Abstract
Since ancient times, plants have been a major source of novel drug molecules and have been used in the treatment of different infectious diseases. Secondary plant metabolites have miraculous healing properties and show potent therapeutic responses when used in combination drug therapy. The prime objective of this review is to summarize the concept of drug combination with special emphasis on the synergistic interactions between plant-derived bioactive phytochemicals with commercially available antimicrobial agents. The study also assesses the roles, importance, and applicability of phytochemicals in the management of different diseases. The review focuses on different aspects of combined antimicrobial activities, the possible mechanisms involved, and the current status of research in the field. The study was conducted based on an extensive literature survey that resulted in the following hypothesis: secondary metabolites derived from plants possess remarkable therapeutic activities. The study was designed as a systematic review that ensures unbiased and accurate representations of the relevant data and information. Jadad scale selection criteria were used for qualitative analysis of the articles to assess them based on the relevant secure score (minimum and maximum scores range between 1 and 5, respectively). Articles with secure scores > 3 were considered for the study. A comprehensive literature survey was conducted using resource databases including PubMed, Google Scholar, Bielefeld Academic Search Engine, Research Gate, Scopus, Medline, and Science Direct up to June 2019. This article contains concise information about the most commonly used bioactive phytochemicals with potent antifungal and antibacterial effects.
Keywords: antimicrobial, antifungal, bioactives, medicinal plants, phytochemicals, synergestic
INTRODUCTION
Our ancestors effectively used natural resources as their sole means of healing infections and injuries. In fact, during the past few decades, plant-derived bioactive materials have played large roles in modern drug discovery. Traditional systems of medicine, such as Ayurveda and Charaka Saṃhita, described a list of 341 plant-derived medicines around 900 BC. Similarly, around 600 BC, Sushruta Samhita described 395 medicinal plants, and around 350 BC, traditional systems of Chinese medicine described 247 medicinal plants and 157 combinations of plant-derived medicines. This literature indicates that plants or plant-derived phytochemicals have remarkable healing capacities for most challenging diseases of society [1]. Until the early 1900s, rational use of most plant-derived bioactive phytochemicals was not achieved due to the deficiency of modern research facilities. The introduction of modern research techniques and research tools provides the scope to explore the medicinal value of designated phytochemicals or bioactive molecules [2].
Combination drug therapy is a promising approach to treat complex diseases, such as cancer, fungal and bacterial infections, inflammation, and others [1-3]. Combination therapy is the use of two or more components together to generate a better therapeutic response against clinical conditions, in which one component may promote the pharmacological action of the other. An alternative drug delivery sometimes used synonymously for combination drug delivery may be defined as one based on the therapeutic value of one chemical entity able to replace another. However, the phenomenon of combined drug interactions is still unpredictable. The most common interactions between two drugs are synergism, antagonism, and summation or additive action. Synergism is a phenomenon in which the pharmacological action of one therapeutically active molecule is increased in the presence of other molecules. The therapeutic activities of plant-derived phytochemicals and their roles in synergism may differ significantly [3]. Despite several controversies, it is possible to make an intense remark on the synergistic activity of therapeutically active phytochemicals in combination with synthetic molecules.
A modern concept like the “isobole method of Berenbaum,” highly efficient technologies like omic technology, pharmacokinetic and pharmacodynamic methods of estimation, biochemical pathway analysis of combinations are the most effective tools for monitoring synergism. The reported literature indicates that there are several mechanisms of synergistic activities [1, 4]. Therefore, the present study has been designed to summarize information about combinations of natural and synthetic compounds that have effective antifungal activity. Information related to compound types and their different mechanisms of action are also discussed.
1. Concept of synergism and its role in the management of infectious diseases
The term synergy comes from the Greek word “synergos,” which means “working together.” The concept of synergy refers to a combination of two or more compounds that generate outcomes greater than the additive impact of those individual compounds. This outcome is the result of interactions in which compounds enhance each other’s performance to achieve a desirable goal. However, if the interaction of bioactive agents or compounds leads to a result less than the sum of the individual effects, this would be designated antagonism. To understand the role and importance of synergism, one should understand the history and the present-day scenario of infectious diseases [5]. Available reports and literature indicate that since they were first recorded, infectious diseases caused by pathogenic bacteria and fungi have remained the most life-threatening issue for society. Infectious diseases affect millions of people worldwide, and a WHO report indicated that approximately 50,000 people die per day globally from these diseases. Hence, the discovery of antimicrobial agents, such as antifungal compounds and antibiotics, was a major achievement in the history of medical sciences [6]. As time went on, new antimicrobial molecules against different active pathogens were introduced to the market. In the last few decades, the irrational and abundant misuse of antimicrobials has allowed clinically important microbes to become resistant to developed antimicrobial agents. Continual efforts by leading scientists are already underway to develop innovative and effective molecules or drugs that can effectively manage infectious diseases [7]. However, the invention of new molecules takes time. Therefore, the lack of new effective molecules and pathogenic resistance to existing molecules is a global health threat.
Several approaches have been taken to manage this situation; of them, multidrug therapy was comparatively more suitable for the effective treatment of infectious diseases [8, 9]. However, the use of synthetic antimicrobials in combination at higher strengths produces more side effects and greater toxicity; therefore, the rational use of multidrug therapy remains controversial.
Plant-derived bioactive phytochemicals have long been used, either singly or in combination, effectively against different life-threatening infectious diseases. Several studies have proposed that natural compounds can potentiate the activity of existing antibiotics and antifungal drugs. In this era, much research has been conducted to explore the medicinal values of different plants or plant-derived products. The results indicate the use of such bioactive materials will help to achieve superior pharmacological and therapeutic outcomes.
MATERIALS AND METHODS
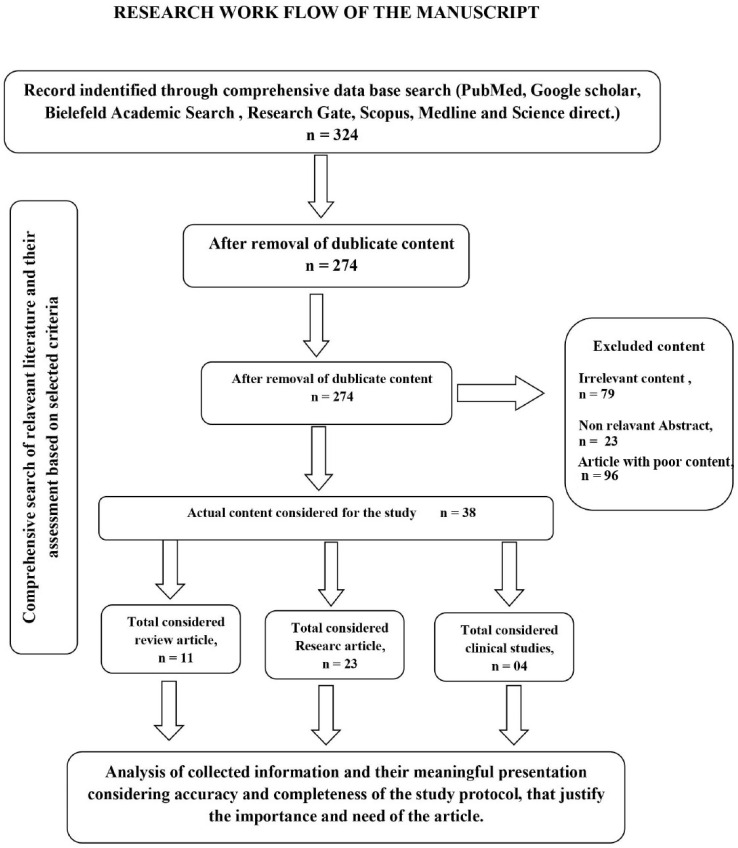
The current review is mainly focused on the role of bioactive phytochemicals in the treatment and management of antimicrobial activities in combination with synthetic drugs and the possibility of improved therapeutic responses. Furthermore, the mechanism involved in antimicrobial action and different strategies involved in disease management were also considered. Keywords, such as antimicrobial, antifungal, antibacterial, clinical trials, combination therapy, summation action, synergistic action, antagonistic effects, natural, plant resources, phytochemicals, bioactive, plant-derived therapeutic agents, synthetic antimicrobial agents, and all their possible combinations, were searched in the databases PubMed, Google Scholar, Bielefeld Academic Search Engine, Research Gate, Scopus, Medline, and Science Direct. Most of the search contents ranged between the year 2000 and June 2017. Literature in the form of abstracts, structured reviews, clinical trial study reports, survey reports, and relevant research articles were collected for this review; however review articles, research articles, and clinical trial reports were prioritized for the write-up. Abstracts, non-relevant articles, repeated articles, and articles with inadequate and irrelevant information were excluded from the study (Fig. 1). Jadad scale selection criteria were used for qualitative analysis of the articles to assess the articles based on their relevant secure scores (minimum and maximum scores range between 1 and 5, respectively). Articles with secure scores > 3 were considered for this study.
Figure 1.
Research work flow.
1. Mechanism of synergism
The role of bioactive phytochemicals on modern therapy can only be justified through exact knowledge and understanding of the mechanisms underlying the therapeutic activity. Based on the latest developments in the fields of classic pharmacological, molecular biological, and clinical techniques, the mechanisms of synergism may be classified as follows.
1) Synergism due to pharmacokinetic or physicochemical interactions
This type of interaction can modify the normal solubility, absorption rate, reabsorption rate, and bioavailability of drugs [10]. Few examples that indicates pharmacokinetic and physicochemical interaction as shown in Table 1.
Table 1.
Synergism due to pharmacokinetic or physicochemical interaction
| Drug A & its mechanism of action | Drug B & its mechanism of action | Reported effects | Possible mechanism of action |
|---|---|---|---|
| Amoxicillin (inhibits bacterial cell-wall synthesis) | Clavulanate (β-lactamase inhibitor) | Antibacterial synergy due to more effective drug distribution or localization | The presence of clavulanate improve the level of amoxicillin at bacterial cell wall by inhibiting its degradation enzyme β-lactamase |
| Sulphamethoxazole (DHPs [Deoxyhypusine synthase] inhibitor) |
Trimethoprim (DHFR [Dihydrofolate reductase] inhibitor) |
No synergism is reported. The only one after other individual effects reflects in the response | Sulphamethoxazole targets the upstream DHPs whereas, trimethoprim targets the downstream DHFR. Trimethoprim act as a backup when the effect of sulphamethoxazolee becomes less effective |
| Erythromycin (inhibit bacterial protein synthesis) | Penicillin (act on bacterial cell wall) |
The combination shows both synergic and additive action due to facilitating action | Presence of penicillin boosts erythromycin penetration into bacterial cells, thereby improve its bioavailability |
2) Synergism due to pharmacodynamics interaction
This type of interaction involves two drugs, which are exerting their activity by joining to the same receptor [11, 12]. Few examples that indicates synergism due to pharmacodynamic interaction are shown in Table 2.
Table 2.
Synergism due to pharmacodynamics interaction
| Drug A & its mechanism of action | Drug B & its mechanism of action | Reported effects | Possible mechanism of action |
|---|---|---|---|
| Cycloserine (inhibits bacterial cell-wall synthesis) | Epigallocatechin gallate (interruption of bacterial cell wall integrity) | Destruction of bacteria cell wall due to the synergistic antibacterial action | The presence of both the component together complements each other. Epigallocatechin disrupts the integrity of bacterial cell wall and cycloserine act as an inhibitor for cell wall synthesis, which hampers the restoration of the cell wall |
| Ampicillin (interrupt bacterial cell-wall synthesis by blocking PBP2A (peptidoglycan transpeptidase) | Daptomycin (break down of bacterial cell membrane structure) |
Strong synergistic antibacterial action | Membrane disruption due to daptomycin, perhaps supported by the presence of ampicillin |
| Artemisinin (disrupts parasite mitochondrial function, modulates host immune function) | Curcumin (generates ROs and produce cytotoxicity for malaria parasites) | Synergic/additiveantimalarial activities | They act at different sites in a non-interfering manner |
| Ampicillin (inhibit bacterial cell-wall synthesis by blocking PBP2A | Imipenem inhibits bacterial cell-wall synthesis by blocking PBP1A, 1B | Synergic/additiveantibacterial effect | Both act at the same active site. Due to the presence of both at relatively high MICs (minimum inhibitory concentration), may make it responsible for the better antibacterial effect |
3) Synergism that overcomes resistance mechanisms of bacteria and fungi
In this type of interaction, the presence of one molecule may improve or facilitate the fundamental mechanism of another chemical entity, which results in better therapeutic activity. This type of synergy occurs when antibiotics are combined with chemical entities that can partly or completely suppress bacterial resistance mechanisms. For example, the combination of penicillin with clavulanic acid (sulbactam or tazobactam) successfully overcomes penicillinase resistance due to three possible reasons: (i) modification of the active site; (ii) modification of antibiotic action by enzymes; and (iii) alteration in the efflux of antibiotics from the cell [1, 10].
Few examples of potential bio-active phytochemicals that played significant role in enchaining therapeutic activity as well as minimizing microbial resistance in combination therapy are shown in the Table 3 [13-35].
Table 3.
List of some bioactive phytochemicals & their role in combination
| Sl no | Combination of active molecules | Observed effect | Mechanism | Ref |
|---|---|---|---|---|
| 1 | Curcumin with Amphotericin B | Antifungal & antibacterial action | A synergistically improve action was observed. In this case, the enzyme inhibitory effect of curcumin mainly reported being responsible for enhanced action | [13] |
| 2 | Garlic oil and allyl alcohol derived from garlic | Potent antifungal and anti-yeast effect | An additive action was observed. Garlic oil was reported to have cell damage capacity which facilitates the action of allyl alcohol, to show potential killing effect by affecting cytosolic components | [14] |
| 3 | Garlic extract with ciprofloxacin | Antibacterial action | The presence of garlic extract improves the inhibitory action of ciprofloxacin | [37] |
| 4 | Rifampicin with nalidixic acid | Anti-microbial action | The combination showed enhanced antimicrobial action may be due to synergistic action. Path of mechanism not reported | [15] |
| 5 | Fluconazole with cardamom oil & boswellia oil | Antifungal activity | Both the combination shows remarkable improved antifungal action may be due to synergism | [16] |
| 6 | Curcumin with fluconazole | Antifungal action against fluconazole-resistant pathogens | The curcumin modulates MDR by inhibiting the transport of fluorescent substrates that are actively effluxes from cells. It improves the sensitivity of fluconazole and, at the same time, practically abolishing cellular growth | [17] |
| 7 | Allicin with ketoconazole | Antifungal activity | The combination demonstrates potential antifungal activity due to synergism | [18] |
| 8 | Thymol with itraconazole (ITR) & fluconazole (FLU) | Potent antifungal action against resistant strain | Thymol enhances the action probably by disruption of the cell wall/membrane integrity mitogen-activated protein kinase (MAPK) system when used in combination with ITR. It probably creates lesions in the plasma membrane and disruption of ergosterol biosynthesis when used with FLU |
[19] |
| 9 | Benzoic acid and its derivatives with fluconazole and itraconazole | Enhance antifungal action against resistant strain | Most effectively enhance the antifungal action of azole derivatives utilizing targeting of an oxidative stress response system | [19] |
| 10 | Caspofung in with ferulic acid | Antifungal action | The combination shows antifungal action due to synergism | [20] |
| 11 | Sulfamethoxazole with myricetin | Synergistic antimicrobial action | The potency of combination increases due to synergism. Myricetin act by DNA binding and induce enzymatic DNA breakage | [21] |
| 12 | Tetracycline with epigallocatechin gallate | Synergistic antimicrobial action | Enhances the activity tetracycline against resistant staphylococcal by impairment of tetracycline efflux pump activity and increased intracellular retention of the drug | [22] |
| 13 | Sulfamethoxazole with proto catechuic acid, ellagic acid, and gallic acid | Synergistic antibacterial and antifungal action | Improve activity may be due to DNA gyrase and topoisomerase IV enzymes | [21] |
| 14 | Sulfadiazine with proto catechuic acid and quercetin | Wide range of antimicrobial action | Reported to have synergism, however, exact mechanisms are unknown | [23] |
| 15 | Kaempferol with norfloxacin and ciprofloxacin | Potent antibacterial action | Combinations show synergism and reported possible mechanism involved may be DNA gyrase and DNA topoisomerase IV | [24] |
| 16 | Ceftazidime with quercetin analogs | Potent antimicrobial action | A possible reported mechanism states that cell wall damage due to leakage of potassium. Both the compound act at the same target either at different sites | [24] |
| 17 | Allium oils with ketoconazole | Fungi static activity | Reported to have synergism. The molecular mechanism was not reported | [18] |
| 18 | Berberine with 5’-methoxyhydnocarpin | Potent antimicrobial action | 5’-methoxyhydnocarpin (1 mg/mL) inhibited the berberine effluxing multidrug pump and thus increased berberine bioavailability. When combined with subinhibitory amounts of berberine, 5’-methoxyhydnocarpin caused complete inhibition of growth at a concentration of 1 mg/mL | [4] |
| 19 | Curcumin with 5-fluorouracil | Anticancer activity | Enhance the capacity of 5-fluorouracil due to synergism. Molecular path not reported | [35] |
| 20 | Quercetin with doxorubicin | Anticancer activity | Quercetin combined with cisplatin, exhibited a proapoptotic effect toward human laryngeal carcinoma cells | [25] |
| 21 | Resveratrol with doxorubicin | Anticancer activity | Resveratrol facilitates doxorubicin uptake by the cells, probably by downregulation of the expression of mrp-1 (mrp-1 belongs to ATP-binding cassette transporter family, involved in multidrug resistance). It acts as an energy-dependent efflux pump whose overexpression causes a decrease in doxorubicin concentration in the cells | [26] |
| 22 | Silibinin with aminoglycosides | Antibacterial action | Improve the action efficacy of aminoglycosides through a significant inhibitory effect on DNA topoisomerase activity due to the formation of complexes that alter enzyme binding | [27] |
| 23 | Kaurenoic acid derivatives with fluconazole | Enhance antifungal action against the fluconazole-resistant strain | Kaurenoic acid derivatives enhance the capacity of fluconazole probably due to inhibition of topoisomerase I | [28] |
| 24 | Glabridin combination with fluconazole | Effectively improve antifungal activity (fungicidal) of fluconazole | Glabridin facilities membrane permeability & damage cell wall, hence increase the performance of fluconazole | [29] |
| 25 | Lactoferrin with fluconazole & itraconazole | Synergistic antifungal activity | Lactoferrin shows synergistic activity in combination with azole derivatives. A possible mechanism may be by promotion or suppression of ergosterol synthesis in the candida cell membrane. Again the iron-chelating function of lactoferrin reported contributing in the synergism | [30] |
| 26 | Nisin with thymol | Synergistic antimicrobial activity | Destabilization of bacterial membrane structure resulting in an increased permeability for nisin which leads to bacterial cell lysis | [31] |
| 27 | Carnosic acid with tetracycline | Antimicrobial activity | Possible mechanism reported inhibiting the MDR pumps | [3] |
4) Synergistic multi-target effects
Synergistic multi-target effects mean that single constituents may show combination effects not only against a single target but also several targets; therefore, they may act in both antagonistic and synergistic ways. Investigation of synergism describes the evidence of multi-targeting that affects gene expression, e.g., methotrexate targets a multitude of genes involved in apoptosis, mismatch repair, cell cycle control, and stress responses [1, 10].
2. Most common methods for in vitro evaluation of synergy
The accurate estimation of synergy between commercial drug molecules or between a drug and bioactive phytochemical based on the results of in vitro testing is essential. Several methods are used to detect synergy. An in-depth understanding of molecular biology, implementation of new clinical and statistical tools for accurate estimation, and introduction of modern technologies, such as omic methods and gene microassay technology, improves the accuracy of in vitro evaluation of synergy. However, for infectious disease concerns, the checkerboard and time-kill curve methods are the two most easily and widely used techniques [36]. In the checkerboard method, microtitration plates are used; for multiple combinations of two antimicrobial agents at different concentrations (equal to, above, and below), their minimal inhibitory concentrations for the microorganism are tested. The combination for which growth is completely inhibited is the effective MIC for the combination [36, 37]. However, the time-kill method assesses the bactericidal activity of the individual and the different concentrations of the drug combinations as a function of time. Tubes containing the individual, as well as the combinations of, compounds with different concentrations ranging from one-quarter to twice the MIC for the bacterial strain are prepared as per NCCLS (National Committee for Clinical Laboratory Standards) guidelines and incubated overnight. Aliquots of samples at 0 h of incubation (reflecting the initial inoculum) and 24 h of incubation (reflecting exposure of bacteria to the compound) are estimated. If a 100-fold or greater decrease in colony count is observed at 24 h by the combination of agents, synergism would be concluded [36-38]. The E-test (Epsilometer test) is another method used for evaluating synergy. It consists of two plastic strips coated with a continuous gradient of each compound on one side. The first compound strip is placed onto an agar plate for 1 h and then removed, and the second compound strip is placed on top of the gradient left behind by the first strip. The MIC of the combination is taken as the value at which the two inhibition zones intersect [38].
Further standardization of these techniques for routine laboratory testing is required for effective and accurate estimation of drug combinations.
DISCUSSION
Combination drug therapy is not a new concept; however, innovative and rational use of phytochemicals to develop safe and effective combination therapies may be the future of infectious disease management. Although the term synergism may be considered a type of combination therapy, in which the presence of one component effectively improves the activities of others, there are several mechanisms. The introduction of modern and improved technologies, statistical tools, and in-depth knowledge of molecular mechanisms may be key to understanding the phenomenon of synergism and its different types. In this article, we have attempted to present a summary of the most effective and often used bioactive compounds in the management of infectious diseases, along with their possible reported mechanisms of action. Again, detailed information about documented synergistic effects of some plant-derived bioactive molecules in combination with synthetic drugs is also discussed. Despite enormous efforts, proper information on the molecular mechanisms underlying the effectiveness of most bioactive phytochemicals is limited. Therefore, a proper understanding of the mechanisms will be the key deciding factor. As far as activities of plant-derived phytochemicals are concerned, in most cases, the appropriate molecular mechanisms remain undefined. Although researchers have reported that different plant extracts show significant therapeutic effects, the lead molecules responsible for their observed activities or their mechanisms of action are yet to be explored. Safe and effective therapies from plant-derived bioactive compounds mainly depend on the toxicity profiles of molecules. Therefore, the concentrations of phytochemicals in a combination and the frequency of use of those combinations are two key factors that should be strictly followed to obtain optimum benefits. Resistance against available synthetic antibacterial and antifungal drugs by pathogenic stains is the most important issue of this era, but it can easily be countered by proper scientific investigations, explanations of the holistic approaches of traditional medicine, and effective utilization of natural resources.
CONCLUSION
This review highlights several aspects of synergism and the role of plant-derived bioactive phytochemicals in developing effective drug combinations for infectious disease management. From the above discussion, it can be concluded that bioactive phytochemicals obtained from different medicinal plants show promising healing capabilities and may effectively treat different diseases. Clear understanding of the molecular mechanisms of such bioactive compounds will enable better disease management. Although the rational design of drug combinations with synergistic effects is challenging, it may become a treatment strategy in the future.
ACKNOWLEDGMENTS
Author would like to acknowledge the management of the Faculty of Pharmaceutical Science, Assam down town University for providing essential facilities and support to complete the study.
Footnotes
CONFLICT OF INTEREST
None.
FUNDING
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
REFERENCES
- 1.Ulrich-Merzenich G, Panek D, Zeitler H, Vetter H, Wagner H. Drug development from natural products: exploiting synergistic effects. Indian J Exp Biol. 2010;48(3):208–19. [PubMed] [Google Scholar]
- 2.Calixto JB. The role of natural products in modern drug discovery. Ann Braz Acad Sci. 2019;91(Suppl 3):e20190105. doi: 10.1590/0001-3765201920190105. [DOI] [PubMed] [Google Scholar]
- 3.Hemaiswarya S, Kruthiventi AK, Doble M. Synergism between natural products and antibiotics against infectious diseases. Phytomedicine. 2008;15(8):639–52. doi: 10.1016/j.phymed.2008.06.008. [DOI] [PubMed] [Google Scholar]
- 4.Ma XH, Zheng CJ, Han LY, Xie B, Jia J, Cao ZW, et al. Synergistic therapeutic actions of herbal ingredients and their mechanisms from molecular interaction and network perspectives. Drug Discov Today. 2009;14(11-12):579–88. doi: 10.1016/j.drudis.2009.03.012. [DOI] [PubMed] [Google Scholar]
- 5.Rani A, Jain S, Dureja P, Kumar R, Kumar A. Synergistic interaction between synthetic and natural products: a promising tool for the development of environmentally safe potent antimicrobial agents. World Appl Sci J. 2009;5:59–63. [Google Scholar]
- 6.Fair RJ, Tor Y. Antibiotics and bacterial resistance in the 21st century. Perspect Medicin Chem. 2014;6:25–64. doi: 10.4137/PMC.S14459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roy A, Choudhury A, Bahadur S, Saha S. Phytoconstituent based mucoadhesive antifungal vaginal formulation: an effective and innovative approach. Biosci Biotechnol Res Commun. 2016;9(4):694–701. doi: 10.21786/bbrc/9.4/17. [DOI] [Google Scholar]
- 8.Tamma PD, Cosgrove SE, Maragakis LL. Combination therapy for treatment of infections with gram-negative bacteria. Clin Microbiol Rev. 2012;25(3):450–70. doi: 10.1128/CMR.05041-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nigam A, Gupta D, Sharma A. Treatment of infectious disease: beyond antibiotics. Microbiol Res. 2014;169(9-10):643–51. doi: 10.1016/j.micres.2014.02.009. [DOI] [PubMed] [Google Scholar]
- 10.Wagner H, Ulrich-Merzenich G. Synergy research: approaching a new generation of phytopharmaceuticals. J Nat Remedies. 2009;9(2):121–41. doi: 10.1016/j.phymed.2008.12.018. [DOI] [PubMed] [Google Scholar]
- 11.Tallarida RJ. Drug synergism: its detection and applications. J Pharmacol Exp Ther. 2001;298(3):865–72. [PubMed] [Google Scholar]
- 12.Chen D, Liu X, Yang Y, Yang H, Lu P. Systematic synergy modeling: understanding drug synergy from a systems biology perspective. BMC Syst Biol. 2015;9:56. doi: 10.1186/s12918-015-0202-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tsao SM, Yin MC. Enhanced inhibitory effect from interaction of curcumin with amphotericin B or fluconazole against Candida species. J Food Drug Anal. 2000;8(3):208–12. doi: 10.38212/2224-6614.2831. [DOI] [Google Scholar]
- 14.Chung I, Kwon SH, Shim ST, Kyung KH. Synergistic antiyeast activity of garlic oil and allyl alcohol derived from alliin in garlic. J Food Sci. 2007;72(9):M437–40. doi: 10.1111/j.1750-3841.2007.00545.x. [DOI] [PubMed] [Google Scholar]
- 15.Amenu D. Antimicrobial activity of medicinal plant extracts and their synergistic effect on some selected pathogens. Am J Ethnomed. 2014;1(1):18–29. [Google Scholar]
- 16.Rabadia A, Kamat S, Kamat D. Antifungal activity of essential oils against fluconazole resistant fungi. Int J Phytomed. 2011;3(4):506–10. [Google Scholar]
- 17.Garcia-Gomes AS, Curvelo JA, Soares RM, Ferreira-Pereira A. Curcumin acts synergistically with fluconazole to sensitize a clinical isolate of Candida albicans showing a MDR phenotype. Med Mycol. 2012;50(1):26–32. doi: 10.3109/13693786.2011.578156. [DOI] [PubMed] [Google Scholar]
- 18.Pyun MS, Shin S. Antifungal effects of the volatile oils from Allium plants against Trichophyton species and synergism of the oils with ketoconazole. Phytomedicine. 2006;13(6):394–400. doi: 10.1016/j.phymed.2005.03.011. [DOI] [PubMed] [Google Scholar]
- 19.Faria NC, Kim JH, Gonçalves LA, Martins Mde L, Chan KL, Campbell BC. Enhanced activity of antifungal drugs using natural phenolics against yeast strains of Candida and Cryptococcus. Lett Appl Microbiol. 2011;52(5):506–13. doi: 10.1111/j.1472-765X.2011.03032.x. [DOI] [PubMed] [Google Scholar]
- 20.Canturk Z. Evaluation of synergistic anticandidal and apoptotic effects of ferulic acid and caspofungin against Candida albicans. J Food Drug Anal. 2018;26(1):439–43. doi: 10.1016/j.jfda.2016.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jayaraman P, Sakharkar MK, Lim CS, Tang TH, Sakharkar KR. Activity and interactions of antibiotic and phytochemical combinations against Pseudomonas aeruginosa in vitro. Int J Biol Sci. 2010;6(6):556–68. doi: 10.7150/ijbs.6.556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sudano Roccaro A, Blanco AR, Giuliano F, Rusciano D, Enea V. Epigallocatechin-gallate enhances the activity of tetracycline in staphylococci by inhibiting its efflux from bacterial cells. Antimicrob Agents Chemother. 2004;48(6):1968–73. doi: 10.1128/AAC.48.6.1968-1973.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sakharkar MK, Jayaraman P, Soe WM, Chow VT, Sing LC, Sakharkar KR. In vitro combinations of antibiotics and phytochemicals against Pseudomonas aeruginosa. J Microbiol Immunol Infect. 2009;42(5):364–70. [PubMed] [Google Scholar]
- 24.Liu MH, Otsuka N, Noyori K, Shiota S, Ogawa W, Kuroda T, et al. Synergistic effect of kaempferol glycosides purified from Laurus nobilis and fluoroquinolones on methicillin-resistant Staphylococcus aureus. Biol Pharm Bull. 2009;32(3):489–92. doi: 10.1248/bpb.32.489. [DOI] [PubMed] [Google Scholar]
- 25.Lewandowska U, Gorlach S, Owczarek K, Hrabec E, Szewczyk K. Synergistic interactions between anticancer chemotherapeutics and phenolic compounds and anticancer synergy between polyphenols. Postepy Hig Med Dosw (Online) 2014;68:528–40. doi: 10.5604/17322693.1102278. [DOI] [PubMed] [Google Scholar]
- 26.Zhu Y, He W, Gao X, Li B, Mei C, Xu R, et al. Resveratrol overcomes gefitinib resistance by increasing the intracellular gefitinib concentration and triggering apoptosis, autophagy and senescence in PC9/G NSCLC cells. Sci Rep. 2015;5:17730. doi: 10.1038/srep17730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.de Oliveira DR, Tintino SR, Braga MF, Boligon AA, Athayde ML, Coutinho HD, et al. In vitro antimicrobial and modulatory activity of the natural products silymarin and silibinin. Biomed Res Int. 2015;2015:292797. doi: 10.1155/2015/292797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.de Andrade Neto JB, da Silva CR, de Sousa Campos R, Nascimento FBSA, Sampaio LS, da Silva AR, et al. Evaluation of synergistic effect of kaurenoic acid derivatives with fluconazole against strains of fluconazole-resistant Candida parapsilosis. Int J Curr Microbiol App Sci. 2015;4(5):68–79. [Google Scholar]
- 29.Liu W, Li LP, Zhang JD, Li Q, Shen H, Chen SM, et al. Synergistic antifungal effect of glabridin and fluconazole. PLoS One. 2014;9(7):e103442. doi: 10.1371/journal.pone.0103442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kobayashi T, Kakeya H, Miyazaki T, Izumikawa K, Yanagihara K, Ohno H, et al. Synergistic antifungal effect of lactoferrin with azole antifungals against Candida albicans and a proposal for a new treatment method for invasive candidiasis. Jpn J Infect Dis. 2011;64(4):292–6. doi: 10.7883/yoken.64.292. [DOI] [PubMed] [Google Scholar]
- 31.Ettayebi K, El Yamani J, Rossi-Hassani B. Synergistic effects of nisin and thymol on antimicrobial activities in Listeria monocytogenes and Bacillus subtilis. FEMS Microbiol Lett. 2000;183(1):191–5. doi: 10.1111/j.1574-6968.2000.tb08956.x. [DOI] [PubMed] [Google Scholar]
- 32.Reuk-ngam N, Chimnoi N, Khunnawutmanotham N, Techasakul S. Antimicrobial activity of coronarin D and its synergistic potential with antibiotics. Biomed Res Int. 2014;2014:581985. doi: 10.1155/2014/581985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cojocneanu Petric R, Braicu C, Raduly L, Zanoaga O, Dragos N, Monroig P, et al. Phytochemicals modulate carcinogenic signaling pathways in breast and hormone-related cancers. Onco Targets Ther. 2015;8:2053–66. doi: 10.2147/OTT.S83597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Aiyegoro OA, Okoh A. Use of bioactive plant products in combination with standard antibiotics: Implications in antimicrobial chemotherapy. J Med Plants Res. 2009;3(13):1147–52. [Google Scholar]
- 35.Wei Y, Yang P, Cao S, Zhao L. The combination of curcumin and 5-fluorouracil in cancer therapy. Arch Pharm Res. 2018;41(1):1–13. doi: 10.1007/s12272-017-0979-x. [DOI] [PubMed] [Google Scholar]
- 36.Sopirala MM, Mangino JE, Gebreyes WA, Biller B, Bannerman T, Balada-Llasat JM, et al. Synergy testing by Etest, microdilution checkerboard, and time-kill methods for pan-drug-resistant Acinetobacter baumannii. Antimicrob Agents Chemother. 2010;54(11):4678–83. doi: 10.1128/AAC.00497-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Orhan G, Bayram A, Zer Y, Balci I. Synergy tests by E test and checkerboard methods of antimicrobial combinations against Brucella melitensis. J Clin Microbiol. 2005;43(1):140–3. doi: 10.1128/JCM.43.1.140-143.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Balouiri M, Sadiki M, Ibnsouda SK. Methods for in vitro evaluating antimicrobial activity: a review. J Pharm Anal. 2016;6(2):71–9. doi: 10.1016/j.jpha.2015.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]