Abstract
Models describing the limits of growth of pathogens under multiple constraints will aid management of the safety of foods which are sporadically contaminated with pathogens and for which subsequent growth of the pathogen would significantly increase the risk of food-borne illness. We modeled the effects of temperature, water activity, pH, and lactic acid levels on the growth of two strains of Listeria monocytogenes in tryptone soya yeast extract broth. The results could be divided unambiguously into “growth is possible” or “growth is not possible” classes. We observed minor differences in growth characteristics of the two L. monocytogenes strains. The data follow a binomial probability distribution and may be modeled using logistic regression. The model used is derived from a growth rate model in a manner similar to that described in a previously published work (K. A. Presser, T. Ross, and D. A. Ratkowsky, Appl. Environ. Microbiol. 64:1773–1779, 1998). We used “nonlinear logistic regression” to estimate the model parameters and developed a relatively simple model that describes our experimental data well. The fitted equations also described well the growth limits of all strains of L. monocytogenes reported in the literature, except at temperatures beyond the limits of the experimental data used to develop the model (3 to 35°C). The models developed will improve the rigor of microbial food safety risk assessment and provide quantitative data in a concise form for the development of safer food products and processes.
Predictive microbiology combines mathematical modeling with experimental data on combinations of factors that influence the growth of food spoilage and/or food-borne pathogenic microorganisms. The models developed are intended to predict the fate of microorganisms in foods. Since the experimental data are usually derived from studies using laboratory media, the models must be validated with data collected under conditions under which food products are customarily stored.
Predictive microbiology models can be divided into kinetic models and probability models. With the former type, one calculates the microbiological life of food products, i.e., the period of time during which the number of microorganisms in the food is less than a specified value. With the latter type, one determines whether a microorganism can grow and identifies storage conditions with a low or nil probability of growth.
Kinetic and probability models may be closely related, because the probability of detectable growth within a specified time period depends on germination, lag, and generation times, i.e., on kinetic parameters. In some cases, a probability model may be derived from a kinetic model by some simple mathematical transformations. For example, in references 33, 35 and 41, a kinetic model was transformed into a probability model by taking the natural logarithm of both sides of the original equation and then replacing one side with the “logit” of a probability, i.e., ln [P/(1 − P)], where P is the probability that growth occurs. Low levels of Listeria monocytogenes, i.e., levels not exceeding 102 to 103 CFU per gram of food at the time of consumption, are considered by many authorities to pose a low risk for most consumers (7, 15). Consequently, some national regulatory authorities, including Germany, The Netherlands, France, and Canada, advocate or have adopted a food safety risk management strategy that involves tolerance of low levels of L. monocytogenes in foods, provided that the organism cannot grow to unacceptable levels during the shelf life of the product (13). Thus, there is a need for a methodology for evaluating rapidly the potential for growth of L. monocytogenes in particular products. Mathematical models to describe the probability of growth of L. monocytogenes in foods can fulfill that need.
Our objective in this study was to develop methods to identify combinations of environmental variables that just permit or just prevent growth. This set of combinations also defines the growth rate of an organism in multidimensional space, the so-called “hyperspace cloud” (4). We also wanted to determine the potential for L. monocytogenes to grow in cold-smoked salmon. We modified the model-fitting procedure described in reference 35 and used nonlinear logistic regression techniques to estimate as many of the cardinal parameters (e.g., Tmin, awmin, etc.) as possible. We tested this model on experimental data from two strains and evaluated the resulting predictive equations by using previously published data from several laboratories (8, 14, 26).
MATERIALS AND METHODS
Culture and inoculum preparation.
Two strains of L. monocytogenes, Scott A and L5, the latter a wild-type strain isolated from cold-smoked salmon, were used. We inoculated two 250-ml Erlenmeyer flasks containing 50 ml of TSB-YE (tryptone soya broth [CM 129; Oxoid] with 0.6% yeast extract [L21; Oxoid]) each with one of the above strains and incubated them with shaking (50 ± 2 rpm) for 18 h at 30°C. Fifty microliters of that culture was transferred to 50 ml of fresh TSB-YE in a 250-ml Erlenmeyer flask, and the incubation was repeated for 18 h at 30°C. Cultures were grown until the late exponential phase of growth, when the optical density (at 540 nm) of the culture was 0.8 (45).
Inoculation procedures.
TSB-YE was used as the basal medium for all experiments but was modified by the addition of NaCl or lactic acid or of HCl or NaOH to adjust the pH. Under aseptic conditions, 100 μl of inoculum was added to 50 ml of culture medium and mixed well, and the pH was measured immediately. Two milliliters of each broth was pipetted into four wells of each of four 24-well plates (4 wells by 6 wells). This inoculum produced turbidity just visible to the unaided eye and was used so that growth would immediately increase the turbidity of the broth without a lag in detection time. Two wells served as negative controls (sterile TSB-YE [pH 7.2]), and another two served as positive controls (TSB-YE [pH 7.2] containing 100 μl of the inoculum), in each well plate. We used two well plates to test 10 different pH levels for each lactic acid concentration in quadruplicate. Replicates were incubated at 4, 10, and 20°C in constant-temperature rooms, at 6 and 8°C in waterbaths, and at 30°C in a laboratory incubator. We also studied water activity, pH, and lactic acid effects in duplicate cultures incubated at 20 and 30°C. Details of the experimental design for each strain are given in Table 1.
TABLE 1.
Experimental designs describing conditions testeda to generate probability-of-growth models
| Both strains
|
Scott A
|
L5
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Temp (°C) | No. of temps tested | Water activity | No. of levels tested | Lactic acid concn (mM) | No. of levels tested | pH | No. of levels tested | No. of conditions tested | pH | No. of levels tested | No. of conditions tested |
| 4, 10, 20, 30 | 4 | 0.993 | 1 | 0 | 1 | 3.9–6.1 | 10 | 40 | 3.9–5.6 | 10 | 40 |
| 4, 10, 20, 30 | 4 | 0.993 | 1 | 10 | 1 | 3.7–6.0 | 10 | 40 | 4.0–5.6 | 10 | 40 |
| 4, 10, 20, 30 | 4 | 0.993 | 1 | 20 | 1 | 4.1–5.9 | 10 | 40 | 4.0–5.6 | 10 | 40 |
| 4, 10, 20, 30 | 4 | 0.993 | 1 | 30 | 1 | 4.1–5.9 | 10 | 40 | 4.2–5.6 | 10 | 40 |
| 4, 10, 20, 30 | 4 | 0.993 | 1 | 50 | 1 | 4.2–6.5 | 10 | 40 | 4.2–5.5 | 10 | 40 |
| 5 | 1 | 0.966 | 1 | 0, 50 | 2 | 6.0 | 1 | 2 | |||
| 5 | 1 | 0.962 | 1 | 100, 150, 200 | 3 | 6.0 | 1 | 3 | |||
| 5 | 1 | 0.960 | 1 | 250, 300 | 2 | 6.0 | 1 | 2 | |||
| 5 | 1 | 0.958 | 1 | 350, 400 | 2 | 6.0 | 1 | 2 | |||
| 5 | 1 | 0.955 | 1 | 500 | 1 | 5.9 | 1 | 1 | |||
| 6 | 1 | 0.993 | 1 | 0 | 1 | 4.4–5.4 | 9 | 9 | 4.5–5.5 | 9 | 9 |
| 8 | 1 | 0.993 | 1 | 0 | 1 | 4.3–5.8 | 6 | 6 | 4.3–5.1 | 6 | 6 |
| 20, 30 | 2 | 0.928 | 1 | 0 | 1 | 5.0–5.6 | 7 | 14 | 5.0–5.5 | 6 | 12 |
| 20, 30 | 2 | 0.928 | 1 | 20 | 1 | 4.9–5.6 | 7 | 14 | 4.9–5.7 | 7 | 14 |
| 20, 30 | 2 | 0.928 | 1 | 50 | 1 | 5.1–5.8 | 7 | 14 | 5.1–5.9 | 7 | 14 |
| 20, 30 | 2 | 0.940 | 1 | 0 | 1 | 4.7–5.3 | 7 | 14 | 4.7–5.3 | 7 | 14 |
| 20, 30 | 2 | 0.940 | 1 | 20 | 1 | 4.7–5.4 | 7 | 14 | 4.7–5.4 | 7 | 14 |
| 20, 30 | 2 | 0.940 | 1 | 50 | 1 | 5.0–5.8 | 7 | 14 | 5.0–5.6 | 7 | 14 |
| 20, 30 | 2 | 0.954 | 1 | 0 | 1 | 4.6–5.3 | 7 | 14 | 4.3–5.3 | 10 | 20 |
| 20, 30 | 2 | 0.954 | 1 | 20 | 1 | 4.7–5.4 | 7 | 14 | 4.7–5.4 | 7 | 14 |
| 20, 30 | 2 | 0.954 | 1 | 50 | 1 | 4.8–5.5 | 7 | 14 | 4.9–5.5 | 7 | 14 |
| 20, 30 | 2 | 0.965 | 1 | 0 | 1 | 4.4–5.2 | 8 | 16 | |||
| 22 | 1 | 0.995 | 1 | 0 | 1 | 4.0–6.8 | 15 | 15 | |||
| 22 | 1 | 0.967 | 1 | 20 | 1 | 4.4–7.4 | 15 | 14 | |||
| 22 | 1 | 0.968 | 1 | 50 | 1 | 4.8–7.8 | 15 | 15 | |||
| 22 | 1 | 0.965 | 1 | 100 | 1 | 4.9–7.7 | 15 | 15 | |||
| 22 | 1 | 0.962 | 1 | 200 | 1 | 5.4–7.7 | 15 | 15 | |||
| 20 | 1 | 0.929–0.993 | 10 | 0, 50 | 2 | 5.5, 5.8, 6.2 | 3 | 60 | |||
| 20 | 1 | 0.936–0.995 | 10 | 0, 50 | 2 | 5.3, 5.7, 6.0 | 3 | 60 | |||
| 20 | 1 | 0.995 | 1 | 0 | 1 | 4.0–6.8 | 15 | 15 | |||
| 20 | 1 | 0.969 | 1 | 20 | 1 | 4.6–7.7 | 15 | 15 | |||
| 20 | 1 | 0.969 | 1 | 50 | 1 | 4.8–7.6 | 15 | 15 | |||
| 21 | 1 | 0.966 | 1 | 100 | 1 | 4.9–7.6 | 15 | 15 | |||
| 21 | 1 | 0.964 | 1 | 200 | 1 | 5.2–7.2 | 14 | 14 | |||
| 21 | 1 | 0.962 | 1 | 450 | 1 | 5.5–6.6 | 11 | 11 | |||
| 30 | 1 | 0.991 | 1 | 0 | 1 | 4.3–4.8 | 6 | 6 | |||
| 20 | 1 | 0.96 | 1 | 200, 250, 300, 350, 400 | 5 | 6.0 | 1 | 5 | |||
| 3.1–35.8 | 30 | 0.995 | 1 | 0 | 1 | 7.3 | 1 | 30 | |||
| 3.1–36.2 | 30 | 7.3 | 1 | 30 | |||||||
Where ranges of the controlling factors are given, levels were chosen to be at approximately regular increments.
Assessment of growth.
Well plate cultures were examined daily for 90 days. “Presumptive growth” was recorded if there was a visible increase in the turbidity of the broths. The day on which growth was first observed was recorded, although that information played no role in the subsequent fitting of the probability model. Growth-positive cultures were mixed, and 0.3 ml was withdrawn for pH measurement in a small capule by using a flat-tip pH probe (model 250A with calomel-sealed flat-tip probe, AEP433; Orion Research Inc., Boston, Mass.). A 0.1-ml aliquot of the culture was streaked onto TSA-YE (tryptone soya agar [CM 131; Oxoid] with 0.6% yeast extract [L21; Oxoid]) and incubated at 30°C for 24 to 48 h. Typical L. monocytogenes colonies were subcultured onto Oxford Formulation Listeria Selective Agar (Oxoid CM856 including selective supplement Oxoid SR140) and incubated at 37°C for 24 to 48 h. If we saw only one colony type on TSA-YE and if colonies typical of Listeria were seen on Oxford Formulation Listeria selective agar, then growth of L. monocytogenes was presumed confirmed. When turbidity did not increase, or if only a deposit formed in the bottom of the well by the end of the incubation period, we used a standardized ecometric technique (29, 30, 33) calibrated to viable counts to identify culture conditions that were lethal to L. monocytogenes, i.e., in which cell numbers declined during incubation. These determinations could not be made using turbidimetric methods alone.
Experimental design.
We conducted three sets of experiments to test the effect of combinations of temperature, pH, and the concentration of lactic acid on the growth of L. monocytogenes. The experimental design covered more than 500 different sets of conditions for each strain tested (see Table 1). The structure of each data set was such that it varied from sparse for certain combinations of factors to more complete in other regions of “factor space.” There were usually four replicates when the experiments were conducted in a water bath, but there was only a single observation for each condition when a temperature gradient incubator was used. Two forms of acidulant, HCl and lactic acid, were used in combination so that inhibition due to pH or to lactic acid could be distinguished. Filter-sterilized 5 M HCl or 4 N NaOH was used to adjust the pH of media containing various levels of lactic acid. Water activity was adjusted with NaCl. The contribution of sodium lactate to the water activity of the media was included in calculations, and the final aw of each medium was measured using a dew point water activity meter (Aqualab CX-2; Decagon Devices, Pullman, Wash.).
Effects of temperature, pH, and lactic acid concentration on growth limits.
Filter-sterilized lactic acid (Univar, analytical-grade reagent; minimum, 88% [wt/wt] Ajax Chemicals) was added to sterile over-strength TSB-YE, prepared in a 1-liter volumetric flask. The broths were made up to final volume with sterile distilled water to yield final lactic acid concentrations of 10, 20, 30, or 50 mM. TSB-YE with no lactic acid was prepared in the same manner. Each medium was adjusted to 10 different pH levels, and each pH-lactic acid combination was dispensed into 25-ml McArtney bottles.
Effects of water activity, pH, and concentration of lactic acid on growth limits.
Three levels of lactic acid, 0, 20, and 50 mM, and four levels of water activity, 0.929, 0.940, 0.954, and 0.965, were selected for additional studies. We prepared a series of over-strength TSB-YE broths of different aws that included NaCl to the desired level. These media were autoclaved at 105°C for 30 min. We added 50 mM sterile lactic acid to the sterile aw-adjusted media in a volumetric flask, and sterile distilled water was added to achieve the required final volume and concentration. At each water activity level, the pH was adjusted to ca. 5.4, 5.7, and 6.1, and 15 ml of the broth was then dispensed into duplicate L-shaped glass tubes (L-tubes) (diameter, 15 mm) designed for use with a temperature gradient incubator (model TN3; Advantec, Toyo Roshi International, Pleasanton, Calif.) L-tubes were incubated at 20°C overnight prior to inoculation.
We also determined growth of L. monocytogenes at different levels of lactic acid, under conditions close to that typical of cold-smoked salmon at 5 and 20°C, i.e., pH ∼6.0 and water activity of ∼0.96. Sterile over-strength TSB-YE plus 4.5% NaCl was prepared in a volumetric flask and made up to final volume with sterile distilled water and filter-sterilized lactic acid (88% [wt/wt]). For experiments at 5°C, lactic acid concentrations of 0 to 400 mM at 50 mM intervals were tested. For experiments at 20°C, lactic acid concentrations of 500 mM and from 200 to 400 mM at 50 mM intervals were tested. Fifty milliliters of each medium was dispensed into separate 250-ml side-arm flasks. All broths were adjusted to pH ∼6.0. Inoculation methods were as described above. The media were incubated at 5 and 20°C in water baths with shaking at ∼33 ± 1 rpm. Growth was assessed turbidimetrically (Spectronic 20D; Spectronic Unicam, Rochester, N.Y.) at 540 nm.
THEORY
Model derivation.
Suitable kinetic models (32, 38) can be converted to a probability model. The most general form of kinetic model employed in those studies is given by equation 1 and has four variables that affect growth, namely, temperature (T [in degrees Celsius]), water activity (aw), pH, and lactic acid concentration ([LAC] [in millimolar units]).
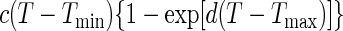 |
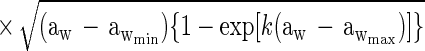 |
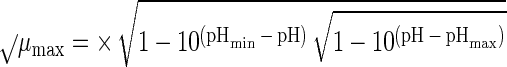 |
1 |
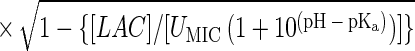 |
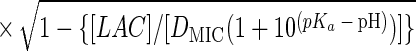 |
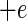 |
where μmax is the maximum specific growth rate, c, d, and k are scale parameters, Tmin is the theoretical minimum temperature for growth, Tmax is the theoretical maximum temperature for growth, awmin is the theoretical minimum water activity for growth, awmax is the theoretical maximum water activity for growth, pH has its usual meaning, pHmin is the theoretical minimum pH for growth, pHmax is the theoretical maximum pH for growth, UMIC is the minimum concentration of undissociated lactic acid which prevents growth, DMIC is the minimum concentration of dissociated lactic acid which prevents growth, pKa is the pH at which levels of undissociated and dissociated acid are equal (3.86 for lactic acid [6]), and e is the error term.
In the thesis of Tienungoon (45), which had four separate data sets, the superoptimal temperature term {1 − exp[d(T − Tmax)]} or superoptimal water activity term {1 − exp[k(aw − awmax)]} or superoptimal pH term (1 − 10pH − pHmax) were sometimes not required because these terms are appropriate only if there are sufficient data to support the estimation of the associated parameters, viz., d, Tmax, k, awmax and pHmax. Similarly, the term involving the dissociated form of lactic acid {1 − [LAC]/[DMIC(1 + 10pKa − pH)]} is needed only if the total [LAC] is very high (e.g., >500 mM). The kinetic experiment that used the L. monocytogenes Scott A strain had a maximum [LAC] of 200 mM and did not require the DMIC in the final model. These considerations allow for some terms to be deleted from the general model, whereas other terms may have to be added in order to produce a good-fitting interface model. A procedure for vetting the terms that are the most appropriate for an interface model is presented below after we describe how we convert a kinetic model to an interface model.
Interface model.
Using an approach proposed previously (35), equation 1 or modifications of it were converted to an interface model for growth/no growth by taking the natural logarithm of both sides and replacing the left-hand side with the logit of the probability, P, that the organism will grow, where logit (P) is a mathematical shorthand for ln[P/(1 − P)]. This operation and substitution result in the following model:
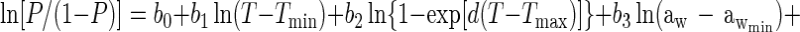 |
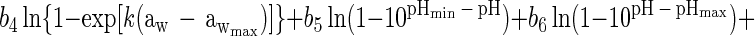 |
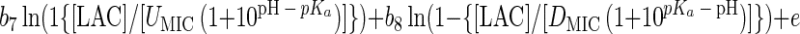 |
2 |
Equation 2 contains nine ‘linear-appearing’ parameters to be estimated, namely b0, b1, b2, b3, b4, b5, b6, b7, and b8. Had the analogy that equation 2 was obtained by log-transformation of equation 1 been strictly followed, then b1 = b2 = 1, and b3 = b4 = b5 = b6 = b7 = b8 = 0.5. However, the analogy is incomplete, and these parameters are treated as free parameters and are estimated with the same maximum likelihood or weighted least-squares procedure that simultaneously produces estimates of Tmin, d, Tmax, awmin, k, awmax, pHmin, pHmax, UMIC, and DMIC. In this respect, the growth/no growth model takes its form from that of equation 1, even though it is not a literal translation of it. Forcing b1 = b2 = 1.0 and b3 = b4 = b5 = b6 = b7 = b8 = 0.5, so that b0 is the only b parameter estimated, resulted in a much poorer fit (results not shown).
Statistical software.
Equation 2 is an example of a nonlinear logistic regression model. It is nonlinear because the expression on the right-hand side contains both linear and nonlinear parameters. It is a logistic regression because the right-hand side of the expression is linked to the response variable P, the probability of growth, by a logit link function. Linear logistic regression is a well-established statistical procedure which can be fitted by a wide variety of available statistical packages, utilizing built-in procedures, e.g., PROC LOGISTIC and PROC GENMOD of SAS (SAS/STAT user's guide, version 6, 4th ed., vol. 2; SAS/STAT software: changes and enhancements, through release 6.11; SAS Institute Inc., Cary, N.C.) or directives, such as in GENSTAT (Genstat S, release 3, manual; Genstat, Downer's Grove, Ill.). Nonlinear logistic regression, however, requires more than a standard procedure or directive. Equation 2 was fitted by adapting code in an example in PROC NLIN of SAS (SAS/STAT user's guide, version 6, p. 1168); see the Appendix for a partial listing of the modified code. The critical change is to use a weight function, which for a binomial probability distribution is nP(1 − P), where P is the probability of growth and n is the number of replicates. The mean value of the nonlinear predictor, the expression involving the growth regulating factors, is linked to the probability P by the logit link function. Convergence to an optimum solution was improved by use of a “loss function” that is described in the same example, although its use is not obligatory.
To identify model terms that were important to include in the model, the stepwise regression feature of PROC LOGISTIC of SAS (SAS/STAT user's guide, version 6) was used. Since that procedure is for linear logistic regression, the nonlinear parameters have to be fixed to constant values. Initially, these constant values were not known and were set to arbitrary values, for which the converged estimates from previously derived kinetic models were employed. Later, these parameters were reestimated with nonlinear logistic regression, using PROC NLIN (SAS/STAT user's guide, version 6) on the updated model, as discussed in the previous paragraph. By this means, we tested whether the model could be improved by the inclusion of terms involving the squares of terms or the cross-products of terms with two or more factors. We investigated the effect of the following 11 terms (with the nonlinear parameters set to the values to which they subsequently converged for the Scott A data set): (i) ln(T − 0.4164), (ii) ln(1 − 103.35 − pH), (iii) ln(aw − 0.9142), (iv) ln{1 − exp[0.536 (T − 48)]}, (v) ln{1 − [LAC]/[23.68(1 + 10pH − 3.86)]}, (vi) ln2(T − 0.4164), (vii) ln2[1 − (103.35 − pH)], (viii) ln2(aw − 0.9142), (ix) ln2{1 − [LAC]/[23.68 (1 + 10pH − 3.86)]}, (x) ln(T − 0.4164) × ln(1 − 103.35 − pH), and (xi) ln[1 − (10pH − 9.5)].
Assessment of model performance.
The receiver operating characteristic (ROC) curve (e.g., see reference 25), the Hosmer-Lemeshow goodness-of-fit statistic (18), and the maximum rescaled R-square statistic (31) were used as measures of goodness of fit of the model developed. The area under the ROC curve, c, is a measure of discrimination, obtained from a plot of sensitivity, i.e., the proportion of observed events that were correctly predicted to be events, against the complement of specificity, i.e., the proportion of nonevents that were correctly predicted to be nonevents. The closer the value of c is to 1, the greater is the discrimination. In epidemiological studies, a c value of >0.7 is considered acceptable discrimination, a c value of >0.8 as excellent discrimination, and a c value of >0.9 as outstanding discrimination (25); however, in epidemiology, usually not all of the factors that influence the response variable are known. For our model, a high degree of discrimination is expected, since the identity and approximate range of values of the important factors that prevent the growth of the organism in the experimental system are well known. The Hosmer-Lemeshow goodness-of-fit statistic, which involves grouping objects into a contingency table and calculating a Pearson chi-square statistic, was proposed (18) as a means of estimating goodness of fit when there is no replication or insufficient replication in any of the subpopulations. Small values of the statistic (large P values) indicate a good fit of the model to the data.
The maximum rescaled R2 for use with binomial error was proposed (31) as a generalization of the coefficient of determination R2 that is commonly used in regression applications involving normally distributed error. The closer the value is to 1, the greater the success in predicting the dependent variable from the independent variables. We also evaluated the model for its ability to predict the results obtained by others (14, 26). Model predictions were compared graphically to the observations of those workers.
We also tested the model on the less complete but more extensive independent published data (more than 1,000 observations) summarized in reference (38). When complete information on environmental conditions was not available, we adopted a “worst-case” strategy and generated predictions assuming that the unspecified variables were at the optimal levels for growth of L. monocytogenes, i.e., 25°C, pH 7, no lactate, and an aw of 0.995. Predictions from the model were made for a P value of 0.5, i.e, 50% probability of growth.
RESULTS
Since we did not have sufficient data to fit all the terms in equation 2, we used a simpler model instead. For example, we collected no data for an aw of >0.997, precluding estimation of awmax. Also, the term representing the effect of dissociated lactic acid (i.e., the one with the parameter DMIC) was not needed to model the present data sets. The parameters Tmax and pHmax of equation 2 had to be fixed to obtain convergence, because insufficient data were available in the high-temperature and high-pH regions. We set these values to Tmax = 48.0°C and pHmax = 9.5 based on work summarized in references 20 and 38. The coefficient of the high-temperature term, d, was fixed at 0.536, the converged value it attained with the kinetic model. This led to the following simplified model.
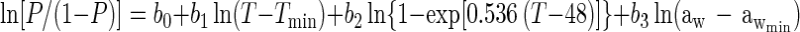 |
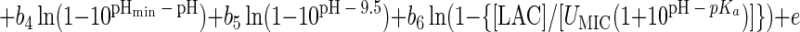 |
3 |
which was tested on the data from both strains.
Using PROC LOGISTIC of SAS (SAS/STAT user's guide, version 6), we examined the effect of the six model terms in equation 3, along with terms for the square of ln(T − Tmin), the square of ln(aw − awmin), the square of ln(1 − 10pHmin − pH), the square of ln(1 − {[LAC]/[UMIC (1 + 10pH − pKa)]}), and the cross-product term ln(T − Tmin) × ln(1 − 10pHmin − pH). The results from the two strains were the same, with the terms ln2(T − Tmin) and ln2(1 − 10pHmin − pH) explaining a statistically significant proportion of the total variation in logit (P), whereas the other three additional terms and the superoptimal pH term ln(1 − 10pH − 9.5) were not significant. This resulted in the following final model, which was used for both data sets:
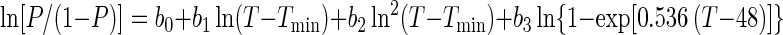 |
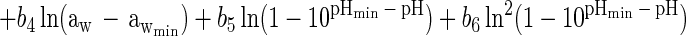 |
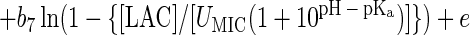 |
4 |
Parameter estimates and their standard errors, obtained with nonlinear logistic regression using PROC NLIN of SAS (SAS/STAT user's guide, version 6) are given in Table 2 for the data from the two strains.
TABLE 2.
Parameter estimates from fitting equation 4 to data for the growth/no growth interface of two strains of L. monocytogenes
| Parameter | Estimated range for strain:
|
|
|---|---|---|
| Scott A | L5 | |
| b0 | −6.023 ± 12.22 | −25.36 ± 28.65 |
| b1 | 19.00 ± 8.131 | 44.12 ± 17.76 |
| b2 | −3.049 ± 1.299 | −7.022 ± 2.660 |
| b3 | 7514 ± 1601 | 10257 ± 1556 |
| b4 | 4.635 ± 0.920 | 8.951 ± 1.241 |
| b5 | 141.0 ± 184.8 | 291.8 ± 357.1 |
| b6 | 240.2 ± 684.9 | 704.1 ± 1766 |
| b7 | 31.98 ± 47.77 | 58.12 ± 58.16 |
| Tmin | 0.4164 ± 1.413 | −1.623 ± 1.919 |
| awmin | 0.9142 ± 0.0047 | 0.9152 ± 0.0028 |
| pHmin | 3.350 ± 0.515 | 3.350 ± 0.495 |
| UMIC | 23.68 ± 32.18 | 25.00 ± 22.80 |
MODEL PERFORMANCE
Goodness of fit.
We compared the modeled growth/no growth interface to the data upon which the model was based (Fig. 1). Since four factors affect growth, it is difficult to display the model in all dimensions, but Fig. 1 is representative of the performance of the models for both strains for all pairs of variables. Objective measures of how well the model describes the data set are summarized in Table 3.
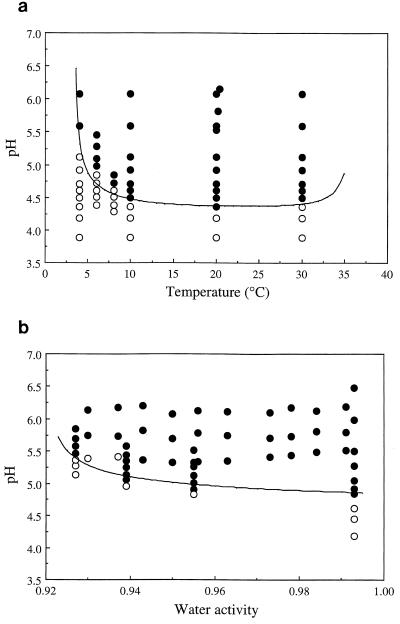
FIG. 1.
Selected growth/no growth interfaces (P = 0.5) predicted by equation 4 for L. monocytogenes, compared to the data used to generate the model. (a) Temperature/pH interface predicted by equation 4 fitted to data for L. monocytogenes Scott A compared to observed growth responses of that strain in TSB-YE with added salt (aw = 0.993 ± 0.001) and without added lactate. Growth (●) or no growth (○) within 90 days is shown. (b) pH/water activity interface predicted by equation 4 fitted to data for L. monocytogenes Scott A compared to observed growth responses of that strain in TSB-YE in the presence of 50 mM lactate at 20°C. Growth (●) or no growth (○) within 90 days from probabilistic and kinetic experiments, respectively, is shown.
TABLE 3.
Performance statistics for assessing logistic regression models
| Statistical parameter | Goodness-of-fit results for strain:
|
|
|---|---|---|
| Scott A | L5 | |
| c (area under ROC curve) | 0.976 | 0.991 |
| Hosmer-Lemeshow (goodness of fit) | 9.01 with 8 df (P = 0.341) | 1.78 with 6 df (P = 0.939) |
| Maximum rescaled R2 | 0.832 | 0.908 |
Comparison to independent data.
We also compared the predictions of the model to other data collected under laboratory conditions (Fig. 2 and 3) and under diverse conditions reported in the literature (data not shown). We collated ∼500 data for growth in laboratory broth and ∼500 data for growth in foods derived from 28 and 60 sources, respectively. Unlike the results shown in Fig. 1 to 3, a comparison to all literature data revealed some cases in which the model predicts growth is not possible where growth has been reported. Of particular note were poor predictions at high (i.e., >35°C) and low (<3°C) temperatures, discussed below.
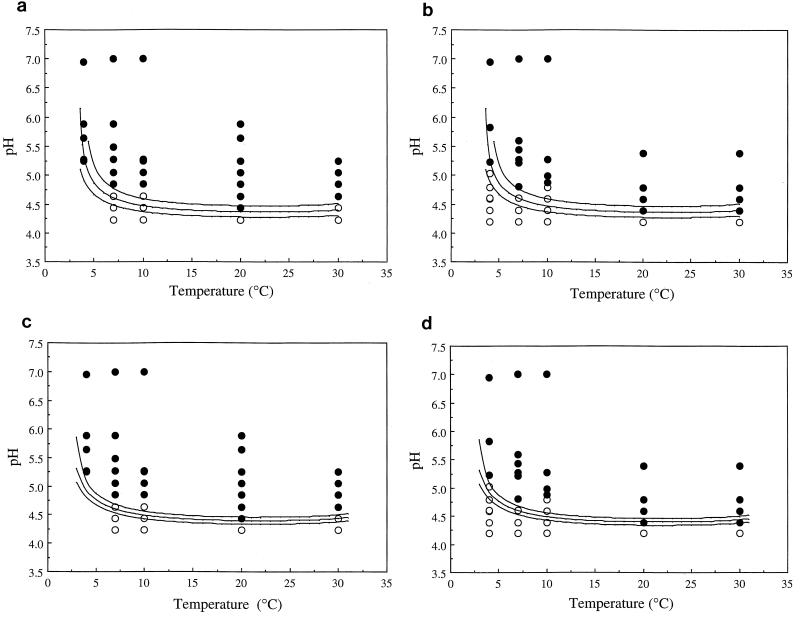
FIG. 2.
Evaluation of the probability models for strains Scott A and L5 fitted to equation 4. The model predictions were compared to the data of George et al. (14) for the effect of temperature and pH on the potential for growth of L. monocytogenes NCTC 10357 (a and c) and Scott A (b and d) in TSB plus 1% glucose plus 0.3% yeast extract (aw, ∼0.995). ●, growth observed; ○, growth not observed. In each panel, the growth/no growth interfaces predicted at P values of 0.1 (lower curve), 0.5 (middle curve), and 0.9 (upper curve) are shown. (a) George et al. data for strain NCTC 10357 compared to data from model for Scott A. (b) George et al. data for strain Scott A compared to data from model for Scott A. (c) George et al. data for strain NCTC 10357 compared to data from model for strain L5. (d) George et al. data for strain Scott A compared to data from model for strain L5.
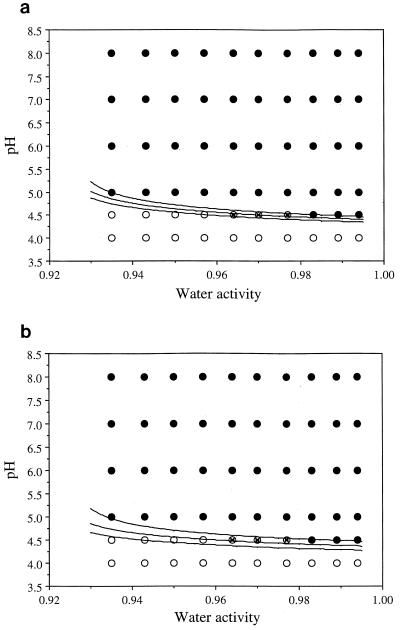
FIG. 3.
Evaluation of the probability models for strains Scott A and L5 fitted to equation 4. The model predictions were compared to the data of McClure et al. (26) for the effect of water activity and pH on the potential for growth of L. monocytogenes NCTC 9863 in TSB at 25°C at three levels of probability of growth: P = 0.1 (lower curve), P = 0.5 (middle curve), and P = 0.9 (upper curve). (a) (Upper) equation 4 fitted to L. monocytogenes Scott A data; (b) (Lower) equation 4 fitted to L. monocytogenes L5 data. Conditions under which growth was reported are indicated by ●, those under which growth was not observed are indicated by ○, and those under which growth was observed in some, but not all, trials are indicated by ⊗−. The abruptness of the predicted transition from a high (P = 0.9) to a low (P = 0.1) probability of growth is illustrated by the closeness of the three predicted boundaries. In both panels, three observations lie within the 10 to 90% probability-of-growth limits.
DISCUSSION
Probability or growth/no growth interface models are of particular interest in managing the safety of foods which occasionally may be contaminated with pathogens and for which subsequent growth of the pathogen would increase the risk of food-borne illness. Definition of all combinations of environmental factors that prevent growth of L. monocytogenes in foods will, in effect, quantify the Hurdle Concept (23, 24). The data underlying the growth/no growth models presented here are based on an observation period of 90 days to ensure that if growth were possible it would have been detected.
Model stability and convergence.
There was a strong similarity between the estimates of the cardinal model parameters Tmin, awmin, pHmin, and UMIC for the two strains (see Table 2). Although it is tempting to interpret these parameters as, respectively, the “true” minimum levels of temperature, pH, water activity (controlled by NaCl), and undissociated lactic acid that permit growth under otherwise optimal conditions, they should be seen as notional. The estimate of one of these parameters, pHmin, converged to 3.35, the lower bound that was specified for that parameter (using the “bounds” directive of SAS PROC NLIN). Relaxing the boundary condition and allowing pHmin to take on lower values resulted in the estimate converging to the new lower bound. In addition, allowing parameter estimates to deviate too far from realistic values caused other parameters to hit bounds. This behavior reflects the fact that nonlinear logistic regression, like its counterpart nonlinear regression with normally distributed error, does not necessarily guarantee convergence to a global optimum. Even when modeling with normally distributed error, a subject for which there is an immense literature (see, for example, references 34 and 42), the behavior of the parameter estimators can be very erratic, with the estimators exhibiting considerable bias and having a highly non-normal distribution. Nonlinear logistic regression is a new field with few published applications, and problems in estimation and interpretation of results should be expected.
Generally, nonlinear logistic regression models are more flexible than models with fixed values of the cardinal parameters, since the extra parameters improve the goodness of fit. Failure to obtain stable solutions in every instance with binomial error distribution is not specific to nonlinear logistic regression but is also experienced using linear logistic regression. This failure usually occurs when the number of trials (or replicates), n, corresponding to each condition (i.e., for a specific combination of temperature, water activity, pH, etc.) is small. Because the information content implicit in binomially distributed error is much lower than that in normally distributed error (i.e., counts are less informative than measures), it may require sample sizes of hundreds for each condition in order to obtain stable outcomes, even for the standard linear logistic model. In our experiments, the number of replicates for each combination of conditions was necessarily small (usually quadruplicate, sometimes only one).
Transition between growth and no growth.
The transition from “likely to grow” conditions (P = 0.9, or 90% likelihood of growth) to “unlikely to grow” conditions (P = 0.1, or 10% likelihood of growth), as predicted from the fitted model, was abrupt as can be seen graphically for combinations of pH and temperatures (Fig. 1a) and pH and water activity (Fig. 1b). The abruptness of the transition between growth or no-growth conditions influenced by pH can be as little as 0.1 to 0.2, which is close to the limit of reproducibility for pH measurements. For temperature and water activity, the transition is much less abrupt, occurring over increments of temperature and water activity that exceed that of measurement or experimental error. Most of the raw data were of the form of all n replicates being observed either to grow or not to grow. For the Scott A strain (521 factor combinations), only four of these combinations gave a response different from “all grew” or “none grew,” while for the L5 strain (541 data points), only seven of these outcomes were not in the “all” or “none” categories. Thus, the experimental data showed an abrupt transition between growth and no growth that is not closely reflected by the results of the mathematical modeling. This abruptness does not indicate inadequate modeling but rather a microbiological reality in which small changes in environmental factors within an experiment may have a strong influence on the position of the interface. These differences are reflected in the predictions of the model at different levels of P.
Validation using other published data.
Both models perform well when their data are compared to the data of George et al. (14), which cover a wide range of pH and temperature (up to 30°C; see Fig. 2) at near-optimal water activity, and of McClure et al. (26), which cover a wide range of pH and water activity (see Fig. 3) at 25°C, which is near optimal for the tolerance of L. monocytogenes to environmental stresses (see, e.g., reference 12). In these comparisons, sufficient details were known about the experimental conditions to use the model for prediction. In contrast, the comparison of model predictions to a collation of growth data from the literature revealed poorer concordance between predictions and observations, with the model generally predicting the growth/no growth interface to lie beyond the region where growth has been reported. This was expected because a worst-case strategy was adopted. For example, inhibitory factors other than those included in the model, such as other acidulants or humectants, preservatives, or other microorganisms, were present in many of the foods tested but could not be included in the model predictions. This led to conservative model predictions. The limitations of using literature data to evaluate model performance have been discussed previously (37, 44).
We found two observations of growth in broths at water activity lower than that at which we observed growth. Those data are from Miller (28), who reported growth of Scott A at an aw of 0.92 with NaCl as a humectant and growth at an aw of 0.90 with glycerol as a humectant, the growth medium being brain heart infusion broth at 28°C and at pH 7.4. The latter observation is consistent with that from reference 12. Many bacteria can tolerate lower water activity when glycerol, which permeates the cell membrane, is the humectant rather than NaCl (17). Under the former conditions, i.e., with NaCl as the humectant, the model for Scott A predicts the probability of growth as 0.38, while the model for L5 predicts the probability of growth as 0.012. At these near-growth-limiting conditions, the predicted response is very sensitive to small differences in input values. For example, the best water activity meters in routine use in food microbiology have a measurement error of the order of ±0.003. Under the conditions reported by Miller (28), if the water activity value were 0.923, the L5 model predicts the probability of growth as 0.49. Thus, either model describes well the observations of Miller (28) within the limits of measurement error.
The minimum pH for the growth of L. monocytogenes was reported to be 4.3 using HCl as the acidulant (12, 14). It was found (45) that L. monocytogenes strains Scott A and L5 were able to grow at pHs as low as 4.23 and 4.25, respectively, in HCl-acidified media. The predictions of the model are consistent with these observations and describe well the limits to growth revealed in Fig. 2 and 3.
Organic acids accentuate the pH inhibition of bacterial growth rates and limits, the magnitude of that inhibition being most dependent on the concentration of the undissociated acid (32, 43, 48), which increases the minimum pH at which growth is observed. The kinetic model from which our interface model is derived explicitly incorporates the assumption that the growth rate is proportional to the concentration of undissociated organic acid (32). We have been unable to identify independent data sets by which to evaluate the predictions of equation 4 for the combined effects of pH and lactic acid.
Bolton and Frank (5) presented data and a modeling approach similar to that described here. Their data describe the effects of water content, brine concentration, and pH on the growth potential of L. monocytogenes. Their experimental media were acidified with lactic acid, but the final concentrations of lactate that resulted at each pH tested were not reported. Thus, a comparison of the predictions of equation 4 to their observations was not attempted.
L. monocytogenes has been reported to grow at temperatures less than 0°C in laboratory media broth (e.g., see references 1 and 46) and in vacuum-packed foods (e.g., see references 3 and 19). However, other reported minimum growth temperatures for L. monocytogenes range from 0.5 to 5.0°C in various broth media (11, 16, 21, 47) and from 3 to 4°C in foods (27, 36, 45). The model describes accurately the data used to generate it, which are mostly in the range of 4 to 30°C (see Fig. 1a). Similarly, for the data shown in Fig. 2 and 3 collected by independent workers (14, 26) under well-controlled conditions, the predictions of equation 4 fitted to L. monocytogenes strains Scott A or L5 provide excellent descriptions of the growth limits of strains Scott A, NCTC 10357, and NCTC 9863. Conversely, while the model predicts well the limits of growth in response to combined pH and temperature constraints in the range of 3 to 35°C reported in the literature (data not shown), at temperatures outside this range the model predictions apparently do not agree well with some of those independent observations. Extrapolation of the model to temperatures above 35°C or below 3°C gave unsatisfactory results for independent data, predicting no growth where it had been reported in several cases, emphasizing that regression models should not be extrapolated beyond the range of the data on which they were based (2). The performance of the model at low temperatures is likely to be of the most practical interest at temperatures in the chill range. Thus, it is essential to resolve whether there are strain differences in the growth potential of L. monocytogenes at chill temperatures or whether there are other factors that increase the growth potential of L. monocytogenes in certain environments. The omission of important factors in a model, and the resultant poor prediction in some cases, has been termed “completeness error” (40). The term containing the parameters d and Tmax was useful in improving the goodness of fit of the model in the suboptimal temperature region but may not be adequate to describe data in the high-temperature region, for which additional experimental data would be needed.
Cold-smoked salmon.
In cold-smoked salmon, water activity, pH, and lactic acid present in the muscle tissue interact to retard microbial growth, while low-temperature storage further extends the shelf life of the product. Typical levels are water activity of 0.96 to 0.98 (with NaCl as a humectant), pH 6, and lactic acid levels of 80 to 100 mM (9), with the recommended storage temperature being typically 5°C. Equation 4 enables prediction of the growth potential of L. monocytogenes under these conditions and, for the most stringent conditions of the ranges indicated above, predicts a probability of growth of 0.58, whereas for the least stringent of that range, P equals 0.69. Results (10) demonstrate that when L. monocytogenes is inoculated onto smoked salmon, growth is possible, but in naturally contaminated salmon, growth was more inhibited, suggesting that other factors, such as smoke components, microbial injury, or the presence of other microorganisms, are important inhibitors of the growth potential of L. monocytogenes.
Conclusions.
The growth or no-growth boundary was previously successfully defined and modeled using only kinetic data (35). In the present study, the good fit to the kinetic data by the probability model is evident (Fig. 1). This may represent an integration of the two extremes, kinetic and probabilistic aspects, of predictive microbiology.
The growth/no growth boundaries at a P value of 0.50 presented in this study may be envisaged as a multidimensional tent enclosing the space where the probability of growth was 100%. The space enclosed by this tent defines part of the hyperspace cloud (4) and is similar to the interpolation region described by Baranyi et al. (2) as the minimum convex polyhedron (MCP). The MCP encompasses the interpolation region containing the combinations of variables tested in a growth rate modeling study. The MCP may provide a rational criterion for designing experiments such that the MCP is maximized to cover the entire growth domain, thus avoiding prediction by extrapolation. Further, if a growth rate model is used to make predictions for extreme conditions, a probability model can supplement that prediction by assessing whether growth is likely. From a model such as equation 4, the position of the interface can be specified by the choice of an appropriate value of P, the probability that growth will occur. Use of a P value of 0.5 may be a suitable choice and is one which we adopt here for exemplification, but more conservative values, such as a P of 0.1 (1 in 10 chance of growth occurring) or less, can be chosen if a greater margin of safety is required.
Though development of probabilistic models received considerable attention in the 1970s and early 1980s (39), until recently there has been greater emphasis on the development of models that predict the rate of microbial growth. Renewed interest in stochastic modeling approaches has aided the development of quantitative microbial risk assessment techniques (22), which aim to describe the most likely levels of exposure to, and the extent of variability in, food-borne microbial risks. The types of models described here, and variations and alternatives such as those described by Bolton and Frank (5), will assist in the development of that science. By evaluating data from our model against a range of independent data sets, we found that the model is valid over a wide range of conditions, but there are some deficiencies that must be resolved before the model can be used with complete confidence.
ACKNOWLEDGMENTS
This work was supported by the Australian Research Council, Meat and Livestock Australia, and TASSAL Ltd., Tasmania, Australia.
We thank F. Grau, Food Science Australia, Brisbane, Australia, for the L. monocytogenes Scott A strain, C. D. Garland, Aquahealth Laboratory, Hobart, Australia, for L. monocytogenes strain L5, a wild-type strain isolated from cold-smoked salmon, and W. T. Ross, Health Canada, for helpful discussions concerning the development of nonlinear logistic regression modeling.
Appendix
The code for performing nonlinear logistic regression using PROC NLIN of SAS (SAS/STAT user's guide, version 6; SAS/STAT software: changes and enhancements, through release 6.11) is as follows: /* DO MLE WITH NONLINEAR LEAST SQUARES*/ proc nlin nohalve sigsq=1 maxiter=20 converge=5.0e-6 data=interfac; /* SET INITIAL PARAMETER VALUES AND THEIR BOUNDS*/ parms b0=0 b1=0 b2=0 b3=0 b4=0 b5=0 b6=0 b7=0 awmin=0.92 Tmin=3.0 pHmin=3.4 Umin=20; bounds −2<Tmin<3.059; bounds 0.88<awmin<0.9279; bounds 3.350<pHmin<3.929; bounds 15.191<Umin<50; /* EXPRESS MODEL*/ y= b0 + b1*log(T-Tmin) + b2*log(T-Tmin)**2 + b3*log(1-exp(0.536*(T-48))) + b4*log(aw-awmin) + b5*log(1-10**(pHmin-pH)) + b6*log(1-10** (pHmin-pH))**2 + b7*log(1-LAC/(Umin*(1+10**(pH-3.86)))); /* CALCULATE EXPECTATION*/ mu=exp(y)/(1+exp(y)); /* CALCULATE WEIGHT AND LOSS FUNCTIONS*/ _weight_=1/(n*mu*(1-mu)); _loss_=(−grow*log(mu) −(n-grow)*log(1-mu))/_weight_; /* MODEL STATEMENT*/ model grow=n*mu; run;
REFERENCES
- 1.Bajard S, Rosso L, Fardel G, Flandrois J P. The particular behaviour of Listeria monocytogenes under sub-optimal conditions. Int J Food Microbiol. 1996;29:201–211. doi: 10.1016/0168-1605(95)00031-3. [DOI] [PubMed] [Google Scholar]
- 2.Baranyi J, Ross T, McMeekin T A, Roberts T A. Effects of parameterisation on the performance of empirical models used in ‘predictive microbiology.’. Food Microbiol. 1996;13:83–91. [Google Scholar]
- 3.Bell R G, Penny N, Moorhead S M. Growth of the psychrotrophic pathogens Aeromonas hydrophila, Listeria monocytogenes and Yersinia enterocolitica on smoked blue cod (Parapercis colias) packed under vacuum or carbon dioxide. Int J Food Sci Technol. 1995;30:515–521. [Google Scholar]
- 4.Boddy L, Wimpenny J W T. Ecological concepts in food microbiology. J Appl Bacteriol Symp. 1992;73(Suppl.):23S–38S. doi: 10.1111/j.1365-2672.1992.tb03622.x. [DOI] [PubMed] [Google Scholar]
- 5.Bolton L F, Frank J F. Defining the growth/no growth interface for Listeria monocytogenes in Mexican-style cheese based on salt, pH and moisture content. J Food Prot. 1999;62:601–609. doi: 10.4315/0362-028x-62.6.601. [DOI] [PubMed] [Google Scholar]
- 6.Budavari S, editor. The Merck index: an encyclopedia of chemicals, drugs, and biologicals. 11th ed. Rahway, N.J: Merck and Co., Inc.; 1989. [Google Scholar]
- 7.Centers for Disease Control and Prevention. Update: multistate outbreak of listeriosis—United States, 1998–1999. Morb Mortal Wkly Rep. 1999;47:1117–1118. [PubMed] [Google Scholar]
- 8.Cole M B, Jones M V, Holyoak C. The effect of pH, salt concentration and temperature on the survival and growth of Listeria monocytogenes. J Appl Bacteriol. 1990;69:63–72. doi: 10.1111/j.1365-2672.1990.tb02912.x. [DOI] [PubMed] [Google Scholar]
- 9.Dalgaard P. Predictive microbiological modelling and seafood quality. In: Luten J, Børresen T, Ohelenschlâger J, editors. Seafood from producer to consumer, integrated approach to quality. Amsterdam, The Netherlands: Elsevier; 1997. pp. 431–443. [Google Scholar]
- 10.Dalgaard P, Jørgensen L V. Predicted and observed growth of Listeria monocytogenes in seafood challenge tests and in naturally contaminated cold-smoked salmon. Int J Food Microbiol. 1998;40:105–115. doi: 10.1016/s0168-1605(98)00019-1. [DOI] [PubMed] [Google Scholar]
- 11.Duh Y-H, Schaffner D W. Modelling the effect of temperature on the growth rate and lag time of Listeria innocua and Listeria monocytogenes. J Food Prot. 1993;56:205–210. doi: 10.4315/0362-028X-56.3.205. [DOI] [PubMed] [Google Scholar]
- 12.Farber J M, Coates F, Daley E. Minimum water activity requirements for the growth of Listeria monocytogenes. Lett Appl Microbiol. 1992;15:103–105. [Google Scholar]
- 13.Food and Agriculture Organization of the United Nations. Report of the FAO expert consultation on the trade impact of Listeria in fish products. FAO fisheries report no. 604. Rome, Italy: Food and Agriculture Organization of the United Nations; 1999. [Google Scholar]
- 14.George S M, Lund B M, Brocklehurst T F. The effect of pH and temperature on initiation of growth of Listeria monocytogenes. Lett Appl Microbiol. 1988;6:153–156. [Google Scholar]
- 15.Gilbert R J, de Louvois J, Donovan T, Hooper W L, Nichols G, Peel R N, Ribeiro C D, Roberts D. Microbiological guidelines for some ready-to-eat foods sampled at the point of sale. PHLS Microbiol Dig. 1996;13:41–43. [Google Scholar]
- 16.Gill C O, Greer G G, Dilts B D. The aerobic growth of Aeromonas hydrophila and Listeria monocytogenes in broths and on pork. Int J Food Microbiol. 1997;35:67–74. doi: 10.1016/s0168-1605(96)01224-x. [DOI] [PubMed] [Google Scholar]
- 17.Gould G W. Drying, raised osmotic pressure and low water activity. In: Gould G W, editor. Mechanisms of action of food preservation procedures. London, United Kingdom: Elsevier Applied Science; 1989. pp. 97–117. [Google Scholar]
- 18.Hosmer D W, Lemeshow S. Applied logistic regression. New York, N.Y: John Wiley & Sons; 1989. [Google Scholar]
- 19.Hudson J A, Mott S J. Growth of Listeria monocytogenes, Aeromonas hydrophila and Yersinia enterocolitica on cold-smoked salmon under refrigeration and mild temperature abuse. Food Microbiol. 1993;10:61–68. doi: 10.1016/0168-1605(93)90055-l. [DOI] [PubMed] [Google Scholar]
- 20.International Commission on Microbiological Specifications for Foods. Micro-organisms in foods, book 5: microbiological specifications of food pathogens. London, United Kingdom: Blackie Academic and Professional; 1996. [Google Scholar]
- 21.Juntilla J T, Niemela S I, Hirn J. Minimum growth temperatures of Listeria monocytogenes and non-haemolytic listeria. J Appl Bacteriol. 1988;65:321–327. doi: 10.1111/j.1365-2672.1988.tb01898.x. [DOI] [PubMed] [Google Scholar]
- 22.Lammerding A M. An overview of microbial food safety risk assessment. J Food Prot. 1997;60:1420–1425. doi: 10.4315/0362-028X-60.11.1420. [DOI] [PubMed] [Google Scholar]
- 23.Leistner L. Hurdle technology applied to meat products of the shelf stable and intermediate moisture food types. In: Simatos D, Multon J L, editors. Properties of water in foods in relation to quality and stability. Dordrecht, The Netherlands: Martinus Nijhoff Publishers; 1985. pp. 309–329. [Google Scholar]
- 24.Leistner L. Allgemeines über Rohwurst. Fleischwirtschaft. 1986;66:290. , 292, 295–300, 359. [Google Scholar]
- 25.Lemeshow S, Le Gall J R. Modeling the severity of illness of ICU patients. JAMA. 1994;272:1049–1055. [PubMed] [Google Scholar]
- 26.McClure P J, Roberts T A, Otto Oguru P. Comparison of the effects of sodium chloride, pH and temperature on the growth of Listeria monocytogenes on gradient plates and in liquid medium. Lett Appl Microbiol. 1989;9:95–99. [Google Scholar]
- 27.Membré J-M, Ross T, McMeekin T A. Behaviour of Listeria monocytogenes under combined chilling processes. Lett Appl Microbiol. 1999;28:216–220. doi: 10.1046/j.1365-2672.1999.00499.x. [DOI] [PubMed] [Google Scholar]
- 28.Miller A. Combined water activity and solute effects on the growth and survival of Listeria monocytogenes Scott A. J Food Prot. 1992;55:414–418. doi: 10.4315/0362-028X-55.6.414. [DOI] [PubMed] [Google Scholar]
- 29.Mossel D A A, Rossem F V, Doopmans M, Hendriks M, Verouden M, Eelderink I. Quality control of solid culture media: a comparison of the classic and the so-called ecometric technique. J Appl Bacteriol. 1980;49:439–454. doi: 10.1111/j.1365-2672.1980.tb04719.x. [DOI] [PubMed] [Google Scholar]
- 30.Mossel D A A, Laarhoven T M G B-V, Ligtenberg-Merdus A M T, Werdler M E B. Quality assurance of selective culture media for bacteria, moulds and yeasts: an attempt at standardization at the international level. J Appl Bacteriol. 1983;54:313–327. doi: 10.1111/j.1365-2672.1983.tb02623.x. [DOI] [PubMed] [Google Scholar]
- 31.Nagelkerke N J D. A note on a general definition of the coefficient of determination. Biometrika. 1991;78:691–692. [Google Scholar]
- 32.Presser K, Ratkowsky D A, Ross T. Modelling the growth rate of Escherichia coli as a function of pH and lactic acid concentration. Appl Environ Microbiol. 1997;63:2355–2360. doi: 10.1128/aem.63.6.2355-2360.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Presser K A, Ross T, Ratkowsky D A. Modelling the growth limits (growth/no growth interface) of E. coli as a function of pH, lactic acid and temperature. Appl Environ Microbiol. 1998;64:1773–1779. doi: 10.1128/aem.64.5.1773-1779.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ratkowsky D A. Nonlinear regression modeling: a unified practical approach. New York, N.Y: Marcel Dekker; 1983. [Google Scholar]
- 35.Ratkowsky D A, Ross T. Modelling the bacterial growth/no growth interface. Lett Appl Microbiol. 1995;20:29–33. [Google Scholar]
- 36.Ross T. A philosophical basis for the development of kinetic models in predictive microbiology. Ph.D. thesis. Hobart, Australia: University of Tasmania; 1993. [Google Scholar]
- 37.Ross T. Indices for performance evaluation of predictive models in food microbiology. J Appl Bacteriol. 1996;81:501–508. doi: 10.1111/j.1365-2672.1996.tb03539.x. [DOI] [PubMed] [Google Scholar]
- 38.Ross T. Predictive food microbiology models in the meat industry. Sydney, Australia: Meat and Livestock Australia; 1999. [Google Scholar]
- 39.Ross T, McMeekin T A. Predictive microbiology—a review. Int J Food Microbiol. 1994;23:241–264. doi: 10.1016/0168-1605(94)90155-4. [DOI] [PubMed] [Google Scholar]
- 40.Ross T, Baranyi J, McMeekin T A. Predictive microbiology and food safety. In: Robinson R, Batt C A, Patel P, editors. Encyclopaedia of food microbiology. London, United Kingdom: Academic Press; 1999. pp. 1699–1710. [Google Scholar]
- 41.Salter M A, Ross T, Ratkowsky D A, McMeekin T A. Modelling the combined temperature and salt (NaCl) limits for growth of a pathogenic Escherichia coli strain using generalised non-linear regression. Int J Food Microbiol. 2000;61:159–167. doi: 10.1016/s0168-1605(00)00352-4. [DOI] [PubMed] [Google Scholar]
- 42.Seber G A F, Wild C J. Nonlinear regression. New York, N.Y: John Wiley & Sons; 1989. [Google Scholar]
- 43.Sorrells K M, Enigl D C, Hatfield J R. Effect of pH, acidulant, time, and temperature on the growth and survival of Listeria monocytogenes. J Food Prot. 1989;52:571–573. doi: 10.4315/0362-028X-52.8.571. [DOI] [PubMed] [Google Scholar]
- 44.Sutherland J P, Bayliss A P, Roberts T A. Predictive modelling of the growth of Staphylococcus aureus: the effects of temperature, pH and sodium chloride. Int J Food Microbiol. 1994;21:217–236. doi: 10.1016/0168-1605(94)90029-9. [DOI] [PubMed] [Google Scholar]
- 45.Tienungoon S. Some aspects of the ecology of Listeria monocytogenes in salmonid aquaculture. Ph.D. thesis. Hobart, Tasmania, Australia: University of Tasmania; 1998. [Google Scholar]
- 46.Walker S J, Archer P, Banks J G. Growth of Listeria monocytogenes at refrigeration temperatures. J Appl Bacteriol. 1990;68:157–162. doi: 10.1111/j.1365-2672.1990.tb02561.x. [DOI] [PubMed] [Google Scholar]
- 47.Wilkins P O, Bourgeois R, Murray R G E. Psychrotrophic properties of Listeria monocytogenes. Can J Microbiol. 1972;18:543–551. doi: 10.1139/m72-087. [DOI] [PubMed] [Google Scholar]
- 48.Young K M, Foegeding P M. Acetic, lactic and citric acids and pH inhibition of Listeria monocytogenes Scott A and the effect on intracellular pH. J Appl Bacteriol. 1993;74:515–520. [PubMed] [Google Scholar]