Abstract
Saccharomyces cerevisiae is often used to produce heterologous proteins that are preferentially secreted to increase economic feasibility. We used N-glycosylation as a tool to enhance protein secretion. Secretion of cutinase, a lipase, and llama VHH antibody fragments by S. cerevisiae or Pichia pastoris improved following the introduction of an N-glycosylation site. When we introduced an N-glycosylation consensus sequence in the N-terminal region of a hydrophobic cutinase, secretion increased fivefold. If an N-glycosylation site was introduced in the C-terminal region, however, secretion increased only 1.8-fold. These results indicate that the use of N glycosylation can significantly enhance heterologous protein secretion.
Eukaryotic cells, such as yeasts, have several quality control systems to ensure that only correctly folded proteins are transported along their secretory pathway (9, 13, 20). These quality control systems are necessary because protein folding in vivo is much more complex than protein folding in vitro. The nascent polypeptide chain emerges from the ribosome and is translocated across the endoplasmic reticulum (ER) membrane, thereby being gradually exposed to the lumen of the ER. Since the polypeptide is not yet folded, the hydrophobic residues are exposed, which, in combination with the high protein concentration in the ER, makes the nascent polypeptide prone to aggregation (8). The cell has several proteins that help to avoid this problem. The best known example of such a protein is the chaperone BiP, the immunoglobulin heavy-chain binding protein (1, 7, 10, 11, 19, 21). If secretory proteins are not correctly folded, they often are retained in the lumen of the ER in BiP-containing clusters (2, 15, 22), thereby preventing further transport along the secretory route. This problem is a well-known bottleneck in the secretion of overexpressed heterologous proteins.
Recently we described the aggregation of a hydrophobic cutinase in the ER in association with BiP (22). The cutinase from Fusarium solani pisi is a lipase with a molecular mass of 21.6 kDa and contains two disulfide bridges (17). This enzyme degrades the cutin layer of plants, enabling penetration by the fungus. Cutinase is active in aqueous solutions, without the need of interfacial activation (26), and is therefore potentially suitable for lipid stain removal applications in the detergent industry (5, 6). However, the natural cutinase has two clear shortcomings: (i) low effective interaction with lipid substrate (on both the molecular and micellar levels) and (ii) sensitivity to anionic detergents. To obtain a cutinase with a higher specific activity, five hydrophobic residues were introduced to increase the enzyme's affinity for its substrates (22). Although wild-type cutinase is secreted efficiently, the hydrophobic cutinase is retained in the ER (22). This hydrophobic cutinase was associated with BiP, probably due to BiP binding to the exposed hydrophobic patches of the modified cutinase (22). Similar phenomena also have been observed with other heterologous proteins in a variety of host organisms (2, 15).
Our objective in this study was to improve the secretion of the hydrophobic cutinase CY028. We hypothesized that N glycosylation could enhance heterologous protein secretion. We used oligosaccharides to shield the hydrophobic patches of the protein during biosynthesis and avoid protein aggregation. We introduced an N-glycosylation site at position 29 to ensure that glycosylation occurs before the hydrophobic stretches are translocated across the ER membrane. The glycosylated hydrophobic cutinase was not retained as ER-localized aggregates but could be transported through the secretory pathway. However, when we introduced an N-glycosylation site at the C terminus of cutinase, the secretion was not significantly increased. This result indicates that the site of glycosylation should occur before the exposed hydrophobic stretches to maximize the secretion enhancement. We also demonstrated the broad application of this strategy by increasing secretion of llama VHH antibody fragments in Saccharomyces cerevisiae and cutinase in Pichia pastoris.
MATERIALS AND METHODS
Strains and genetic constructs.
Cutinase constructs were expressed in S. cerevisiae VW cen.pk111-32D (leu2) or in P. pastoris GS115 (Invitrogen). Different cutinase constructs were used: wild-type cutinase CY000 (25), hydrophobic cutinase CY028 (Gly82Ala, Ala85Phe, Val184Ile, Ala185Leu, Leu189Phe), glycosylated wild-type cutinase CY047 (Ala29Ser), N-region-glycosylated hydrophobic cutinase CY181 (Ala29Ser, Gly82Ala, Ala85Phe, Val184Ile, Ala185Leu, Leu189Phe), and C-region-glycosylated hydrophobic cutinase CY182 (Gly82Ala, Ala85Phe, Val184Ile, Ala185Leu, Leu189Phe, Arg211Asn). In S. cerevisiae, expression was controlled by the GAL7 promoter and the protein was directed to the secretion pathway by the invertase signal sequence. To ensure genetic stability, the constructs were integrated into the chromosomal rRNA gene locus; the construct contained a leu2 gene, which enabled growth on medium lacking leucine (25).
In P. pastoris, expression was controlled by the AOX1 promoter and the protein was directed to the secretion pathway by the invertase signal. These constructs were integrated into the his4 locus to ensure genetic stability. The mutant cutinases were constructed and provided by C. Visser, Unilever Research Laboratory, Vlaardingen, The Netherlands.
The constructs used for expression of the antibody fragments have been described previously (24). These antibodies are devoid of light chains and are referred to as heavy chain immunoglobulin G; therefore, their binding domains consist only of the variable domains of the heavy chains, referred to as VHH (12). These antibody fragments were raised against the dye RR-6 and expressed in S. cerevisiae (24).
Cutinase activity assay.
The cutinase activity assay was performed as described previously (25). p-Nitrophenyl butyrate (Sigma) was used as a substrate, and the increase in absorbance was measured spectrophotometrically at 405 nm. The specific activity of cutinase was determined with olive oil as the substrate and was expressed in specific lipase units (SLUs); 1 SLU corresponds to 1 μmol of free fatty acid released per min.
Statistical methods.
The significance of the cutinase measurements was determined by analysis of variance followed by a t test.
Western blotting.
Western blotting was performed as described before (22).
Endo H treatment.
We used the endoglycosidase H (endo H) deglycosylation kit from Boehringer Mannheim (catalog no. 1 836 579), according to the manufacturer's instructions, to deglycosylate high-mannose-type asparagine-linked glycan chains on glycoproteins.
Electron microscopy.
S. cerevisiae cells were taken up by capillary action in single dry cellulose capillary tubes 5 to 10 mm long (14). After filling, the capillary tube was submerged in 1-hexadecane and cut into about 2-mm pieces, which were subsequently sandwiched in aluminum specimen holders. This sandwich was inserted in a holder of a high-pressure freezer from Leica. After high-pressure freezing, the sandwich was put into liquid nitrogen. The two specimen holders were separated under liquid nitrogen. The capillary tube, still attached to one of the specimen aluminum holders, was freed from adhering 1-hexadecane under liquid nitrogen by gently scraping with a fine needle. Thereafter, the capillary tube attached to the specimen holder was transferred to a 2.0-ml Saf-T-seal free-standing tube (Biozyme) that contained frozen substitution medium (0.3% uranyl acetate and 0.01% glutaraldehyde in anhydrous methanol) and a miniature transfer basket (14). This vial was placed in a CS-auto substitution apparatus (23) at −90°C for 48 h. Subsequently the temperature was raised to −40°C at a speed of 3°C/h. The freeze-substitution medium was exchanged for methanol, and the specimen holders were removed from the vial. The capillary tubes were infiltrated with an increasing concentration of Lowicryl HM20 (4): 25% for 2 h, 50% for 2 h, 75% for 15 h, 100% for 1 h, 100% for 72 h, 100% for 48 h, and fresh made 100% for 48 h. The capillary tubes were embedded in 100% Lowicryl HM20. Polymerization was done at −40°C for 48 h with a UV source attachment (4). After 1 day of curing under UV light at room temperature, ultrathin sections were labeled with immunogold to locate the enzyme cutinase as described before (22).
Pulse-chase.
Yeast cells were grown in shake flasks to the mid-logarithmic phase (optical density at 600 nm [OD600] = 1) in YPGal (1% yeast extract, 2% Bacto Peptone [Difco], 2% galactose). The cells were resuspended in 1 ml of YNB (0.67% yeast nitrogen base without amino acids; Difco) supplemented with amino acids (20 mg of each per liter) and with 2% galactose as the carbon source, to an OD600 of 20. After incubation for 30 min at 30°C, the cells were labeled for 10 min with 15 μCi of [35S]methionine-cysteine from Amersham. Radioactivity was chased by washing the cells in 1 ml of YNB–2% galactose, resuspending the cells in YNB–2% galactose and adding 200 μl of a nonradioactive mixture of methionine (100 mM) and cysteine (50 mM). At the indicated time points 200 μl of cells was lysed by adding 120 μl of 1.85 M NaOH–7.5% β-mercaptoethanol, followed by an incubation on ice for 10 min. The labeled proteins were precipitated by adding 120 μl of 50% trichloroacetic acid and incubation on ice for 10 min. The precipitated proteins were pelleted by centrifugation at 13,000 × g for 10 min. The pellet was washed with 500 μl of 1 M Tris and resuspended in 400 μl of a solution containing 25 mM imidazole, 2.5 mM EDTA, and 2% sodium dodecyl sulfate (SDS), pH 6.8. For solubilization the samples were boiled for 7 min and diluted in 1 ml of INET (50 mM imidazole, 140 mM NaCl, 5 mM EDTA, 1% Triton X-100 [pH 8.0]). After centrifugation at 13,000 × g for 10 min the supernatant was isolated and diluted in INET to a final volume of 5 ml, for immunoprecipitation.
Immunoprecipitation.
Ten microliters of rabbit anticutinase serum was added to the labeled proteins and incubated overnight at 4°C in an orbital shaker at 10 rpm. Twenty-five microliters of protein A coupled to Sepharose (protein A-Sepharose CL-4B; Pharmacia) in radioimmunoprecipitation assay buffer (70 μg in 500 μl) was added, and this mixture was incubated at 4°C for 2 h. The immunocomplexes were washed three times with INET, and 30 μl of sample buffer was added. The samples were boiled for 5 min and centrifuged for 1 min at 13,000 × g. An SDS–12.5% polyacrylamide gel electrophoresis (PAGE) gel was loaded with 20 μl of the supernatant. After amplification (Amersham Amplify) the gels were dried and exposed to Hyperfilm (Amersham).
RESULTS
Production of extracellular cutinase (glyco)variants.
After 24 h wild-type cutinase (CY000) was secreted at a level of 51 mg/liter (standard deviation [SD] = 3.6 mg/liter) and the glycosylated variant (CY047) was secreted at 53 mg/liter (SD = 13 mg/liter). The hydrophobic cutinase (CY028) was secreted at 8 mg/liter (SD = 1.1 mg/liter), but this level was increased dramatically to 41 mg/liter (SD = 5.0 mg/liter) when this cutinase was glycosylated at the N-terminal region (CY181). When the glycosylation site was placed at the C-terminal region the secretion level reached 14 mg/liter (SD = 2.0 mg/liter) (CY182). The C terminus of cutinase is not buried inside the molecule (18) and is accessible for glycosylation after folding.
The specific activity of CY000, with olive oil as the substrate, was 330 SLU/mg; the hydrophobic CY028 cutinase has a specific activity of 1,100 SLU/mg. The glycosylated CY181 cutinase has a specific activity of 900 SLU/mg; glycosylated CY182 cutinase has a specific activity of 440 SLU/mg. These results demonstrate that the introduction of an N-glycosylation site at the N terminus has almost no influence on the specific activity of the hydrophobic cutinase.
Influence of glycosylation on secretion of cutinase (glyco)variants.
We also measured cutinase secretion in the presence of tunicamycin (5 μg/ml), an N-glycosylation inhibitor, and found that the secretion levels of CY181 (5.2 mg/liter [SD = 2.5 mg/liter]) and CY028 (3.4 mg/liter [SD = 0.7 mg/liter]) were comparable, indicating that the inhibition of N glycosylation of CY181 decreases the secretion level back to that of CY028. Thus, the glycosylation of CY181 was responsible for the increased secretion, rather than the A29S mutation. The inhibition of glycosylation of the CY047 cutinase had virtually no effect on secretion, (27 mg/liter [SD = 7.6 mg/liter], whereas CY000 was secreted at 28 mg/liter [SD = 0.3 mg/liter]), and suggests that glycosylation of CY047 is not important for efficient secretion. All of the cutinase variants were secreted at lower levels when the cells were grown in the presence of tunicamycin, probably because of the decreased growth rate of the yeast cells and the inhibition of glycosylation of endogenous glycoproteins.
Determination of glycosylation by endo H treatment.
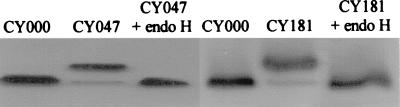
The introduced N-glycosylation sequence in CY047 and CY181 cutinase resulted in a mobility shift on an SDS-PAGE gel relative to CY000 cutinase (Fig. 1). A small amount of CY047 and CY181 had the same mobility as CY000 cutinase (Fig. 1), indicating that not all of the secreted CY047 and CY181 cutinase was glycosylated. After treatment with endo H, only N-acetylglucosamine (GlcNAc) is still attached to the Asn residue of CY047 and of CY181. This results in the same mobility as that of CY000 cutinase and led us to conclude that the observed mobility shift of CY047 and CY181 is due to N-linked glycosylation.
FIG. 1.
Determination of N glycosylation of CY047 and CY181. Ten-microliter aliquots of supernatant of YPGal-grown yeast cultures, containing the respective cutinase variants, were subjected to cleavage of the attached sugars by using the endo H deglycosylation kit (catalog no. 1 836 579; Boehringer Mannheim) according to the manufacturer's instructions. The proteins were separated on an SDS–12% PAGE gel. As a control CY000, CY047, and CY181 cutinases without endo H treatment were loaded on the gel.
Secretion efficiency of cutinase (glyco)variants determined by pulse-chase.
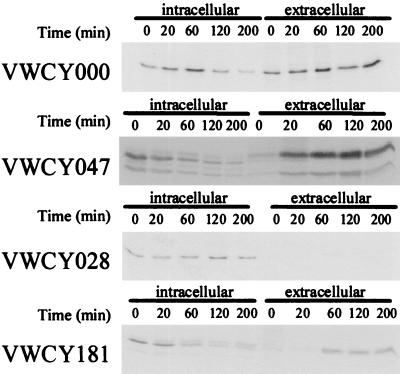
We performed pulse-chase experiments to determine the relative amounts of intra- and extracellular cutinase (Fig. 2). The amount of intracellular wild-type cutinase (VWCY000) decreases and the amount of extracellular cutinase increases rapidly; even at t = 0, wild-type labeled extracellular cutinase is present. This result implies that CY000 cutinase is secreted rapidly and efficiently. The glycosylated form of the wild-type cutinase (CY047) has the same secretion characteristics as CY000; the double band is due to the fact that the glycosylation of this cutinase is not 100% efficient. This result implies that glycosylation is not necessary for secretion of wild-type cutinase. The hydrophobic mutant, CY028, was not secreted during the chase period of 200 min, and no degradation was detected. The N-terminal glycosylated variant of CY028 (CY181) was efficiently secreted. We detected three intracellular forms of CY181, representing different states of glycosylation. With time, the intracellular pool of labeled CY181 decreases as the fully glycosylated CY181 is secreted into the medium. This result implies that glycosylation is required for the efficient secretion of the hydrophobic cutinase.
FIG. 2.
Pulse-chase analysis of cutinase (glyco)variants. Cutinase-expressing cells were labeled for 10 min with 15 μCi of [35S]methionine-cysteine for 10 min. At the indicated time points samples were taken and prepared for immunoprecipitation as described in Materials and Methods. Precipitated complexes were subjected to SDS-PAGE, and autoradiography was performed.
Localization of intracellular CY028 and CY181 cutinase.
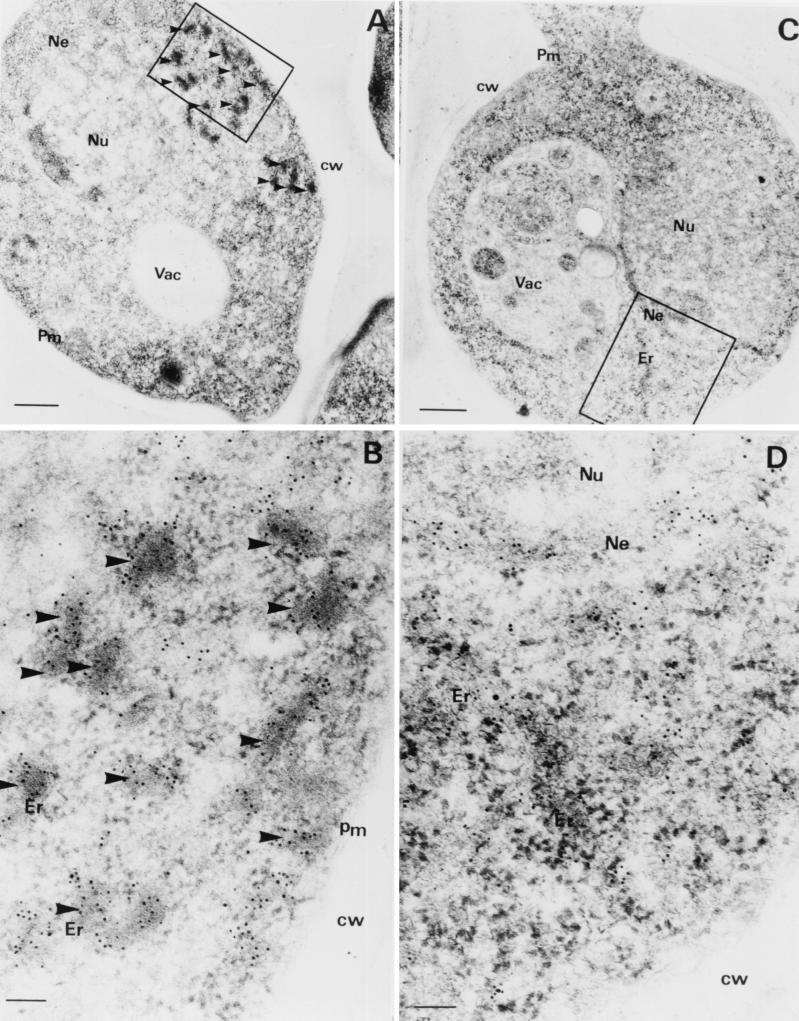
We compared the intracellular localization of the CY181 and CY028 cutinases. The ER in CY181-producing cells had a normal morphology (Fig. 3C and D), whereas the ER in the CY028-producing cells was full of cutinase aggregates and showed a swollen and distorted morphology (Fig. 3A and B). The electron-dense aggregates of cutinase were not found in the CY181 cutinase-producing cells. Instead, cutinase was homogeneously dispersed in ER structures around the nucleus (Fig. 3C and D). The CY181 cutinase-producing yeast cells have a morphology similar to that of CY000 cutinase-producing cells. These findings suggest that CY181 cutinase does not aggregate in the ER as CY028 cutinase does.
FIG. 3.
Immunogold labeling of CY028 and CY181 cutinase-producing S. cerevisiae strains. S. cerevisiae cells were cryofixed and freeze-substituted as described in Materials and Methods. (A and B) CY028 cutinase-producing cells were labeled with anticutinase and goat antirabbit antibodies conjugated with 6-nm-diameter-gold particles. (C and D) CY181 cutinase-producing cells were labeled with anti-cutinase and goat antirabbit antibodies conjugated with 6-nm-diameter-gold particles. CY028 cutinase is located in large electron-dense structures (A and B), whereas CY181 cutinase is distributed homogenously around the nucleus and in structures most likely representing the ER (C and D). Bars represent 500 nm in panels A and C and 100 nm in panels B and D. Arrowheads indicate CY028 cutinase aggregates. Abbreviations: Pm, plasma membrane; Ne, nuclear envelope; cw, cell wall; Nu, nucleus; Vac, vacuole.
Production of cutinase variants in P. pastoris.
In the methylotrophic yeast P. pastoris, CY000 cutinase is secreted at levels of 160 mg/liter (SD = 73 mg/liter) and CY028 cutinase is secreted at levels of 60 mg/liter (SD = 2.8 mg/liter). The CY181, which is N glycosylated in the N-terminal region, is secreted at levels of 120 mg/liter (SD = 30 mg/liter), indicating that N glycosylation also can enhance the cutinase secretion in P. pastoris. The absolute amounts of secreted cutinase are higher than those in S. cerevisiae, probably due to the very strong inducible AOX promoter. The relative level of secreted CY181 cutinase is 80% of that of CY000 cutinase secretion and is similar to the improvement of secretion observed in S. cerevisiae.
Production of N-glycosylated llama VHH antibody fragments by S. cerevisiae.
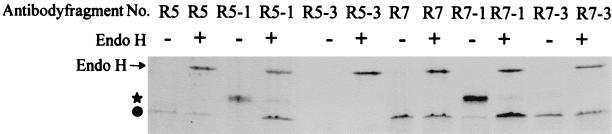
The secretion of llama VHH antibody fragments by S. cerevisiae also improved when an N-glycosylation site was introduced. The secretion of antibody fragments R5 and R7 increased following introduction of an N-glycosylation site (Fig. 4). Glycosylation was determined with endo H treatment, which resulted in a higher electrophoretic mobility of the VHHs. The improvement of secretion was more evident when the glycosylation site was constructed at position 82 (Fig. 4, R5 versus R5-1 and R7 versus R7-1) than when it was at position 110, near the C terminus (Fig. 4, R5 versus R5-3 and R7 versus R7-3).
FIG. 4.
Secretion levels of (glycosylated) antibody fragments by S. cerevisiae. Yeast cells transformed with the indicated antibody fragment construct were grown overnight in yeast extract–peptone–d-glucose diluted 1:10 in YPGal for induction of the antibody fragment gene. After 24 h, antibody fragment production was measured by subjecting 10 μl of culture supernatant to SDS-PAGE with or without treatment with the endo H deglycosylation kit (catalog no. 1 836 579; Boehringer Mannheim) according to the manufacturer's instructions. Symbols: ★, glycosylated antibody fragment; ●, unglycosylated antibody fragment.
DISCUSSION
We improved the secretion of heterologous proteins by introducing an N-glycosylation site. We had found that a hydrophobic cutinase (CY028) was secreted at 10% of the wild-type cutinase secretion level in continuous cultures of S. cerevisiae (22). The majority of the CY028 was retained in ER-derived vesicles in an aggregated form, in association with BiP. This retention is often the fate of overexpressed, heterologous proteins (15, 22). We hypothesized that the introduction of an N-glycosylation consensus sequence would improve the secretion efficiency of the CY028 cutinase since glycosylation of human cytomegalovirus (27) and of a membrane protein in CHO cells (9) increased their secretion.
We identified the positions of potential N-glycosylation sites by using molecular models based on the X-ray structure of cutinase (18). The N glycosylation could not interfere with the correct folding of the cutinase, which precluded introduction of a glycosylation site in one of the central β-sheets, and the sugar groups could not cover the active site of the molecule. If the N-glycosylation site is placed near the N terminus of the proteins, then the core glycosylation should occur before the hydrophobic patches of the CY028 cutinase emerge into the lumen of the ER, where they are prone to aggregation. To minimize interference with the three-dimensional conformation of the cutinase, we substituted only one amino acid (the alanine at position 29 was replaced with a serine), instead of three amino acids, to create an N-glycosylation site.
CY181 was not found in electron-dense aggregates, like CY028, and resulted in an ER morphology comparable to that of the CY000 cutinase-producing cells. CY181 may not form aggregates, as CY028 does (Fig. 3), thereby reducing the aggregated structures that can act as a target for BiP binding. The CY182 cutinase with the N-glycosylation site in the C-terminal region is not as efficiently secreted as CY181. We interpret this result to mean that N-terminal glycosylation is more effective than C-terminal glycosylation in enhancing secretion. N glycosylation also improves secretion of CY028 cutinase by P. pastoris.
N glycosylation at the N terminus does not significantly decrease the specific activity (CY028, 1,100 SLU/mg; CY181, 900 SLU/mg), although N glycosylation at the C terminus does (CY182, 440 SLU/mg). We hypothesize that the decrease of specific activity of CY182 is due to the importance of the C terminus of cutinase in the interaction with the substrate.
Our strategy of introducing an N-glycosylation site also improves the secretion of llama VHH antibody fragments by S. cerevisiae (Fig. 4) and does not alter the specific activity of these antibody fragments. The secretion of these antibody fragments increases when a glycosylation site is located at position 82, but a glycosylation site located near the C terminus, at position 110, does not enhance secretion. These results are similar to those with cutinase, in which an N-glycosylation site located N terminal of the hydrophobic stretches has a more pronounced effect on secretion than does a site that is C terminal of these stretches. Although this difference could be due to less-efficient glycosylation at the C terminus of the antibody fragments (Fig. 4, R5-3 and R7-3), we think it is more likely that glycosylation must occur before hydrophobic portions of the polypeptide are translocated into the ER, where they are prone to aggregation.
When no glycosylation sites can be engineered, an alternative would be to construct a prosequence, containing an N-glycosylation site, at the N terminus of the protein. If a KEX2 cleavage site is constructed between the protein and the prosequence, the Golgi-localized KEX2 protease will cleave the fusion product. In Aspergillus niger this approach was shown to be feasible (3); a similar strategy might also work in yeast.
In conclusion, we have shown that introducing an N-glycosylation site, preferably at the N terminus of the protein, can enhance heterologous protein secretion. This strategy might be useful when heterologous proteins are impaired in secretion due to aggregation. The site of glycosylation should be determined carefully; it should not interfere with the folding of the protein and should not cover the active site of the molecule.
ACKNOWLEDGMENTS
We thank Han Peeters for his work on molecular modeling of cutinase, M. C. D. van der Burg-Koorevaar for the purification of the cutinase variants and the determination of the specific activities, Robert Doornbos for help with the statistical analysis, and John Chapman for critically reading the manuscript.
REFERENCES
- 1.Blond-Elguindi S, Cwirla S E, Dower W J, Lipshutz R J, Sprang S R, Sambrook J F, Gething M J H. Affinity panning of a library of peptides displayed on bacteriophages reveals the binding specificity of BiP. Cell. 1993;75:717–728. doi: 10.1016/0092-8674(93)90492-9. [DOI] [PubMed] [Google Scholar]
- 2.Bole D G, Hendershot L, Kearney J F. Posttranslational association of immunoglobulin heavy chain binding protein with nascent heavy chain in nonsecreting and secreting hybridomas. J Cell Biol. 1986;102:1558–1566. doi: 10.1083/jcb.102.5.1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Broekhuijsen M P, Mattern I E, Contreras R, Kinghorn J R, van den Hondel C A M J J. Secretion of heterologous proteins by Aspergillus niger: production of active human interleukin-6 in a protease-deficient mutant by KEX2-like processing of a glucoamylase-hIL6 fusion protein. J Biotechnol. 1993;31:135–145. doi: 10.1016/0168-1656(93)90156-h. [DOI] [PubMed] [Google Scholar]
- 4.Carlemalm E, Garavito R M, Villiger W. Resin development for electron microscopy and an analysis of embedding at low temperature. J Microsc. 1982;126:123–143. doi: 10.1111/j.1365-2818.1982.tb00358.x. [DOI] [PubMed] [Google Scholar]
- 5.Egmond, M. R., H. W. T. M. van der Hijden, W. Musters, H. Peters, and C. T. Verrips. 1994. Modified cutinases, DNA, vector and host. Patent WO 94/14963.
- 6.Egmond, M. R., H. W. T. M. van der Hijden, W. Musters, H. Peters, and C. T. Verrips. 1994. Modified cutinases, DNA, vector and host. Patent WO 94/14964.
- 7.Gething M J, McCammon K, Sambrook J. Expression of wild-type and mutant forms of influenza hemagglutinin: the role of folding and intracellular transport. Cell. 1986;46:939–950. doi: 10.1016/0092-8674(86)90076-0. [DOI] [PubMed] [Google Scholar]
- 8.Gething M J, Sambrook J. Protein folding in the cell. Nature. 1992;355:33–45. doi: 10.1038/355033a0. [DOI] [PubMed] [Google Scholar]
- 9.Guan J-L, Machamer C E, Rose J K. Glycosylation allows cell-surface transport of an anchored secretory protein. Cell. 1985;42:489–496. doi: 10.1016/0092-8674(85)90106-0. [DOI] [PubMed] [Google Scholar]
- 10.Haas I G. BiP—a heat shock protein involved in immunoglobulin chain assembly. Curr Top Microbiol Immunol. 1991;167:71–82. doi: 10.1007/978-3-642-75875-1_4. [DOI] [PubMed] [Google Scholar]
- 11.Haas I G, Wabl M. Immunoglobulin heavy chain binding protein. Nature. 1983;306:387–389. doi: 10.1038/306387a0. [DOI] [PubMed] [Google Scholar]
- 12.Hamers-Casterman C, Atarhouch T, Muyldermans S, Robinson G, Hamers C, Songa E B, Bendahman N, Hamers R. Naturally-occurring antibodies devoid of light-chains. Nature. 1993;363:446–448. doi: 10.1038/363446a0. [DOI] [PubMed] [Google Scholar]
- 13.Hammond C, Helenius A. Quality control in the secretory pathway: retention of a misfolded viral membrane glycoprotein involves cycling between the ER, intermediate compartment, and Golgi apparatus. J Cell Biol. 1994;126:41–52. doi: 10.1083/jcb.126.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hohenberg H, Mannweiter K, Muller M. High-pressure freezing of cell suspensions in cellulose capillary tubes. Modified cutinases, DNA, vector and host. J Microsc. 1994;175:34–43. doi: 10.1111/j.1365-2818.1994.tb04785.x. [DOI] [PubMed] [Google Scholar]
- 15.Hurtley S M, Bole D G, Hoover L H, Helenius A, Copeland C S. Interactions of misfolded influenza virus hemagglutinin with binding protein (BiP) J Cell Biol. 1989;108:2117–2126. doi: 10.1083/jcb.108.6.2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim P S, Bole D, Arvan P. Transient aggregation of nascent thyroglobulin in the endoplasmic reticulum: relationship to the molecular chaperone, BiP. J Cell Biol. 1992;118:541–549. doi: 10.1083/jcb.118.3.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kolattukudy P E. Cutinases from fungi and pollen. In: Borgstrom B, Brockman H, editors. Lipases. Amsterdam, The Netherlands: Elsevier Science; 1984. pp. 471–504. [Google Scholar]
- 18.Martinez C, de Geus P, Lauwereys M, Matthysens G, Cambillau C. Fusarium solani cutinase is a lipolytic enzyme with a catalytic serine accessible to solvent. Nature. 1992;356:615–618. doi: 10.1038/356615a0. [DOI] [PubMed] [Google Scholar]
- 19.Normington K, Kohno K, Kozutsumi Y, Gething M J, Sambrook J. S. cerevisiae encodes an essential protein homologous in sequence and function to mammalian BiP. Cell. 1989;57:1223–1236. doi: 10.1016/0092-8674(89)90059-7. [DOI] [PubMed] [Google Scholar]
- 20.Parlati F, Dominguez M, Bergeron J J M, Thomas D Y. Saccharomyces cerevisiae CNE1 encodes an endoplasmic reticulum (ER) membrane protein with sequence similarity to calnexin and calreticulin and functions as a constituent of the ER quality control apparatus. J Biol Chem. 1995;270:244–253. doi: 10.1074/jbc.270.1.244. [DOI] [PubMed] [Google Scholar]
- 21.Rose M D, Misra L M, Vogel J P. KAR2, a karyogamy gene, is the yeast homolog of the mammalian BiP/GRP78 gene. Cell. 1989;57:1211–1221. doi: 10.1016/0092-8674(89)90058-5. [DOI] [PubMed] [Google Scholar]
- 22.Sagt C M J, Müller W H, Boonstra J, Verkleij A J, Verrips C T. Impaired secretion of a hydrophobic cutinase by Saccharomyces cerevisiae correlates with an increased association with immunoglobulin heavy-chain binding protein (BiP) Appl Environ Microbiol. 1998;64:316–324. doi: 10.1128/aem.64.1.316-324.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sitte H, Neumann K, Edelmann L. Cryofixation and cryosubstitution for routine work in transmission electron microscopy. In: Müller M, Becker R P, Boyde A, Wolosewick J J, editors. Science of biological specimen preparation. Chicago, Ill: SEM Inc., AMF O'Hare; 1985. pp. 103–108. [Google Scholar]
- 24.van der Linden R H J, Frenken L G J, de Geus B, Harmsen M M, Ruuls R C, Stok W, de Ron L, Wilson S, Davis P, Verrips C T. Comparison of physical chemical properties of llama VHH antibody fragments and mouse monoclonal antibodies. Biochim Biophys Acta. 1999;1431:37–46. doi: 10.1016/s0167-4838(99)00030-8. [DOI] [PubMed] [Google Scholar]
- 25.van Gemeren I A, Musters W, van den Hondel C A M J J, Verrips C T. Construction and heterologous expression of a synthetic copy of the cutinase cDNA from Fusarium solani pisi. J Biotechnol. 1995;40:155–162. doi: 10.1016/0168-1656(95)00041-n. [DOI] [PubMed] [Google Scholar]
- 26.Verger R, de Haas G H. Interfacial enzyme kinetics of lipolysis. Annu Rev Biophys Bioeng. 1979;5:77–117. doi: 10.1146/annurev.bb.05.060176.000453. [DOI] [PubMed] [Google Scholar]
- 27.Zheng Z, Maidji E, Tugizov S, Pereira L. Mutations in the carboxyl-terminal hydrophobic sequence of human cytomegalovirus glycoprotein B alter transport and protein chaperone binding. J Virol. 1996;70:8029–8040. doi: 10.1128/jvi.70.11.8029-8040.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]