Abstract
Short-term synaptic plasticity represents a fundamental mechanism in neural information processing and is regulated by neuromodulators. Here, using field recordings from the CA1 region of adult rat hippocampal slices, we show that excitatory synaptic transmission is suppressed by strong but not moderate activation of adenosine A1 receptors by 2-Chloro-N6-cyclopentyladenosine (CCPA) more in the dorsal than the ventral hippocampus; in contrast, both mild and strong activation of GABAB receptors by baclofen (1 μM, 10 μM) suppress synaptic transmission more in the ventral than the dorsal hippocampus. Using a 10-pulse stimulation train of variable frequency, we found that CCPA modulates short-term synaptic plasticity independently of the suppression of synaptic transmission in both segments of the hippocampus and at stimulation frequencies greater than 10 Hz. However, specifically regarding the paired-pulse ratio (PPR) and frequency facilitation/depression (FF/D) we found significant drug action before but not after adjusting conditioning responses to control levels. Activation of GABABRs by baclofen suppressed synaptic transmission more in the ventral than the dorsal hippocampus. Furthermore, relatively high (10 μM) but not low (1 μM) baclofen concentration enhanced both PPR and FF in both hippocampal segments at stimulation frequencies greater than 1 Hz, independently of the suppression of synaptic transmission by baclofen. These results show that A1Rs and GABABRs control synaptic transmission more effectively in the dorsal and the ventral hippocampus, respectively, and suggest that these receptors modulate PPR and FF/D at different frequency bands of afferent input, in both segments of the hippocampus.
Keywords: Hippocampus, dorsoventral, septotemporal, longitudinal axis, short-term synaptic plasticity, adenosine receptors, GABAb receptors, neuromodulation, in vitro, rat
Introduction
Neuromodulation is a variety of physiological processes implicated in regulating synaptic efficacy and neuronal excitability (Katz and Edwards, 1999; Nadim and Bucher, 2014), thereby flexibly altering the flow of information in neural circuits and determining brain state and behavior (Marder et al., 2014; McCormick et al., 2020; O’Callaghan et al., 2021). A basic function of neuromodulation is to modify the strength of synapses and the properties of short-term synaptic plasticity, that is, the synaptic dynamics (Ito and Schuman, 2008; Nadim and Bucher, 2014).
Short-term synaptic plasticity is a major category of activity-dependent changes in synaptic efficacy, encompassing several phenomena of transient changes in synaptic transmission, lasting from tens of milliseconds to tens of minutes (Jackman and Regehr, 2017; Zucker and Regehr, 2002). For instance, a widely studied form of short-term synaptic plasticity is the so-called paired-pulse facilitation or depression, which consists of a change (i.e. increase or decrease, respectively) in the second versus the first response of a pair of synaptic responses evoked by pairing two stimuli (paired-pulse stimulation) applied to presynaptic fibers in fast succession. Here, we will refer to this form of short-term synaptic plasticity with the term paired-pulse ratio (PPR). The specific effect of paired-pulse stimulation, that is, facilitation or depression, and the magnitude of induced changes depends on several factors including the constitutive properties of a synapse and the specific brain region where synapses are located, the interstimulus interval, the ratio between Ca2+ and Mg2+ in the extracellular milieu, whether synapses have undergone long-term changes, the age (Dobrunz and Stevens, 1997; Dumas and Foster, 1998; Jackman et al., 2016; Manabe et al., 1993; Papatheodoropoulos and Kostopoulos, 1998; Zucker and Regehr, 2002). Another form of short-term synaptic plasticity is frequency facilitation or depression (FF/D) which is evident during short bursts of presynaptic activity of varying frequency (Abbott et al., 1997; Jackman et al., 2016; Markram and Tsodyks, 1996).
Phenomena of short-term synaptic plasticity are thought to play important roles in neural information processing performed across at a relative fast time scale, including temporal filtering, dynamic gain control, temporal selectivity, and synaptic input diversification (Dobrunz and Stevens, 1999; Lisman, 1997; Motanis et al., 2018; Rotman et al., 2011; Thomson, 2000). Furthermore, short-term synaptic plasticity is involved in processing ongoing neural activity (Klausnitzer and Manahan-Vaughan, 2008; Yang and Xu-Friedman, 2015; for recent reviews, see Abbott and Regehr, 2004; Jackman and Regehr, 2017). Therefore, the properties of short-term synaptic plasticity can critically be involved to diversify or specialize information processing in neural networks (Carrillo-Reid et al., 2015; Dayan, 2012; Giocomo and Hasselmo, 2007; Marder, 2012; McCormick and Nusbaum, 2014) and short-term synaptic plasticity may importantly be implicated in transient brain activity and related functions such as short-term memory and working memory (Devaraju et al., 2017; Le Barillier et al., 2015; Pals et al., 2020). Importantly, neuromodulation can significantly change the properties of short-term synaptic plasticity (Gonzalez-Burgos et al., 2005; Ito and Schuman, 2007; Kirby et al., 1995; Reis et al., 2019).
The hippocampus is an elongated brain structure involved in spatial and temporal navigation, memory processing and emotionality (Buzsaki and Moser, 2013; Eichenbaum et al., 2016; Gray and McNaughton, 2003). Remarkably, the functions of hippocampus are segregated along its longitudinal axis (or septotemporal axis, which corresponds to dorsal-ventral axis in rodents and anterior-posterior axis in primates). The concept of functional segregation along the hippocampus states that different segments along the hippocampus, usually represented by the dorsal and the ventral hippocampus, participate to varying degrees to hippocampus-dependent behaviors (Bannerman et al., 2014; Strange et al., 2014). More specifically, existing evidence shows that the dorsal hippocampus has an increased involvement in information processing underlying spatial learning and memory (Jung et al., 1994; Maurer et al., 2005; Moser et al., 1993), while the ventral hippocampus has been linked to anxiety-related behaviors (Bannerman et al., 2002; Kjelstrup et al., 2002; Pentkowski et al., 2006), stress-induced disfunctions and social interactions (McHugh et al., 2004; Okuyama et al., 2016). In addition to functional segregation revealed at the level of behavior, a relatively recently developed body of research shows that significant specializations exist along the longitudinal axis of the hippocampus also at the level of intrinsic neuronal network. This intrinsic diversification includes gene expression profiles (Cembrowski et al., 2016b; Dong et al., 2009; Floriou-Servou et al., 2018; Lee et al., 2017; Thompson et al., 2008), principal cell properties (Cembrowski et al., 2016a; Dougherty et al., 2012; Dubovyk and Manahan-Vaughan, 2018; Honigsperger et al., 2015; Maggio and Segal, 2009; Milior et al., 2016; Papatheodoropoulos et al., 2002), and long-term synaptic plasticity (Babiec et al., 2017; Dubovyk and Manahan-Vaughan, 2018; Grigoryan et al., 2012; Kouvaros and Papatheodoropoulos, 2016b; Maggio and Segal, 2007; Maruki et al., 2001; Milior et al., 2016; Moschovos and Papatheodoropoulos, 2016; Papatheodoropoulos and Kostopoulos, 2000a; Reis et al., 2019; Schreurs et al., 2017; Tidball et al., 2017). Moreover, remarkable dorsoventral differences have been also found in forms of short-term synaptic plasticity, namely PPR and FF/D. More specifically, dorsal versus ventral CA1 hippocampal synapses show higher scores of PPR (Babiec et al., 2017; Dubovyk and Manahan-Vaughan, 2018; Maruki et al., 2001; Milior et al., 2016; Papatheodoropoulos, 2015; Papatheodoropoulos and Kostopoulos, 2000b; Tidball et al., 2017), and the dorsal CA1 synapses prominently display FF instead of FD that characterizes the corresponding ventral synapses (Koutsoumpa and Papatheodoropoulos, 2019, 2021; Papaleonidopoulos et al., 2017).
Recent evidence shows that neuromodulation play significant roles in diversifying the functions of the local neuronal network along the hippocampus (Dubovyk and Manahan-Vaughan, 2018; Grigoryan and Segal, 2013; Maggio and Segal, 2007; Malik and Johnston, 2017; Mlinar and Corradetti, 2018; Papaleonidopoulos et al., 2018; Reis et al., 2019). Interestingly, working memory which may engage changes in short-term synaptic plasticity (Devaraju et al., 2017; Le Barillier et al., 2015; Pals et al., 2020) and is amenable to neuromodulation (Cardoso-Cruz et al., 2014; McHugh et al., 2008) may involve a distinct participation of the dorsal (posterior) and ventral (anterior) hippocampus, as recent evidence suggests (Hauser et al., 2020; Li et al., 2022). However, despite the plethora of evidence regarding dorsoventral differences in short-term synaptic plasticity, little is known regarding the actions of neuromodulation on short-term synaptic plasticity along the hippocampus. For instance, μ-opioid receptors and GABAA receptors are involved in shaping FF/D in the dorsal but not ventral hippocampus (Koutsoumpa and Papatheodoropoulos, 2019), and beta-adrenergic receptors modulate synaptic responses evoked by theta-burst stimulation only in the dorsal hippocampus (Papaleonidopoulos and Papatheodoropoulos, 2018).
Neuromodulators affect short-term synaptic plasticity mainly by regulating neurotransmitter release from presynaptic terminals (Cheng et al., 2018; Miller, 1998; Mukunda and Narayanan, 2017). In the hippocampus, transmitter release at excitatory synapses is very efficiently controlled by the neuromodulator adenosine (Cunha, 2001; Sebastião and Ribeiro, 2014) by acting at presynaptic A1 receptors (A1Rs) (Reddington et al., 1982; Sebastião et al., 1990; Thompson et al., 1992). Similarly, GABA controls excitatory synaptic transmission in the hippocampus acting at presynaptic GABAB receptors (GABABRs) (Ulrich and Bettler, 2007; Vizi and Kiss, 1998). In addition, A1Rs (Brager and Thompson, 2003; Dunwiddie and Haas, 1985; Klausnitzer and Manahan-Vaughan, 2008; Lupica et al., 1992; Trompoukis and Papatheodoropoulos, 2020) and GABABRs (Trompoukis and Papatheodoropoulos, 2020) modulate some forms of short-term synaptic plasticity in the hippocampus and other brain regions and may significantly contribute in diversify short-term synaptic plasticity along the long axis of the hippocampus. However, how adenosine receptors and GABAB receptors modulate frequency-dependent short-term synaptic dynamics in the dorsal and the ventral hippocampus remains largely unclear.
In the present study, we examined the actions of adenosine receptors and GABABRs on two forms of short-term synaptic plasticity, namely the PPR and FF/D. It should be noted that short-term synaptic plasticity is distinguished from short-term synaptic potentiation, which is an initial phase of synaptic potentiation, decays in an activity-dependent manner, can last for several minutes to hours, and is followed by a stable phase of long-term potentiation (Volianskis et al., 2015). We studied PPR and FF/D using a frequency stimulation protocol consisting of brief 10-pulse trains applied at the presynaptic fibers at different frequencies, from 0.1 to 100 Hz. PPR was studied by measuring the changes induced in the second response in a train, while the FF/D was studied by measuring the steady-state response, which was represented by the mean value of the last three responses (8th–10th). We found significant adenosine receptor-mediated and GABABR-mediated effects on basal excitatory synaptic transmission and its short-term plastic changes induced during repetitive activation.
Methods
Animals and hippocampal slice preparation
Hippocampal slices were prepared from male Wistar rats 3–4 months old. Rats were kept at the Laboratory of Experimental Animals of the Department of Medicine, University of Patras (license No: EL-13-BIOexp-04) under stable conditions of temperature (20°C–22°C) and light–dark cycle (12/12 h), and they had free access to food and water. The treatment of animals and all experimental procedures used in this study were conducted in accordance with the European Communities Council Directive Guidelines for the care and use of Laboratory animals (2010/63/EU – European Commission). Furthermore, the treatment of experimental animals and all experimental procedures have been approved by the Protocol Evaluation Committee of the Department of Medicine of the University of Patras and the Directorate of Veterinary Services of the Achaia Prefecture of Western Greece Region (reg. number: 187531/626, 26/06/2018). The number of animals that would be required in the study was determined using the G*power software. We prepared transverse 500-μm-thick slices from the dorsal and the ventral segment of hippocampus as previously described (Papatheodoropoulos and Kostopoulos, 2000a; Koutsoumpa and Papatheodoropoulos, 2019). Briefly, following decapitation under conditions of deep animal anaesthesia with diethyl-ether, the brain was removed from the cranium and placed in ice-cold (2°C–4°C) standard artificial cerebrospinal fluid (ASCF) containing, in mM, 124 NaCl, 4 KCl, 2 CaCl2, 2 MgSO4, 26 NaHCO3, 1.25 NaH2PO4 and 10 glucose. ACSF was equilibrated with 95% O2 and 5% CO2 gas mixture at a pH of 7.4. Τhen, the two hippocampi were removed from the brain and positioned on a McIlwain tissue chopper where 500-µm-thick slices were prepared by cutting hippocampus transversely to its long axis (Figure 1(a)). Slices were prepared from the two segments of the hippocampus extending between 0.5 and 3.5 mm from each end of the structure. Slices were immediately transferred to an interface type recording chamber where they were maintained at a constant temperature of 30°C±0.5°C, continuously perfused with ACSF of the same composition as above described, at a perfusion rate of ~1.5 ml/min. Slices were continuously humidified with a mixed gas consisting of 95% O2 and 5% CO2. The slices were left for at least one and a half hours to recover, and then stimulation and recording were started.
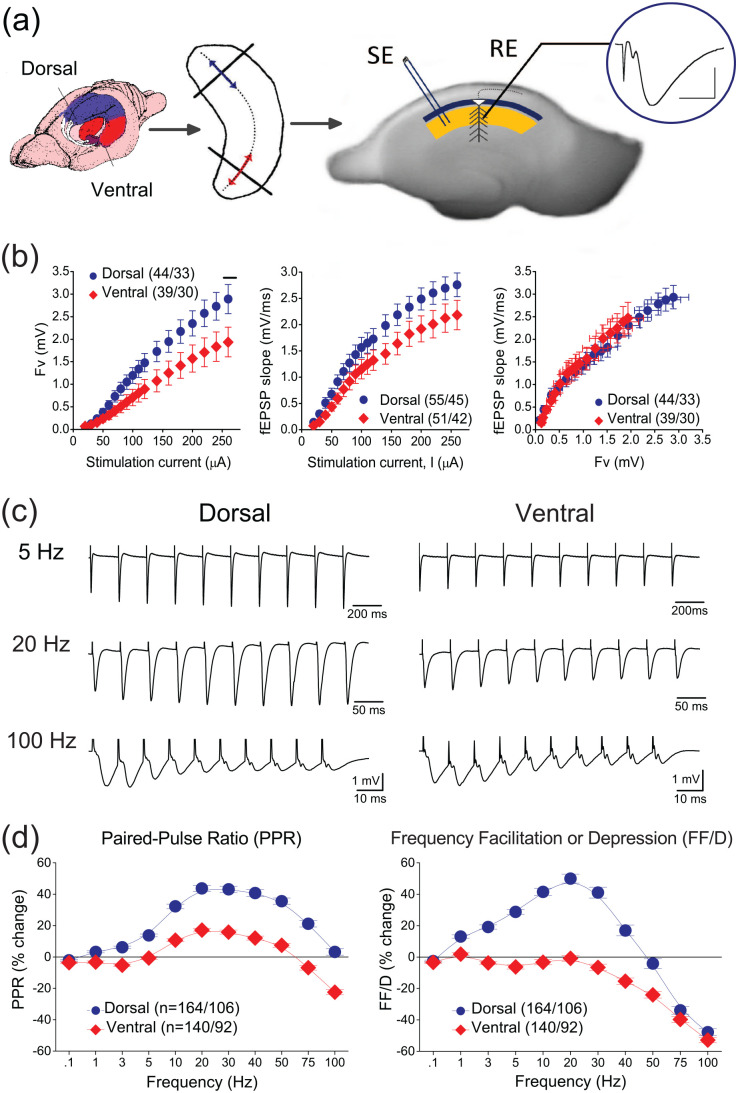
Figure 1.
(a) Methods used to prepare dorsal and ventral hippocampal slices. Schematic drawing of the hippocampus in the rat brain and the portions of the dorsal and ventral hippocampus used to prepare slices (lines with arrowheads) transversely to the long axis of the structure are shown in the left and middle panels, respectively. In the right panel is shown a photograph of a ventral hippocampal slice illustrating the method used to stimulate Schaffer collaterals and record fEPSP (trace inside circle) in the stratum radiatum (yellow region), below stratum pyramidale (dark blue band) where pyramidal cell bodies are located. The extension of colored regions delineates the CA1 hippocampal subfield. SE, stimulation electrode; RE, recording electrode. Calibration bars: 1 mV, 5 ms. (b) Baseline measures in dorsal and ventral hippocampal slices. Input-output curves constructed by plotting fiber volley (Fv) and fEPSP as a function of stimulation current intensity (left and middle graph, respectively), and fEPSP as a function of Fv (right graph). Fv was significantly larger in dorsal than in ventral slices only at high stimulation current intensities (horizontal line in left graph; independent t-test, p < 0.05). (c) Examples of responses evoked by the stimulation frequency protocol, applied in dorsal and ventral hippocampal slices. Stimulation frequency consisted of a train of 10 pulses delivered at varying frequency. These examples illustrate synaptic responses (fEPSPs) elicited by stimulation trains delivered at three different frequencies: 5 Hz, 20 Hz, and 100 Hz. These two slices (dorsal and ventral) were obtained from the same right hippocampus of a rat. (d) Collective results, obtained under basal conditions from dorsal and ventral hippocampal slices, regarding the second and steady-state responses evoked by a stimulation train plotted as a function of stimulation frequency; the percent changes induced in the second and steady-state responses represent two forms of short-term synaptic plasticity: the paired-pulse ratio (PPR) and the frequency facilitation or depression (FF/D), respectively. The results presented in these diagrams correspond to the results for the 2nd and the average of 8th–10th responses, shown in Supplementary Figure 1 (which presents the percent changes of fEPSPs as a function of stimulation pulses). Data were obtained from 164 dorsal slices prepared from 106 rats and 140 ventral slices obtained from 92 rats. PPR ratio was significantly higher in the dorsal versus ventral hippocampus for all stimulation frequencies greater than 0.1 Hz (independent t-test, p < 0.001). Furthermore, the dorsal hippocampus showed frequency facilitation for stimulation frequencies 1–40 Hz and frequency depression at higher frequencies, while the ventral hippocampus consistently showed frequency depression; significant dorsoventral differences in FF/D were found for stimulation frequencies 1–50 Hz (independent t-test, p < 0.001). Results for additional statistical tests are given in the main text.
Electrophysiology, data processing and analysis
Evoked field excitatory postsynaptic potentials (fEPSPs) were recorded from the CA1 stratum radiatum after electrical stimulation of the Schaffer collaterals. Electrical stimulation consisted of constant current pulses of 100 μs in duration and variable amplitude (20–260 μA). We applied electrical current pulses using a home-made bipolar platinum/iridium wire electrode with a wire diameter of 25 μm and an inter-wire distance of 100 μm; wire was purchased from World Precision Instruments, USA. Recordings of fEPSPs were performed using a 7-μm-thick carbon fiber electrode (Kation Scientific, Minneapolis, USA), which was positioned 300–400 μm apart the stimulation electrode. Baseline stimulation was delivered every 30 s using a stimulation current intensity that elicited a fEPSP with a slope of about 1 mV/ms. We systematically made input–output curves between stimulation current intensity and fEPSP. We studied short-term changes of fEPSP using a frequency stimulation protocol as previously described (Koutsoumpa and Papatheodoropoulos, 2019; Papaleonidopoulos et al., 2017). Specifically, the frequency stimulation protocol consisted of a sequence of 10 consecutive pulses delivered at varying frequency between 0.1 and 100 Hz; this pattern is similar to the spike trains that normally occur in hippocampal pyramidal cells (Fenton and Muller, 1998). Stimulation trains of different frequency were applied at a random fashion during each experiment. Furthermore, consecutive trains of pulses were separated by 2-min-long intervals. We applied frequency stimulation at baseline stimulation current intensity, that is, at a stimulation current intensity producing a subthreshold fEPSP with a slope of about 1 mV/ms. In some cases, in which the first (conditioning) fEPSP in a train caused the appearance of a population spike, we slightly reduced the intensity of the stimulation current so that the fEPSP became subthreshold. Under these conditions (i.e. subthreshold conditioning fEPSP), subsequent (conditioned) fEPSPs in a train did not evoke population spike. Considering that drugs may affected the amplitude of fEPSP and that the magnitude of fEPSP significantly determines the pattern of short-term changes in conditioned fEPSPs (Koutsoumpa and Papatheodoropoulos, 2019), we applied frequency stimulation in drug condition also after adjusting conditioning fEPSP to control levels to counteract the direct effect of drugs on synaptic transmission. In this way, we can discriminate between drug actions on mechanisms of short-term synaptic plasticity and secondary drug effects on short-term synaptic plasticity through change in synaptic transmission. Signals were amplified 500 times, band-pass filtered at 0.5 Hz–2kHz using Neurolog amplifiers (Digitimer Limited, UK), digitized at 10 kHz and stored on a computer disk for offline analysis using the CED 1401-plus interface and the Signal6 software (Cambridge Electronic Design, Cambridge, UK). To quantify fEPSP, we measured the maximum slope of its initial rising phase. The effect of frequency stimulation on fEPSP was quantified as the percent change of each of the nine consecutive evoked responses with respect to the first fEPSP in a train. Steady-state response was estimated by averaging the responses evoked by the last three pulses in a train (i.e. 8th–10th). The data about fEPSP changes induced during the application of 10-pulse trains are presented either as a function of the number of stimulus pulses, in different graphs for the different stimulation frequencies (Supplementary Figures), or as a function of the stimulus frequency, only for the PPR and FF/D corresponding to second and steady-state responses, respectively (graphs in main Figures).
Drugs
The following drugs were used: the selective A1R agonist 2-Chloro-N6-cyclopentyladenosine (CCPA, 0.2–5 μM); the selective A1R antagonist 8-Cyclopentyl-1,3-dipropylxanthine (DPCPX, 150–500 nM); the selective A2AR antagonist 4-(2-[7-Amino-2-(2-furyl)[1,2,4]a][1,3,5]triazin-5-ylamino] (ZM241385, 200 nΜ); the selective agonist of GABABRs baclofen (1 and 10 μM), and the selective antagonist of GABABR 3-[[(3,4-Dichlorophenyl)methyl]amino]propyl] diethoxymethyl)phosphinic acid (CGP52432, 10 μM); the specific antagonist of N-methyl-D-aspartate (NMDA) receptors 3-((R)-2-Carboxypiperazin-4-yl)-propyl-1-phosphonic acid (CPP, 10 μΜ). Drugs were first prepared as stock solutions and then dissolved in standard medium, and bath applied to the tissue. Stock solutions of CCPA, CGP52432, CPP and baclofen were prepared in distilled water, whereas stock solutions of DPCPX and ZM241385 were prepared in dimethyl sulfoxide (DMSO) at a concentration that when diluted for bath application the final volume of DMSO was lower than 0.05%. Stock solutions in water were maintained at 4°C while solutions in DMSO were prepared in aliquots and kept at −20°C. Stock solutions were diluted in standard medium to the desired concentrations the day of the experiment. DPCPX, ZM241385, CGP52432 and baclofen were purchased from Tocris Cookson Ltd, UK; CCPA was obtained from Sigma-Aldrich, Germany.
Statistics
For statistical comparisons, we used the univariate full factorial general linear model (GLM) and the parametric two-tailed paired and independent t-test. To statistically study the action of the drugs on the synaptic transmission, we used the average value of the last 5 min under drug condition with the average value of the last 5 min of control condition, in each slice. The values in the text and figures express mean ± SEM. The number of slices and animals used is given throughout the text (slices/animals). The statistics were performed using the number of slices. The IBM SPSS Statistics 27 software package was used for all statistical analyses.
Results
Basal synaptic transmission, PPR and FF/D in the dorsal and ventral hippocampus
We compared input–output curves between the dorsal and ventral CA1 hippocampal field. Neither fiber volley (Fv) (UNIANOVA, F = 1.116, p > 0.1) nor fEPSP (UNIANOVA, F = 0.372, p > 0.5) significantly differ between the two segments of the hippocampus (Figure 1(b)). However, at relatively strong stimulation current intensities Fv was found larger in dorsal versus ventral hippocampal slices (240–260 μA, horizontal line in left graph; independent t-test at individual stimulation current intensities, F = 0.752 and F = 0.991 for 240 and 260 μA, respectively, p < 0.05). These results are similar to those reported previously (Kouvaros and Papatheodoropoulos, 2016a; Grigoryan and Segal, 2016; Milior et al., 2016). However, other studies have reported similar Fv in the two segments of the hippocampus (Kouvaros and Papatheodoropoulos, 2016b) or an increased fEPSP in the dorsal hippocampus, especially at high intensities of presynaptic stimulation (Trompoukis and Papatheodoropoulos, 2020). These discrepancies could probably result from small variations in the cutting angle that has been used in different studies to prepare hippocampal slices. The cutting angle may affect the number of fibers (expressed by Fv) that are kept intact within a slice, therefore affecting the size of Fv and fEPSP. It should, however, be noted that most studies have shown that the ratio between EPSP and Fv does not significantly differ between dorsal and ventral hippocampal slices, as also reported here.
Regarding the two forms of short-term synaptic plasticity, which we examined in this study, that is, PPR and FF/D, we found significant dorsoventral differences under basal conditions (Figure 1(c)-(d) and Supplementary Figure 1). Specifically, the dorsal hippocampus showed continuous paired-pulse facilitation across stimulation frequencies from 1 to 75 Hz (increase in PPR; paired t-test, p < 0.001). In contrast, the ventral hippocampus showed paired-pulse facilitation at stimulation frequencies 10–50 Hz (paired t-test, p < 0.001), which was significantly lower compared with the dorsal hippocampus (independent t-test, p < 0.001) and paired-pulse depression at lower (1–3 Hz) and higher (75–100 Hz) stimulation frequencies (paired t-test, p < 0.001); at 5 Hz, which signals frequency transition, we did not observe significant change in PPR (paired t-test, p > 0.05). Regarding FF/D, the dorsal hippocampus displayed significant facilitation at 1–40 Hz (paired t-test, p < 0.001) and depression at higher frequencies (50–100 Hz, paired t-test, p < 0.001). In contrast, the ventral hippocampus responded to frequency stimulation with depression of the steady-state response at 3–100 Hz but not at 20 Hz (paired t-test, p < 0.001). At the highest stimulation frequencies used (75–100 Hz), the magnitude of frequency depression was similar between the dorsal and ventral hippocampus (independent t-test, p > 0.05). The responses to the entire stimulation train delivered at different stimulation frequencies are presented in Supplementary Figure 1. These results are generally in agreement with previous observations (Koutsoumpa and Papatheodoropoulos, 2019, 2021; Miliou et al., 2021; Papaleonidopoulos et al., 2017). In should be noted that the changes induced in the conditioned fEPSPs in a train, including the second and steady-state responses, depend not only on the stimulation frequency but also on the magnitude of the conditioning (first) fEPSP (Creager et al., 1980; Dobrunz and Stevens, 1997; Harris and Cotman, 1983; Koutsoumpa and Papatheodoropoulos, 2019; Papatheodoropoulos, 2015). Therefore, some minor discrepancies in basal PPR and FF/D that may occur between studies may be due to moderately different initial stimulation conditions.
Modulation of basal synaptic transmission by endogenous adenosine
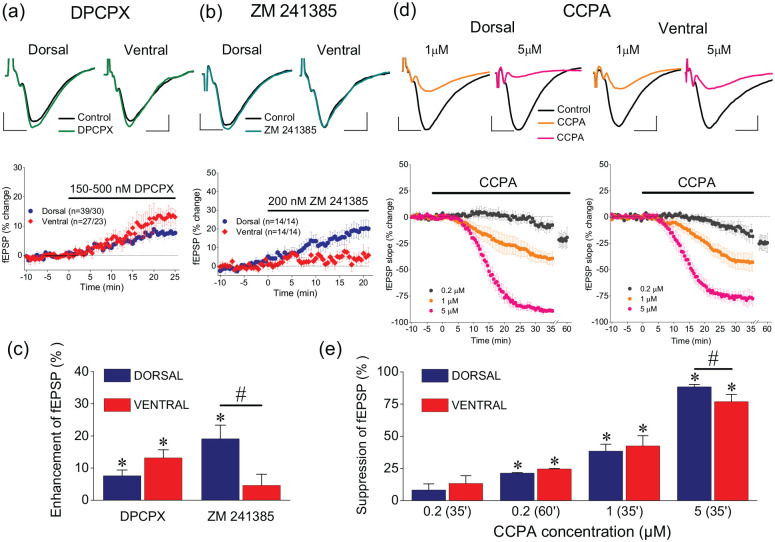
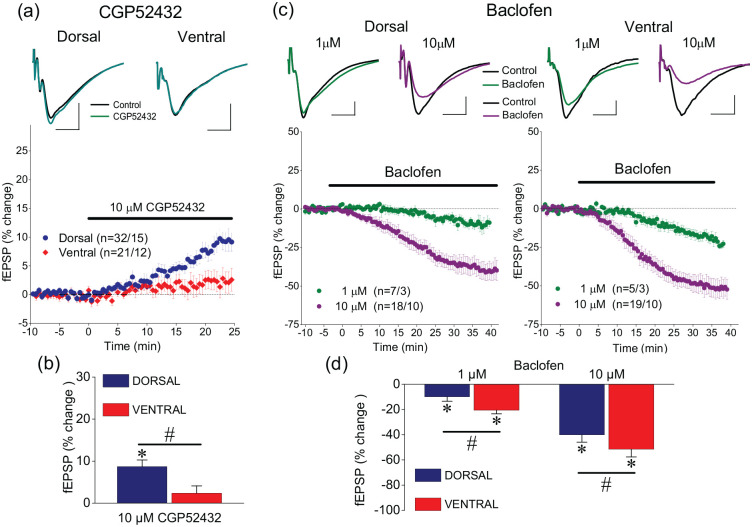
We first studied possible tonic activation of A1Rs and A2ARs by endogenous adenosine using selective receptor antagonists. We perfused slices with either 150 nM or 500 nM DPCPX. We found that 150 nM DPCPX increased fEPSP in both the dorsal (n = 32/23, paired t-test, t30 = −4.58, p < 0.05) and the ventral hippocampus (n = 21/17, paired t-test, t30 = −2.1, p < 0.05) similarly (independent t-test, t51 = −0.752, p > 0.05). Likewise, 500 nM DPCPX increased fEPSP in both the dorsal (n = 7/7, paired t-test, t6 = −2.6, p < 0.05) and the ventral hippocampus (n = 6/6, paired t-test, t5 = −2.1, p < 0.05) similarly (independent t-test, t11 = −1.95, p > 0.05). We did not find any significant difference on DPCPX effects between the two drug concentrations either in the dorsal (independent t-test, t37 = −0.887, p > 0.05) or the ventral hippocampus (independent t-test, t25 = −0.948, p > 0.05); thus, the results obtained with the two drug concentrations were pooled. Overall, we found that DPCPX significantly increased fEPSP in both the dorsal (paired t-test, t37 = −5.019, p < 0.0005) and the ventral hippocampus (paired t-test, t25 = −2.211, p < 0.05) similarly (independent t-test, t51 = 0.62, p > 0.05) (Figure 2(a), (c)). These results are consistent with previous observations (Reis et al., 2019), considering the magnitude of fEPSP to which the effect of DPCPX was studied. Application of 200 nM ZM241385 significantly increased fEPSP in the dorsal (paired t-test, t13 = −3.528, p < 0.005) but not the ventral hippocampus (paired t-test, t13 = −1.284, p > 0.05; independent t-test between the two segments of the hippocampus, F = 0.412, t26 = 2.537, p > 0.05) (Figure 2(b), (c)).
Figure 2.
The control of synaptic transmission by adenosinergic neuromodulation differs between the dorsal and the ventral hippocampus. (a) Example fEPSP traces before and during application of the specific antagonist of A1Rs DPCPX, 150–500 nM (upper panel) and the time course of DPCPX action on fEPSP (lower panel) in dorsal and ventral hippocampal slices. (b) Example fEPSP traces before and during application of the specific antagonist of A2ARs ZM241385, 200 nM (upper panel) and the time course of ZM241385 action on fEPSP (lower panel) in dorsal and ventral hippocampal slices. (c) Blockade of A1Rs by DPCPX significantly enhances fEPSP in the dorsal and the ventral hippocampus, similarly, while blockade of A2ARs by ZM241385 significantly increased fEPSP only in the dorsal hippocampus. (d) Example fEPSP traces before and during application of 1 μM or 5 μM CCPA (upper traces) and the time course of drug action; CCPA was used at the concentrations of 0.2 μM, 1 μM and 5 μM. Calibration bars in panels (a), (b) and (d): 0.5 mV, 5 ms. Note that 0.2 μM CCPA was applied for longer time (i.e. 60 min, last 5 min shown in the two graphs) than higher drug concentrations, to reach steady state. (e) Exogenous application of CCPA produced a concentration-dependent suppression of fEPSP in both segments of the hippocampus; however, at the highest drug concentration used (5 μM), the suppression of fEPSP was significantly stronger in the dorsal than ventral hippocampus. Asterisks in (c) and (e) denote statistically significant drug effects (paired t-test, at p < 0.05), and hash symbol is denoting significant differences of drug effects between the dorsal and ventral hippocampus (independent t-test, at p < 0.05).
A1Rs control basal synaptic transmission in the dorsal and the ventral hippocampus
Then, we studied the effects of the selective A1R agonist CCPA using three different concentrations, namely 0.2 μM, 1 μM and 5 μM. The relatively lower concentrations (0.2–1 μM) fall within the range of adenosine concentrations in the brain extracellular fluid (Dunwiddie and Diao, 1994; Hagberg et al., 1987; Zetterström et al., 1982), while the relatively higher concentration (5 μM) may represent the increased brain adenosine concentration that occurs during periods of intense neuronal activity (Winn et al., 1980). We found that application of CCPA produced a concentration-dependent suppression of fEPSP in both segments of the hippocampus (Figure 2(d) & (e)). Specifically, CCPA significantly suppressed fEPSP in both the dorsal and the ventral hippocampus when applied at the concentration of 0.2 μM for 60 min (paired t-test, n = 9/5, p < 0.05 and n = 7/4, p < 0.005, in the dorsal and ventral hippocampus, respectively), when applied at the concentration of 1 μM for 35 min (paired t-test, n = 16/14, p < 0.001 and n = 18/14, p < 0.001, in dorsal and ventral hippocampus, respectively), and when applied at the concentration 5 μM for 35 min (paired t-test, n = 9/3, p < 0.001 and n = 5/3, p < 0.05, in the dorsal and ventral hippocampus, respectively). The effect of CCPA was similar in the two segments of the hippocampus for drug concentrations of 0.2 μM and 1 μM (independent t-test, p > 0.05). However, the suppressive effect of 5 μM CCPA was significantly higher in the dorsal than the ventral hippocampus (independent t-test, p < 0.01) (Figure 2(e)). The results obtained with lower CCPA concentrations, that is, 0.2 μΜ and 1 μΜ, confirm the results of a recent study (Reis et al., 2019) in which 2-chloroadenosine was used to activate A1Rs, while the higher effects of 5 μM CCPA in the dorsal compared with the ventral hippocampus are similar to those reported previously using adenosine (Lee et al., 1983).
NMDA receptors do not participate in PPR or FF/D
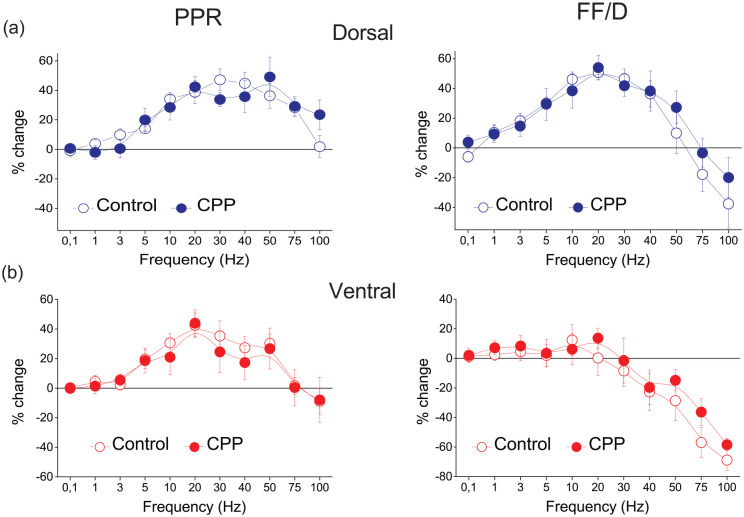
NMDA receptors are widely involved in phenomena of long-term synaptic plasticity (Park et al., 2014; Volianskis et al., 2015), and they may also participate in regulating forms of short-term synaptic plasticity (Bouvier et al., 2018; Davies and Collingridge, 1996; Papatheodoropoulos, 2015). Therefore, before examining the effects of adenosine receptors and GABAB receptors on PPR and FF/D, we sought to determine whether NMDA receptors are involved in these forms of short-term synaptic plasticity. We found that NMDA receptors did not significantly contribute to short-term changes of fEPSP induced during application of frequency stimulation (Figure 3). Specifically, CPP did not significantly change PPR or FF/D in either the dorsal (n = 5/3; paired t-test, p > 0.05) or the ventral hippocampus (n = 5/3; paired t-test, p > 0.05). Also, CPP did not significantly affect basal synaptic transmission either in the dorsal (paired t-test, p > 0.05) or the ventral hippocampus (paired t-test, p > 0.05).
Figure 3.
NMDA receptors are not involved in either PPR or FF/D, in the dorsal (a) or the ventral hippocampus (b). Results for PPR and FF/D are shown under blockade of NMDA receptors by 10 μM CPP (dorsal hippocampus, n = 5/3; ventral hippocampus, n = 5/3). Blockade of NMDA receptors produced no significant change in either PPR or FF/D in either the dorsal (paired t-test, p > 0.05) or the ventral hippocampus (paired t-test, p > 0.05).
Endogenous adenosine does not modulate PPR or FF/D
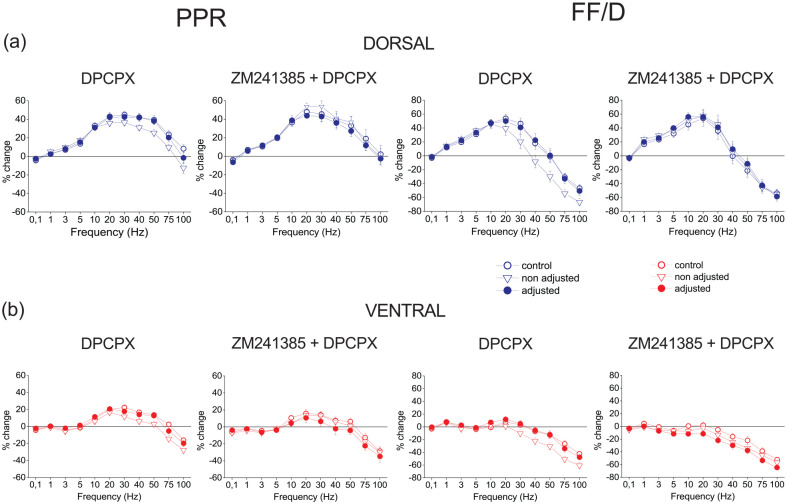
Considering that endogenous adenosine controls baseline synaptic transmission more in the dorsal than the ventral hippocampus acting on A1Rs and that removal of this tonic activation of A1Rs may affect the properties of short-term synaptic plasticity, we examined whether tonic A1R activation by endogenous adenosine may also differently modulate short-term synaptic plasticity in the two segments of the hippocampus. We found that DPCPX did not significantly affect conditioned responses either in the dorsal (GLM, multivariate analysis of variance (MANOVA), F90,4276.34 = 0.846, Wilk’s Λ = 0.887, p > 0.5 and F90,4052.53 = 0.792, Wilk’s Λ = 0.889, p > 0.5, before and after the adjustment of conditioning fEPSP to control levels, respectively; see Methods) or the ventral hippocampus (GLM, MANOVA, F90,3306.47 = 1.108, Wilk’s Λ = 0.817, p > 0.2 and F90,3299.69 = 0.815, Wilk’s Λ = 0.862, p > 0.5, before and after the adjustment of fEPSP to control levels, respectively) (Figure 4, Supplementary Figure 2). We further confirmed the absence of effects of DPCPX on short-term synaptic plasticity by looking at the PPR and FF/D in the dorsal hippocampus, before (F10,461 = 0.622, p > 0.5 and F10,461 = 0.420, p > 0.5, for the PPR and FF/D, respectively) and after the adjustment of fEPSP (F10,395 = 0.466 p > 0.5 and F10,395 = 0.147, p > 0.5, for the PPR and FF/D, respectively), and in the ventral hippocampus before (F10,351 = 1.421, p > 0.1 and F10,351 = 0.919, p > 0.5, for the PPR and FF/D, respectively) and after adjusting fEPSP to control levels (F10,349 = 1.148, p > 0.1 and F10,349 = 0.617, p > 0.5, for the PPR and FF/D, respectively) (Figure 4). Similarly, application of A2AR antagonist ZM241385 (200 nM) did not significantly influenced short-term synaptic plasticity either in the dorsal (F90,2044.96 = 0.701, Wilk’s Λ = 0.814, p > 0.5 and F90,2044.96 = 0.724, Wilk’s Λ = 0.808, p > 0.5, before and after the adjustment of fEPSP to control levels, respectively) or the ventral hippocampus (F90,2044.96 = 0.934, Wilk’s Λ = 0.761, p > 0.5 and F90,2044.96 = 0.942, Wilk’s Λ = 0.759, p > 0.5, before and after the adjustment of fEPSP to control levels, respectively), (Figure 4, Supplementary Figure 3). Accordingly, 200 nM ZM241385 did not significantly affect the PPR and FF/D in the dorsal hippocampus (F10,308 = 0.183, p > 0.5 and F10,308 = 0.098, p > 0.5, for the PPR and FF/D, respectively, after response adjustment) and the ventral hippocampus (F10,308 = 0.204, p > 0.5 and F10,308 = 0.293, p > 0.5, for the PPR and FF/D, respectively, after response adjustment).
Figure 4.
Neither A1Rs nor A2ARs tonically modulate PPR or FF/D in either the dorsal (a) or the ventral hippocampus (b). Results on PPR and FF/D are shown under blockade of A1Rs by 150–500 nM DPCPX (dorsal hippocampus, n = 18/16; ventral hippocampus, n = 17/16) or under blockade of both A1Rs and A2ARs (by 200 nM ZM241385) (dorsal hippocampus, n = 15/15; ventral hippocampus, n = 15/15). Data under drug conditions were obtained before (open triangles) and after (filled circles) adjusting fEPSP to control levels.
Then, considering the possible interaction between A1Rs and A2ARs (Cunha, 2001), we further examined the effects of DPCPX in the presence of ZM241385 and found that blockade of A2ARs did not reveal any significant effect of subsequent application of DPCPX either in the dorsal or the ventral hippocampus (GLM, MANOVA, F90,1895.75 = 0.727, Wilk’s Λ = 0.794, p > 0.5 and F90,1373.51 = 0.703, Wilk’s Λ = 0.737, p > 0.5, after the adjustment of fEPSP) (Figure 4, Supplementary Figure 3). These results were corroborated by observations on the PPR and FF/D in both the dorsal (F10,308 = 0.401, p > 0.5 and F10,308 = 0.106, p > 0.5, for the PPR and FF/D, respectively, after response adjustment) and the ventral hippocampus (F10,307 = 0.426, p > 0.5 and F10,307 = 0.453, p > 0.5, for the PPR and FF/D, respectively, after response adjustment).
A1Rs modulate PPR and FF/D in the dorsal and ventral hippocampus
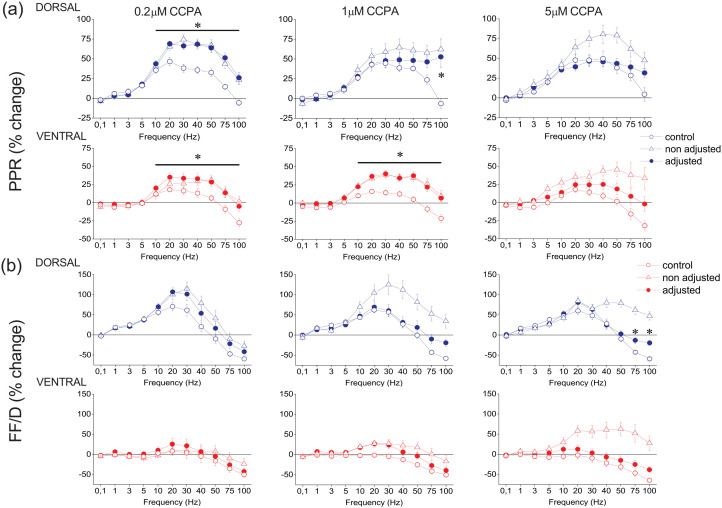
Then we studied the effects of application of CCPA on short-term synaptic plasticity at three drug concentrations, 0.2 μM, 1 μM and 5 μM (Supplementary Figure 4, Supplementary Figure 5, and Supplementary Figure 6, for 0.2, 1 and 5 μM, respectively). Considering all conditioned responses in a train, without adjusting conditioning responses, we found that all CCPA concentrations significantly modulated conditioned responses both in the dorsal (GLM, MANOVA, F90,1366.73 = 2.11, Wilk’s Λ = 0.414, p < 0.001, F90,3265.78 = 2.342, Wilk’s Λ = 0.654, p < 0.001 and F90,3360.73 = 2.038, Wilk’s Λ = 0.697, p < 0.001 for 0.2 μM, n = 11/5; 1 μM, n = 24/18; and 5 μM, n = 14/6, respectively) and the ventral hippocampus (GLM, MANOVA, F90,993.70 = 1.895, Wilk’s Λ = 0.341, p < 0.001, F90,3672.72 = 1.234, Wilk’s Λ = 0.817 p < 0.05 and F90,2119.56 = 1.943, Wilk’s Λ = 0.584, p < 0.001 for 0.2 μM, n = 9/5; 1 μM, n = 26/15; and 5 μM, n = 10/6, respectively). We confirmed these results by examining the PPR and FF/D in both the dorsal and the ventral hippocampus (Figure 5). Specifically, all three CCPA concentrations significantly increased the facilitation of the PPR in the dorsal (GLM, MANOVA, F10,208 = 8.404, p < 0.001, F10,488 = 5.882, p < 0.001 and F10,502 = 2.800, p < 0.005 for 0.2, 1 and 5 μM, respectively) and the ventral hippocampus (GLM, MANOVA, F10,154 = 3.169, p < 0.005, F10,548 = 4.385, p < 0.001 and F10,319 = 4.164, p < 0.001 for 0.2, 1 and 5 μM, respectively). Similarly, all three CCPA concentrations significantly modulated FF/D in the dorsal hippocampus (GLM, MANOVA, F10,208 = 3.14, p < 0.005, F10,488 = 7.251, p < 0.001 and F10,502 = 7.505, p < 0.001 for 0.2, 1 and 5 μM, respectively). However, in the ventral hippocampus, CCPA significantly modulated FF/D at 1 μM and 5 μM (GLM, MANOVA, F10,548 = 2.689, p < 0.005 and F10,502 = 7.505, p < 0.001, for 1 and 5 μM, respectively) but not 0.2 μM (F10,154 = 0.722, p > 0.5).
Figure 5.
CCPA modulates PPR and FF/D in the dorsal and the ventral hippocampus. The effects of CCPA on PPR and FF/D are shown in panels (a) and (b), respectively. Data under drug conditions were obtained before (open triangles) and after (filled circles) adjusting conditioning fEPSP to control levels (after their reduction by CCPA). CCPA was applied at the concentration of 0.2 μM (dorsal hippocampus, n = 11/5 and ventral hippocampus, n = 9/5), at the concentration of 1 μM (dorsal hippocampus, n = 24/18 and ventral hippocampus, n = 26/15) and at the concentration of 5 μM (dorsal hippocampus, n = 14/6 and ventral hippocampus, n = 10/6). Asterisks indicate statistically significant differences between control and drug conditions after adjusting conditioning fEPSP (paired t-test, at p < 0.05). Note that significant drug effects occur at stimulation frequencies greater than 10 Hz. The results of the statistical comparison between control and “non-adjusted” condition are described in the main text.
The significant modulatory effect of CCPA on short-term synaptic plasticity was maintained in the dorsal hippocampus even after increasing the stimulation current intensity to counteract the depressant effect of CCPA on synaptic transmission. Specifically, after adjusting the conditioning fEPSP to control levels, CCPA significantly modulated conditioned responses in the dorsal hippocampus (GLM, MANOVA, F90,1298.90 = 2.098, Wilk’s Λ = 0.398, p < 0.001, F90,2302.69 = 1.459, Wilk’s Λ = 0.687, p < 0.005 and F90,1882.18 = 1.619, Wilk’s Λ = 0.603, p < 0.001 for 0.2 μM, n = 11/5; 1 μM, n = 24/18; and 5 μM, n = 14/6, respectively). In the ventral hippocampus, however, CCPA significantly modulated conditioned responses after adjustment of the conditioning fEPSP and at relatively lower drug concentrations, that is, 0.2 and 1 μM (F90,1149.69 = 1.587, Wilk’s Λ = 0.452, p < 0.005 and F90,2777.45 = 1.35, Wilk’s Λ = 0.748, p < 0.05 for 0.2 μM, n = 9/5 and 1 μM, n = 26/15, respectively) but not at 5 μM (F90,1570.20 = 1.238, Wilk’s Λ = 0.628, n = 10/6, p > 0.05). Furthermore, when we adjusted conditioning fEPSP to control levels, the effects of CCPA depended on both the CCPA concentration and the stimulation time. More specifically, after adjusting fEPSP, 0.2 μM and 1 μM but not 5 μM CCPA, significantly modulated the PPR in the dorsal (F10,198 = 7.370, p < 0.001 and F10,346 = 4.349, p < 0.001, for 0.2 μM and 1 μM, respectively) and the ventral hippocampus (F10,176 = 1.915, p < 0.05 and F10,416 = 3.725, p < 0.001, for 0.2 μM and 1 μM, respectively) (Figure 5). In contrast, CCPA did not significantly affect FF/D either in the dorsal (F10,198 = 1.389, p > 0.1 and F10,346 = 1.396, p > 0.1, for 0.2 μM and 1 μM, respectively) or the ventral hippocampus (F10,176 = 0.110, p > 0. 5 and F10,416 = 1.254, p > 0.1, for 0.2 μM and 1 μM, respectively). Yet, 5 μM CCPA significantly modulated FF/D in the dorsal hippocampus, at high stimulation frequencies (75–100 Hz), (F10,284 = 2.274, p < 0.05). Summarizing, we found that generally CCPA significantly modified conditioned responses in both segments of the hippocampus regardless of whether conditioning responses were adjusted or not; however, specifically regarding the PPR and FF/D we found significant drug action before but not after adjusting conditioning responses to control levels.
Modulation of basal synaptic transmission PPR and FF/D by GABABRs
Then, we examined the effects of endogenous and exogenous activation of GABABRs on synaptic transmission and short-term synaptic plasticity in the dorsal and ventral hippocampus. Figure 6(a)–(b) shows that blockade of GABABRs by 10 μM CGP52432 significantly increases fEPSP in the dorsal (n = 32/15, paired t-test, p < 0.05) but not the ventral hippocampus (n = 21/12, paired t-test, p > 0.05). In contrast, exogenous activation of GABABRs by 1 μM and 10 μM baclofen led to a greater suppression of fEPSP in the ventral compared with the dorsal hippocampus (Figure 6(a) and (c)). These data corroborated previous observations (Kouvaros and Papatheodoropoulos, 2016b; Trompoukis and Papatheodoropoulos, 2020) and suggested that excitatory synaptic transmission is tonically controlled by endogenous GABA in the dorsal hippocampus only and that under conditions of relatively enhanced activation of GABABRs, synaptic transmission is curtailed more in the ventral than the dorsal hippocampus.
Figure 6.
Tonic GABABR activation controls synaptic transmission only in the dorsal hippocampus while the effectiveness of exogenous GABABR activation is higher in the ventral than the dorsal hippocampus. (a–b) Blockade of GABABRs by 10 μM CGP52432 increases fEPSP in the dorsal hippocampus only. (c–d) Activation of GABABRs by 1 and 10 μM baclofen suppresses fEPSP more in the ventral than the dorsal hippocampus. Calibration bars in panels (a) and (c): 0.5 mV, 5 ms. Asterisks are denoting statistically significant drug effects (paired t-test, at p < 0.05), and hash symbols are denoting significant differences of drug effects between the dorsal and ventral hippocampus (independent t-test, at p < 0.05).
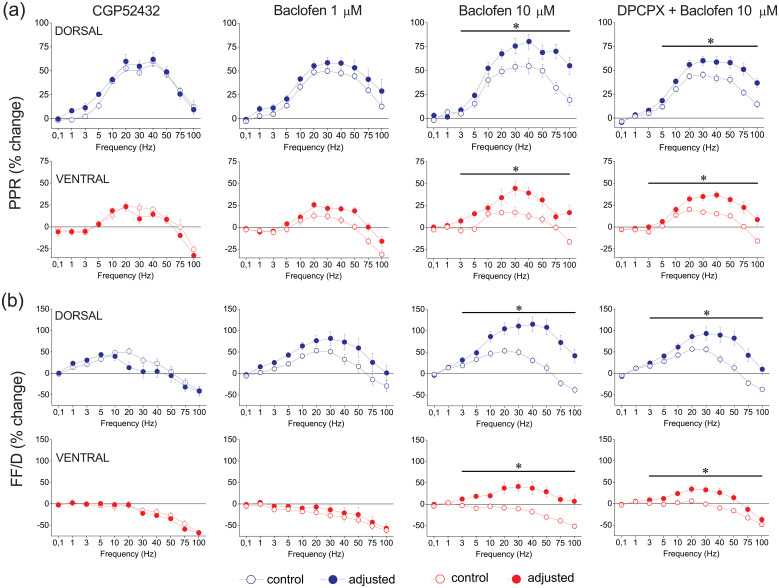
Considering the existence of dorsal-ventral difference in tonic GABABR-mediated action on basal synaptic transmission, we wondered whether a difference between the two segments of the hippocampus exists also for GABABR action on short-term synaptic plasticity. Considering all conditioned responses, we found that before adjusting conditioning fEPSP to control levels, CGP52432 significantly modified short-term synaptic plasticity in the dorsal hippocampus (F90,1298.90 = 1.295, Wilk’s Λ = 0.558, n = 10/4, p < 0.05) but not the ventral hippocampus (F90,695.27 = 0.890, Wilk’s Λ = 0.477, n = 6/3, p > 0.5) (Supplementary Figure 7). However, although these results were confirmed by the drug effects on PPR and FF/D in the ventral hippocampus (F90,109 = 0.777, p > 0.5 and F90,109 = 0.525, p > 0.5, for the PPR and FF/D, respectively), they could not be confirmed in the dorsal hippocampus where none of the two responses were significantly affected by the drug (F90,198 = 0.691, p > 0. 5 and F90,198 = 1.435, p > 0.1, for the PPR and FF/D, respectively). After adjusting conditioning fEPSP, we found that CGP52432 did not significantly affect short-term synaptic plasticity either in the dorsal (F90,105.21 = 0.994, Wilk’s Λ = 0.015, p > 0.5) or the ventral hippocampus (F90,105.21 = 0.856, Wilk’s Λ = 0.024, p > 0.5) (Supplementary Figure 7 and Figure 7).
Figure 7.
GABABRs modulate PPR or FF/D in the dorsal and the ventral hippocampus. Results on PPR and FF/D to stimulation train are shown in panels (a) and (b), respectively. Graphs arranged in columns show results obtained under blockade of GABABRs (10 μM CGP52432; dorsal hippocampus, n = 10/4 and ventral hippocampus, n = 6/3), activation of GABABRs by 1 μM baclofen (Baclofen 1 μM; dorsal hippocampus, n = 7/3 and ventral hippocampus, n = 9/3), 10 μM baclofen (Baclofen 10 μM; dorsal hippocampus, n = 11/6 and ventral hippocampus, n = 13/9), and application of 10 μM baclofen in the presence of 150 nM DPCPX (DPCPX + Baclofen 10 μM; dorsal hippocampus, n = 19/12 and ventral hippocampus, n = 20/12). Data under drug conditions were obtained after adjusting conditioning responses to control levels (after their reduction by baclofen). These results were similar to those obtained without adjusting conditioning responses (see Supplementary Figures 8 and 9). Asterisks denote statistically significant difference between control and drug conditions (paired t-test, p < 0.05).
Then, we studied the effect of exogenous activation of GABABRs on short-term synaptic plasticity in the two segments of the hippocampus. We found that application of baclofen differently modulated short-term synaptic plasticity in the dorsal and ventral hippocampus, depending on the drug concentration. Low baclofen concentration, 1 μM, did not significantly change short-term synaptic plasticity either in the dorsal (n = 7/3, F90,851.27 = 1.011, Wilk’s Λ = 0.502, p > 0.1 and F90,851.27 = 0.972, Wilk’s Λ = 0.515, p > 0.5, before and after the adjustment of fEPSP, respectively) or the ventral hippocampus (n = 9/3, F90,552.85 = 1.121, Wilk’s Λ = 0.321, p > 0.1 and F90,552.85 = 0.756, Wilk’s Λ = 0.455, p > 0.5, before and after the adjustment of fEPSP, respectively) (Supplementary Figure 8). These results were confirmed by those regarding PPR and FF/D in the dorsal (F10,132 = 0.256, p > 0.5 and F10,132 = 0.599, p > 0.5, for the PPR and FF/D, respectively, after adjustment of fEPSP) and the ventral hippocampus (F10,88 = 1.587, p > 0.1 and F10,88 = 0.083, p > 0.5, for the PPR and FF/D, respectively, after adjustment of fEPSP) (Figure 7, 1 μM).
In contrast to low baclofen concentration, high baclofen concentration (10 μM) significantly modified short-term synaptic plasticity in both the dorsal hippocampus (n = 11/6, F90,2940.23 = 1.979, Wilk’s Λ = 0.671, p < 0.001 and F90,2044.96 = 1.817, Wilk’s Λ = 0.594, p < 0.001, before and after the adjustment of fEPSP, respectively) and the ventral hippocampus (n = 13/9, F90,2791.01 = 2.077, Wilk’s Λ = 0.644, p < 0.001 and F90,2635.02 = 1.907, Wilk’s Λ = 0.652, p < 0.001, before and after the adjustment of fEPSP, respectively) by dramatically enhancing frequency facilitation or reverting frequency depression into facilitation (Supplementary Figure 9). These results were confirmed by those regarding the PPR and FF/D in both the dorsal (F10,308 = 3.643, p < 0.001 and F10,308 = 5.155, p < 0.001, for the PPR and FF/D, respectively, after adjustment of fEPSP) and the ventral hippocampus (F10,395 = 3.850, p < 0.001 and F10,395 = 5.227, p < 0.001, for the PPR and FF/D, respectively, after adjustment of fEPSP) (Figure 7, 10 μM). Eventually, exogenous activation of GABABRs by relatively high but not low baclofen concentrations significantly modulated short-term synaptic plasticity in both segments of the hippocampus, regardless of whether conditioning responses were adjusted or not. Here, we could emphasize the switching of frequency depression into facilitation across a wide range of stimulation frequencies (3–100 Hz) in the ventral hippocampus, produced by activation of GABABRs.
Finally, considering that activation of GABABRs may lead to activation of A1Rs (Zhang et al., 2003), we examined whether modulation of short-term synaptic plasticity by GABABRs interferes with activation of A1Rs. Thus, we applied 10 μM baclofen under blockade of A1Rs by 150 nM DPCPX. We observed that DPCPX did not occluded the effect of baclofen on short-term synaptic plasticity and 10 μM baclofen significantly modified short-term synaptic plasticity in both the dorsal (n = 19/12, F20,1020.0 = 2.69, Wilk’s Λ = 0.902, p < 0.001, after adjusting) and the ventral hippocampus (n = 20/12, F20,878 = 3.765, Wilk’s Λ = 0.848, p < 0.001, after adjusting) (Supplementary Figure 10). More specifically, 10 μM baclofen, in the presence of DPCPX, significantly modulated the PPR and FF/D in both the dorsal (F10,510 = 3.533, p < 0.001 and F10,511 = 3.970, p < 0.001, for the PPR and FF/D, respectively, after adjustment of fEPSP) and the ventral hippocampus (F20,440 = 4.408, p < 0.001 and F10,440 = 2.479, p < 0.001, for the PPR and FF/D, respectively, after adjustment of fEPSP) (Figure 7, DPCPX + Baclofen 10 μM). However, under blockade of A1Rs baclofen failed to eliminate the depression of high-frequency steady-state responses in the ventral hippocampus.
Discussion
In this study, we compared the effects of A1Rs, A2ARs and GABABRs on baseline synaptic transmission and short-term synaptic plasticity in the dorsal and ventral hippocampus of adult rats.
The main findings of the present study are the following:
Endogenous adenosine tonically controls synaptic transmission through A1Rs in the dorsal and ventral hippocampus, similarly, and through A2ARs in the dorsal but not the ventral hippocampus; however, endogenous adenosine does not tonically modulate PPR or FF/D in either segment of the hippocampus.
Exogenous A1R activation by high CCPA concentrations suppresses synaptic transmission more in the dorsal than the ventral hippocampus.
CCPA modulates short-term synaptic plasticity in both segments of the hippocampus independently of the suppression of synaptic transmission; yet CCPA modulates PPR but not FF/D after the depressant effect of CCPA on synaptic transmission was counteracted.
Endogenous GABABR activation tonically controls synaptic transmission in the dorsal but not the ventral hippocampus without affecting PPR or FF/D in either segment of the hippocampus.
Exogenous GABABR activation (by baclofen) suppresses synaptic transmission more in the ventral than the dorsal hippocampus and modulates PPR or FF/D in the two segments of the hippocampus, similarly, and in a A1R-independent manner.
We found that A1Rs mediate a similar tonic control of excitatory synaptic transmission in the two segments of the hippocampus, a finding that is in good agreement with previously reported observations made at a comparable level of synaptic activation (Reis et al., 2019; Trompoukis and Papatheodoropoulos, 2020). In contrast, we found that A2ARs tonically control excitatory synaptic transmission in the dorsal hippocampus only. This may sound paradox given the well-established action of A2ARs to enhance excitatory synaptic transmission (Cunha et al., 1994; Sebastião and Ribeiro, 1992); however, A2ARs also promote presynaptic GABA release (Cunha and Ribeiro, 2000), are likely involved in the anticonvulsant action of adenosine (Dunwiddie and Masino, 2001) and thus, it may contribute dampening postsynaptic depolarizations (but see also Rombo et al., 2015). Present evidence of tonic activity of A2ARs in the dorsal but not the ventral hippocampus is consistent with previous findings (Kouvaros and Papatheodoropoulos, 2016a) but is inconsistent with a previous report showing absence of tonic activity of A2ARs in the mouse hippocampus (Reis et al., 2019). This discrepancy may be related with the different species of experimental animal that have been used, since the study by Reis and colleagues was performed in mice, while the present study was performed in rats. In addition to tonic activity of endogenous adenosine, we also found that application of high CCPA concentration suppresses synaptic transmission more in the dorsal than the ventral hippocampus, confirming previous results (Lee et al., 1983) and can tentatively be explained by the higher density of A1Rs in the dorsal versus the ventral hippocampus (Lee et al., 1983; Reis et al., 2019). The present study provides the first comparative results of the effects of A1R activation on short-term synaptic plasticity in the dorsal and the ventral hippocampus. We also confirm previous observations on the higher suppressive effect of GABABR activation on synaptic transmission in the dorsal versus the ventral hippocampus (Trompoukis and Papatheodoropoulos, 2020) that corroborate histochemical data (Dubovyk and Manahan-Vaughan, 2018).
Results from previous studies have suggested that adenosinergic modulation is differentiated along the longitudinal axis of the hippocampus. Thus, in addition to an increased expression of A1Rs (Lee et al., 1983; Reis et al., 2019) and A2ARs (Reis et al., 2019) in the dorsal compared with the ventral segment of the hippocampus, some functional aspects of the adenosinergic system have also been found to differ along the septotemporal axis of the hippocampus. A1Rs control excitatory synaptic transmission more effectively in the dorsal than the ventral hippocampus (Lee et al., 1983) (and present results), contribute to resting membrane properties of CA1 pyramidal cells in the dorsal but not the ventral hippocampus (Kim and Johnston, 2015), control the induction of long-term potentiation in the ventral, not the dorsal, hippocampus (Reis et al., 2019), and they also have a higher contribution to transient heterosynaptic depression in the dorsal compared with the ventral hippocampus (Trompoukis and Papatheodoropoulos, 2020). A2Rs contribute to suppression of synaptic transmission and enhancement of neuronal excitation which is induced under coactivation of NMDA receptors and metabotropic glutamate receptor-5 in the dorsal but not the ventral hippocampus (Kouvaros and Papatheodoropoulos, 2016a), control the induction of long-term synaptic potentiation in the dorsal but not the ventral hippocampus (Reis et al., 2019) and facilitate the induction of epileptogenesis in the dorsal hippocampus under conditions of A1R blockade (Moschovos et al., 2012). Furthermore, in keeping with results from other studies (Reis et al., 2019; Trompoukis and Papatheodoropoulos, 2020), we found that endogenous adenosine tonically inhibit excitatory synaptic transmission in the dorsal and the ventral hippocampus.
Adenosine is a basic modulator of neuronal activity, implicated in several normal and pathological conditions including sleep, homeostatic synaptic plasticity, hypoxia/ischemia and epilepsy (Cunha, 2001; Dias et al., 2013; Dunwiddie and Masino, 2001; Sebastião and Ribeiro, 2014). For instance, increased release of adenosine occurs under conditions of intense synaptic activity (Lloyd et al., 1993) and intense neuronal activity associated with epileptic seizures (Schrader et al., 1980; Winn et al., 1980). Therefore, the present results that show that increased adenosine concentrations suppress excitatory synaptic transmission more in the dorsal than the ventral hippocampus may suggest that under conditions of relatively strong neuronal activation A1Rs mediate a greater curtail of synaptic transmission in the dorsal than the ventral hippocampus before the local network gets very excited. In this way, adenosine and A1Rs may act in a homeostatic manner to compensate for the increases in neuronal activity and stabilize local network activity, more in the dorsal than in the ventral segment of the hippocampus.
We found that endogenous adenosine did not tonically modulate PPR or FF/D in either segment of the hippocampus, though it modulates basal synaptic transmission. For instance, we found that DPCPX did not significantly affect PPR in dorsal or ventral hippocampal slices, while it produced an increase in basal synaptic transmission in both segments of the hippocampus. These results are indicative of a tonic activity of A1Rs, which, however, does not affect paired-pulse facilitation, and contradict findings from a previous study (Reis et al., 2019) which showed that DPCPX inhibits paired-pulse facilitation in the ventral hippocampus, where A1Rs were found to be tonically activated. The apparent discrepancy between the two studies can be interpreted in terms of transmitter release probability. Activation of A1R reduces the probability of transmitter release (Manabe et al., 1993), which is inversely related to the magnitude of paired-pulse facilitation (Dobrunz and Stevens, 1997). Therefore, tonic activation of A1Rs by endogenous adenosine is expected to increase paired-pulse facilitation. However, this effect of A1Rs may be absent if the baseline transmitter release probability is already low. For instance, in our study slices were perfused with a reduced ratio Ca2+/Mg2+, which keeps the probability of transmitter release low (Manabe et al., 1993), thereby limiting the effect of endogenous adenosine on paired-pulse facilitation, which thus remains insensitive to DPCPX. On the other hand, under conditions of high Ca2+/Mg2+ ratio, as occurs in the study by Reis et al. (2019), the baseline probability of transmitter release is relatively high allowing for a contribution of endogenous adenosine (via A1Rs) to paired-pulse facilitation, which is thus reduced by blocking A1Rs with DPCPX.
In contrast to the lack of tonic action of endogenous adenosine, activation of A1Rs by low CCPA concentrations (0.2 μM), which are equal or slightly higher than endogenous extracellular adenosine concentrations in CA1 region of rat hippocampal slices (0.14–0.2 μM) (Dunwiddie and Diao, 1994), significantly modulates PPR and FF/D suggesting that a tonic control of synaptic transmission can occur at very low ambient levels of adenosine. We found that both A1Rs and GABABRs modulate short-term synaptic plasticity in the dorsal and the ventral hippocampus, by enhancing facilitation and/or reducing depression of the conditioned responses. To some extent, these effects may result from the suppression of conditioning response produced by these receptors, given that the magnitude of synaptic facilitation is inversely related to the magnitude of the conditioning response (Creager et al., 1980; Dobrunz and Stevens, 1997; Harris and Cotman, 1983). In particular, simple forms of short-term synaptic plasticity are thought to depend mainly on presynaptic calcium-dependent mechanisms that control the probability of transmitter release and the speed of recovery from transmitter depletion (Jackman and Regehr, 2017; von Gersdorff and Borst, 2002; Zucker and Regehr, 2002). However, both A1Rs and GABABRs significantly modified short-term synaptic plasticity also after adjusting conditioning synaptic response to control levels, suggesting that activation of these receptors may directly impact on mechanisms that determine short-term synaptic plasticity in the hippocampus (Dunwiddie and Haas, 1985). In addition to presynaptic mechanisms, postsynaptic mechanisms may also be involved in some of the effects of exogenous activation of A1Rs or GABABRs. For instance, activation of these receptors in CA1 pyramidal cells leads to a hyperpolarization of the resting membrane potential through activation of G-protein-coupled inwardly rectifying potassium (GIRK) channels (Kim and Johnston, 2015; Luscher et al., 1997). A hyperpolarized resting membrane potential produced by the continuous presence of an agonist for A1Rs or GABABRs (CCPA or baclofen) can lead to an increase in driving force for flow of cation current (specifically for the sodium ion) at excitatory synapses likely resulting in an increase in response amplitude during frequency stimulation. Interestingly, in the case of A1Rs, the enhancing effect of CCPA was seen with a lower drug concentration in the dorsal compared with the ventral hippocampus; A1R-mediated activation of GIRK channels is higher in the dorsal than in ventral hippocampus (Kim and Johnston, 2015).
Extending existing evidence, we show that A1Rs can modify short-term synaptic plasticity at a wide range of agonist concentrations and in a generally similar fashion in the dorsal and the ventral hippocampus. Yet, one point to note is that though CCPA modifies short-term synaptic plasticity at both low and high concentrations, however, at high levels of CCPA, resembling adenosine concentrations that are normally seen in conditions of intense synaptic and neuronal activity (Lloyd et al., 1993; Winn et al., 1980), transmission is facilitated at the beginning but not when repetitive activity reaches a steady state. This may represent a mechanism by which adenosine signals the onset of repetitive activation of afferent input and concurrently prevents the risk of runaway excitation on local neuronal network. In contrast to A1Rs, GABABRs, which also suppress excitatory synaptic transmission in the hippocampus, require an increased activation to modify short-term synaptic plasticity, suggesting that GABABR controls the transmission of “online” information only under conditions of intense neuronal activity.
In conclusion, the present finding shows that despite significant dorsal-ventral differences in the action of A1Rs and GABABRs on baseline synaptic transmission, these receptors permit the synaptic amplification of “online” neuronal information, by means of short-term synaptic plasticity, in a similar fashion in the two segments of the hippocampus. Furthermore, these modulatory actions occur in a frequency-depended manner that differs between the two neurotransmitter receptors. A1R modifies PPR and FF/D at relatively high stimulation frequencies (>10 Hz), while GABABR modulates PPR and FF/D at stimulation frequencies greater than 1 Hz. Thus, GABABRs modulate short-term synaptic plasticity at a wider frequency range compared with A1Rs. Accordingly, a specific pattern of actions of A1Rs and GABABRs on short-term synaptic plasticity can emerge from the present results. Specifically, a wide range of ambient levels of adenosine may modulate short-term synaptic plasticity of relatively high-frequency inputs via A1Rs activation. In contrast, only intense activation of GABABRs steadily amplifies synaptic input over a wide range of frequency.
Supplemental Material
Supplemental material, sj-eps-1-bna-10.1177_23982128221106315 for Septotemporal variation in modulation of synaptic transmission, paired-pulse ratio and frequency facilitation/depression by adenosine and GABAB receptors in the rat hippocampus by Maria A. Samara, George D. Oikonomou, George Trompoukis, Georgia Madarou, Maria Adamopoulou and Costas Papatheodoropoulos in Brain and Neuroscience Advances
Supplemental material, sj-eps-10-bna-10.1177_23982128221106315 for Septotemporal variation in modulation of synaptic transmission, paired-pulse ratio and frequency facilitation/depression by adenosine and GABAB receptors in the rat hippocampus by Maria A. Samara, George D. Oikonomou, George Trompoukis, Georgia Madarou, Maria Adamopoulou and Costas Papatheodoropoulos in Brain and Neuroscience Advances
Supplemental material, sj-eps-2-bna-10.1177_23982128221106315 for Septotemporal variation in modulation of synaptic transmission, paired-pulse ratio and frequency facilitation/depression by adenosine and GABAB receptors in the rat hippocampus by Maria A. Samara, George D. Oikonomou, George Trompoukis, Georgia Madarou, Maria Adamopoulou and Costas Papatheodoropoulos in Brain and Neuroscience Advances
Supplemental material, sj-eps-3-bna-10.1177_23982128221106315 for Septotemporal variation in modulation of synaptic transmission, paired-pulse ratio and frequency facilitation/depression by adenosine and GABAB receptors in the rat hippocampus by Maria A. Samara, George D. Oikonomou, George Trompoukis, Georgia Madarou, Maria Adamopoulou and Costas Papatheodoropoulos in Brain and Neuroscience Advances
Supplemental material, sj-eps-4-bna-10.1177_23982128221106315 for Septotemporal variation in modulation of synaptic transmission, paired-pulse ratio and frequency facilitation/depression by adenosine and GABAB receptors in the rat hippocampus by Maria A. Samara, George D. Oikonomou, George Trompoukis, Georgia Madarou, Maria Adamopoulou and Costas Papatheodoropoulos in Brain and Neuroscience Advances
Supplemental material, sj-eps-5-bna-10.1177_23982128221106315 for Septotemporal variation in modulation of synaptic transmission, paired-pulse ratio and frequency facilitation/depression by adenosine and GABAB receptors in the rat hippocampus by Maria A. Samara, George D. Oikonomou, George Trompoukis, Georgia Madarou, Maria Adamopoulou and Costas Papatheodoropoulos in Brain and Neuroscience Advances
Supplemental material, sj-eps-6-bna-10.1177_23982128221106315 for Septotemporal variation in modulation of synaptic transmission, paired-pulse ratio and frequency facilitation/depression by adenosine and GABAB receptors in the rat hippocampus by Maria A. Samara, George D. Oikonomou, George Trompoukis, Georgia Madarou, Maria Adamopoulou and Costas Papatheodoropoulos in Brain and Neuroscience Advances
Supplemental material, sj-eps-7-bna-10.1177_23982128221106315 for Septotemporal variation in modulation of synaptic transmission, paired-pulse ratio and frequency facilitation/depression by adenosine and GABAB receptors in the rat hippocampus by Maria A. Samara, George D. Oikonomou, George Trompoukis, Georgia Madarou, Maria Adamopoulou and Costas Papatheodoropoulos in Brain and Neuroscience Advances
Supplemental material, sj-eps-8-bna-10.1177_23982128221106315 for Septotemporal variation in modulation of synaptic transmission, paired-pulse ratio and frequency facilitation/depression by adenosine and GABAB receptors in the rat hippocampus by Maria A. Samara, George D. Oikonomou, George Trompoukis, Georgia Madarou, Maria Adamopoulou and Costas Papatheodoropoulos in Brain and Neuroscience Advances
Supplemental material, sj-eps-9-bna-10.1177_23982128221106315 for Septotemporal variation in modulation of synaptic transmission, paired-pulse ratio and frequency facilitation/depression by adenosine and GABAB receptors in the rat hippocampus by Maria A. Samara, George D. Oikonomou, George Trompoukis, Georgia Madarou, Maria Adamopoulou and Costas Papatheodoropoulos in Brain and Neuroscience Advances
Acknowledgments
The authors thank Nicoleta Spiropoulou for animal care.
Footnotes
Author contributions: M.S., G.O., G.T., G.M. and M.A. performed the experiments and analyzed the data to a degree expressed by their relative position appearing in the article. C.P. designed and supervised the research, supported the data analysis, performed the statistical analysis, and prepared, wrote, and edited the whole manuscript.
Data statement: All the datasets generated and analyzed during this study are kept in the Physiology Lab, Department of Medicine, University of Patras, and they are available from the corresponding author on reasonable request.
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research has been co-financed by the European Union and Greek national funds through the Operational Program Competitiveness, Entrepreneurship and Innovation, under the call RESEARCH – CREATE – INNOVATE (project code: T2EDK – 02075).
ORCID iDs: Maria Α. Samara  https://orcid.org/0000-0002-3063-7921
https://orcid.org/0000-0002-3063-7921
Georgia Madarou  https://orcid.org/0000-0002-7684-7262
https://orcid.org/0000-0002-7684-7262
Costas Papatheodoropoulos  https://orcid.org/0000-0002-7860-9583
https://orcid.org/0000-0002-7860-9583
Supplemental material: Supplemental material for this article is available online.
References
- Abbott LF, Regehr WG. (2004) Synaptic computation. Nature 431(7010): 796–803. [DOI] [PubMed] [Google Scholar]
- Abbott LF, Varela JA, Sen K, et al. (1997) Synaptic depression and cortical gain control. Science 275(5297): 220–224. [DOI] [PubMed] [Google Scholar]
- Babiec WE, Jami SA, Guglietta R, et al. (2017) Differential regulation of NMDA receptor-mediated transmission by SK channels underlies dorsal-ventral differences in dynamics of Schaffer collateral synaptic function. Journal of Neuroscience 37(7): 1950–1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannerman DM, Deacon RM, Offen S, et al. (2002) Double dissociation of function within the hippocampus: Spatial memory and hyponeophagia. Behavioral Neuroscience 116(5): 884–901. [DOI] [PubMed] [Google Scholar]
- Bannerman DM, Sprengel R, Sanderson DJ, et al. (2014) Hippocampal synaptic plasticity, spatial memory and anxiety. Nature Reviews. Neuroscience 15(3): 181–192. [DOI] [PubMed] [Google Scholar]
- Bouvier G, Larsen RS, Rodríguez-Moreno A, et al. (2018) Towards resolving the presynaptic NMDA receptor debate. Current Opinion in Neurobiology 51(1): 1–7. [DOI] [PubMed] [Google Scholar]
- Brager DH, Thompson SM. (2003) Activity-dependent release of adenosine contributes to short-term depression at CA3-CA1 synapses in rat hippocampus. Journal of Neurophysiology 89(1): 22–26. [DOI] [PubMed] [Google Scholar]
- Buzsaki G, Moser EI. (2013) Memory, navigation and theta rhythm in the hippocampal-entorhinal system. Nature Neuroscience 16(2): 130–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardoso-Cruz H, Dourado M, Monteiro C, et al. (2014) Activation of dopaminergic D2/D3 receptors modulates dorsoventral connectivity in the hippocampus and reverses the impairment of working memory after nerve injury. Journal of Neuroscience 34(17): 5861–5873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrillo-Reid L, Lopez-Huerta VG, Garcia-Munoz M, et al. (2015) Cell assembly signatures defined by short-term synaptic plasticity in cortical networks. International Journal of Neural Systems 25(7): 1550026. [DOI] [PubMed] [Google Scholar]
- Cembrowski MS, Bachman JL, Wang L, et al. (2016. a) Spatial gene-expression gradients underlie prominent heterogeneity of CA1 pyramidal neurons. Neuron 89(2): 351–368. [DOI] [PubMed] [Google Scholar]
- Cembrowski MS, Wang L, Sugino K, et al. (2016. b) Hipposeq: A comprehensive RNA-seq database of gene expression in hippocampal principal neurons. ELife 5(1): e14997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Q, Song SH, Augustine GJ. (2018) Molecular mechanisms of short-term plasticity: Role of synapsin phosphorylation in augmentation and potentiation of spontaneous glutamate release. Frontiers in Synaptic Neuroscience 10(1): 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creager R, Dunwiddie T, Lynch G. (1980) Paired-pulse and frequency facilitation in the CA1 region of the in vitro rat hippocampus. Journal of Physiology 299: 409–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunha RA. (2001) Adenosine as a neuromodulator and as a homeostatic regulator in the nervous system: Different roles, different sources and different receptors. Neurochemistry International 38(2): 107–125. [DOI] [PubMed] [Google Scholar]
- Cunha RA, Johansson B, van der Ploeg I, et al. (1994) Evidence for functionally important adenosine A2a receptors in the rat hippocampus. Brain Research 649(1): 208–216. [DOI] [PubMed] [Google Scholar]
- Cunha RA, Ribeiro JA. (2000) Purinergic modulation of [(3)H]GABA release from rat hippocampal nerve terminals. Neuropharmacology 39(2): 1156–1167. [DOI] [PubMed] [Google Scholar]
- Davies CH, Collingridge GL. (1996) Regulation of EPSPs by the synaptic activation of GABAB autoreceptors in rat hippocampus. Journal of Physiology 496(Pt 2): 451–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dayan P. (2012) Twenty-five lessons from computational neuromodulation. Neuron 76(1): 240–256. [DOI] [PubMed] [Google Scholar]
- Devaraju P, Yu J, Eddins D, et al. (2017) Haploinsufficiency of the 22q11.2 microdeletion gene Mrpl40 disrupts short-term synaptic plasticity and working memory through dysregulation of mitochondrial calcium. Molecular Psychiatry 22 (9): 1313–1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dias RB, Rombo DM, Ribeiro JA, et al. (2013) Adenosine: Setting the stage for plasticity. Trends in Neurosciences 36(4): 248–257. [DOI] [PubMed] [Google Scholar]
- Dobrunz LE, Stevens CF. (1997) Heterogeneity of release probability, facilitation, and depletion at central synapses. Neuron 18(6): 995–1008. [DOI] [PubMed] [Google Scholar]
- Dobrunz LE, Stevens CF. (1999) Response of hippocampal synapses to natural stimulation patterns. Neuron 22(1): 157–166. [DOI] [PubMed] [Google Scholar]
- Dong HW, Swanson LW, Chen L, et al. (2009) Genomic-anatomic evidence for distinct functional domains in hippocampal field CA1. Proceedings of the National Academy of Sciences of the United States of America 106(28): 11794–11799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougherty KA, Islam T, Johnston D. (2012) Intrinsic excitability of CA1 pyramidal neurones from the rat dorsal and ventral hippocampus. Journal of Physiology 590(22): 5707–5722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubovyk V, Manahan-Vaughan D. (2018) Less means more: The magnitude of synaptic plasticity along the hippocampal dorso-ventral axis is inversely related to the expression levels of plasticity-related neurotransmitter receptors. Hippocampus 28(2): 136–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumas TC, Foster TC. (1998) Late developmental changes in the ability of adenosine A1 receptors to regulate synaptic transmission in the hippocampus. Brain Research 105(1): 137–139. [PubMed] [Google Scholar]
- Dunwiddie TV, Diao L. (1994) Extracellular adenosine concentrations in hippocampal brain slices and the tonic inhibitory modulation of evoked excitatory responses. The Journal of Pharmacology and Experimental Therapeutics 268(2): 537–545. [PubMed] [Google Scholar]
- Dunwiddie TV, Haas HL. (1985) Adenosine increases synaptic facilitation in the in vitro rat hippocampus: Evidence for a presynaptic site of action. Journal of Physiology 369(1): 365–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunwiddie TV, Masino SA. (2001) The role and regulation of adenosine in the central nervous system. Annual Review of Neuroscience 24: 31–55. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H, Amaral DG, Buffalo EA, et al. (2016) Hippocampus at 25. Hippocampus 26(10): 1238–1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenton AA, Muller RU. (1998) Place cell discharge is extremely variable during individual passes of the rat through the firing field. Proceedings of the National Academy of Sciences of the United States of America 95(6): 3182–3187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floriou-Servou A, von Ziegler L, Stalder L, et al. (2018) Distinct proteomic, transcriptomic, and epigenetic stress responses in dorsal and ventral hippocampus. Biological Psychiatry 84(7): 531–541. [DOI] [PubMed] [Google Scholar]
- Giocomo LM, Hasselmo ME. (2007) Neuromodulation by glutamate and acetylcholine can change circuit dynamics by regulating the relative influence of afferent input and excitatory feedback. Molecular Neurobiology 36(2): 184–200. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Burgos G, Kroener S, Seamans JK, et al. (2005) Dopaminergic modulation of short-term synaptic plasticity in fast-spiking interneurons of primate dorsolateral prefrontal cortex. Journal of Neurophysiology 94(6): 4168–4177. [DOI] [PubMed] [Google Scholar]
- Gray JA, McNaughton N. (2003) The Neuropsychology of Anxiety: An Enquiry into the Function of the Septo-hippocampal System. Oxford: Oxford University Press. [Google Scholar]
- Grigoryan G, Korkotian E, Segal M. (2012) Selective facilitation of LTP in the ventral hippocampus by calcium stores. Hippocampus 22(7): 1635–1644. [DOI] [PubMed] [Google Scholar]
- Grigoryan G, Segal M. (2013) Prenatal stress alters noradrenergic modulation of LTP in hippocampal slices. Journal of Neurophysiology 110(1): 279–285. [DOI] [PubMed] [Google Scholar]
- Grigoryan G, Segal M. (2016) Ryanodine-mediated conversion of STP to LTP is lacking in synaptopodin-deficient mice. Brain Structure & Function 221(4): 2393–2397. [DOI] [PubMed] [Google Scholar]
- Hagberg H, Andersson P, Lacarewicz J, et al. (1987) Extracellular adenosine, inosine, hypoxanthine, and xanthine in relation to tissue nucleotides and purines in rat striatum during transient ischemia. Journal of Neurochemistry 49(1): 227–231. [DOI] [PubMed] [Google Scholar]
- Harris EW, Cotman CW. (1983) Effects of acidic amino acid antagonists on paired-pulse potentiation at the lateral perforant path. Experimental Brain Research 52(3): 455–460. [DOI] [PubMed] [Google Scholar]
- Hauser J, Llano López LH, Feldon J, et al. (2020) Small lesions of the dorsal or ventral hippocampus subregions are associated with distinct impairments in working memory and reference memory retrieval, and combining them attenuates the acquisition rate of spatial reference memory. Hippocampus 30(9): 938–957. [DOI] [PubMed] [Google Scholar]
- Honigsperger C, Marosi M, Murphy R, et al. (2015) Dorsoventral differences in Kv7/M-current and its impact on resonance, temporal summation and excitability in rat hippocampal pyramidal cells. Journal of Physiology 593(7): 1551–1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito HT, Schuman EM. (2007) Frequency-dependent gating of synaptic transmission and plasticity by dopamine. Front Neural Circuits 1(1): 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito HT, Schuman EM. (2008) Frequency-dependent signal transmission and modulation by neuromodulators. Frontiers in Neuroscience 2(2): 138–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackman SL, Regehr WG. (2017) The mechanisms and functions of synaptic facilitation. Neuron 94(3): 447–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackman SL, Turecek J, Belinsky JE, et al. (2016) The calcium sensor synaptotagmin 7 is required for synaptic facilitation. Nature 529(7584): 88–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung MW, Wiener SI, McNaughton BL. (1994) Comparison of spatial firing characteristics of units in dorsal and ventral hippocampus of the rat. Journal of Neuroscience 14(12): 7347–7356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz P, Edwards D. (1999) Beyond Neurotransmission. New York: Oxford University Press. [Google Scholar]
- Kim CS, Johnston D. (2015) A1 adenosine receptor-mediated GIRK channels contribute to the resting conductance of CA1 neurons in the dorsal hippocampus. Journal of Neurophysiology 113(7): 2511–2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby MT, Hampson RE, Deadwyler SA. (1995) Cannabinoids selectively decrease paired-pulse facilitation of perforant path synaptic potentials in the dentate gyrus in vitro. Brain Research 688(1–2): 114–120. [DOI] [PubMed] [Google Scholar]
- Kjelstrup KG, Tuvnes FA, Steffenach HA, et al. (2002) Reduced fear expression after lesions of the ventral hippocampus. Proceedings of the National Academy of Sciences of the United States of America 99(16): 10825–10830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klausnitzer J, Manahan-Vaughan D. (2008) Frequency facilitation at mossy fiber-CA3 synapses of freely behaving rats is regulated by adenosine A1 receptors. Journal of Neuroscience 28(18): 4836–4840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koutsoumpa A, Papatheodoropoulos C. (2019) Short-term dynamics of input and output of CA1 network greatly differ between the dorsal and ventral rat hippocampus. BMC Neuroscience 20(1): 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koutsoumpa A, Papatheodoropoulos C. (2021) Frequency-dependent layer-specific differences in short-term synaptic plasticity in the dorsal and ventral CA1 hippocampal field. Synapse (New York, N.Y.) 75(7): e22199. [DOI] [PubMed] [Google Scholar]
- Kouvaros S, Papatheodoropoulos C. (2016. a) Major dorsoventral differences in the modulation of the local CA1 hippocampal network by NMDA, mGlu5, adenosine A2A and cannabinoid CB1 receptors. Neuroscience 317: 47–64. [DOI] [PubMed] [Google Scholar]
- Kouvaros S, Papatheodoropoulos C. (2016. b) Theta burst stimulation-induced LTP: Differences and similarities between the dorsal and ventral CA1 hippocampal synapses. Hippocampus 26(12): 1542–1559. [DOI] [PubMed] [Google Scholar]
- Le Barillier L, Léger L, Luppi PH, et al. (2015) Genetic deletion of melanin-concentrating hormone neurons impairs hippocampal short-term synaptic plasticity and hippocampal-dependent forms of short-term memory. Hippocampus 25(11): 1361–1373. [DOI] [PubMed] [Google Scholar]
- Lee AR, Kim JH, Cho E, et al. (2017) Dorsal and ventral hippocampus differentiate in functional pathways and differentially associate with neurological disease-related genes during postnatal development. Frontiers in Molecular Neuroscience 10: 331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KS, Reddington M, Schubert P, et al. (1983) Regulation of the strength of adenosine modulation in the hippocampus by a differential distribution of the density of A1 receptors. Brain Research 260(1): 156–159. [DOI] [PubMed] [Google Scholar]
- Li J, Cao D, Dimakopoulos V, et al. (2022) Anterior-posterior hippocampal dynamics support working memory processing. Journal of Neuroscience 42(3): 443–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisman JE. (1997) Bursts as a unit of neural information: Making unreliable synapses reliable. Trends in Neurosciences 20(1): 38–43. [DOI] [PubMed] [Google Scholar]
- Lloyd HG, Lindström K, Fredholm BB. (1993) Intracellular formation and release of adenosine from rat hippocampal slices evoked by electrical stimulation or energy depletion. Neurochemistry International 23(2): 173–185. [DOI] [PubMed] [Google Scholar]
- Lupica CR, Proctor WR, Dunwiddie TV. (1992) Presynaptic inhibition of excitatory synaptic transmission by adenosine in rat hippocampus: Analysis of unitary EPSP variance measured by whole-cell recording. Journal of Neuroscience 12(10): 3753–3764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luscher C, Jan LY, Stoffel M, et al. (1997) G protein-coupled inwardly rectifying K+ channels (GIRKs) mediate postsynaptic but not presynaptic transmitter actions in hippocampal neurons. Neuron 19(3): 687–695. [DOI] [PubMed] [Google Scholar]
- Maggio N, Segal M. (2007) Striking variations in corticosteroid modulation of long-term potentiation along the septotemporal axis of the hippocampus. Journal of Neuroscience 27(21): 5757–5765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maggio N, Segal M. (2009) Differential corticosteroid modulation of inhibitory synaptic currents in the dorsal and ventral hippocampus. Journal of Neuroscience 29(9): 2857–2866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malik R, Johnston D. (2017) Dendritic GIRK channels gate the integration window, plateau potentials, and induction of synaptic plasticity in dorsal but not ventral CA1 neurons. Journal of Neuroscience 37(14): 3940–3955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manabe T, Wyllie DJ, Perkel DJ, et al. (1993) Modulation of synaptic transmission and long-term potentiation: Effects on paired pulse facilitation and EPSC variance in the CA1 region of the hippocampus. Journal of Neurophysiology 70(4): 1451–1499. [DOI] [PubMed] [Google Scholar]
- Marder E. (2012) Neuromodulation of neuronal circuits: Back to the future. Neuron 76(1): 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marder E, O’Leary T, Shruti S. (2014) Neuromodulation of circuits with variable parameters: Single neurons and small circuits reveal principles of state-dependent and robust neuromodulation. Annual Review of Neuroscience 37: 329–346. [DOI] [PubMed] [Google Scholar]
- Markram H, Tsodyks M. (1996) Redistribution of synaptic efficacy between neocortical pyramidal neurons. Nature 382(6594): 807–810. [DOI] [PubMed] [Google Scholar]
- Maruki K, Izaki Y, Nomura M, et al. (2001) Differences in paired-pulse facilitation and long-term potentiation between dorsal and ventral CA1 regions in anesthetized rats. Hippocampus 11(6): 655–661. [DOI] [PubMed] [Google Scholar]
- Maurer AP, Vanrhoads SR, Sutherland GR, et al. (2005) Self-motion and the origin of differential spatial scaling along the septo-temporal axis of the hippocampus. Hippocampus 15(7): 841–852. [DOI] [PubMed] [Google Scholar]
- McCormick DA, Nestvogel DB, He BJ. (2020) Neuromodulation of brain state and behavior. Annual Review of Neuroscience 43: 391–415. [DOI] [PubMed] [Google Scholar]
- McCormick DA, Nusbaum MP. (2014) Editorial overview: Neuromodulation: Tuning the properties of neurons, networks and behavior. Current Opinion in Neurobiology 29: iv–vii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHugh SB, Deacon RM, Rawlins JN, et al. (2004) Amygdala and ventral hippocampus contribute differentially to mechanisms of fear and anxiety. Behavioral Neuroscience 118(1): 63–78. [DOI] [PubMed] [Google Scholar]
- McHugh SB, Niewoehner B, Rawlins JN, et al. (2008) Dorsal hippocampal N-methyl-D-aspartate receptors underlie spatial working memory performance during non-matching to place testing on the T-maze. Behavioural Brain Research 186(1): 41–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milior G, Castro MA, Sciarria LP, et al. (2016) Electrophysiological properties of CA1 pyramidal neurons along the longitudinal axis of the mouse hippocampus. Science Report 6: 38242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miliou A, Papaleonidopoulos V, Trompoukis G, et al. (2021) Septotemporal variation in beta-adrenergic modulation of short-term dynamics in the hippocampus. IBRO Neuroscience Reports 11: 64–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller RJ. (1998) Presynaptic receptors. Annual Review of Pharmacology and Toxicology 38: 201–227. [DOI] [PubMed] [Google Scholar]
- Mlinar B, Corradetti R. (2018) Differential modulation of CA1 impulse flow by endogenous serotonin along the hippocampal longitudinal axis. Hippocampus 28(3): 217–225. [DOI] [PubMed] [Google Scholar]
- Moschovos C, Kostopoulos G, Papatheodoropoulos C. (2012) Endogenous adenosine induces NMDA receptor-independent persistent epileptiform discharges in dorsal and ventral hippocampus via activation of A2 receptors. Epilepsy Research 100(1–2): 157–167. [DOI] [PubMed] [Google Scholar]
- Moschovos C, Papatheodoropoulos C. (2016) The L-type voltage-dependent calcium channel long-term potentiation is higher in the dorsal compared with the ventral associational/commissural CA3 hippocampal synapses. Neuroscience Research 106: 62–65. [DOI] [PubMed] [Google Scholar]
- Moser E, Moser MB, Andersen P. (1993) Spatial learning impairment parallels the magnitude of dorsal hippocampal lesions, but is hardly present following ventral lesions. Journal of Neuroscience 13(9): 3916–3925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motanis H, Seay MJ, Buonomano DV. (2018) Short-term synaptic plasticity as a mechanism for sensory timing. Trends in Neurosciences 41(10): 701–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukunda CL, Narayanan R. (2017) Degeneracy in the regulation of short-term plasticity and synaptic filtering by presynaptic mechanisms. Journal of Physiology 595(8): 2611–2637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadim F, Bucher D. (2014) Neuromodulation of neurons and synapses. Current Opinion in Neurobiology 29: 48–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Callaghan C, Walpola IC, Shine JM. (2021) Neuromodulation of the mind-wandering brain state: The interaction between neuromodulatory tone, sharp wave-ripples and spontaneous thought. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences 376(1817): 20190699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuyama T, Kitamura T, Roy DS, et al. (2016) Ventral CA1 neurons store social memory. Science 353(6307): 1536–1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pals M, Stewart TC, Akyürek EG, et al. (2020) A functional spiking-neuron model of activity-silent working memory in humans based on calcium-mediated short-term synaptic plasticity. PLoS Computational Biology 16(6): e1007936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papaleonidopoulos V, Kouvaros S, Papatheodoropoulos C. (2018) Effects of endogenous and exogenous D1/D5 dopamine receptor activation on LTP in ventral and dorsal CA1 hippocampal synapses. Synapse (New York, N.Y.) 72(8): e22033. [DOI] [PubMed] [Google Scholar]
- Papaleonidopoulos V, Papatheodoropoulos C. (2018) β-adrenergic receptors reduce the threshold for induction and stabilization of LTP and enhance its magnitude via multiple mechanisms in the ventral but not the dorsal hippocampus. Neurobiology of Learning and Memory 151: 71–84. [DOI] [PubMed] [Google Scholar]
- Papaleonidopoulos V, Trompoukis G, Koutsoumpa A, et al. (2017) A gradient of frequency-dependent synaptic properties along the longitudinal hippocampal axis. BMC Neuroscience 18(1): 79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papatheodoropoulos C. (2015) Striking differences in synaptic facilitation along the dorsoventral axis of the hippocampus. Neuroscience 301: 454–470. [DOI] [PubMed] [Google Scholar]
- Papatheodoropoulos C, Asprodini E, Nikita I, et al. (2002) Weaker synaptic inhibition in CA1 region of ventral compared to dorsal rat hippocampal slices. Brain Research 948(1–2): 117–121. [DOI] [PubMed] [Google Scholar]
- Papatheodoropoulos C, Kostopoulos G. (1998) Development of a transient increase in recurrent inhibition and paired-pulse facilitation in hippocampal CA1 region. Brain Research 108(1–2): 273–285. [DOI] [PubMed] [Google Scholar]
- Papatheodoropoulos C, Kostopoulos G. (2000. a) Decreased ability of rat temporal hippocampal CA1 region to produce long-term potentiation. Neuroscience Letters 279(3): 177–180. [DOI] [PubMed] [Google Scholar]
- Papatheodoropoulos C, Kostopoulos G. (2000. b) Dorsal-ventral differentiation of short-term synaptic plasticity in rat CA1 hippocampal region. Neuroscience Letters 286(1): 57–60. [DOI] [PubMed] [Google Scholar]
- Park P, Volianskis A, Sanderson TM, et al. (2014) NMDA receptor-dependent long-term potentiation comprises a family of temporally overlapping forms of synaptic plasticity that are induced by different patterns of stimulation. Philosophical Transactions of the Royal Society B: Biological Sciences 369(1633): 20130131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pentkowski NS, Blanchard DC, Lever C, et al. (2006) Effects of lesions to the dorsal and ventral hippocampus on defensive behaviors in rats. European Journal of Neuroscience 23(8): 2185–2196. [DOI] [PubMed] [Google Scholar]
- Reddington M, Lee KS, Schubert P. (1982) An A1-adenosine receptor, characterized by [3H] cyclohexyladenosine binding, mediates the depression of evoked potentials in a rat hippocampal slice preparation. Neuroscience Letters 28(3): 275–279. [DOI] [PubMed] [Google Scholar]
- Reis SL, Silva HB, Almeida M, et al. (2019) Adenosine A1 and A2A receptors differently control synaptic plasticity in the mouse dorsal and ventral hippocampus. Journal of Neurochemistry 151(2): 227–237. [DOI] [PubMed] [Google Scholar]
- Rombo DM, Newton K, Nissen W, et al. (2015) Synaptic mechanisms of adenosine A2A receptor-mediated hyperexcitability in the hippocampus. Hippocampus 25(5): 566–580. [DOI] [PubMed] [Google Scholar]
- Rotman Z, Deng PY, Klyachko VA. (2011) Short-term plasticity optimizes synaptic information transmission. Journal of Neuroscience 31(41): 14800–14809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrader J, Wahl M, Kuschinsky W, et al. (1980) Increase of adenosine content in cerebral cortex of the cat during bicuculline-induced seizure. European Journal of Physiology 387(3): 245–251. [DOI] [PubMed] [Google Scholar]
- Schreurs A, Sabanov V, Balschun D. (2017) Distinct properties of long-term potentiation in the dentate gyrus along the dorsoventral axis: Influence of age and inhibition. Science Report 7(1): 5157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebastiao AM, Ribeiro JA. (1992) Evidence for the presence of excitatory A2 adenosine receptors in the rat hippocampus. Neuroscience Letters 138(1): 41–44. [DOI] [PubMed] [Google Scholar]
- Sebastiao AM, Ribeiro JA. (2014) Neuromodulation and metamodulation by adenosine: Impact and subtleties upon synaptic plasticity regulation. Brain Research 1621: 102–113. [DOI] [PubMed] [Google Scholar]
- Sebastião AM, Stone TW, Ribeiro JA. (1990) The inhibitory adenosine receptor at the neuromuscular junction and hippocampus of the rat: Antagonism by 1,3,8-substituted xanthines. British Journal of Pharmacology 101(2): 453–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strange BA, Witter MP, Lein ES, et al. (2014) Functional organization of the hippocampal longitudinal axis. Nature Reviews Neuroscience 15(10): 655–669. [DOI] [PubMed] [Google Scholar]
- Thompson CL, Pathak SD, Jeromin A, et al. (2008) Genomic anatomy of the hippocampus. Neuron 60(6): 1010–1021. [DOI] [PubMed] [Google Scholar]
- Thompson SM, Haas HL, Gahwiler BH. (1992) Comparison of the actions of adenosine at pre- and postsynaptic receptors in the rat hippocampus in vitro. Journal of Physiology 451: 347–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson AM. (2000) Molecular frequency filters at central synapses. Progress in Neurobiology 62(2): 159–196. [DOI] [PubMed] [Google Scholar]
- Tidball P, Burn HV, Teh KL, et al. (2017) Differential ability of the dorsal and ventral rat hippocampus to exhibit group I metabotropic glutamate receptor-dependent synaptic and intrinsic plasticity. Brain and Neuroscience Advances 1(1): 2398212816689792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trompoukis G, Papatheodoropoulos C. (2020) Dorsal-ventral differences in modulation of synaptic transmission in the hippocampus. Frontiers in Synaptic Neuroscience 12: 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulrich D, Bettler B. (2007) GABA(B) receptors: Synaptic functions and mechanisms of diversity. Current Opinion in Neurobiology 17(3): 298–303. [DOI] [PubMed] [Google Scholar]
- Vizi ES, Kiss JP. (1998) Neurochemistry and pharmacology of the major hippocampal transmitter systems: Synaptic and nonsynaptic interactions. Hippocampus 8(6): 566–607. [DOI] [PubMed] [Google Scholar]
- Volianskis A, France G, Jensen MS, et al. (2015) Long-term potentiation and the role of N-methyl-d-aspartate receptors. Brain Research 1621: 5–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Gersdorff H, Borst JG. (2002) Short-term plasticity at the calyx of Held. Nature Reviews Neuroscience 3(1): 53–64. [DOI] [PubMed] [Google Scholar]
- Winn HR, Welsh JE, Rubio R, et al. (1980) Changes in brain adenosine during bicuculline-induced seizures in rats. Effects of hypoxia and altered systemic blood pressure. Circulation Research 47(4): 568–567. [DOI] [PubMed] [Google Scholar]
- Yang H, Xu-Friedman MA. (2015) Skipped-stimulus approach reveals that short-term plasticity dominates synaptic strength during ongoing activity. Journal of Neuroscience 35(21): 8297–8307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zetterström T, Vernet L, Ungerstedt U, et al. (1982) Purine levels in the intact rat brain. Studies with an implanted perfused hollow fibre. Neuroscience Letters 29(2): 111–115. [DOI] [PubMed] [Google Scholar]
- Zhang JM, Wang HK, Ye CQ, et al. (2003) ATP released by astrocytes mediates glutamatergic activity-dependent heterosynaptic suppression. Neuron 40(5): 971–982. [DOI] [PubMed] [Google Scholar]
- Zucker RS, Regehr WG. (2002) Short-term synaptic plasticity. Annual Review of Physiology 64: 355–405. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-eps-1-bna-10.1177_23982128221106315 for Septotemporal variation in modulation of synaptic transmission, paired-pulse ratio and frequency facilitation/depression by adenosine and GABAB receptors in the rat hippocampus by Maria A. Samara, George D. Oikonomou, George Trompoukis, Georgia Madarou, Maria Adamopoulou and Costas Papatheodoropoulos in Brain and Neuroscience Advances
Supplemental material, sj-eps-10-bna-10.1177_23982128221106315 for Septotemporal variation in modulation of synaptic transmission, paired-pulse ratio and frequency facilitation/depression by adenosine and GABAB receptors in the rat hippocampus by Maria A. Samara, George D. Oikonomou, George Trompoukis, Georgia Madarou, Maria Adamopoulou and Costas Papatheodoropoulos in Brain and Neuroscience Advances
Supplemental material, sj-eps-2-bna-10.1177_23982128221106315 for Septotemporal variation in modulation of synaptic transmission, paired-pulse ratio and frequency facilitation/depression by adenosine and GABAB receptors in the rat hippocampus by Maria A. Samara, George D. Oikonomou, George Trompoukis, Georgia Madarou, Maria Adamopoulou and Costas Papatheodoropoulos in Brain and Neuroscience Advances
Supplemental material, sj-eps-3-bna-10.1177_23982128221106315 for Septotemporal variation in modulation of synaptic transmission, paired-pulse ratio and frequency facilitation/depression by adenosine and GABAB receptors in the rat hippocampus by Maria A. Samara, George D. Oikonomou, George Trompoukis, Georgia Madarou, Maria Adamopoulou and Costas Papatheodoropoulos in Brain and Neuroscience Advances
Supplemental material, sj-eps-4-bna-10.1177_23982128221106315 for Septotemporal variation in modulation of synaptic transmission, paired-pulse ratio and frequency facilitation/depression by adenosine and GABAB receptors in the rat hippocampus by Maria A. Samara, George D. Oikonomou, George Trompoukis, Georgia Madarou, Maria Adamopoulou and Costas Papatheodoropoulos in Brain and Neuroscience Advances
Supplemental material, sj-eps-5-bna-10.1177_23982128221106315 for Septotemporal variation in modulation of synaptic transmission, paired-pulse ratio and frequency facilitation/depression by adenosine and GABAB receptors in the rat hippocampus by Maria A. Samara, George D. Oikonomou, George Trompoukis, Georgia Madarou, Maria Adamopoulou and Costas Papatheodoropoulos in Brain and Neuroscience Advances
Supplemental material, sj-eps-6-bna-10.1177_23982128221106315 for Septotemporal variation in modulation of synaptic transmission, paired-pulse ratio and frequency facilitation/depression by adenosine and GABAB receptors in the rat hippocampus by Maria A. Samara, George D. Oikonomou, George Trompoukis, Georgia Madarou, Maria Adamopoulou and Costas Papatheodoropoulos in Brain and Neuroscience Advances
Supplemental material, sj-eps-7-bna-10.1177_23982128221106315 for Septotemporal variation in modulation of synaptic transmission, paired-pulse ratio and frequency facilitation/depression by adenosine and GABAB receptors in the rat hippocampus by Maria A. Samara, George D. Oikonomou, George Trompoukis, Georgia Madarou, Maria Adamopoulou and Costas Papatheodoropoulos in Brain and Neuroscience Advances
Supplemental material, sj-eps-8-bna-10.1177_23982128221106315 for Septotemporal variation in modulation of synaptic transmission, paired-pulse ratio and frequency facilitation/depression by adenosine and GABAB receptors in the rat hippocampus by Maria A. Samara, George D. Oikonomou, George Trompoukis, Georgia Madarou, Maria Adamopoulou and Costas Papatheodoropoulos in Brain and Neuroscience Advances
Supplemental material, sj-eps-9-bna-10.1177_23982128221106315 for Septotemporal variation in modulation of synaptic transmission, paired-pulse ratio and frequency facilitation/depression by adenosine and GABAB receptors in the rat hippocampus by Maria A. Samara, George D. Oikonomou, George Trompoukis, Georgia Madarou, Maria Adamopoulou and Costas Papatheodoropoulos in Brain and Neuroscience Advances