“Artificial intelligence is one of the most profound things we’re working on as humanity. It is more profound than fire or electricity.” - Sundar Pichai
Can Machines Think?
It was 72-years-ago that the British mathematician Alan Turing had posed a provocative question “Can machines think?” in his landmark 1950 paper “Computing Machinery and Intelligence”. He also described a test to evaluate the humanness of the computer.[1] The Turing Test is a 3-player imitation game in which a computer tries to fool a human interrogator into thinking that it’s a person.[1] Seven decades later, despite all the advances in artificial intelligence (AI), no computer has conclusively passed the Turing Test. Despite the failure to conquer the bar of artificial general intelligence prescribed by Turing, computers have come to become an inseparable part of our lives and times. Perhaps, AI is not meant to evolve like a human mind. As Stuart Russell and Peter Norvig pointed out, “Aeronautical engineering texts do not define the goal of their field as making machines that fly so exactly like pigeons that they can fool other pigeons.” The evolution of AI has opened yet unfathomable new paradigms in several domains that intricately affect us, including medicine.
The Bloom Beyond the Dark AI Winter
Unimate, the first industrial robot arm, Eliza, the natural language processor to mimic human communication, and Shakey, the first electronic person were the significant milestones in the early evolution of AI from 1950 to 1970.[2] The period 1970-2000 is referred to as AI winter when skepticism and the prohibitive cost leashed the rapid development of AI.[2] Despite the general winter, the application of AI in medicine saw some progress in these years. A consultation program for glaucoma based on casual-associational network (CASNET), MYCIN, and EMYCIN which guided decision-making for antibiotics and DXplain to generate symptoms-based differential diagnosis evolved during this period.[2]
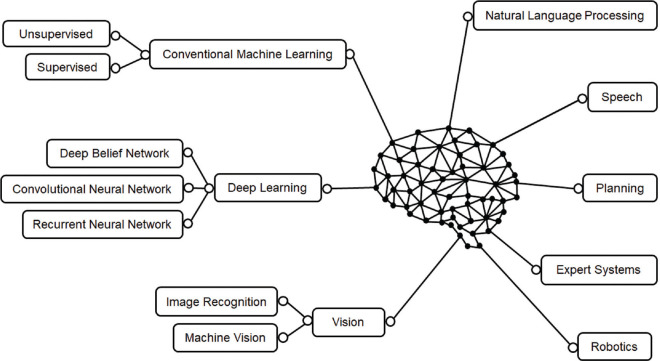
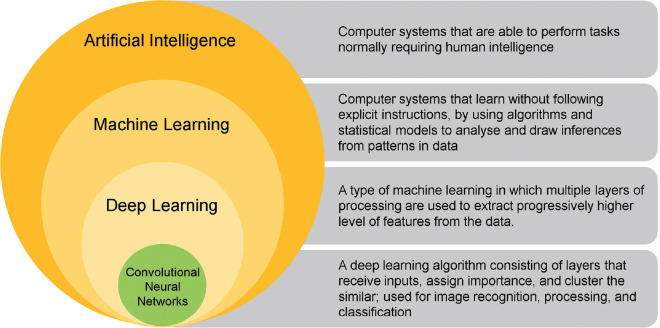
Advances in computational power, newer learning algorithms, availability of massive computer-readable data from electronic medical records, wearable health devices, accessible funding, and the positive influence of the passionate thought leaders have resulted in a seminal transformation in AI and systematic exploitation of the potential of machine learning (ML) in the last two decades. There are essentially three types of AI – artificial narrow intelligence (weak AI), artificial general intelligence, and artificial superintelligence.[3] Based on likeness to the human mind, AI and AI-enabled devices can be classified as 1. reactive machines, 2. limited memory machines, 3. theory of mind AI, and 4. self-aware AI.[3] AI has itself evolved into several unique but inter-connected subfields [Fig. 1]. The domain of ML has radically expanded to include deep learning (DL) and neural networks [Fig. 2].
Figure 1.
Various domains of artificial intelligence
Figure 2.
Deep learning is a type of machine learning. A convolutional neural network is a variant of deep learning
Convolutional neural network (CNN) is an advanced multilayered variant of DL, which simulates interconnected neurons of the human brain to analyze an input image to recognize patterns.[2] Le-NET, AlexNet, VGG, GoogLeNet, and ResNet are some of the CNN algorithms.[2]
IBM Watson was developed to answer the quiz show Jeopardy!. In 2011, it competed on Jeopardy! against champions Brad Rutter and Ken Jennings, winning the prize of $1 million. It is based on DeepQA (which “deeply analyzes natural language input to better find, synthesize, deliver, and organize relevant answers and their justifications”)[4] and leads the pack in clinical applications of AI. Watson Sugar. IQ helps optimize diabetes management.[4] Watson for Oncology (WFO) supports 12 common cancers accounting for 80% of overall cancer incidence. WFO, together with Watson for Genomics, Watson Clinical Trials Matching, and Watson for Drug Discovery, is helping to make cancer care knowledge-powered, evidence-based, and target-driven to optimize the outcomes.[5] Current real-world applications of AI in medicine include disease diagnosis (based on input X-ray, computed tomography, magnetic resonance imaging, histopathology, clinical photographs, electrocardiogram, etc), triage, risk stratification, risk prediction, AI-assisted clinical decision-making, personalized treatment, virtual nursing assistant, target drug development and repurposing, and gene editing.[6]
AI and the Eye
Ophthalmic applications of AI are not new. The CASNET-based glaucoma consultation program in 1976 was demonstrated the feasibility of applying the ML aspect of AI in clinical practice.[7] Ophthalmology, being an image-based and data-rich specialty of medicine, possibly has the widest scope for the application of AI. DL algorithms in ophthalmology are designed centered on disease-based learning, where the clinician assigns specific known characteristics of the disease on the image canvas for the machine to recognize and learn, and the output is verifiable against the identifiers of the disease recognizable by expert human observers.[8] The Google Brain DL algorithm intriguingly taught itself to correctly identify the recognition attributes of DR in anonymized images, and this is termed image-based (“black box”) learning.[9] The most promising AI tools are currently in the field of the retina – for diabetic retinopathy (DR), age-related macular degeneration (AMD), and retinopathy of prematurity (ROP). There are AI models applicable to glaucoma, keratoconus, cataract, and other anterior segment diseases and oculoplastic surgery.[8] This issue of the Indian Journal of Ophthalmology carries publications on AI-applications in glaucoma, ultrasonography, and the acceptability of AI-based retinal screening.[10,11,12]
Diabetic retinopathy
Community-based screening, timely referral, and appropriate treatment constitute the robust three-pronged strategy to prevent blindness from DR. Availability of adequate screening resources, however, remains a challenge. The application of AI can help screen large populations with minimal manpower and maximal efficiency, and accurately identify the pre-defined referrable disease for expedited treatment and thus, can have far-reaching consequences. Abràmoff et al.[13] reported a sensitivity of 96.8% and specificity of 87.0% with an area under the receiver operating characteristic curve (AUC) of 0.980 in the detection of referable DR. Gargeya et al.[14] cross-validated the results and found comparable results with two other datasets. Gulshan et al.[15] reported excellent results using a Google AI-based DL system with an AUC was 0.990 and 0.991 for two different datasets. Ting et al.[16] validated the application of AI in DR screening using real-world data from 10 datasets in 6 countries with an AUC of 0.936, sensitivity of 90.5% and specificity of 91.6% in detecting referable DR and an AUC of 0.958, sensitivity of 100%, and specificity of 91.1% in detecting vision-threatening DR. In a prospective study, Abràmoff et al.[17] achieved 87.2% sensitivity and 90.7% specificity. Their IDx-DR (Digital Diagnostics, Corville, IA, USA) was the first US Food and Drug Administration (FDA)-approved autonomous AI device in medicine, designed to detect diabetic retinopathy and diabetic macular edema.[18] Topcon TRC-NW400 (Topcon, Tokyo, Japan) digital fundus camera is IDx-DR incorporated.[18] EyeArt (Eyenuk, Woodland Hills, Ca, USA) is a FDA-approved autonomous AI to detect more-than-mild and vision-threatening DR and works with with two non-mydriatic fundus cameras - Canon CR-2 AF and the Canon CR-2 Plus AF (Canon, Irvine, CA, USA).[18] EyeArt achieved a high 96% sensitivity and a modest 88% specificity in detecting more-than-mild DR as compared to expert observers. It demonstrated 92% sensitivity and 94% specificity in detecting vision-threatening DR.[18]
Age-related macular degeneration and other macular diseases
With AMD becoming a looming public health problem, the application for AI for screening of referrable AMD is of immediate interest. Ting et al.,[16] using the DR screening image set, demonstrated 0.932 AUC, 93.20% sensitivity, and 88.70% specificity in detecting referable AMD. A diagnostic accuracy ranging from 88.4% and 91.6%, with an AUC between 0.94 and 0.96 was reported using image segmentation with the AREDS data.[19] Grassmann et al.[20] reported 84.2% sensitivity in the detection of any AMD.
The large-scale availability of optical coherence tomography (OCT) images, relative consistency of OCT scans, and intricate structural details that OCT images provide make these an ideal fodder for DL in macular diseases.[16] Beginning with the automated classification of AMD using the two-dimensional OCT images, then on to segmentation of retinal anatomical boundaries and finally combining the power of segmentation and classification to identify standard retinal lesions, DL is now used to triage patients with macular pathologies (choroidal neovascularization, macular edema, drusen, geographic atrophy, epiretinal membrane, vitreomacular traction, macular hole, central serous chorioretinopathy, etc.), for triage and referral (urgent, semi-urgent, routine, observation) in the setting of an unsupervised virtual clinic with accuracy comparable to that of experts.[16]
Retinopathy of prematurity
The management of retinopathy of prematurity is based on timely screening. While expert- and telemedicine-based screening are established strategies, a fully automated DL system was able to accurately identify plus disease with an AUC of 0.98.[21] The i-ROP DL system matched the consensus diagnosis more often than six of the eight international experts.[22] It could also assign an objective severity score for monitoring of disease progression, regression, and response to treatment over time.[22] In being able to detect pre-plus or worse disease, DL algorithm had 100% sensitivity and 94% specificity when compared to the experts.[22] Homegrown 3 nethra camera (Forus Health Incorporated, Bengaluru, Karnataka, India) has also demonstrated high performance in accurately identifying the ROP stage in retinal images.[23]
Glaucoma
Glaucoma is a potentially blinding disease that is amenable to screening. DL algorithms have been trained to identify glaucoma-like disc and to recognize glaucomatous nerve fiber layer damage on wide-angle OCTs,[24] detect early visual field loss,[25] and recognize progression earlier than conventional strategies. A prediction tool that uses intraocular pressure and visual field data to project trajectories of clinical scenarios at various levels of target IOPs is available.[26] Future possibilities include the identification of optic discs associated with the potential loss of visual field and recognition of serial structural changes in the optic disc over time.
Anterior segment disorders
Goh et al.[27] have explored the application of AI for cataract screening using ophthalmic imaging such as fundus photographs or slit lamp images and to automate the selection of best-fit intraocular lens. Wu et al.[28] have provided an elaborate compilation of the scope and range of anterior segment AI applications [Fig. 3]. Data from a Scheimpflug camera has been used to detect keratoconus or identify preclinical keratoconus,[29,30] and to assess corneal power after refractive surgery.[31] There are attempts to perform AI-based eyelid and periorbital measurements, and to plan the surgery for horizontal strabismus.[32]
Figure 3.
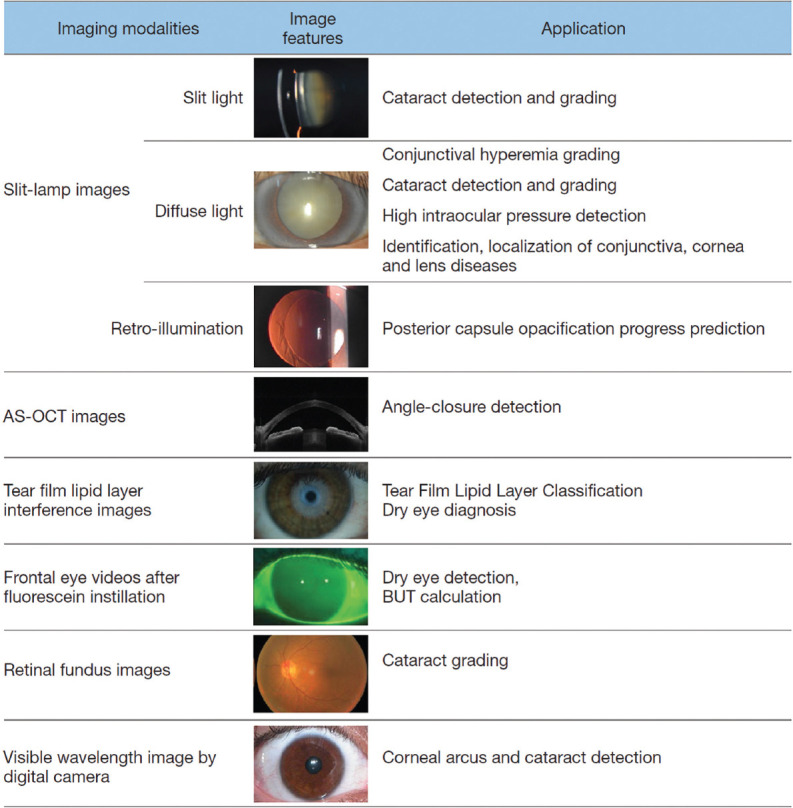
Anterior segment applications of artificial intelligence (reproduced from Wu X, Liu L, Zhao L, Guo C, Li R, Wang T, Yang X, et al. Application of artificial intelligence in anterior segment ophthalmic diseases: diversity and standardization. Ann Transl Med 2020;8:714)
Challenges
In ophthalmology, AI has the potential to universalize patient access to disease screening and diagnosis, help triage referral, expedite treatment as appropriate, and monitor outcomes. However, the real-life challenges to large-scale clinical application of AI in ophthalmology include “1. availability of validated AI solution suitable for practical application by a combination of DL systems with clinically acceptable performance and ability to receive images of varying quality from commonly used devices in a standard clinical setting; 2. resolution of issues of the non-homogeneous patient population, acquisition standards, and image quality; 3. dealing with interobserver variation in clinical findings and agreement between the experts regarding the threshold for referral and intervention; 4. establishing a fair quotient of trust (on part of the patient and the treating ophthalmologist) in AI-based system for critical decision-making and potential medico-legal liability and acceptability; 5. establishing standardized reporting format and consensus criteria for diagnosis/referral/triage, and 6. negotiating technological challenges and gray areas”.[33]
Great Power, Greater Responsibility
The World Health Organization has identified six key areas of ethical concern: “1. Protecting human autonomy, 2. promoting human well-being and safety and the public interest, 3. ensuring transparency, explainability, and intelligibility, 4. fostering responsibility and accountability, 5. ensuring inclusiveness and equity, and 6. promoting AI that is responsive and sustainable.”[34] As emphasized by Gerke et al.,[35] “Informed consent, uncompromised data protection and privacy, adequate cyber resilience and cybersecurity, inherent algorithmic fairness, built-in transparency and regulatory oversight, high standards of safety and effectiveness, and provision for liability” are the key ethical and legal elements that need to be incorporated to create a system with high level of public trust.
Will AI take over the role of ophthalmologists and make them redundant? It is a very unlikely scenario. On the contrary, AI could help perform baseline screening of a large number of patients with resource-efficiency, and bring in a new set of patients (who would otherwise have been missed) with referable/treatable disease to the ophthalmologist. If played well, it is likely to be a win-win game for all the stakeholders.
In 2022, we are unenviably positioned at the road divide – a physician-based medical practice as it exists today versus the attraction of AI. Do we continue to trudge through the familiar territory, or risk taking the least traveled but a potentially exciting route? A middle path with an optimal blend of traditional personal care and technology-driven personalized ophthalmology, where artificial intelligence does not cast a deep shadow on the art of medicine yet maximizes the efficiency and precision, maybe the ideal sweet spot.
“Some people call this artificial intelligence, but the reality is this technology will enhance us. So instead of artificial intelligence, I think we’ll augment our intelligence.” – Ginni Rometty
References
- 1.Turing AM. Computing machinery and intelligence. Mind. 1950;236:433–60. [Google Scholar]
- 2.Kaul V, Enslin S, Gross SA. History of artificial intelligence in medicine. Gastrointest Endosc. 2020;92:807–12. doi: 10.1016/j.gie.2020.06.040. [DOI] [PubMed] [Google Scholar]
- 3. [Last accessed on 2022 Mar 10]. Available from: https://codebots.com/artificial-intelligence/the-3-types-of-ai-is-the-third-even-possible .
- 4. [Last accessed on 2022 Mar 10]. Available from: https://researcher.watson.ibm.com/researcher/view_group_subpage.php?id=2159 .
- 5. [Last accessed on 2022 Mar 10]. Available from: https://healthcare-digital.com/technology-and-ai/four-ways-which-watson-transforming-healthcare-sector .
- 6.Basu K, Sinha R, Ong A, Basu T. Artificial intelligence: How is it changing medical sciences and its future? Indian J Dermatol. 2020;65:365–70. doi: 10.4103/ijd.IJD_421_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weiss S, Kulikowski CA, Safir A. Glaucoma consultation by computer. Comput Biol Med. 1978;8:25–40. doi: 10.1016/0010-4825(78)90011-2. [DOI] [PubMed] [Google Scholar]
- 8.Ting DSW, Pasquale LR, Peng L, Campbell JP, Lee AY, Raman R, et al. Artificial intelligence and deep learning in ophthalmology. Br J Ophthalmol. 2019;103:167–75. doi: 10.1136/bjophthalmol-2018-313173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. [Last accessed 2022 Mar 10]. Available from: https://www.aao.org/eyenet/article/artificial-intelligence .
- 10.Ramesh PV, Subramaniam T, Ray P, Devadas AK, Ramesh SV, Ansar SM, et al. Utilizing human intelligence in artificial intelligence for detecting glaucomatous fundus images using human-in-the-loop machine learning. Indian J Ophthalmol. 2022;70:1131–8. doi: 10.4103/ijo.IJO_2583_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shah P, Mishra D, Shanmugam M, Vighnesh MJ, Jayaraj H. Acceptability of artificial intelligence-based retina screening in general population. Indian J Ophthalmol. 2022;70:1140–4. doi: 10.4103/ijo.IJO_1840_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Adithya VK, Baskaran P, Aruna S, Mohankumar A, Hubschman JP, Shukla AG, et al. Development and validation of an offline deep learning algorithm to detect vitreoretinal abnormalities on ocular ultrasound. Indian J Ophthalmol. 2022;70:1145–9. doi: 10.4103/ijo.IJO_2119_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abràmoff MD, Lou Y, Erginay A, Clarida W, Amelon R, Folk JC, et al. Improved automated detection of diabetic retinopathy on a publicly available dataset through integration of deep learning. Invest Ophthalmol Vis Sci. 2016;57:5200–6. doi: 10.1167/iovs.16-19964. [DOI] [PubMed] [Google Scholar]
- 14.Gargeya R, Leng T. Automated identification of diabetic retinopathy using deep learning. Ophthalmology. 2017;124:962–9. doi: 10.1016/j.ophtha.2017.02.008. [DOI] [PubMed] [Google Scholar]
- 15.Gulshan V, Peng L, Coram M, Stumpe MC, Wu D, Narayanaswamy A, et al. Development and validation of a deep learning algorithm for detection of diabetic retinopathy in retinal fundus photographs. JAMA. 2016;316:2402–10. doi: 10.1001/jama.2016.17216. [DOI] [PubMed] [Google Scholar]
- 16.Ting DSW, Cheung CY, Lim G, Tan GSW, Quang ND, Gan A, et al. Development and validation of a deep learning system for diabetic retinopathy and related eye diseases using retinal images from multiethnic populations with diabetes. JAMA. 2017;318:2211–23. doi: 10.1001/jama.2017.18152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Abramoff MD, Lavin PT, Birch M, Shah N, Folk JC. Pivotal trial of an autonomous AI-based diagnostic system for detection of diabetic retinopathy in primary care offices. NPJ Digit Med. 2018;28:1–39. doi: 10.1038/s41746-018-0040-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. [Last accessed on 2022 Mar 10]. Available from: https://www.reviewofophthalmology.com/article/artificial-intelligence-the-big-questions .
- 19.Burlina PM, Joshi N, Pekala M, Pacheco KD, Freund DE, Bressler NM. Automated grading of age-related macular degeneration from color fundus images using deep convolutional neural networks. JAMA Ophthalmol. 2017;135:1170–6. doi: 10.1001/jamaophthalmol.2017.3782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grassmann F, Mengelkamp J, Brandl C, Harsch S, Zimmermann ME, Linkohr B, et al. A deep learning algorithm for prediction of age-related eye disease study severity scale for age-related macular degeneration from color fundus photography. Ophthalmology. 2018;125:1410–20. doi: 10.1016/j.ophtha.2018.02.037. [DOI] [PubMed] [Google Scholar]
- 21.Brown JM, Campbell JP, Beers A, Chang K, Ostmo S, Chan RVP, et al. Automated diagnosis of plus disease in retinopathy of prematurity using deep convolutional neural networks. JAMA Ophthalmol. 2018;136:803–10. doi: 10.1001/jamaophthalmol.2018.1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brown JM, Campbell JP, Beers A. Fully automated disease severity assessment and treatment monitoring in retinopathy of prematurity using deep learning. Proceedings Volume 10579, Medical Imaging 2018: Imaging Informatics for Healthcare, Research, and Applications. 2018 [Google Scholar]
- 23.Chen JS, Coyner AS, Ostmo S, Sonmez K, Bajimaya S, Pradhan E, et al. Deep learning for the diagnosis of stage in retinopathy of prematurity: Accuracy and generalizability across populations and cameras. Ophthalmol Retina. 2021;5:1027–35. doi: 10.1016/j.oret.2020.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Christopher M, Belghith A, Weinreb RN, Bowd C, Goldbaum MH, Saunders LJ, et al. Retinal nerve fiber layer features identified by unsupervised machine learning on optical coherence tomography scans predict glaucoma progression. Invest Ophthalmol Vis Sci. 2018;59:2748–56. doi: 10.1167/iovs.17-23387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang M, Pasquale LR, Shen LQ, Boland MV, Wellik SR, De Moraes CG, et al. Reversal of glaucoma hemifield test results and visual field features in glaucoma. Ophthalmology. 2018;125:352–60. doi: 10.1016/j.ophtha.2017.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kazemian P, Lavieri MS, Van Oyen MP, Andrews C, Stein JD. Personalized prediction of glaucoma progression under different target intraocular pressure levels using filtered forecasting methods. Ophthalmology. 2018;125:569–77. doi: 10.1016/j.ophtha.2017.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goh JHL, Lim ZW, Fang X, Anees A, Nusinovici S, Rim TH, et al. Artificial intelligence for cataract detection and management. Asia Pac J Ophthalmol (Phila) 2020;9:88–95. doi: 10.1097/01.APO.0000656988.16221.04. [DOI] [PubMed] [Google Scholar]
- 28.Wu X, Liu L, Zhao L, Guo C, Li R, Wang T, et al. Application of artificial intelligence in anterior segment ophthalmic diseases: Diversity and standardization. Ann Transl Med. 2020;8:714. doi: 10.21037/atm-20-976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kovács I, Miháltz K, Kránitz K, Juhász É, Takács Á, Dienes L, et al. Accuracy of machine learning classifiers using bilateral data from a Scheimpflug camera for identifying eyes with preclinical signs of keratoconus. J Cataract Refract Surg. 2016;42:275–83. doi: 10.1016/j.jcrs.2015.09.020. [DOI] [PubMed] [Google Scholar]
- 30.Hidalgo IR, Rodriguez P, Rozema JJ, Dhubhghaill SN, Zakaria N, Tassignon MJ, et al. Evaluation of a machine-learning classifier for keratoconus detection based on Scheimpflug tomography. Cornea. 2016;35:827–32. doi: 10.1097/ICO.0000000000000834. [DOI] [PubMed] [Google Scholar]
- 31.Koprowski R, Lanza M, Irregolare C. Corneal power evaluation after myopic corneal refractive surgery using artificial neural networks. Biomed Eng Online. 2016;15:1–2. doi: 10.1186/s12938-016-0243-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.de Almeida JD, Silva AC, Teixeira JA, Paiva AC, Gattass M. Surgical planning for horizontal strabismus using support vector regression. Comput Biol Med. 2015;63:178–86. doi: 10.1016/j.compbiomed.2015.05.025. [DOI] [PubMed] [Google Scholar]
- 33.Gunasekeran DV, Wong TY. Artificial intelligence in ophthalmology in 2020: A Technology on the cusp for translation and implementation. Asia Pac J Ophthalmol (Phila) 2020;9:61–6. doi: 10.1097/01.APO.0000656984.56467.2c. [DOI] [PubMed] [Google Scholar]
- 34.Ethics and governance of artificial intelligence for health. [Last accessed on 2022 Mar 10]. Available from: https://www.who.int/teams/health-ethics-governance/emerging-technologies/big-data-and-artificial-intelligence .
- 35.Gerke S, Minssen T, Cohen G. Ethical and legal challenges of artificial intelligence-driven healthcare. Artificial Intelligence in Healthcare. 2020:295–336. [Google Scholar]