Abstract
Pulmonary hypertension due to left heart disease (PH‐LHD) is regarded as the most prevalent form of pulmonary hypertension (PH). Indeed, PH is an independent risk factor and predicts adverse prognosis for patients with left heart disease (LHD). Clinically, there are no drugs or treatments that directly address PH‐LHD, and treatment of LHD alone will not also ameliorate PH. To target the underlying physiopathological alterations of PH‐LHD and to develop novel therapeutic approaches for this population, animal models that simulate the pathophysiology of PH‐LHD are required. There are several available models for PH‐LHD that have been successfully employed in rodents or large animals by artificially provoking an elevated pressure load on the left heart, which by transduction elicits an escalated pressure in pulmonary artery. In addition, metabolic derangement combined with aortic banding or vascular endothelial growth factor receptor antagonist is also currently applied to reproduce the phenotype of PH‐LHD. As of today, none of the animal models exactly recapitulates the condition of patients with PH‐LHD. Nevertheless, the selection of an appropriate animal model is essential in basic and translational studies of PH‐LHD. Therefore, this review will summarize the characteristics of each PH‐LHD animal model and discuss the advantages and limitations of the different models.
Keywords: animal model, left ventricular failure, metabolic syndrome, pulmonary hypertension due to left heart disease, pulmonary vascular remodeling
This schematic overview (created with BioRender.com) illustrates that PH‐LHD animal models are established by (1) pressure overload‐induced LV failure including aortic banding (AoB), transverse aortic constriction (TAC), pulmonary vein banding (PVB) and left atrium stenosis (LAS); (2) ischemic heart failure via ligation of left coronary artery; and (3) metabolic disturbance induced by high fat diet (HFD) alone or with pressure overload‐induced LV failure, or the combination of olanzapine and pressure overload‐induced LV failure, or by genetic predisposition of cardiometabolic syndrome and SU5416.
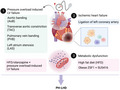
1. INTRODUCTION
Pulmonary hypertension (PH) is a progressive cardiovascular disorder of multiple etiologies that typically ends in death due to right heart failure (HF). Based on the World Health Organization (WHO) classification of PH, it is divided into 5 groups, of which PH due to left heart disease (PH‐LHD) is the commonest. PH‐LHD is described as a PH subtype with mean pulmonary artery pressure (mPAP) >25 mmHg and pulmonary artery wedge pressure (PAWP) >15 mmHg, 1 although the threshold values of PAWP are controversial. 2
HF caused by LHD is mainly manifested as reduced ejection fraction (HFrEF), with preserved ejection fraction (HFpEF) and left‐sided heart valve disease (VHD). 3 The occurrence of PH in patients with LHD is a sign of disease progression. 4 Nevertheless, therapeutic agents directly targeting patients with PH‐LHD are not available. Although there have been some studies supporting the treatment of PH‐LHD with targeted drugs for PAH, none has been applied to clinical treatment of PH‐LHD. 5 , 6 , 7 , 8 , 9 , 10 , 11 , 12 , 13 , 14 , 15 , 16 Effective treatments for LHD do not seem to improve PH‐LHD, 17 because PH‐LHD is divided hemodynamically into pure postcapillary PH and combined pre‐ and postcapillary PH. 18 , 19 The biggest difference lies in pure postcapillary PH simply being a bruise in the pulmonary veins, while the latter tends to occur with pulmonary artery vascular remodeling. Normally, they are difficult to distinguish from each other on the basis of clinical phenotype or symptoms. Some studies found that diastolic pulmonary vascular pressure gradient 20 , 21 , 22 , 23 , 24 , 25 may be indicative of whether PH‐LHD is accompanied by pulmonary vascular remodeling.
Hence, more in‐depth studies are necessary to address the pathophysiology of PH‐LHD and its pathogenic cellular and molecular signaling mechanisms, such as the increase in capillary pressure in the lungs caused by LHD affecting the dysregulation of endothelial homeostasis. 26 Not much is known about the complex lesions and disease progression of PH‐LHD, and an animal model that fully reproduces the pathological progression of human PH‐LHD is still unavailable. Typically, PH‐LHD animal models are mostly simulated models of PH due to HF. Moreover, the majority of patients with PH‐LHD have metabolic syndrome manifestations, 27 and recently there have been in vivo experiments to generate animal models of PH by simulating the metabolic syndrome of PH‐LHD. Such studies will broaden our knowledge of PH‐LHD with animal models that more closely approximate the disease, which will in turn lead to the development of better model options for preclinical treatments. The purpose of this review is to summarize the animal models of PH‐LHD available at present and to outline the advantages and limitations of each model.
2. PH FROM PRESSURE OVERLOAD‐INDUCED LEFT‐VENTRICULAR (LV) FAILURE
LV failure can be induced by several pressure overload entities. The banding position for PH‐LHD in animal models is summarized in Figure 1, which will be further discussed, and Table 1 lists the advantages and pitfalls of all PH‐LHD animal models for various animal species. The left ventricle is highly sensitive to a rapid increment in afterload stress. In the majority of models, this causes a sudden rise in LV afterload that induces acute left HF, which may result in death in some animals. However, the phenotype of hemodynamics, including mPAP and/or right ventricular systolic pressure (RVSP), is different among various animal species. The main type commonly used at present is aortic banding or ascending aortic banding models.
FIGURE 1.
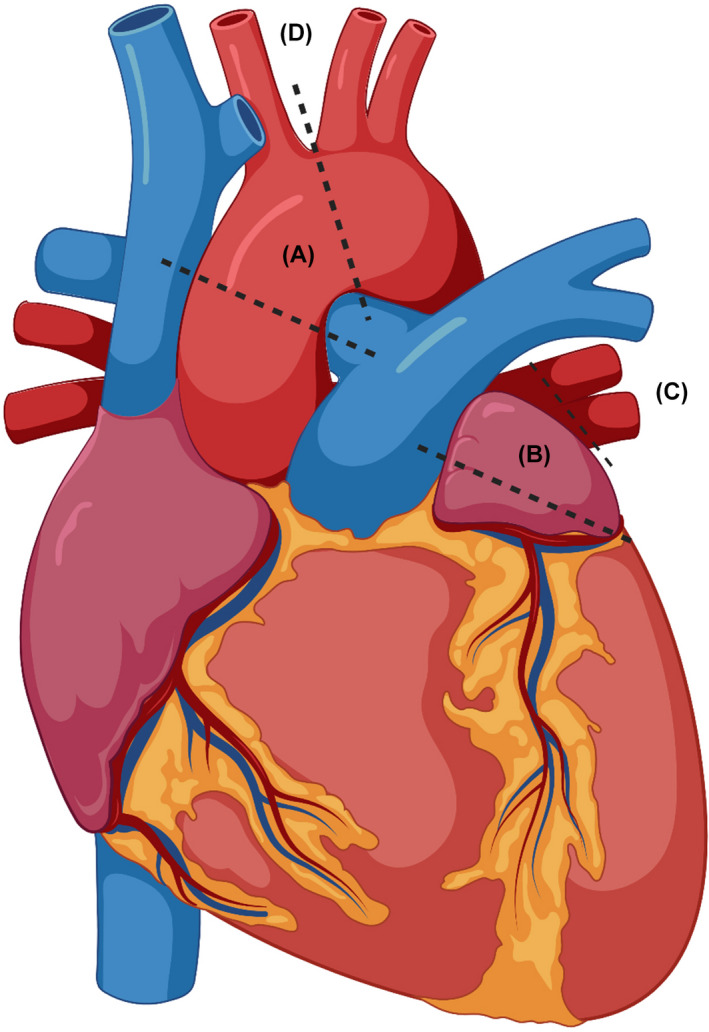
Overview of the banding for PH‐LHD animal models. (A) Aortic banding; (B) left atrium; (C) pulmonary vein; (D) transverse aortic constriction. Created with BioRender.com
TABLE 1.
Animal models of PH‐LHD
| Method | Strengths | Limitations | Time | RVSP/mPAP | Species |
|---|---|---|---|---|---|
| Aortic banding (AoB) |
Simple; reproducible; commonly used; simulates HFpEF; low cost |
Sudden initial afterload increased predisposition to left heart failure and death; difficult to induce severe RV failure; PH due to aortic stenosis is uncommon in human patients |
7 wk 39 ; |
RVP (cmH2O) ≈35 34 ; RVSP (mmHg) ≈36 36 ; ≈40 39 ; mPAP (mmHg) ≈24 41 ; ≈16 33 ; ≈30 35 ; ≈33 37 ; ≈42 38 ; ≈18 86 ; ≈32 87 ; ≈22. 85 |
Rat 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 85 , 86 ; kitten 87 |
| Transverse aortic constriction (TAC) |
Especially for mice; simulates HFpEF; low cost |
Sudden initial afterload increased predisposition to left heart failure and death; infrequent; PH due to aortic stenosis is uncommon in human patients |
6 wk 5 ; |
RVSP (mmHg) ≈40. 47 |
Mouse 5 , 46 , 47 |
| Descending aorta implanted by stent |
Large animal; simulates HFpEF |
Operative difficulties; high cost; long time |
5 months 43 |
RVSP (mmHg) 51. 43 |
Swine 43 |
| Left atrial stenosis |
Less complicated; low cost; mimics PH‐LHD caused by mitral valve lesions |
Surgery bleeding; difficult to estimate LA pressure |
10 wk 52 |
RVSP (mmHg) 41. 52 |
Rat 52 , 53 |
| Pulmonary vein banding |
Large animal; simulation of congenital pulmonary vein stenosis; low mortality rate; reproducible |
Complicated; high cost |
10 wk 88 ; 3 months 50 |
mPAP (mmHg) ≈40, 88 ≈33. 50 |
Swine 50 , 88 , 89 , 90 |
| Ligation of left coronary artery |
Mimics HFrEF‐PH; low cost; simple |
Difficult to control the area of infarcts (size of pathological infarction only sufficient to develop HFrEF‐PH) |
5 wk 60 ; 8 wk 92 ; 17 d 64 |
RVSP (mmHg) ≈52 58 ; ≈25. 64 |
Rat 58 , 60 , 61 , 62 , 91 , 92 ; swine 64 |
| High‐fat diets (HFD) |
Simple; mimics HFpEF‐PH |
Long time |
16 wk 71 |
RVSP (mmHg) ≈40 65 ; ≈32. 71 |
Mouse 65 , 66 , 71 |
| Combined metabolic syndrome and pressure overload‐induced PH‐LHD |
Mimics EFpHF‐PH; closer to clinical patients with PH‐LHD; double hit |
Complicated phenotype; high cost |
9 wk 68 |
mPAP (mmHg) ≈30. 68 |
Rat 68 |
| Combined metabolic syndrome and SU5416‐induced PH‐LHD |
Mimic EFpHF‐PH; double hit; closer to clinical patients with PH‐LHD |
Complicated phenotype; high cost |
14 wk 71 , 93 |
RVSP (mmHg) |
Rat 71 , 93 |
2.1. Aortic banding (AoB)
AoB is a simple, ancient, and common surgical procedure in which a band is clamped around the aorta to increase afterload. In its early state, it was mainly used in studies focusing on cardiac dysfunction in hypertension, 28 myocardia hypertrophy, 29 or cardiac pressure‐overload hypertrophy. 30 In 2001, Backs et al. applied the AoB model to study congestive HF. 31 The surgical procedure consisted mainly of ascending aortic binding in Wistar rats weighing about 90 g. The material used was a tantalum hemostatic clip (internal diameter 0.71 mm), and 4 weeks after surgery, it was evident that the left and right ventricular weight increased, and pulmonary stasis was noticeable compared with the sham group. Although RVSP and mPAP were not measured when the AoB rat model was first described, they could be predicted to be elevated based on the changes in right ventricular remodeling.
In principle, AoB mimics increased LV afterload, which passively causes an increase in LV ejection pressure and pressure conduction, leading to increased pulmonary venous pressure, eventually leading to chronic right HF and PH. In 2007, Hentschel et al. further modified an AoB animal model with a 0.8 mm diameter titanium clip, and hemodynamics were examined 9 weeks later. Compared with the sham group, the AoB rat model exhibited elevated mPAP, pulmonary vascular resistance (PVR), and left atrial pressure. 32 In 2009, this research team used this rat model to study PH‐LHD and found that inhaled nitric oxide improved pulmonary hemodynamics and pulmonary edema in group 2 PH. 33 This animal model was further promoted, and aortic banding in rats is at present the most commonly used animal model to study PH‐LHD, 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 although the timing of the banding varies depending on the operator's experience.
Due to its simplicity, feasibility, and high reproductivity, AoB is commonly used in rat models to produce mild or medium PH‐LHD depending on the aortic banding time according to the hemodynamics characteristics summarized in Table 1. Aortic banding is usually utilized in young rats. Given that the chance of death due to acute left HF after ligation might increase in older rats, the rats are generally required to be 80–100 g. As the rats grow, the banding becomes tighter, which causes severe LHD and PH.
In recent years, attempts to develop large animal models of left ventricular pressure afterload have been emerging. Gyöngyösi et al. 43 implanted a bare metal stent (9 mm in diameter) into the descending aorta (immediately below the aortic arch) of pigs by left heart catheter insertion. At a 5‐month follow‐up, there was a significant increase in mPAP, RVSP, right ventricle mass, and LV hypertrophy as well as a trend toward decreased right heart function. Of note, this is a large animal model with a minimally invasive percutaneous method to slowly induce LV hypertrophy and secondary PH, which adds great translational value for novel target identification due to its simulation of human conditions.
2.2. Transverse aortic constriction (TAC)
Another common model for pressure‐load‐induced HF in mice is TAC. In 1991, Rockman et al. 44 first applied this model to the study of cardiomyocyte hypertrophy in mice by the ligation of transverse aorta using a 27 G blunt needle positioned between the innominate artery and the left carotid artery, which over a 6‐week period established a mouse PH model caused by left HF with medium hemodynamic compromise (RVSP 35–40 mmHg). 45 , 46 After TAC in mice, left ventricular insufficiency, right ventricular hypertrophy, increased RVSP, and elevated mPAP develop over time, accompanied by an increased degree of pulmonary fibrosis and infiltration of inflammatory cells. 47 It has been demonstrated that the use of riociguat (a soluble guanylate cyclase agonist) or sildenafil (phosphodiesterase 5 inhibitor) improves pulmonary vascular remodeling and right heart function in TAC‐induced PH‐LHD. 5 TAC in mice is not extremely challenging operationally and has a relatively high successful rate (80%–90%) with practice. In line with aortic banding, mice undergoing TAC also experience a sudden elevation in LV pressure, and acute left HF is more likely to occur if older mice undergo the procedure, so it is recommended to use younger mice, about 3–4 months of age. 45
2.3. Pulmonary vein banding (PVB)
To establish PVB model, the confluence of 2 main pulmonary veins is in principle nonrestrictively banded by surgery to narrow the pulmonary veins in experimental animals, causing obstruction of the pulmonary circulation flow and enhanced pulmonary venous afterload, followed by an increase in PAP and the eventual development of right HF. In 1972, Silove et al. applied a calf model of PVB to the study of progressive PH. 48 In 1990, LaBourene et al. first used pulmonary vein ligation in piglets to study fibrosis of pulmonary veins. 49 On this basis, in 2014, Pereda et al. first utilized this PVB surgery in a porcine model to simulate a part of the disease physiopathological process in PH‐LHD. 50 In the same year they described for the first time a swine model of pulmonary vein tying, they were the first to use large animals in the study of postcapillary PH as well as combined pre‐ and postcapillary or active PH. 51
This model is generally applied to large animals, such as pigs. The observation period after surgery is usually 4 months, during which right heart catheterization is performed regularly, and it is the most common large animal model currently mimicking group 2 PH with medium hemodynamic compromise (mPAP 33–40 mmHg). The advantage of pulmonary vein narrowing is that it can simulate an animal model of PH caused by congenital pulmonary vein stenosis. The disadvantage lies in the high expense, and the procedure requires multiple operators.
2.4. Left atrium stenosis (LAS)
In 2017, Yoshitaka et al. proposed the first LAS surgery in rat and successfully established a new PH‐LHD rat model for the study of arterialization of the internal pulmonary veins. 52 Those rats phenocopied medium PH‐LHD as evidenced by an increase of RVSP to 41 mmHg. The model is operated by opening the chest and ligating left atrium using a larger clip under direct vision. 53 One study found that LAS surgery simulated pulmonary venous arterialization and PH caused by the mitral stenosis. Echocardiographic findings post‐modeling revealed a significant increase in mitral inflow velocity in rats receiving LAS surgery compared with those without, which is the first study to apply this model in PH caused by heart valve disease. 53 It was also verified that increased left atrial area on computed tomography (CT) or magnetic resonance imaging (MRI) can predict the diagnosis of PH‐LHD in patients. 54 , 55 The LAS model is mainly applied in small animals of rats, which likewise adds a new option for the study of the molecular mechanisms of pulmonary venous arterialization.
3. ISCHEMIC HEART FAILURE‐INDUCED PH
Myocardial infarction models are the most widely used models in studies of cardiac ischemia, in which left anterior descending coronary artery was commonly ligated to induce myocardial infarction. This has been applied to the study of ventricular function since as early as the 1970s. 56 However, the application of this model to the study of PH‐LHD has drawn attention in recent years. In 1998, Quang et al. 57 found that endothelin A receptor blockade did not abolish pulmonary fibrosis and vasculature remodeling in a rat model of myocardial infarction despite improvement in mPAP. 58 , 59 The reason for coronary artery ligation inducing PH might be that it simulates HF caused by low cardiac output or low ejection fraction, which mimics the manifestation of PH. Normally, it takes approximately 5–8 weeks to develop PH for this model.
This model is commonly used in small animals such as rats 60 , 61 , 62 or mice, 63 but can also be applied to large animals such as swine. 64 The left coronary circumflex artery can also be chosen for ligation, although it is less frequently selected compared with the left anterior descending coronary artery. This PH‐LHD rodent models by ligation of left coronary artery displayed a medium to severe hemodynamic compromise with an increase of RVSP to the range of 40–52 mmHg, although swine exhibited only a mild PH phenotype developed by this method owing to a short observation period and species‐specific physiology. The main disadvantage of left anterior descending ligation is the low postoperative survival rate, mainly due to surgery‐induced cardiac arrhythmias in animals, bleeding, or death caused by pneumothorax. This model requires rigorous training and experience to ensure a high survival rate for animals undergoing the procedure. The strengths of a myocardial infarction‐induced PH model are attributable to its recapitulation of HFrEF in humans, especially for patients with myocardial infarction resulting in myocardial cell necrosis, HF, and, finally, group 2 PH.
4. METABOLIC MODELS OF PH‐LHD
Metabolic syndrome is observed in the majority of patients with PH‐LHD 27 and includes hypertension, hyperlipidemia, and diabetes mellitus. Animal models of metabolic syndrome are new models that have been gradually developed in recent years compared with aforementioned PH models induced by HF.
4.1. High‐fat diets (HFD)
In 2016, Meng et al. 65 first described that HFD could induce PH‐LHD in animals, reporting development of heart failure with manifestations of PH in mice on a HFD for 20 weeks. This noninvasive method is simple and convenient, but it takes time for the mice to develop the phenotype of mild to medium PH‐LHD. Of note, on genetic screening for preclinical HFD‐induced PH models, epidermal growth factor receptor (EGFR) was identified to associate with PH susceptibility induced by HFD. 66 However, further investigation into the role of EGFR in the development of PH and its interplay with metabolic crosstalk per se in this model is warranted to prove a cause‐and‐effect relationship. The HFD‐induced PH‐LHD mouse model, mostly seen in PH associated with HFpEF, is the classic model for studying this type of disease, while large animal models of PH associated with obesity‐induced LHD also exist, such as in cattle. 67
4.2. Combined metabolic syndrome and pressure overload‐induced PH‐LHD
In 2019, Ranchoux et al. first described a complicated model of PH‐LHD in rats together with AoB causing left ventricular diastolic dysfunction followed by HFD or intraperitoneal injection of olanzapine for 9 weeks. 68 This model combines metabolic syndrome and left ventricular diastolic dysfunction for the first time, and the experimental rats exhibit increased PVR and pulmonary vascular remodeling, more closely simulating PH caused by HFpEF. Their study showed that metabolic syndrome significantly promoted diastolic dysfunction and remodeling of pulmonary vasculature in rats. Moreover, they confirmed that metformin not only improved the inflammatory environment of PH‐LHD, but also mitigated pulmonary vascular remodeling in this PH‐LHD model. 69 However, the hemodynamic characteristics demonstrated a mild elevation of mPAP to approximately 30 mmHg at the end point, slightly higher than that of the AoB‐only model, despite obvious lung vasculature remodeling as evidenced by a vascular wall thickness triple that in control rats. This would suggest this model might be appropriate for the study of mild PH‐LHD rather than severe cases, and metformin may be a new promising therapeutic agent for those patients with mild PH‐LHD accompanied by metabolic syndrome.
4.3. Combined metabolic syndrome and SU5416‐induced PH‐LHD
In 2016, Lai et al. first used a genetic rat model to study PH‐LHD. They generated a double‐hit rat model, combining metabolic syndrome as a consequence of obese ZSF1 with double‐leptin receptor defect and pulmonary endothelial impairment by vascular endothelial growth factor receptor antagonist SU5416 for the first time, and found that RVSP was significantly increased to around 40 mmHg and the hemodynamic characteristics in rats were similar to the clinical features of human HFpEF‐induced PH, 70 indicating this model might be appropriate for the study of medium PH‐LHD. It is currently the only genetic rat model that can be used in the study of PH‐LHD with metabolic syndrome. Importantly, treprostinil together with metformin is able to improve HFpEF‐induced PH, alongside ameliorating metabolic syndrome. 71
5. DIFFERENCES BETWEEN ANIMAL MODELS AND HUMAN PH‐LHD
The distinctions between animal models and PH‐LHD patients are not well studied. Some studies differ with regard to the timing of modeling in the AoB model, with one study reporting 25 days to produce right heart dysfunction, 37 but most of the AoB models are established at 9 weeks post‐banding, which corresponds to the long time it takes for patients to develop PH‐LHD and the right heart failure as a late response to left heart failure. PH‐LHD patients with right heart failure sometimes manifest clinical signs of remission, when in fact the disease is further aggravated. The AoB model mainly recapitulates PH due to aortic stenosis. Nevertheless, its application to the study of other subtypes of PH‐LHD (e.g., PH‐LHD due to congenital cardiomyopathies) is challenging. For the mouse model of myocardial infarction‐induced PH, the difference lies in the infarction area in the mouse model being relatively fixed, while there is no strict pattern of infarction region in patients with PH‐LHD. Finally, in most patients, PH‐LHD is accompanied by metabolic syndrome, but it remains to be elucidated whether it is the metabolic syndrome affecting left or right heart function or LHD causing the metabolic syndrome. However, in the PH‐LHD metabolic disorder model, a high‐fat diet is usually applied to induce metabolic derangement, which might be inconsistent with the patients' conditions.
6. CONCLUSION AND PROSPECTS
Currently, therapies to improve or treat the long‐term prognosis of PH‐LHD are limited, with the diseased subpopulation occupying the largest proportion in PH. Despite the increasing understanding of the subgroup classification of PH‐LHD and the continued development of animal models, many challenges remain in uncovering the pathophysiological mechanisms and in developing effective treatments for the disease because of the complexity of LHD, PH, and the metabolic disorders in PH‐LHD. In preclinical studies of PH‐LHD, none of the current available animal models, including aortic banding, left coronary artery ligation, and the recent combination of metabolic syndrome with aortic banding or vascular endothelial growth factor receptor blocker (Figure 2), can fully characterize PH‐LHD and simulate the development of the disease. Although an attempt to apply an animal model of volume loading‐induced heart failure 72 to the study of PH‐LHD has been proposed, its incompatibility with the pathological process of the disease does not yet allow its mainstreaming. Considering PH‐LHD caused by myxomatous mitral valve disease occurring in dogs, 73 its predominant echocardiography findings do not support its establishment as a stable model. Although metabolomics has been employed to in vivo models of PH challenged by monocrotaline, hypoxia, or SU5416/hypoxia at the tissue or cellular level or in vitro models (such as pulmonary arterial smooth muscle cells under the stimulus of hypoxia), 74 , 75 , 76 , 77 , 78 to date, the metabolic reprogramming of animal models mimicking PH‐LHD remains unveiled. Hence the application of metabolomics to the PH‐LHD would provide critical information for our understanding of the metabolic mechanisms underlying PH‐LHD, especially with metabolic perturbations.
FIGURE 2.
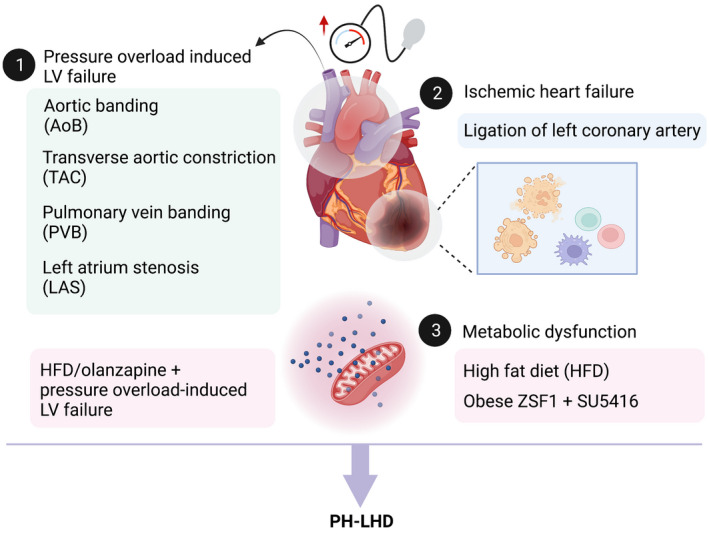
Currently available animal models of PH‐LHD. At present, PH‐LHD animal models are established by (1) pressure overload‐induced LV failure including aortic banding (AoB), transverse aortic constriction (TAC), pulmonary vein banding (PVB), and left atrium stenosis (LAS); (2) ischemic heart failure via ligation of left coronary artery; and (3) metabolic disturbance induced by high‐fat diet (HFD) alone or with pressure overload‐induced LV failure, or the combination of olanzapine and pressure overload‐induced LV failure, or by genetic predisposition of cardiometabolic syndrome and SU5416. Created with BioRender.com
Aside from the triggers of overload‐induced LV failure, ischemic heart failure, or metabolic dysfunction, the genetic predisposition to PH‐LHD at a large scale will require further investigation with the aid of next‐generation sequencing, such as bone morphogenetic protein receptor 2 (BMPR2) as a pivotal predisposing gene for pulmonary arterial hypertension (PAH, group 1 PH). It is reported that BMPR2‐deficient rats or Bmpr2+/ R899X knock‐in mice display a spontaneous pulmonary vascular remodeling and have been widely applied to explore the pathogenesis of PAH. 79 , 80 The generation of a new genetic rodent model might represent a promising tool to study the pathogenesis of PH‐LHD and whether it has a synergistic effect with other factors (such as metabolic dysfunction). In fact, the role of epigenetic regulation (e.g., DNA methylation, 81 histone acetylation, 82 and noncoding RNAs 83 ) has been investigated in multiple PH animal models induced by either monocrotaline or hypoxia. However, its role in animal models of PH‐LHD remains largely unknown. Furthermore, the study of specific gene profiling of PH‐LHD in comparison with other PH subtypes or LHD would be of great benefit to the discovery of potential targets and, thus, the generation of PH‐LHD induced by genetic factors either alone or by double hits. Given that sex hormones are associated with insulin resistance and metabolic syndrome, 84 future studies on PH‐LHD evaluating whether there exists a gender bias and investigating the detrimental or protective role of sex hormones are warranted. In addition, the differences in right ventricular adaptation/decompensation pattern of PH‐LHD and other PH subtypes should be scrutinized as well, since LHD might affect the right ventricle much earlier.
A more stable and reproducible animal model is a requisite for the identification of novel and effective therapeutic approaches for PH‐LHD treatment, and the development of an appropriate preclinical model of PH‐LHD is urgently needed, which would allow us to better understand this disease and thus accelerate the development of new therapies.
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
AUTHOR CONTRIBUTIONS
S.‐F. L. conceived and wrote the original draft manuscript and provided funding support. Y. Yan conceived and revised the manuscript.
ACKNOWLEDGMENTS
None.
Liu S‐F, Yan Y. Animal models of pulmonary hypertension due to left heart disease. Anim Models Exp Med. 2022;5:197–206. doi: 10.1002/ame2.12214
Funding information
The study was funded by the China Scholarship Council (CSC) (no. 202108080221).
Contributor Information
Shao‐Fei Liu, Email: shaofei.liu@charite.de.
Yi Yan, Email: yi.yan@med.uni-muenchen.de.
REFERENCES
- 1. Simonneau G, Gatzoulis MA, Adatia I, et al. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol. 2013;62:D34‐D41. doi: 10.1016/j.jacc.2013.10.029 [DOI] [PubMed] [Google Scholar]
- 2. Xu D, Zhang H, Cheng H, et al. Pulmonary hypertension due to left heart disease with pulmonary arterial wedge pressure ≤15mmHg. Herz. 2021;46:209‐214. doi: 10.1007/s00059-020-04983-3 [DOI] [PubMed] [Google Scholar]
- 3. Fang JC, DeMarco T, Givertz MM, et al. World Health Organization Pulmonary Hypertension group 2: pulmonary hypertension due to left heart disease in the adult—a summary statement from the Pulmonary Hypertension Council of the International Society for Heart and Lung Transplantation. J Heart Lung Transplant. 2012;31:913‐933. doi: 10.1016/j.healun.2012.06.002 [DOI] [PubMed] [Google Scholar]
- 4. Dzudie A, Kengne AP, Thienemann F, Sliwa K. Predictors of hospitalisations for heart failure and mortality in patients with pulmonary hypertension associated with left heart disease: a systematic review. BMJ Open. 2014;4:e004843. doi: 10.1136/bmjopen-2014-004843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Pradhan K, Sydykov A, Tian X, et al. Soluble guanylate cyclase stimulator riociguat and phosphodiesterase 5 inhibitor sildenafil ameliorate pulmonary hypertension due to left heart disease in mice. Int J Cardiol. 2016;216:85‐91. doi: 10.1016/j.ijcard.2016.04.098 [DOI] [PubMed] [Google Scholar]
- 6. Kido K, Coons JC. Efficacy and safety of the use of pulmonary arterial hypertension pharmacotherapy in patients with pulmonary hypertension secondary to left heart disease: a systematic review. Pharmacotherapy. 2019;39:929‐945. doi: 10.1002/phar.2314 [DOI] [PubMed] [Google Scholar]
- 7. Han B, Wang Q. Study on the clinical efficacy of specific phosphodiesterase inhibitor in patients with pulmonary hypertension due to left heart disease. Exp Ther Med. 2018;16:1175‐1186. doi: 10.3892/etm.2018.6310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bermejo J, Yotti R, García‐Orta R, et al. Sildenafil for improving outcomes in patients with corrected valvular heart disease and persistent pulmonary hypertension: a multicenter, double‐blind, randomized clinical trial. Eur Heart J. 2018;39:1255‐1264. doi: 10.1093/eurheartj/ehx700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jiang R, Wang L, Zhu CT, et al. Comparative effectiveness of sildenafil for pulmonary hypertension due to left heart disease with HFrEF. Hypertens Res. 2015;38:829‐839. doi: 10.1038/hr.2015.73 [DOI] [PubMed] [Google Scholar]
- 10. Moghaddam N, Swiston JR, Tsang MYC, et al. Impact of targeted pulmonary arterial hypertension therapy in patients with combined post‐ and precapillary pulmonary hypertension. Am Heart J. 2021;235:74‐81. doi: 10.1016/j.ahj.2021.01.003 [DOI] [PubMed] [Google Scholar]
- 11. Vachiéry JL, Delcroix M, Al‐Hiti H, et al. Macitentan in pulmonary hypertension due to left ventricular dysfunction. Eur Respir J. 2018;51:1701886. doi: 10.1183/13993003.01886-2017 [DOI] [PubMed] [Google Scholar]
- 12. Hoendermis ES, Liu LCY, Hummel YM, et al. Effects of sildenafil on invasive haemodynamics and exercise capacity in heart failure patients with preserved ejection fraction and pulmonary hypertension: a randomized controlled trial. Eur Heart J. 2015;36:2565‐2573. doi: 10.1093/eurheartj/ehv336 [DOI] [PubMed] [Google Scholar]
- 13. Wu X, Yang T, Zhou Q, Li S, Huang L. Additional use of a phosphodiesterase 5 inhibitor in patients with pulmonary hypertension secondary to chronic systolic heart failure: a meta‐analysis. Eur J Heart Fail. 2014;16:444‐453. doi: 10.1002/ejhf.47 [DOI] [PubMed] [Google Scholar]
- 14. Bonderman D, Ghio S, Felix SB, et al. Riociguat for patients with pulmonary hypertension caused by systolic left ventricular dysfunction: a phase IIb double‐blind, randomized, placebo‐controlled, dose‐ranging hemodynamic study. Circulation. 2013;128:502‐511. doi: 10.1161/CIRCULATIONAHA.113.001458 [DOI] [PubMed] [Google Scholar]
- 15. Simon MA, Vanderpool RR, Nouraie M, et al. Acute hemodynamic effects of inhaled sodium nitrite in pulmonary hypertension associated with heart failure with preserved ejection fraction. JCI Insight. 2016;1:e89620. doi: 10.1172/jci.insight.89620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sun D, Yang W, Wang Z, Gao B. Efficacy of beraprost sodium combined with sildenafil and its effects on vascular endothelial function and inflammation in patients experiencing left heart failure complicated with pulmonary arterial hypertension. Med Sci Monit. 2021;27:e928413. doi: 10.12659/MSM.928413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Domingo E, Grignola JC, Trujillo P, Aguilar R, Roman A. Proximal pulmonary arterial wall disease in patients with persistent pulmonary hypertension after successful left‐sided valve replacement according to the hemodynamic phenotype. Pulm Circ. 2019;9:2045894018816972. doi: 10.1177/2045894018816972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ibe T, Wada H, Sakakura K, et al. Combined pre‐ and post‐capillary pulmonary hypertension: the clinical implications for patients with heart failure. PLoS One. 2021;16:e0247987. doi: 10.1371/journal.pone.0247987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gerges C, Gerges M, Fesler P, et al. In‐depth haemodynamic phenotyping of pulmonary hypertension due to left heart disease. Eur Respir J. 2018;51:1800067. doi: 10.1183/13993003.00067-2018 [DOI] [PubMed] [Google Scholar]
- 20. Ibe T, Wada H, Sakakura K, et al. Pulmonary hypertension due to left heart disease: the prognostic implications of diastolic pulmonary vascular pressure gradient. J Cardiol. 2016;67:555‐559. doi: 10.1016/j.jjcc.2015.07.015 [DOI] [PubMed] [Google Scholar]
- 21. Dragu R, Hardak E, Ohanyan A, Adir Y, Aronson D. Prognostic value and diagnostic properties of the diastolic pulmonary pressure gradient in patients with pulmonary hypertension and left heart disease. Int J Cardiol. 2019;290:138‐143. doi: 10.1016/j.ijcard.2019.05.016 [DOI] [PubMed] [Google Scholar]
- 22. Caravita S, Faini A, Carolino D'Araujo S, et al. Clinical phenotypes and outcomes of pulmonary hypertension due to left heart disease: role of the pre‐capillary component. PLoS One. 2018;13:e0199164. doi: 10.1371/journal.pone.0199164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Nagy AI, Venkateshvaran A, Merkely B, Lund LH, Manouras A. Determinants and prognostic implications of the negative diastolic pulmonary pressure gradient in patients with pulmonary hypertension due to left heart disease. Eur J Heart Fail. 2017;19:88‐97. doi: 10.1002/ejhf.675 [DOI] [PubMed] [Google Scholar]
- 24. Howard C, Rangajhavala K, Safdar Z. Pulmonary artery diastolic pressure gradient as an indicator of severity of illness in patients with pulmonary hypertension related to left‐sided heart disease. Ther Adv Respir Dis. 2015;9:35‐41. doi: 10.1177/1753465815573373 [DOI] [PubMed] [Google Scholar]
- 25. Sugimoto K, Yoshihisa A, Nakazato K, et al. Significance of pulmonary vascular resistance and diastolic pressure gradient on the new definition of combined post‐capillary pulmonary hypertension. Int Heart J. 2020;61:301‐307. doi: 10.1536/ihj.19-476 [DOI] [PubMed] [Google Scholar]
- 26. Dayeh NR, Ledoux J, Dupuis J. Lung capillary stress failure and arteriolar remodelling in pulmonary hypertension associated with left heart disease (Group 2 PH). Prog Cardiovasc Dis. 2016;59:11‐21. doi: 10.1016/j.pcad.2016.05.002 [DOI] [PubMed] [Google Scholar]
- 27. Ussavarungsi K, Thomas CS, Burger CD. Prevalence of metabolic syndrome in patients with pulmonary hypertension. Clin Respir J. 2017;11:721‐726. doi: 10.1111/crj.12406 [DOI] [PubMed] [Google Scholar]
- 28. Schunkert H, Jackson B, Tang SS, et al. Distribution and functional significance of cardiac angiotensin converting enzyme in hypertrophied rat hearts. Circulation. 1993;87:1328‐1339. doi: 10.1161/01.cir.87.4.1328 [DOI] [PubMed] [Google Scholar]
- 29. Kromer EP, Riegger GA. Effects of long‐term angiotensin converting enzyme inhibition on myocardial hypertrophy in experimental aortic stenosis in the rat. Am J Cardiol. 1988;62:161‐163. doi: 10.1016/0002-9149(88)91389-6 [DOI] [PubMed] [Google Scholar]
- 30. Bruckschlegel G, Holmer SR, Jandeleit K, et al. Blockade of the renin‐angiotensin system in cardiac pressure‐overload hypertrophy in rats. Hypertension. 1995;25:250‐259. doi: 10.1161/01.hyp.25.2.250 [DOI] [PubMed] [Google Scholar]
- 31. Backs J, Haunstetter A, Gerber SH, et al. The neuronal norepinephrine transporter in experimental heart failure: evidence for a posttranscriptional downregulation. J Mol Cell Cardiol. 2001;33:461‐472. doi: 10.1006/jmcc.2000.1319 [DOI] [PubMed] [Google Scholar]
- 32. Hentschel T, Yin N, Riad A, et al. Inhalation of the phosphodiesterase‐3 inhibitor milrinone attenuates pulmonary hypertension in a rat model of congestive heart failure. Anesthesiology. 2007;106:124‐131. doi: 10.1097/00000542-200701000-00021 [DOI] [PubMed] [Google Scholar]
- 33. Yin N, Kaestle S, Yin J, et al. Inhaled nitric oxide versus aerosolized iloprost for the treatment of pulmonary hypertension with left heart disease. Crit Care Med. 2009;37:980‐986. doi: 10.1097/CCM.0b013e3181962ce6 [DOI] [PubMed] [Google Scholar]
- 34. Huang W, Liu H, Pan Y, Yang H, Lin J, Zhang H. Mechanical stretching of the pulmonary vein mediates pulmonary hypertension due to left heart disease by regulating SAC/MAPK pathway and the expression of IL‐6 and TNF‐alpha. J Cardiothorac Surg. 2021;16:127. doi: 10.1186/s13019-021-01471-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zhuang R, Wu J, Lin F, et al. Fasudil preserves lung endothelial function and reduces pulmonary vascular remodeling in a rat model of endstage pulmonary hypertension with left heart disease. Int J Mol Med. 2018;42:1341‐1352. doi: 10.3892/ijmm.2018.3728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zhang H, Huang W, Liu H, Zheng Y, Liao L. Mechanical stretching of pulmonary vein stimulates matrix metalloproteinase‐9 and transforming growth factor‐beta1 through stretch‐activated channel/MAPK pathways in pulmonary hypertension due to left heart disease model rats. PLoS One. 2020;15:e0235824. doi: 10.1371/journal.pone.0235824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wang Q, Guo YZ, Zhang YT, et al. The effects and mechanism of atorvastatin on pulmonary hypertension due to left heart disease. PLoS One. 2016;11:e0157171. doi: 10.1371/journal.pone.0157171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hunt JM, Bethea B, Liu X, et al. Pulmonary veins in the normal lung and pulmonary hypertension due to left heart disease. Am J Physiol Lung Cell Mol Physiol. 2013;305:L725‐736. doi: 10.1152/ajplung.00186.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lambert M, Mendes‐Ferreira P, Ghigna MR, et al. Kcnk3 dysfunction exaggerates the development of pulmonary hypertension induced by left ventricular pressure overload. Cardiovasc Res. 2021;117:2474‐2488. doi: 10.1093/cvr/cvab016 [DOI] [PubMed] [Google Scholar]
- 40. Zhang YT, Xue JJ, Wang Q, et al. Dehydroepiandrosterone attenuates pulmonary artery and right ventricular remodeling in a rat model of pulmonary hypertension due to left heart failure. Life Sci. 2019;219:82‐89. doi: 10.1016/j.lfs.2018.12.056 [DOI] [PubMed] [Google Scholar]
- 41. Chen IC, Tan MS, Wu BN, et al. Statins ameliorate pulmonary hypertension secondary to left ventricular dysfunction through the Rho‐kinase pathway and NADPH oxidase. Pediatr Pulmonol. 2017;52:443‐457. doi: 10.1002/ppul.23610 [DOI] [PubMed] [Google Scholar]
- 42. Dai ZK, Tan MS, Chai CY, et al. Upregulation of endothelial nitric oxide synthase and endothelin‐1 in pulmonary hypertension secondary to heart failure in aorta‐banded rats. Pediatr Pulmonol. 2004;37:249‐256. doi: 10.1002/ppul.10413 [DOI] [PubMed] [Google Scholar]
- 43. Gyöngyösi M, Pavo N, Lukovic D, et al. Porcine model of progressive cardiac hypertrophy and fibrosis with secondary postcapillary pulmonary hypertension. J Transl Med. 2017;15:202. doi: 10.1186/s12967-017-1299-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Rockman HA, Ross RS, Harris AN, et al. Segregation of atrial‐specific and inducible expression of an atrial natriuretic factor transgene in an in vivo murine model of cardiac hypertrophy. Proc Natl Acad Sci USA. 1991;88:8277‐8281. doi: 10.1073/pnas.88.18.8277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. deAlmeida AC, van Oort RJ, Wehrens XH. Transverse aortic constriction in mice. J Vis Exp. 2010;(38):1729. doi: 10.3791/1729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Chen JJ, Ma WM, Yuan JL, Cui LQ. PM2.5 exposure aggravates left heart failure induced pulmonary hypertension. Acta Cardiol. 2019;74:238‐244. doi: 10.1080/00015385.2018.1488568 [DOI] [PubMed] [Google Scholar]
- 47. Chen Y, Guo H, Xu D, et al. Left ventricular failure produces profound lung remodeling and pulmonary hypertension in mice: heart failure causes severe lung disease. Hypertension. 2012;59:1170‐1178. doi: 10.1161/HYPERTENSIONAHA.111.186072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Silove ED, Tavernor WD, Berry CL. Reactive pulmonary arterial hypertension after pulmonary venous constriction in the calf. Cardiovasc Res. 1972;6:36‐44. doi: 10.1093/cvr/6.1.36 [DOI] [PubMed] [Google Scholar]
- 49. LaBourene JI, Coles JG, Johnson DJ, et al. Alterations in elastin and collagen related to the mechanism of progressive pulmonary venous obstruction in a piglet model. A hemodynamic, ultrastructural, and biochemical study. Circ Res. 1990;66:438‐456. doi: 10.1161/01.res.66.2.438 [DOI] [PubMed] [Google Scholar]
- 50. Pereda D, García‐Alvarez A, Sánchez‐Quintana D, et al. Swine model of chronic postcapillary pulmonary hypertension with right ventricular remodeling: long‐term characterization by cardiac catheterization, magnetic resonance, and pathology. J Cardiovasc Transl Res. 2014;7:494‐506. doi: 10.1007/s12265-014-9564-6 [DOI] [PubMed] [Google Scholar]
- 51. Aguero J, Ishikawa K, Hadri L, et al. Characterization of right ventricular remodeling and failure in a chronic pulmonary hypertension model. Am J Physiol Heart Circ Physiol. 2014;307:H1204‐H1215. doi: 10.1152/ajpheart.00246.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Fujimoto Y, Urashima T, Kawachi F, et al. Pulmonary hypertension due to left heart disease causes intrapulmonary venous arterialization in rats. J Thorac Cardiovasc Surg. 2017;154:1742–1753.e8. doi: 10.1016/j.jtcvs.2017.06.053 [DOI] [PubMed] [Google Scholar]
- 53. Xiong PY, Baba S, Nishioka N, et al. Left atrial stenosis induced pulmonary venous arterialization and group 2 pulmonary hypertension in rat. J Vis Exp. 2018;(141):e58787. doi: 10.3791/58787 [DOI] [PubMed] [Google Scholar]
- 54. Currie BJ, Johns C, Chin M, et al. CT derived left atrial size identifies left heart disease in suspected pulmonary hypertension: derivation and validation of predictive thresholds. Int J Cardiol. 2018;260:172‐177. doi: 10.1016/j.ijcard.2018.02.114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Aviram G, Rozenbaum Z, Ziv‐Baran T, et al. Identification of pulmonary hypertension caused by left‐sided heart disease (World Health Organization Group 2) based on cardiac chamber volumes derived from chest CT imaging. Chest. 2017;152:792‐799. doi: 10.1016/j.chest.2017.04.184 [DOI] [PubMed] [Google Scholar]
- 56. Pfeffer MA, Pfeffer JM, Fishbein MC, et al. Myocardial infarct size and ventricular function in rats. Circ Res. 1979;44:503‐512. doi: 10.1161/01.res.44.4.503 [DOI] [PubMed] [Google Scholar]
- 57. Nguyen QT, Cernacek P, Calderoni A, et al. Endothelin A receptor blockade causes adverse left ventricular remodeling but improves pulmonary artery pressure after infarction in the rat. Circulation. 1998;98:2323‐2330. doi: 10.1161/01.cir.98.21.2323 [DOI] [PubMed] [Google Scholar]
- 58. Nguyen QT, Colombo F, Rouleau JL, Dupuis J, Calderone A. LU135252, an endothelin(A) receptor antagonist did not prevent pulmonary vascular remodelling or lung fibrosis in a rat model of myocardial infarction. Br J Pharmacol. 2000;130:1525‐1530. doi: 10.1038/sj.bjp.0703466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Jiang BH, Tardif JC, Shi Y, Dupuis J. Bosentan does not improve pulmonary hypertension and lung remodeling in heart failure. Eur Respir J. 2011;37:578‐586. doi: 10.1183/09031936.00053710 [DOI] [PubMed] [Google Scholar]
- 60. Nguyen QT, Nsaibia MJ, Sirois MG, et al. PBI‐4050 reduces pulmonary hypertension, lung fibrosis, and right ventricular dysfunction in heart failure. Cardiovasc Res. 2020;116:171‐182. doi: 10.1093/cvr/cvz034 [DOI] [PubMed] [Google Scholar]
- 61. Reichert K, Colantuono B, McCormack I, et al. Murine left anterior descending (LAD) coronary artery ligation: an improved and simplified model for myocardial infarction. J Vis Exp. 2017;(122):55353. doi: 10.3791/55353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Ravi Y, Selvendiran K, Naidu SK, et al. Pulmonary hypertension secondary to left‐heart failure involves peroxynitrite‐induced downregulation of PTEN in the lung. Hypertension. 2013;61:593‐601. doi: 10.1161/HYPERTENSIONAHA.111.00514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Dayeh NR, Tardif JC, Shi Y, et al. Echocardiographic validation of pulmonary hypertension due to heart failure with reduced ejection fraction in mice. Sci Rep. 2018;8:1363. doi: 10.1038/s41598-018-19625-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Houweling B, Merkus D, Sorop O, Boomsma F, Duncker DJ. Role of endothelin receptor activation in secondary pulmonary hypertension in awake swine after myocardial infarction. J Physiol. 2006;574:615‐626. doi: 10.1113/jphysiol.2006.107060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Meng Q, Lai YC, Kelly NJ, et al. Development of a mouse model of metabolic syndrome, pulmonary hypertension, and heart failure with preserved ejection fraction. Am J Respir Cell Mol Biol. 2017;56:497‐505. doi: 10.1165/rcmb.2016-0177OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Kelly NJ, Radder JE, Baust JJ, et al. Mouse genome‐wide association study of preclinical group II pulmonary hypertension identifies epidermal growth factor receptor. Am J Respir Cell Mol Biol. 2017;56:488‐496. doi: 10.1165/rcmb.2016-0176OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Krafsur GM, Neary JM, Garry F, et al. Cardiopulmonary remodeling in fattened beef cattle: a naturally occurring large animal model of obesity‐associated pulmonary hypertension with left heart disease. Pulm Circ. 2019;9:2045894018796804. doi: 10.1177/2045894018796804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Ranchoux B, Nadeau V, Bourgeois A, et al. Metabolic syndrome exacerbates pulmonary hypertension due to left heart disease. Circ Res. 2019;125:449‐466. doi: 10.1161/CIRCRESAHA.118.314555 [DOI] [PubMed] [Google Scholar]
- 69. Mulkareddy V, Simon MA. Metformin in pulmonary hypertension in left heart disease. Front Med. 2020;7:425. doi: 10.3389/fmed.2020.00425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Lai Y‐C, Tabima DM, Dube JJ, et al. SIRT3‐AMP‐activated protein kinase activation by nitrite and metformin improves hyperglycemia and normalizes pulmonary hypertension associated with heart failure with preserved ejection fraction. Circulation. 2016;133:717‐731. doi: 10.1161/CIRCULATIONAHA.115.018935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Wang L, Halliday G, Huot JR, et al. Treatment with treprostinil and metformin normalizes hyperglycemia and improves cardiac function in pulmonary hypertension associated with heart failure with preserved ejection fraction. Arterioscler Thromb Vasc Biol. 2020;40:1543‐1558. doi: 10.1161/ATVBAHA.119.313883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Alvarez DF, King JA, Townsley MI. Resistance to store depletion‐induced endothelial injury in rat lung after chronic heart failure. Am J Respir Crit Care Med. 2005;172:1153‐1160. doi: 10.1164/rccm.200506-847OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Suzuki R, Yuchi Y, Kanno H, et al. Pulmonary vascular resistance estimated by echocardiography in dogs with myxomatous mitral valve disease and pulmonary hypertension probability. Front Vet Sci. 2021;8:771726. doi: 10.3389/fvets.2021.771726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Zhao JH, He YY, Guo SS, et al. Circulating plasma metabolomic profiles differentiate rodent models of pulmonary hypertension and idiopathic pulmonary arterial hypertension patients. Am J Hypertens. 2019;32:1109‐1117. doi: 10.1093/ajh/hpz121 [DOI] [PubMed] [Google Scholar]
- 75. Li M, Riddle S, Zhang H, et al. Metabolic reprogramming regulates the proliferative and inflammatory phenotype of adventitial fibroblasts in pulmonary hypertension through the transcriptional corepressor C‐terminal binding protein‐1. Circulation. 2016;134:1105‐1121. doi: 10.1161/CIRCULATIONAHA.116.023171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Graham BB, Kumar R, Mickael C, et al. Vascular adaptation of the right ventricle in experimental pulmonary hypertension. Am J Respir Cell Mol Biol. 2018;59:479‐489. doi: 10.1165/rcmb.2018-0095OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Izquierdo‐Garcia JL, Arias T, Rojas Y, et al. Metabolic reprogramming in the heart and lung in a murine model of pulmonary arterial hypertension. Front Cardiovasc Med. 2018;5:110. doi: 10.3389/fcvm.2018.00110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. He YY, Xie XM, Zhang HD, et al. Identification of hypoxia induced metabolism associated genes in pulmonary hypertension. Front Pharmacol. 2021;12:753727. doi: 10.3389/fphar.2021.753727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Ranchoux B, Antigny F, Rucker‐Martin C, et al. Endothelial‐to‐mesenchymal transition in pulmonary hypertension. Circulation. 2015;131:1006‐1018. doi: 10.1161/CIRCULATIONAHA.114.008750 [DOI] [PubMed] [Google Scholar]
- 80. Hautefort A, Mendes‐Ferreira P, Sabourin J, et al. Bmpr2 mutant rats develop pulmonary and cardiac characteristics of pulmonary arterial hypertension. Circulation. 2019;139:932‐948. doi: 10.1161/CIRCULATIONAHA.118.033744 [DOI] [PubMed] [Google Scholar]
- 81. Yan YI, He YY, Jiang X, et al. DNA methyltransferase 3B deficiency unveils a new pathological mechanism of pulmonary hypertension. Sci Adv. 2020;6:eaba2470. doi: 10.1126/sciadv.aba2470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Zhao L, Chen CN, Hajji N, et al. Histone deacetylation inhibition in pulmonary hypertension: therapeutic potential of valproic acid and suberoylanilide hydroxamic acid. Circulation. 2012;126:455‐467. doi: 10.1161/CIRCULATIONAHA.112.103176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Omura J, Habbout K, Shimauchi T, et al. Identification of long noncoding RNA H19 as a New biomarker and therapeutic target in right ventricular failure in pulmonary arterial hypertension. Circulation. 2020;142:1464‐1484. doi: 10.1161/CIRCULATIONAHA.120.047626 [DOI] [PubMed] [Google Scholar]
- 84. Muller M, Grobbee DE, den Tonkelaar I, Lamberts SW, van der Schouw YT. Endogenous sex hormones and metabolic syndrome in aging men. J Clin Endocrinol Metab. 2005;90:2618‐2623. doi: 10.1210/jc.2004-1158 [DOI] [PubMed] [Google Scholar]
- 85. Chen IC, Lin JY, Liu YC, et al. The beneficial effects of angiotensin‐converting enzyme II (ACE2) activator in pulmonary hypertension secondary to left ventricular dysfunction. Int J Med Sci. 2020;17:2594‐2602. doi: 10.7150/ijms.48096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Yin J, Kukucka M, Hoffmann J, et al. Sildenafil preserves lung endothelial function and prevents pulmonary vascular remodeling in a rat model of diastolic heart failure. Circ Heart Fail. 2011;4:198‐206. doi: 10.1161/CIRCHEARTFAILURE.110.957050 [DOI] [PubMed] [Google Scholar]
- 87. Wallner M, Eaton DM, Berretta RM, et al. A feline HFpEF model with pulmonary hypertension and compromised pulmonary function. Sci Rep. 2017;7:16587. doi: 10.1038/s41598-017-15851-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Duin RWB, Stam K, Cai Z, et al. Transition from post‐capillary pulmonary hypertension to combined pre‐ and post‐capillary pulmonary hypertension in swine: a key role for endothelin. J Physiol. 2019;597:1157‐1173. doi: 10.1113/JP275987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Bikou O, Ishikawa K, Fish KM, Zarragoikoetxea I, Hajjar RJ, Aguero J. Modeling pulmonary hypertension: a pig model of postcapillary pulmonary hypertension. Methods Mol Biol. 2018;1816:367–383. doi: 10.1007/978-1-4939-8597-5_29 [DOI] [PubMed] [Google Scholar]
- 90. García‐Álvarez A, Pereda D, García‐Lunar I, et al. Beta‐3 adrenergic agonists reduce pulmonary vascular resistance and improve right ventricular performance in a porcine model of chronic pulmonary hypertension. Basic Res Cardiol. 2016;111:49. doi: 10.1007/s00395-016-0567-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Jasmin JF, Mercier I, Hnasko R, et al. Lung remodeling and pulmonary hypertension after myocardial infarction: pathogenic role of reduced caveolin expression. Cardiovasc Res. 2004;63:747‐755. doi: 10.1016/j.cardiores.2004.05.018 [DOI] [PubMed] [Google Scholar]
- 92. Jasmin JF, Calderone A, Leung TK, Villeneuve L, Dupuis J. Lung structural remodeling and pulmonary hypertension after myocardial infarction: complete reversal with irbesartan. Cardiovasc Res. 2003;58:621‐631. doi: 10.1016/s0008-6363(03)00290-6 [DOI] [PubMed] [Google Scholar]
- 93. Satoh T, Wang L, Espinosa‐Diez C, et al. Metabolic syndrome mediates ROS‐miR‐193b‐NFYA‐dependent downregulation of soluble guanylate cyclase and contributes to exercise‐induced pulmonary hypertension in heart failure with preserved ejection fraction. Circulation. 2021;144:615‐637. doi: 10.1161/CIRCULATIONAHA.121.053889 [DOI] [PMC free article] [PubMed] [Google Scholar]


