i3.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhang X, Zhou Y, Chen C, Fang W, Cai X, Zhang X, et al. Hepatitis B virus reactivation in cancer patients with positive Hepatitis B surface antigen undergoing PD-1 inhibition. J Immunother Cancer. 2019;7(1):322. doi: 10.1186/s40425-019-0808-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shih CA, Chen WC. Prevention of hepatitis B reactivation in patients requiring chemotherapy and immunosuppressive therapy. World J Clin Cases. 2021;9(21):5769–5781. doi: 10.12998/wjcc.v9.i21.5769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sagnelli C, Pisaturo M, Calò F, Martini S, Sagnelli E, Coppola N. Reactivation of hepatitis B virus infection in patients with hemo-lymphoproliferative diseases, and its prevention. World J Gastroenterol. 2019;25:3299–3312. doi: 10.3748/wjg.v25.i26.3299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lau G, Yu ML, Wong G, Thompson A, Ghazinian H, Hou JL, et al. APASL clinical practice guideline on hepatitis B reactivation related to the use of immunosuppressive therapy. Hepatol Int. 2021;15(5):1031–1048. doi: 10.1007/s12072-021-10239-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Akiyama S, Cotter TG, Sakuraba A. Risk of hepatitis B virus reactivation in patients with autoimmune diseases undergoing non-tumor necrosis factor-targeted biologics. World J Gastroenterol. 2021;27(19):2312–2324. doi: 10.3748/wjg.v27.i19.2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cantini F, Boccia S, Goletti D, Iannone F, Leoncini E, Panic N, et al. HBV Reactivation in Patients Treated with Antitumor Necrosis Factor-Alpha (TNF-α) Agents for Rheumatic and Dermatologic Conditions: A Systematic Review and Meta-Analysis. Int J Rheumatol. 2014;2014:926836. doi: 10.1155/2014/926836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Burns EA, Muhsen IN, Anand K, Xu J, Umoru G, Arain AN, et al. Hepatitis B Virus Reactivation in Cancer Patients Treated With Immune Checkpoint Inhibitors. J Immunother. 2021;44(3):132–139. doi: 10.1097/CJI.0000000000000358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Godbert B, Petitpain N, Lopez A, Nisse YE, Gillet P. Hepatitis B reactivation and immune check point inhibitors. Dig Liver Dis. 2021;53(4):452–455. doi: 10.1016/j.dld.2020.08.041. [DOI] [PubMed] [Google Scholar]