Abstract
Introduction
Lower limb motor dysfunction is common in patients with stroke, and usually caused by brain neural connectivity disorder. Previous studies have shown that the whole-body vibration training (WBVT) significantly improves the lower limb motor function in patients with stroke and may promote nerve remodelling. The prior purpose of this study is to explore effects of WBVT on lower limb motor function and neuroplasticity in patients with stroke.
Methods
A single-blind randomised controlled trial will be conducted. Sixty patients with stroke will be recruited and allocated randomly to WBVT, routine rehabilitation training (RRT) and control group (CG). The WBVT and RRT interventions will be implemented as five 25 min sessions weekly for continuous 12 weeks; the CG will remain daily habitual living styles and routine treatments, in community or hospital, and will also receive telephone follow-up and health-related lectures. Transcranial magnetic stimulation will be used to assess neural plasticity while lower limb motor function is assessed using indicators of strength, walking ability and joint activity. The assessments will be conducted at the period of baseline, week 6, week 12 as well as on 4 and 8 weeks, respectively, after intervention completion.
Ethics and dissemination
This study has been approved by the Shanghai University of Sport Research Ethics Committee (102772021RT067) and will provide data on the effects of WBVT relative to RRT in terms of the improvement of stroke patients’ lower limb motor function and neural plasticity. The results of this study will be disseminated via publications in peer-reviewed journals and presentations at international conference.
Trial registration number
ChiCTR2200055143.
Keywords: stroke, sports medicine, rehabilitation medicine
Strengths and limitations of this study.
This protocol presents a rigorous design of a randomised controlled trial that aims to explore the effects of whole-body vibration training (WBVT) on lower limb motor function and neuroplasticity in patients who had a stroke.
An accurate measurement tool and multiple indicators will be used to judge the effects of WBVT on neuroplasticity in patients who had a stroke.
The patients will come from one geographic area, which limits the generalisability.
Introduction
Stroke is prevalent and associated with high disability, recurrence and mortality rates.1 The latest global burden of disease study demonstrates that the overall lifetime risk of stroke in China is 39.9%, ranking the first in the world, stroke is also the leading cause among diseases of lost years of life in China.2 Patients with stroke often have a variety of sequelae. The most common is hemiplegia, which is characterised by numbness, weakness of one limb and spasticity. It significantly reduces patients’ abilities to perform daily activities and impacts their quality of life.3 Lower limb motor function can be restored to a limited extent in more than 70% of hemiplegic patients, and most of such cannot obtain a good gait or walking speed.4 Lower limb motor dysfunction after stroke is caused by central nervous system injuries, resulting in abnormal movement patterns.5 Its main characteristics are poor muscle strength,6 spasticity,5 joint instability,7 associated reactions8 and synergy movement.9 Thus, the improvement of affected patients’ muscle strength, balance ability and walking ability is critical in restoring their lower-limb motor function.
Neural plasticity generally refers to the nervous system’s inherent abilitties to make structural and functional changes to adapt to changes in the internal and external environments.10 Changes in neural plasticity after stroke have been shown to be the foundation of the recovery of motor function.11 After unilateral stroke, the neural plasticity includes two aspects: (a) changes in neural synaptic connections and (b) changes in the excitability of various structures. Functional recovery after stroke is related to changes in the motor cortex and other regions regarding the brain’s anatomical structure and function.12 The presence of lesions on one side of the cerebral hemisphere reduces or inhibits the excitability of the motor cortex on that side, while the cortex of the contralateral hemisphere will be hyperexcitable.13 Therefore, maladaptive neural plasticity exists, which regards to the hindered functional recovery of the development of an unwanted symptom, such as compensatory movement pattern, delayed-onset involuntary abnormal movement.14 The primary motor cortex (M1) provides the main outputs to the descending motor system and autonomous motor commands. Such mechanism is closely linked to somatosensory and spatial processing in the parietal lobe, premotor cortex and supplementary motor area (SMA); therefore, changes in M1’s excitability will affect motor function.15 M1 is also defined as the scalp site where the minimum stimulation intensity causes the maximum motor evoked potential (MEP) of the muscle. Thus, changes in the MEP reflect the conditions of motor function.16 The pre-SMA, located in between the prefrontal lobe and motor system, is responsible for functions such as language and idea generation, action recognition, working memory maintenance, learning and the execution of action sequences.17 The enhancement of pre-SMA activity has been found to alleviate M1 disorders in patients with stroke, and changes in connectivity between motor areas may contribute to the improvements of the patients’ motor function.18 Therefore, the recovery of lower limb motor function in patients with stroke correlates to the functional connectivity between cerebral hemispheres as well as the normalisation of the bilateral sensorimotor cortical network.
Exercise intervention therapy has the advantages of compliance, minimal side effects and strong operability. It has become an important means of rehabilitation for patients with stroke.19 Functional recovery after stroke is a complex process. The repeated sensory input is among the most effective means of improving the cortical structure and body function.20 In addition, early intervention, task-oriented training and repetitive intensity are also determinants of motor function recovery after stroke.21 The repeated performance of specific actions during exercise has been found to construct motor memory, which is a form of brain plasticity improvement.22 In addition to improving muscle strength and joint activity, exercise intervention therapy can aid the improvement of neural plasticity and brain function, thereby promoting the recovery of motor function.23 However, patients’ defected motor function and executive function will potentially make it difficult for them to effectively remember and perform complex rehabilitation. In that way, the effectiveness of rehabilitation is compromised.24 Thus, the key to an effective rehabilitation is to enable patients to exercise as much as possible only if within their limited range of physical activity.
Whole-body vibration training (WBVT) is an exercise or treatment method used in sport, physiotherapy and rehabilitation.25 26 During WBVT, people sit, stand or exercise on a vibrating platform that generate vibration.27 WBVT was found to activate the muscle spindles, thereby inducing reflex muscle activation.28 It has also been found to effectively improve the lower limb muscle strength,29 spasticity,30 walking ability31 and balance32 of many people, including patients with stroke33 In addition, a review of the clinical application of WBVT in patients with chronic stroke showed that its main effects include promotion of muscle contraction, stimulation of the proprioceptive system and improvement of motor control ability.34 WBVT has also been shown to increase oxygen consumption and promote the release of vasodilators in patients with stroke, without additional effects on the heart rates or blood pressure.35 Thus, a period of WBVT can improve blood perfusion on the affected side in patients with stroke36 In addition, a transcranial magnetic stimulation (TMS) study revealed significant changes in cortical excitability after vibration training in healthy people.37 The convergence of evidence from several experimental studies suggests that WBVT induces the reorganisation of sensory motor processes in healthy people’s brain.29 It may also promote functional recovery after stroke by enhancing the proprioceptive afferents of the central nervous system.24 A previous TMS study demonstrated that after a period of WBVT, patients with stroke have lower motor thresholds and higher MEP amplitudes, along with improved activation of flexors.32 The study concluded that WBVT is a suitable non-pharmacological therapy to promote the recovery of neural plasticity and motor function in patients with stroke, even if the patients were in the chronic phase.32 Thus, WBVT can effectively improve the motor function of patients with stroke and may also have a strong effect on brain neural plasticity. However, limited research on this topic has been conducted, and scholars have reached different conclusions. In addition, the type and parameter of effective WBVT have not been explicitly identified; further research is needed.
In sum, little research has examined the application of WBVT for the rehabilitation of patients with stroke, and especially the positive and negative impacts of WBVT on these patients’ brain function. Thus, this randomised controlled trial (RCT) was designed to examine the impacts of WBVT on stroke patients’ lower limb motor function and neural plasticity. Lower limb motor function will be evaluated by isokinetic muscle strength and other assessment method, and TMS will be used to examine changes in neural plasticity.
Aims and objectives
This study aims to determine the effect of 12 weeks of WBVT on stroke patients’ lower limb motor function and neural plasticity and explore the difference between WBVT and routine rehabilitation training after 6 and 12 weeks of training. In addition, this study will evaluate and compare it after 4 and 8 weeks of stopping training. The feasibility of a future full-scale RCT will be assessed.
The study objectives are to:
clarify the effects of WBVT on stroke patients’ lower limb motor function and neural plasticity.
Analyse the training effects and maintenance times of the two training methods.
Explore the facilitators, barriers and contextual factors influencing the implementation of WBVT.
Test the acceptability of the data collection procedures used.
Methods and design
Study design
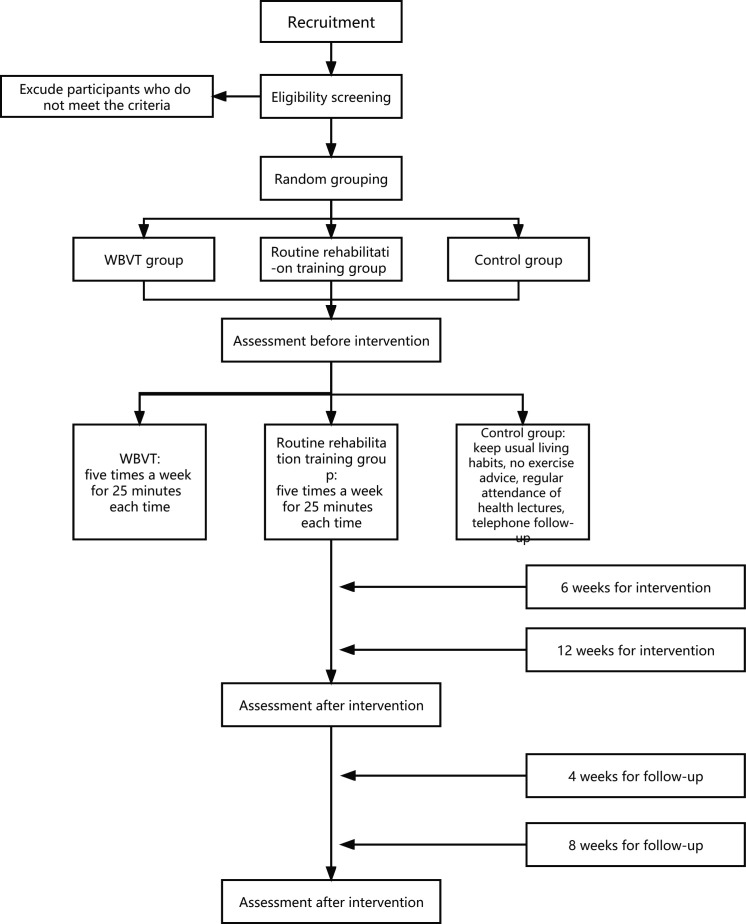
This study was designed as a prospective single-blind RCT. Eligible participants with stroke will be assigned randomly to the WBVT group (WBVG), routine rehabilitation training group (RRTG) and control group (CG) at a ratio of 1:1:1. The CG will maintain daily living and routine treatment, in community or hospital, and will also receive telephone follow-up and lectures. On this basis, the WBVG and RRTG will receive exercise interventions in the Sports Laboratory of Shanghai University of Sport, Shanghai, China. The interventions will be implemented five times a week for 12 weeks and 25 min a day. The training will be arranged from Monday to Friday. Participants will be evaluated at baseline, after 6 and 12 weeks of intervention, and 4 and 8 weeks after intervention termination (figure 1). This research protocol has been approved by the research ethics committee of Shanghai University of Sport (no. 102772021RT067). The study is scheduled to begin in September 2022 and continue until January 2023.
Figure 1.
Diagram of study flow. WBVT, whole-body vibration training.
Participants
Participants in Shanghai will be recruited through community outreach, from outpatient clinics, with media advertising, and by telephone. All participants will follow their routine medication and physical therapy/massage regimens during the study period. They will provide written informed consent before inclusion in the study. Before and after the intervention, data on participants’ demographic and clinical characteristics will be collected and analysed (table 1).
Table 1.
Demographic and clinical characteristics of participants
| WBVG | RRTG | CG | |
| Age | |||
| Gender | |||
| BMI | |||
| Time of illness | |||
| Stroke type | |||
| Affected side | |||
| Whether auxiliary equipment is used | |||
| FMA score | |||
| Berg score | |||
| TUG | |||
| MoCA score | |||
| SF-36 |
BMI, body mass index; CG, control group; FMA, Fugl-Meyer assessment; MoCA, Montreal Cognitive Assessment; RRTG, routine rehabilitation training group; SF-36, MOS Item Short from Health Survey; TUG, timed up and go; WBVG, whole-body vibration training group.
Inclusion and exclusion criteria
The inclusion criteria will be: (a) the included cases met the inclusion criteria of stroke in the classification scheme of various cerebrovascular diseases formulated by the Fourth National Academic Conference on cerebrovascular diseases in 1995 and were confirmed by cranial CT or MRI, (b) Brunnstrom stage IV, (c) ability to stand and walk without the help of another person, (d) stable medical condition, (e) aged 50–75 years, (f) duration of illness ≥3 months, (g) no serious organ disease, (h) no vibration training experience. The exclusion criteria will be: (a) it does not meet the diagnostic criteria of stroke in the classification scheme of various cerebrovascular diseases formulated by the fourth national cerebrovascular disease academic conference in 1995, and there is no head CT or MRI confirmation, (b) other nervous system disease, (c) severe skeletal muscle or cardiovascular disease, (d) severe lumbar disc herniation, (e) dysfunction or failure of the heart, lung, liver, kidney or other major organs, (f) other serious disease or exercise contraindication and (g) vibration training experience.
Sample size
The sample size has been estimated using the G*power statistical software (V.3.1.9.2 for Windows 7×64; Franz Faul, Kiel University, Germany), used widely for this purpose. In this part of the study, the sample size was estimated by F tests: analysis of variance (ANOVA): related measures, between factors: computer required sample size. Under the significance level of 0.05 and repeated-measures ANOVA setting of 80% efficacy, the total number of subjects needed was determined to be 42 (14 per group). Considering a 20% loss rate, we plan to recruit 60 subjects (20 per group).
Randomisation
Eligible participants will be randomised into WBVG, RRTG and CG at 1:1:1 ratio after consenting and baseline assessment. Excel software will be used to code the subjects in 1–60 according to the recruitment time, and then use the formula ‘=RAND ()’ to generate the corresponding random sequence. By sorting the random sequence and then grouping it, 60 subjects will be randomly grouped. These tasks will be completed by professional computer workers blinded to recruitment and allocation after the completion of recruitment.
Interventions
WBVT intervention
Because of the stroke characteristics, patients usually have weak muscle strength in the lower limbs and poor balance, they will better accept low-frequency vibration training,38 which has been shown to be more likely to induce changes in brain nerve excitation.14 The WBVT intervention will be implemented using a vibrating platform (I-vib5050A; Bodygreen, Taiwan) that generates vertical vibrations and has an adjustable frequency range (6–12 Hz). During WBVT sessions, the subjects will wear shoes to stand on a vibrating paltform. The vibration frequency will be increased in a stepwise manner in three phases (weeks 1–4, 5–8 and 9–12) over the 12-week intervention period. The training will consist of adaptation to the vibration (6 Hz, 7 Hz and 7 Hz, respectively, in phases 1–3) with 5 min of static standing, 1 min of rest, two rounds of 5 min of rhythmic half-squat to standing practice (alternation of 60° knee flexion and standing for 5 s each) with vertical vibration (8 Hz, 10Hz and 12 Hz, respectively, in phases 1–3) and 1 min rest between rounds, 5 min of vertical vibration (8 Hz, 10 Hz and 12 Hz, respectively, in phases 1–3) under traction created by the placement of a~4-cm-thick towel under the front sole of the foot to bend the patient’s ankle back and pull the calf muscles with 1 min rest between rounds, and a final 5 min of standing with vibration (6 Hz, 7 Hz and 7 Hz, respectively, in phases 1–3). The peak-to-peak displacement will be maintained at 4 mm in all phases. The participants will be monitored continuously during training, and training will be terminated immediately on complaint of any abnormal condition, such as panic, chest tightness, dizziness or pain (table 2).
Table 2.
Whole-body vibration training schedule
| Time | Vibration time (min) | Schedule (min) | Vibration frequency (Hz) |
| Phase I | |||
| Weeks 1 and 2 | 25 | 5-5-5-5-5 | 6–8 |
| Weeks 3 and 4 | 25 | 5-5-5-5-5 | 6–8 |
| Phase II | |||
| Weeks 5 and 6 | 25 | 5-5-5-5-5 | 7–10 |
| Weeks 7 and 8 | 25 | 5-5-5-5-5 | 7–10 |
| Phase III | |||
| Weeks 9 and 10 | 25 | 5-5-5-5-5 | 7–12 |
| Weeks 11 and 12 | 25 | 5-5-5-5-5 | 7–12 |
Routine rehabilitation exercise intervention
The routine rehabilitation exercise intervention will consist of in situ alternate leg lifting with the feet at shoulder width (while in a safe, stable position and with the help of both hands/arms), in situ squatting (to 60–90°, increasingly gradually according to the patient’s condition) with the feet at shoulder width (while holding a protective rod), in situ heel lifting while on a step with the feet at shoulder width (while holding a protective rod) and walking on a treadmill equipped with safety handrails (table 3). Their exercise intensity will be monitored using the Borg scale (table 4).39
Table 3.
Routine rehabilitation training
| Phase | Exercise | Repetitions/duration |
| I: weeks 1–4 | ||
| Alternating in-situ leg lifts | Two rounds of 30 s, inter-round interval to complete recovery | |
| In-situ squats | Two rounds of 8–10 repetitions, inter-round interval to complete recovery | |
| Step heel lifts | Two rounds of 15 repetitions, inter-round interval to complete recovery | |
| Walking | 5 min | |
| II: weeks 5–8 | ||
| Alternating in-situ leg lifts | Three rounds of 30 s, inter-round intervals to complete recovery | |
| In-situ squats | Three rounds of 8–10 repetitions, inter-round intervals to complete recovery | |
| Step heel lifts | Three rounds of 15 repetitions, inter-round intervals to complete recovery | |
| Walking | 10 min | |
| III: weeks 9–12 | ||
| Alternating in-situ leg lifts | Three rounds of 30 s, inter-round intervals to complete recovery | |
| In-situ squats | Four rounds of 8–10 repetitions, inter-round intervals to complete recovery | |
| Step heel lifts | Four rounds of 15 repetitions, inter-round intervals to complete recovery | |
| Walking | 10 min |
Table 4.
Borg scale
| Level | Description |
| 6 | No exertion at all |
| 7 | Extremely light |
| 8 | Light |
| 9 | Very light (easy, slow walking at a comfortable pace) |
| 10 | This is the effort level where you can’t hear your breath |
| 11 | You are able to easily talk and you can run at this level for a long time |
| 12 | Light (you are building aerobic endurance) |
| 13 | Somewhat hard (you are making quite an effort; you feel tired but can continue) |
| 14 | You start to hear your breath, but are not gasping for air |
| 15 | You can talk, but it is more challenging, you use one- or two-word answers |
| 16 | Hard (this is considered to be your steady state) |
| 17 | Very hard (very strenuous and you are very fatigued) |
| 18 | Your breathing is vigorous, you can’t talk, you are gasping for air |
| 19 | Extremely hard (you are counting the minutes until it ends) |
| 20 | Maximal exertion |
Control group
These participants will be requested to maintain their original habits of lifestyle. They will receive usual care including usual stroke services available to the participants, including but not limited to, medical consultations offered by hospital, rehabilitation services by community-based organisations.
Participants in the CG will receive telephone follow-up and health lectures but will not receive any specific exercise training from the study scheme.
The specific intervention details of the three groups are shown in table 5.40
Table 5.
Exercise intervention TIDieR40
| Item number | Brief name | Group | ||
| WBVG | RRTG | CG | ||
| 1 | Why | WBVT | Routine rehabilitation training | Control |
| 2 | What | 12 weeks training under the guidance of professionals | Maintenance of usual living habits, no exercise advice, regular attendance of health lectures, telephone follow-up | |
| 3 | What (content) | 25 min exercise, five times/week | Attendance of fortnightly health lectures, monthly telephone interviews | |
| 4 | What (procedure) | Evaluation at baseline, 6 weeks and 12 weeks, and 4 and 8 weeks after intervention termination, reporting of results to participants so that they can understand the physical changes occurring | ||
| 5 | Who (administrators) | WBVT coach is a professional rehabilitation physician, assessments performed by Shanghai University of Sport PhD students | Routine rehabilitation training coach is a professional rehabilitation physician, assessments performed by Shanghai University of Sport PhD students | Health lectures and telephone interviews performed by Shanghai University of Sport PhD students (College of Physical Education and Training) |
| 6 | How | The exercise interventions will take place in a stationary gymnasium, the instructors will direct the whole group face to face | The health lectures will be held in the conference room of the College of Physical Education and Training, Shanghai University of Sport | |
| 7 | When and how much | See table 2 | See table 3 | Health lectures, 30–50 min; interviews, 10 min |
| 8 | How well | Participants will receive regular feedback, including physical and psychological data and reports on their motor skills learning performance; they will be kept up to date on their progress and status to keep them engaged | ||
CG, control group; RRTG, routine rehabilitation training group; TIDieR, Template for intervention Description and Replication; WBVG, whole-body vibration training group; WBVT, whole-body vibration training.
Transcranial magnetic stimulation (TMS) protocol
Electromyographic recording
Surface electromyograms will be recorded from the rectus femoris (RF) muscle with 9-mm-diameter Ag-AgCl surface electrodes. The electrode will be placed on the muscle belly of the RF, and the reference electrode will be located above the patella (figure 2). The signal will be amplified (1000×), bandpass filtered (2–2.5 kHz; Intronix Technologies Model), digitised at 5 kHz by an analogue–digital interface (Micro1401; Cambridge Electronics Design, Cambridge, UK) and saved for offline analysis.
Figure 2.
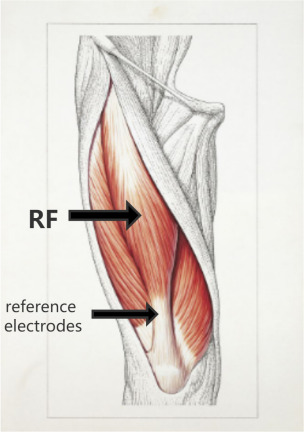
EMG acquisition site. RF, rectus femoris.
TMS
TMS will be applied to the bilateral M1 with a figure-of-eight-shaped coil (7 cm external loop diameter) connected to two single-pulse monophasic stimulators (Magstim, Whitland, Dyfeld, UK). The M1 hotspot will be defined as the scalp location inducing the largest peak–peak MEP amplitude in the contralateral RF muscle. The handle of the test stimulus (TS) coil will be angled posteriorly 30–45° from the midsagittal line. TS1mV will be defined as the lowest TMS intensity required to generate MEPs of 1 mV in the relaxed RF muscle in at least 5 of 10 trials. The resting motor threshold (RMT) will be defined as the lowest TMS intensity required to generate MEPs>50 V in at least 5 of 10 trials with the target muscle completely relaxed.41
Isokinetic strength assessment protocol
Due to the particularities of the participants’ conditions, for safety reasons and based on previous isokinetic muscle strength research, the angular velocity for isokinetic strength testing in both lower limbs will 60°/s. The testing instrument will be warmed up and debugged before assessment. The assessment will be performed after an adaptability exercise with the participant’s body fixed and his or her hands placed in front of the chest. The test action will be repeated five times with intervening 90 s rest intervals. The average peak torque of the flexor and extensor muscles of the knee joint will be taken as the measure of strength. The peak torque is the gold-standard measure for isokinetic assessment and has shown high degrees of accuracy and repeatability.42
Outcomes
Primary outcome
Neural plasticity
MEP amplitude
MEPs will be recorded during TMS. MEP amplitudes will be measured as peak-to-peak values.
Short-interval intracortical inhibition
The intensity of the conditioning stimulus (CS) is 80% RMT or 90% RMT, the intensity of TS is 1 MV. The interstimulus intervals (ISIs) will be 2, 3 and 4 ms. Each block will contain 40 trials in random order.43 44
M1–pre-SMA connectivity
To investigate changes in connectivity between the left M1 and pre-SMA after long-term exercise training, the two high-power Magstim 200 devices and two figure-of-eight coil sites will be performed with TMS. Coil placement will be performed as in a similar hemispheric study to avoid overlap.45 The smaller CS coil will be placed over the right hemisphere to induce a medially directed current in the brain and will be used to stimulate the pre-SMA. The TS coil will be placed over the leg representation of the left hemisphere for the induction of a posterior–anterior current in the brain. The CS will be delivered by an octagonal coil (50 mm diameter) to stimulate the pre-SMA. Its intensity will be 110% or 90% of the RMT. The angle between the placement direction and the scalp midline will be 45° to induce a front-to-back current.46 The TS (M1) intensity will be set to evoke a resting MEP with the same TMS coil. The ISIs will be 6, 8, 10, 30, 40 and 50 ms. The strength of the CS will be changed for each block and complete the order pseudo-random for each subject block. Each block will contain 60 trials. Separate TS will be collected 10 times. The MEP of each ISI will be collected 10 times, for a total of 70 measurements. Each block will contain 70 trials in random order.
Lower-limb motor function
Peak torque
Participants’ lower limb flexion and extension muscle strength will be measured by using the Biodex isokinetic testing system (Biodex Medical System 4, New York)47 at all assessment timepoints.
Brunnstrom stage
The Brunnstrom approach is a set of treatment methods for dyskinesia after central nervous system injury developed by Swedish physiotherapist Signe Brunnstrom. Motor function recovery is divided into six stages, with muscle tension increasing gradually from low to high and joint reaction, joint movement and spasm gradually becoming significant. With the completion of common motion, separation motion and fine motion appear until they completely return to normal.48
Fugl-Meyer assessment
Fugl-Meyer assessment (FMA) is a simplified, time-saving means of evaluating upper and lower limb motor function. The index comprises upper limb (66 points) and lower limb (34 points) items (total, 100 points). Higher scores reflect better functional recovery. FMA scores can be used to characterise the severity of dyskinesia in stroke patients. Only the lower limb FMA items will be applied in this study. The passive range of motion of each joint of each participant will be determined before FMA. During the assessment, the non-hemiplegic side will be evaluated first, followed by the hemiplegic side.49
Timed up-and-go test
The timed up-and-go test is used to assess patients’ mobility, balance, walking ability and fall risk. The participant will sit in a standard armchair with his or her back touching the chair and arms on the armrests. Assistive devices for walking will be placed near the chair. He or she will then be asked to walk to a sign placed at a distance of 3 m at a safe and normal speed, turn around, walk back to the chair and sit down. The test is complete when the participant’s hip touches the seat, and the time taken to complete it (in seconds) will be recorded.50
Berg balance test
The Berg balance test includes 14 actions, with performance scored on a 0–4 scale (total possible score, 56). Higher scores reflect better balance function. Scores of 0–20 indicate that a patient is safe with wheelchair use, scores of 21–40 indicate that the patient should use an assistive device to walk, and scores of 41–56 indicate that the patient can walk independently; thus, scores <40 indicate a fall risk.51
Patients and public involvement
Participants have not been involved in the study recruitment. The author conceived the initial research questions and outcome measures and modified according to the telephone interviews with patients and their guardians by a research assistant. In order to assure the safety and feasibility of the intervention, 10 patients with stroke will be invited to learn and practise the WBVT and routine rehabilitation training before designing the RCT. WBVT and routine rehabilitation training were revised based on the exercise performance and feedback provided by the participants. The burden of the intervention will be assessed by patients and their advisors through face-to-face interviews before signing informed consent. The findings of the study will be disseminated to the participants and their guardians.
Statistical analysis
The statistical analysis will be performed by designated members of the research group who will be blinded to participants’ group allocations. All statistical analyses will be conducted using IBM SPSS V.24.0. All quantitative data will be summarised and presented using appropriate descriptive statistics, and baseline data from the WBVG, RRTG and CG will be analysed using the independent-samples t test. To explore the effects of the training interventions on stroke patients’ motor function and neural plasticity, repeated-measures ANOVA will be used to examine differences in outcomes between and within groups at all assessment timepoints (table 6).
Table 6.
Overview of the analysis of differences among study groups
| Group | Baseline | 12 weeks | 4 weeks after intervention | F (P value) group effect | F (P value) interaction effect | |
| FMA | WBVT RRT Control |
|||||
| TUG | WBVT RRT Control |
|||||
| Berg | WBVT RRT Control |
|||||
| Brunnstrom | WBVT RRT Control |
|||||
| Peak torque | WBVT RRT Control |
|||||
| Mep amplitude | WBVT RRT Control |
|||||
| SICI | WBVT RRT Control |
|||||
| M1-pre- SMA | WBVT RRT Control |
|||||
| MoCA | WBVT RRT Control |
|||||
| SF-36 | WBVT RRT Control |
FMA, Fugl-Meyer assessment; M1, primary motor cortex; MoCA, Montreal Cognitive Assessment; pre-SMA, pre-supplementary motor area; RRT, routine rehabilitation training; SF-36, the MOS item short from health survey; SICI, short-interval intracortical inhibition; TUG, time up and go; WBVT, whole-body vibration training.
Ethics and dissemination
All individuals who meet the study criteria will be required to sign an informed consent from prior to enrollment in the study. This study protocol has been approved by the Shanghai University of Sport Research Ethics Committee (102772021RT067). Study findings will be disseminated via publication in peer-reviewed journals and presentations at international conferences.
Supplementary Material
Footnotes
Contributors: MZ: data curation, writing—original draft preparation, writing—reviewing and editing. JW: visualisation, investigation. MZ and JW have the same contribution to the article. XW: conceptualisation, methodology, SoftwarePriya.
Funding: This study was supported by grants from the Research supported by The Program for Overseas High-level talents at Shanghai Institutions of Higher Learning (TP2020063).
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were involved in the design, or conduct, or reporting, or dissemination plans of this research. Refer to the Methods section for further details.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Patient consent for publication
Consent obtained directly from patient(s)
References
- 1.Benjamin EJ, Virani SS, Callaway CW, et al. Heart disease and stroke Statistics-2018 update: a report from the American heart association. Circulation 2018;137:e67–492. 10.1161/CIR.0000000000000558 [DOI] [PubMed] [Google Scholar]
- 2.Brief report on stroke prevention and treatment in China,2019. Chinese J Cerebrovasc Dis 2020:272–81. doi:CNKI:SUN:NXGB.0.2020-05-009 [Google Scholar]
- 3.Wist S, Clivaz J, Sattelmayer M. Muscle strengthening for hemiparesis after stroke: a meta-analysis. Ann Phys Rehabil Med 2016;59:114–24. 10.1016/j.rehab.2016.02.001 [DOI] [PubMed] [Google Scholar]
- 4.Zhu Y. Data from: Effects of Modified Constraint-induced Movement Therapyon Walking Ability and Gait in Stroke Patients with Hemiplegic. Shanghai University of Sport, PhD dissertation, 2016. [Google Scholar]
- 5.Broderick P, Horgan F, Blake C, et al. Mirror therapy for improving lower limb motor function and mobility after stroke: a systematic review and meta-analysis. Gait Posture 2018;63:208–20. 10.1016/j.gaitpost.2018.05.017 [DOI] [PubMed] [Google Scholar]
- 6.Ramsay JW, Barrance PJ, Buchanan TS, et al. Paretic muscle atrophy and non-contractile tissue content in individual muscles of the post-stroke lower extremity. J Biomech 2011;44:2741–6. 10.1016/j.jbiomech.2011.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oh D-S, Choi J-D. The effect of motor imagery training for trunk movements on trunk muscle control and proprioception in stroke patients. J Phys Ther Sci 2017;29:1224–8. 10.1589/jpts.29.1224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kline TL, Schmit BD, Kamper DG. Exaggerated interlimb neural coupling following stroke. Brain 2007;130:159–69. 10.1093/brain/awl278 [DOI] [PubMed] [Google Scholar]
- 9.Fulk GD, Ludwig M, Dunning K, et al. Estimating clinically important change in gait speed in people with stroke undergoing outpatient rehabilitation. J Neurol Phys Ther 2011;35:82–9. 10.1097/NPT.0b013e318218e2f2 [DOI] [PubMed] [Google Scholar]
- 10.Liang Y. Data from: Effect of Pro Kin balance training combined with suspension training on lower limb motor function of stroke patients. Shengyang Sports University, MA thesis, 2021. [Google Scholar]
- 11.Qi L. Data from: Effects of repetitive transcranial magnetic stimulation on lower extremity motor function in patients after stroke. Qingdao University, MA thesis, 2019. [Google Scholar]
- 12.Wang L, Yu C, Chen H, et al. Dynamic functional reorganization of the motor execution network after stroke. Brain 2010;133:1224–38. 10.1093/brain/awq043 [DOI] [PubMed] [Google Scholar]
- 13.Corti M, Patten C, Triggs W. Repetitive transcranial magnetic stimulation of motor cortex after stroke: a focused review. Am J Phys Med Rehabil 2012;91:254–70. 10.1097/PHM.0b013e318228bf0c [DOI] [PubMed] [Google Scholar]
- 14.Dimyan MA, Cohen LG. Neuroplasticity in the context of motor rehabilitation after stroke. Nat Rev Neurol 2011;7:76–85. 10.1038/nrneurol.2010.200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Graziano MSA, Aflalo TN. Mapping behavioral repertoire onto the cortex. Neuron 2007;56:239–51. 10.1016/j.neuron.2007.09.013 [DOI] [PubMed] [Google Scholar]
- 16.Wang J. Data from: TMS modulation effect:Individualized precise- localization of hand motor area and evaluation of its aftereffect. Shanghai University of Sport, PhD dissertation, 2020. [Google Scholar]
- 17.Nachev P, Wydell H, O'neill K, et al. The role of the pre-supplementary motor area in the control of action. Neuroimage 2007;36 Suppl 2:T155–63. 10.1016/j.neuroimage.2007.03.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lazaridou A, Astrakas L, Mintzopoulos D, et al. fMRI as a molecular imaging procedure for the functional reorganization of motor systems in chronic stroke. Mol Med Rep 2013;8:775–9. 10.3892/mmr.2013.1603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang Y. China stroke report 2019 (Chinese version) (1). Chinese Journal of Stroke 2020;15:1037–43. doi:CNKI:SUN:ZUZH.0.2020-10-001 [Google Scholar]
- 20.Ward NS, Cohen LG. Mechanisms underlying recovery of motor function after stroke. Arch Neurol 2004;61:1844–8. 10.1001/archneur.61.12.1844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rensink M, Schuurmans M, Lindeman E, et al. Task-oriented training in rehabilitation after stroke: systematic review. J Adv Nurs 2009;65:737–54. 10.1111/j.1365-2648.2008.04925.x [DOI] [PubMed] [Google Scholar]
- 22.Classen J, Liepert J, Wise SP, et al. Rapid plasticity of human cortical movement representation induced by practice. J Neurophysiol 1998;79:1117–23. 10.1152/jn.1998.79.2.1117 [DOI] [PubMed] [Google Scholar]
- 23.Zhang ZG, Chopp M. Neurorestorative therapies for stroke: underlying mechanisms and translation to the clinic. Lancet Neurol 2009;8:491–500. 10.1016/S1474-4422(09)70061-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lo SHS, Chau JPC, Choi KC, Choi KC, et al. Feasibility of a ballet-inspired low-impact at-home workout programme for adults with stroke: a mixed-methods exploratory study protocol. BMJ Open 2021;11:e045064. 10.1136/bmjopen-2020-045064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rauch F, Sievanen H, Boonen S, et al. Reporting whole-body vibration intervention studies: recommendations of the International Society of musculoskeletal and neuronal interactions. J Musculoskelet Neuronal Interact 2010;10:193–8. 10.1080/0305006910270105 [DOI] [PubMed] [Google Scholar]
- 26.van Heuvelen MJG, Rittweger J, Judex S, et al. Reporting guidelines for whole-body vibration studies in humans, animals and cell cultures: a consensus statement from an international group of experts. Biology 2021;10:965. 10.3390/biology10100965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wuestefeld A, Fuermaier ABM, Bernardo-Filho M, et al. Towards reporting guidelines of research using whole-body vibration as training or treatment regimen in human subjects-a delphi consensus study. PLoS One 2020;15:e0235905. 10.1371/journal.pone.0235905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huang M, Miller T, Ying M, et al. Whole-body vibration modulates leg muscle reflex and blood perfusion among people with chronic stroke: a randomized controlled crossover trial. Sci Rep 2020;10:1–11. 10.1038/s41598-020-58479-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Celletti C, Suppa A, Bianchini E, et al. Promoting post-stroke recovery through focal or whole body vibration: criticisms and prospects from a narrative review. Neurol Sci 2020;41:11–24. 10.1007/s10072-019-04047-3 [DOI] [PubMed] [Google Scholar]
- 30.Chan K-S, Liu C-W, Chen T-W, et al. Effects of a single session of whole body vibration on ankle plantarflexion spasticity and gait performance in patients with chronic stroke: a randomized controlled trial. Clin Rehabil 2012;26:1087–95. 10.1177/0269215512446314 [DOI] [PubMed] [Google Scholar]
- 31.Hilgers C, Mündermann A, Riehle H, et al. Effects of whole-body vibration training on physical function in patients with multiple sclerosis. NeuroRehabilitation 2013;32:655–63. 10.3233/NRE-130888 [DOI] [PubMed] [Google Scholar]
- 32.El-Shamy SM. Effect of whole-body vibration on muscle strength and balance in diplegic cerebral palsy: a randomized controlled trial. Am J Phys Med Rehabil 2014;93:114–21. 10.1097/PHM.0b013e3182a541a4 [DOI] [PubMed] [Google Scholar]
- 33.Sañudo B, Taiar R, Furness T, et al. Clinical approaches of whole-body vibration exercises in individuals with stroke: a narrative revision. Rehabil Res Pract 2018;2018:1–8. 10.1155/2018/8180901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Murillo N, Valls-Sole J, Vidal J, et al. Focal vibration in neurorehabilitation. Eur J Phys Rehabil Med 2014;50:231–42. 10.1186/1743-0003-11-47 [DOI] [PubMed] [Google Scholar]
- 35.Liao L-R, Ng GYF, Jones AYM, et al. Cardiovascular stress induced by whole-body vibration exercise in individuals with chronic stroke. Phys Ther 2015;95:966–77. 10.2522/ptj.20140295 [DOI] [PubMed] [Google Scholar]
- 36.Alashram AR, Annino G, Annino G. Effects of whole-body vibration on motor impairments in patients with neurological disorders: a systematic review. Am J Phys Med Rehabil 2019;98:1084–98. 10.1097/PHM.0000000000001252 [DOI] [PubMed] [Google Scholar]
- 37.Binder C, Kaya AE, Liepert J. Vibration prolongs the cortical silent period in an antagonistic muscle. Muscle Nerve 2009;39:776–80. 10.1002/mus.21240 [DOI] [PubMed] [Google Scholar]
- 38.Issurin VB, Tenenbaum G. Acute and residual effects of vibratory stimulation on explosive strength in elite and amateur athletes. J Sports Sci 1999;17:177–82. 10.1080/026404199366073 [DOI] [PubMed] [Google Scholar]
- 39.Kamps A, Schüle K. Cyclic movement training of the lower limb in stroke rehabilitation. Neurol Rehabil 2005;11:1–12. [Google Scholar]
- 40.Hoffmann TC, Glasziou PP, Boutron I, et al. Better reporting of interventions: template for intervention description and replication (TIDieR) checklist and guide. BMJ 2014;348:g1687. 10.1136/bmj.g1687 [DOI] [PubMed] [Google Scholar]
- 41.O'Shea J, Sebastian C, Boorman ED, et al. Functional specificity of human premotor-motor cortical interactions during action selection. Eur J Neurosci 2007;26:2085–95. 10.1111/j.1460-9568.2007.05795.x [DOI] [PubMed] [Google Scholar]
- 42.Pontes SS, de Carvalho ALR, Almeida KdeO, et al. Effects of isokinetic muscle strengthening on muscle strength, mobility, and gait in post-stroke patients: a systematic review and meta-analysis. Clin Rehabil 2019;33:381–94. 10.1177/0269215518815220 [DOI] [PubMed] [Google Scholar]
- 43.Kujirai T, Caramia MD, Rothwell JC, et al. Corticocortical inhibition in human motor cortex. J Physiol 1993;471:501–19. 10.1113/jphysiol.1993.sp019912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ziemann U, Rothwell JC, Ridding MC. Interaction between intracortical inhibition and facilitation in human motor cortex. J Physiol 1996;496:873–81. 10.1113/jphysiol.1996.sp021734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ye Z, Altena E, Nombela C, et al. Selective serotonin reuptake inhibition modulates response inhibition in parkinson's disease. Brain 2014;137:1145–55. 10.1093/brain/awu032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang Y, Cao N, Lin Y, et al. Hemispheric differences in functional interactions between the dorsal lateral prefrontal cortex and ipsilateral motor cortex. Front Hum Neurosci 2020;14:202. 10.3389/fnhum.2020.00202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nascimento LR, Teixeira-Salmela LF, Polese JC, et al. Strength deficits of the shoulder complex during isokinetic testing in people with chronic stroke. Braz J Phys Ther 2014;18:268–75. 10.1590/bjpt-rbf.2014.0030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Iii F, John W, Merkas J. The effect of exercise training on gait, balance, and physical fitness asymmetries in persons with chronic neurological conditions: a systematic review of randomized controlled trials. Frontiers in Physiology 2020;11:1316. 10.3389/fphys.2020.585765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fernandez-Gonzalo R, Fernandez-Gonzalo S, Turon M, et al. Muscle, functional and cognitive adaptations after flywheel resistance training in stroke patients: a pilot randomized controlled trial. J Neuroeng Rehabil 2016;13:1–11. 10.1186/s12984-016-0144-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Munari D, Pedrinolla A, Smania N, et al. High-intensity treadmill training improves gait ability, VO2peak and cost of walking in stroke survivors: preliminary results of a pilot randomized controlled trial. Eur J Phys Rehabil Med 2018;54:408–18. 10.23736/S1973-9087.16.04224-6 [DOI] [PubMed] [Google Scholar]
- 51.Gordon CD, Wilks R, McCaw-Binns A. Effect of aerobic exercise (walking) training on functional status and health-related quality of life in chronic stroke survivors: a randomized controlled trial. Stroke 2013;44:1179–81. 10.1161/STROKEAHA.111.000642 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.