Abstract
Purpose:
We aimed to assess the impact of drinking water (500 and 1000 mL) on corneal biomechanics and determine the level of association between changes in intraocular pressure and variations in the different biomechanical properties of the cornea.
Methods:
A total of 39 healthy young adults ingested either 1000 mL (n = 21) or 500 mL (n = 18) of tap water in 5 min. The CorVis ST system was used to assess corneal biomechanics at baseline and at 15, 30, and 45 min after water ingestion.
Results:
Water drinking induced statistically significant changes in the deformation amplitude (P < 0.001, η² = 0.166), highest concavity time (P = 0.012, η² = 0.093), peak distance (P < 0.001, η² = 0.171), time and velocity of the first applanation (P < 0.001, η² = 0.288 and P = 0.016, η² = 0.087, respectively), and time and velocity of the second applanation (P = 0.030, η² = 0.074 and P = 0.001, η² = 0.132, respectively), being independent of the amount of water ingested (P > 0.05 in all cases). There were significant associations between changes in intraocular pressure and some parameters of corneal biomechanics
Conclusion:
Small variations in whole-body hydration status alter different biomechanical properties of the cornea, with these changes being associated with intraocular pressure levels. These findings indicate that whole-body hydration status can be considered for the diagnosis and management of different ocular conditions.
Keywords: Corneal biomechanics, Corvis, hydration, water drinking
Human physiology is altered by internal and external factors that must be compensated for by immediate or long-term adaptations to preserve homeostasis.[1] In regard to ocular physiology, several aspects, such as circadian variations,[2] psychological background,[3,4] physical exercise,[5] and diet,[6] have been demonstrated to affect different ocular indices. Remarkably, the short-term effects of hydration on ocular physiology have been investigated, showing that water loading induces significant changes in axial length[7] and intraocular pressure.[8] In addition, whole-body hydration status is associated with tear osmolality[9] and different corneal properties,[10] suggesting that hydration status must be taken into account by ophthalmologists and optometrists when interpreting clinical signs.
In this regard, an acute reduction of central corneal thickness has been observed after corneal dehydration.[11] However, a recent investigation suggested that water intake decreases viscosity and cancels out the effect of the increase in thickness.[12] Moreover, the refractive index of the cornea is negatively associated with its water content, and the ablation effects of excimer laser techniques are affected by corneal hydration levels.[10] Interestingly, recent animal studies have demonstrated that corneal biomechanical properties are modulated as a function of corneal hydration levels.[13,14,15]
Based on the previously mentioned scientific evidence associated with the impact of water intake on different ocular parameters,[7,8] we decided to explore the effects of different levels of whole-body hydration on corneal biomechanics by using recent technological developments for the analysis of corneal biomechanics. Therefore, the main objectives of the present study were (i) to assess the short-term effects of water loading on the biomechanical properties of the cornea, (ii) to determine the influence of the amount of water ingested (500 mL vs. 1000 mL), and (iii) to explore whether the changes in the different parameters of corneal biomechanics are associated with the intraocular pressure (IOP) variation caused by water loading. As water is a major constituent of the eye[16] and changes in corneal hydration have been demonstrated to induce variations in central corneal thickness,[11] we hypothesized that (i) drinking a considerable amount of water would alter the biomechanical properties of the cornea (as it has been shown for other ocular indices),[7,8] (ii) these changes would be dependent on the amount of water ingested,[13] and (iii) the IOP variations induced by water intake would be associated with changes in the biomechanical properties of the cornea.[17]
Methods
Participants and ethical approval
The sample size was calculated based upon an a priori power analysis using GPower 3.1 software.[18] As this study was the first of its nature and because of the lack of applicable data, this analysis was based on an assumed effect size of 0.20, an alpha of 0.05, and a power of 0.80. This analysis projected a minimum sample size of 36. A total of 39 healthy young adults took part in this study and were randomly assigned to one of the two experimental conditions (1000 mL (n = 21) and 500 mL (n = 18)) [see Table 1 for a description of the experimental sample]. All participants were free of any ocular or systemic disease and had no history of refractive surgery and orthokeratology. In addition, we did not include people who wore contact lenses. This study was conducted in accordance with the Declaration of Helsinki, and permission was obtained from the University of Granada Institutional Review Board (IRB approval: 438/CEIH/2017). Informed consent was obtained from all participants included in the study.
Table 1.
Descriptive (mean±standard deviation) characteristics of the experimental sample
| 1000-mL group | 500-mL group | Total sample | |
|---|---|---|---|
| Sample size | 21 | 18 | 39 |
| Age (years) | 22.4±5.0 | 22.7±5.3 | 22.5±5.1 |
| Gender (males/females) | 9/12 | 7/11 | 16/23 |
| Central corneal thickness (µm) | 564.8±33.2 | 565.4±29.3 | 565.1±31.1 |
| Intraocular pressure (mm Hg) | 16.0±2.7 | 16.3±2.6 | 16.2±2.7 |
Instrument
We used the corneal visualization Scheimpflug technology (CorVis ST; Oculus, Wetzlar, Germany), which provides repeatable and accurate measurements of the biomechanical properties of the cornea.[15,19,20] This instrument is a non-contact tonometer, which is synchronized with an ultra-high-speed Scheimpflug camera that allows one to take 4330 images/s and 8.5-mm horizontal corneal coverage to determine the corneal response to an air puff pulse (see Hon and Lam[19] for a description). The analysis of the corneal deformation caused by the air puff indentation provides different parameters related to corneal biomechanics, including indices (time, velocity, and length) of the flattened cornea in the first applanation (inward applanation). Due to the viscoelastic properties of the cornea, it rebounds from the point of highest concavity to another point of applanation (the second applanation, outward applanation) and then returns to its natural convex curvature. Other indices commonly analyzed with this apparatus are the maximum deformation amplitude of the cornea at the highest concavity, the distance of the two apexes of the cornea at the highest concavity (peak distance (PD)), time from the start of deformation until the time at which the highest concavity of the cornea is reached (highest concavity time), and the central curvature radius at the highest concavity (highest concavity radius).[19,21] In addition, the CorVis ST measures central corneal thickness (CCT) and IOP.
Experimental design and procedure
We used a mixed design to assess the influence of ingesting 1000 and 500 mL of water on corneal biomechanics. The within-participants factor was the point of measure (baseline, 15 min, 30 min, and 45 min), whereas the between-participants factor was the amount of water ingested (1000 and 500 mL). We considered the following indices given by the Corvis ST system as dependent variables: non-corrected IOP; biomechanically corrected IOP (bIOP); central corneal thickness (CCT); deformation amplitude (DA); highest concavity (HC) time; peak distance (PD); highest concavity curvature (HC radius); and time, length and velocity of the first and second applanations (A1 time, A1 length, and A1 velocity and A2 time, A2 length, and A2 velocity). Only the right eye was assessed. All measurements were taken by the same examiner, and the Corvis ST readings with alignment errors were discarded.
The participants were asked to abstain from any food or liquid 2 h prior to the test.[7,8] The participants were asked to refrain from alcohol and caffeine-based drinks 12 h before presenting to the laboratory and to sleep at least 7 h the preceding night. Corneal biomechanics were measured immediately before the ingestion of water (baseline measurement). After this first measurement, they were asked to drink either 1000 or 500 mL of tap water in 5 min, with the amount of water ingested being chosen in a random manner. After this, corneal biomechanics were evaluated 15, 30, and 45 min after water intake.
Statistical analysis
Before any statistical analysis, the normal distribution of the data (Shapiro–Wilk test) and the homogeneity of variances (Levene’s test) were confirmed (P > 0.05). We performed separate mixed analyses of variance (ANOVAs) for each of the dependent variables, considering the point of measure as the only within-participants factor and water intake as the only between-participants factor. Additionally, we performed bivariate correlations between the IOP changes after 15, 30, and 45 min of water intake and the changes of the different indices of the corneal biomechanics at the same points of measure. We reported Cohen’s d and eta-squared (2) as effect size indices, and post hoc tests were corrected using the Holm–Bonferroni procedure. The level of statistical significance was set at 0.05.
Results
Table 2 shows the descriptive values for all the variables for corneal biomechanics assessed at the different points of measure in both experimental conditions.
Table 2.
Average±standard deviation values for corneal biomechanics indices at the different points in both experimental conditions
| Baseline | 15 min | 30 min | 45 min | |||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|||||
| 1000 mL | 500 mL | 1000 mL | 500 mL | 1000 mL | 500 mL | 1000 mL | 500 mL | |
| IOP (mm Hg) | 16.0±2.8 | 16.3±2.6 | 17.9±3.6 | 17.5±3.7 | 17.6±4.1 | 17.8±4.2 | 17.5±3.9 | 17.1±3.3 |
| bIOP (mm Hg) | 15.4±2.1 | 15.8±2.0 | 17.6±16.7 | 16.7±2.9 | 16.8±3.1 | 16.8±3.4 | 16.9±2.8 | 16.4±2.7 |
| CCT (µm) | 564.8±33.2 | 565.4±29.3 | 563.1±32.5 | 567.0±24.3 | 565.5±34.4 | 567.9±29.1 | 562.5±31.6 | 564.7±27.3 |
| A1 time (ms) | 7.46±0.41 | 7.51±0.36 | 7.74±0.53 | 7.71±0.51 | 7.72±0.56 | 7.74±0.53 | 7.72±0.55 | 7.65±0.46 |
| A1 length (mm) | 0.13±0.01 | 0.13±0.01 | 0.13±0.01 | 0.13±0.01 | 0.13±0.01 | 0.13±0.01 | 0.13±0.01 | 0.13±0.01 |
| A1 velocity (m/s) | 0.14±0.02 | 0.13±0.02 | 0.13±0.02 | 0.13±0.02 | 0.13±0.02 | 0.13±0.02 | 0.13±0.02 | 0.13±0.02 |
| A2 time (ms) | 21.89±0.40 | 21.79±0.39 | 21.71±0.31 | 21.74±0.42 | 21.77±0.39 | 21.72±0.44 | 21.78±0.36 | 21.86±0.40 |
| A2 length (mm) | 0.39±0.06 | 0.37±0.05 | 0.38±0.06 | 0.37±0.06 | 0.38±0.07 | 0.36±0.06 | 0.38±0.06 | 0.39±0.04 |
| A2 velocity (m/s) | -0.28±0.04 | -0.27±0.03 | -0.25±0.03 | -0.26±0.03 | -0.26±0.03 | -0.26±0.04 | -0.27±0.03 | -0.27±0.04 |
| HC time (ms) | 17.16±0.54 | 17.06±0.32 | 16.99±0.28 | 16.94±0.40 | 16.86±0.41 | 16.91±0.45 | 16.89±0.41 | 16.89±0.24 |
| HC radius (mm) | 7.84±1.06 | 8.20±0.82 | 8.02±1.21 | 7.86±0.92 | 7.89±0.90 | 8.13±1.01 | 7.80±1.04 | 8.16±0.92 |
| PD (mm) | 5.02±0.32 | 5.07±0.31 | 4.89±0.34 | 4.91±0.39 | 4.92±0.40 | 4.95±0.40 | 4.92±0.38 | 5.02±0.34 |
| DA (mm) | 1.05±0.11 | 1.02±0.11 | 0.98±0.10 | 1.00±0.13 | 1.00±0.11 | 1.00±0.13 | 1.01±0.11 | 1.02±0.11 |
Abbreviations: IOP=non-corrected intraocular pressure; bIOP=biomechanically corrected intraocular pressure; CCT=central corneal thickness; A1 time=time of the first applanation; A1 length=length of the first applanation; A1 velocity=velocity of the first applanation; A2 time=time of the second applanation; A2 length=length of the second applanation; A2 velocity=velocity of the second applanation; HC time=time for reaching the highest concavity; HC radius=central curvature radius at the highest concavity; PD=distance of the two apexes of the cornea at the highest concavity; DA=maximum deformation amplitude of the cornea at the highest concavity
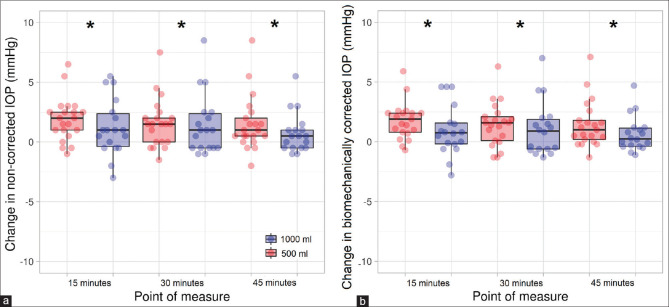
There was a main effect of the point of measure for the IOP and bIOP values (F3,111 = 11.39, P < 0.001, h² = 0.232 and F3,111 = 8.71, P < 0.001, h² = 0.185, respectively). However, the amount of water intake or the interaction point of measure × water intake did not reach statistical significance for either the IOP (F1,37 = 0.01, P = 0.926 and F3,111 = 0.77, P = 0.515, respectively) or bIOP values (F1,37 = 0.10, P = 0.754 and F3,111 = 1.36, P = 0.259, respectively). Post hoc tests for the IOP and bIOP values showed a statistically significant IOP rise 15 min (corrected P < 0.001, d = 0.76 and corrected P = 0.001, d = 0.66, respectively), 30 min (corrected P < 0.001, d = 0.67 and corrected P = 0.002, d = 0.63, respectively) and 45 min (corrected P = 0.004, d = 0.57 and corrected P = 0.002, d = 0.61, respectively) after water intake in comparison to the baseline measurement [Fig. 1]. For its part, no statistically significant differences for either the point of measure (F3,111 = 1.43, P = 0.238), water intake (F1,37 = 0.06, P = 0.815) or the interaction point of measure × water intake (F3,111 = 0.40, P = 0.752) were found for CCT.
Figure 1.
Scatterplot and boxplot of the effect of water intake on intraocular pressure. The difference between the measurement taken after water ingestion and the baseline measurement for the non-corrected intraocular pressure values are displayed in (a) whereas the biomechanically corrected intraocular pressure values are shown in panel (b) * denotes statistically significant differences in comparison to the baseline measurement (corrected P < 0.05). The whiskers represent the interquartile range, and the horizontal lines indicate the median value
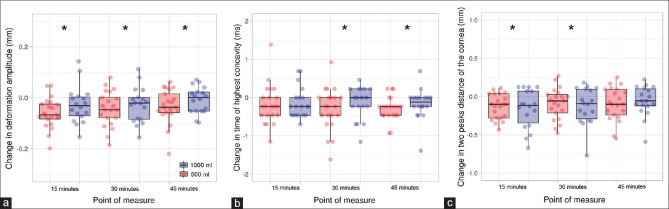
The analysis of the DA showed a statistically significant effect for the point of measure (F3,111 = 7.55, P < 0.001, h² = 0.166), whereas no differences were obtained for the water intake of the interaction point of measure × water intake (F1,37 = 0.01, P = 0.996 and F3,111 = 0.99, P = 0.399, respectively). Post hoc tests revealed that there were statistically significant differences for the comparisons at baseline versus 15 min (corrected P < 0.001, d = 0.67), baseline versus 30 min (corrected P = 0.011, d = 0.53), and 15 min versus 30 min (corrected P = 0.044, d = 0.43) [Fig. 2, panel a]. The HC time exhibited statistical significance for the main effect of the point of measure (F3,111 = 3.82, P = 0.012, h² = 0.093), whereas the main effect of water intake and the interaction point of measure × water intake were far from reaching statistical significance (F1,37 = 0.07, P = 0.788 and F3,111 = 0.39, P = 0.758; respectively). Post hoc tests demonstrated that the HC time was slower 30 and 45 min after water intake in comparison to the baseline measurement (corrected P = 0.043, d = 0.44 and corrected P = 0.002, d = 0.62, respectively) [Fig. 2, panel b]. The PD demonstrated a statistically significant effect for the point of measure (F3,111 = 7.77, P < 0.001, h² = 0.171). However, the main factor for water intake and the interactive effect of point of measure × water intake did not show statistical significance (F1,37 = 0.20, P = 0.654 and F3,111 = 0.56, P = 0.646, respectively). Post hoc tests showed a shorter PD for the measurements taken 15 and 30 min after water intake when compared with the baseline reading (corrected P < 0.001, d = 0.68 and corrected P < 0.020, d = 0.49, respectively) [Fig. 2, panel c]. The HC radius did not yield differences for either the point of measure (F3,111 = 0.20, P = 0.900), water intake (F1,37 = 0.48, P = 0.491) or interaction (F3,111 = 2.10, P = 0.104).
Figure 2.
Scatterplot and boxplot of the effect of water intake on deformation amplitude (a), time from starting until highest concavity is reached (b), and distance between the two peaks of the cornea at highest concavity (c). * denotes statistically significant differences in comparison to the baseline measurement (corrected P < 0.05). The whiskers represent the interquartile range, and the horizontal lines indicate the median value
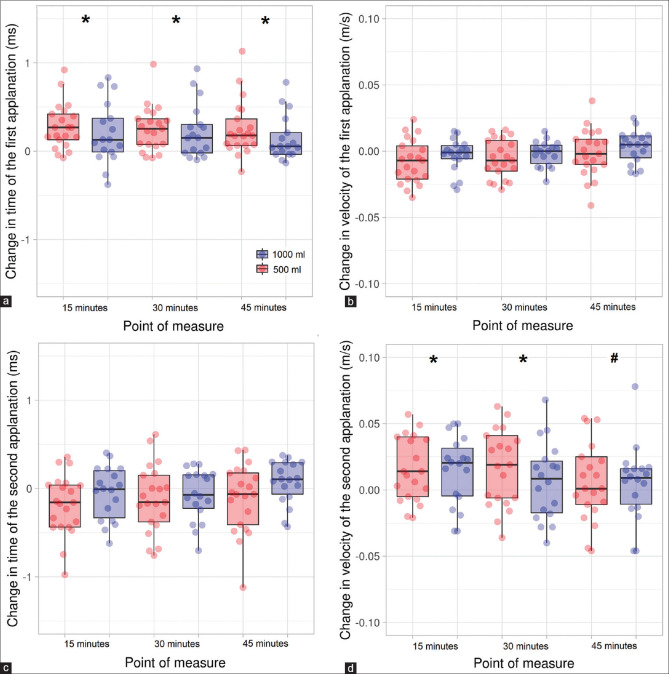
The A1 time was sensitive to the point of measure (F3,111 = 15.30, P < 0.001, h² = 0.288), but no effects were found for the factors of water intake and the interaction point of measure × water intake (F1,37 = 0.02, P = 0.961 and F3,111 = 0.84, P = 0.476, respectively). Post hoc comparisons exhibited that the A1 time was greater 15, 30, and 45 min after water intake in comparison to the baseline measurement (corrected P < 0.001 in the three cases and d = 0.81, 0.88, and 0.70, respectively) [Fig. 3, panel a]. The A1 length did not reach statistical significance for any of the two factors (F3,111 = 1.17, P = 0.325 and F1,37 = 0.03, P = 0.873 for the point of measure and water intake, respectively), as well as for the interaction (F3,111 = 0.70, P = 0.555). For its part, the A1 velocity yielded statistical significance for the point of measure (F3,111 = 3.58, P = 0.016, h² = 0.087), whereas the water intake (F1,37 = 0.11, P = 0.739) and the interaction (F3,111 = 0.47, P = 0.706) were far from showing any significance. Post hoc tests between the different points of measure were statistically insignificant (all with a corrected P > 0.05) [Fig. 3, panel b].
Figure 3.
Scatterplot and boxplot of the effect of water intake on time of the first applanation (a), velocity of the first applanation (b), time of the second applanation (c), and velocity of the second applanation (d). * and # denote statistically significant differences in comparison to the measurements at baseline and 15 min, respectively (corrected P < 0.05). The whiskers represent the interquartile range, and the horizontal lines indicate the median value
In regard to the second applanation, the A2 time revealed a significant effect for the point of measure (F3,111 = 3.103, P = 0.030, h² = 0.074), but no differences were observed for the water intake (F1,37 = 0.01, P = 0.910) or the interaction (F3,111 = 1.572, P = 0.200). Post hoc analyses between the different points of measure did not yield statistically significant results (all with a corrected P > 0.05) [Fig. 3, panel c]. A2 length showed no significant effects for the point of measure (F3,111 = 1.79, P = 0.153), the water intake (F1 37 = 0.39, P = 0.535), and the interaction (F3,111 = 1.42, P = 0.241). Lastly, the analysis of A2 velocity exhibited a statistically significant effect for the point of measure (F3,111 = 5.746, P = 0.001, h² = 0.132), whereas no differences were found for either the water intake (F1,37 = 0.03, P = 0.872) or the interaction (F3,111 = 0.83, P = 0.481). Post hoc comparisons evidenced differences for the comparisons at baseline versus 15 and 30 min (corrected P = 0.008 and 0.044, and d = 0.55 and 0.43), as well as 15 versus 45 min (corrected P = 0.025, d = 0.48) [Fig. 3, panel d].
Additionally, we performed correlational analyses between the changes in IOP and changes in the different biomechanical parameters of the cornea, observing a strong positive association between IOP and A1 time, whereas a strong negative correlation between IOP and A2 time and PD and DA was observed. Moderate positive correlations were found between the changes in IOP and the changes in A1 length and A2 velocity. All correlations are displayed in Table 3.
Table 3.
Pearsonxs product-moment correlation coefficients (Pearson r [P value]) of changes in intraocular pressure with changes in corneal biomechanical parameters at the different points of measure
| Changes in intraocular pressure (mm Hg) | |||
|---|---|---|---|
|
| |||
| After 15 min of water intake | After 30 min of water intake | After 45 min of water intake | |
| CCT (µm) | −0.191 (0.243) | −0.180 (0.273) | −0.090 (0.588) |
| A1 time (ms) | 0.977 (<0.001) *** | 0.975 (<0.001) *** | 0.966 (<0.001) *** |
| A1 length (mm) | 0.503 (0.001) ** | 0.590 (<0.001) *** | 0.632 (<0.001) *** |
| A1 velocity (m/s) | −0.366 (0.022) * | −0.253 (0.120) | −0.406 (0.010) * |
| A2 time (ms) | −0.735 (<0.001) *** | −0.664 (<0.001) *** | −0.769 (<0.001) *** |
| A2 length (mm) | 0.060 (0.719) | 0.255 (0.117) | 0.281 (0.083) |
| A2 velocity (m/s) | 0.544 (<0.001) *** | 0.564 (<0.001) *** | 0.576 (<0.001) *** |
| HC time (ms) | 0.210 (0.199) | −0.019 (0.910) | 0.106 (0.520) |
| HC radius (mm) | 0.193 (0.293) | 0.019 (0.923) | 0.248 (0.128) |
| PD (mm) | −0.786 (<0.001) *** | −0.871 (<0.001) *** | −0.813 (<0.001) *** |
| DA (mm) | −0.735 (<0.001) *** | −0.673 (<0.001) *** | −0.724 (<0.001) *** |
Abbreviations: CCT=central corneal thickness; A1 time=time of the first applanation; A1 length=length of the first applanation; A1 velocity=velocity of the first applanation; A2 time=time of the second applanation; A2 length=length of the second applanation; A2 velocity=velocity of the second applanation; HC time=time for reaching the highest concavity; HC radius=central curvature radius at the highest concavity; PD=distance of the two apexes of the cornea at the highest concavity; DA=maximum deformation amplitude of the cornea at the highest concavity. *, **, and *** denote statistically significant differences (P<0.05, 0.01, and 0.001, respectively)
Discussion
Our data demonstrated that the corneal biomechanical response is sensitive to whole-body hydration status, with 500 mL of water being sufficient to alter some corneal biomechanical parameters. In particular, DA, HC time, and PD, as well the time and velocity of the first and second applanations, were influenced by the ingestion of water. Moreover, our results revealed an acute intraocular pressure rise after water loading, which was found for both the non-corrected IOP and bIOP values, with the change in IOP being meaningfully associated with changes in some corneal biomechanical indices, such as A1 time, A1 length, A2 time, A2 velocity, PD, and DA. Taken together, these findings may be of relevance for the diagnosis and management of ocular conditions, such as glaucoma or corneal ectasias, as clinical decisions are based on the biomechanical properties of the cornea.
The primary objective was to determine the effects of drinking 500 and 1000 mL of water on the different corneal biomechanical parameters given by the Corvis ST system. In this regard, animal studies have evidenced that the biomechanical properties of the cornea are sensitive to the manipulation of the levels of corneal hydration with invasive techniques.[13,14,15] In humans, there is only one study that has assessed the changes in corneal hysteresis, as measured by an ocular response analyzer, caused by the water-loading test, observing no significant changes in this parameter.[22] However, the incorporation of the Corvis ST system allows for a more accurate visualization and evaluation of the corneal deformation process; therefore, it has opened up new possibilities in laboratory and clinical settings. Here, we found that some corneal biomechanical parameters are influenced by water ingestion. In particular, drinking either 500 or 1000 mL of water induced a reduction of the DA, HC time, and PD, suggesting that whole-body hydration status influences the spatial and temporal profiles of corneal deformation. These results are in line with the study by Read and Collins,[7] who found that the hydration level modifies the axial length, and it may be of relevance due to the changes in hydration that occur throughout the day.
The IOP variations observed in the present study converge with previous investigations (~1–2 mm Hg),[7,23] and in agreement with most studies,[7,23,24] our results also yielded IOP increments that were maintained 45 min after water intake. However, this finding is contrary to the result obtained by Ulas et al.,[22] who found an IOP increase 10 min after water intake, returning to baseline levels after 20 min. Somewhat surprisingly, the increases in the IOP caused by the manipulation of whole-body hydration status were roughly similar after drinking 500 or 1000 mL of water. Similarly, the corneal biomechanics were sensitive to water intake regardless of the amount of water, suggesting that relatively small changes in the hydration status may have an impact on ocular physiology, specifically on IOP and some biomechanical properties of the cornea.
Our finding may be of special relevance in clinical and research settings due to the necessity of obtaining repeatable measures of ocular physiology for the diagnosis and management of different eye conditions. Delving into the study of how whole-body hydration status alters different biomechanical properties of the cornea will help to develop clinical guidelines and protocols regarding what conditions affecting the biomechanical properties of the cornea should be examined by eye care professionals. For example, the level of hydration of the cornea determines the success of excimer laser ablation,[25,26] and importantly, the diagnosis and management of keratoconus are based on the biomechanical properties of the cornea (DA, HC time, PD, HC radius, and time, as well as the length and velocity of the first and second applanations).[27,28] Additionally, IOP and corneal biomechanics are associated with the onset and progression of glaucoma, with changes in these indices leading to adopting different interventional strategies.[17,29] Moreover, the level of corneal hydration is known to affect optical quality.[10] Therefore, eye care specialists should be aware that small variations in whole-body hydration status can influence the diagnosis and follow-up of different ocular conditions, as well as the success of laser refractive surgery, as corneal hydration status at the time of the intervention may affect the refractive correction and magnitude of myopic regression.[25,30]
Furthermore, we aimed to explore the association between IOP variations and corneal biomechanical changes that occur after drinking water. Our data showed that the change in A1 time was strongly and positively related to the IOP changes 15, 30, and 45 min after water ingestion (Pearson r = 0.966–0.977), whereas the A2 time revealed a negative correlation (Pearson r = −0.664 to −0.769). The analysis of the DA and PD revealed that they were negatively associated with IOP (Pearson r = −0.673 to −0.735 and −0.786 to −0.871, respectively). This result agrees with Kling and Marcos,[15] who reported that corneal deformation is highly sensitive to IOP variations by using in vitro testing. For its part, changes in CCT were far from showing any association with IOP variations, corroborating the lack of correlation between CCT and IOP peak and fluctuation during the water drinking test shown in earlier studies with glaucoma patients.[31] Based on the present outcomes, the incorporation of corneal biomechanical parameters that control IOP is mandatory to minimize erroneous clinical decisions[32] as IOP is sensitive to multiple lifestyle habits, such as physical exercise,[33,34] caffeine consumption,[35] mental stress,[36] or wearing a tight necktie.[37]
The present study reveals that drinking water influences corneal biomechanics and IOP, with the changes in some biomechanical properties of the cornea being highly associated with the IOP changes. However, our investigation is not exempt from limitations. First, glaucoma patients have shown an inaccurate functioning of the autoregulatory mechanisms of ocular hemodynamics, showing greater IOP responsiveness to water loading.[8] Therefore, future studies should consider including glaucoma patients. Second, the corneal biomechanics are altered in individuals with corneal ectasias.[38] Thus, the impact of drinking water on the biomechanical properties of the cornea may vary in this clinical population. Lastly, we consider it interesting to assess the possible influence of manipulating whole-body hydration status on the diagnosis and management of ocular conditions that are based on corneal biomechanics.[28]
Conclusion
The ingestion of a relatively low amount of water (500 mL) alters different parameters of corneal biomechanics in young healthy adults. Our results indicate that the changes in the A1 time, A1 length, A2 time, A2 velocity, PD, and DA are significantly associated with IOP fluctuations 15, 30, and 45 min after water intake. Taken together, the current findings highlight the importance of considering corneal biomechanical indices that are independent of IOP as small IOP variations may provoke erroneous clinical decisions for the diagnosis and management of different ocular conditions.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgements
The authors thank all the participants who selflessly collaborated in this investigation.
References
- 1.Sherwood L. Human Physiology: From Cells to Systems. 9th ed. Boston, MA: Cengage Learning; 2015. [Google Scholar]
- 2.Read SA, Collins MJ, Iskander DR. Diurnal variation of axial length, intraocular pressure, and anterior eye biometrics. Investig Ophthalmol Vis Sci. 2008;49:2911–8. doi: 10.1167/iovs.08-1833. [DOI] [PubMed] [Google Scholar]
- 3.Szakáts I, Sebestyén M, Németh J, Birkás E, Purebl G. The role of health anxiety and depressive symptoms in dry eye disease. Curr Eye Res. 2016;41:1044–9. doi: 10.3109/02713683.2015.1088955. [DOI] [PubMed] [Google Scholar]
- 4.Gillmann K, Hoskens K, Mansouri K. Acute emotional stress as a trigger for intraocular pressure elevation in Glaucoma. BMC Ophthalmol. 2019;19:69. doi: 10.1186/s12886-019-1075-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wyl?gała A. The effects of physical exercises on ocular physiology: A review. J Glaucoma. 2016;25:e843–9. doi: 10.1097/IJG.0000000000000454. [DOI] [PubMed] [Google Scholar]
- 6.Boeing H, Bechthold A, Bub A, Ellinger S, Haller D, Kroke A, et al. Critical review: Vegetables and fruit in the prevention of chronic diseases. Eur J Nutr. 2012;51:637–63. doi: 10.1007/s00394-012-0380-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Read S, Collins M. Water drinking influences eye length and IOP in young healthy subjects. Exp Eye Res. 2010;91:180–5. doi: 10.1016/j.exer.2010.04.015. [DOI] [PubMed] [Google Scholar]
- 8.Susanna R, Clement C, Goldberg I, Hatanaka M. Applications of the water drinking test in glaucoma management. Clin Exp Ophthalmol. 2017;45:625–31. doi: 10.1111/ceo.12925. [DOI] [PubMed] [Google Scholar]
- 9.Walsh NP, Fortes MB, Esmaeelpour M. Influence of modest changes in whole-body hydration on tear fluid osmolarity: Important considerations for dry eye disease detection. Cornea. 2011;30:1517. doi: 10.1097/ICO.0b013e31821ddd3a. author reply 1517-8. [DOI] [PubMed] [Google Scholar]
- 10.Sherwin JC, Kokavec J, Thornton SN. Hydration, fluid regulation and the eye: In health and disease. Clin Exp Ophthalmol. 2015;43:749–64. doi: 10.1111/ceo.12546. [DOI] [PubMed] [Google Scholar]
- 11.Sabetti L, Renzetti A, D'Alessandri L, Balestrazzi E. Eventual error caused by dehydration with pachometry. Ophthalmologica. 2001;215:97–101. doi: 10.1159/000050837. [DOI] [PubMed] [Google Scholar]
- 12.Terai N, Raiskup F, Haustein M, Pillunat LE, Spoerl E. Identification of biomechanical properties of the cornea: The ocular response analyzer. Curr Eye Res. 2012;37:553–62. doi: 10.3109/02713683.2012.669007. [DOI] [PubMed] [Google Scholar]
- 13.Seiler TG, Shao P, Frueh BE, Yun SH, Seiler T. The influence of hydration on different mechanical moduli of the cornea. Graefes Arch Clin Exp Ophthalmol. 2018;256:1653–60. doi: 10.1007/s00417-018-4069-7. [DOI] [PubMed] [Google Scholar]
- 14.Singh M, Han Z, Li J, Vantipalli S, Aglyamov SR, Twa MD, et al. Quantifying the effects of hydration on corneal stiffness with noncontact optical coherence elastography. J Cataract Refract Surg. 2018;44:1023–31. doi: 10.1016/j.jcrs.2018.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kling S, Marcos S. Contributing factors to corneal deformation in air puff measurements. Investig Ophthalmol Vis Sci. 2013;54:5078–85. doi: 10.1167/iovs.13-12509. [DOI] [PubMed] [Google Scholar]
- 16.Fischbarg J. Water channels and their roles in some ocular tissues. Mol Aspects Med. 2012;33:638–41. doi: 10.1016/j.mam.2012.07.016. [DOI] [PubMed] [Google Scholar]
- 17.Kotecha A. What biomechanical properties of the cornea are relevant for the clinician? Surv Ophthalmol. 2007;52(Suppl 6):109–14. doi: 10.1016/j.survophthal.2007.08.004. [DOI] [PubMed] [Google Scholar]
- 18.Faul F, Erdfelder E, Lang AG, Buchner A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. 2007;39:175–91. doi: 10.3758/bf03193146. [DOI] [PubMed] [Google Scholar]
- 19.Hon Y, Lam AKC. Corneal deformation measurement using scheimpflug noncontact tonometry. Optom Vis Sci. 2013;90:1–8. doi: 10.1097/OPX.0b013e318279eb87. [DOI] [PubMed] [Google Scholar]
- 20.Ali NQ, Patel DV, McGhee CNJ. Biomechanical responses of healthy and keratoconic corneas measured using a noncontact scheimpflug-based tonometer. Investig Ophthalmol Vis Sci. 2014;55:3651–9. doi: 10.1167/iovs.13-13715. [DOI] [PubMed] [Google Scholar]
- 21.Nemeth G, Hassan Z, Csutak A, Szalai E, Berta A, Modis L. Repeatability of ocular biomechanical data measurements with a scheimpflug-based noncontact device on normal corneas. J Refract Surg. 2014;29:558–63. doi: 10.3928/1081597X-20130719-06. [DOI] [PubMed] [Google Scholar]
- 22.Ulaş F, Balbaba M, Çelebi S. Effects of a water-loading test on intraocular pressure and corneal hysteresis in young healthy subjects. J Glaucoma. 2014;23:101–4. doi: 10.1097/IJG.0b013e318264ce7c. [DOI] [PubMed] [Google Scholar]
- 23.Brucculeri M, Hammel T, Harris A, Malinovsky V, Martin B. Regulation of intraocular pressure after water drinking. J Glaucoma. 1999;8:111–6. [PubMed] [Google Scholar]
- 24.Hatanaka M, Alencar LM, De Moraes CG, Susanna R. Reproducibility of intraocular pressure peak and fluctuation of the water-drinking test. Clin Exp Ophthalmol. 2013;41:355–9. doi: 10.1111/j.1442-9071.2012.02882.x. [DOI] [PubMed] [Google Scholar]
- 25.Kim WS, Jo JM. Corneal hydration affects ablation during laser in situ keratomileusis surgery. Cornea. 2001;20:394–7. doi: 10.1097/00003226-200105000-00011. [DOI] [PubMed] [Google Scholar]
- 26.Feltham MH, Stapleton F. The effect of water content on the 193 nm excimer laser ablation. Clin Exp Ophthalmol. 2002;30:99–103. doi: 10.1046/j.1442-6404.2002.00496.x. [DOI] [PubMed] [Google Scholar]
- 27.Johnson RD, Nguyen MT, Lee N, Hamilton DR. Corneal biomechanical properties in normal, forme fruste keratoconus, and manifest keratoconus after statistical correction for potentially confounding factors. Cornea. 2011;30:516–23. doi: 10.1097/ICO.0b013e3181f0579e. [DOI] [PubMed] [Google Scholar]
- 28.Vinciguerra R, Ambrósio R, Elsheikh A, Roberts CJ, Lopes B, Morenghi E, et al. Detection of keratoconus with a new biomechanical index. J Refract Surg. 2016;32:803–10. doi: 10.3928/1081597X-20160629-01. [DOI] [PubMed] [Google Scholar]
- 29.Garcia-Porta N, Fernandes P, Queiros A, Salgado-Borges J, Parafita-Mato M, González-Méijome JM. Corneal biomechanical properties in different ocular conditions and new measurement techniques. ISRN Ophthalmol 2014. 2014 doi: 10.1155/2014/724546. 724546. doi: 10.1155/2014/724546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mcalinden C. Corneal refractive surgery: Past to present. Clin Exp Optom. 2012;95:386–98. doi: 10.1111/j.1444-0938.2012.00761.x. [DOI] [PubMed] [Google Scholar]
- 31.Furlanetto RL, Facio AC, Jr, Hatanaka M, Susanna Junior R. Correlation between central corneal thickness and intraocular pressure peak and fluctuation during the water drinking test in glaucoma patients. Clinics. 2010;65:967–70. doi: 10.1590/S1807-59322010001000007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Roberts CJ. Importance of accurately assessing biomechanics of the cornea. Curr Opin Ophthalmol. 2016;27:285–91. doi: 10.1097/ICU.0000000000000282. [DOI] [PubMed] [Google Scholar]
- 33.Vera J, Jiménez R, Redondo B, Cárdenas D, Bryon R, García-ramos A, et al. Acute intraocular pressure responses to high- intensity interval-training protocols in men and women. J Sports Sci. 2019;37:803–9. doi: 10.1080/02640414.2018.1527674. [DOI] [PubMed] [Google Scholar]
- 34.Vera J, Jiménez R, Redondo B, Torrejón A, De Moraes CGCG, García-Ramos A. Effect of the level of effort during resistance training on intraocular pressure. Eur J Sport Sci. 2019;19:394–401. doi: 10.1080/17461391.2018.1505959. [DOI] [PubMed] [Google Scholar]
- 35.Vera J, Redondo B, Molina R, Bermúdez J, Jiménez R. Effects of caffeine on intraocular pressure are subject to tolerance: A comparative study between low and high caffeine consumers. Psychopharmacology (Berl) 2018;236:811–9. doi: 10.1007/s00213-018-5114-2. [DOI] [PubMed] [Google Scholar]
- 36.Jiménez R, Vera J. Effect of examination stress on intraocular pressure in university students. Appl Ergon. 2018;67:252–8. doi: 10.1016/j.apergo.2017.10.010. [DOI] [PubMed] [Google Scholar]
- 37.Tally P, O'Brien PD. Does extended wear of a tight necktie cause raised intraocular pressure? J Glaucoma. 2005;14:508–10. doi: 10.1097/01.ijg.0000185435.08051.cb. [DOI] [PubMed] [Google Scholar]
- 38.Ma J, Wang Y, Wei P, Jhanji V. Biomechanics and structure of the cornea: Implications and association with corneal disorders. Surv Ophthalmol. 2018;63:851–61. doi: 10.1016/j.survophthal.2018.05.004. [DOI] [PubMed] [Google Scholar]