Abstract
Background
Cerebral venous disorder may have a harmful effect on ischaemic stroke; however, the underlying mechanism remains to be elucidated. Although Dl-3-n-butylphthalide is a multitarget agent for antiischaemic stroke, its neuroprotective role in brain ischaemia accompanied by brain venous disturbance remains unclear. In this study, we induced cerebral venous disturbance by the occlusion of bilateral external jugular veins (EJVs) to explore the potential mechanism of the adverse effects of cerebrovenous disorders in cerebral infarction and explore the protective effect of Dl-3-n-butylphthalide on cerebral infarction accompanied through cerebral venous disturbance.
Methods
Cerebral venous disturbance was induced in Sprague-Dawley rats through the permanent occlusion of bilateral EJVs, and cerebral ischaemic stroke was induced through the permanent occlusion of the right cortical branches of the middle cerebral artery. 2,3,5-triphenyltetrazolium chloride staining, MRI, Evans blue extravasation and behavioural test were performed to evaluate infarction volume, cerebral blood flow (CBF), blood–brain barrier (BBB) integrity and neurological function. Immunofluorescence staining and western blot analysis were performed to detect loss of neuron, endothelial cells, pericytes and tight junctions.
Results
Bilateral EJVs occlusion did not cause cerebral infarction; however, it increased the infarction volume compared with the simple middle cerebral artery occlusion (MCAO) group, accompanied by severe neuron loss, worse neurological function, lower CBF, increased EJVs pressure, exacerbated Evans blue extravasation and brain oedema, as well as attenuated angiogenesis. Dl-3-n-butylphthalide displayed a neuroprotective effect in rats with MCAO accompanied by EJVs occlusion by reducing neuron loss, accelerating CBF restoration, promoting angiogenesis and relieving BBB damage.
Conclusion
Bilateral EJVs occlusion did not significantly affect normal rats but aggravated brain damage in the case of ischaemic stroke. Dl-3-n-butylphthalide treatment plays a neuroprotective role in rats with MCAO accompanied by EJVs occlusion, mainly due to the promotion of CBF restoration and BBB protection.
Keywords: cerebral Infarction, veins
Introduction
Good collateral has always been considered a predictor of small ischaemic core lesions and a key factor for the prognosis of patients with acute ischaemic stroke (AIS), and numerous clinical trials have used perfusion imaging to select eligible patients for endovascular thrombectomy.1 2 However, a large ischaemic core lesion with good collateral was also seen in clinical practice.3 Moreover, the no-reflow phenomenon and other complications, such as brain oedema and haemorrhage, often confuse clinicians.4
The cerebral venous system is a component of brain blood circulation, which can drain out cerebral blood, regulate intracranial pressure and maintain the integrity of the blood–brain barrier (BBB). Meanwhile, venous blood plays an important role in blood storage, for 70%–80% of the total intracerebral blood in the venous system.5 A previous study reported that spontaneous intracranial hypertension was associated with complete or partial internal jugular vein occlusion.6 Venous was confirmed to play an essential role in maintaining the normal function of the central nervous system as a series of diseases were reported to be associated with venous abnormalities, including cerebral oedema,7 high-altitude headache,8 transient monocular blindness,9 epilepsy10 and cortical dysplasia.11 Yura et al 12 reported that the bilateral occlusion of the external jugular veins (EJVs) would significantly increase the infarct area in a rat middle cerebral artery occlusion (MCAO) model. With the evidence and obstacles of the no-reflow phenomenon and the unresolved adverse effects after recanalisation, although few studies have been carried out, researchers have begun to consider the venous system as an influencing factor. Van den Wijngaard et al found that the slow and poor extent of cortical vein filling were related to poor clinical outcomes.13 Delayed cortical vein filling during the late venous phase on dynamic CT angiography was thought to be a marker of poor reperfusion after recombinant tissue plasminogen activator (rtPA) treatment in AIS patients.14 AIS patients with ipsilateral venous drainage deficiency are more likely to develop early fatal oedema.15 Evidence has established that venous drainage disorders could aggravate ischaemic stroke; however, the mechanism of adverse effects caused by venous circulation defects on ischaemic stroke is unknown, and there are no reports on the treatment of AIS accompanied by venous drainage obstacles.
Dl-3-n-butylphthalide (NBP) is a small-molecular and multitarget agent used to treat ischaemic stroke, with the effects of cerebral artery vasodilation,16 angiogenesis promotion,17 enhancement of axonal growth and neurogenesis,18 and anti-inflammatory effects.19 While it remains unclear whether NBP has the same neuroprotective effect in patients with AIS who are accompanied by venous circulation disturbance. As over 20% of people live with abnormalities in vein structure,20 21 it is worth exploring the potential treatment of ischaemic stroke accompanied by cerebral venous disturbance.
Methods and materials
Animals
Three hundred and ten 8-week-old male Sprague-Dawley rats (weighing 300–380 g) were purchased from the Animal Experiment Centre of Southern Medical University (Guangzhou, China). All animal experiments were performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. The rats were kept with free food and water under a 12 hours/12 hours light/dark cycle. The ambient temperature and humidity were maintained at 22°C±1°C and 50%±10%, respectively. Animals were randomly assigned to the sham-operated control group (Sham, n=67), EJV occlusion group (EJVO), n=67, MCAO (n=67), EJVO plus MCAO group (EJVO +MCAO, n=67), and EJVO plus MCAO treated with dl-3-butylphthalide group (EJVO +MCAO+ NBP, n=67).
Surgery and drug administration
To induce cerebral venous circulation dysfunction, bilateral EJVO was performed as previously described.12 Briefly, rats were anaesthetised with 10% chloral hydrate (350 mg/kg, intraperitoneally), and a small midline incision was made in the neck to expose the bilateral EJV. The bilateral EJV were cut-off with double-ligated silk sutures. Permanent distal MCAO was performed as previously described.22 In brief, a 1 cm transverse incision was made between the right eye and the right ear under deep anaesthesia with an intraperitoneal injection of 10% chloral hydrate (350 mg/kg). Then, the temporal muscles were separated and retracted, followed by a 3 mm diameter craniotomy to explore the cortical branches of the MCA using a mini skull drill (RWD Life Science, Shenzhen, China). The dura was incised with a needle and the right distal MCA was exposed and occluded by electrocoagulation without damaging the brain tissue. Finally, the muscles and skin were sutured separately. In the sham-operation control group, the EJV and MCA were not occluded. In the EJVO group, the MCA was not occluded. In the MCAO group, the EJV was not occluded. To measure the pressure of the EJV, an intravenous indwelling needle was inserted into the left EJV to monitor the pressure of the vein dynamically. To avoid the breakage of the inserted needle by rats, we created a tunnel from the anterior neck to the head. Isoflurane was inhaled using a mask to maintain the sedative state of the rats. Continuous indwelling needles were not placed in rats in the Sham and EJVO groups as the needles were pulled out after recording the venous pressure. NBP-treated rats received daily tail vein injection of NBP solution (5 mg/kg/day) for seven consecutive days, being initiated 1 hour after the operation. 0.9%saline, at the same dose, was injected into the rats in other groups as controls at the corresponding time points.
Neurobehavioral examination
Forelimb grasping force
The grasping force exerted by both forelimbs was calculated using a grasping force tester. Animals were placed on the tester, their tails were pulled back gently with the hindlimb leaving the tester, and the grasp force was recorded automatically by the tester. Each rat was measured four times during one test, and the average force value was taken to reduce experimental error while avoiding the exhaustion caused by several tests.
Balance beam test
Motor coordination was assessed using the balance beam test. For 3 days before surgery, the rats were trained to traverse an elevated narrow beam (2.5 cm in width, 1.5 m in length, and 1.0 m in height) to enter a safe location at the opposite end of the beam. The test was conducted on days 1, 3, 7 and 14 after the operation, and the performance of the rats was analysed by a blinded investigator using a six-point rating scale.
Adhesive-removal test
Animals were trained for 3 days before inducing MCAO, and the test procedure was carried out according to a previous study.23 The technique involved the application of two small pieces of adhesive tape with an area of 1 cm2 to both forelimbs. The time taken to remove the adhesive tape was recorded in each of the four trials within 120 s.
MRI and assessment of cerebral blood flow
Twenty-five rats were scanned with MRI at five time points: before the operation, immediately, day 3, day 7 and day 14 after the operation. MRI was performed using a Discovery 750 3.0T scanner with an 8-channel wrist coil (GE Healthcare, Mliwaukee, WI, USA). The detailed scan parameters for the three dimensional arterial spin labeling (3D ASL) series are described in our previous study.24 Cerebral blood flow (CBF) maps were generated automatically with the use of a commercially available scanner software programme (Functool 3D ASL, Software version 4.5, GE Medical Systems, Milwaukee, Wisconsin, USA). The cortex of the infarction lesions was defined as regions of interest (ROIs) to measure CBF values.
Measurement of EJV pressure
The EJV pressure was recorded using the Medlab biological signal acquisition and processing system (Zhongshi Technology, China). To estimate dynamic changes in the EJV pressure, the pressure was measured before EJV ligation, immediately after ligation and 1 hour, 2 hours, 4 hours, 12 hours, 24 hours, 3 days and 7 days after EJV ligation.
2, 3, 5-triphenyltetrazolium chloride staining and the measurement of infarct volume
Rats were sacrificed at 24 hours and 72 hours after the operation, and the brains were quickly preserved at −20°C for 10 min. The brains were cut into 2 mm coronal sections and stained with 2% 2, 3, 5-triphenyltetrazolium chloride (TTC, Synbio Technologies, China) as previously described. The total infarct volume was calculated as the sum of each slice infarction as measured by ImageJ (NIH, Bethesda, MD, USA). The infarct volume was defined as V%=volume of the infarct area/volume of the contralateral area ×100% to avoid mismeasurement due to oedema.
Brain edema assessment
The rats’ brains were quickly removed after deep anaesthesia with 10% chloral hydrate (400 mg/kg, intraperitoneal injection). The olfactory bulb, cerebellum and lower brainstem were discarded, and the ipsilateral hemisphere was weighed before and after drying at 65°C for 72 hours. Brain oedema was evaluated as brain water content, which was defined as (1-dry weight/wet weight)×100%.
BBB permeability
For the analysis of BBB permeability, the rats were injected with 4% Evans blue (EB; 2 mL/kg, Aladdin) for 3 hours through their tail veins. The animals were then perfused with 400 mL of iced 0.9% saline, and the brains were removed. Each brain was cut into five coronal sections. The ipsilateral and contralateral cortices were homogenised in N, N-dimethylformamide (2 µL/g wet weight tissue, Aladdin). After EB was extracted by grinding, the mixture was centrifuged for 45 min at 15 000 rpm. The supernatants were collected, and the concentration of EB was quantified by measuring the absorbance at 620 nm minus the background calculated from the baseline absorbance between 500 and 740 nm. Briefly, the formula was used for permeability evaluation: EB value = A620nm - (A500nm + A740nm)/2.
Western blots
Ischaemic cortical tissue was lysed in a whole-cell lysis assay (containing protease inhibitors, phosphatase inhibitors, and PMSF, KeyGEN BioTECH, China). The total protein concentration was assessed using a BCA protein assay kit (CWBIO, China). Equal amounts of brain tissue proteins were subjected to sodium dodecyl sulface-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to a polyvinylidene fluoride membrane. The membranes were blocked with 5% skim milk powder (BIOFROXX, Germany), incubated with a primary antibody, and then incubated with the appropriate horseradish peroxidase (HRP)-conjugated secondary antibody. The membranes were exposed to an ECL detection solution (Thermo Scientific, USA). The primary antibodies used in the experiment were rabbit monoclonal anti-PDGFRβ (Abcam, ab32570, 1:4000), goat polyclonal anti-desmin (Abcam, ab80503, 1:1000), rabbit monoclonal anti-occludin (Abcam, ab167161, 1:10000),rabbit polyclonal anti-TJP1 (Novus, NBP1-85047, 1:250), mouse monoclonal anti-β-actin (ProMab, 20270, 1:1000), rabbit polyclonal anti-NeuN (Invitrogen, PA5-78639, 1:1000), and goat polyclonal anti-CD31 (R&D systems, AF 3628, 1:400). The secondary antibodies used for western blotting were HRP-conjugated goat anti-rabbit secondary antibody (Beyotime, A0208, 1:1000, Lot: 081619190201), HRP-conjugated donkey anti-goat secondary antibody (Beyotime, A0181, 1:1000, Lot: 100818181217), and HRP-conjugated goat anti-mouse secondary antibody (Beyotime, A0216, 1:1000, Lot: 081619190201).
Immunofluorescent staining and analyses
The animals were transcardially perfused with cryogenic 0.9% saline followed by 4% paraformaldehyde, and then the brains were removed immediately and fixed in 4% paraformaldehyde for 24 hours. Coronal blocks from the optic chiasm to the posterior level of the hypothalamus were prepared for paraffin embedding. The tissue was cut into 4 µm coronal slices for immunofluorescence staining. The sections were treated with microwave heat antigen retrieval in Tris-EDTA (Servicebio, pH 8.8) and blocked with 5% donkey serum (Solarbio) after dewaxing and rehydration. For CD31 immunofluorescence, a 0.1% trypsin digestion solution (Solarbio) at 37°C was used for antigen retrieval. Then, the sections were incubated with primary antibody diluted in a blocking solution at 4°C overnight. The primary antibodies used for immunofluorescent staining were as follows: rabbit monoclonal anti-PDGFRβ (Abcam, ab32570, 1:100), goat polyclonal anti-desmin (Abcam, ab80503, 1:100), rabbit monoclonal antioccludin (Thermo Fisher, 71–1500, 1:100), rabbit polyclonal anti-ZO-1 (Thermo Fisher,61–7300, 1:100), rabbit polyclonal anti-NeuN (Invitrogen, PA5-78639, 1:100), and goat polyclonal anti-CD31 (R&D systems, AF 3628, 1:20). After that, sections were washed with 0.01 M PBS and incubated with the corresponding secondary antibody (DyLight 488 AffiniPure Donkey Anti-Rabbit, Earthox, 1:400, DyLight 488 AffiniPure Donkey Anti-Goat, Earthox, 1:200, CyTM3-conjugated AffiniPure Donkey Anti-Goat, Servicebio, GB21404, 1:300) and DAPI (4,6-diamino-2-phenylindole, Biyotime). Stained sections were covered with antifading mounting medium (Solarbio) and imaged using a fluorescence microscope (Leica). Image post-analysis was performed using ImageJ software.
HE staining
HE staining was used for the histological feature analysis. The paraffin-embedded sections were dewaxed with xylene three times (each for 10 min) and dehydrated with gradient alcohol (100%, 95%, 85%, 75% and 50%, each for 10 min), followed by distilled water for 5 min. Sections were then incubated with haematoxylin for 20 min at room temperature, followed by 10 s colour separation and eosin staining for 2 min. The sections were then washed with running water, dehydrated with 95% alcohol (1 min), and then dehydrated with 100% alcohol (1 min). Xylene was used to clear the sections, and neutral balsam was sealed. To observe the results, the slices were imaged by light microscopy.
Statistical analysis
Data analysis was conducted using SPSS V.23.0 (SPSS IBM). Data are presented as mean±SE, and one-way analysis of variance (ANOVA) was performed to analyse the differences between the various groups. The least significant difference procedure was performed to evaluate differences between groups. The repeated-measures ANOVA was conducted to compare the differences in CBF and neurological function between the groups. The threshold for statistical significance was set at p<0.05.
Results
NBP reduced the increased infarct volume caused by venous circulation disturbance
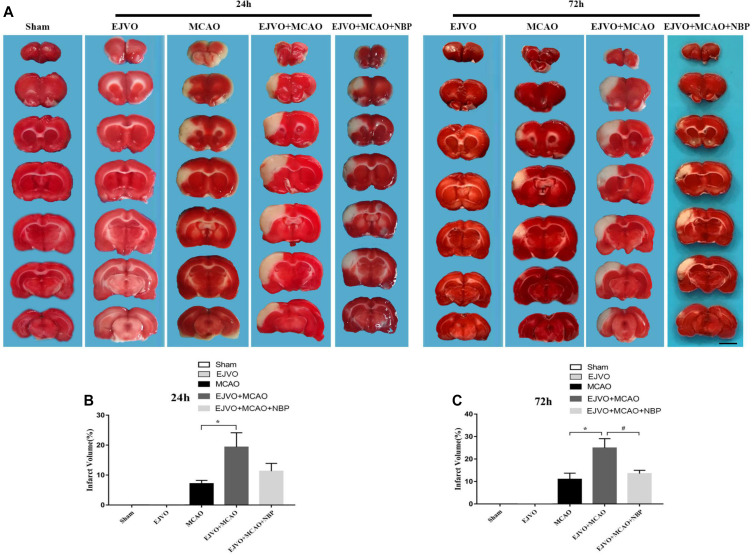
To investigate whether the occlusion of bilateral EJV would increase the volume of an infarct, TTC staining was performed to evaluate the ischaemic area. Two time points were selected (24 and 72 hours after MCAO) for measurement. The results showed significant enlargement of the infarct volume in MCAO rats, which was accompanied by venous circulation disturbance at both time points. In rats with MCAO accompanied by venous destruction, NBP treatment reduced the infarct area 3 days postoperatively (figure 1).
Figure 1.
Cerebral venous circulation disturbance increased infarction volume, and NBP could reduce infarct area after 3 days of treatment. (A) Infarction volume was evaluated by TTC staining 24 hours and 72 hours postoperation in groups of MCAO, EJVO+MCAO and EJVO+MCAO+ NBP. (B) Quantification of infarction volume at 24 hours after operation. (C) Quantification of infarction volume at 72 hours after operation. Data are presented as mean±SD; n=5 per group. *P<0.05, EJVO+MCAO vs MCAO; #P<0.05, EJVO+MCAO + NBP vs EJVO+MCAO. Scale bar=5 mm. EJVO, external jugular vein occlusion; MACO, middle cerebral artery occlusion; NBP, Dl-3-n-butylphthalide; TTC, 2, 3, 5-triphenyltetrazolium chloride.
NBP decreased the aggravated deterioration of neurological function caused by venous circulation disturbance
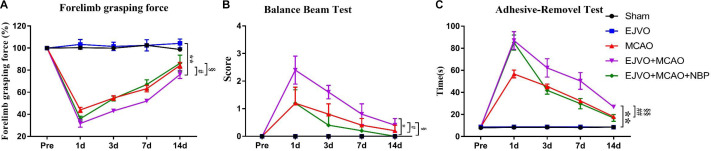
To assess neurological impairment after MCAO and the therapeutic effect of NBP, and to investigate whether cerebral venous drainage blocking would affect the neurological function of rats, we dynamically recorded motor and sensory functions in each animal. The bilateral occlusion of the EJV did not significantly influence the neurobehavioral score while venous circulation disturbance could aggravate the impairment of both motor and sensory function after the rats suffered cortical infarction. MCAO rats with or without venous circulation disturbance had significant neurological impairment compared with the sham rats, and rats with cortical infarction accompanied by venous circulation disorder suffered more severe brain damage, which was mainly manifested in smaller forelimb grasping force (figure 2A), higher balance beam test scores (figure 2B), and longer adhesive-removal time (figure 2C). Rats receiving intravenous injections of NBP showed better behavioural scores than the untreated rats (figure 2A–C).
Figure 2.
Cerebral venous circulation disturbance aggravate deterioration of neurological function, and NBP treatment contributed to improved neurological function in rats of MCAO accompanied by EJVO. (A) Quantification analysis of forelimb grasping force by repeated measurement ANOVA. (B) Quantification analysis of balance beam test score by repeated measurement ANOVA. (C) Quantification analysis of balance beam test score by repeated measurement ANOVA. Data are presented as mean±SD; n=5 per group. *P<0.05, EJVO +MCAO vs sham; **P<0.01, EJVO +MCAO vs sham; #p<0.05, EJVO +MCAO vs MCAO; ##p<0.01, EJVO +MCAO vs MCAO; §P<0.05, EJVO +MCAO + NBP vs EJVO +MCAO; §§p<0.01, EJVO +MCAO + NBP vs EJVO +MCAO. ANOVA, analysis of variance; EJVO, external jugular vein occlusion; MACO, middle cerebral artery occlusion; NBP, Dl-3-n-butylphthalide.
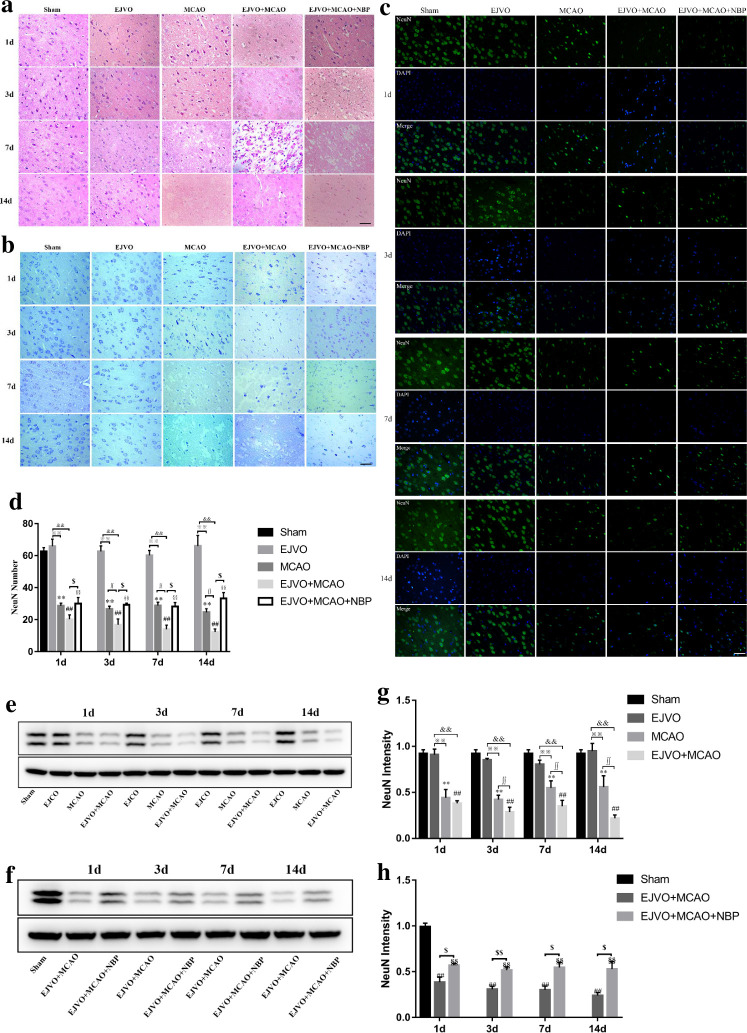
NBP reduce neuron damage in rate after MCAO and EJVO
HE staining was performed to observe the morphology of the neurons. Representative images are shown in figure 3A. As the disease progresses, the nuclear membrane breaks down and the cytoplasm mixes with the contents of the nucleus, hinting at neuronal cell damage. Rats that underwent MCAO showed more obvious neuronal damage than those in the sham group. In the EJVO+MCAO group, neuronal cell damage occurred in the early stage and suffered serious damage. In the NBP-treated group, the degree of neuronal damage was mild, and nuclear rupture occurred in the later stage compared with the non-treated EJVO+MCAO group.
Figure 3.
Disturbance of venous circulation aggravates neuron injury after MCAO, and NBP played a neuroprotective role in rats of MCAO accompanied by EJVO. (A) Representative H&E staining showing neuron cells from first to 14th day in infarction area or corresponding area in the sham, EJVO, MCAO, EJVO +MCAO and EJVO +MCAO+ NBP groups. (B) Representative Nissl staining showing neuron cells from first to 14th day in infarction area or corresponding area in the sham, EJVO, MCAO, EJVO +MCAO and EJVO +MCAO+ NBP groups. (C) Neuron cells were labelled with NeuN antibody (green) and merged with DAPI (blue) in ischaemic area or corresponding regions of sham or EJVO group. (D) Changes of neuron cells as measured by NeuN positive cell in the different time points after operation with immunofluorescence staining. (E, F) Western blots showed NueN expression after operation treated with NBP or not. (G, H) Quantification analysis of relative expression of NeuN level. Data are presented as mean±SD, n=5 per group for immunofluorescence staining and n=3 per group for Western bolt. **P<0.01, MCAO vs Sham; ##p<0.01, EJVO +MCAO vs sham; §§p<0.01, EJVO +MCAO + NBP vs sham; ※※p<0.01, MCAO vs EJVO; &&p<0.01, EJVO +MCAO vs EJVO; ∫∫p<0.05, EJVO +MCAO vs MCAO; $p<0.05, EJVO +MCAO + NBP vs EJVO +MCAO; $$p<0.01, EJVO +MCAO + NBP vs EJVO +MCAO. Scale bar=50 µm. EJVO, external jugular vein occlusion; MACO, middle cerebral artery occlusion; NBP, Dl-3-n-butylphthalide.
Nissl bodies in neurons were observed with the help of Nissl staining. As shown in figure 3B, rats in the sham group and EJVO groups displayed almost intact Nissl bodies while among those in the MCAO, EJVO +MCAO, and EJVO +MCAO+ NBP groups, Nissl staining revealed that the arrangement of neurons was disordered, and a large number of dark neurons appeared in the early stage. The number of Nissl-positive cells was lower than that in the sham group. Compared with the EJVO +MCAO group, the MCAO and EJVO +MCAO+ NBP groups preserved more Nissl-positive cells, which indicated that EJVO aggravated neuronal damage in rats with the MCAO group, while NBP treatment protected part of the neurons.
To analyse preserved neuronal cells in the ischaemic area, we used specific immunofluorescence staining of NeuN to label neurons. Figure 3C shows neurons in the regions of the cortical infarct. Rats’ neurons remained similar after the EJVO operation compared with the sham group. MCAO caused a significant loss of neuronal cells, and rats in the EJVO +MCAO group suffered a more severe neuronal loss than the ones in the MCAO group, with the difference being statistically significant from the third day after operation. Among rats with MCAO accompanied by venous disturbance, those treated with NBP showed milder injury compared with the placebo group (figure 3D). NeuN protein concentration was verified by western blotting to be consistent with the immunofluorescence (figure 3E–H). These findings suggest that once cerebral infarction occurs, concomitant venous circulation disorder aggravates neuronal damage. However, NBP treatment had a protective effect in rats with MCAO accompanied by venous disturbance.
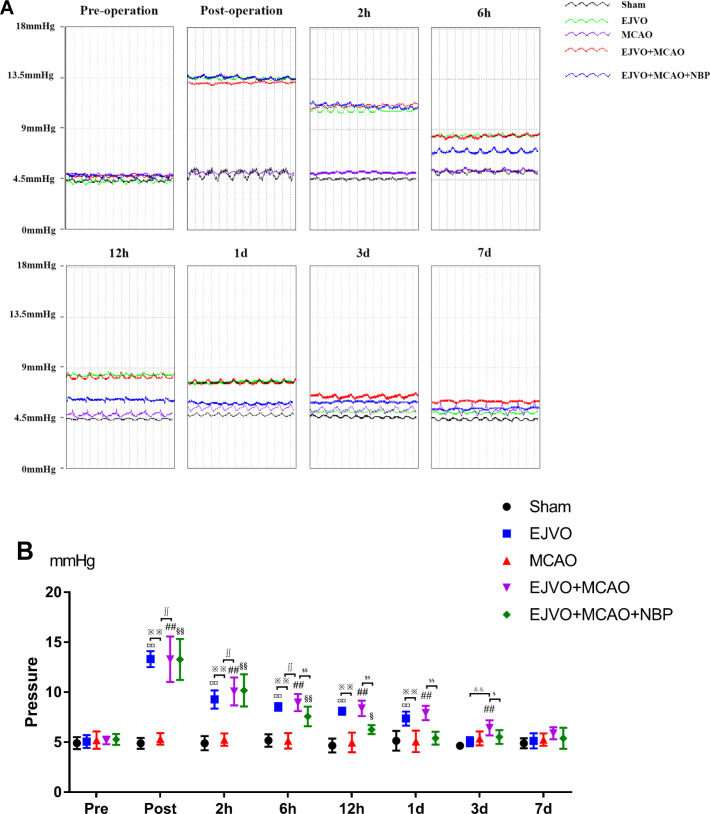
NBP reduced the increased pressure of the distal end of the ligated vein
The pressure of the EJV was measured at eight time points (preoperation, immediately, 2 hours, 6 hours, 12 hours, 1 day, 3 days and 7 days after the operation, as shown in figure 5. The bilateral ligation of the EJV could induce increased EVJ’s pressure immediately after the operation, and the elevated pressure began to decrease 2 hours after the operation and returned to normal levels on the third day. A single MCAO operation does not lead to an increase in EJV pressure. In the EJVO +MCAO and EJVO +MCAO+ NBP groups, we detected a significant increase in EJV pressure after the operation, similar to the EJVO group. This high EJV pressure status lasted for at least 3 days in the EJVO +MCAO group, whereas in the NBP-treated groups, it only lasted for less than 1 day. NBP treatment may help restore venous circulation and reduce EJV pressure in the early stage in rats with EJVO plus MCAO.
Figure 5.
Cerebral venous circulation disturbance increased external jugular veins pressure, and NBP could decline the pressure in early stage. (A) External jugular veins’ pressure of difference time points of preoperation and postoperation each group. (B) Quantification of external jugular pressure. ¤¤P<0.01, EJVO (immediately post-, 2 hours, 6 hours, 12 hours and 1 day after operation) vs EJVO (preoperation); ## P<0.01, EJVO +MCAO (immediately post-, 2 hours, 6 hours, 12 hours, 1 day and 3 days after operation) vs EJVO +MCAO (preoperation); §§p<0.01, EJVO +MCAO+ MCAO (immediately post-, 2 hours and 6 hours after operation) vs EJVO +MCAO+ NBP (preoperation); $p<0.05, EJVO +MCAO+ MCAO (12 hours after operation) vs EJVO +MCAO+ NBP (preoperation); ∫∫p<0.01, EJVO +MCAO+ NBP vs EJVO +MCAO in corresponding time points; ※※p<0.05, MCAO vs EJVO in corresponding time points; &&p<0.05, EJVO vs EJVO +MCAO in corresponding time points. EJVO, external jugular vein occlusion; MACO, middle cerebral artery occlusion; NBP, Dl-3-n-butylphthalide.
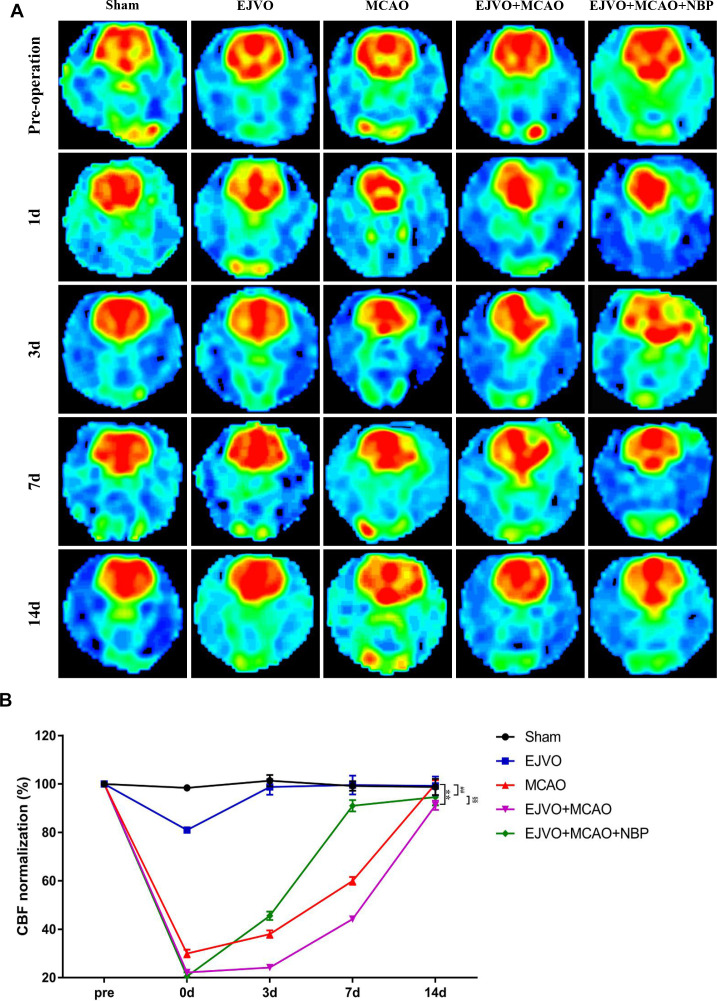
NBP promote the recovery of decreased CBF after MCAO of rats with venous circulation disturbance
In this study, 3D ASL was used for the dynamic assessment of CBF in rats. Ischaemic cortical areas (or corresponding brain areas in non-MCAO rats) were designated as ROIs and CBF values were measured at five time-points: preoperation, immediately after the operation, and the 3rd, 7th and 14th day after the operation. CBF in the ROI decreased immediately after the EJVO operation but returned to normal on the 3rd day. Rats in the MCAO group showed a significant decline in CBF, and the decline in EJVO +MCAO was more severe. Compared with the EJVO +MCAO group, rats in the NBP-treated group presented a quicker restoration of CBF. The results showed that rats with MCAO suffered an extreme decrease in CBF, among which EJVO delayed the recovery of CBF. NBP treatment accelerated CBF restoration in EJVO +MCAO rats. The CBF map and normalised quantitative analysis are shown in figure 6A, B.
Figure 6.
Cerebral venous circulation disturbance delayed recovery of CBF after MCAO, and NBP promoted CBF restoration. (A) CBF maps were generated from ASL and EJVO +MCAO group showed a lower CBF than MCAO group. NBP treatment could accelerate CBF recovery after rats underwent MCAO operation plus with EJVO. (B) Quantitative analysis showing changes of CBF in infarct area from first to 14th day. data are presented as mean±SD; n=5 per group. **P<0.01, EJVO +MCAO vs Sham; ##p<0.01, MCAO vs sham; §§p<0.01, EJVO +MCAO + NBP vs EJVO +MCAO. CBF, cerebral blood flow; EJVO, external jugular vein occlusion; MACO, middle cerebral artery occlusion; NBP, Dl-3-n-butylphthalide.
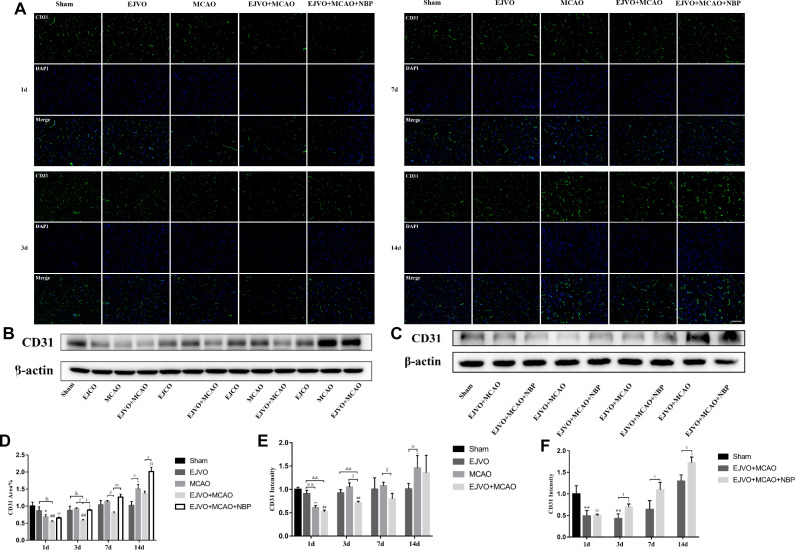
NBP promoted microvascular angiogenesis of MCAO rats with venous circulation disturbance
To explore the effect of venous circulation disturbance on angiogenesis after cerebral infarction, we counted the rate of CD31 (a classical endothelial cell marker) coverage in brain sections by immunofluorescence and evaluated the total ischaemic brain tissue concentration of CD31 by western blot. The results showed a slight decline in the EJVO group but the difference was not statistically significant (figure 4). Significant reduction in CD31 was detected on the first day after MCAO, whether or not it was accompanied by venous circulatory disturbance. On the third day after the operation, the simple MCAO group presented a prominent improvement in CD31 extremely back to the normal level; however, the EJVO +MCAO group showed a mild increase in CD31. Statistically significant differences remained between the EJVO +MCAO versus sham, EJVO, and MCAO groups. Then, on the seventh day, the CD31 level in the EJVO +MCAO group returned to normal; however, it was still significantly lower than that in the MCAO group. When the CD31 level in the MCAO group was higher than that in the sham group on the 14th day, the CD31 of the EJVO +MCAO group increased but not significantly. Among rats with MCAO accompanied by venous disturbance, NBP treatment significantly increased vascular endothelial density. Angiogenesis, as the third classification of collateral in vivo, is conducive to the restoration of blood flow. The results of our study suggest that venous circulation disturbance caused by bilateral EJVO would hinder angiogenesis in the infarction area, and NBP treatment could promote angiogenesis in these rats.
Figure 4.
Disturbance of venous circulation decrease microvascular angiogenesis, and NBP treatment could improve angiogenesis. (A) Immunofluorescence staining of CD31 positive cells in the microvessels in the region of ischaemia from first to 14th day after operation in the sham, EJVO, MCAO, EJVO +MCAO and EJVO +MCAO+ NBP groups. (B, C) Western blots of expression of CD31. (D) Quantification of microvassels density measured by immunofluorescence staining of CD31. (E, F) Quantification analysis of relative expression of CD31 level measured by Western blot. Data are presented as mean±SD, n=5 per group for immunofluorescence staining and n=3 per group for Western bolt. *P<0.05, MCAO vs sham; **P<0.01, MCAO vs Sham; ## P<0.01, EJVO +MCAO vs sham; §p<0.05, EJVO +MCAO + NBP vs sham; §§p<0.01, EJVO +MCAO + NBP vs sham; ※P<0.05, MCAO vs EJVO; ※※P<0.01, MCAO vs EJVO; & p<0.05, EJVO +MCAO vs EJVO; &&p<0.01, EJVO +MCAO vs EJVO; ∫∫p<0.05, EJVO +MCAO vs MCAO; $p<0.05, EJVO +MCAO + NBP vs EJVO +MCAO; $$p<0.01, EJVO +MCAO + NBP vs EJVO +MCAO. Scale bar=50 µm. EJVO, external jugular vein occlusion; MACO, middle cerebral artery occlusion; NBP, Dl-3-n-butylphthalide.
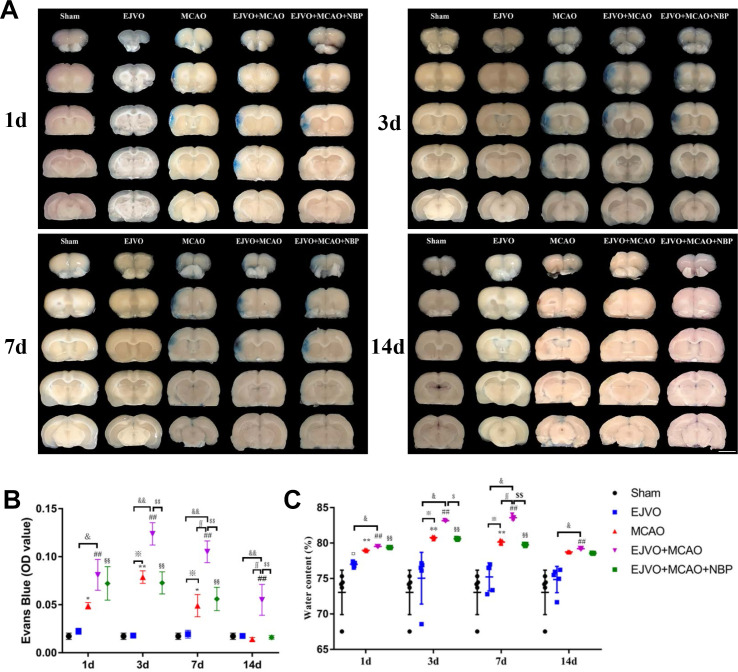
NBP decreased the aggravated damage of BBB by venous circulation disturbance
First, we used EB extravasation to assess BBB integrity. After EB circulation for 3 hour, the brain was sliced for photographing and measuring the absorbance, as displayed in figure 7A. Rats with bilateral EJVO did not display obvious EB extravasation in our study, while MCAO rats with bilateral EJVO aggravated BBB damage to a large extent after MCAO (figure 7B). EB extravasation of MCAO alone was restored to normal 14 days after the operation, while it remained at a high level in the EJVO +MCAO group throughout the study period. In rats with MCAO accompanied by EJVO, NBP treatment attenuated EB extravasation caused by MCAO plus EJVO (figure 7A and B).
Figure 7.
Cerebral venous circulation disturbance aggravate the damage of blood brain barrier, and NBP could reduce BBB damage in EJVO +MCAO+ NBP group. (A) Representative images showing EB extravasation from first to 14th day after operation in the sham, EJVO, MCAO, EJVO +MCAO and EJVO +MCAO+ NBP groups. (B) Quantification analysis of absorbance of EB extravasation. (C) Quantification analysis of ipsilateral brain tissue water content. data are presented as mean±SD; n=5 per group. ¤P<0.05, EJVO vs sham; *p<0.05, MCAO vs sham; **p<0.01, MCAO vs Sham; ##p<0.01, EJVO +MCAO vs sham; §§p<0.01, EJVO +MCAO + NBP vs sham; ※p<0.05, MCAO vs EJVO; &p<0.05, EJVO +MCAO vs EJVO; &&p<0.01, EJVO +MCAO vs EJVO; ∫∫p<0.05, EJVO +MCAO vs MCAO; $p<0.05, EJVO +MCAO + NBP vs EJVO +MCAO; $$p<0.01, EJVO +MCAO + NBP vs EJVO +MCAO. Scale bar=5 mm. BBB, blood-brain barrier; EB, Evans blue; EJVO, external jugular vein occlusion; MACO, middle cerebral artery occlusion; NBP, Dl-3-n-butylphthalide.
The brain water content was measured to evaluate the brain oedema. Water content was briefly increased in the bilateral EJVO on the first day after operation, and then returned to normal rapidly (figure 7C), suggesting that acute cerebral venous circulation disorder may cause transient brain oedema. In the simple MCAO group, brain oedema was detected from the first to the seventh day after the operation compared with the sham group. In addition, in the MCAO group accompanied by venous circulation disturbance, the rats developed worse brain oedema than simple MCAO rats, which hinted at the adverse effects of venous circulation disturbance on brain oedema. Moreover, when the brain water content of simple MCAO rats returned to normal levels on the 14th day after operation, the EJVO +MCAO group had a significantly higher water content than the sham group. NBP treatment reduced brain oedema after 3 days of intervention in the rats with MCAO accompanied by venous circulation disturbance.
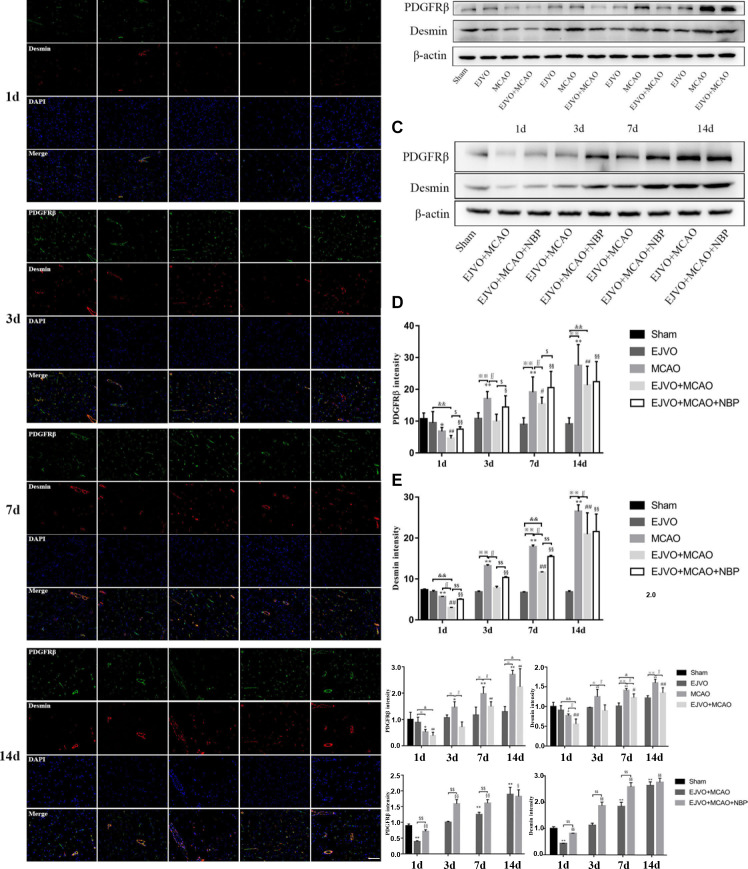
NBP decreased the destruction of BBB integrity caused by MCAO and EJVO
Pericytes are a type of cells that surround endothelial cells and are essential components of the BBB. Pericytes are also distributed in venules and arterioles, which have the ability to contract and migrate. To investigate changes in pericytes in our animal model, PDGFRβ and desmin were selected to label pericytes. Regardless of the presence of venous distortion, rats that underwent MCAO showed a decrease in pericytes 1 d after the operation. On the first day, EJVO+MCAO rats showed significantly lower PDGFRβ and desmin levels than MCAO rats. There was a prominent improvement in pericytes in the MCAO group from 3 days after the operation, which was far beyond the normal level, while in the EJVO+MCAO group, both PDGFRβ and desmin increased to the normal level at 3 days after surgery, with a significantly lower level than that in the MCAO group. All rats that underwent MCAO displayed an increase in the number of pericytes in the ischaemic region after 7 days, and the increase in simple MCAO rats was more prominent. NBP-treated rats reduced the loss of pericytes at an early stage and increased PDGFRβ and desmin levels to a greater extent than in the non-treated group during the following procedure (figure 8).
Figure 8.
Cerebral venous circulation disturbance aggravated pericytes loss, and NBP treatment could protect pericyte from severe loss in rats of MCAO accompanied by EJVO. (A) the expression of pericytes labelled by colocalisaton of PDGFRβ (green) and desmin (red) in the ischaemic area or corresponding region. (B, C) Expression of PDGFRβ and desmin detected by Western blot. (D) Quantification analysis of PDGFRβ analysed by immunofluorescence staining. (E) quantification analysis of desmin analysed by immunofluorescence staining. (F, H) Quantification analysis of PDGFRβ analysed by Western blot. (G, I) Quantification analysis of desmin analysed by Western blot. Data are presented as mean±SD, n=5 per group for immunofluorescence staining and n=3 per group for Western bolt. *P<0.05, MCAO vs sham; **p<0.01, MCAO vs sham; #p<0.05, EJVO +MCAO vs Sham; ##p<0.01, EJVO +MCAO vs sham; §p<0.05, EJVO +MCAO + NBP vs sham; §§p<0.01, EJVO +MCAO + NBP vs sham; ※p<0.05, MCAO vs EJVO; ※※p<0.01, MCAO vs EJVO; &p<0.05, EJVO +MCAO vs EJVO; &&p<0.01, EJVO +MCAO vs EJVO; ∫∫p<0.05, EJVO +MCAO vs MCAO; $p<0.05, EJVO +MCAO + NBP vs EJVO +MCAO; $$p<0.01, EJVO +MCAO + NBP vs EJVO +MCAO. Scale bar=50 µm. EJVO, external jugular vein occlusion; MACO, middle cerebral artery occlusion; NBP, Dl-3-n-butylphthalide.
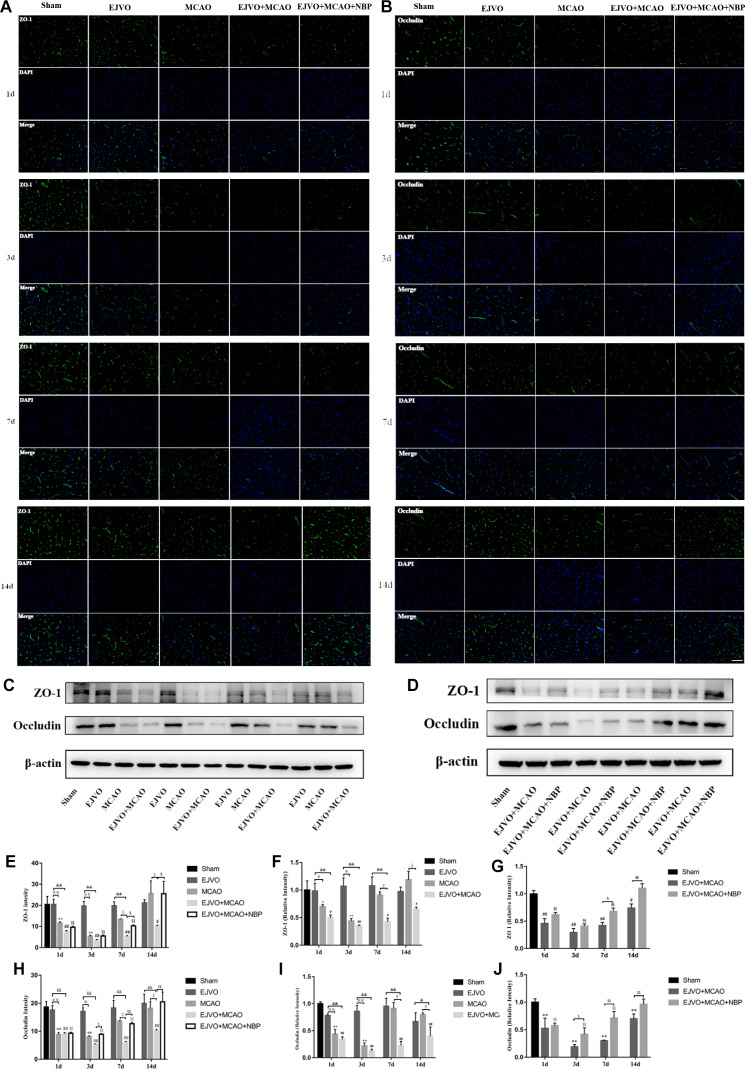
In addition, the levels of tight junction proteins, including ZO-1 and occluding, were significantly decreased after MCAO; however, no difference was detected in the EJVO group throughout the study period. By comparing ZO-1 levels between MCAO rats with or without venous outflow disorder, we found a statistically significant decrease in the EJVO+MCAO group beginning at 7 days after operation, and ZO-1 remained at a low level for 14 days while it was restored to normal in the simple MCAO group. We then tested the effect of NBP and found a significant improvement in ZO-1 in the treated group of MCAO rats accompanied by venous distortion, especially 7 days after the operation (figure 9).
Figure 9.
Cerebral venous circulation disturbance accelerate BBB damage, and NBP attenuated damage of BBB caused by MCAO plus with EJVO. (A) Expression of ZO-1 in the ischaemic area or corresponding region measured by immunofluorescence staining. (B) Expression of occludin in the ischaemic area or corresponding region measured by immunofluorescence staining. (C, D) Expression of ZO-1 and occludin detected by Western blot in the ischaemic brain tissue or corresponding area in the sham or EJVO group. (E) Quantification analysis of ZO-1 analysed by immunofluorescence staining. (F, G) Quantification analysis of ZO-1 analysed by Western blot. (H) Quantification analysis of occludin analysed by immunofluorescence staining. (I, J) Quantification analysis of occludin analysed by Western blot. data are presented as mean±SD, n=5 per group for immunofluorescence staining and n=3 per group for Western bolt. *P<0.05, MCAO vs sham; **p<0.01, MCAO vs sham; #p<0.05, EJVO +MCAO vs Sham; ##p<0.01, EJVO +MCAO vs sham; §§P<0.01, EJVO +MCAO + NBP vs sham; ※p<0.05, MCAO vs EJVO; ※※p<0.01, MCAO vs EJVO; &p<0.05, EJVO +MCAO vs EJVO; &&p<0.01, EJVO +MCAO vs EJVO; ∫∫p<0.05, EJVO +MCAO vs MCAO; $p<0.05, EJVO +MCAO + NBP vs EJVO +MCAO; $$p<0.01, EJVO +MCAO + NBP vs EJVO +MCAO. Scale bar=50 µm. BBB, blood-brain barrier; EJVO, external jugular vein occlusion; MACO, middle cerebral artery occlusion; NBP, Dl-3-n-butylphthalide.
For occludin, the same declining trend was detected after MCAO, as well as the more severe loss of occludin in the EJVO+MCAO group. Rats in the NBP-treated group presented a higher occlusion level after 3 days (figure 8). These results showed that venous distortion accelerated BBB breakdown after MCAO, and NBP treatment showed a protective effect on the BBB in MCAO rats accompanied by venous disturbance.
Discussion
Ischaemic stroke is a serious threat to human health because of its high morbidity, disability and mortality. The pathological causes of the disease lie in the death of neurons caused by the sudden interruption of blood flow, ischaemic-reperfusion injury, brain oedema and so on. Very early blood recovery is the most effective way to reverse nerve injury after cerebral infarction, but no-reflow often causes problems for medical workers. Clinical studies have found that venous circulation affects the prognosis of stroke patients.5 15 In the current study, we discovered that blocking the outflow channel of intracranial blood would increase the infarct area of MCAO rats, accompanied by the aggravation of motor function and sensory function. The malignant effect might be related to the aggravation of BBB damage and delay of angiogenesis in rats after cortical cerebral infarction. NBP treatment significantly improved neurological function in rats with MCAO accompanied by venous disturbance. This study provides preclinical evidence suggesting that brain venous circulation function is an important factor for ischaemic stroke prognosis, and NBP treatment may have a protective effect on ischaemic rats accompanied by brain venous circulation disorder.
Clinical and preclinical research has demonstrated that the infarction area is the most important prognostic factor after ischaemic stroke.25 In the current study, we occluded the anterior branch of the distal right MCA and detected the infarct volume by TTC staining. The results showed that cortical cerebral infarction was found in all animals undergoing MCAO, accompanied by a decline in neurological function. Compared with simple MCAO, rats with MCAO accompanied by EJVO had a larger infarct size and aggravated neuron loss. Meanwhile, MCAO rats with EJVO displayed worse neurological function, presented as a lower forelimb grasping force, a higher balance beam test score and increased adhesive-removal test time. Previous clinical research revealed that over 20% of people have abnormal development of intracranial veins, and most of them remain asymptomatic.20 21 Yu et al considered that venous collaterals may be sufficient to prevent cerebral venous congestion and oedema in the case of normal intracranial pressure, while abnormalities of cerebral venous drainage would accelerate and aggravate brain oedema in the case of large MCA infarction.15 The current findings of our study are consistent with those of previous studies showing that the occlusion of bilateral EJVs would not lead to neuron loss or behavioural decline; however, in the setting of MCAO, bilateral EJVO could significantly exacerbate neuronal damage. Therefore, we concluded that brain venous disturbance would aggravate ischaemic stroke injury.
In this study, we detected a slight decline in CBF in EJVO rats immediately after bilateral EJVO; however, this decline was not statistically significant, as we performed analysis by repeated-measures ANOVA during the study period. CD31 levels remained normal throughout the study period in the EJVO group. Compared with the sham group, a prominent decrease in CBF was detected in rats that underwent MCAO, regardless of EJVO. In addition, rats with MCAO accompanied by EJVO displayed a lower CBF value and a later CBF restoration in the infarct cortex than the simple MCAO rats. Published research reported that angiogenesis occurred 1–2 weeks after the onset of infarction.26 In our current study, vascular endothelial cells in the simple MCAO group were restored to the sham group 3 days after the operation and were significantly higher than those in the sham group 14 days after surgery, which was consistent with the findings of previous studies. In the EJVO +MCAO group, lower endothelial cells remained within 3 days and showed a non-significant increment on the seventh and 14th days compared with the sham group. We considered that the disturbance of venous circulation could aggravate the damage of microvasculature and delay angiogenesis and CBF restoration.
In this study, we found that the occlusion of bilateral EJVs did not reduce the number of pericytes but a significant decrease in pericytes was detected in the early stage after MCAO and returned to an extremely high level on the third day. Additionally, we found worse brain oedema and EB extravasation in EJVO+MCAO rats. Pericytes are a type of mural cell that envelope endothelial cells and play important roles in BBB maintenance, intracranial pressure regulation, endothelial proliferation and blood vessel maturation.27 28 Researchers observed the ultrastructure of the brain vessels after ischaemia and found that pericytes separated from the endothelial cells for migration after 1–2 hours of hypoxia.29 Ghori A et al reported that pericytes drop off from the microvasculature within 24 hours post-MCAO, leading to increased vascular permeability.30 An in vitro experiment revealed that rat brain pericytes increased the expression of occludin by the Ang-1/Tie-2 pathway.31 In addition, endothelial cell survival of pericytes is promoted by the upregulation of VEGF-A.32 The in vivo experiment demonstrated that the injection of blood-derived MSC-like pericytes could rescue ischaemic hindlimb tissue by enhancing revascularisation.33 Our current results show that in the EJVO+MCAO group, a more severe loss of pericytes and later increment was detected. This is at least part of the cause of serious brain oedema and EB extravasation. Venous drainage disturbances may cause BBB damage after MCAO.
Tight junctions contain a series of proteins located between endothelial cells, playing a significant role in the formation of continuous blood vessel structures and maintaining the integrity of the BBB.34 Tight junctions were associated with increased BBB permeability.35 In this study, the levels of tight junction proteins, including ZO-1 and occludin, were significantly decreased after ischaemic stroke, accompanied by brain oedema and EB extravasation. Lower ZO-1 and occludin levels and more consistent EB extravasation, as well as more severe brain oedema, were detected in the EJVO+MCAO group than in the MCAO group. We can speculate from the current results that EJVO alone would not degenerate BBB integrity, while in the case of MCAO, EJVO would deteriorate BBB permeability.
Published studies have demonstrated that NBP reduces the infarct area, decreases neuronal cell death, preserves the BBB, and improves neurological function.17 Preclinical studies have shown that NBP could increase CBF after MCAO.36 The mechanism by which NBP increases CBF may involve the dilation of the cerebral artery after acute or chronic cerebral ischaemia.16 In addition, NBP has been shown to upregulate the expression of vascular endothelial growth factor and angiopoietin-1 to promote angiogenesis.17 In addition, the neuroprotective effects of NBP on anti-inflammatory,37 antithrombotic38 and mitochondrial dysfunction39 have been confirmed in previous studies. Although the multifunction of NBP in cerebral ischaemia has been determined, the effectiveness of NBP in AIS accompanied by brain venous disturbance is still not known and is worth exploring. In our current study, we found a prominent protective effect of NBP on rats with MCAO combined with EJVO. The treated rats showed reduced infarct size, decreased brain oedema, attenuated EB extravasation, and improved neurological function accompanied by decreased neuron loss. We found an improvement in CBF and ameliorated angiogenesis in the NBP-treated group compared with the vehicle group. We speculate that the protective effects of NBP in our animal model may contribute, at least in part, to its enhancement of CBF. Furthermore, pericytes were protected from a great deal of loss by NBP at the early stage after surgery and were increased with a greater number in later stages, which may promote the repair of BBB and angiogenesis. Additionally, we found that the levels of tight junction proteins, another key component of the BBB, increased with NBP treatment. Therefore, we believe that NBP also has a significant effect on BBB protection in MCAO rats with venous circulation disturbance. Based on our findings, we speculate that NBP-mediated CBF restoration and BBB damage alleviation may contribute to nervous protection. Furthermore, we found that NBP could improve venous circulation as a faster recovery of EJV pressure was obtained in the NBP-treated group. The mechanism by which NBP reduces venous pressure remains unclear. A previous study reported that NBP dilates the main artery supplying the brain tissue, promotes the opening of the leptomeningeal anastomotic branches and promotes angiogenesis. It was also found in the chronic cerebral hypoperfusion model of rats that NBP treatment can help to dilate the vertebral artery and promote the recovery of CBF. We speculate that NBP plays a role in reducing venous pressure by increasing the diameter of the drainage veins and promoting the opening of anastomotic branches. Further research is required to elucidate the mechanism of adverse effects of venous circulation disturbance on AIS and explore potential therapeutic targets.
Conclusion
We revealed that bilateral EJVO did not significantly affect the normal rats but aggravated brain damage in the case of ischaemic stroke. NBP treatment plays a neuroprotective role in rats with MCAO accompanied by EJVO mainly due to the promotion of CBF restoration, EJV’s pressure decline and BBB protection.
Acknowledgments
We are grateful to staff of the Institute of Laboratory Animal Science of Jinan University for their support and care of the animals. We are also grateful to Medical School of Jinan University for providing us with the necessary experimental equipment. We would like to thank Editage (www.editage.cn) for English language editing.
Footnotes
KS, XZ and XX contributed equally.
Contributors: All authors have contributed significantly to this manuscript, and KS, XZ and XX contributed equally to this work and share first authorship. And corresponding author is professor LH. Contributions of each author are listed as follow: KS, Study design, experiment implementation, and manuscript writing. XZ, experiment implementation, and manuscript revision. XX, experiment implementation, and manuscript revision. RZ, statistical analysis. JL, MRI scanning and data collection. GC, manuscript revision. LH, study design and manuscript revision. LH is is responsible for the overall content as guarantor. The guarantor accepts full responsibility for the finished work and the conduct of the study, had access to the data, and controlled the decision to publish.
Funding: This study was funded by the National Natural Science Foundation of China (No. 81971120) and the Science and Technology Planning Project of Guangdong Province of China (No 2017A02021520081971120).
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement
Data are available on reasonable request.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
The protocol was approved by the Ethics Committee of the Institute of Laboratory Animal Science of Jinan University.
References
- 1. Albers GW, Marks MP, Kemp S, et al. Thrombectomy for stroke at 6 to 16 hours with selection by perfusion imaging. N Engl J Med 2018;378:708–18. 10.1056/NEJMoa1713973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Campbell BCV, Mitchell PJ, Kleinig TJ, et al. Endovascular therapy for ischemic stroke with perfusion-imaging selection. N Engl J Med 2015;372:1009–18. 10.1056/NEJMoa1414792 [DOI] [PubMed] [Google Scholar]
- 3. Campbell BCV, Christensen S, Tress BM, et al. Failure of collateral blood flow is associated with infarct growth in ischemic stroke. J Cereb Blood Flow Metab 2013;33:1168–72. 10.1038/jcbfm.2013.77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kloner RA, King KS, Harrington MG. No-Reflow phenomenon in the heart and brain. Am J Physiol Heart Circ Physiol 2018;315:H550–62. 10.1152/ajpheart.00183.2018 [DOI] [PubMed] [Google Scholar]
- 5. Tong L-S, Guo Z-N, Ou Y-B, et al. Cerebral venous collaterals: a new Fort for fighting ischemic stroke? Prog Neurobiol 2018;163-164:172–93. 10.1016/j.pneurobio.2017.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Alperin N, Lee SH, Mazda M, et al. Evidence for the importance of extracranial venous flow in patients with idiopathic intracranial hypertension (IIH). Acta Neurochir Suppl 2005;95:129–32. 10.1007/3-211-32318-x_28 [DOI] [PubMed] [Google Scholar]
- 7. Sagoo RS, Hutchinson CE, Wright A, et al. Magnetic resonance investigation into the mechanisms involved in the development of high-altitude cerebral edema. J Cereb Blood Flow Metab 2017;37:319–31. 10.1177/0271678X15625350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wilson MH, Davagnanam I, Holland G, et al. Cerebral venous system and anatomical predisposition to high-altitude headache. Ann Neurol 2013;73:381–9. 10.1002/ana.23796 [DOI] [PubMed] [Google Scholar]
- 9. Hsu H-Y, Chao A-C, Chen Y-Y, et al. Reflux of jugular and retrobulbar venous flow in transient monocular blindness. Ann Neurol 2008;63:247–53. 10.1002/ana.21299 [DOI] [PubMed] [Google Scholar]
- 10. Enslin JMN, Lefeuvre D, Taylor A. Developmental venous anomaly with contralateral impaired venous drainage in a 17-year-old male. A case report. Interv Neuroradiol 2013;19:67–72. 10.1177/159101991301900110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rasalkar DD, Paunipagar BK. Developmental venous anomaly associated with cortical dysplasia. Pediatr Radiol 2010;40 Suppl 1:S165. 10.1007/s00247-010-1654-2 [DOI] [PubMed] [Google Scholar]
- 12. Yura S, Sako K, Yonemasu Y. [The effects of disturbance of cerebral venous drainage on focal cerebral blood flow and ischemic cerebral edema]. No To Shinkei 1990;42:269–75. [PubMed] [Google Scholar]
- 13. van den Wijngaard IR, Wermer MJH, Boiten J, et al. Cortical venous filling on dynamic computed tomographic angiography: a novel predictor of clinical outcome in patients with acute middle cerebral artery stroke. Stroke 2016;47:762–7. 10.1161/STROKEAHA.115.012279 [DOI] [PubMed] [Google Scholar]
- 14. Bhaskar S, Bivard A, Stanwell P, et al. Association of cortical vein filling with clot location and clinical outcomes in acute ischaemic stroke patients. Sci Rep 2016;6:38525. 10.1038/srep38525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Yu W, Rives J, Welch B, et al. Hypoplasia or occlusion of the ipsilateral cranial venous drainage is associated with early fatal edema of middle cerebral artery infarction. Stroke 2009;40:3736–9. 10.1161/STROKEAHA.109.563080 [DOI] [PubMed] [Google Scholar]
- 16. Qin C, Zhou P, Wang L, et al. Dl-3-N-butylphthalide attenuates ischemic reperfusion injury by improving the function of cerebral artery and circulation. J Cereb Blood Flow Metab 2019;39:2011-2021. 10.1177/0271678X18776833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zhou P-T, Wang L-P, Qu M-J, et al. Dl-3-N-butylphthalide promotes angiogenesis and upregulates sonic hedgehog expression after cerebral ischemia in rats. CNS Neurosci Ther 2019;25:748–58. 10.1111/cns.13104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sun Y, Cheng X, Wang H, et al. dl-3-n-butylphthalide promotes neuroplasticity and motor recovery in stroke rats. Behav Brain Res 2017;329:67–74. 10.1016/j.bbr.2017.04.039 [DOI] [PubMed] [Google Scholar]
- 19. Yang C-S, Guo A, Li Y, et al. Dl-3-n-butylphthalide reduces neurovascular inflammation and ischemic brain injury in mice. Aging Dis 2019;10:964–76. 10.14336/AD.2019.0608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lichtenstein D, Saïfi R, Augarde R, et al. The internal jugular veins are asymmetric. usefulness of ultrasound before catheterization. Intensive Care Med 2001;27:301–5. 10.1007/s001340000792 [DOI] [PubMed] [Google Scholar]
- 21. Alper F, Kantarci M, Dane S, et al. Importance of anatomical asymmetries of transverse sinuses: an MR venographic study. Cerebrovasc Dis 2004;18:236–9. 10.1159/000079960 [DOI] [PubMed] [Google Scholar]
- 22. Wang Y-Y, Niu R-Z, Wang J-D, et al. Establishment of brain ischemia model in tree shrew. Brain Res 2019;1718:194–200. 10.1016/j.brainres.2019.05.011 [DOI] [PubMed] [Google Scholar]
- 23. Bouet V, Boulouard M, Toutain J, et al. The adhesive removal test: a sensitive method to assess sensorimotor deficits in mice. Nat Protoc 2009;4:1560–4. 10.1038/nprot.2009.125 [DOI] [PubMed] [Google Scholar]
- 24. Jing Z, Shi C, Zhu L, et al. Chronic cerebral hypoperfusion induces vascular plasticity and hemodynamics but also neuronal degeneration and cognitive impairment. J Cereb Blood Flow Metab 2015;35:1249–59. 10.1038/jcbfm.2015.55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cheng X, Yeung PKK, Zhong K, et al. Astrocytic endothelin-1 overexpression promotes neural progenitor cells proliferation and differentiation into astrocytes via the JAK2/STAT3 pathway after stroke. J Neuroinflammation 2019;16:227. 10.1186/s12974-019-1597-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zhang J, Liu M, Huang M, et al. Ginsenoside F1 promotes angiogenesis by activating the IGF-1/IGF1R pathway. Pharmacol Res 2019;144:292–305. 10.1016/j.phrs.2019.04.021 [DOI] [PubMed] [Google Scholar]
- 27. Cheng J, Korte N, Nortley R, et al. Targeting pericytes for therapeutic approaches to neurological disorders. Acta Neuropathol 2018;136:507–23. 10.1007/s00401-018-1893-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Berthiaume A-A, Grant RI, McDowell KP, et al. Dynamic remodeling of pericytes in vivo maintains capillary coverage in the adult mouse brain. Cell Rep 2018;22:8–16. 10.1016/j.celrep.2017.12.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Duz B, Oztas E, Erginay T, et al. The effect of moderate hypothermia in acute ischemic stroke on pericyte migration: an ultrastructural study. Cryobiology 2007;55:279–84. 10.1016/j.cryobiol.2007.08.009 [DOI] [PubMed] [Google Scholar]
- 30. Ghori A, Freimann FB, Nieminen-Kelhä M, et al. Ephrinb2 activation enhances vascular repair mechanisms and reduces brain swelling after mild cerebral ischemia. Arterioscler Thromb Vasc Biol 2017;37:867–78. 10.1161/ATVBAHA.116.308620 [DOI] [PubMed] [Google Scholar]
- 31. Hori S, Ohtsuki S, Hosoya K-ichi, et al. A pericyte-derived angiopoietin-1 multimeric complex induces occludin gene expression in brain capillary endothelial cells through Tie-2 activation in vitro. J Neurochem 2004;89:503–13. 10.1111/j.1471-4159.2004.02343.x [DOI] [PubMed] [Google Scholar]
- 32. Franco M, Roswall P, Cortez E, et al. Pericytes promote endothelial cell survival through induction of autocrine VEGF-A signaling and Bcl-w expression. Blood 2011;118:2906–17. 10.1182/blood-2011-01-331694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Blocki A, Wang Y, Koch M, et al. Sourcing of an alternative pericyte-like cell type from peripheral blood in clinically relevant numbers for therapeutic angiogenic applications. Mol Ther 2015;23:510–22. 10.1038/mt.2014.232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Pandit R, Chen L, Gotz J. The blood-brain barrier: physiology and strategies for drug delivery. Adv Drug Deliv Rev 2019. 10.1016/j.addr.2019.11.009 [DOI] [PubMed] [Google Scholar]
- 35. Feng S, Zou L, Wang H, et al. RhoA/ROCK-2 pathway inhibition and tight junction protein upregulation by catalpol suppresses lipopolysaccaride-induced disruption of blood-brain barrier permeability. Molecules 2018;23:2371. 10.3390/molecules23092371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Li W, Wei D, Xie X, et al. Dl-3-n-Butylphthalide regulates the Ang-1/Ang-2/Tie-2 signaling axis to promote neovascularization in chronic cerebral hypoperfusion. Biomed Pharmacother 2019;113:108757. 10.1016/j.biopha.2019.108757 [DOI] [PubMed] [Google Scholar]
- 37. Xu HL, Feng YP. Inhibitory effects of chiral 3-n-butylphthalide on inflammation following focal ischemic brain injury in rats. Acta Pharmacol Sin 2000;21:433–8. [PubMed] [Google Scholar]
- 38. Wang X, Wang L, Sheng X, et al. Design, synthesis and biological evaluation of hydrogen sulfide releasing derivatives of 3-n-butylphthalide as potential antiplatelet and antithrombotic agents. Org Biomol Chem 2014;12:5995–6004. 10.1039/C4OB00830H [DOI] [PubMed] [Google Scholar]
- 39. Huang J-Z, Chen Y-Z, Su M, et al. dl-3-n-Butylphthalide prevents oxidative damage and reduces mitochondrial dysfunction in an MPP(+)-induced cellular model of Parkinson's disease. Neurosci Lett 2010;475:89–94. 10.1016/j.neulet.2010.03.053 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available on reasonable request.