Abstract
Background
The objective of this study was to validate an animal model for dry eye during and after the administration of 1% ophthalmic atropine sulfate (OAS) in New Zealand white (NZW) rabbits.
Methods
OAS (1%) was applied three times per day to 30 eyes of 15 healthy NZW rabbits. Sacrifice, enucleation, and lacrimal gland removal took place on days 15, 21, and 30 (OAS group). A second group (n = 5) was used as control. Clinical evaluations took place on days 3, 10, 15, 18, 21, 24 and 30. The primary endpoints were: Schirmer I test, tear break‐up time (TBUT), and corneal fluorescein staining. As secondary endpoints, clinical changes including intraocular pressure, and histopathology were evaluated.
Results
While OAS was administered, the Schirmer I test showed a statistically significant reduction for OAS group versus control (p < 0.001), and versus basal production (p < 0.001). TBUT showed statistically significant differences between groups (days 3 and 10; p = 0.001) and versus basal values (day 3; p < 0.001). Fluorescein staining showed a statistically significant difference (day 3; p = 0.001). The most frequent clinical finding was conjunctival hyperemia (76.9% OAS vs. 20% control). For histopathology, all OAS subjects presented some degree of inflammation (86.7% minimal; 13.3% mild) whereas the control presented only 30% minimal inflammation. Goblet cell density showed no difference.
Conclusions
The effectiveness of the OAS dry eye model in NZW rabbits as reported in previous studies was confirmed, provided that the application of the drug is maintained throughout the intervention; it is not a viable model after OAS administration is suspended.
Keywords: atropine sulfate, dry eye, rabbit model, safety, Schirmer I test, TBUT
Administration of 1% ophthalmic atropine sulfate (OAS) three times per day (t.i.d.) for 14 days to 30 eyes of 15 healthy New Zealand white (NZW) rabbits. No eye drops were applied in the control group, n = 5 NZW rabbits (1). Clinical evaluations: Schirmer I test, tear break‐up time (TBUT), corneal fluorescein staining, indirect fundoscopy and the intraocular pressure, took place on days: 3, 10, 15, 18, 21, 24 and 30 (2). Histopathology evaluations (cornea, conjunctiva, ciliary body, retina, optic nerve, lacrimal glands, and goblet cell density) were evaluated on days: 15, 21, and 30 (3).
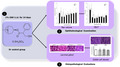
1. INTRODUCTION
Tear film stability is one of the most important characteristics of a healthy ocular surface, since it allows it to perform some of its main functions, such as building an adequate refractive surface, as well as providing protection and moisture to the cornea. When the homeostasis of this layer is lost, dry eye disease (DED) appears. 1
DED is a multifactorial disease that affects millions of people around the world, resulting in costs calculated in at 3.8 billion dollars annually in the United States alone. 2 It is a symptomatic disease characterized by a vicious circle that includes tear film instability, hyperosmolarity, and inflammation of the ocular surface. 3 It is one of the main causes of ophthalmologic consultation due to the associated pain, constraints on performing everyday activities, reduction of vision and even depression associated with the disease. 1
The use of in vivo preclinical models to study this disease allows for a detailed evaluation of the pathological mechanisms that cause the clinical presentation of this condition, as well as the exploration of relevant risk factors and efficacy of lubricant eyedrops. 4 , 5
Multiple animal models have been described and created to emulate different kinds of DED. For instance, for evaluation of non‐Sjögren dry eye, models include a stress desiccating technique where elevated air flow with low relative humidity, with or without muscarinic blockage, have been used. Previously described dry eye models also include use of specific mouse strains such as C57BL/6 and/or surgical techniques such as injection of lacrimal glands with concanavalin A and extirpation of the nictitating membrane and lacrimal glands. 1 , 4 , 5 Meanwhile, Sjögren's syndrome has been evaluated using mouse strains such as C57BL/6, CD25KO, NOD.B10.H2b, MRL/lpr, and Meibomian gland disfunction has been studied in primate, rabbit, and mouse models either by evaluation of HR‐1, GHA y GHR KO mouse strains, by generating the obstruction of these glands’ orifices after exposure to a lipid deficient diet, or through the use of chemical interventions with compounds like isotretinoin and epinephrine. 1 , 4
However, these models can be expensive, generally difficult to use, require hard‐to‐attain special animal strains and specialized equipment, and frequently require long periods of time for the dry eye signs to arise. 4 Finally, the administration of antimuscarinic medications such as atropine sulfate and the subsequent neural control interruption of lacrimal secretion has also been used as a DED model. The cholinergic antagonist receptor (M3) atropine has been used in previous works to decrease aqueous production and modify tear stability in rabbits. 6 Systemic administration of these compounds has not been viable or effective; however, its topical application has been reported as a simple and useful model for ocular lubricant evaluation, during the intervention period. 1 , 4 , 7
Burgalassi and collaborators have described the use of 1% ophthalmic atropine sulfate (OAS) in New Zealand white (NZW) rabbits as a dry eye model. With the objective of evaluating the protective activity of ocular lubricants against signs associated with DED, OAS was applied three times a day (t.i.d.) for 5 days in addition to one of the studied lubricants. 8 Similarly, Crawford and collaborators used this model to evaluate a formulation's efficacy for DE treatment through the application of OAS and either the studied lubricant or its vehicle for a total of 13 days. 9 Other authors, such as El‐Shazly and collaborators, 10 and Bucolo and collaborators, 6 , 7 also applied 1% OAS t.i.d. to NZW rabbits for the duration of their studies, either alone or concomitantly with the drugs whose efficacy was to be tested. In all these studies, a decrease in tear volume production (evaluated through Schirmer I) and the alteration of the integrity of the ocular surface (as evidenced via corneal staining assessment) were observed during the exposure to OAS. 10
The effect of 1% OAS has also been studied previously in dogs. Laus and collaborators evaluated the lacrimal production of 20 animals during a 14‐day intervention with application of one drop of 1% OAS once a day (q.d.). Schirmer I tests were conducted 1 hour before and every 3 hours after the instillation. Furthermore, the tear production continued to be evaluated daily using the same method for 16 consecutive days. The results showed that even though the tear production recovered gradually after the suspension of the drug, a difference in tear production volume was still evident during the last period of the study. 11
Likewise, Hollingsworth and collaborators studied 19 dogs exposed to one eye drop of OAS q.d. for 14 days. During this period, there was a progressive reduction of tear production until the last visit, 1 day after the last instillation. In addition, during a control visit 5 weeks after the last application, even when tear volume production showed a tendency to recovery, it was still statistically significantly difference to basal values. 12
Based on this evidence, the purpose of the present study was to validate a dry eye rabbit model during and after administration of 1% ophthalmic atropine sulfate in NZW rabbits.
2. METHODS
2.1. Animals and experimental procedures
All procedures adhered to the guidelines from the Association for Research in Vision and Ophthalmology (ARVO) resolution on the use of animals in ophthalmic and vision research, and approval was obtained from the Institutional Animal Care and Use Committee of Laboratorios Sophia, SA de CV (CICUALLS, approval number: PREC0620/PI‐08/13/20). A total of 20 male NZW rabbits, 2‐3 months of age and weighing between 2 and 3 kg were acclimatized for at least 7 days before the experiment under a 12/12‐hour light/dark cycle with free access to food and water. To estimate the number of animals required, a resource equation method was implemented to calculate the sample size needed to obtain reliable data and with the purpose of avoiding the use of a number of animals greater than that strictly necessary to obtain reliable data. 13 , 14 , 15 Eye drops containing 1% atropine sulphate (Atro Ofteno®, Laboratorios Sophia, SA de CV, Zapopan, Mexico) were administered into the lower conjunctival sac of both eyes t.i.d. for 14 days, in the OAS group (n = 15). No eye drops were applied in the normal control group (n = 5). To assess the degree of DE 48 hours after OAS instillation, tear volume (Schirmer I), TBUT, and corneal fluorescein staining were used as primary endpoints. The OAS group was further divided into three subgroups (5 animals/subgroup), being sacrificed on days 15, 21 and 30.
2.2. Ophthalmological examination
An ophthalmic eligibility screening with a slit lamp (Luxvision®, Class I Type B, Doral FL, USA) and fluorescein staining was performed to ensure there were no clinical exclusion criteria present (presence of secretion, conjunctival hyperemia, corneal or conjunctival lacerations, corneal degeneration, lens opacity, fundus alterations, etc.) or any pathological findings in the indirect fundoscopy performed with a 78D lens (Ocular Instruments, Bellevue, WA, USA). Examinations were performed on all eyes before the study began and on days 1, 3, 10, 15, 18, 21, 24, and 30 using the following tests.
Tear volume (Schirmer I). Schirmer I test (without anesthesia) was performed to measure the rise length of the tear using Schirmer strips (Tear‐Flo™, HUB Pharmaceuticals, UK). The strips were carefully placed in the lower fornix for a period of 5 minutes, and the wetted area was then measured in millimeters.
Tear breakup time (TBUT). Invasive TBUT was performed after the instillation of one drop of sodium fluorescein (4 mg, BioGlo™, HUB Pharmaceuticals, UK) diluted with 5 mL of 0.5% ophthalmic tetracaine (Ponti Ofteno®, Laboratorios Sophia, SA de CV, Zapopan, Mexico) in both rabbit eyes. A slit lamp with cobalt blue light filter was used to enhance fluorescein patterns; TBUT was determined by timing the appearance of the first dark spot or streak on the cornea after the last blink.
Corneal fluorescein staining. After the TBUT evaluation, the corneal surface was observed with the slit lamp biomicroscope. The numbers of stained corneal quadrants (superior temporal, inferior temporal, superior nasal, and inferior nasal) were recorded. Surface dye staining was graded from 0 to III (0 = no staining, I = mild, II = moderate, and III = severe) in accordance with the percentage of affected area (National Eye Institute/Workshop on Clinical Trials in Dry eyes). 16
Clinical observations. The anterior and posterior segments were evaluated by indirect fundoscopy, and the intraocular pressure (IOP) was measured with a Goldmann tonometer (Luxvision®, YZ30, Doral FL, USA).
Histopathological evaluations. The animals were euthanized on days 15 (OAS group, n = 5), 21 (OAS group, n = 5), and 30 (OAS group, n = 5; control group n = 5) by CO2 administration. Eyes were enucleated and lacrimal glands removed. Enucleated eyes and extracted lacrimal glands were fixed and preserved separately in 4% paraformaldehyde for subsequent paraffin embedding, section preparation and staining with hematoxylin and eosin (HE), as well as with periodic acid‐Shiff (PAS). Histological changes in the following structures were evaluated by microscopy (40x): cornea, conjunctiva, ciliary body, retina, optic nerve, and lacrimal glands. The goblet cell density in the conjunctiva was evaluated with PAS staining, and the average number of goblet cells was calculated with a 40x objective (ocular 18 × 22) as a percentage of epithelial cells. A minimum of 100 cells were counted in the same anatomical portion of the conjunctiva at the margin of the cornea. Both margins were evaluated. The higher value was selected for analysis.
2.3. Statistical analyses
Analyses of data were carried out using SPSS 19.0 software for Windows (SPSS Inc., Chicago, IL, USA). Microsoft® Office Excel 2016 was used for data processing. Since measurements obtained from right and left eyes are usually correlated, 15 , 17 data from both eyes were averaged to give a single data point per subject. 18 , 19 All data are expressed as mean ±standard deviation (SD) unless indicated otherwise. Statistical significance was determined using a Mann‐Whitney U test for continuous data, and Pearson's chi‐square test or Fisher's exact test for categorical data. All statistical analyses performed in this study with p values ≤ 0.05 were considered statistically significant.
3. RESULTS
No baseline significant differences were found among the two experimental groups. Data were obtained from 40 eyes of 20 NZW rabbits. No signs of ocular inflammation or adverse events were observed.
3.1. Tear production (Schirmer I test)
No significant differences in the mean value ±SD were identified between the control group and the OAS group at baseline (18.10 ± 4.08 mm vs. 20.00 ± 4.07 mm, p = 0.315). Lacrimal production was evaluated during the period of product instillation (14 days) on days 3 and 10, and after OAS discontinuation on days 15, 18, 21, 24 and 30. Only for the first two evaluations (days 3 and 10) was a significant difference found between the control group and OAS‐exposed subjects (24.40 ± 4.42 mm vs. 13.00 ± 4.70 mm, p = 0.001; 21.80 ± 4.01 mm vs. 12.83 ± 4.49 mm, p = 0.005, respectively). No difference between groups was present on days 10 through 30 (see Figure 1).
FIGURE 1.
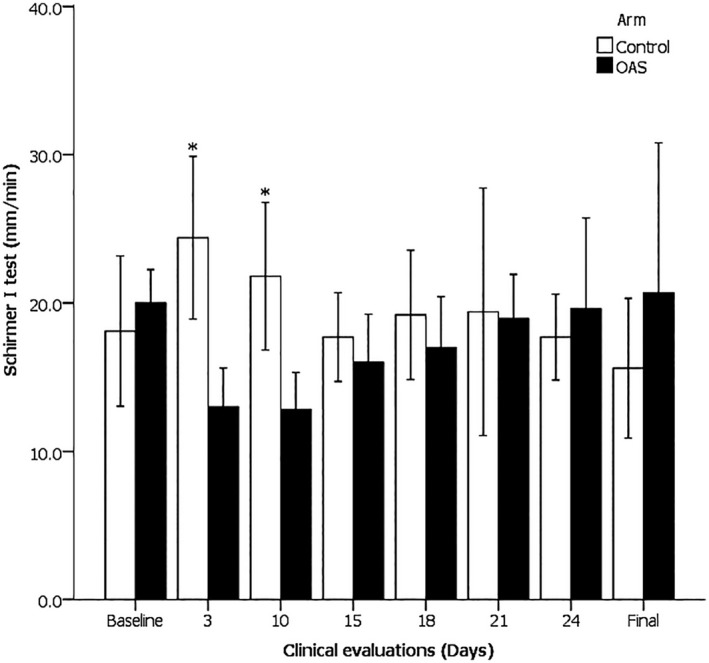
Tear production of the control and 1% ocular atropine sulfate (OAS) groups at measurement times in response to topically applied atropine (3 and 10), and 1‐15 d after treatment (15‐final). On days 3 and 10, Control > OAS, *p ≤ 0.005. Mean value ± 95% CI
Likewise, it was only for the first two evaluations on days 3 and 10 that a significant difference was observed when comparing these values to the basal data (6.30 ± 7.05 mm versus −7.00 ± 3.97 mm, p = 0.003; 3.70 ± 6.68 mm vs. −7.17 ± 2.89 mm; p = 0.016, respectively). The lacrimal production was not different from the basal value after 7 or 14 days subsequent to the suspension of OAS application.
3.2. Tear break‐up time (TBUT)
Similarly to the Schirmer I test, one of the other primary endpoints, the only TBUT evaluations in which a statistically significant difference between the control group and subjects exposed to OAS was seen were those taking place on days 3 and 10 (37.20 ± 8.37 seconds vs. 13.93 ± 4.44 seconds, p = 0.001; 21.20 ± 3.33 seconds versus 11.10 ± 2.20 seconds, p = 0.001, respectively). The TBUT was not different in OAS subjects compared to the control group on day 15 nor after 7 or 14 days of the suspension of OAS application (see Figure 2).
FIGURE 2.
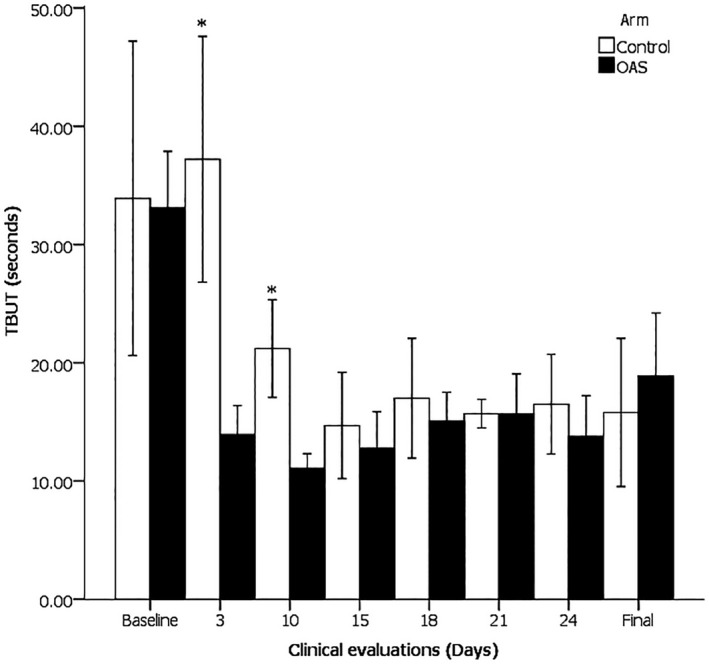
Tear break‐up time (TBUT), in response to topically applied atropine (3 and 10), and 1‐15 d after treatment (15‐final). On days 3 and 10, Control > OAS, *p = 0.001. Mean value ± 95% CI
Once again, comparing the TBUT at every test point to the basal value, only on the third day was any significant difference found (3.33 ± 6.39 seconds vs. −19.20 ± 8.95 seconds, p = 0.001). The lacrimal production was not different from the basal value on day 15 nor after 7 or 14 days subsequent to the suspension of OAS application.
3.3. Corneal fluorescein staining
During the intervention, OAS subjects only presented a statistically significant difference compared to control on the third day of the study (p = 0.001). At this time point, 80% of OAS cases presented mild staining (grade I) and 20% presented moderate staining (grade II) versus 20% mild (grade I) cases for the control. On day 10, 40% of OAS subjects exhibited mild staining, 40% moderate (grade II) and 6.7% severe (grade III) versus 60% mild (grade I) staining for the control group. However, this difference was not statistically significant (p = 0.261). At the last time point on day 30, 50% of OAS cases presented with mild (grade I) staining versus 100% of control group cases showing no staining (grade 0); at none of the other time points were data significant (see Table 1).
TABLE 1.
Corneal Fluorescein Staining
| Clinical examination | Control | OAS | p value | ||||
|---|---|---|---|---|---|---|---|
| No staining | Mild | No staining | Mild | Moderate | Severe | ||
| Baseline | 100 | 0 | 100 | 0 | 0 | 0 | … |
| 3 d under treatment | 80 | 20 | 0 | 80 | 20 | 0 | 0.001 a |
| 10 d under treatment | 40 | 60 | 13.3 | 40 | 40 | 6.7 | 0.261 a |
| 1 d after treatment | 60 | 40 | 53.3 | 46.7 | 0 | 0 | 0.604 b |
| 4 d after treatment | 80 | 20 | 40 | 60 | 0 | 0 | 0.182 b |
| 7 d after treatment | 100 | 0 | 70 | 30 | 0 | 0 | 0.264 b |
| 10 d after treatment | 80 | 20 | 80 | 20 | 0 | 0 | 0.778 b |
| 14 d after treatment | 100 | 0 | 70 | 30 | 0 | 0 | 0.083 b |
Comparison of corneal fluorescein staining at different time points between control and 1% ocular atropine sulfate (OAS) groups. Values are percentages.
Pearson's chi‐squared test.
Fisher's exact test.
3.4. Clinical observations
3.4.1. Anterior segment evaluation
A total of 23 findings were reported during the anterior segment evaluations (13 for OAS and 10 for control, p > 0.05). The most frequently reported finding was mild conjunctival hyperemia (76.9% for OAS vs. 20% for control); followed by mild secretion (15.4% for OAS vs. 50% for control). Other observations included epithelial irregularity (7.7%, OAS), inflamed nictitating membrane (20%, control) and chemosis (10%, control) (see Table 2).
TABLE 2.
Clinical and Histopathological Findings
| Clinical observations | Control | OAS | p value |
|---|---|---|---|
| IOP, mmHg | |||
| Baseline | 9.30 ± 0.57 | 8.97 ± 0.64 | 0.321 a |
| 14 d after treatment | 8.20 ± 1.20 | 8.20 ± 1.44 | 0.752 a |
| Anterior segment evaluation | |||
| 1 d after treatment | Mild secretion | Mild hyperemia | 0.159 b |
| 7 d after treatment | Chemosis | Mild hyperemia and mild secretion | 0.392 b |
| 14 d after treatment | Mild hyperemia | None | 1.000 c |
| Incidence of adverse events | Negative | Negative | … |
| Histopathology* | |||
| Goblet cell density, % | 22.40 ± 1.95 | 21.47 ± 5.93 | 0.357 a |
| Cornea | Negative | Corneal cartilaginous metaplasia | 0.250 c |
| Conjunctiva, ciliary body, retina, and optic nerve | Negative | Negative | … |
| Lacrimal gland | Minimal and occasional edema, minimal and occasional congestion, and interlobular lymphocytic infiltration | Minimal and occasional edema, minimal and mild occasional congestion, minimal degree of inflammation, and interlobular lymphocytic infiltration. | <0.050 b |
Safety evaluations for control group (n = 5 NZW) and 1% ocular atropine sulfate (OAS) group (n = 15 NZW).
Mann‐Whitney U test.
Pearson's chi‐squared test.
Fisher's Exact test.
All findings were considered outside normal limits or related to fixation and preservation methods.
3.4.2. Intraocular pressure (IOP)
All the evaluated eyes presented IOP values within normal ranges. Ocular tonometry showed no significant differences in the IOP between control and OAS eyes at baseline (9.30 ± 0.57 mmHg vs. 8.97 ± 0.64 mmHg, p = 0.321) and at 30 days (8.20 ± 1.20 mmHg vs. 8.20 ± 1.44 mmHg, p = 0.752) (see Table 2).
3.4.3. Posterior segment evaluation
No alterations were observed at any time for any group during this study.
3.4.4. Ocular histopathology
The reported changes were all considered within normal limits or as incidental findings (one case of corneal cartilaginous metaplasia considered not related to the intervention and the occasional presence of changes related to fixation and preservation methods) in the following structures for either group: cornea, conjunctiva, ciliary body, retina, and optic nerve (see Table 2).
3.4.5. Lacrimal gland histopathology
In total, 23.3% of OAS versus 60% of control cases presented minimal and occasional edema (p = 0.052); while 86.7% OAS cases showed minimal and 3.3% mild congestion versus 30% minimal and occasional congestion in control (p = 0.001). Other studied variables such as fibrosis, degeneration, and necrosis were absent in histopathological evaluation.
Regarding inflammation, in total, 86.7% of OAS cases presented a minimal degree of inflammation, while 13.3% showed mild inflammation (p = 0.0001). All OAS cases presented interlobular lymphocytic infiltration versus 40% in control (p = 0.001). Even when these findings proved statistically significant, the pathologist described these changes as very minor, within normal limits, and not clinically significant (see Figure 3).
FIGURE 3.
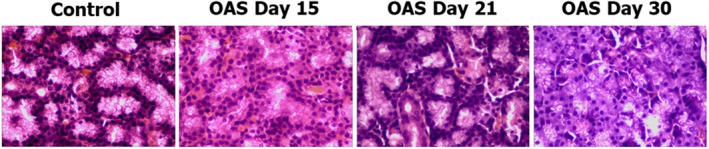
Representative examples of lacrimal gland histopathology in each group (HE‐staining, 40x). Minimal interlobular lymphocytic infiltration can be observed in OAS groups
3.4.6. Goblet cell density
There were no statistically significant differences between control group and OAS subjects. The mean density ±SD of goblet cells was 22.40 ± 1.95 cells versus 21.47 ± 5.93 cells, respectively (p = 0.357) (see Table 2). Goblet cell density was not different between the control and OAS groups on day 15 nor on days 21 or 30 (see Figure 4).
FIGURE 4.
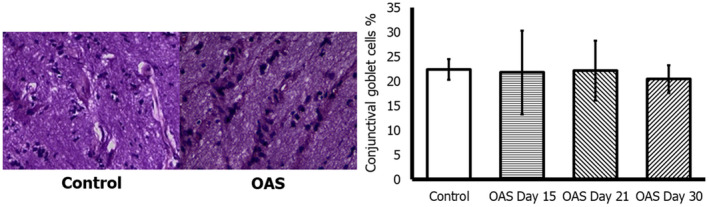
Up: Conjunctival staining of control and 1% ocular atropine sulfate (OAS) groups at day 30. Down: Goblet cell density (%) for each group (PAS staining, 40x). Mean value ± SD
4. DISCUSSION
DED is a multifactorial disease that impacts millions of lives around the world. It is an extremely important disease given its prevalence and the clinical and economic impacts on those who suffer from it. As a result, great efforts being made to find new ways to understand and treat its physiopathology. In vivo preclinical models have been created to study it, allowing a detailed evaluation of its etiology, risk factors, and even the efficacy of formulations used to treat it. 1 One of these models is the neural control interruption of lacrimal secretion through administration of antimuscarinic medications. 4 Topically applied 1% OAS has been used successfully in NZW rabbits to emulate DED 6 , 7 , 8 , 9 , 10 ; likewise, in other species such as dogs, this effect has been corroborated and reported as lasting up to 5 weeks after the last exposure to OAS. 11 , 12 As discussed before, animal studies have been an important part of research endeavors for dry eye. However, simple, and easy to replicate models are still scarce and not readily available to every researcher. NZW rabbits are a very convenient species, which has been used previously as a successful model for antimuscarinic tear production reduction. 6 , 7 , 8 , 9 , 10 However, even though this model has been used previously, available publications regarding the NZW rabbit model describe only the administration of OAS with concurrent instillation of any treatments for dry eye being evaluated, and do not include evaluation of the effects of OAS and the appearance of dry eye signs after the suspension of the drug. Since in other species such as dogs, the effect of OAS has been observed up to 5 weeks after the cessation of the intervention, this study explored the possibility of evaluating the dry eye manifestations derived from OAS application in NZW rabbits beyond its discontinuance in order to potentially evaluate dry eye treatments without risking the appearance of OAS‐related adverse events such as superficial keratitis, papillary conjunctivitis, contact dermatitis, and lid edema, which could diminish the reliability of the results obtained. 20 In order to avoid the risk of confounding variables altering the result of a particular study, and since the known adverse events of OAS on the ocular have been reported and listed broadly in the literature, 20 this study was performed to validate a dry eye preclinical model not only during, but after the cessation of the administration of 1% OAS in NZW rabbits.
The justification for this line of experimentation came from evidence of delayed responses in other species to the dry eye effects of OAS several weeks after its suspension. If this could be replicated in NZW rabbits, this would potentially provide an accessible, unbiased, and unaltered dry eye animal model that could be used to reliably evaluate potential treatments for DED.
The evaluated endpoints were tear production (Schirmer I test), TBUT, and corneal fluorescein staining. For the Schirmer I test, a statistically significant difference was found for the first and second evaluations (p values = 0.001 and 0.005, respectively); both of which took place while the investigated product was being instilled t.i.d. on the ocular surface of the experimental animals. However, for the rest of the evaluations, after cessation of OAS application, tear production did not appear to suffer any significant decrease compared to baseline or to the control group.
Likewise, TBUT was significantly shorter for OAS groups during the first and second evaluations, on the third and tenth days of OAS application (p = 0.001); but no difference was observed between baseline and the control group against OAS subjects for the rest of the tests.
Finally, it was only for the first evaluation on day 3 that any difference in corneal fluorescein staining between groups was noted. For none of the other tests was any difference observed.
Concerning the rest of the clinical evaluations, one of the differences worth noting between groups and basal values was an increase in the incidence of mild hyperemia (76.9% for OAS vs. 20% for control), a finding that correlates to the expected adverse events previously reported in the literature for atropine sulfate.
Ocular histopathological findings were considered either within normal ranges or as incidental findings related to the fixation methods, while lacrimal gland histopathological analysis revealed a 100% incidence of inflammation related findings, such as some degree (86.7% minimal and 13.3% mild) of interlobular lymphocytic infiltration in OAS exposed animals versus 40% in the control group. Regardless of how these results are interpreted, extrapolation to a clinical setting must be undertaken with caution, since the pathologist in this study considered these findings not clinically significant. The minor extent of alterations was considered within normal limits.
Unfortunately, as evidenced by the data obtained from the Schirmer I test, TBUT and corneal fluorescein staining, the dry eye signs produced by OAS lasted as long as the instillation of the solution took place, and no longer than that. The findings of this study confirm that a 1% OAS application to the ocular surface of NZW rabbits provides a suitable DED model while active instillation takes place. Furthermore, the other results here presented attest to the satisfactory safety profile of the product, since only mild alterations were demonstrated in the histopathology results and any clinical changes induced by it were speedily reversible.
Limitations of this study include: the reduced number of tests and clinical evaluations during the administration of the product in comparison to the whole intervention span; and obtaining the control group's lacrimal glands on day 30, as opposed to at the same time points to the OAS groups, which would have provided a more homogeneous comparison between groups. However, the study was designed as such in order to avoid using a larger number of animals. Also, since no changes were observed, it is therefore safe to infer that tests at other time points would have shown similar results.
5. CONCLUSION
The administration of 1% OAS on the ocular surface of NZW rabbits delivers an effective dry eye model, provided that the instillation of this solution is continuous throughout the experimental intervention at hand. This practical and easy to replicate model demonstrated changes in the Schirmer I test, TBUT and corneal fluorescein staining on the ocular surface of animals exposed to 1% OAS. It does not require the acquisition of hard to attain and generally expensive strains of animals, changes are seen practically immediately after the onset of the intervention, and there is no need for specialized equipment not accessible in many laboratory facilities. However, this study proved that the dry eye signs induced by this topical formulation in NZW rabbits last only while its administration is taking place. Therefore, this model should not be considered as a first choice when the risk of presenting the topical adverse events commonly reported for 1% atropine sulfate or any of its excipients could potentially skew the results of the evaluated variables.
CONFLICT OF INTEREST
ASR, EYCG, JMME, AANS, OOM, LBD and PMV are employees of Laboratorios Sophia, SA de CV. The sponsor provided support in the form of salaries for authors. This does not alter our adherence to the Good Publication Practice guidelines for pharmaceutical companies (GPP3) polices on sharing data and materials. The authors report no other conflicts of interest in this work.
AUTHORS’ CONTRIBUTIONS
All authors contributed significantly to this study, study conceptualization, to data analysis, drafting or revising the article, read and approved the final manuscript, and agree to be accountable for all aspects of the work.
6. ACKNOWLEDGEMENTS
None.
Sánchez‐Ríos A, Correa‐Gallegos EY, Medina‐Espinoza JM, et al. Validation of a preclinical dry eye model in New Zealand white rabbits during and following topical instillation of 1% ophthalmic atropine sulfate. Anim Models Exp Med. 2022;5:266–273. doi: 10.1002/ame2.12218
Funding information
This study was sponsored by Laboratorios Sophia, SA de CV (Zapopan, Jalisco, Mexico).
REFERENCES
- 1. Bron AJ, de Paiva CS, Chauhan SK, et al. TFOS DEWS II pathophysiology report. Ocul Surf. 2017;15(3):438‐510. 10.1016/j.jtos.2017.05.011 [DOI] [PubMed] [Google Scholar]
- 2. Clayton JA. Dry eye. N Engl J Med. 2018;378(23):2212‐2223. 10.1056/NEJMra1407936 [DOI] [PubMed] [Google Scholar]
- 3. Shah M, Cabrera‐Ghayouri S, Christie LA, Held KS, Viswanath V. Translational preclinical pharmacologic disease models for ophthalmic drug development. Pharm Res. 2019;36(4):58. 10.1007/s11095-019-2588-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Barabino S, Dana MR. Animal models of dry eye: a critical assessment of opportunities and limitations. Invest Ophthalmol vis Sci. 2004;45(6):1641‐1646. 10.1167/iovs.03-1055 [DOI] [PubMed] [Google Scholar]
- 5. Zheng W, Ma M, Du E, et al. Therapeutic efficacy of fibroblast growth factor 10 in a rabbit model of dry eye. Mol Med Rep. 2015;12(5):7344‐7350. 10.3892/mmr.2015.4368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bucolo C, Musumeci M, Salomone S, et al. Effects of topical fucosyl‐lactose, a milk oligosaccharide, on dry eye model: an example of nutraceutical candidate. Front Pharmacol. 2015;6:280. 10.3389/fphar.2015.00280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bucolo C, Fidilio A, Fresta CG, et al. Ocular pharmacological profile of hydrocortisone in dry eye disease. Front Pharmacol. 2019;10:1240. 10.3389/fphar.2019.01240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Burgalassi S, Panichi L, Chetoni P, Saettone MF, Boldrini E. Development of a simple dry eye model in the albino rabbit and evaluation of some tear substitutes. Ophthalmic Res. 1999;31(3):229‐235. 10.1159/000055537 [DOI] [PubMed] [Google Scholar]
- 9. Crawford K, Schuh C, Schuh J, Hsu H. Effects of ALY688 on atropine‐induced dry eye in rabbits. Invest Ophthalmol vis Sci. 2019;60(9):305. [Google Scholar]
- 10. El‐Shazly AH, El‐Gohary AA, El‐Shazly LH, El‐Hossary GG. Comparison between two cyclooxygenase inhibitors in an experimental dry eye model in albino rabbits. Acta Pharm. 2008;58(2):163‐173. 10.2478/v10007-008-0009-0 [DOI] [PubMed] [Google Scholar]
- 11. Laus L, Sanches R, Bolzan A, Mendes‐Vicenti F, Pereira G. Short and long term effects of 1% atropine drops on tear production in mixed breed dogs. R Bras Ci Vet. 2000;7(2):120‐122. 10.4322/rbcv.2015.194 [DOI] [Google Scholar]
- 12. Hollingsworth SR, Canton DD, Buyukmihci NC, Farver TB. Effect of topically administered atropine on tear production in dogs. J Am Vet Med Assoc. 1992;200(10):1481‐1484. [PubMed] [Google Scholar]
- 13. Charan J, Kantharia ND. How to calculate sample size in animal studies? J Pharmacol Pharmacother. 2013;4(4):303‐306. 10.4103/0976-500X.119726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Festing MF. Reduction of animal use: experimental design and quality of experiments. Lab Anim. 1994;28(3):212‐221. 10.1258/002367794780681697 [DOI] [PubMed] [Google Scholar]
- 15. Anderson AJ, Vingrys AJ. Small samples: does size matter? Invest Ophthalmol vis Sci. 2001;42(7):1411‐1413. [PubMed] [Google Scholar]
- 16. Lemp MA. Report of the national eye institute/industry workshop on clinical trials in dry eyes. CLAO J. 1995;21(4):221‐232. [PubMed] [Google Scholar]
- 17. Armstrong RA. Statistical guidelines for the analysis of data obtained from one or both eyes. Ophthalmic Physiol Opt. 2013;33(1):7‐14. 10.1111/opo.12009 [DOI] [PubMed] [Google Scholar]
- 18. Gilger BC. Concerns with analysis of correlated eye data. Vet Ophthalmol. 2011;14(3):214. 10.1111/j.1463-5224.2011.00893.x [DOI] [PubMed] [Google Scholar]
- 19. Ray WA, O'Day DM. Statistical analysis of multi‐eye data in ophthalmic research. Invest Ophthalmol Vis Sci. 1985;26(8):1186‐1188. [PubMed] [Google Scholar]
- 20. Highlights of Prescribing Information, Atropine Sulfate Ophthalmic Solution, USP 1%. https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/206289s000lbl.pdf. Accessed January 3, 2022.


