Abstract
Background
Venous access is an essential part of caring for the sick neonate. However, problems such as contamination of fluids with bacteria, endotoxins and particulates have been associated with intravenous infusion therapy. Intravenous in‐line filters claim to be an effective strategy for the removal of bacteria, endotoxins and particulates associated with intravenous therapy in adults and are increasingly being recommended for use in neonates.
Objectives
To determine the effect of intravenous in‐line filters on morbidity and mortality in neonates.
Search methods
We used the standard search strategy of the Cochrane Neonatal Review Group. We searched the electronic databases MEDLINE (from 1966 to May, 2015), EMBASE (from 1980 to May, 2015), CINAHL (from 1982 to May 2015) and the Cochrane Central Register of Controlled Trials (CENTRAL; 2015, Issue 5). We did not impose any language restrictions. Further searching included cross references, abstracts, conferences, symposia proceedings, expert informants and journal handsearching.
Selection criteria
We included randomised controlled trials (RCTs) or quasi‐RCTs that compared the use of intravenous in‐line filters with placebo or nothing in neonates.
Data collection and analysis
We followed the procedures of the Cochrane Neonatal Review Group throughout. We checked titles and abstracts identified from the search. We obtained the full text of all studies of possible relevance. We independently assessed the trials for their methodological quality and subsequent inclusion in the review. We contacted authors for further information as needed. Statistical analysis followed the procedures of the Cochrane Neonatal Review Group.
Main results
There were four eligible studies that recruited a total of 704 neonates. This review of low to very low quality evidence found that the use of in‐line filters compared with unfiltered fluids for intravenous infusion had no statistically significant difference in effectiveness on overall mortality (typical RR 0.87, 95% CI 0.52 to 1.47; typical RD ‐0.01, 95% CI ‐0.06 to 0.04; two studies, 530 infants), proven and suspect septicaemia (typical RR 0.86, 95% CI 0.59 to 1.27; typical RD ‐0.02, 95% CI ‐0.09 to 0.04; two studies, 530 infants), or other secondary outcomes (including local phlebitis and thrombus, necrotising enterocolitis, duration of cannula patency, length of stay in hospital, number of catheters inserted and financial costs).
Authors' conclusions
There is insufficient evidence to recommend the use of intravenous in‐line filters to prevent morbidity and mortality in neonates.
Plain language summary
Intravenous in‐line filters for preventing morbidity and mortality in neonates
Review question: Does the use of in‐line filters on intravenous lines reduce morbidity and mortality in neonates?
Background: Preterm or sick newborn infants are often fed with nutrients and fluids that are delivered directly into a vein. This intravenous delivery can be associated with infection, toxins released by bacteria, and tiny particles that may be in the fluids, such as rubber and plastic, going into the blood. In adults, placing a filter in the intravenous line has been reported to be effective in reducing such risks, and filters are increasingly being recommended for use in newborn infants.
Study characteristics: The review authors searched the medical literature and identified four eligible studies that recruited a total of 704 newborns.
Key findings: Septicaemia and illness, deaths or problems with the intravenous lines were no different with or without a filter.
Conclusions: There is insufficient evidence to recommend the use of intravenous in‐line filters to prevent morbidity and mortality in newborn infants.
Summary of findings
Summary of findings for the main comparison. Intravenous in‐line filter for preventing morbidity and mortality in neonates.
| Intravenous in‐line filter for preventing morbidity and mortality in neonates | ||||||
| Patient or population: patients with preventing morbidity and mortality in neonates Settings: Intervention: Intravenous in‐line filter | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Intravenous in‐line filter | |||||
| Mortality during hospitalisation number of infants with event | 112 per 1000 | 98 per 1000 (58 to 165) | RR 0.87 (0.52 to 1.47) | 530 (2 studies) | ⊕⊕⊕⊝ moderate1,2,3 | |
| Risk of proven septicaemia per infant number of infants with the event | 174 per 1000 | 150 per 1000 (103 to 222) | RR 0.86 (0.59 to 1.27) | 530 (2 studies) | ⊕⊕⊕⊝ moderate1,3,4 | |
| Suspected septicaemia number of infants with the event | 159 per 1000 | 91 per 1000 (29 to 288) | RR 0.57 (0.18 to 1.81) | 88 (1 study) | ⊕⊕⊝⊝ low1,3,4,5 | |
| Localised thrombi number of infants with event | 45 per 1000 | 9 per 1000 (0 to 184) | RR 0.2 (0.01 to 4.05) | 88 (1 study) | ⊕⊕⊝⊝ low1,3,4,5 | |
| Proven necrotising enterocolitis number of infants with the event | 45 per 1000 | 9 per 1000 (0 to 184) | RR 0.2 (0.01 to 4.05) | 88 (1 study) | ⊕⊕⊝⊝ low1,3,5 | |
| Composite thrombus, proven or unproven sepsis, necrotising enterocolitis number of infants with event | 477 per 1000 | 181 per 1000 (91 to 367) | RR 0.38 (0.19 to 0.77) | 88 (1 study) | ⊕⊕⊕⊝ moderate1,3,4 | |
| Localised phlebitis number of infants with the event | 16 per 1000 | 19 per 1000 (6 to 60) | RR 1.22 (0.4 to 3.77) | 641 (3 studies) | ⊕⊕⊝⊝ low1,3,5 | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 All studies were at high risk of bias 2 Large difference in relative risk estimates between studies 3 Unable to assess publication bias as <10 studies were included in the meta‐analysis 4 Single study 5 Low event rates
Background
Description of the condition
Venous access is an essential part of caring for the sick neonate; however, problems associated with intravenous infusion therapy include contamination of fluids with bacteria, endotoxins and particulates (Bethune 2001; Evans 2006).
Infusion therapy carries a risk for catheter‐associated septicaemia (Evans 2006; Geiss 1992). Infection can originate from the catheter tubing, the ports, at the cannula site or from contaminated infusion fluid. Factors cited as increasing the risk for catheter‐related infection include type of intravenous fluid, for example total parenteral nutrition solutions or solutions with high concentrations of dextrose (Pearson 1996). While not all infections lead to septicaemia, immunocompromised patients such as neonates, are at greater risk, and infection becomes a major problem (Ng 1989). In adult patients, use of the bacterial retention filter lead to fewer clinically significant bacteremias (Quercia 1986).
Contamination of intravenous administration sets with gram‐negative bacteria has been reported to lead to rapid proliferation of endotoxins (Bethune 2001). In adults, endotoxins have been implicated in several serious disease processes, including respiratory distress syndrome (Parsons 1989), septic shock (Glauser 1991), multiple organ failure, endotoxic shock, and systemic inflammatory response syndrome (Casale 1990; Glauser 1991; Suffredini 1989a). Cardiovascular changes such as increased heart rate, decreased vascular resistance and depressed left ventricular function (Suffredini 1989b), and increased intestinal permeability (O'Dwyer 1988) have also been reported. Periventricular leukomalacia is an ischaemic lesion of the periventricular white matter that is primarily seen in premature neonates (Hill 1992; Volpe 2001). Animal studies have demonstrated the development of periventricular leukomalacia in the brains of newborn kittens following injection of endotoxins (Gilles 1977) and it has been postulated that endotoxins may be involved in the pathogenesis of a proportion of cases of periventricular leukomalacia in the human neonate (Volpe 2001).
Particulate matter may cause localised phlebitis (Marshall 1987). The duration of cannulation has been found to contribute to the development of infusion‐related phlebitis (Maki 1991), and this may require the cannula to be replaced. Frequent cannula change is an added cost to treatment and may cause the patient pain and distress (Chee 2002).
Adverse systemic effects of particulate matter including granulomata formation in the lung (Marshall 1987) and ischaemic necrosis, are a common finding in necrotising enterocolitis (Ballance 1990). Garvan examined intravenous fluids available in Australia, England, Europe and the United States of America for the presence of particulates. Microscopic analysis found rubber particles, crystals, cellulose fibres, fungal spores, starch granules, and a crustacean claw (Garvan 1964). More recent studies found glass fragments from the opening of glass ampoules (Shaw 1985), and particles from rubber stoppers and intravenous equipment (Kirkpatrick 1988). Inorganic elements such as calcium, silicon, aluminium, lead and iron, that may have originated from the manufacture and packaging processes (Backhouse 1987), have also been found. Positively‐charged in‐line filters are reported to be effective in the retention of endotoxins (Barnett 1996).
Description of the intervention
There are two main intravenous filter pore sizes; the 0.22 micron filter is used for aqueous solutions, and the 1.2 micron filter is recommended for larger molecule solutions such as lipids. The 0.22 micron filter has also been reported to remove air, micro‐organisms and particulate matter. In addition, endotoxin retention is reportedly achieved by using a positively charged filter membrane; toxic macro‐molecules are released by gram‐negative bacteria and are claimed to be effective for up to 96 hours (Bethune 2001).
How the intervention might work
Intravenous in‐line filters were conceived and first utilised in the 1960s for the retention of particulate contamination. Since then, filter systems have been further refined. Intravenous in‐line filters are currently claimed to be an effective strategy for the removal of bacteria, endotoxins and particulates associated with intravenous therapy in adults (Ball 2003; Kunac 1999), and more favourable patient outcomes, such as shorter hospital length of stay (Koekenberg 1983). Several adult studies have shown that intravenous in‐line filtration significantly reduces the incidence (Chee 2002; Roberts 1994) and delays the onset of phlebitis (Chee 2002; Allcutt 1983; Roberts 1994), resulting in extended‐line survival (Roberts 1994), fewer recannulations (Chee 2002) and lower costs.The use of in‐line filters in adults has been shown to be effective in the removal of particulates,especially those precipitates caused from the administration of drugs, such as antibiotics (Ball 2003; Chee 2002; Roberts 1994). In addition, a large randomised trial found that in‐line filtration reduced severe complications and length of stay in the paediatric intensive care unit (Jack 2012).
Why it is important to do this review
Several authors have challenged the benefits of using intravenous in‐line filters in the adult population. The Centre for Disease Control and Prevention recommends the filtration of all infusates during the manufacturing process in preference to the use of intravenous in‐line filters as a more cost‐effective and practical way to remove particulates (O'Grady 2011; Newell 1998; Pearson 1996). In support of this recommendation, Friedland 1985, reported that some solutions caused a reduction in flow rate or clogging of the filter and that certain drugs, such as antibiotics, may be retained in the filters, causing a reduction in potency. There are no known adverse effects from the use of intravenous in‐line filters. The need to change the filters when they become blocked may lead to the need for increased manipulation of the intravenous administration set, increasing the risk of bacterial contamination. However, blocking of the filter is claimed to be indicative of complications such as those associated with microprecipitation occurring within the intravenous line which is a potentially harmful source of particulate matter which may then become dislodged entering the venous circulation (Bethune 2001). Friedland 1985, also argued that filters could not reduce the risk of infection caused by contaminants entering the line below the in‐line filter. A study by Newell 1998, found no difference in the rate of septicaemia between children in an oncology unit who had filters fitted and those who did not. They concluded from their results that the added cost of using intravenous in‐line filters was not warranted.
Intravenous in‐line filters are recommended for use in neonates (Bethune 2001; Kunac 1999). Therefore, the aim of this review is to systematically assess the evidence on the effectiveness of in‐line filters on intravenous lines in neonates.
Objectives
To determine the effect of in‐line filters on intravenous lines on morbidity and mortality in neonates.
We will carry out the following prespecified subgroup analyses.
Type of filter (approximate diameter 0.2 micron, 1.2 micron).
Gestation: term and preterm (defined as < 37 weeks) or extreme preterm (defined as < 30 weeks gestation).
Type of intravenous line (central or peripheral).
Type of intravenous fluid (crystalloid solutions, total parenteral nutrition, antibiotics, lipids).
Methods
Criteria for considering studies for this review
Types of studies
We included RCTs or quasi‐RCTs in the review.
Types of participants
Neonates with intravenous infusions who were randomised in the neonatal period (< 29 days post delivery).
Types of interventions
Intravenous in‐line filter versus placebo or nothing.
Types of outcome measures
Primary outcomes
Mortality from any cause.
Proven septicaemic infection; positive bacterial or fungal blood culture.
Secondary outcomes
Localised phlebitis; redness, inflammation and tenderness at location of cannula.
Duration of cannula patency (days).
Number of catheters inserted.
Suspected septicaemic infection; clinical symptoms consistent with septicaemia but not proven.
Thrombus, local (cannula insertion site); diagnosed by ultrasound.
Thrombus, systemic; diagnosed by ultrasound.
Proven necrotising enterocolitis; Bell's stage two or greater.
Suspected necrotising enterocolitis; clinical symptoms consistent with necrotising enterocolitis, but not proven.
Periventricular leukomalacia; cystic changes in the periventricular areas.
Neurodevelopment assessed up to two years corrected age as measured by a validated assessment tool.
Financial costs.
Length of stay in hospital (days).
Adverse effects reported in the trials (if any).
Search methods for identification of studies
Electronic searches
We searched the electronic databases of the Cochrane Central Register of Controlled Trials (CENTRAL; Issue 5, 2015); MEDLINE (from 1966 to May, 2015), EMBASE (from 1980 to May 2015), and CINAHL (from 1982 to May 2015). We did not impose any language restrictions. We used the following MeSH terms: infant OR newborn AND text terms 'intravenous catheter' OR 'infusion filter' OR 'filtration' OR 'in‐line filter' OR 'infusions' OR 'endotoxins' OR 'bacterial' OR 'particulate contamination', OR 'phlebitis' OR 'infection', OR 'intravenous infusion'.
Searching other resources
We examined the references in all studies identified as potentially relevant. We searched the abstracts from the annual meetings of the Pediatric Academic Societies (1993 to 2014), the European Society for Pediatric Research (1995 to 2014), the UK Royal College of Paediatrics and Child Health (2000 to 2014) and the Perinatal Society of Australia and New Zealand (2000 to 2014). We did not identify any new trials. We also searched clinical trials registries for ongoing or recently completed trials (clinicaltrials.gov; controlled‐trials.com; and who.int/ictrp).
Data collection and analysis
We followed the procedures of the Cochrane Neonatal Review Group throughout.
Selection of studies
Two review authors (JF and RR) screened the title and abstract of all studies identified by the above search strategy. We reassessed the full text of any potentially eligible reports and excluded those studies that did not meet all of the inclusion criteria. We discussed all disagreements until we achieved consensus.
Data extraction and management
We used a data collection form to aid extraction of relevant information from each included study. Two review authors (JF and RR) extracted the data separately. The two reviewers (JF and RR) independently assessed the trials for their methodological quality and subsequent inclusion in the review. We discussed all disagreements until we achieved consensus. If data from the trial reports were insufficient, we contacted the investigators for further information.
Assessment of risk of bias in included studies
Two review authors planned to independently assess trials for methodological quality. We evaluated the following issues and enter the findings into the 'Risk of bias' tables (Higgins 2011):
Sequence generation (checking for possible selection bias): For each included study, we categorised the method used to generate the allocation sequence as:Low risk (any truly random process e.g. random number table; computer random number generator);High risk (any non random process e.g. odd or even date of birth; hospital or clinic record number);Unclear risk.
Allocation concealment (checking for possible selection bias): For each included study, we categorised the method used to conceal the allocation sequence as:Low risk (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes);High risk (open random allocation; unsealed or non‐opaque envelopes; alternation; date of birth);Unclear risk.
Blinding (checking for possible performance bias): For each included study, we categorised the methods used to blind study participants and personnel from knowledge of which intervention a participant received. We planned to categorise blinding separately for different outcomes or classes of outcomes. We categorised the methods as:Low risk, high risk, or unclear risk for participants;Low risk, high risk, or unclear risk for personnel;Low risk, high risk, or unclear risk for outcome assessors.
Incomplete outcome data (checking for possible attrition bias through withdrawals, dropouts, protocol deviations): For each included study and for each outcome, we described the completeness of data including attrition and exclusions from the analysis. We noted whether attrition and exclusions were reported, the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information was reported or supplied by the trial authors, we planned to re‐include missing data in the analyses. We categorised the methods as:Low risk (< 20% missing data);High risk (≥ 20% missing data);Unclear risk.
Selective reporting bias: For each included study, we described how we investigated the possibility of selective outcome reporting bias and what we found. We assessed the methods as:Low risk (where it is clear that all of the study’s pre‐specified outcomes and all expected outcomes of interest to the review have been reported);High risk (where not all the study's pre‐specified outcomes have been reported; one or more reported primary outcomes were not pre‐specified; outcomes of interest are reported incompletely and so cannot be used; study fails to include results of a key outcome that would have been expected to have been reported);Unclear risk.
Other sources of bias: For each included study, we described any important concerns we had about other possible sources of bias (e.g. whether there was a potential source of bias related to the specific study design or whether the trial was stopped early due to some data‐dependent process). We assessed whether each study was free of other problems that could put it at risk of bias as:Low risk;High risk;Unclear risk.
Measures of treatment effect
We followed the procedures of the Cochrane Neonatal Review Group for statistical analysis. We expressed dichotomous data as risk ratios (RRs) with 95% confidence intervals (CIs), risk differences (RDs) with 95% CIs, and number needed to treat (NNT) for dichotomous outcomes. We analysed continuous variables using weighted mean differences (WMDs) and 95% CIs. We estimated the heterogeneity of studies using the I2 statistic.
Unit of analysis issues
The unit of analysis was the participating infant in individually randomised trials, except for outcomes where an individual infant may have experienced the event more than once. These include: rates of proven sepsis (Figure 1) in which case the unit of analysis will be the number of events over a given time period.
1.
Forest plot of comparison: 1 Intravenous in‐line filter versus no filter, outcome: 1.7 Proven necrotising enterocolitis.
Assessment of heterogeneity
If more than one trial was included in a meta‐analysis, we examined the treatment effects of individual trials and heterogeneity between trial results by inspecting the forest plots. We calculated the I² statistic for each analysis to quantify inconsistency across studies and describe the percentage of variability in effect estimates that may be due to heterogeneity rather than sampling error. If we detected moderate (I² > 50%) heterogeneity, we explored the possible causes (for example, differences in study design, participants, interventions, or completeness of outcome assessments) in sensitivity analyses.
Data synthesis
We used the fixed‐effects model in Review Manager 5.3 (RevMan 2014) for meta‐analysis.
Results
Description of studies
See tables: Characteristics of included studies.
Results of the search
Four published studies met the inclusion criteria. We excluded one trial. A total of 704 neonates were included in these studies (range 63 to 442). The details of each of these four studies are given in the table of Characteristics of included studies.
Included studies
van den Hoogen 2006 evaluated the effect of using 0.22 micron Pall Posidyne ELD96TM in‐line filters versus no filter in 442 neonates on mortality, sepsis, phlebitis, number of catheter days and financial cost. The manufacturer recommends using this filter for the elimination of particles, microbes, air and endotoxins. All intravenous fluids in the study group (with the exception of lipids which were administered through a 1.2 micron LipiporTM filter) were given through the 0.22 micron in‐line filter, and the administration sets were changed every four days. The intravenous sets in the control group and the LipiporTM filters (that are not able to retain endotoxins) were changed daily. The filters were positioned at the distal end of the intravenous administration catheter after a series of stopcocks. The authors noted that this construction guaranteed that all clear fluids, including intravenous medication were administered via the filter. The LipiporTM filter was placed distally to the 0.22 micron in‐line filter. No information on cannula site preparation was provided.
van Lingen 2004 studied 88 neonates to evaluate the effectiveness of the 0.22 micron Pall Posidyne ELD96TM in‐line filters with no filters to prevent complications such as bacteraemia, phlebitis, extravasation, thrombosis, septicaemia and necrotising enterocolitis in neonates who required an intravenous catheter. van Lingen 2004 also evaluated the economic impact of the use of intravenous in‐line filters. All intravenous fluids in the study group (with the exception of lipids, blood or blood products) were given through the in‐line filter and the administration sets were changed every four days. The intravenous sets in the control group were changed daily. In the study group, bacterial cultures were obtained at the time of change from both sides of the discarded filter and from the lipid solution. For the control group, bacterial cultures were obtained from the intravenous fluids every four days. In addition, catheter tips were cultured after removal. Blood was cultured only when sepsis was suspected. No information was provided on cannula site preparation. van Lingen 2004 reported that four patients in the control group died from "causes unrelated to catheter usage" i.e. necrotising enterocolitis, pulmonary bleeding, severe intraventricular haemorrhage, circulatory insufficiency. However, these four neonates were included in the mortality outcome in this review.
Thomas 1989 assessed the effect of in‐line filters on duration of cannula patency in 63 neonates requiring intravenous fluids. Thomas 1989 used a 0.2 micron CathivexTM filter that is only recommended for the removal of particulates and air. The filters in the study group were positioned before the cannulae, except where fluids such as blood, plasma protein fraction, fresh frozen plasma or emulsions were being administered. On these occasions, the filter was positioned upstream of the three way tap used for adding such fluids to the primary infusion line. In the control group, an extension set was substituted for the filter. This was included as it provided an equal number of connections and manipulations in the lines for both groups. The intravenous lines and filters were changed every 24 hours in the control and study groups. Cannula site preparation was limited to swabbing the skin with isopropyl alcohol. Following cannulation, the site was covered with a sterile dressing. No extra cannula site care was performed (such as application of antibiotic cream or spray) during the study. Cannula life was assessed by duration of patency and volume of intravenous fluid passed by the site. Nursing staff observations of the infusion site were used to subjectively determine the end point. Nursing staff routinely checked and recorded the condition of the intravenous cannula sites and the volume of fluid delivered every hour. No information was provided on the length of the study period.
Bennion 1991 assessed the effect of intravenous in‐line filters on serum gentamicin level results, incidence of necrosed areas at the infiltration site, and cost of administration sets, with and without an intravenous in‐line filter in 111 neonates. Bennion 1991 used a 0.22 micron Pall Posidyne ELD96TM that was used by van Lingen 2004 and van den Hoogen 2006. The intravenous sets were changed every four days in the treatment group, and daily in the control group. A record of the administration sets was made each day together with serum gentamicin level results for each baby and any necrosed areas that developed. Serum gentamicin levels were checked on the third dose of the antibiotic. No information was provided on cannula site preparation.
All studies were single centre studies. All four studies used 0.2 micron filters. The Bennion 1991 and Thomas 1989 studies used peripheral catheters, and the van Lingen 2004 and van den Hoogen 2006 studies used central venous (percutaneous or umbilical) catheters to deliver the intravenous fluids. While all the studies compared the use of in‐line filters with no in‐line filter, the outcomes that were measured varied.
There were no other identified studies that assessed whether intravenous in‐line filters prevent morbidity and mortality in neonates.
Excluded studies
There was one excluded study (see Characteristics of excluded studies). Jack 2012 was a single centre, prospective RCT, that randomised 807 critically ill children admitted to a paediatric intensive care unit, either to control (n = 406) or filter group (n = 401), with the latter receiving in‐line filtration. The study included subjects less than 18 years of age (mean age 5 to 6 years). The primary endpoint was reduction in the rate of overall complications, which included the occurrence of systemic inflammatory response syndrome, sepsis, organ failure (circulation, lung, liver, kidney) and thrombosis. Secondary objectives were a reduction in the length of stay in the paediatric intensive care unit and overall hospital stay. Duration of mechanical ventilation and mortality were also analysed.
Risk of bias in included studies
Details of the methodological quality of each trial are given in the table Characteristics of included studies.
Allocation
Only the study of van Lingen 2004 was randomised (computer‐generated randomisation with sealed numbered envelopes that were opened on admission of each neonate). Three studies (Bennion 1991; Thomas 1989; van den Hoogen 2006) were quasi‐randomised (alternate allocation).
Blinding
A placebo was not used in any of the four studies and, therefore, there was no blinding of the intervention. Information on outcome measurements was inadequately described in all of the studies.
Incomplete outcome data
The four studies examined short‐term outcomes and accounted for all neonates in the intervention and control groups. Thirteen per cent of the neonates in the van den Hoogen 2006 study were excluded from the study following randomisation because of incomplete data, either because the infant died or the patient was discharged soon after birth.
Effects of interventions
See: Table 1
Comparison: Intravenous in‐line filter versus placebo
We did not identify any studies for this comparison.
Comparison: Intravenous in‐line filter versus no filter
We identified four studies for this comparison (Bennion 1991; Thomas 1989; van Lingen 2004; van den Hoogen 2006).
Primary outcomes
1) Mortality from any cause during hospitalisation (Outcome 1.1)
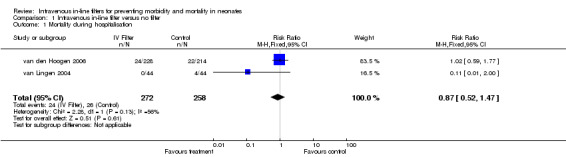
Mortality was reported in two of the studies. For the van Lingen 2004 study of 88 infants, there were four deaths in the control group and none in the treatment group. For the van den Hoogen 2006 study of 442 infants, there were 22 deaths in the control group and 24 in the treatment group. There was no statistical difference for mortality in either of the studies (typical RR 0.87, 95% CI 0.52 to 1.47; typical RD ‐0.01, 95% CI ‐0.06 to 0.04; 2 studies, 530 infants) (Figure 2)
2.
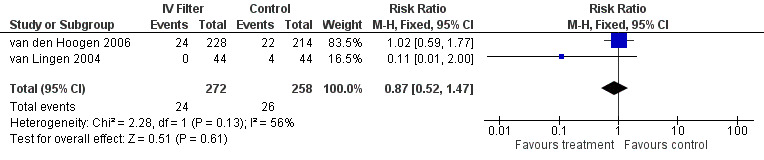
Forest plot of comparison: 1 Intravenous in‐line filter versus no filter, outcome: 1.1 Mortality during hospitalisation.
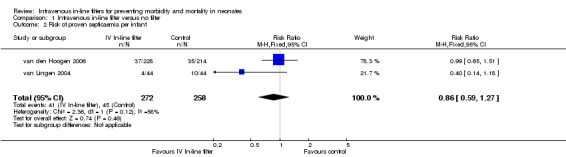
2) Proven septicaemic infection (positive bacterial or fungal blood culture) (Outcomes 1.2 and 1.3)
Proven septicaemic infection was reported as number of infants with events in two of the studies. There was no statistical difference found in the van Lingen 2004 study of 88 infants and in the van den Hoogen 2006 study of 442 infants (typical RR 0.86, 95% CI 0.59 to 1.27; typical RD ‐0.02, 95% CI ‐0.09 to 0.04; 2 studies, 530 infants) (Figure 3).
3.
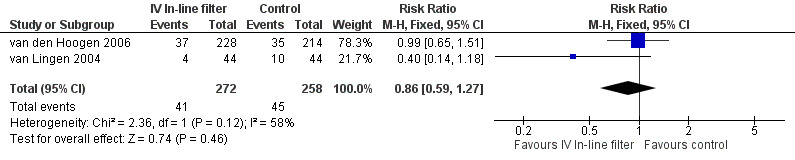
Forest plot of comparison: 1 Intravenous in‐line filter versus no filter, outcome: 1.2 Risk of proven septicaemia per infant.
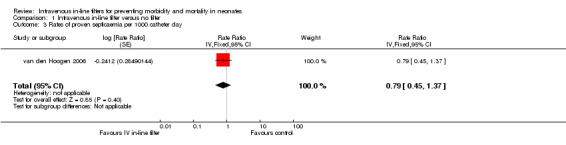
One study van den Hoogen 2006 reported rates of proven septicaemic infection as number of events per group (intravenous in‐line filter group; 22 events: control group; 28 events) over a specified follow‐up period (intravenous in‐line filter group; 11 days: control group; 10 days), rate ratio ( 0.86 [0.49, 1.51] (Rate Ratio 0.86, 95% CI 0.49 to 1.51) and found no difference in rates (Figure 4).
4.
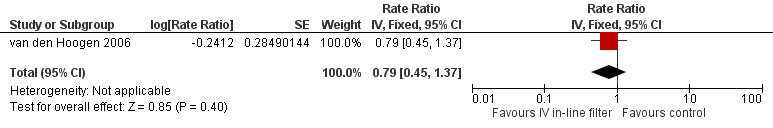
Forest plot of comparison: 1 Intravenous in‐line filter versus no filter, outcome: 1.3 Rates of proven septicaemia per 1000 catheter days.
Secondary outcomes
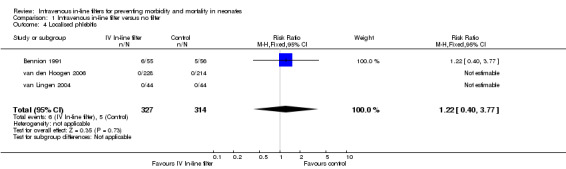
1) Localised phlebitis (redness, inflammation and tenderness at location of cannula) (Outcome 1.4)
Localised phlebitis was reported in three studies. Bennion 1991 reported the incidence of localised necrosis (undefined) in 111 infants with no statistical difference between the treatment and control groups. Phlebitis did not occur in the van den Hoogen 2006 or van Lingen 2004 studies (typical RR 1.22, 95% CI 0.40 to 3.77; typical RD 0.01, 95% CI ‐0.05 to 0.08; 3 studies, 641 infants) (Figure 5).
5.
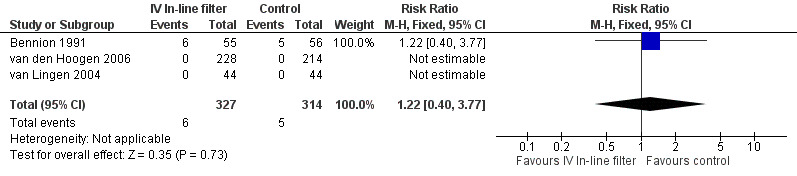
Forest plot of comparison: 1 Intravenous in‐line filter versus no filter, outcome: 1.4 Localised phlebitis.
Thomas 1989 reported the incidence of 'tissuing' (undefined by authors, but regarded as infiltration or leaking of fluids into the area surrounding the vein) related to number of cannulations, rather than number of neonates and, therefore, could not be included in the above analysis. There were 59 incidences of phlebitis from 81 cannulations in the treatment group (n = 30) compared to 67 incidences of phlebitis from 86 cannulations in the control group (n = 33). There was no difference between the treatment and control groups.
2) Duration of cannula patency
Three studies reported on duration of cannula patency. In the van Lingen 2004 study, the total duration for the catheters remaining in place for all neonates in the study group (n = 44) was a total of 525 patient days (mean 8.1 days per neonate). In the control group, the total duration for the catheters remaining in place for all neonates (n = 44) was a total of 493 patient days (mean 8.8 days per neonate). A difference between the treatment and control groups was not found.
In the Thomas 1989 study, the median duration of catheter patency in the treatment group was 59 hours compared to 49 hours in the control group. The authors reported a statistical difference between the two groups (log rank test Chi2 = 4.024, P < 0.05) with a median increase in cannula patency of 20% in the treatment group compared to the control group. van den Hoogen 2006 compared the number of catheter days for umbilical venous, percutaneous and central venous catheters and found no difference between the treatment and control groups.
3) Number of catheters inserted
Two studies reported on the number of catheters inserted (Thomas 1989; van Lingen 2004). There was no difference between the treatment and control groups. In the van Lingen 2004 study there was a total of 65 intravenous catheter insertions. These were reported as 23 percutaneous and 42 umbilical central venous catheter insertions (43 first, 17 second and 5 third) for the neonates in the study group (n = 44). There were 56 (40 percutaneous and 16 umbilical) catheter insertions (42 first, 12 second and 2 third) for the neonates in the control group (n = 44). For the Thomas 1989 study there was a total of 81 catheter (peripheral) insertions in the study group (n = 30) compared to 86 catheter (peripheral) insertions in the control group (n = 33). Standard deviations were not provided and, therefore, a weighted mean difference could not be performed.
4) Suspected septicaemic infection (clinical symptoms consistent with septicaemia, but not proven) (Outcome 1.5)
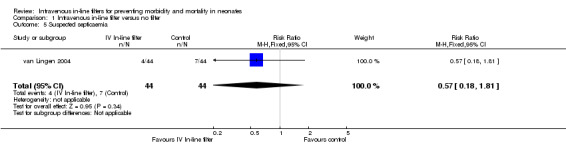
The van Lingen 2004 study of 88 infants was the only study to report suspected septicaemic infection and found no difference (typical RR 0.57, 95% CI 0.18 to 1.81; typical RD ‐0.07, 95% CI ‐0.21 to 0.07; 1 study, 88 infants) (Figure 6).
6.
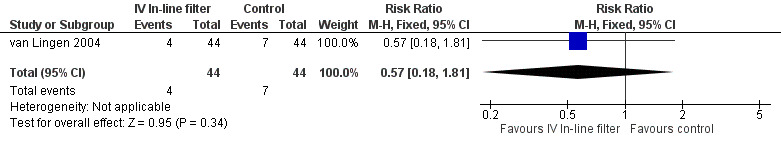
Forest plot of comparison: 1 Intravenous in‐line filter versus no filter, outcome: 1.5 Suspected septicaemia.
5) Local thrombosis (cannula insertion site, diagnosed by ultrasound) (Outcome 1.6)
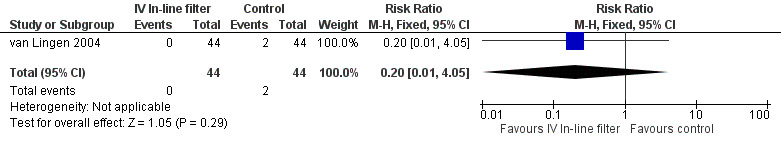
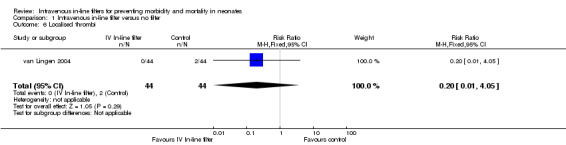
Local thrombosis was only reported in the van Lingen 2004 study. No significant difference was found (typical RR 0.20, 95% CI 0.01 to 4.05; typical RD ‐0.05, 95% CI ‐0.12 to 0.03; 1 study, 88 infants) (Figure 7).
7.
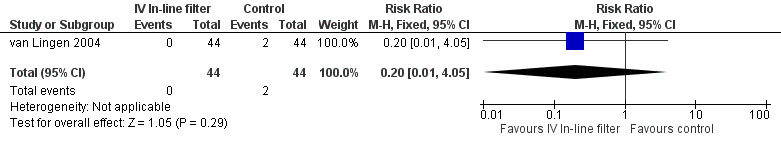
Forest plot of comparison: 1 Intravenous in‐line filter versus no filter, outcome: 1.6 Localised thrombi.
6) Systemic thrombus (diagnosed by ultrasound)
No data were available.
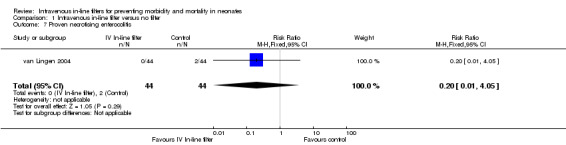
7) Proven necrotising enterocolitis (Bell's stage two or greater) (Outcome 1.7)
Proven necrotising enterocolitis was only reported in the van Lingen 2004 study, and there were no significant differences (typical RR 0.20, 95% CI 0.01 to 4.05; typical RD ‐0.05, 95% CI ‐0.12 to 0.03; 1 study, 88 infants) (Figure 1).
8) Suspected necrotising enterocolitis (clinical symptoms consistent with necrotising enterocolitis, but not proven) No data were available.
9) Periventricular leukomalacia (cystic changes in the periventricular areas)
No data were available.
10) Neurodevelopment (assessed up to two years corrected age, as measured by a validated assessment tool)
No data were available.
11) Financial costs
van den Hoogen 2006 compared the costs of disposable materials per patient per four days. In the study group, two filters (clear fluid and lipid) and intravenous sets were changed every 96 hours and the intravenous sets in the control group were changed daily. The total cost per neonate in the control group was EUR 241.76 and EUR 238.63 in the study group, showing that the cost of using an in‐line filter was compensated by the reduced consumption of intravenous administration sets. van den Hoogen 2006 also used filters for fat emulsions that had to be changed daily. Without the inclusion of these filters in the calculation, costs of disposable materials in the in‐line filter group would have been much lower at EUR 107.73 over the four day period, less than half that in the control group. This study also found that the time necessary for changing the intravenous administration sets was significantly longer in the non‐filter group: the mean time was 14 minutes plus +/‐ 7 minutes in the non‐filter group, compared to 10 minutes +/‐ 5 minutes in the filter group (P = 0.000).
For the van Lingen 2004 study, costs attributable to patients in both control and study groups were calculated on a 'cost of disposables' basis during a standard eight day stay. Additionally, the time taken for line change was calculated by 'direct assessment', and an estimate of the relative nursing costs was built into the analysis. In the study group, filters and intravenous sets were changed every 96 hours and in the control group, the intravenous sets were changed daily. However, unlike the van den Hoogen 2006 study, lipid filters were not included in the calculation. The total cost per neonate in the control group was EUR 85.75 and for the study group was EUR 37.44, showing a saving of EUR 48.31 per neonate, over a period of eight days.
The Bennion 1991 study reported the average cost of an administration system as approximately GBP 17.28 per day for the control group compared to GBP 8.84 per day in the study group (showing a daily saving of GBP 8.44 in the treatment group). This was calculated by dividing the total cost of the equipment used by the number of cot days occupied by infants in each group. The intravenous sets were changed every 96 hours in the control group and daily in the study group.
12) Adverse affects reported in the trials
In the Bennion 1991 study, precipitate was found to clog the filter and the flow of intravenous fluids. The intravenous in‐line filter also became blocked in one patient in the van den Hoogen 2006 study; that was reported to be possibly due to the administration of very high glucose concentrations (50%).
There were no data available to be able to perform subgroup analyses on type of filter, gestation, type of intravenous line, or type of intravenous fluid.
13) Composite thrombus, proven or unproven sepsis and necrotising enterocolitis.
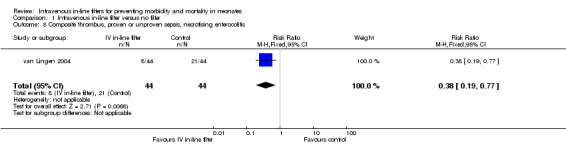
A composite outcome for thrombi, proven or unproven sepsis, and necrotising enterocolitis was not prespecified in this review and is therefore reported post hoc. van Lingen 2004 reported a statistically significant reduction in the combined risk of thrombi proven sepsis, or NEC (typical RR 0.38, 95% CI 0.19 to 0.77; typical RD ‐0.30, 95% CI ‐0.48 to ‐0.11) (Figure 8).
8.
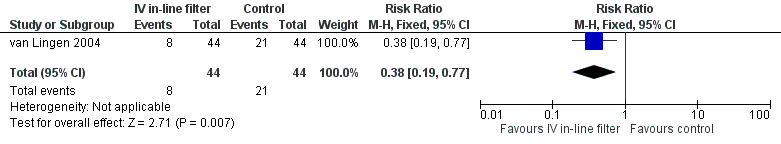
Forest plot of comparison: 1 Intravenous in‐line filter versus no filter, outcome: 1.8 Composite thrombus, proven or unproven sepsis, necrotising enterocolitis.
Discussion
Summary of main results
There was no statistically significant difference in any of the outcomes of mortality, proven or suspected septicaemia, localised phlebitis, localised thrombi, necrotising enterocolitis, length of stay, duration of cannula patency or number of catheters inserted between infants receiving intravenous therapy using in‐line intravenous filters or those receiving intravenous therapy without in‐line intravenous filters.
A composite outcome for thrombi, proven or unproven sepsis, sepsis and necrotising enterocolitis was not prespecified in this review and is therefore reported post hoc. van Lingen 2004 reported a statistically significant reduction in the combined risk of thrombi proven sepsis, or NEC (RR 0.38 (95% CI 0.19 to 0.77), RD ‐0.30 (95% CI ‐0.48 to ‐0.11), or just over two thirds reduction in risk in favour of intravenous in‐line filtration.
Cost savings were reported by Bennion 1991, van Lingen 2004 and van den Hoogen 2006. We found no data available from RCTs that compared periventricular leukomalacia, incidence of systemic thrombus, and neurodevelopmental outcomes.
Overall completeness and applicability of evidence
Low to moderate quality evidence from four randomised trials is insufficient to support or refute the use of intravenous in‐line filters in preventing line‐related morbidity and mortality in neonates.
In‐line intravenous filters combined with less frequent changing of the intravenous sets (every four days) were not effective in reducing the risk of morbidity, mortality, or adverse events compared with daily changing of unfiltered intravenous administration sets.
Quality of the evidence
Four trials of low to moderate quality were included in the review (Bennion 1991; Thomas 1989; van Lingen 2004; van den Hoogen 2006). (Refer Risk of bias in included studies and Table 1).
Although blinding of the intervention is not likely to be easily achieved, future studies could improve quality by performing and reporting blinded outcome assessment, randomisation/allocation sequences, and pre‐registration of trial protocols using trial registries.
Authors' conclusions
Implications for practice.
There is insufficient evidence to recommend the use of intravenous in‐line filters to prevent morbidity or mortality in neonates. However, cost savings were found with the less frequent changing of the intravenous sets when a 0.2 micron intravenous in‐line filter (that removes endotoxins) was used, without an increase in adverse outcomes.
Implications for research.
Further trials are needed to be able to assess the effectiveness of 0.22 micron positively charged 96‐hour intravenous in‐line filters and 1.2 micron intravenous in‐line filters used for lipid administration for use in term and preterm neonates. These trials should include the outcomes of periventricular leukomalacia, necrotising enterocolitis, systemic and local thrombus and local phlebitis.
What's new
| Date | Event | Description |
|---|---|---|
| 8 July 2015 | New search has been performed | This updates the review "Intravenous in‐line filters for preventing morbidity and mortality in neonates" Foster 2006. |
| 22 May 2014 | New citation required but conclusions have not changed | Search updated in May 2015, no new trials identified. New additional author. |
History
Protocol first published: Issue 2, 2005 Review first published: Issue 2, 2006
| Date | Event | Description |
|---|---|---|
| 20 January 2013 | Amended | Contact details updated. |
| 2 April 2011 | New search has been performed | This updates the review "Intravenous in‐line filters for preventing morbidity and mortality in neonates (Foster 2006). Updated search in April 2011 did not identify any new studies. Conclusions remain the same. |
| 15 February 2011 | Amended | Contact details updated. |
| 4 December 2008 | New search has been performed | This updates the review "Intravenous in‐line filters for preventing morbidity and mortality in neonates" published in The Cochrane Library, Issue 2, 2006 (Foster 2006). One eligible trial was found and has been included in this review. |
| 18 September 2008 | Amended | Converted to new review format. |
Acknowledgements
Editorial support of the Cochrane Neonatal Review Group has been funded with federal funds from the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, Department of Health and Human Services, USA, under Contract NO. HHSN267200603418C.
Data and analyses
Comparison 1. Intravenous in‐line filter versus no filter.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality during hospitalisation | 2 | 530 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.52, 1.47] |
| 2 Risk of proven septicaemia per infant | 2 | 530 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.59, 1.27] |
| 3 Rates of proven septicaemia per 1000 catheter day | 1 | Rate Ratio (Fixed, 95% CI) | 0.79 [0.45, 1.37] | |
| 4 Localised phlebitis | 3 | 641 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.22 [0.40, 3.77] |
| 5 Suspected septicaemia | 1 | 88 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.57 [0.18, 1.81] |
| 6 Localised thrombi | 1 | 88 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.2 [0.01, 4.05] |
| 7 Proven necrotising enterocolitis | 1 | 88 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.2 [0.01, 4.05] |
| 8 Composite thrombus, proven or unproven sepsis, necrotising enterocolitis | 1 | 88 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.38 [0.19, 0.77] |
1.1. Analysis.
Comparison 1 Intravenous in‐line filter versus no filter, Outcome 1 Mortality during hospitalisation.
1.2. Analysis.
Comparison 1 Intravenous in‐line filter versus no filter, Outcome 2 Risk of proven septicaemia per infant.
1.3. Analysis.
Comparison 1 Intravenous in‐line filter versus no filter, Outcome 3 Rates of proven septicaemia per 1000 catheter day.
1.4. Analysis.
Comparison 1 Intravenous in‐line filter versus no filter, Outcome 4 Localised phlebitis.
1.5. Analysis.
Comparison 1 Intravenous in‐line filter versus no filter, Outcome 5 Suspected septicaemia.
1.6. Analysis.
Comparison 1 Intravenous in‐line filter versus no filter, Outcome 6 Localised thrombi.
1.7. Analysis.
Comparison 1 Intravenous in‐line filter versus no filter, Outcome 7 Proven necrotising enterocolitis.
1.8. Analysis.
Comparison 1 Intravenous in‐line filter versus no filter, Outcome 8 Composite thrombus, proven or unproven sepsis, necrotising enterocolitis.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Bennion 1991.
| Methods | Parallel, quasi‐randomised controlled trial | |
| Participants | 111 neonates receiving IV fluids via peripheral venous line Gestation: 28 ‐ 37 weeks Birth weight: 1170 ‐ 3240 gm No exclusions |
|
| Interventions | Experimental group: 0.22 micron intravenous in‐line filter (N = 55) Control group: No filter or placebo. Administration set changed daily in control group and every 96 hours in treatment group (N = 56) | |
| Outcomes | 1. Effect of IV in‐line filters on gentamicin level results 2. Incidence of necrosed area at infiltration site 3. Cost of administration sets when an in‐line filter is used, compared to the cost when a filter is not used 4. Duration of IV in progress 5. Duration of IV infusion in progress | |
| Notes | Sample size calculation: Not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Each baby that required IV infusion was entered into the study with alternate babies having an in‐line filter |
| Allocation concealment (selection bias) | High risk | No |
| Blinding (performance bias and detection bias) All outcomes | High risk | No |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Unclear risk | We were unable to obtain the study protocol |
Thomas 1989.
| Methods | Parallel, quasi‐randomised controlled trial | |
| Participants | 63 neonates requiring IV fluids via peripheral venous line Mean gestation: 34.13 ‐ 34.91 weeks Mean birth weight: 2230 ‐ 2350 gm No exclusions |
|
| Interventions | Experimental group: 0.2 micron intravenous filter (N = 30) Control group: No filter but extension set substituted for filter (N = 33) Administration sets changed every 24 hours in treatment and control groups | |
| Outcomes | 1. Duration of cannula patency 2. Extravasation 3. Leaking at infusion site 4. Number of catheter insertions | |
| Notes | Sample size calculation: Not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Each baby that required intravenous infusion was entered into the study with alternate babies having an in‐line filter |
| Allocation concealment (selection bias) | High risk | No |
| Blinding (performance bias and detection bias) All outcomes | High risk | No |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Unclear risk | We were unable to obtain the study protocol |
van den Hoogen 2006.
| Methods | Parallel, quasi‐randomised controlled trial | |
| Participants | 442 neonates requiring IV fluids via an umbilical or percutaneous central venous catheter or a catheter inserted in the subclavian or femoral vein. All neonates admitted to the neonatal intensive care unit and required fluids via a central venous catheter were eligible Gestation: 25 ‐ 43 weeks Birth weight: 600 ‐ 4640 gm |
|
| Interventions | Experimental group: 0.22 micron intravenous filter (N = 228) Control group: No filter (N = 214) Catheter tips cultured after removal Administration set changed daily in the control group. Administration set changed every 96 hours in treatment group | |
| Outcomes | 1. Mortality 2. Phlebitis: defined as 'signs of local infection and a positive culture from the infected site' 2. Proven Sepsis: defined as 'occurrence of clinical signs of local infection and a positive blood culture' 3. Number of catheter days 4. Length of stay in hospital 5. Financial costs |
|
| Notes | Sample size calculation: Not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Each baby that required intravenous infusion was entered into the study with alternate babies having an in‐line filter |
| Allocation concealment (selection bias) | High risk | No |
| Blinding (performance bias and detection bias) All outcomes | High risk | No |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data for 65 infants were excluded post randomisation 'because the patient was discharged soon after birth, died after a few days, or because of incomplete data (approximately 14% loss to follow‐up). |
| Selective reporting (reporting bias) | Unclear risk | We were unable to obtain the study protocol. There was incomplete reporting on reasons for catheter removal and reinsertion for both treatment and control group |
van Lingen 2004.
| Methods | Parallel, randomised controlled trial | |
| Participants | 88 neonates requiring IV fluids via an umbilical or percutaneous central venous line. Premature infants with respiratory distress syndrome (RDS) and term infants with asphyxia or pneumonia/septicaemia were eligible Gestation: 26.3 ‐ 42.3 weeks Birth weight: 585 ‐ 4100 gm Ineligible if congenital malformation present and infants < 26 weeks gestation |
|
| Interventions | Experimental group: 0.22 micron IV filter (N = 44) Control group: No filter or placebo (N = 44) Full blood count on admission Catheter tips cultured after removal Administration set changed daily in the control group. Administration set changed every 96 hours in treatment group | |
| Outcomes | 1. Phlebitis 2. Extravasation 3. Thrombosis 4. Proven sepsis: 'characteristic clinical symptoms' positive blood culture, and abnormal tests, (leucocytosis, leucopenia, granulocytopenia, C‐Reactive Protein (CRP) > 10 mg/L) 5. Unproven sepsis: 'characteristic clinical symptoms' and negative blood culture and abnormal tests 6. Necrotising enterocolitis 7. Duration of cannula patency 7. Number of catheter insertions 8. Duration of catheter insertion 9. Secondary septicaemia | |
| Notes | Sample size calculation: To achieve a reduction in infection rate from 30% to 5% by using an in‐line filter with power of 80% and at 5% significance would require at least 36 infants in each group | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation was carried out by one of the neonatologists who did not participate in the study |
| Allocation concealment (selection bias) | Low risk | Sealed numbered envelopes were opened on admission of the neonate; neonates allocated to either study or control group |
| Blinding (performance bias and detection bias) All outcomes | High risk | No |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not reported |
| Selective reporting (reporting bias) | Unclear risk | Routinely measured outcomes are reported |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Jack 2012 |
Jack 2012: Single centre, prospective, randomised controlled trial that randomised 807 critically ill children admitted to paediatric intensive care unit either to control (n = 406) or filter group (n = 401), with the latter receiving in‐line filtration The study included subjects less than 18 years of age (mean age 5 to 6 years). The primary endpoint was reduction in the rate of overall complications, which included the occurrence of systemic inflammatory response syndrome, sepsis, organ failure (circulation, lung, liver, kidney) and thrombosis. Secondary objectives were a reduction in the length of stay in the paediatric intensive care unit and overall hospital stay. Duration of mechanical ventilation and mortality were also analysed Analysis demonstrated a significant reduction in the overall complication rate (n = 166 [40.9 %] vs n = 124 [30.9 %]; P = 0.003) for the filter group. In particular, the incidence of systemic inflammatory response syndrome was significantly lower (n = 123 [30.3 %] vs n = 90 [22.4 %]; P = 0.01). Moreover the length of stay in paediatric intensive care unit (3.89 [95 % confidence interval 2.97‐4.82] vs 2.98 [2.33‐3.64]; P = 0.025) and duration of mechanical ventilation (14.0 [5.6‐22.4] vs 11.0 [7.1‐14.9] h; P = 0.028) were significantly reduced. |
IV: intravenous
Differences between protocol and review
Nil.
Contributions of authors
Jann Foster (JF), Robyn Richards (RR) and Marian Showell (MS) independently assessed studies for inclusion in this review. JF and RR wrote the review with the assistance of MS and LJ. JF acts as guarantor for the review.
Sources of support
Internal sources
No sources of support supplied
External sources
-
Eunice Kennedy Shriver National Institute of Child Health and Human Development National Institutes of Health, Department of Health and Human Services, USA.
Editorial support of the Cochrane Neonatal Review Group has been funded with Federal funds from the Eunice Kennedy Shriver National Institute of Child Health and Human Development National Institutes of Health, Department of Health and Human Services, USA, under Contract No. HHSN275201100016C.
Declarations of interest
JF: There are no potential conflicts of interest.
RR: There are no potential conflicts of interest.
MS: There are no potential conflicts of interest.
LJ: There are no potential conflicts of interest.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Bennion 1991 {published data only}
- Bennion D, Martin K. In‐line filtration. Paediatric Nursing 1991;June:20‐21. [Google Scholar]
Thomas 1989 {published data only}
- Thomas PH. In‐line terminal filtration of intravenous fluids and its effect on cannula patency in neonates. Proceedings ‐ Guild of Hospital Pharmacy 2004;26:3‐10. [Google Scholar]
van den Hoogen 2006 {published data only}
- Hoogen A, Krediet TG, Uiterwaal C, Bolenius J, Gerards LJ, Fleer A. In‐line filters in central venous catheters in a neonatal intensive care unit. Journal of Perinatal Medicine 2006;34(1):71‐74. [PUBMED: 16489888] [DOI] [PubMed] [Google Scholar]
van Lingen 2004 {published data only}
- Lingen RA, Baerts W, Marquering AC, Ruijs GJ. The use of in‐line intravenous filters in sick newborn infants. Acta Paediatrica 2004;93(5):658‐62. [PUBMED: 15174791] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Jack 2012 {published data only}
- Jack T, Boehne M, Brent BE, Hoy L, Köditz H, Wessel A, et al. In‐line filtration reduces severe complications and length of stay on pediatric intensive care unit: a prospective, randomized, controlled trial. Intensive Care Medicine 2012;38(6):1008‐16. [PUBMED: 22527062] [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
Allcutt 1983
- Allcutt DA, Lort D, McCollum CN. Final inline filtration for intravenous infusions: a prospective hospital study. British Journal of Surgery 1983;70(2):111‐13. [PUBMED: 6824894] [DOI] [PubMed] [Google Scholar]
Backhouse 1987
- Backhouse CM, Ball PR, Booth S, Kelshaw MA, Potter SR, McCollum CN. Particulate contaminants of intravenous medications and infusions. Journal of Pharmacy and Pharmacology 1987;39(4):241‐5. [PUBMED: 2884285] [DOI] [PubMed] [Google Scholar]
Ball 2003
- Ball PA. Intravenous in‐line filters: filtering the evidence. Current Opinion in Cinical Nutrition and Metabolic Care 2003;6(3):319‐25. [PUBMED: 12690266] [DOI] [PubMed] [Google Scholar]
Ballance 1990
- Ballance WA, Dahms BB, Shenker N, Kliegman RM. Pathology of neonatal necrotizing enterocolitis: a ten year experience. Journal of Pediatrics 1990;117(1 Pt 2):S6‐13. [PUBMED: 2362230] [DOI] [PubMed] [Google Scholar]
Barnett 1996
- Barnett MI, Cosslett AG. Endotoxin retention capabilities of positively charged nylon and positively charged polysulphone membrane intravenous filters. Pharmacy and Pharmacology Communications 1996;2(7):319‐20. [Google Scholar]
Bethune 2001
- Bethune K, Allwood M, Grainger C, Wormleighton C, British Pharmaceutical Nutrition Group Working Party. Use of filters during the preparation and administration of parenteral nutrition: position paper and guidelines prepared by a British pharmaceutical nutrition group working party. Nutrition 2001;17(5):403‐8. [PUBMED: 11377134] [DOI] [PubMed] [Google Scholar]
Casale 1990
- Casale TB, Ballas ZK, Kaliner MA, Keahey TM. The effects of intravenous endotoxin on various host‐effector molecules. Journal of Allergy and Clinical Immunology 1990;85(1 Pt 1):45‐51. [PUBMED: 2137152] [DOI] [PubMed] [Google Scholar]
Chee 2002
- Chee S, Tan W. Reducing infusion phlebitis in Singapore hospitals using extended life end‐line filters. Journal of Infusion Nursing 2002;25(2):95‐104. [PUBMED: 11984223] [DOI] [PubMed] [Google Scholar]
Evans 2006
- Evans C, Dixon A. Intravenous therapy: practice issues. Infant 2006;2(4):133‐9. [Google Scholar]
Friedland 1985
- Friedland G. Infusion‐related phlebitis‐is the in‐line filter the solution?. New England Journal of Medicine 1985;312(2):113‐5. [PUBMED: 3964914] [DOI] [PubMed] [Google Scholar]
Garvan 1964
- Garvan JM, Gunner BW. The harmful effects of particles in intravenous fluids. Medical Journal of Australia 1964;12:1‐6. [PUBMED: 14175312] [DOI] [PubMed] [Google Scholar]
Geiss 1992
- Geiss HK, Batzer A, Sonntag HG. Investigations on the retention of microorganisms by inline‐filters for intravenous infusions in ICU patients. Hygiene Und Medizin 1992;17:421‐6. [Google Scholar]
Gilles 1977
- Gilles FH, Averill DR Jr, Kerr CS. Neonatal endotoxin encephalopathy. Annals of Neurology 1977;2(1):49‐56. [PUBMED: 409336] [DOI] [PubMed] [Google Scholar]
Glauser 1991
- Glauser MP, Zanetti G, Baumgartner JD, Cohen J. Septic shock: pathogenesis. Lancet 1991;338(8769):732‐6. [PUBMED: 1679876] [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hill 1992
- Hill A, Volpe JJ. Textbook of Neonatology. 2nd Edition. London: Churchill Livingstone, 1992. [Google Scholar]
Kirkpatrick 1988
- Kirkpatrick CJ. Particulate matter in intravenous fluids: the importance for medicine. Krankenhauspharmazie 1988;9:487‐90. [Google Scholar]
Koekenberg 1983
- Koekenberg H. A case for I.V. filtration. Infusion 1983;7:76‐82. [Google Scholar]
Kunac 1999
- Kunac DL, Ball PA, Broadbent RS. In‐line intravenous filtration in neonates‐help not hindrance. Australian Journal of Hospital Pharmacy 1999;29:321‐27. [Google Scholar]
Maki 1991
- Maki DG, Ringer M. Risk factors for infusion‐related phlebitis with small peripheral venous catheters. A randomized controlled trial. Annals of Internal Medicine 1991;114(10):845‐54. [PUBMED: 2014945] [DOI] [PubMed] [Google Scholar]
Marshall 1987
- Marshall L, Lloyd G. Intravenous fluid infiltration. Care of the Critically Ill 1987;3:10‐17. [Google Scholar]
Newell 1998
- Newell F, Ranson K, Robertson J. Use of in‐line filters in pediatric intravenous therapy. Journal of Intravenous Nursing 1998;21(3):166‐70. [PUBMED: 9652276] [PubMed] [Google Scholar]
Ng 1989
- Ng PC, Herrington RA, Beane CA, Ghoneim AT, Dear PR. An outbreak of acinetobacter septicaemia in a neonatal intensive care unit. Journal of Hospital Infection 1989;14(4):363‐8. [PUBMED: 2575636] [DOI] [PubMed] [Google Scholar]
O'Dwyer 1988
- O'Dwyer ST, Michie HR, Zeigler TR, Revhaug A, Smith RJ, Wilmore DW. A single dose of endotoxin increases intestinal permeability in healthy humans. Archives of Surgery 1988;123(12):1459‐64. [PUBMED: 3142442] [DOI] [PubMed] [Google Scholar]
O'Grady 2011
- O'Grady NP, Alexander M, Burns LA, Dellinger EP, Garland J, Heard SO, et al. Healthcare Infection Control Practices Advisory Committee. Guidelines for the prevention of intravascular catheter‐related infections. Centers for Disease Control and Prevention 2011; Vol. http://www.cdc.gov/hicpac/BSI/BSI‐guidelines‐2011.html:1‐59.
Parsons 1989
- Parsons PE, Worthen GS, Moore EE, Tate RM, Henson PM. The association of circulating endotoxin with the development of adult respiratory distress syndrome. American Review of Respiratory Diseases 1989;140(2):294‐301. [PUBMED: 2764364] [DOI] [PubMed] [Google Scholar]
Pearson 1996
- Pearson ML. Guideline for prevention of intravascular device‐related infections: Part 1. Intravascular device‐related infections: an overview. The Hospital Infection Control Practices Advisory Committee. American Journal of Infection Control 1996;24(4):262‐77. [DOI] [PubMed] [Google Scholar]
Quercia 1986
- Quercia RA, Hills SW, Klimek JJ, McLaughlin JC, Nightingale CH, Drezner AD, et al. Bacteriologic contamination of intravenous infusion delivery systems in an intensive care unit. American Journal of Medicine 1986;80(3):364‐8. [PUBMED: 3513558] [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Roberts 1994
- Roberts GW, Holmes MD, Staugas RE, Day RA, Finlay CF, Pitcher A. Peripheral intravenous line survival and phlebitis prevention in patients receiving intravenous antibiotics: heparin/hydrocortisone versus in‐line filters. The Annals of Pharmacotherapy 1994;28(1):11‐6. [PUBMED: 8123947] [DOI] [PubMed] [Google Scholar]
Shaw 1985
- Shaw NJ, Lyall EG. Hazards of glass ampoules. British Medical Journal (Clinical Research Ed) 1985;291(6506):1390. [PUBMED: 3933681] [DOI] [PMC free article] [PubMed] [Google Scholar]
Suffredini 1989a
- Suffredini AF, Harpel PC, Parrillo JE. Promotion and subsequent inhibition of plasminogen activation after administration of intravenous endotoxin to normal subjects. New England Journal of Medicine 1989;320(18):1165‐72. [PUBMED: 2496309] [DOI] [PubMed] [Google Scholar]
Suffredini 1989b
- Suffredini AF, Fromm RE, Parker MM, Brenner M, Kovacs JA, Wesley RA, et al. The cardiovascular response of normal humans to the administration of endotoxin. New England Journal of Medicine 1989;321(5):280‐7. [PUBMED: 2664516] [DOI] [PubMed] [Google Scholar]
Volpe 2001
- Volpe JJ. Neurobiology of periventricular leukomalacia in the premature infant. Pediatric Research 2001;50(5):553‐62. [PUBMED: 11641446] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Foster 2006
- Foster JP, Richards R, Showell MG. Intravenous in‐line filters for preventing morbidity and mortality in neonates. Cochrane Database of Systematic Reviews 2006, Issue 2. [DOI: 10.1002/14651858.CD005248.pub2] [DOI] [PubMed] [Google Scholar]


