Abstract
Object
Early‐life neglect has irreversible emotional effects on the central nervous system. In this work, we aimed to elucidate distinct functional neural changes in medial prefrontal cortex (mPFC) of model rats.
Methods
Maternal separation with early weaning was used as a rat model of early‐life neglect. The excitation of glutamatergic and GABAergic neurons in rat mPFC was recorded and analyzed by whole‐cell patch clamp.
Results
Glutamatergic and GABAergic neurons of mPFC were distinguished by typical electrophysiological properties. The excitation of mPFC glutamatergic neurons was significantly increased in male groups, while the excitation of mPFC GABAergic neurons was significant in both female and male groups, but mainly in terms of rest membrane potential and amplitude, respectively.
Conclusions
Glutamatergic and GABAergic neurons in medial prefrontal cortex showed different excitability changes in a rat model of early‐life neglect, which can contribute to distinct mechanisms for emotional and cognitive manifestations.
Keywords: early‐life neglect model, GABAergic, glutamatergic, maternal separation with early weaning, medial prefrontal cortex, neuronal excitability
Early‐life neglect have irreversible emotional effects to central nervous system. Herein this work, we were aimed to decipher distinct functional neural changes in medial prefrontal cortex of model rats. Maternal separation with early weaning were taken as rat model of early‐life neglect. The excitation of glutamatergic and GABAergic neurons in rat mPFC were recorded and analyzed by whole‐cell patch‐clamp. Glutamatergic and GABAergic neurons of mPFC were distinguished by typical electrophysiological properties. The excitation of mPFC Glutamatergic neurons were significant increase in male groups, while the excitation of mPFC GABAergic neurons were more significant in female groups. Glutamatergic and GABAergic neurons in medial prefrontal cortex showed different excitability changes in rat model of early‐life neglect, which can contribute to distinct mechanisms for emotional and cognitive manifestations.
1. INTRODUCTION
Childhood is a critical period for brain development. Early‐life neglect has been a social trend during global urbanization, leading to negative effects in both urban and rural areas. 1 Exposure to early‐life neglect induces early‐life stress (ELS), which has extensive influences on the central nervous system, including behavior, emotion, and cognition, and could increase the risk of long‐lasting effects on brain development 2 and changes in personal characteristic, and may cause symptoms including cognitive impairment, 3 anxiety, depression, addiction, 4 and impulsivity. 5 These effects are the result of gene × environment interactions.
Literature reports that multiple brain areas contribute to the pathogenesis of clinical manifestations, such as the amygdala, fusiform gyrus, insula, striatum, and prefrontal cortex. 6 Different brain areas and neural circuits participate in behavioral or pathological manifestations. It has been reported that dopamine and receptors in caudate‐putamen of rodent striatum might contribute to object recognition behavior. 3 Orbital frontal cortex (OFC) and medial prefrontal cortex (mPFC) play important roles in impulsivity. 7 mPFC is an essential cortex area for complex cognition and emotion modulation, connected to diverse brain areas and forming afferent and efferent neural pathways. 8 , 9 Liu et al. found that oligodendrocytes and myelination impairment of mouse mPFC played a role in social life stress. 10 In previous work, we demonstrated changes in rat medial prefrontal cortex (mPFC) of an early‐life neglect model. 11 Chen et al. reported that the excitation/inhibition balance of mPFC layer V also contributed to depression and anxiety‐like emotional disorders of rats. 12 Intrinsic excitability of mPFC neurons acts as a neural basis of physiological functions, as well as their information integration and processing. In pathological states, the functional changes of neural excitability are considered neural mechanisms of symptom and behavioral phenotype.
In this research, we take advantage of maternal separation with early weaning (MSEW) as a rat model for early‐life neglect, and decipher different neural excitability of GABAergic and glutamatergic neurons in rat mPFC by whole‐cell patch clamp. Our findings suggest different neuronal excitability alterations of rat mPFC GABAergic and glutamatergic neurons in an early‐life neglect model, which may be considered as potential therapeutic targets in the future.
2. MATERIAL AND METHODS
2.1. Animals
Four adult specific‐pathogen‐free (SPF) Sprague–Dawley female rats and 2 male rats were acquired from Beijing Huafukang Bioscience Co. Ltd. For rat breeding, every cage contained 1 male rat and 2 female rats. All the animals were housed at room temperature ~24–26°C, 12‐h/12‐h light/dark cycle, with food and drink ad libitum. This project was approved by the Institutional Animal Care and Use Committee of ILAS (ILAS‐QC‐2017‐002). All the experiment procedures were conducted in accordance with the ethical guidelines, 3R rules, and national standard. 13
2.2. Early‐life neglect model of rats
Maternal separation with early weaning (MSEW) was regarded as an early‐life neglect model of rats. Methods were described previously by George et al. 14 Twenty litters, half male and half female, were obtained after breeding. From postnatal day (PND) 0, litters were randomly divided into 4 groups (each group n = 5): female MSEW group, female control group, male MSEW group, and male control group. Control mouse groups were undisturbed and weaned normally until PND 21. MSEW were separated from the dam every day as follows: ~PND 2‐PND 5, separated for 4 h each day; ~PND 6‐PND 16, separated for 8 hours each day; PND 17, weaned. Separated litters were kept in separate rat cages, without bedding. Temperature was kept at 32°C with heating pads. After weaning, litters were group‐housed with the same sex and group.
2.3. Patch‐clamp electrophysiology
2.3.1. Brain slice preparation
Whole‐cell patch clamp of rat acute brain slices was used to examine the excitation of mPFC neurons. Five rats and 10 cells were recorded for each group. For patch‐clamp slice preparation, rats were anesthetized by pentobarbital sodium intraperitoneally. The whole brain was rapidly dissected and submerged in oxygenated (95% O2/5% CO2), ice‐cold high‐sucrose cutting solution, containing (in mM): KCl 2.5, NaH2PO4 1.25, CaCl2 0.5, MgSO4 10, NaHCO3 26, glucose 10, sucrose 230 (pH 7.4, 300–310 mOsm). With a Leica VT1000S vibrating microtome (Leica Instruments, Germany), 250 μm coronal slices of medial prefrontal cortex (mPFC) were made. Brain slices were transferred to incubation chamber with 32°C oxygenated artificial cerebrospinal fluid (ACSF), containing (in mM): NaCl 126, KCl 2.5, MgCl2 1.3, NaH2PO4 1.2, CaCl2 2.4, NaHCO3 18, glucose 10 (pH 7.4, 300–310 mOsm). Slices were equilibrated for more than 0.5 h at room temperature before recording.
2.3.2. Whole‐cell patch‐clamp recording
The excitation of mPFC neurons was examined by whole‐cell patch‐clamp recording. Brain slices were transferred to a recording chamber under an Olympus BX51 microscope (Olympus, Germany), while cell images were taken with an infrared differential interference contract camera. During recording, slices were perfused 2 ml/min with regular ACSF. Borosilicate glass capillary tubes (Sutter 150‐86‐10, USA) were pulled by PC‐10 pipette puller (Narishige, Japan). The resistance of glass pipettes was ~5–8 MΩ, filled with K+‐Met‐sulfonate internal solution containing (in mM): K+‐Met‐sulfonate 140.5, NaCl 7.5, HEPES hemisodium salt 10, Mg‐ATP 2, Na‐GTP 0.2 (pH 7.33, 300–310 mOsm). Data were recorded by a Multiclamp 700 B amplifier (Molecular Devices, Sunnyvale, CA), filtered at 10 kHz and sampled at 50 kHz, and later analyzed with pClamp 10.6 software (Molecular Devices, USA). Recordings were discarded if the series resistance increased by more than 20% during the course of the recordings. ACSF and solutions were applied to the slice via a peristaltic pump (Longer Pump, China).
To distinguish different cell types of rat mPFC, we run the firing test protocol under current‐clamp mode. A 100‐pA, 1‐second current was injected in the neuron, and the number and frequency of firing was measured. To examine the excitability of different cells, a single and fast depolarizing current was injected to trigger a single action potential (AP) (30 ms, 100pA). To trigger a series of APs, a series of 600‐ms current pulses was injected, with intensities between −200 and 400 pA in 100‐pA increments. The rest membrane potential, single action potential, and number of multiple action potentials were analyzed and plotted as done previously. 15 , 16
2.4. Statistics
All data are presented as mean ± standard deviation (SD). Data were analyzed with unpaired t‐test between 2 groups, and 2‐way analysis of variation (ANOVA) for multiple groups. Graphpad Prism 8 software was used for data analysis, and p < 0.05 was considered significant.
3. RESULTS
3.1. Electrophysiological properties of different cell types in mPFC
To study different mechanisms of mPFC neurons during MSEW, we used whole‐cell patch‐clamp test to analyze mPFC neural functions in layer II/III (Figure 1A). The medial prefrontal cortex consists of glutamatergic and GABAergic neurons, which compose the 2 most basic circuits of mPFC, respectively the excitatory and inhibitory synapses. 17 To separate these 2 types of mPFC neurons, before whole‐cell states, these 2 cell types can be preliminarily distinguished by cell shape under phase‐contrast microscopy. Glutamatergic neurons were almost pyramidal neurons in mPFC, appearing as a triangle‐like shape with one main neurite (Figure 1E, left panel). GABAergic neurons in mPFC have an oval shape, and are smaller than glutamatergic ones (Figure 1F, left panel). Under whole‐cell patch‐clamp recording, these 2 cell types have distinct intrinsic electrophysiological properties in terms of neural rest membrane potential, innate action potential firing frequency, and halfwidth. 18 Our results show that the rest membrane potential of glutamatergic neurons was −72.84 ± 3.003 mV, while that of GABAergic neurons was −67.65 ± 3.066 mV (Figure 1B). With injection of depolarizing current during whole‐cell recording, the intrinsic firing frequencies were typically 2.600 ± 2.702 Hz for glutamatergic neurons and 18.80 ± 4.817 Hz for GABAergic neurons (Figure 1C,E,F, middle panel). When triggering a single action potential, the halfwidth of glutamatergic neurons was 2.800 ± 0.3000 ms, and 1.580 ± 0.04472 ms for GABAergic neurons (Figure 1D–F, right panel). On the basis of the above 3 characteristics, glutamatergic neurons and GABAergic neurons were distinguished from the mPFC brain slices for further patch‐clamp recordings.
FIGURE 1.
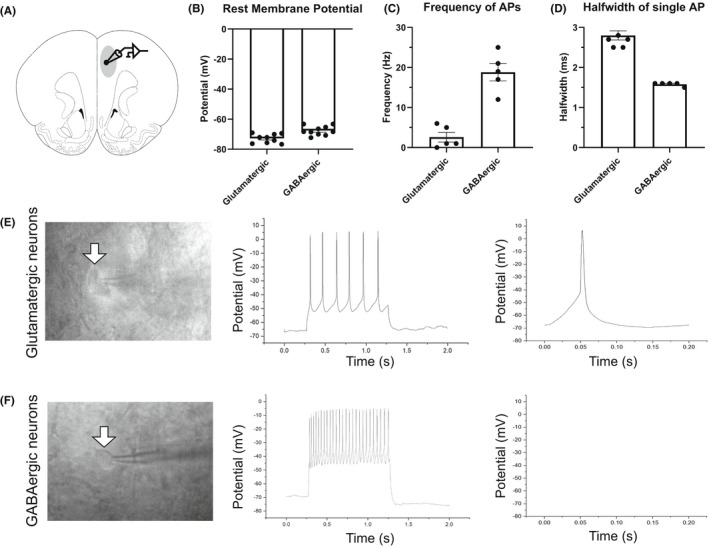
Electrophysiological properties of putative glutamatergic and GABAergic neurons in medial prefrontal cortex. (A) Schematic coronal section of a rat brain, showing the medial prefrontal cortex (mPFC). Recording electrodes were placed in layer II/III of mPFC. Excitation of neurons were recorded by whole‐cell patch clamp. (B–D) Summary histograms demonstrating different electrophysiological properties of mPFC glutamatergic and GABAergic neurons, regarding rest membrane potentials (B), action potential firing frequency (C), and halfwidth (D). (E) Typical example of a putative glutamatergic neuron from mPFC. Left, pyramidal shape of a mPFC glutamatergic neuron. Middle, multiple action potentials triggered by 1‐ms depolarizing current of 100 pA. Right, a typical single action potential from the neuron in left panel. (F) Typical example of a putative GABAergic neuron from mPFC. Left, oval shape of a mPFC GABAergic neuron. Middle, multiple action potentials triggered by 1 ms depolarizing current of 100 pA. Right, a single action potential from the neuron in left panel.
3.2. MSEW generated functional changes in mPFC glutamatergic neurons
Under whole‐cell current clamp, the rest membrane potential (RMP) of mPFC glutamatergic neurons was decreased in male rats but not female rats, from −70.34 ± 5.212 mV in the male control group to −63.37 ± 6.295 mV in the male MSEW group, facilitating the activation of these neurons (Figure 2A, Table 1). Similarly, when triggering a single action potential (AP) in these mPFC glutamatergic neurons, the threshold of the male MSEW group was increased compared with the male control group, from −42.85 ± 1.878 to −47.04 ± 2.157 mV, which also facilitated the triggering of an action potential (Figure 2B). Taken together, the alterations in RMP and AP threshold showed that male MSEW rats produced an action potential more easily.
FIGURE 2.
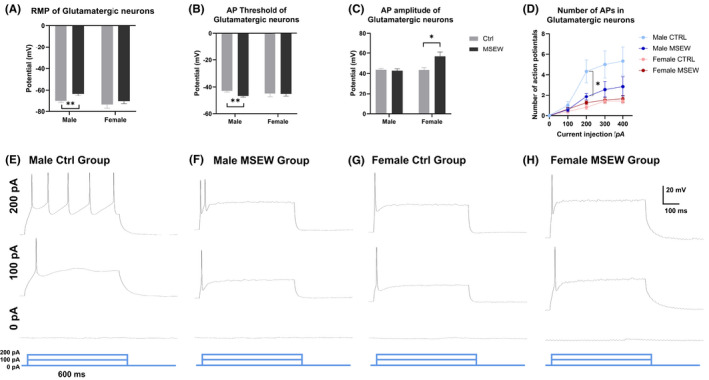
Distinct alterations in glutamatergic neuronal excitation between male and female rats. (A) Altered rest membrane potentials of mPFC glutamatergic neurons between male control and male MSEW group. (B) Altered threshold of a single action potential of mPFC glutamatergic neurons between male control and male MSEW group. (C) Increased amplitude of a single action potential of mPFC glutamatergic neurons in female MSEW group. (D) Multiple action potentials of 4 groups. Decreased number of APs between male groups. (E–H) Representative multiple action potentials of the 4 groups (*p < .05; **p < .01, MSEW group compared with control group. N = 10 cells for each group).
TABLE 1.
Summary of intrinsic physiological properties of mPFC glutamatergic neurons
| Male control | Male MSEW | Female control | Female MSEW | |
|---|---|---|---|---|
| Rest membrane potential (mV) | −70.34 ± 5.212 | −63.37 ± 6.295 ** | −73.70 ± 11.65 | −70.61 ± 7.900 |
| Voltage threshold (mV) | −42.85 ± 1.878 | −47.04 ± 2.157b | −44.76 ± 8.785 | −45.02 ± 6.365 |
| Action potential amplitude (mV) | 43.62 ± 3.016 | 42.77 ± 4.928 | 43.40 ± 6.991 | 56.75 ± 12.06 a |
Note: Data are shown as mean ± SD from 10 mPFC cells from 5 rats.
Control group compared with MSEW group. p < 0.05.
Control group compared with MSEW group. p < 0.01.
To comprehensively analyze the intrinsic excitation, we also injected a longer depolarizing current of 600 ms to trigger multiple action potentials. Results demonstrated that in male, but not female, rats, MSEW modeling altered the number of APs, causing alterations in firing frequencies and refractory periods (Figure 2D–F). This result is consistent with our previous research, 11 where we demonstrated that selectively in male MSEW rats, but not female mice, mPFC glutamatergic neurons exhibited a decrement in the number of multiple action potentials triggered by a series of 600‐ms depolarizing current (0, 100, 200, 300, 400 pA). This decreased excitability between male MSEW rats and male control rats contributed to the anxiety‐like behavior of male MSEW rats.
For female rats, MSEW modeling only increased the amplitude of a single AP (43.40 ± 6.991 mV in the female control group, and 56.75 ± 12.06 mV in the female MSEW group), without other changes in electrophysiological properties (Figure 2C,G,H).
3.3. Different alterations in excitability of mPFC GABAergic neurons
Different from the above‐mentioned functional changes in mPFC glutamatergic neurons, MSEW modeling led to multiple changes in mPFC GABAergic neurons.
Under whole‐cell current clamp, the rest membrane potential (RMP) of mPFC GABAergic neurons was decreased in female rats but not male rats, from −69.35 ± 2.669 mV in the female control group to −64.17 ± 4.602 mV in the female MSEW group, facilitating the activation of these neurons (Figure 3A, Table 2).
FIGURE 3.
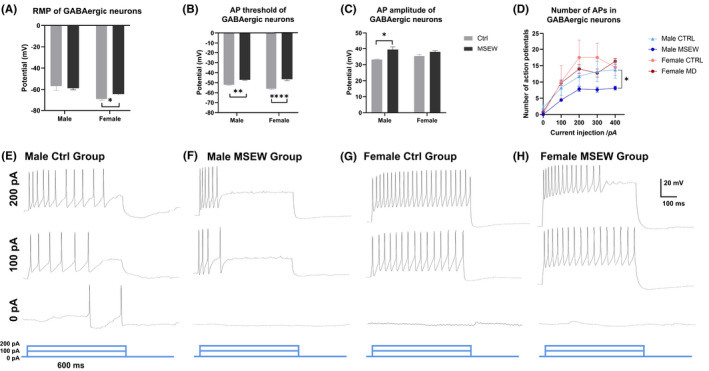
Distinct alterations in GABAergic neuronal excitation between male and female rats. (A) Altered rest membrane potentials of mPFC GABAergic neurons between female control and female MSEW group. (B) Altered threshold of a single action potential of mPFC GABAergic neurons between control and MSEW groups. (C) Increased amplitude of a single action potential of mPFC glutamatergic neurons in male MSEW group. (D) Multiple action potentials of 4 groups. Decreased number of APs in between male groups. (E–H) Representative multiple action potentials of the 4 groups (*p < .05; **p < .01; ****p < .0001, MSEW group compared with control group. N = 10 cells for each group).
TABLE 2.
Summary of intrinsic physiological properties of mPFC GABAergic neurons
| Male control | Male MSEW | Female control | Female MSEW | |
|---|---|---|---|---|
| Rest membrane potential (mV) | −56.78 ± 12.03 | −58.77 ± 5.081 | −69.35 ± 2.669 | −64.17 ± 4.602 a |
| Voltage threshold (mV) | −52.02 ± 0.6987 | −47.11 ± 1.474 ** | −55.97 ± 1.786 | −46.51 ± 2.803 *** |
| Action potential amplitude (mV) | 33.20 ± 0.5492 | 39.40 ± 5.648 a | 35.35 ± 2.143 | 37.93 ± 1.661 |
Note: Data are shown as mean ± SD from 10 mPFC cells from 5 rats.
Control group compared with MSEW group. p < 0.05.
Control group compared with MSEW group. p < 0.01.
Control group compared with MSEW group. p < 0.0001.
For the thresholds of a single action potential, MSEW modeling decreased the threshold in both male and female groups: in male groups, −52.02 ± 0.6987 mV in the male control group and −47.11 ± 1.474 mV in the male MSEW group; in female groups, −55.97 ± 1.786 mV in the female control group and −46.51 ± 2.803 mV in the female MSEW group (Figure 3B).
For the amplitude of a single action potential, MSEW modeling only increased the amplitude in the male group, from 33.20 ± 0.5492 mV in the male control group to 39.40 ± 5.648 mV in the male MSEW group (Figure 3C). To comprehensively analyze intrinsic excitation, we also injected a longer depolarizing current of 600 ms to trigger multiple action potentials. Results demonstrated that in male, but not female, rats, MSEW modeling altered the number of APs, which caused alterations in firing frequency and refractory periods (Figure 3D–F).
4. DISCUSSION
In this work, we chose maternal separation with early weaning (MSEW) as a rodent model of early‐life neglect. Since early‐life stress has shown increasing impact on brain development and physiological functions, we tried to find related brain areas, cell types, and neural electrophysiological mechanisms.
The excitability of neurons can be verified by different aspects. 19 Under the current mode of whole‐cell patch clamp, the firing response of the mPFC neurons to injected depolarizing currents was examined. Rheobase refers to the minimum injected current that can trigger AP. Other membrane properties of AP can also be measured, such as rest membrane potential and input resistance. Besides, for the first AP in a series of multiple APs, the threshold, amplitude, halfwidth, and afterhyperpolarization (AHP) can also be analyzed.
Medial prefrontal cortex (mPFC) consists of excitatory glutamatergic neurons and inhibitory GABAergic neurons. 10 , 20 , 21 Different types of neurons compose excitatory or inhibitory synapses. In our results, male MSEW rats had facilitated glutamatergic neurons, while the GABAergic neurons were relatively stable. In female rats, glutamatergic neurons were stable (except for higher AP amplitude), while triggering of AP in GABAergic neurons was not easy as the threshold changed. Taken together, we found that, although the results looked controversial between male/female and glutamatergic/GABAergic, the overall excitatory/inhibitory (E/I) ratio had similar trends in both male and female rats. 12 Regarding the E/I ratio, the results were similar, but by different implementation: male rats by more excited glutamatergic neurons, female rats by less excited GABAergic neurons. In the future, the exact E/I ratio can be examined by ratio of (m/s) EPSC frequency to (m/s) IPSC frequency. 22
Sex differences in the nervous system due to estrous cycle, sex hormones, and their receptors have gained increasing attention in recent years. 23 Yousuf et al. demonstrated that infralimbic estradiol could enhance neuronal excitability of female rats, 24 which could be a putative reason for the sexually dimorphic effects. Potent estrogen, 17β‐estradiol (E2), can be directly infused into the mPFC area, and has been demonstrated to facilitate the excitation of mPFC neurons. In our research, we found that, in an MSEW rat model, female rats showed decreased excitation in GABAergic neurons and increased AP amplitude of glutamatergic neurons. Combining these 2 alterations, it can be inferred that the excitation of mPFC as a whole can be increased by estrogen. In terms of E/I ratio, estrogen might result in increased excitatory synapse and decreased inhibitory synapse.
In summary, our research found that an early‐life neglect model of rats had different alterations of electrophysiological properties in mPFC glutamatergic and GABAergic neurons: male MSEW rats mainly by glutamatergic neurons, and female MSEW rats by GABAergic neurons.
AUTHOR CONTRIBUTIONS
All listed authors meet the requirements for authorship. Chuan Qin conceived and guided this work. Yu Zhang and Xiuping Sun designed and performed the experiments. Yu Zhang wrote the manuscript. Changsong Dou and Ling Zhang performed and analyzed the electrophysiological data. Xianglei Li managed the animals and performed the modeling. All authors have read and approved the manuscript.
FUNDING INFORMATION
Young Elite Scientist Sponsorship Program by CAST (2019QNRC001); CAMS Innovation Fund for Medical Sciences (CIFMS) (No. 2021‐I2M‐1‐034); National Natural Science Foundation of China (31970510)
CONFLICT OF INTEREST
Yu Zhang is an Editorial Board member of AMEM and a coauthor of this article. To minimize bias, they were excluded from all editorial decision‐making related to the acceptance of this article for publication. Chuan Qin is the Editor‐in‐Chief of AMEM and a co‐author of this article. They were excluded from editorial decision‐making related to the acceptance and publication of this article.
ACKNOWLEDGMENTS
This work is supported by Young Elite Scientist Sponsorship Program by CAST (2019QNRC001); CAMS Innovation Fund for Medical Sciences (CIFMS) (No. 2021‐I2M‐1‐034); National Natural Science Foundation of China (31970510).
Zhang Y, Sun X, Dou C, Li X, Zhang L, Qin C. Distinct neuronal excitability alterations of medial prefrontal cortex in early‐life neglect model of rats. Anim Models Exp Med. 2022;5:274‐280. doi: 10.1002/ame2.12252
Yu Zhang and Xiuping Sun contributed equally to this work.
REFERENCES
- 1. Matjasko JL, Herbst JH, Estefan LF. Preventing adverse childhood experiences: the role of etiological, evaluation, and implementation research. Am J Prev Med. 2022;62(6S1):S6‐S15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Miguel PM, Pereira LO, Silveira PP, Meaney MJ. Early environmental influences on the development of children's brain structure and function. Dev Med Child Neurol. 2019;61(10):1127‐1133. [DOI] [PubMed] [Google Scholar]
- 3. Sinani A, Vassi A, Tsotsokou G, Nikolakopoulou M, Kouvelas ED, Mitsacos A. Early life stress influences basal ganglia dopamine receptors and novel object recognition of adolescent and adult rats. IBRO Neurosci Rep. 2022;12:342‐354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Baracz SJ, Robinson KJ, Wright AL, et al. Oxytocin as an adolescent treatment for methamphetamine addiction after early life stress in male and female rats. Neuropsychopharmacology. 2022. Epub ahead of print. 10.1038/s41386-022-01336-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sanchez EO, Bangasser DA. The effects of early life stress on impulsivity. Neurosci Biobehav Rev. 2022;137:104638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Palamarchuk IS, Vaillancourt T. Integrative brain dynamics in childhood bullying victimization: cognitive and emotional convergence associated with stress psychopathology. Front Integr Neurosci. 2022;16:782154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dalley JW, Ersche KD. Neural circuitry and mechanisms of waiting impulsivity: relevance to addiction. Philos Trans R Soc Lond B Biol Sci. 2019;374(1766):20180145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mcklveen JM, Myers B, Flak JN, et al. Role of prefrontal cortex glucocorticoid receptors in stress and emotion. Biol Psychiatry. 2013;74(9):672‐679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mcklveen JM, Myers B, Herman JP. The medial prefrontal cortex: coordinator of autonomic, neuroendocrine and behavioural responses to stress. J Neuroendocrinol. 2015;27(6):446‐456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Liu J, Dietz K, Deloyht JM, et al. Impaired adult myelination in the prefrontal cortex of socially isolated mice. Nat Neurosci. 2012;15(12):1621‐1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sun X, Zhang Y, Li X, Liu X, Qin C. Early‐life neglect alters emotional and cognitive behavior in a sex‐dependent manner and reduces glutamatergic neuronal excitability in the prefrontal cortex. Front Psych. 2020;11:572224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chen Y, Zheng Y, Yan J, et al. Early life stress induces different behaviors in adolescence and adulthood may related with abnormal medial prefrontal cortex excitation/inhibition balance. Front Neurosci. 2021;15:720286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Macarthur Clark JA, Sun D. Guidelines for the ethical review of laboratory animal welfare People's Republic of China National Standard GB/T 35892‐2018 [Issued 6 February 2018 Effective from 1 September 2018]. Animal Model Exp Med. 2020;3(1):103‐113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. George ED, Bordner KA, Elwafi HM, Simen AA. Maternal separation with early weaning: a novel mouse model of early life neglect. BMC Neurosci. 2010;11:123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zhang Y, Jiang YY, Shao S, et al. Inhibiting medial septal cholinergic neurons with DREADD alleviated anxiety‐like behaviors in mice. Neurosci Lett. 2017;638:139‐144. [DOI] [PubMed] [Google Scholar]
- 16. Liu Y, Lai S, Ma W, et al. CDYL suppresses epileptogenesis in mice through repression of axonal Nav1.6 sodium channel expression. Nat Commun. 2017;8(1):355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rossignol E. Genetics and function of neocortical GABAergic interneurons in neurodevelopmental disorders. Neural Plast. 2011;2011:649325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dupuis JP, Feyder M, Miguelez C, et al. Dopamine‐dependent long‐term depression at subthalamo‐nigral synapses is lost in experimental parkinsonism. J Neurosci. 2013;33(36):14331‐14341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lei Y, Wang J, Wang D, et al. SIRT1 in forebrain excitatory neurons produces sexually dimorphic effects on depression‐related behaviors and modulates neuronal excitability and synaptic transmission in the medial prefrontal cortex. Mol Psychiatry. 2020;25(5):1094‐1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zhang W, Daly KM, Liang B, et al. BDNF rescues prefrontal dysfunction elicited by pyramidal neuron‐specific DTNBP1 deletion in vivo. J Mol Cell Biol. 2017;9(2):117‐131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zhang C, Chen RX, Zhang Y, et al. Reduced GABAergic transmission in the ventrobasal thalamus contributes to thermal hyperalgesia in chronic inflammatory pain. Sci Rep. 2017;7:41439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bassetti D, Luhmann HJ, Kirischuk S. Presynaptic GABAB receptor‐mediated network excitation in the medial prefrontal cortex of Tsc2(+/−) mice. Pflugers Arch. 2021;473(8):1261‐1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wang C, Zhang Y, Shao S, Cui S, Wan Y, Yi M. Ventral hippocampus modulates anxiety‐like behavior in male but not female C57BL/6J mice. Neuroscience. 2019;418:50‐58. [DOI] [PubMed] [Google Scholar]
- 24. Yousuf H, Smies CW, Hafenbreidel M, et al. Infralimbic estradiol enhances neuronal excitability and facilitates extinction of cocaine seeking in female rats via a BDNF/TrkB mechanism. Front Behav Neurosci. 2019;13:168. [DOI] [PMC free article] [PubMed] [Google Scholar]


