Abstract
Importance
COVID-19 mRNA vaccine-associated myocarditis has previously been described; however specific features in the adolescent population are currently not well understood.
Objective
To describe myocarditis adverse events following immunisation reported following any COVID-19 mRNA vaccines in the adolescent population in Victoria, Australia.
Design
Statewide, population-based study.
Setting
Surveillance of Adverse Events Following Vaccination in the Community (SAEFVIC) is the vaccine-safety service for Victoria, Australia.
Participants
All SAEFVIC reports of myocarditis and myopericarditis in 12–17-year-old COVID-19 mRNA vaccinees submitted between 22 February 2021 and 22 February 2022, as well as accompanying diagnostic investigation results where available, were assessed using Brighton Collaboration criteria for diagnostic certainty.
Exposures
Any mRNA COVID-19 vaccine.
Main outcomes/Mmeasure
Confirmed myocarditis as per Brighton Collaboration criteria (levels 1–3).
Results
Clinical review demonstrated definitive (Brighton level 1) or probable (level 2) diagnoses in 75 cases. Confirmed myocarditis reporting rates were 8.3 per 100 000 doses in this age group. Cases were predominantly male (n=62, 82.7%) and post dose 2 (n=61, 81.3%). Rates peaked in the 16–17-year-old age group and were higher in males than females (17.7 vs 3.9 per 100 000, p=<0.001).
The most common presenting symptoms were chest pain, dyspnoea and palpitations. A large majority of cases who had a cardiac MRI had abnormalities (n=33, 91.7%). Females were more likely to have ongoing clinical symptoms at 1-month follow-up (p=0.02).
Conclusion
Accurate evaluation and confirmation of episodes of COVID-19 mRNA vaccine-associated myocarditis enabled understanding of clinical phenotypes in the adolescent age group. Any potential vaccination and safety surveillance policies needs to consider age and gender differences.
Keywords: COVID-19, cardiology, adolescent health
WHAT IS ALREADY KNOWN ON THIS TOPIC
Previous case studies suggest that there is an association between COVID-19 mRNA vaccines and increased rates of myocarditis, most commonly in the following 2 weeks.
Recommendations on use of COVID-19 mRNA vaccines in different age groups vary globally and may not take into account their different risk profiles.
WHAT THIS STUDY ADDS
Incidence of myocarditis post COVID-19 mRNA vaccines are higher after the second dose and appears to differ by age and gender, with adolescent and young adult males being at a higher risk.
Females were more likely to have ongoing clinical symptoms at 1-month follow-up compared with males.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE AND/OR POLICY
Potential policy and safety surveillance adjustments may need to take into account age and gender differentials in myocarditis when reviewing COVID-19 primary (two-dose) vaccine roll-out to the adolescent population.
Introduction
Australia has utilised two mRNA vaccines as part of its COVID-19 vaccine strategy in 12–17-year olds, namely Comirnaty BNT162b2 COVID-19 (Pfizer-BioNTech) and Spikevax mRNA-1273 (Moderna).1 Comirnaty was initially provisionally licensed by the Australian regulator, the Therapeutic Goods Administration (TGA) for those aged 16 years and older, and administered from 25 January 2021 in adults 18+ years. Spikevax received provisional TGA approval for administration in individuals from 18 years from 9 August 2021. Following the availability of age-specific clinical trial data, further approvals were added for the 12–15-year-old group for Comirnaty (22 July 2021) and 12–17-year old for Spikevax (4 September 2021).
Of particular interest in the young adult population is postvaccination myocarditis and pericarditis causally associated with COVID-19 mRNA vaccines. These adverse events of special interest (AESI) were first flagged in Israel, which implemented Comirnaty at a population level as soon as it was available (20 December 2020).2 In a linked electronic health record observational case–control study design from Israel’s largest healthcare insurer, Barda et al described an elevated risk of myocarditis following Comirnaty (risk ratio (RR): 3.24; 95% CI: 1.55 to 12.44), while noting a substantially higher risk following COVID-19 disease (RR: 18.28; 95% CI: 3.95 to 25.12).3
Subsequent postlicensure observational military and report-based case studies confirmed the highest risk group for post mRNA COVID-19 vaccine myocarditis in young males (<24 years old) following the second vaccine dose.4–6 Both the spontaneous Vaccine Adverse Event Reporting System and active Vaccine Safety Datalink Surveillance System in the USA have confirmed a myocarditis signal safety,7 along with similar findings in Canada, the UK and various Nordic countries.6
The case phenotype includes chest pain as the most common symptom, but also includes fever, shortness of breath and other non-specific symptoms such as headache, myalgia and vomiting. Common investigation findings include a raised troponin, abnormal ECG with ST or T wave changes and late gadolinium enhancement or myocardial oedema seen on cardiac MRI.8–11
Due to this AESI signal, the risk, clinical manifestations and follow-up of myocarditis and pericarditis following mRNA COVID-19 in younger populations has been of particular interest. Some regions (eg, Hong Kong) are only administering a single dose of mRNA vaccine in adolescents aged 12–17 years and notably, there is limited use of Spikevax in this age group internationally. Spikevax is not currently licensed by the US Food and Drug Administration (FDA) for 12–17-year olds, while Canada and several European countries have issued preferential recommendations for Comirnaty over Spikevax for young adults.12 This study describes clinical presentation and evaluation of myocarditis AESI following mRNA COVID-19 vaccination in 12–17-year-old adolescents in Victoria, Australia.
Methods
Victoria is a south-eastern Australian state with a population of approximately 6.6 million.13 Adverse events following immunisation (AEFI) are spontaneously reported by patients, caregivers or healthcare providers to Surveillance of Adverse Events Following Vaccination in the Community (SAEFVIC), the state-wide vaccine safety service.14 SAEFVIC comprises central reporting enhanced passive and active surveillance systems integrated with clinical services and has been operating since 2007.
Identified reports of myocarditis and myopericarditis in 12–17-year-old vaccinees submitted to SAEFVIC between 22 February 2021 and 22 February 2022 were assessed. The majority of these were practitioner reported. Myocarditis and myopericarditis reports (henceforth summarised as myocarditis) were systematically followed up and diagnostic test results (where available) obtained to confirm the diagnoses, including potential alternative causes such as viral, autoimmune or medication-related myocarditis. Data gathered included a list of symptoms such as chest pain, shortness of breath, dizziness and fatigue (table 1), as well as investigations undertaken by treating clinicians, including ECG, cardiac biomarkers, echocardiogram and cardiac MRI scans. All troponin levels obtained were high sensitivity troponin assays, with troponin levels reported as fold increase from the upper limit of normal to facilitate comparison between different assays.
Table 1.
Brighton collaboration criteria for myocarditis
| Level 1: ‘definitive’ case | Level 2: ‘probable case’ | Level 3: ‘possible case’ |
| Histopathologic examination of myocardial tissue (autopsy or endomyocardial biopsy) showed myocardial inflammation OR ≥1 new finding of
AND ≥1 New cMRI findings consistent with
OR Echocardiogram (ECHO) abnormalities biomarkers ≥1 new finding of as per level 2 case |
Clinical symptoms and exclusion as per Level three case AND Elevated myocardial biomarkers ≥1 new finding of
OR Echocardiogram (ECHO) abnormalities ≥1 new finding of
OR ECG abnormalities≥1 new finding of
|
Presence of ≥1 new or worsening of the following clinical symptoms:
OR Presence of ≥2 new or worsening of the following clinical symptoms:
AND ≥1 new supported finding of inflammation Elevated CRP/ESR or D-Dimer AND Presence of ≥1 new abnormal ECG such as:
AND
|
AV, atrioventricular; cMRI, cardiac MRI; PAC, premature atrial complex; PVC, premature ventricular complex.
Once data were available, each case was categorised by at least two independent experts utilising the Brighton Collaboration definition with graded levels of certainty (table 1).15 All reports were forwarded to the national regulator, the TGA, who report weekly on spontaneous AEFI reports at a national level.16 17
Cases where vaccination was the most likely cause of their diagnosis were followed up after 1 month to answer a series of questions about ongoing symptoms and clinical management. Cases were declared lost to follow-up after three unsuccessful attempts to contact.
Patient and Public Involvement
Statistical analysis
Data were analysed using Microsoft PowerBI (V.2.91.701.0) with 90% Poisson CIs calculated for rates. Vaccine doses administered and population estimates were obtained from the Australian Immunisation Registry.18 Age groups were defined by the vaccine roll-out in Victoria, whereby 16–17-year-olds were eligible for vaccination prior to 12–15-year olds. Mood’s median test was used to compare median fold increase in troponin levels.
Results
As of 22 February 2022, 454 974 12–17-year-old Victorians had received 871 689 mRNA doses (782 964 Comirnaty and 88 725 Spikevax). This equated to approximately 97.9% first dose and 93.7% second dose coverage of this age cohort.
At this timepoint, there were 75 reports of confirmed myocarditis (44 myocarditis, 31 myopericarditis) as per Brighton Collaboration level 1 (definitive) or 2 (probable) criteria. This equates to a rate of 8.3 per 100 000 doses (90% CI: 6.8 to 10.1) in this age group (table 1).
Cases were predominantly in males (n=62, 82.7%) and post dose 2 (n=61, 81.3%). Presentation was temporally related to Comirnaty in 63 (84.10) and to Spikevax in 12 (16.0%) cases, respectively. Higher rates were observed in the 16–17-year-old age group, and in males compared with females (p<0.001; table 2).
Table 2.
Count and rate of cases by sex, age group, dose number and Brighton Collaboration level
| Male | Female | Total | |||||
| Count | Rate per 100 000 doses (90% CI) | Count | Rate per 100 000 doses (90% CI) | Count | Rate per 100 000 doses (90% CI) | ||
| Total | 62 | 13.6 (10.9 to 16.8) | 13 | 2.9 (1.7 to 4.7) | 75 | 8.3 (6.8 to 10.1) | |
| Age group (years) | 12–15 | 34 | 11.4 (8.4 to 15.2) | 7 | 2.4 (1.1 to 4.6) | 41 | 7.0 (5.3 to 9.1) |
| 16–17 | 28 | 17.7 (12.6 to 24.2) | 6 | 3.9 (1.7 to 7.6) | 34 | 10.8 (8.0 to 14.4) | |
| Dose | 1 | 10 | 4.4 (2.4 to 7.5) | 4 | 1.8 (0.6 to 4.2) | 14 | 3.2 (1.9 to 4.9) |
| 2 | 52 | 24.2 (19.0 to 30.5) | 9 | 4.3 (2.3 to 7.5) | 61 | 14.4 (11.5 to 17.8) | |
| Brighton collaboration level | Definitive | 27 | 5.9 (4.2 to 8.2) | 3 | 0.7 (0.2 to 1.8) | 30 | 3.3 (2.4 to 4.5) |
| Probable | 35 | 7.7 (5.7 to 10.2) | 10 | 2.3 (1.2 to 3.8) | 45 | 5.0 (3.8 to 6.4) | |
Of the 75 cases in the study period, 5 of them were considered by the treating clinician to have an alternative cause for myocarditis more likely than COVID-19 vaccination. One of these cases had evidence of historical COVID-19 infection, with a positive COVID-19 respiratory PCR 3 weeks prior to receiving first dose Comirnaty vaccination, – with subsequent onset of myocarditis 2 days later.
For the 70 cases related to COVID-19 mRNA vaccination, onset of symptoms ranged from 0 to 49 days after vaccination, with a median of 2 days (IQR: 1–3 days), in males from 0 to 34 days (median 2 days) and in females from 1 to 49 days (median 2 days). Fifty-one (77.1%) cases required hospital admission with a median length of stay of two nights. No cases required intensive care unit (ICU) admission and no deaths were recorded. All admissions were discharged to home.
All cases had chest pain as a presenting symptom. Other common symptoms included dyspnoea (21 cases, 30%), palpitations (14,20%) and diaphoresis. Thirty-three (47.1%) cases had concomitant non-specific symptoms such as dizziness, vomiting and fatigue (table 3).
Table 3.
Symptoms, laboratory, ECG and imaging data
| Symptoms (n=70), n (%) | Count |
| Chest pain | 70 (100%) |
| Palpitations | 14 (20.0%) |
| Dyspnoea | 21 (30.0%) |
| Diaphoresis | 6 (8.6%) |
| Non-specific symptoms (dizziness, vomiting, fatigue) | 33 (47.1%) |
| Laboratory test | Value |
| Troponin (n=70), median fold increase | 138.3 (IQR 56.9–315.0) |
| Testing/imaging | Count |
| ECG (n=70), n (%) | |
| Abnormal | 49 (70.0%) |
| Normal | 21 (30.0%) |
| Abnormal ECG findings or arrhythmias (n=49) | |
| ST-wave or T-wave changes/elevation | 28 (57.1%) |
| ST segment depression in AVR | 9 (18.4%) |
| PR depression without reciprocal ST depression | 7 (14.3%) |
| AV node conduction delay | 3 (6.1%) |
| T wave inversion | 3 (6.1%) |
| Other | 9 (18.4%) |
| Echocardiogram (n=68) | |
| Normal function | 62 (91.2%) |
| Abnormal function | 8 (8.8%) |
| Systolic dysfunction | 5 (62.5%) |
| Wall motion abnormalities | 2 (25.0%) |
| LV strain | 1 (12.5%) |
| Cardiac MRI (n=36) | |
| Abnormal findings, n (%) | 33 (91.7%) |
| Late gadolinium enhancement | 32 (97.0%) |
| Myocardial oedema | 20 (60.6%) |
| Other abnormality on T2 imaging | 9 (27.3%) |
| Pericardial effusion or inflammation | 5 (15.2%) |
| Fibrosis | 2 (2.4%) |
LV, Left ventricular.
ECG abnormalities were observed in 49 (70.0%) cases, with the most common finding being ST-elevation. An echocardiogram was performed in 70 cases and was abnormal in 8 (11.4%). An initial diagnostic cardiac MRI was performed in 36 cases with abnormalities documented in the majority of these (33 cases, 91.7%) (table 3).
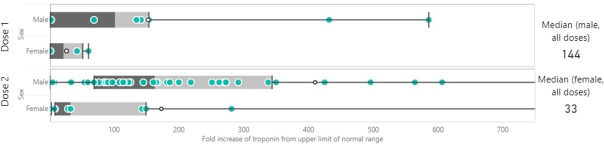
There was a trend for males towards higher and more variable increases in troponin, with a median fold rise of 144 times above normal levels compared with females at 33 times above normal (p=0.222; figure 1).
Figure 1.
Peak troponin differential between males and females. aScale on x axis has been truncated for ease of interpretation. Male dose extends to 2909.09.
Follow-up at 1 month was completed for 64 of the 70 cases where COVID-19 vaccination was the most likely cause of their diagnosis (table 4). The remaining six cases were lost to follow-up and excluded from analysis. Symptoms remained in 50.0% of those who participated in follow-up, with a higher percentage of females having ongoing symptoms (44.6 vs 87.5%, p=0.02) including chest pain (48.0 vs 71.4%, p=0.01; table 4).
Table 4.
One-month follow-up outcomes, by sex (n=64)
| Male (n=56) | Female (n=8) | Total (n=64) | χ2 statistic (p value) | |
| Count, % | Count, % | Count, % | ||
| Ongoing symptoms | 25, 44.6 | 7, 87.5 | 32, 50.0 | 5.14 (p=0.02) |
| Chest pain | 12, 48.0 | 5, 71.4 | 17, 53.1 | 6.05 (p=0.01) |
| Fatigue | 11, 44.0 | 4, 57.1 | 15, 46.9 | 3.60 (p=0.58) |
| Palpitations | 6, 24.0 | 3, 42.8 | 9, 28.1 | 4.16 (p=0.41) |
| Dyspnoea | 6, 24.0 | 3, 42.8 | 9, 28.1 | 4.16 (p=0.41) |
Discussion
We describe 75 cases of myocarditis reported following COVID-19 mRNA vaccination in the adolescent age group. Our rate of cases was similar, although slightly higher than other international studies in Israel, the UK and the USA.17
This rate likely reflects concerted public health messaging and awareness of the AESI, coupled with the combination of active and passive surveillance methodology to ascertain these cases. While this may have led to increased reporting rates, the robust diagnostic criteria requiring laboratory and imaging tests to confirm myocarditis, together with tight COVID-19-related environmental restrictions that significantly reduced the chance of COVID-19 infection-related myocarditis, indicate that this is likely to represent an accurate rate.
Clinical findings were also similar to other international cohorts of adolescents. Dionne et al 18 described similar symptom prevalence in a cohort of myocarditis patients <19 years.18 There was also similar cardiac MRI abnormalities noted in similar cohorts.19 In contrast to our findings, the cohort from Truong et al had a high number of patients (18.7%) requiring ICU admission, although only two required inotropic support.8 It is likely that the difference in ICU admission criteria, rather than true clinical severity accounts for this discrepancy.
Unique to this study is the clear clinical phenotypic differences identified between sexes. Males tended to present with symptoms and a significantly elevated peak troponin level. Just under half had no residual symptoms 4 weeks after initial onset. In contrast, females tended to have had a much lower median peak troponin level, with similar rates post dose 1 and 2. Symptoms were still experienced by a majority of females 4 weeks after onset. These differences may support a potential impact of testosterone as a risk factor for stronger cardiac inflammation in myocarditis, but with a potentially beneficial impact on duration of symptoms.20
Our data indicate a higher rate of cases between the age of 16 and 17 years and a tapering in rate for the 12–15-year-old age group. Further analysis is needed and ongoing to compare rates of myocarditis between Comirnaty and Spikevax when sufficient doses are administered, although there is currently no discernible difference in case severity or clinical presentation.
These observations suggest that any potential immunisation policy and vaccine safety surveillance adjustments may need to take into account these age and gender differentials. For example, it has implications for younger age groups, including those between 5 and 11 years - a reduced dose (10 mcg) Comirnaty vaccine was TGA approved and rolled out starting 10th January 2022 across Australia.
No COVID-19 vaccine clinical trials to date have been sufficiently powered to detect an AESI such as myocarditis. However, with millions of doses administered worldwide in this age group at time of writing, real-world phase IV data will help inform the important risk-benefit discussion regarding COVID-19 vaccines in younger children, who from background rates of myocarditis (all causes) would be expected to have fewer cases than adolescents.
Limitations
This study is based on clinical data compiled by SAEFVIC as part of vaccine safety surveillance. A passive vaccine safety surveillance system may under-report potential cases of myocarditis. Furthermore, there was lack of clinical data on some cases (eg, investigations not performed), making full description of clinical phenotype, evaluation and diagnosis more challenging. While patient-reported symptoms were included as part of the evaluation, Brighton diagnostic criteria were used as a benchmark to reduce recall bias. The accuracy of diagnosis was also maintained by ensuring that available patient clinical information were reviewed by at least two independent medical specialists to verify reported myocarditis cases and reduce misclassification.
Conclusion
Rates of myocarditis in the adolescent population differ by dose and sex in the 12–17-year-old age group. With the vaccine roll-out in younger children and adolescents in mind, these clinical phenotypic differences should be considered for future COVID-19 vaccine recommendations including ‘booster’ doses.
Supplementary Material
Footnotes
Collaborators: SAEFVIC: Hannah Morgan, Greta Goldsmith, Samar Hikmat, Josh Osowicki, Linny Phuong, Mel Addison, Louise Dempsey, Adele Harris, Georgie Lewis, Bianca Penak, Laura Voss, Jaimee Craft, Victoria Scott, Lois Tham. Department of Health (Victoria): Anna Power, Ngaree Blow, Elise Virah Sawmy, Eleanor Duckworth, Michelle Wolthuizen, Naveen Tenneti, Nick White, Melodie Heland, Sally Gordon. VicSIS paediatric clinic sites: Jane Standish (Barwon Health), Kathleen McCloskey (Barwon Health), Brooke Doherty (Barwon Health), Catie Fleming (Austin Health), Jeremy Carr (Monash Health), Matthew O’Brien (Monash Health), Paxton Loke (Monash Health), Ciara Earley (Monash Health), David Tran (Northern Health), Shane O’Dea (Northern Health), Lianne Cox (Western Health), Yoko Asakawa (Western Health), Teresa Lazzaro (RCH), Kirsten Perrett (RCH), Shidan Tosif (RCH), Wonie Uahwatanasakul (RCH) and all other medical, nursing and administrative staff that support the VicSIS clinics. All other hospital sites and clinicians involved in providing information on cases.
Contributors: DRC, NWC and JB conceived and designed the study. HJC, ER and HM performed statistical analyses and data management. PS assisted with data collection. All authors were involved in drafting, editing and reviewing the article. All corresponding authors meet the authorship criteria. DRC is the study’s guarantor.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Contributor Information
the SAEFVIC and VicSIS investigators:
Greta Goldsmith, Samar Hikmat, Josh Osowicki, Linny Phuong, Mel Addison, Louise Dempsey, Adele Harris, Georgie Lewis, Bianca Penak, Laura Voss, Jaimee Craft, Victoria Scott, Lois Tham, Anna Power, Ngaree Blow, Elise Virah Sawmy, Eleanor Duckworth, Michelle Wolthuizen, Naveen Tenneti, Nick White, Melodie Heland, Sally Gordon, Jane Standish, Kathleen McCloskey, Brooke Doherty, Catie Fleming, Jeremy Carr, Matthew O’Brien, Paxton Loke, Ciara Earley, David Tran, Shane O’Dea, Lianne Cox, Yoko Asakawa, Teresa Lazzaro, Kirsten Perrett, Shidan Tosif, and Wonie Uahwatanasakul
Data availability statement
No data are available.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
Follow-up of cases was undertaken as part of public health AEFI management. SAEFVIC data are part of a clinical quality registry that forms part of Victoria’s vaccine safety surveillance program.
References
- 1. Operation COVID Shield . National COVID vaccine campaign plan, 2021. Available: https://www.health.gov.au/sites/default/files/documents/2021/08/op-covid-shield-national-covid-vaccine-campaign-plan.pdf
- 2. Ministry of Health Government of Israel . Surveillance of myocarditis (inflammation of the heart muscle) cases between December 2020 and may 2021, 2021. Available: https://www.gov.il/en/departments/news/01062021-03
- 3. Barda N, Dagan N, Ben-Shlomo Y, et al. Safety of the BNT162b2 mRNA Covid-19 vaccine in a nationwide setting. N Engl J Med Overseas Ed 2021;385:1078–90. 10.1056/NEJMoa2110475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Montgomery J, Ryan M, Engler R, et al. Myocarditis following immunization with mRNA COVID-19 vaccines in members of the US military. JAMA Cardiol 2021;6:1202. 10.1001/jamacardio.2021.2833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kim HW, Jenista ER, Wendell DC, et al. Patients with acute myocarditis following mRNA COVID-19 vaccination. JAMA Cardiol 2021;6:1196. 10.1001/jamacardio.2021.2828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Patone M, Mei XW, Handunnetthi L, et al. Risks of myocarditis, pericarditis, and cardiac arrhythmias associated with COVID-19 vaccination or SARS-CoV-2 infection. Nat Med 2022;28:410–22. 10.1038/s41591-021-01630-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Centers for Disease Control and Prevention . ACIP presentation slides: November 2-3, 2021 meeting, 2021. Available: https://www.cdc.gov/vaccines/acip/meetings/slides-2021-11-2-3.html
- 8. Truong DT, Dionne A, Muniz JC, et al. Clinically suspected myocarditis temporally related to COVID-19 vaccination in adolescents and young adults: suspected myocarditis after COVID-19 vaccination. Circulation 2022;145:345–56. 10.1161/CIRCULATIONAHA.121.056583 [DOI] [PubMed] [Google Scholar]
- 9. Oster ME, Shay DK, Su JR, et al. Myocarditis cases reported after mRNA-based COVID-19 vaccination in the US from December 2020 to August 2021. JAMA 2022;327:331–40. 10.1001/jama.2021.24110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Manfredi R, Bianco F, Bucciarelli V, et al. Clinical profiles and CMR findings of young adults and pediatrics with acute myocarditis following mRNA COVID-19 vaccination: a case series. Vaccines 2022;10:169. 10.3390/vaccines10020169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kildegaard H, Lund LC, Højlund M, et al. Risk of adverse events after covid-19 in Danish children and adolescents and effectiveness of BNT162b2 in adolescents: cohort study. BMJ 2022;377:e068898. 10.1136/bmj-2021-068898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gellad WF. Myocarditis after vaccination against covid-19. BMJ 2021;375:n3090. 10.1136/bmj.n3090 [DOI] [PubMed] [Google Scholar]
- 13. Clothier HJ, Crawford NW, Kempe A, et al. Surveillance of adverse events following immunisation: the model of SAEFVIC, Victoria. Commun Dis Intell Q Rep 2011;35:294–8. [PubMed] [Google Scholar]
- 14. Sexson Tejtel SK, Munoz FM, Al-Ammouri I, et al. Myocarditis and pericarditis: case definition and guidelines for data collection, analysis, and presentation of immunization safety data. Vaccine 2022;40:1499–511. 10.1016/j.vaccine.2021.11.074 [DOI] [PubMed] [Google Scholar]
- 15. Therapeutic Goods Administration (TGA) . Reporting suspected side effects associated with a COVID-19 vaccine, 2021. Available: https://www.tga.gov.au/reporting-suspected-side-effects-associated-covid-19-vaccine
- 16. Therapeutic Goods Administration (TGA) . COVID-19 vaccine weekly safety report - 14.04.2022, 2022. Available: https://www.tga.gov.au/periodic/covid-19-vaccine-weekly-safety-report
- 17. Services Australia . Australian immunisation register. Australian government, 2021. Available: https://www.servicesaustralia.gov.au/australian-immunisation-register
- 18. Dionne A, Sperotto F, Chamberlain S, et al. Association of myocarditis with BNT162b2 messenger RNA COVID-19 vaccine in a case series of children. JAMA Cardiol 2021;6:1446–50. 10.1001/jamacardio.2021.3471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Jain SS, Steele JM, Fonseca B, et al. COVID-19 Vaccination-Associated myocarditis in adolescents. Pediatrics 2021;148:e2021053427. 10.1542/peds.2021-053427 [DOI] [PubMed] [Google Scholar]
- 20. Barcena ML, Jeuthe S, Niehues MH, et al. Sex-Specific differences of the inflammatory state in experimental autoimmune myocarditis. Front Immunol 2021;12:686384. 10.3389/fimmu.2021.686384 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No data are available.