Summary
Background
Vaccine derived poliovirus (VDPV) remains a major barrier to polio eradication, and recent growing emergences are concerning. This paper presents the global epidemiology of circulating VDPV (cVDPV) by exploring associations between demographic and socioeconomic factors with its recent rise.
Methods
Data on reported cVDPV cases and isolates between January 1 2016 and June 30 2021 were compiled from EPIWATCH, an open-source observatory for outbreak scanning and analysis, the World Health Organisation (WHO) and ProMed, and analysed descriptively. Reports containing cVDPV case information were included while duplicates and defective links were excluded. Data collection occurred from April 5 2021 to July 16 2021. To identify factors associated with cVDPV, a retrospective case-control study comparing socioeconomic profiles of countries which reported cVDPV with those that did not was undertaken with weighted logistic regression analysis.
Findings
cVDPV caused by serotype 2 poliovirus was the predominant strain (95%) of 1818 total human cVDPV cases reported. Of 40 countries reporting cVDPV cases or isolates, 22 (55%) had polio vaccination coverages below 80%. Low vaccination coverage (Adjusted OR = 83·41, 95% CI: [5·01, 1387·71], p = 0·0020) was found to be associated with increased odds of reporting cVDPV after adjusting for confounding effects of GDP per capita, female adult literacy rates, maternal mortality rate, and Global Peace Index.
Interpretation
Our findings reinforce the importance of maintaining high levels of vaccination, as risk of re-emergence rises when immunity wanes. Interventions to increase vaccination and standards of living in developing countries, coupled with robust surveillance are required if humanity hopes to eradicate polio in the near future.
Funding
This research was supported by the MRFF 2021 Frontier Health and Medical Research Grant (ID RFRHPI000280), Department of Health, the Australian Government.
Keywords: Vaccine derived poliovirus, Poliovirus, Epidemiology, Global health, Vaccination
Research in context.
Evidence before this study
Vaccine-derived poliovirus (VDPV) is a major barrier against global polio eradication. Although VDPV cases have been present historically, the rapid growth with divergent epidemiology of circulating VDPV (cVDPV) observed in recent years has been flagged as a major concern by the World Health Organisation. Searching PubMed, Google Scholar, Medline, and Cochrane for studies up to July 2021 with the terms “polio”, “vaccine derived polio”, “cVDPV”, “vaccination”, “eradication”, “epidemiology”, “factors”, there is little doubt that low population immunity to polio due to low vaccination coverage increases the risk of cVDPV. These studies have also elucidated associations between low vaccine uptake and factors such as poverty, maternal education levels, conflict, and vaccine hesitancy. However, these socioeconomic and demographic factors have not been examined in the context of cVDPV. Notably, there was also a gap in epidemiological reporting on new cVDPV outbreaks post-2016. In response, this study aims to describe the current shift in epidemiology of cVDPV, and explore socioeconomic and demographic which may have given rise to these emergences.
Added value of this study
Through this study, we quantified the rise in cVDPV case numbers, finding a total of 1818 human cVDPV cases reported in the approximate five year period of study. cVDPV caused by serotype 2 poliovirus (cVDPV2) was the predominant strain, accounting for 95% of total reported cVDPV. The high proportion of countries reporting cVDPV cases or isolates with subpar vaccination is a likely cause for the rise in cases. We have also demonstrated a strong relationship between low population vaccination coverage and increased cVDPV incidence, and found a statistically significant relationship between higher GDP per capita and increased cVDPV reporting, likely attributable to better surveillance activities in wealthier health systems.
Implications of all the available evidence
These findings reinforce the importance of maintaining high levels of vaccination, as risk of re-emergence is high when population immunity wanes. The threat of cVDPV must not be underestimated and must be treated with urgency, especially if we hope to eradicate polio in the near future.
Alt-text: Unlabelled box
Introduction
Poliomyelitis, commonly known as “polio”, refers to an acute infectious disease caused by the poliovirus, a single stranded RNA enterovirus, which results in damage to motor neurons of the spinal cord and brainstem.1 There are three main types of poliovirus: Type 1, 2, and 3. Polio most commonly affects children under five years, with the potential to cause acute flaccid paralysis (AFP), which may result in irreversible paralysis or death. Fortunately, with the advent and subsequent widespread adoption of polio vaccines, the global health burden of polio has since dramatically reduced, and only two wild polio-endemic nations remain – Afghanistan and Pakistan. Despite the success in reducing wild poliovirus transmission, one major barrier in the form of vaccine-derived poliovirus (VDPV) remains; global VDPV cases currently outnumber that of wild poliovirus (WPV).2
The oral polio vaccine (OPV) is one of the two main types of vaccines currently in use, the other being the inactivated polio vaccine (IPV). The OPV, like the IPV, is effective in inducing humoral immunity against poliovirus in the host;3 it also has some advantages over the IPV including its ease of administration, low cost per dose, and efficacy in interrupting viral transmission through inducing intestinal mucosal immunity.3 However, given that it contains live virus, it has the potential to cause VDPV, which is one of the rare risks associated with OPV use.2
VDPVs occur when excreted virus from an individual immunised with OPV is allowed to circulate and mutate, and eventually, the virus develops sufficient neurovirulence and transmissibility to cause paralytic disease in a patient.2 The Global Polio Eradication Initiative defines VDPV as: “OPV virus strains that are >1% divergent (or 10 nucleotide (NT) changes, for types 1 and 3) or >0·6% divergent ( 6 NT changes, for type 2) from the corresponding OPV strain in the complete VP1 genomic region”.4 In addition to direct transmission of OPV, because the OPV (like the wild type virus) is excreted in the faeces, it can persist in waterways.
VDPVs can be further subdivided into the following categories:4
-
1.
Circulating VDPV (cVDPV): where evidence of human-to-human transmission in the community is present. Isolates must either be from i) at least two individuals (not necessarily AFP cases) who are not direct contacts; ii) from one individual and one or more environmental samples; or iii) from two or more environmental samples collected more than two months apart or from more than one distinct collection site
-
2.
Immune-deficiency associated VDPV (iVDPV): where VDPV is isolated from patients with primary immunodeficiencies (PID)
-
3.
Ambiguous VDPV (aVDPV): VDPV isolates (human or environmental), without evidence of circulation and from individuals with no known immunodeficiency. May be reclassified as “circulating” if genetically linked isolates are found subsequently
cVDPV will be the focus of this study given its recent growth and spread.
One of the first recorded outbreaks of cVDPV occurred between 2000 and 2001 in Haiti and the Dominican Republic, which resulted in 21 cases of cVDPV1 and two fatalities.5 Other cVDPV outbreaks have been reported in various countries since then. From 2000 to 2016, 24 outbreaks of cVDPV have occurred, with approximately 760 cases.6
While wild poliovirus type 2 was last detected in 1999,2 vaccine-related type 2 viruses continued to cause most cVDPV outbreaks and vaccine associated paralytic poliomyelitis (VAPP); thus, routine use of OPV containing type 2 carried more risk than benefit.7 In response, the World Health Assembly endorsed a phased cessation of the trivalent OPV (tOPV), known as the “Switch”.8 The “Switch” occurred in April to May 2016, and involved a coordinated global “Switch” from the tOPV (contains all three strains) to the bivalent OPV (bOPV; contains only type 1 and 3). Type 2 containing OPVs would only be used in outbreak responses and must be handled with strict protocols to prevent spread and emergence.7,8
IPV was also introduced into national immunisation schedules to mediate risk of post-“Switch” type 2 re-emergence. Countries were encouraged to introduce at least one dose of IPV into national immunisation schedules before the “Switch”, by the end of 2015. This would help to reduce risk of type 2 re-emergence, as well as facilitate better control in the event of future outbreaks.9
Increased susceptibility to type 2 poliovirus outbreaks in the immediate aftermath of the “Switch” was expected,10 as new birth cohorts born post-“Switch” would only have humoral immunity against type 2 from the IPV, but not primary intestinal immunity against type 2 poliovirus.8 However, the developing trend of emerging cVDPV outbreaks since 2016, increasing in incidence and with evidence of international spread is concerning,2,6 and begs the question as to whether risk-mitigating strategies were adequately aggressive. In 2020 alone, a total of 1048 cVDPV cases were reported in 26 different countries,6 many of whom had previously been declared polio-free.
Low immunisation coverage in the community has been widely established as the leading cause of polio events and outbreaks.9 Several factors have been suggested to predispose to low vaccine coverage or uptake, which include poverty,11, 12, 13, 14 low levels of maternal education13 and household literacy,13,14 vaccine hesitancy, and conflict.15 However, these individual factors have not been studied rigorously in the context of cVDPV.
Thus, this study examines the global epidemiology of cVDPV from 2016 to 2021 in order to identify divergent epidemiological patterns. An exploration of the associations between demographic and socioeconomic factors and VDPV that have been delineated previously will be undertaken in the context of the recent growth in cVDPV incidence.
Methods
Study design and data sources
A retrospective case-control study comparing socioeconomic profiles of countries which reported cVDPV with those that did not was conducted, and the epidemiology of globally reported WPV and cVDPV between January 1 2016 and June 30 2021 was examined.
Data about total reported confirmed cases and environmental isolates of WPV and cVDPV from January 1 2016 to June 30 2021 were retrieved from EPIWATCH,16 the World Health Organisation (WHO),6 the Global Polio Eradication Initiative (GPEI),17 and ProMed.18 Data collection from databases occurred between April 5 2021 and July 16 2021, where data about cases reported between January 1 2016 and June 30 2021 were obtained from the aforementioned sources. EPIWATCH is an open-source observatory for outbreak scanning and analysis developed by the Kirby Institute in the University of New South Wales (UNSW) which utilises open-source data such as news reports and social media to rapidly detect epidemic signals before validation by traditional public health surveillance.16 Where available, more detailed demographic data such as vaccination status, age and sex of the cases were also retrieved.
Estimates of vaccination coverage (Percentage of one-year-old who have received three doses of polio vaccine in a given year (Pol3) coverage) were obtained from WHO/UNICEF.19 Data pertaining to socioeconomic indicators including GDP per capita,20 literacy rates,21,22 maternal mortality ratio per 100,000 live births,23 and sanitation24 were obtained from the World Bank.
Country data for the Global Peace Index (GPI) were obtained from the 2021 report by the Institute for Economics and Peace (IEP).25 The GPI is a measure devised by the IEP to quantify how peaceful a country is, by scoring several socio-political indicators, such as the number and duration of internal conflicts and the level of violent crime.
All data used was public domain and de-identified, thus ethics approval was not required. This paper was prepared in accordance to reporting guidelines as stipulated by STROBE.
Procedures
Data were extracted from EPIWATCH using the search terms “polio”, “poliomyelitis”, “acute flaccid paralysis”, “AFP”, “wild poliovirus”, “WPV”, “vaccine derived poliovirus”, “VDPV”, “cVDPV”, “outbreak”, and “epidemic”. Table 1 outlines the inclusion and exclusion criteria for information obtained from EPIWATCH;16 news items were excluded if duplicates, defective links, or irrelevant articles were found. All “Poliomyelitis updates” from ProMed26 were accessed to supplement data from EPIWATCH.
Table 1.
Inclusion & exclusion criteria.
| Inclusion Criteria | Exclusion Criteria |
|---|---|
Reports about:
|
|
“AFP” refers to acute flaccid paralysis, “cVDPV” refers to circulating vaccine-derived poliovirus, and “WPV” refers to wild polio virus.
A line list dataset was created containing information about location, date of reporting, date of AFP onset, strain type, and where available, vaccination history, age, sex, clinical presentation, and reasons for vaccine refusal. Date of reporting was chosen in analysis instead of date of onset of illness due to limited available data pertaining to the latter. Microsoft Excel 2019 was used to map outbreaks and produce epidemic curves to identify geo-temporal patterns.
A retrospective case-control study was also conducted to identify factors associated with cVDPV incidence. The main outcome variable was whether or not a country reported cVDPV cases within the stipulated period of study. Variables were coded as either “case” or “control” countries.
A “case” country was one which experienced cVDPV events or outbreaks between January 1 2016 and June 30 2021, as defined by WHO.26 “Case” countries were ones which reported:
-
a.
AFP case(s) with confirmed cVDPV isolates; and/or
-
b.
Asymptomatic or non-paralytic polio case(s) with confirmed cVDPV isolates; and/or
-
c.
Environmental sample(s) from which cVDPV was isolated
Based on the World Bank classification,27 a complete list of low income (LIC), lower-middle income (LMIC), and upper-middle income countries (UMIC) was obtained. There were no high income country (HIC) “case” countries, and thus HIC were not considered for controls to maximise socioeconomic congruency between case and controls. “Control” countries were selected from countries which did not report any cVDPV isolates between January 1 2016 and June 30 2021 from the aforementioned list. Countries with missing data and/or which had outlying data points were excluded from selection. There were a total of 40 “case” and 40 “control” countries. Supplementary Table A contains a full list of the countries considered for analysis.
Statistical analysis
Descriptive statistics of the cleaned dataset was used to describe the sociodemographic profiles of polio cases to examine any trends in age, sex, and strain type. It was also conducted to illustrate the sociodemographic characteristics of “case” and “control” countries.
Considering large discrepancies of population size between countries, we adopted weighting in the regression analyses. Data for population size were obtained from the World Bank.28 The weight (population at risk in each country) was normalised by dividing them by the total population in all countries and then multiplied by 80 (number of countries). To avoid spurious precision29 (very high precision that is higher than possible due to some error in calculation) in the estimates, we adopted importance weights (by using “iweight” option in Stata) in the logistic regression models.
There was a small number (only seven) of potential explanatory variables compared to the sample size (N = 80), so the backward elimination method was adopted to decide the final model after adjusting for confounders. Initially, univariable weighted logistic regression models were fitted and any variable giving p<0·25 were considered as a candidate for the base model. From the base model, we first removed the explanatory variable with highest p-value that is greater than 0·05. To check for confounding effects of the excluded variable, we compared the odds ratio of vaccine coverage (the main effect) before and after removing the variable. If the difference was greater than 5%, the variable was considered as a confounder and kept in the model. If it was not a confounder, we compared the precision (p-value) of the main effect. If removal of the variable did not reduce the precision of the main effect we excluded it, otherwise we left it in the model. We did this for all the relevant variables to arrive at the final model.
Variables considered for univariable analysis were: Pol3 vaccination coverage,19 GDP per capita,20 adult literacy rates21 (measure of education), female adult literacy rates22 (measure of maternal education), maternal mortality rate23 (measure of healthcare infrastructure), the percentage of population using at least basic sanitation services24 (measure of sanitation), and the Global Peace Index25 (GPI; measure of the state of peace). Vaccination coverage was coded into “low (<80%)” or “high (80%)” as the herd immunity threshold for polio is approximately 80%.30
Adult literacy and female literacy rates were highly correlated ( = 0·989, n = 80, p<0·01). To avoid multicollinearity, only female literacy was included in the base model.
Following the model building, we found all but the variable “sanitation” to be strong confounders of the main effect. Removal of sanitation also enhanced the precision of the main effect. Thus, the final multivariable model consisted of vaccine coverage, GDP per capita, female literacy rates, maternal mortality rate and GPI.
The IBM® SPSS® Statistics 26 software31 and R(version 3·6·0)/R Studio32 were used for the aforementioned analyses, with confidence intervals set at 95% and significance threshold at p<0·05. The weighted logistic regression models were fitted using Stata 17.
Role of the funding source
The funders of this study had no role in study design, data collection, data analysis, data interpretation, or writing of this report. All authors had access to the dataset in this study. YL had the final responsibility for the decision to submit for publication.
Results
Geo-temporal trends
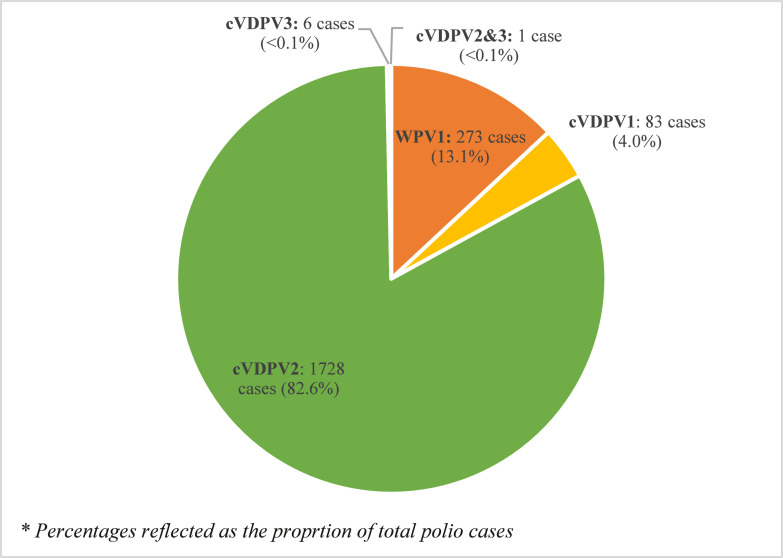
From January 1 2016 to June 30 2021, a total of 2091 human polio cases were reported. 273 cases (13·1%) were caused by WPV1 and 1818 (86·9%) by cVDPV; cVDPV2 accounted for majority (95·0%) of cVDPV, with 1728 cases. There were also 83 cVDPV1 cases, 6 cVDPV3 cases, and 1 case which had a combination of cVDPV2 and cVDPV3. This is illustrated in Figure 1.
Figure 1.
Total reported human polio cases globally by strain type, 01/01/2016 – 30/06/2021.
“WPV1” refers to wild poliovirus serotype 1 (orange), “cVDPV1” refers to circulating vaccine-derived poliovirus serotype 1 (yellow), “cVDPV2” refers to circulating vaccine-derived poliovirus serotype 2 (green), “cVDPV3” refers to circulating vaccine-derived poliovirus serotype 3 (blue), and “cVDPV2&3” refers to a case where both cVDPV2 and cVDPV3 were isolated from a single individual (brown). Percentages are based on the total reported wild poliovirus and circulating vaccine-derived poliovirus cases in the period of study.
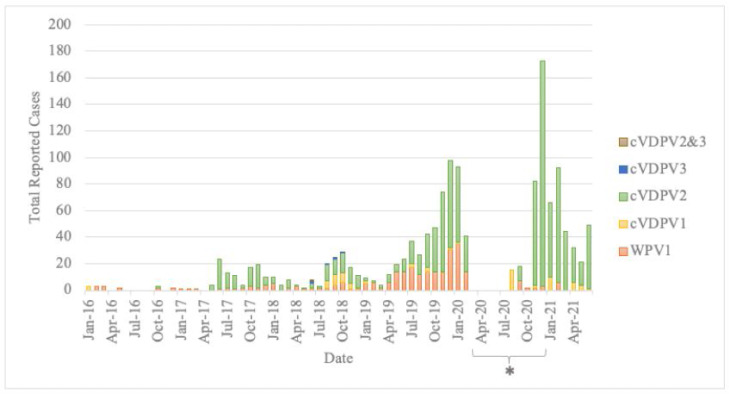
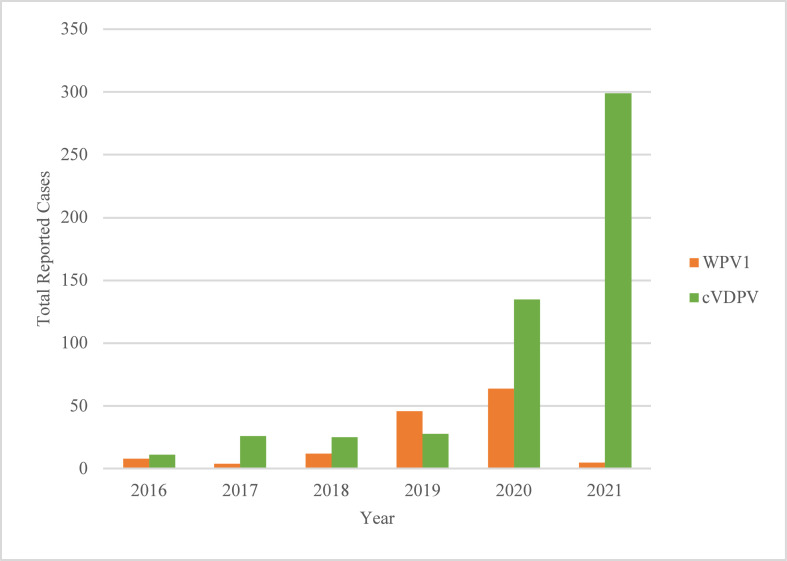
Figure 2 is an epidemic curve plotting the number of reported human cases of polio on a monthly time-scale, sorted by strain type. The trend in total cases reported from January to June in each year of study is shown in Figure 3.
Figure 2.
Epidemic Curve of reported human polio cases globally, 01/01/2016 – 30/06/2021.
“WPV1” refers to wild poliovirus serotype 1, “cVDPV1” refers to circulating vaccine-derived poliovirus serotype 1, “cVDPV2” refers to circulating vaccine-derived poliovirus serotype 2, “cVDPV3” refers to circulating vaccine-derived poliovirus serotype 3, and “cVDPV2&3” refers to a case where both cVDPV2 and cVDPV3 were isolated from a single individual. Colours represent the serotype of poliovirus reported: WPV1 (orange), cVDPV1 (yellow), cVDPV2 (green), cVDPV3 (blue), and cVDPV2&3 (brown).
*There was a reporting gap from ProMed between March and December 2020; retrospectively, a total of 3 cVDPV1 and 684 cVDPV2 AFP cases were reported in this period.
Figure 3.
Total Reported human Polio Cases Globally, Year-to-Date, January to June, 2016 – 2021.
“WPV1” refers to wild poliovirus serotype 1 (orange), and “cVDPV” (green) refers to all three serotypes of circulating vaccine-derived poliovirus. Total reported cases in each year from 2016 to 2021 between January 1 to June 30 are shown in this figure.
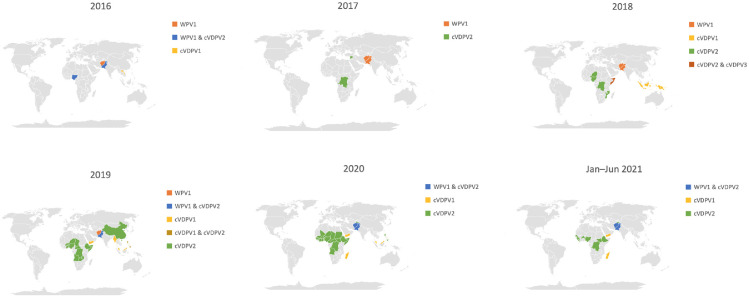
The geographical distribution of human polio cases from 2016 to 2021 is mapped in Figure 4. Of note, a geographical expansion of cVDPV2, especially in the central African region, is observed. cVDPV1 cases were reported in Laos (2016), Indonesia (2018), Papua New Guinea (2018), Yemen (2019 – 2021), Malaysia (2019 – 2020), Myanmar (2019), and Madagascar (2020 – 2021); all cVDPV3 cases were part of an outbreak limited to Somalia.
Figure 4.
Global distribution of human Polio Cases annually, 01/01/2016 – 30/06/2021.
“WPV1” refers to wild poliovirus serotype 1, “cVDPV1” refers to circulating vaccine-derived poliovirus serotype 1, “cVDPV2” refers to circulating vaccine-derived poliovirus serotype 2, “cVDPV3” refers to circulating vaccine-derived poliovirus serotype 3. Where two serotypes are mentioned (e.g. “WPV1&cVDPV2”), it signifies that both serotypes were reported in the country in that year.
WPV1 was the only type of wild poliovirus observed, and was confined to the endemic nations of Pakistan and Afghanistan from 2016 to 2021, and Nigeria in 2016. A complete list of countries which reported cases is seen in Supplementary Table B.
Affected age groups and sex
Information pertaining to age and/or sex were only available for 235 cases in the period of study and included for analysis. In terms of age (Table 2), majority of cases occurred in the “0-3 years old” age group for WPV1 (86·3%) and cVDPV2 (75·8%). In contrast, a bimodal distribution was observed for cVDPV1, with peaks in the “0-3 years old” and “>5 years old” age groups. Both the youngest (2 months) and oldest (156 months) patients fell in the WPV1 group. WPV1 also had the lowest median age (18 months) while cVDPV1 had the highest (40 months).
Table 2.
The number of reported polio cases globally with known age, 01/01/2016 – 30/06/2021.
| Age range | All Strain Types (N = 235*) | WPV1 (N = 190) | cVDPV1 (N = 12) | cVDPV2 (N = 33) |
|---|---|---|---|---|
| 0-3 years old | 83.0% (N = 195) | 86.3% (N = 164) | 50.0% (N = 6) | 75.8% (N = 25) |
| 3-5 years old | 8.1% (N = 19) | 6.8% (N = 13) | 8.3% (N = 1) | 15.2% (N = 5) |
| >5 years old | 8.9% (N = 21) | 6.8% (N = 13) | 41.7% (N = 5) | 9.1% (N = 3) |
| Minimum | 2 months | 2 months | 3 months | 9 months |
| Maximum | 156 months | 156 months | 132 months | 132 months |
| Mean | 28 months | 26 months | 53 months | 35 months |
| Median | 21 months | 18 months | 40 months | 26 months |
| Std. Dev. | 28.2 months | 26.7 months | 42.7 months | 26.1 months |
“WPV1” refers to wild poliovirus serotype 1, “cVDPV1” refers to circulating vaccine-derived poliovirus serotype 1, and “cVDPV2” refers to circulating vaccine-derived poliovirus serotype 2.
* Information about age was only available for 235 out of 2091 total reported human polio cases (273 WPV1, 1818 cVDPV) and included for analysis.
For sex (Supplementary Table C), cases were relatively equally distributed amongst females (36·6%) and males (48·5%).
Demographic & socioeconomic associated with cVDPV: case-control study
A total of 40 countries reported human cVDPV cases and/or environmental cVDPV isolates from 2016 to 2021, of which 19 were low income (LIC), 19 were lower-middle income (LMIC), and two were upper-middle income countries (UMIC). These countries were compared against 40 other control countries with similar economic profiles (Supplementary Tables A, D).
Pol3 vaccination coverage
There was a clear contrast between polio vaccination coverage in “case” and “control” countries. While only 4 “control” countries (10%) had vaccination rates below the 80% herd immunity threshold, 22 “case” countries (55%) had low vaccination coverage below 80%.
Weighted logistic regression analysis
The outcome variable for logistic regression was whether or not a country reported cVDPV in the study period. Weighted differences for variables can be found in Supplementary Table E. Results of univariable and multivariable analyses are summarised in Table 3.
Table 3.
Factors associated with cVDPV incidence in a country by weighted logistic regression.
| Unadjusted (univariable) |
Adjusted* (multivariable) |
|||||
|---|---|---|---|---|---|---|
| Variable | Odds Ratio | P value | 95% Confidence Interval | Odds Ratio | P value | 95% Confidence Interval |
|
GDP Per Capita (per US$1000) |
1·27 | 0·0020 | 1·09–1·47 | 1·76 | 0.0003 | 1·30–2·38 |
|
Maternal Mortality Rate (per 1,000,000 live births) |
1·00 | 0·23 | 1·00–1·00 | 1·00 | 0·19 | 1·00–1·01 |
| Adult Literacy Rate | 2·83 | 0·45 | 0·19–42.76 | Not available | Not available | Not available |
| Female Literacy Rate | 2·68 | 0·40 | 0·27–26·26 | 0·02 | 0·27 | 0·00–17·80 |
|
Vaccination coverage: Low (<80%) (ref = high (≥80%)) |
17·69 | 0·012 | 1·90–164·19 | 83·41 | 0·0020 | 5·01–1387·71 |
|
Global Peace Index score (higher = less peaceful) |
0·63 | 0·46 | 0·18–2·18 | 0·13 | 0·13 | 0·01–1·80 |
| Sanitation | 1·18 | 0·86 | 0·17–8·06 | Not available | Not available | Not available |
Adult literacy rate was excluded from the base model to avoid multicollinearity as adult literacy rate and female literacy rate were highly correlated ( = 0·989, n = 80, p<0·01). Following model building, all variables but “sanitation” were found to be strong confounders of vaccination coverage, the main effect, and removal of “sanitation” enhanced the precision of the main effect. Thus, the final multivariable model consisted of vaccine coverage, GDP per capita, female literacy rates, maternal mortality rate and GPI, adjusting for the confounding effects of the latter four variables on vaccine coverage.
In univariable logistic regression, only GDP per capita (per US$1000) and vaccination coverage were found to be significant at p<0·05. Countries with lower vaccination rates (<80%) were approximately 18 times more likely (OR = 17·69, 95% CI: [1·90, 164·19], p = 0·012) to report cVDPV compared to those with high vaccination coverage. A higher GDP per capita was also associated with increased odds of reporting cVDPV; the odds of reporting cVDPV was 1·27 times that of not reporting cVDPV for every US$1000 increment in GDP per capita (OR = 1·27, 95% CI: [1·09, 1·47], p = 0·0020). Unadjusted (univariable) results for maternal mortality rate, literacy rates, global peace index, and sanitation were not statistically significant.
Vaccination coverage remained statistically significant after adjusting for confounding effects of GDP per capita, maternal mortality rate, female literacy rate, and GPI in the multivariable logistic regression model. The odds of a country with low vaccination coverage (<80%) reporting cVDPV was 83 times greater (AOR = 83·41, 95% CI: [5·01, 1387·71], p = 0·0020) than that of control countries. GDP per capita was also statistically significant in the multivariable model; a higher GDP Per Capita was also associated with increased odds of reporting cVDPV (AOR = 1·76, 95% CI: [1·30, 2·38], p = 0·0003). Maternal mortality rate, female literacy rate and GPI were not found to be significant in the multivariable model, and sanitation was not included in the model as it was not a confounder.
Discussion
In our study, the global epidemiology of cVDPV from January 2016 to June 2021 was examined to identify demographic and socioeconomic factors giving rise to cVDPV incidence. The strong relationship (AOR = 83·41, 95% CI: [5·01, 1387·71], p = 0·0020) between low vaccination coverage and cVDPV reporting found in our study clearly reinforces that low vaccination coverage is a major factor giving rise to cVDPV emergence. The effect of subpar vaccination coverage was also evident in the fact that most countries which reported cVDPV (55%) had vaccination rates below the herd immunity threshold of 80%.30 Noteworthy is that vaccination data used in this study were nationwide estimates; vaccination coverage may not be uniform throughout a country and may in fact be much lower than reflected in outbreak areas, given that these areas typically involve underserved communities.
While some of the initial growth in cVDPV incidence observed in 2016 to 2017 may be attributable to the “Switch” away from the tOPV,10 the case incidence observed now may likely a result of waning population immunity and inadequate risk-mitigation in underserved communities. The presence of new emergences in countries which have maintained polio-free status for years (e.g. Papua New Guinea) also serves as a warning against complacency in other similar nations; surveillance systems must be kept robust to ensure confidence in the quality of VDPV data.
Poverty is frequently linked in literature to higher incidence of polio, given that it perpetuates other cited risk factors such as sanitation and access to healthcare.11 Our finding that countries with higher GDP Per Capita were associated with increased odds of reporting cVDPV (AOR = 1·76, 95% CI: [1·30, 2·38], p = 0·0003) was thus surprising, as it appeared contradictory to existing literature. However, this can be accounted for as public health surveillance systems tend to be more robust in wealthier nations,33 thus allowing for greater ability to detect and report polio cases.
Most countries included in analysis were also mainly low or lower-middle income countries. Therefore, it may also be the case where while low to lower-middle income countries adopt a disproportionate burden of infectious disease,11 when conducting analyses within these country income groups, the impact of wealth becomes more tenuous compared to when also considering high income countries. Additionally, GDP Per Capita, while an adequate general marker of country wealth, fails to account for the presence of income disparity within a nation; high levels of income inequality exist in many developing countries,34 of which many were considered for analysis in this study.
Unexpectedly, maternal mortality rate, female adult literacy rate, global peace index, and access to sanitation services were all not found to be significant in both univariable and multivariable regression models. This was surprising as a higher level of maternal education13,14 has been cited as a reason for low vaccination uptake in literature, while conflict15 and poor sanitation11 have been flagged to be associated with polio incidence. While our findings do not rule out an effect on cVDPV incidence, they suggest that these variables may be more prominent in shaping vaccination uptake, as suggested in literature, rather than on cVDPV incidence directly.
The impact of vaccine hesitancy was also apparent through data collection from news reports through EPIWATCH. Anecdotally, some patients who had not received vaccinations cited distrust in the vaccine and authorities, where some families in Pakistan resorted to “fake finger marking” or hiding their child to avoid vaccination.35 Several news reports of misinformation that polio vaccines kill children spreading through social media36 giving rise to vaccine refusal were also found. Vaccine hesitancy is a major challenge which undermines eradication efforts already hampered by resource and access difficulties. To tackle issues such as vaccine hesitancy, partnership with local authorities is required to address reasons for vaccine refusal.37 Engaging and involving local stakeholders in the administration of vaccines may also aid in fostering trust and the sustainability of vaccination programmes. Better quality data is required to monitor and manage threats against health workers in high conflict areas, and investment into local communities is paramount to ensure the sustainability of vaccination programmes.38
Finally, the finding of proportionately more cVDPV1 cases in older children (41·7%) compared to cVDPV2 (9·1%) and WPV1 (6·8%) is interesting and warrants further investigation with a larger and more complete dataset. It is unknown whether this is a true finding, or just an artifact resulting from sampling bias due to limited information available (N=235) especially since the minimum and maximum age of polio patients across all strain types were similar. Notably, a sizable number of children above the age of 5 (42 out of 235) were affected by polio; immunisation programmes are typically targeted at children under the age of 5. Expanding target age groups during supplementary immunisation activities may thus be worth considering especially in vulnerable regions.39
This study was not without limitations. One key limitation of our study stemmed from the use of Pol3 coverage to reflect immunisation status. In light of the 2016 “Switch”, routine immunisation schedules would no longer include the tOPV, and immunity against type 2 poliovirus in birth cohorts born post-“Switch” would solely originate from humoral immunity induced by the IPV. While the IPV may have played a role in reducing paralytic disease caused by cVDPV2,3,7,8 it would not have as much of an effect on halting transmission due to its limited impact on intestinal mucosal immunity.3 Although low Pol3 coverage would most likely predispose to cVDPV emergence, the relationship may not work the other way, as populations with high rates of bOPV may still be highly vulnerable to cVDPV2 transmission. Care must therefore be taken against over-generalisation when interpreting the results of this study. Pol3 also does not consider the impact of supplementary immunisation activities (SIAs), which play an important role in outbreak management especially in high-risk nations.
The impact of introducing the novel oral polio vaccine type 2 (nOPV2) into the WHO Emergency Use Listing Procedure (EULP) in early 2021 must also be considered in future study of cVDPV2 epidemiology. The nOPV2, a modified form of the original monovalent OPV2 (mOPV2), has been shown to confer considerable immunity while at the same time being more genetically stable with a lower risk of cVDPV2 emergence.40 With its addition to the EULP and use as a primary vaccine option in response to cVDPV2 outbreaks, the nOPV2 holds much promise as a replacement of the mOPV2 in controlling and preventing further cVDPV2 outbreaks.40
Additionally, the inclusion criteria for a “case” country meant that countries which reported just one case (e.g. China, Indonesia) were considered, without accounting for the extent of outbreaks or the strength of response to outbreaks; this may have resulted in skew. Reasons for an outbreak are also multifactorial and country-dependent;41 this study was a broad overview and other factors such as geography and type of vaccination used prior to the “Switch” were not accounted for in analysis.
Another important factor that was not quantitatively studied was the effect of COVID-19, which likely had its own flow-on effects. Disruptions to usual anti-polio interventions due to redirection of polio resources to the COVID-19 response have been documented; for example, over 40 million children in Pakistan were found to have missed their routine immunisations in 2020.42 More indirect effects of COVID-19, such as the loss of economic stability, could also precipitate into high risk of cVDPV due to increased poverty levels and poorer standards of living secondary to that.
In conclusion, cVDPV transmission is one major barrier in the race to eradicate polio. Low vaccination was found to be strongly associated with an increased likelihood of reporting cVDPV, and higher GDP per capita was found to increase the odds of reporting cVDPV. Interventions at the local level to increase vaccination and standards of living, coupled with frequent evaluation to test the robustness of surveillance systems should be undertaken if humanity hopes to eradicate all forms of polioviruses in the near future.
Contributors
The study concept and design were formulated by YL and CRM. Literature search was completed by YL. Data sourcing and collection was undertaken by YL. Data access and verification, and formal statistical analysis were done by YL, BR, MK, and XC. The manuscript was written by YL, with editorial input from CRM, XC, and MK.
Data sharing statement
Data collected for this study is available on request with publication; requests should be directed to the corresponding author (YL).
Declaration of interests
All authors declare no competing interests.
Acknowledgements
The authors would like to thank Mrs Ashley Quigley and Dr Mark Raphael for kindly providing related material on poliomyelitis for reference, and the Biosecurity team at the Kirby Institute, UNSW, for their ongoing intellectual input and support.
Funding
This research was supported by the MRFF 2021 Frontier Health and Medical Research Grant (ID RFRHPI000280), Department of Health, the Australian Government.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.eclinm.2022.101508.
Appendix. Supplementary materials
References
- 1.De Jesus NH. Epidemics to eradication: the modern history of poliomyelitis. Virology. 2007;4(1):1–18. doi: 10.1186/1743-422X-4-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Burki T. Vaccine-derived poliovirus cases exceed wild types. Lancet Infect Dis. 2019;19(2):140. doi: 10.1016/S1473-3099(19)30012-X. [DOI] [PubMed] [Google Scholar]
- 3.Xiao Y, Daniell H. Long-term evaluation of mucosal and systemic immunity and protection conferred by different polio booster vaccines. Vaccine. 2017;35(40):5418–5425. doi: 10.1016/j.vaccine.2016.12.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Global Polio Eradication Initiative. Classification of vaccine-derived polioviruses: GPEI guidelines. 2016. https://polioeradication.org/wp-content/uploads/2016/09/Reporting-and-Classification-of-VDPVs_Aug2016_EN.pdf. Accessed 4 April 2021.
- 5.Kew O, Morris-Glasgow V, Landaverde M, et al. Outbreak of poliomyelitis in Hispaniola associated with circulating type 1 vaccine-derived poliovirus. Science. 2002;296(5566):356–359. doi: 10.1126/science.1068284. [DOI] [PubMed] [Google Scholar]
- 6.World Health Organization. World Health Organisation extranet dataset. 2021. https://extranet.who.int/polis/public/CaseCount.aspx. Accessed 14 June 2021.
- 7.Pan American Health Organization. Inactivated Polio Vaccine (IPV) introduction. https://www.paho.org/hq/dmdocuments/2014/IPV-IntroductionFAQ-e.pdf. Accessed 24 March 2022.
- 8.Garon J, Seib K, Orenstein WA, Ramirez Gonzalez A, Chang Blanc D, Zaffran M, Patel M. Polio endgame: the global switch from tOPV to bOPV. Expert Rev Vaccin. 2016;15(6):693–708. doi: 10.1586/14760584.2016.1140041. [DOI] [PubMed] [Google Scholar]
- 9.World Health Organization Polio vaccines: WHO position paper, January 2014. Wkly Epidemiol Record Relevé épidémiologique hebdomadaire. 2014;89(09):73–92. [PubMed] [Google Scholar]
- 10.Thompson KM, Duintjer Tebbens RJ. Modeling the dynamics of oral poliovirus vaccine cessation. J Infect Dis. 2014;210(suppl_1):S475–S484. doi: 10.1093/infdis/jit845. [DOI] [PubMed] [Google Scholar]
- 11.Noori N, Drake JM, Rohani P. Comparative epidemiology of poliovirus transmission. Sci Rep. 2017;7(1):1–12. doi: 10.1038/s41598-017-17749-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Landoh DE, Ouro-Kavalah F, Yaya I, et al. Predictors of incomplete immunization coverage among one to five years old children in Togo. BMC Public Health. 2016;16(1):1–7. doi: 10.1186/s12889-016-3625-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Khan MT, Zaheer S, Shafique K. Maternal education, empowerment, economic status and child polio vaccination uptake in Pakistan: a population based cross sectional study. BMJ Open. 2017;7(3) doi: 10.1136/bmjopen-2016-013853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Taylor S, Khan M, Muhammad A, et al. Understanding vaccine hesitancy in polio eradication in northern Nigeria. Vaccine. 2017;35(47):6438–6443. doi: 10.1016/j.vaccine.2017.09.075. [DOI] [PubMed] [Google Scholar]
- 15.Shah SFA, Ginossar T, Weiss D. “This is a Pakhtun disease”: Pakhtun health journalists’ perceptions of the barriers and facilitators to polio vaccine acceptance among the high-risk Pakhtun community in Pakistan. Vaccine. 2019;37(28):3694–3703. doi: 10.1016/j.vaccine.2019.05.029. [DOI] [PubMed] [Google Scholar]
- 16.NHMRC Centre for Research Excellence . EPIWATCH; 2017. Integrated Systems for Epidemic Response.https://iser.med.unsw.edu.au/epiwatch Accessed 14 May 2021. [Google Scholar]
- 17.Global Polio Eradication Initiative. Polio now – this week. 2021. https://polioeradication.org/polio-today/polio-now/this-week/. Accessed 2 July 2021.
- 18.ProMED-mail. Poliomyelitis update (13): global (cVDPV2 Nigeria, Egypt environmental). 2021. https://promedmail.org/promed-post/?id=20210702.8489890. Accessed 15 July 2021.
- 19.World Health Organization. WHO-UNICEF estimates of Pol3 coverage. 2020. https://apps.who.int/immunization_monitoring/globalsummary/timeseries/tswucoveragepol3.html. Accessed 25 June 2021.
- 20.World Bank. GDP per capita (current US$). 2021. https://data.worldbank.org/indicator/NY.GDP.PCAP.CD. Accessed 26 June 2021.
- 21.World Bank. Literacy rate, adult total (% of peoples ages 15 and above). 2020. https://data.worldbank.org/indicator/SE.ADT.LITR.ZS. Accessed 26 June 2021.
- 22.World Bank. Literacy rate, adult female (% of peoples ages 15 and above). 2020. https://data.worldbank.org/indicator/SE.ADT.LITR.FE.ZS. Accessed 26 June 2021.
- 23.World Bank. Maternal mortality ratio (modelled estimate, per 100,000 live births). 2019. https://data.worldbank.org/indicator/SH.STA.MMRT. Accessed 2 July 2021.
- 24.World Bank. People using at least basic sanitation services (% of population). 2021. https://data.worldbank.org/indicator/SH.STA.BASS.ZS. Accessed 26 June 2021.
- 25.Institute for Economics & Peace . Institute for Economics & Peace; Sydney: 2021. Global Peace Index 2021: Measuring Peace in a Complex World. [Google Scholar]
- 26.World Health Organization. Standard operating procedures: responding to a poliovirus event or outbreak (2020).
- 27.World Bank. World bank country and lending groups. 2021. https://datahelpdesk.worldbank.org/knowledgebase/articles/906519-world-bank-country-and-lending-groups. Accessed 17 May 2021.
- 28.World Bank. Population, total. 2020. https://data.worldbank.org/indicator/SP.POP.TOTL. Accessed 20 April 2022.
- 29.Condon RE. Spurious precision. Surgery. 2003 May;133(5):588. doi: 10.1067/msy.2003.130. [DOI] [PubMed] [Google Scholar]
- 30.Plans-Rubió P. Evaluation of the establishment of herd immunity in the population by means of serological surveys and vaccination coverage. Hum Vaccin Immunother. 2012;8(2):184–188. doi: 10.4161/hv.18444. [DOI] [PubMed] [Google Scholar]
- 31.IBM Corporation . IBM Corp; New York: 2019. IBM SPSS Statistics for Macintosh, Version 26.0. [Google Scholar]
- 32.R Core Team . R Core Team; 2021. R: A Language and Environment for Statistical Computing. [Google Scholar]
- 33.Nsubuga P, Nwanyanwu O, Nkengasong JN, Mukanga D, Trostle M. Strengthening public health surveillance and response using the health systems strengthening agenda in developing countries. BMC Public Health. 2010 Dec;10(1):1–5. doi: 10.1186/1471-2458-10-S1-S5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ravallion M. Income inequality in the developing world. Science. 2014 May 23;344(6186):851–855. doi: 10.1126/science.1251875. [DOI] [PubMed] [Google Scholar]
- 35.ProMED-mail. Poliomyelitis update (59): Pakistan (KP, PB). 2019. https://promedmail.org/promed-post/?id=20190715.6569236. Accessed 10 June 2021.
- 36.ProMED-mail. Poliomyelitis update (60): Pakistan, negative impact of social media. 2018. https://promedmail.org/promed-post/?id=20181219.6214362. Accessed 5 June 2021.
- 37.World Health Organization . World Health Organization; Geneva: 2020. Immunization Agenda 2030: a Global Strategy to Leave No One Behind. [Google Scholar]
- 38.Druce P, Bogatyreva E, Siem FF, et al. Springer; 2019. Approaches to Protect and Maintain Health Care Services in Armed Conflict–Meeting SDGs 3 and 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tebbens RJD, Kalkowska DA, Wassilak SG, Pallansch MA, Cochi SL, Thompson KM. The potential impact of expanding target age groups for polio immunization campaigns. BMC Infect Dis. 2014;14(1):1–17. doi: 10.1186/1471-2334-14-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wahid R, Mercer L, Gast C, De Leon T, Sáez-Llorens X, Fix A, Macadam A, Stephens L, Chumakov K, Smits SL, Murreddu M. Evaluating stability of attenuated Sabin and two novel type 2 oral poliovirus vaccines in children. npj Vaccines. 2022;7(1):1. doi: 10.1038/s41541-022-00437-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Phillips D.E., Dieleman J.L., Lim S.S., Shearer J. Determinants of effective vaccine coverage in low and middle-income countries: a systematic review and interpretive synthesis. BMC Health Serv Res. 2017;17(1):1–7. doi: 10.1186/s12913-017-2626-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Haqqi A, Zahoor S, Aftab MN, et al. COVID-19 in Pakistan: impact on global polio eradication initiative. J Med Virol. 2021 doi: 10.1002/jmv.26240. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.