Abstract
Photosensitizers (PSs) are critical substances with considerable potential for use in non-invasive photomedicine. Type I PSs, which generate reactive radical species by electron transfer from the excited state induced via photoirradiation, attracted much attention because of their suitability for photodynamic therapy (PDT) irrespective of the oxygen concentration. However, most organic PSs are type II, which activates only oxygen, generating singlet oxygen (1O2) via energy transfer from the triplet state. Here, we proposed a strategy to form type I supramolecular PSs (SPSs) utilizing the charge-separated state induced by self-assembly. This was demonstrated using a supramolecular assembly of fluorescein, which is a type II PS in the monomeric state; however, it changes to a type I SPS via self-assembly. The switching mechanism from type II to I via self-assembly was clarified using photophysical and electrochemical analyses, with the type I SPS exhibiting significant PDT effects on cancer cells. This study provides a promising approach for the development of type I PSs based on supramolecular assemblies.
Keywords: supramolecular assembly, fluorescein, type I photosensitizer, reactive oxygen species (ROS), charge separation
1. Introduction
Photodynamic therapy (PDT) is a promising approach for treating cancer and microbial infections.1−3 The photosensitization reaction in PDT proceeds only in the region irradiated with light, generating reactive oxygen species (ROS) and radical species.4 As the chemical process occurs within a few tens of nanometers, PDT exhibits spatiotemporal selectivity and is minimally invasive. However, due to the lack of an ideal photosensitizer (PS) and methods of selective transport and/or activation at the disease site, PDT is only applied in treating a limited number of diseases.5,6 One of the problems is the low PDT efficiency toward cancer cells.7,8 Most PSs proceed with type II photosensitization reactions that activate only oxygen, generating 1O2 via energy transfer from the triplet state. Type II PDT is oxygen-dependent and thus ineffective for cancer cells under hypoxic conditions.9 Cancer cells exhibit significantly high proliferative capacities and use excessive amounts of oxygen, resulting in a hypoxic microenvironment surrounding the tumor.10 Therefore, PSs enabling PDT under hypoxic conditions are required. In contrast to type II PSs, type I PSs generate various active radical species [e.g., superoxide (O2•–), hydroxy radical (•OH)] via electron transfer from the excited state even in a hypoxic environment and hence exhibit potential for non-oxygen-dependent PDT.11 Therefore, research regarding the development of type I PSs has accelerated.12−15
Supramolecular assemblies are of considerable interest in the field of nanomedicine because they may be prepared using facile “bottom-up” methods and tuned at the molecular level.16−19 Nano-sized supramolecular assemblies, such as porphyrin-peptide assembled nanodots20 and pillararene,21 demonstrated the potential in photomedicine. Numerous supramolecular assemblies based on small molecules were designed to overcome the various limitations of PDT.22−29 These materials exhibit cancer-selective, biocompatible, and light-absorbing properties due to the molecular-level design of the constituents and functional molecule hybridization via non-covalent bonds. However, type I supramolecular PSs (SPSs) are still rare. To the best of our knowledge, there is only one method for their preparation. Yoon and co-workers showed that phthalocyanines with electron-donating amine groups self-assembled to function as type I SPSs.30 The proximity of the electron-donating group to the triplet state PS enables rapid reductive quenching, yielding a radical anion that induces electron transfer to oxygen or substrates. Therefore, the hybridization of PSs and electron-donating groups may give type I SPSs.31−33 However, this strategy requires the introduction of electron-donating substituents onto the PS backbone, which may result in a complex synthesis and challenges in further functionalization. The development of another method for producing a type I SPS without such limitations may result in more efficient PDT.
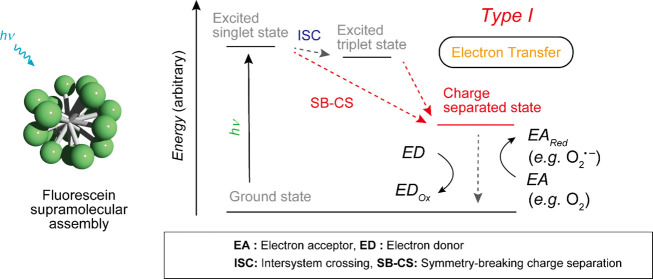
In this study, we proposed a simple strategy to prepare a type I SPS via a charge-separated (CS) state of a PS and showed that fluorescein which is a type II PS in the monomeric state changes to a type I SPS via self-assembly (Figure 1). The CS state induced by self-assembly is crucial for the generation of a type I SPS. Generally, in the excited triplet states of organic molecules, type II photosensitization reactions proceed preferentially. We assumed that if the CS state, which is prone to electron transfer, is somehow stabilized over the triplet state, the type II reaction is suppressed, and the type I reaction preferentially proceeds (Figure 1). Inspired by our previous studies of rhodamine and cyanine dyes forming a CS state via self-assembly and acquiring photosensitizing function,34,35 we speculated that in various molecules, CS states could be stabilized in the self-assembly states. Furthermore, certain type II organic PSs could be transformed into type I SPSs via self-assembly. In fact, the supramolecular assembly of fluorescein, which is a typical type II PS in the monomer state, functions as a type I PS (Figure 1) exhibiting significant PDT effects on cancer cells.
Figure 1.
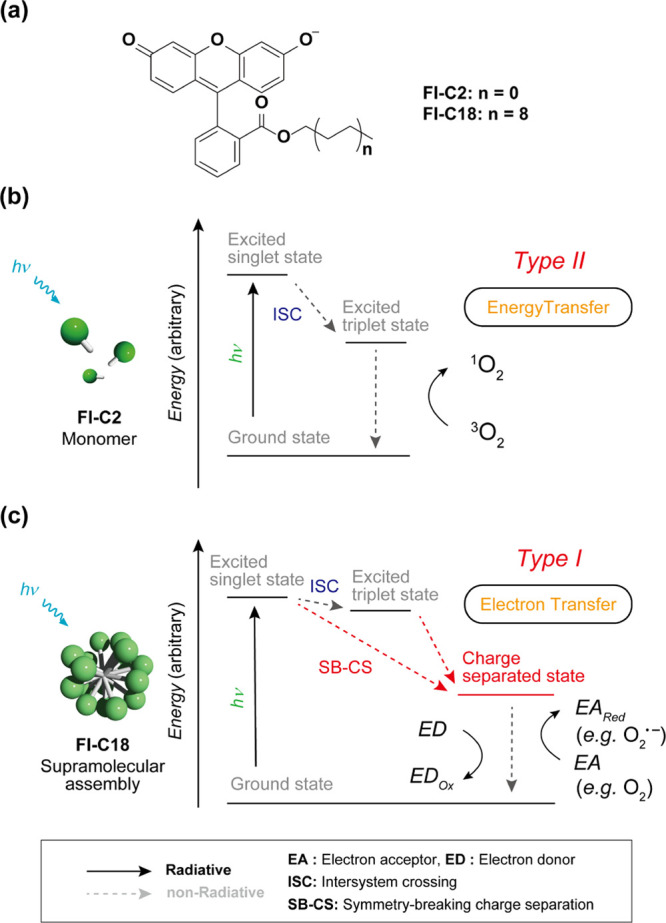
(a) Chemical structures of Fl-C2 and Fl-C18. (b,c) (b) Type II and (c) Type I photosensitization mechanisms of (b) Fl-C2 (monomer state) and (c) Fl-C18 (assembly state), respectively. The radiative decay processes from excited states are omitted for clarity.
2. Results and Discussion
2.1. Self-Assembly Properties of Fluoresceins
Fluorescein was used as a representative constituent of a type I SPS (Figure 1a). It is a typical fluorescent dye used as a chemical probe.36,37 Fluoresceins generally exhibit type II photosensitizing functions and are used in chromophore-assisted light inactivation.38,39 The energy level of the CS state of fluorescein may be lower than that of the T1 state, and the CS state can be generated by symmetry-breaking charge separation (SB–CS) and charge carrier migration in the assembly.40 Amphiphilic Fl-C18 was synthesized to form the self-assembly of fluorescein in water (Figure 1a and Scheme S2). For comparison, hydrophilic Fl-C2, with a short ethyl chain, was also synthesized (Scheme S1). The absorption and fluorescence spectra of Fl-C2 and Fl-C18 in the monomer state in dimethyl sulfoxide (DMSO) revealed that Fl-C2 and Fl-C18 are suitable for evaluating the changes in photophysical properties via self-assembly in water. The absorption and fluorescence spectra of Fl-C2 and Fl-C18 are almost identical [Figure S1, λabs: 530 (Fl-C2) and 531 (Fl-C18) nm, λem: 556 (Fl-C2) and 556 (Fl-C18) nm], indicating that the difference in alkyl chain length hardly affects the photophysical properties.
To evaluate the self-assembly properties in water, absorption and fluorescence spectra were measured (Figure 2a,b). The absorption spectrum of Fl-C2 exhibits a similar shape to that in DMSO (λabs: 494 nm) whereas that of Fl-C18 reveals a decrease in absorbance, with the maximal absorption band split and broadening (λabs: 508 nm). This splitting is due to the H- and J-type fluorescein aggregate mixtures,41 and the broadening suggests random aggregation. The fluorescence of Fl-C18 is significantly lower than that of Fl-C2 [λem: 520 (Fl-C2) and 517 (Fl-C18) nm], with quantum yields of 0.75 and 0.008 for Fl-C2 and Fl-C18, respectively. The average aggregate size of Fl-C18 determined by dynamic light scattering (DLS) is 103 nm, and transmission electron microscopy (TEM) observation reveals the formation of spherical aggregates (Figure 2c,d).
Figure 2.
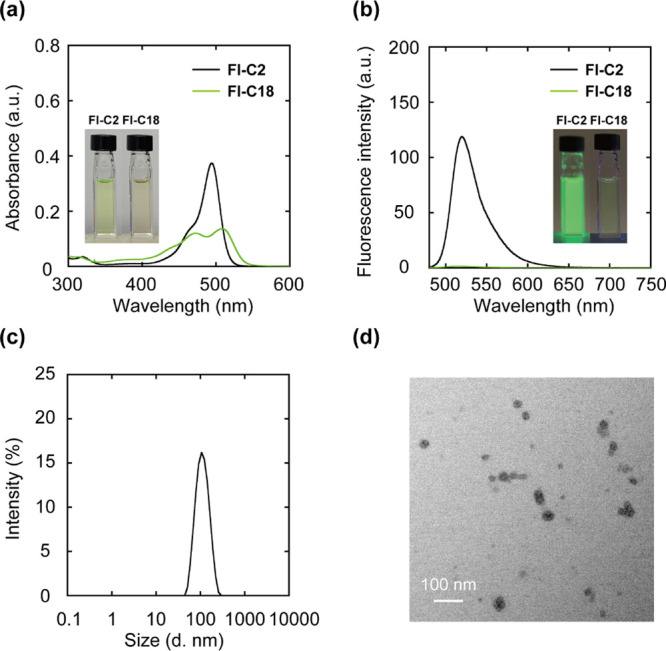
(a) UV–vis and (b) PL spectra of Fl-C2 and Fl-C18. Experimental conditions: [Fl-C2] = [Fl-C18] = 5.0 μM, 1.0 mM phosphate buffer solution, rt, excitation wavelength: 460 nm. (c) Size distribution of the supramolecular assembly of Fl-C18 determined using DLS (Z-average: 103 nm, PDI: 0.10). (d) TEM image of the Fl-C18 supramolecular assemblies. Experimental conditions: [Fl-C2] = [Fl-C18] = 1.0 μM, 1.0 mM phosphate buffer solution, rt.
2.2. ROS Generation by Fl-C2 and Fl-C18
The photosensitizing functions of Fl-C2 and Fl-C18 were evaluated using ROS detection probes such as 9,10-anthracenediyl-bis(methylene)-dimalonic acid (ABDA)42 and dihydrorhodamine 123 (DHR123)43 (Figure S2). First, we performed studies using ABDA to detect singlet oxygen (1O2) produced by energy transfer from the PS to molecular oxygen (type II photosensitization reaction). Light irradiation (460 nm, 300 W, Xe lamp) of the Fl-C2/ABDA mixture results in a time-dependent decrease in the fluorescence intensity of ABDA, suggesting that monomeric Fl-C2 functions as a type II PS (Figure 3a). Time-dependent decrease in fluorescence intensity of ABDA did not occur without the Fl derivatives (Figure S3). The generation of 1O2 by Fl-C2 was also confirmed by phosphorescence emission of 1O2 (λem: 1270 nm) (Figure S4). Conversely, there is no decrease in the fluorescence intensity of ABDA with Fl-C18 (Figure 3b). The residual ratios of ABDA after 30 min photoirradiation are <0.01 and 1.0 for Fl-C2 and Fl-C18, respectively (Figure S5). These results indicate that the type II photosensitization reaction of Fl-C18 is almost suppressed. Subsequently, the ROS generated by Fl-C18 were examined using DHR123, which converts to emissive rhodamine 123 (Rh123) upon oxidation (Figure S2b) and superoxide dismutase (SOD),44 which is a quencher of superoxide (O2•–) generated by type I photosensitization. As shown in Figure S6a,c,e, with Fl-C2, there is no change in the increasing Rh123 fluorescence intensity regardless of the presence of SOD. Conversely, with Fl-C18, the rate of increase in the fluorescence intensity of Rh123 is slightly decreased (Figure S6b,d,f), suggesting that Fl-C18 generates superoxides in an aqueous solution via a type I photosensitization reaction. In addition, to confirm the generation of O2•– by Fl-C18, we examined the reduction of cytochrome c by photoirradiation (Figure S7). It is known that cytochrome c is reduced by O2•– to afford reduced cytochrome c whose characteristic absorption band is at 550 nm.45 After photoirradiation to the mixture of Fl-C18 and cytochrome c, the absorption band at 550 nm was increased (ΔA550nm: 0.11). However, under the existence of SOD, the absorption band intensity was hardly changed (ΔA550nm: 0.04). These results support the O2•– generation via Fl-C18.
Figure 3.
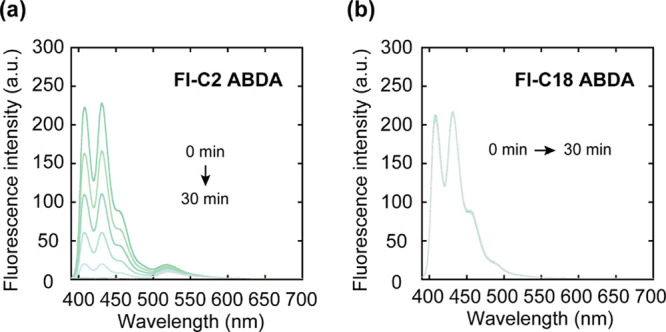
Fluorescent spectra of the mixtures after photoirradiation (λex: 360 nm). (a) Fl-C2 and ABDA and (b) Fl-C18 and ABDA. Experimental conditions: [Fl-C2] = [Fl-C18] = [ABDA] = 5.0 μM, 1.0 mM phosphate buffer solution, rt. Irradiation light: 460 nm, 300 W, Xe lamp.
Electron spin resonance (ESR) spectroscopy was performed using spin-trapping reagents 4-hydroxy-2,2,6,6-tetramethylpiperidine (4-OH-TEMP)46 and 1-hydroxy-3-carboxy-2,2,5,5-tetramethylpyrrolidine (CPH)47 to examine 1O2 and O2•– generation. 4-OH-TEMP and CPH react with 1O2 or O2•– to generate ESR-detectable 4-hydroxy-2,2,6,6-tetramethylpiperidine 1-oxyl (4-OH-TEMPO) and CP (CP•) radicals, respectively (Figure S8). Figure 4a displays the ESR spectra of Fl-C2/4-OH-TEMP and Fl-C18/4-OH-TEMP mixtures after 1 min of photoirradiation, which show clear and no ESR signals, respectively. Therefore, Fl-C2 generates 1O2 after photoirradiation, but Fl-C18 does not. Subsequently, we examined the spin-trapping reagent CPH for O2•– detection. The distinct triplet ESR signal of CP• is observed for the Fl-C18/CPH mixture after photoirradiation (Figure 4b). The supramolecular assembly of Fl-C18 promotes electron transfer to molecular oxygen, and the CS state would be generated. The ESR spectra are consistent with the results obtained using the ROS chemical probes. Therefore, the photosensitization mechanism of fluorescein is clearly altered by self-assembly.
Figure 4.
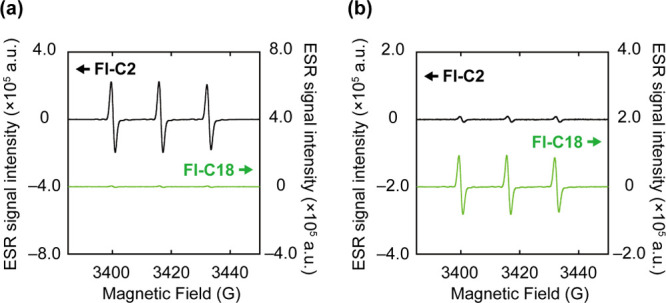
ESR spectra of the mixtures with spin-trapping reagents after photoirradiation. (a) 4-OH-TEMP or (b) CPH with Fl-C2 (upper) or Fl-C18 (lower). Experimental conditions: [Fl-C2] = [Fl-C18] = 5.0 μM, [4-OH-TEMP] = [CPH] = 50 μM, 1.0 mM phosphate buffer solution, rt.
2.3. Mechanistic Study of Self-Assembly-Induced Switching of ROS Generation
Nanosecond laser flash photolysis was performed to elucidate the mechanistic details of the Fl-C2 and Fl-C18 photosensitizing reactions.48 The nanosecond transient absorption (TA) spectra of Fl-C2 and Fl-C18 were measured after nanosecond laser excitation at 460 nm in a deaerated aqueous buffer solution (Figure S9). For Fl-C2, a TA band at 550 nm with a relatively long lifetime (t1/2 = 7.2 μs) is observed (Figure 5a), and the lifetime is significantly shortened in the presence of oxygen (t1/2 = 1.1 μs), which is attributed to the triplet state of Fl-C2.49,50 Conversely, the spectrum of Fl-C18 showed no TA band at 550 nm (Figure S10a). Instead of triplet formation, the spectrum of Fl-C18 exhibits a new TA band at 430 nm (Figure 5b), which is not observed for Fl-C2 (Figure S10b). The lifetime (>10 μs) is sufficient for chemical reactions, and the transient species may be in a CS state involving the photoreaction. The signal is assigned to the intermolecular CS state (neutral radical or radical dianion species) of Fl-C18 in the supramolecular assembly. To directly observe the Fl-C18 CS state, the ESR spectroscopy of the photo-irradiated Fl-C18 at 173 K was performed. The ESR spectrum showed a clear signal corresponding to the Fl-C18 radical at 3420 G (Figure S11).51 Therefore, the CS state is generated in Fl-C18, which is critical in the switching of the photosensitizing mechanism via self-assembly.
Figure 5.
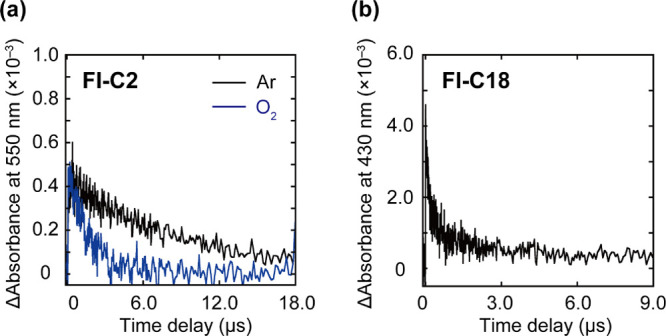
TA decay profiles of (a) Fl-C2 and (b) Fl-C18 at (a) 550 and (b) 430 nm. (a) Black and blue lines are TA decay profiles under Ar and O2 atmospheres, respectively. Experimental conditions: [Fl-C2] = 50 μM, [Fl-C18] = 100 μM, 1.0 mM phosphate buffer solution (pH 7.4), rt. Excitation light: 460 nm.
The energy levels of the triplet and CS states of Fl-C2 and Fl-C18 were estimated using spectroscopic and electrochemical techniques to confirm our hypothesis. The phosphorescence spectra of Fl-C2 and Fl-C18 at 77 K are shown in Figure S12. The 0–0 bands of phosphorescence were observed at 621 and 626 nm for Fl-C2 and Fl-C18, respectively, with energy levels of the triplet states of 2.0 eV for Fl-C2 and Fl-C18. The energy levels of CS states were determined by electrochemical analysis (Figures S13 and S14) to be 1.6 and 1.3 eV for Fl-C2 and Fl-C18, respectively. The energy level of the CS state is lower than that of the triplet state (Table 1). The proposed energy diagram of Fl-C18 is shown in Figure S15. Fl-C18 forms the S1 state after photoirradiation and converted into the CS state directly or via a triplet state through SB–CS. Conversely, Fl-C2 mainly loses the absorbed energy via fluorescence and proceeds via intersystem crossing to the triplet state.
Table 1. Optical and Electrochemical Properties of Fl-C2 and Fl-C18.
| Compound | Eox (V vs Ag/AgCl) | Ered (V vs Ag/AgCl) | S1 (eV) | T1 (eV) | ΔEcs (eV) |
|---|---|---|---|---|---|
| Fl-C2 | 0.70 | –0.85 | 2.2 | 2.0 | 1.6 |
| Fl-C18 | 0.54 | –0.80 | 2.2 | 2.0 | 1.3 |
2.4. PDT Effects of Fl-C2 and Fl-C18
We investigated the potential of Fl-C18 as an anticancer PDT. Fl-C2 or Fl-C18 and molecular probes (mitochondria and lysosome trackers) were incubated with PC9-luc cells (human adenocarcinoma lung cancer cells) with genes encoding firefly luciferase52 to examine the cellular localization of Fl-C2 and Fl-C18. Confocal laser scanning microscopy (CLSM) images are shown in Figure 6. The CLSM images of Fl-C2 (100 nM) and MitoTracker (250 nM) are well matched, indicating that Fl-C2 localizes in mitochondria (Figure 6b). The CLSM images of Fl-C18 (100 nM) and LysoTracker (75 nM) correspond, revealing Fl-C18 localization in lysosomes (Figure 6a). Pearson correlation coefficients of Fl-C2/Lysotracker, Fl-C2/Lysotracker, Fl-C18/Mitotracker, and Fl-C18/Mitotracker images are summarized in Figure S16. The average Pearson correlation coefficients of Fl-C2/Lysotracker and Fl-C18/Lysotracker images are 0.39 and 0.64, respectively. The good correlation between Fl-C18 and Lysotracker images indicates that Fl-C18 tends to localize in lysosomes. The average Pearson correlation coefficients of Fl-C2/Mitotracker (0.79) and Fl-C2/Mitotracker (0.44) images also indicate the localization of Fl-C2 in mitochondria. Fl-C18 would enter cells via endocytosis and localize in the lysosomes. Indeed, dynasore, an inhibitor for clathrin-mediated endocytosis, decreased the mean fluorescence intensity of PC9-luc cells [dynasore(−): 1.05 × 104, dynasore(+): 0.63 × 104] (Figure S17). Lysosomes are an emerging target for PDT due to their close relationship with apoptosis and necrosis.53,54 Both the localized organelles with the PSs (Fl-C2: mitochondria, Fl-C18: lysosome) are effective targets in PDT.55 The cellular uptake of Fl-C2 and Fl-C18 after 12 h incubation (5.0 μM) was evaluated by Fl spectra. 20% Fl-C2 and 9% Fl-C18 entered the cells after incubation (Figure S18). The intracellular concentration of Fl-C18 was estimated to 2.2-fold smaller than that of Fl-C2.
Figure 6.
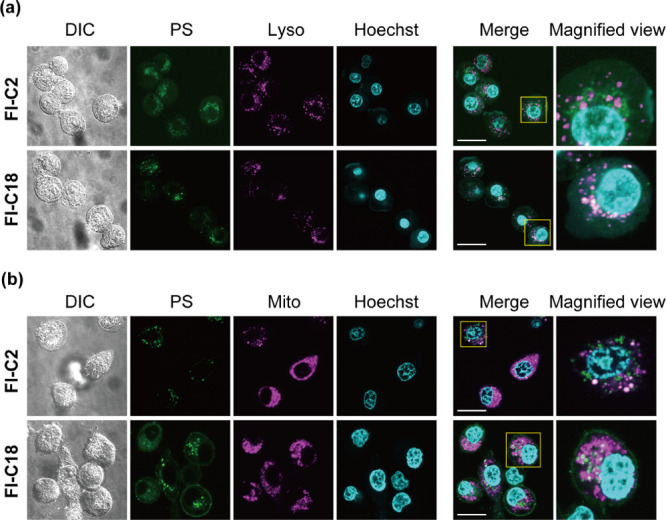
CLSM images of PC9-luc cells with (a) Fl-C2, Fl-C18, LysoTracker, and Hoechst and (b) Fl-C2, Fl-C18, MitoTracker, and Hoechst. The images show differential interference contrast, dye (Fl-C2 or Fl-C18), tracker, Hoechst, merge, and the magnified view of merged images from left to right. Scale bar: 20 μm. Experimental conditions: [Fl-C2] = [Fl-C18] = 100 nM, [MitoTracker] = 250 nM, [LysoTracker] = 75 nM, and [Hoechst] = 500 nM, 37 °C.
Subsequently, the PDT effects of Fl-C2 (1.0 μM) and Fl-C18 (1.0 μM) were evaluated based on the luciferase activity of the PC9-luc cells (Figure 7). Without photoirradiation, the luciferase activities hardly decrease (Fl-C2: 89.4 ± 4.3%, Fl-C18: 109.7 ± 4.1%), indicating that these compounds show no cytotoxicity. In addition, photoirradiation of the PC9-luc cells without the PSs, using a high-power light-emitting diode (LED) at a wavelength of 490–500 nm (3 W cyan high-power LED, 10 min), also shows no cytotoxicity (109.5 ± 7.1%). Fl-C2 and Fl-C18 exhibited light-intensity-dependent PDT effects. After light irradiation (120 J/cm2) of PC9-luc cells with Fl-C2, the luciferase activity decreased to 62.4 ± 3.9%. The rate of the decrease is light intensity-dependent, indicating the PDT effect of Fl-C2 on the cells. The effect is insufficient and almost saturated at 90 J/cm2 light intensity (luciferase activity: 64.1 ± 13.8%). Conversely, the PDT effect of Fl-C18 increases depending on the power of light irradiation at ≤ 120 J/cm2. After irradiation with 120 J/cm2 light, the luciferase activity decreased to 8.3 ± 2.7%, indicating that most PC9-luc cells were dead. The CCK-8 assay of PC9-luc cells showed the same tendency with the luciferase assay (Figure S19). Under the hypoxic condition (O2 concentration 2–5%), Fl-C18 also showed a higher PDT effect than Fl-C2. The cell viability using Fl-C2 and Fl-C18 was 70 and 43% after photoirradiation (90 J/cm2), respectively (Figure S20), under the O2 depletion condition. The PDT effect of Fl-C18 is significantly improved compared to that of Fl-C2 under both normoxia and hypoxia conditions despite the low intracellular concentration. Although the localization organelles of Fl-C2 and Fl-C18 differ,56 the significant improvement would be due to the change in the type of photosensitization reaction as Fl-C18 functioned as a type I SPS in PC9-luc cells. Finally, we conformed the intracellular O2•– generation by Fl-C2 and Fl-C18 using dihydroethidium (DHE) which specifically detects of O2•– and hydrogen peroxide (H2O2).57 The mean fluorescence intensity of the PC9-luc cells treated with Fl-C18 (1.0 μM) was increased after photoirradiation (before: 2.44 × 103, after: 1.02 × 104) (Figure S21). This indicates that Fl-C18 generated O2•– inside PC9-luc cells. On the other hand, in the case of Fl-C2, the mean fluorescence intensity was hardly changed after photoirradiation (before: 1.79 × 103, after: 1.27 × 103). These results support that Fl-C2 and Fl-C18 functioned as type II and type I PSs in the cells, respectively.
Figure 7.
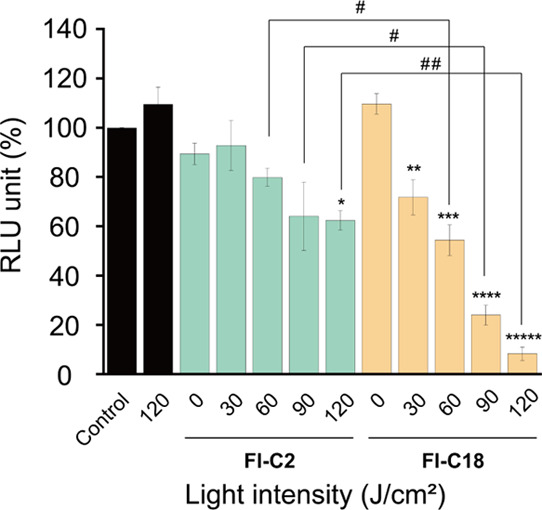
Luciferase activity in PC9-luc cells PDT-treated with Fl-C2 and Fl-C18 in relative light units: the activities decrease depending on the light irradiation [n = 3, *p < 0.001, **p < 0.05, ***p < 0.005, ****p < 0.0001 vs control, #p < 0.05, ##p < 0.0005 (two-tailed unpaired t-test)].
3. Conclusions
In this study, we proposed a development strategy for type I SPSs based on the induction of charge separation via self-assembly. Fluorescein, a conventional dye, functioned as a type II PS in the monomer state, with self-assembly switching the type of photosensitization observed. Spectroscopic and electrochemical analyses showed that the supramolecular assembly formed the CS state via photoirradiation, with the transition reasonable in terms of energy levels. Furthermore, Fl-C18 showed a light-intensity-dependent PDT effect on human adenocarcinoma lung cancer cells, which was sufficiently improved compared with that of Fl-C2. As there are numerous molecules that exhibit the potential to form a CS state during assembly, molecules without type I photosensitizing properties in the monomer state may function as type I SPSs. This phenomenon implies that various organic dyes function as a type I PS by simple self-assembly. This strategy using the CS state for the development of a type I PS may be applied not only in supramolecular assembly but also in monomeric organic PSs. This study provides a novel strategy for developing type I SPSs and contributes to efficient PDT.
Acknowledgments
This work was performed at Osaka University and financially supported by a grant-in-aid for Young Scientists (JSPS KAKENHI Grant no. JP18K14189 and 21K14601), AMED (grant nos. JP19lm0203007 and JP19lm0203014), the Takeda Science Foundation, the Kowa Life Science Foundation, the Senri Life Science Foundation, and the Kato Memorial Bioscience Foundation. We appreciate Y. Murakami for technical support for TEM observation. We also thank Dr. Y. Morimoto and Dr. K. Oohora for technical support for ESR measurement. Finally, we appreciate Dr. T. Nakagawa for kind support for measurement of transition absorption spectra.
Glossary
Abbreviations
- PDT
photodynamic therapy
- ROS
reactive oxygen species
- PS
photosensitizer
- SPS
supramolecular photosensitizer
- CS
charge separated
- SB–CS
symmetry-breaking charge separation
- DMSO
dimethylsulfoxide
- DLS
dynamic light scattering
- TEM
transmission electron microscopy
- ABDA
9,10-anthracenediyl-bis(methylene)-dimalonic acid
- DHR123
dihydrorhodamine 123
- Rh123
rhodamine123
- 4-OH-TEMP
4-hydroxy-2,2,6,6-tetramethylpiperidine
- CPH
1-hydroxy-3-carboxy-2,2,5,5-tetramethylpyrrolidine
- 4-OH-TEMPO
4-hydroxy-2,2,6,6-tetramethylpiperidine 1-oxyl
- ESR
electron spin resonance
- SOD
superoxide dismutase
- CLSM
confocal laser microscopy
- DHE
dihydroethidium
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/jacsau.2c00243.
Materials, experimental methods, synthetic procedures, characterization data, and photophysical and electrochemical data (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Dolmans D. E. J. G. J.; Fukumura D.; Jain R. K. Photodynamic therapy for cancer. Nat. Rev. Cancer 2003, 3, 380–387. 10.1038/nrc1071. [DOI] [PubMed] [Google Scholar]
- Castano A. P.; Mroz P.; Hamblin M. R. Photodynamic therapy and anti-tumour immunity. Nat. Rev. Cancer 2006, 6, 535–545. 10.1038/nrc1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celli J. P.; Spring B. Q.; Rizvi I.; Evans C. L.; Samkoe K. S.; Verma S.; Pogue B. W.; Hasan T. Imaging and photodynamic therapy: Mechanisms, monitoring, and optimization. Chem. Rev. 2010, 110, 2795–2838. 10.1021/cr900300p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan W.; Huang P.; Chen X. Overcoming the Achilles’ heel of photodynamic therapy. Chem. Soc. Rev. 2016, 45, 6488–6519. 10.1039/c6cs00616g. [DOI] [PubMed] [Google Scholar]
- Juarranz Á.; Jaén P.; Sanz-Rodríguez F.; Cuevas J.; González S. Photodynamic therapy of cancer. Basic principles and applications. Clin. Transl. Oncol. 2008, 10, 148–154. 10.1007/s12094-008-0172-2. [DOI] [PubMed] [Google Scholar]
- Lovell J. F.; Liu T. W. B.; Chen J.; Zheng G. Activatable photosensitizers for imaging and therapy. Chem. Rev. 2010, 110, 2839–2857. 10.1021/cr900236h. [DOI] [PubMed] [Google Scholar]
- Zhao X.; Liu J.; Fan J.; Chao H.; Peng X. Recent progress in photosensitizers for overcoming the challenges of photodynamic therapy: from molecular design to application. Chem. Soc. Rev. 2021, 50, 4185–4219. 10.1039/d0cs00173b. [DOI] [PubMed] [Google Scholar]
- Zhang W.; Huo F.; Cheng F.; Yin C. Employing an ICT-FRET Integration Platform for the Real-Time Tracking of SO2 Metabolism in Cancer Cells and Tumor Models. J. Am. Chem. Soc. 2020, 142, 6324–6331. 10.1021/jacs.0c00992. [DOI] [PubMed] [Google Scholar]
- Pervaiz S.; Olivo M. Art and science of photodynamic therapy. Clin. Exp. Pharmacol. Physiol. 2006, 33, 551–556. 10.1111/j.1440-1681.2006.04406.x. [DOI] [PubMed] [Google Scholar]
- Dougherty T. J.; Gomer C. J.; Henderson B. W.; Jori G.; Kessel D.; Korbelik M.; Moan J.; Peng Q. Photodynamic therapy. J. Natl. Cancer Inst. 1998, 90, 889–905. 10.1093/jnci/90.12.889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baptista M. S.; Cadet J.; Di Mascio P.; Ghogare A. A.; Greer A.; Hamblin M. R.; Lorente C.; Nunez S. C.; Ribeiro M. S.; Thomas A. H.; Vignoni M.; Yoshimura T. M. Type I and type II photosensitized oxidation reactions: guidelines and mechanistic pathways. Photochem. Photobiol. 2017, 93, 912–919. 10.1111/php.12716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M.; Xia J.; Tian R.; Wang J.; Fan J.; Du J.; Long S.; Song X.; Foley J. W.; Peng X. Near-infrared light-initiated molecular superoxide radical generator: Rejuvenating photodynamic therapy against hypoxic tumors. J. Am. Chem. Soc. 2018, 140, 14851–14859. 10.1021/jacs.8b08658. [DOI] [PubMed] [Google Scholar]
- Liu J.-n.; Bu W.; Shi J. Chemical Design and Synthesis of Functionalized Probes for Imaging and Treating Tumor Hypoxia. J. Chem. Rev. 2017, 117, 6160–6224. 10.1021/acs.chemrev.6b00525. [DOI] [PubMed] [Google Scholar]
- Zhou Z.; Song J.; Nie L.; Chen X. Reactive oxygen species generating systems meeting challenges of photodynamic cancer therapy. Chem. Soc. Rev. 2016, 45, 6597–6626. 10.1039/c6cs00271d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loftus L. M.; Al-Afyouni K. F.; Rohrabaugh T. N. Jr; Gallucci J. C.; Moore C. E.; Rack J. J.; Turro C. Unexpected Role of Ru(II) Orbital and Spin Contribution on Photoinduced Ligand Exchange: New Mechanism To Access the Photodynamic Therapy Window. J. Phys Chem. Chem. C 2019, 123, 10291–10299. 10.1021/acs.jpcc.9b01576. [DOI] [Google Scholar]
- Li X.; Lee S.; Yoon J. Supramolecular photosensitizers rejuvenate photodynamic therapy. Chem. Soc. Rev. 2018, 47, 1174–1188. 10.1039/c7cs00594f. [DOI] [PubMed] [Google Scholar]
- Xie Z.; Fan T.; An J.; Choi W.; Duo Y.; Ge Y.; Zhang B.; Nie G.; Xie N.; Zheng T.; Chen Y.; Zhang H.; Kim J. S. Emerging combination strategies with phototherapy in cancer nanomedicine. Chem. Soc. Rev. 2020, 49, 8065–8087. 10.1039/d0cs00215a. [DOI] [PubMed] [Google Scholar]
- Kwon N.; Kim H.; Li X.; Yoon J. Supramolecular agents for combination of photodynamic therapy and other treatments. Chem. Sci. 2021, 12, 7248–7268. 10.1039/d1sc01125a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun L.; Wang J.; Yang B.; Wang X.; Yang G.; Wang X.; Jiang Y.; Wang T.; Jiang J. Assembled small organic molecules for photodynamic therapy and photothermal therapy. RSC Adv. 2021, 11, 10061–10074. 10.1039/d1ra00579k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou Q.; Abbas M.; Zhao L.; Li S.; Shen G.; Yan X. Biological photothermal nanodots based on self-assembly of peptide–porphyrin conjugates for antitumor therapy. J. Am. Chem. Soc. 2017, 139, 1921–1927. 10.1021/jacs.6b11382. [DOI] [PubMed] [Google Scholar]
- Zhu H.; Wang H.; Shi B.; Shangguan L.; Tong W.; Yu G.; Mao Z.; Huang F. Supramolecular peptide constructed by molecular Lego allowing programmable self-assembly for photodynamic therapy. Nat. Commun. 2019, 10, 2412. 10.1038/s41467-019-10385-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webber M. J.; Langer R. Drug delivery by supramolecular design. Chem. Soc. Rev. 2017, 46, 6600–6620. 10.1039/c7cs00391a. [DOI] [PubMed] [Google Scholar]
- Sato K.; Hendricks M. P.; Palmer L. C.; Stupp S. I. Peptide supramolecular materials for therapeutics. Chem. Soc. Rev. 2018, 47, 7539–7551. 10.1039/c7cs00735c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheetham A. G.; Chakroun R. W.; Ma W.; Cui H. Self-assembling prodrugs. Chem. Soc. Rev. 2017, 46, 6638–6663. 10.1039/c7cs00521k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shigemitsu H.; Hamachi I. Supramolecular assemblies responsive to biomolecules toward biological applications. Chem.—Asian J. 2015, 10, 2026–2038. 10.1002/asia.201500563. [DOI] [PubMed] [Google Scholar]
- Busseron E.; Ruff Y.; Moulin E.; Giuseppone N. Supramolecular self-assemblies as functional nanomaterials. Nanoscale 2013, 5, 7098–7140. 10.1039/c3nr02176a. [DOI] [PubMed] [Google Scholar]
- Hu F.; Mao D.; Kenry; Cai X.; Wu W.; Kong D.; Liu B. A Light-Up Probe with Aggregation-Induced Emission for Real-Time Bio-orthogonal Tumor Labeling and Image-Guided Photodynamic Therapy. Angew. Chem., Int. Ed. 2018, 57, 10182–10186. 10.1002/anie.201805446. [DOI] [PubMed] [Google Scholar]
- Li Q.; Li Y.; Min T.; Gong J.; Du L.; Phillips D. L.; Liu J.; Lam J. W. Y.; Sung H. H. Y.; Williams I. D.; Kwok R. T. K.; Ho C. L.; Li K.; Wang J.; Tang B. Z. Time-Dependent Photodynamic Therapy for Multiple Targets: A Highly Efficient AIE-Active Photosensitizer for Selective Bacterial Elimination and Cancer Cell Ablation. Angew. Chem., Int. Ed. 2020, 59, 9470–9477. 10.1002/anie.201909706. [DOI] [PubMed] [Google Scholar]
- Ji C.; Gao Q.; Dong X.; Yin W.; Gu Z.; Gan Z.; Zhao Y.; Yin M. A Size-Reducible Nanodrug with an Aggregation-Enhanced Photodynamic Effect for Deep Chemo-Photodynamic Therapy. Angew. Chem., Int. Ed. 2018, 57, 11384–11388. 10.1002/anie.201807602. [DOI] [PubMed] [Google Scholar]
- Li X.; Lee D.; Huang J.-D.; Yoon J. Phthalocyanine-assembled nanodots as photosensitizers for highly efficient type I photoreactions in photodynamic therapy. Angew. Chem., Int. Ed. 2018, 57, 9885–9890. 10.1002/anie.201806551. [DOI] [PubMed] [Google Scholar]
- Chen D.; Xu Q.; Wang W.; Shao J.; Huang W.; Dong X. Type I photosensitizers revitalizing photodynamic oncotherapy. Small 2021, 17, 2006742. 10.1002/smll.202006742. [DOI] [PubMed] [Google Scholar]
- Ding H.; Yu H.; Dong Y.; Tian R.; Huang G.; Boothman D. A.; Sumer B. D.; Gao J. Photoactivation switch from type II to type I reactions by electron-rich micelles for improved photodynamic therapy of cancer cells under hypoxia. J. Controlled Release 2011, 156, 276–280. 10.1016/j.jconrel.2011.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang Z.; Dai J.; Yu M.; Li J.; Shen P.; Hu R.; Lou X.; Zhao Z.; Tang B. Z. Type I photosensitizers based on phosphindole oxide for photodynamic therapy: apoptosis and autophagy induced by endoplasmic reticulum stress. Chem. Sci. 2020, 11, 3405–3417. 10.1039/d0sc00785d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shigemitsu H.; Tani Y.; Tamemoto T.; Mori T.; Li X.; Osakada Y.; Fujitsuka M.; Kida T. Aggregation-induced photocatalytic activity and efficient photocatalytic hydrogen evolution of amphiphilic rhodamines in water. Chem. Sci. 2020, 11, 11843–11848. 10.1039/d0sc04285d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shigemitsu H.; Tamemoto T.; Ohkubo K.; Mori T.; Osakada Y.; Fujitsuka M.; Kida T. A cyanine dye based supramolecular photosensitizer enabling visible-light-driven organic reaction in water. Chem. Commun. 2021, 57, 11217–11220. 10.1039/d1cc04685c. [DOI] [PubMed] [Google Scholar]
- Tanaka K.; Miura T.; Umezawa N.; Urano Y.; Kikuchi K.; Higuchi T.; Nagano T. Rational design of fluorescein-based fluorescence probes. Mechanism-based design of a maximum fluorescence probe for singlet oxygen. J. Am. Chem. Soc. 2001, 123, 2530–2536. 10.1021/ja0035708. [DOI] [PubMed] [Google Scholar]
- Park S.-H.; Kwon N.; Lee J.-H.; Yoon J.; Shin I. Synthetic ratiometric fluorescent probes for detection of ions. Chem. Soc. Rev. 2020, 49, 143–179. 10.1039/c9cs00243j. [DOI] [PubMed] [Google Scholar]
- Surrey T.; Elowitz M. B.; Wolf P.-E.; Yang F.; Nédélec F.; Shokat K.; Leibler S. Chromophore-assisted light inactivation and self-organization of microtubules and motors. Proc. Natl. Acad. Sci. U.S.A. 1998, 95, 4293–4298. 10.1073/pnas.95.8.4293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson K.; Rajfur Z.; Vitriol E.; Hahn K. Chromophore-assisted laser inactivation in cell biology. Trends Cell Biol. 2008, 18, 443–450. 10.1016/j.tcb.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paziewske-Nowak A.; Dawgul M.; Pijanowska D. G. Comparative Study on Voltammetric and Spectrofluorimetric Methods for Fluorescein Detection. Int. J. Electrochem. Sci. 2019, 14, 3764–3776. [Google Scholar]
- Das S.; Chattopadhyay A. P.; De S. Controlling J aggregation in fluorescein by bile salt hydrogels. J. Photochem. Photobiol. A: Chem. 2008, 197, 402–414. 10.1016/j.jphotochem.2008.02.003. [DOI] [Google Scholar]
- Entradas T.; Waldron S.; Volk M. The detection sensitivity of commonly used singlet oxygen probes in aqueous environments. J. Photochem. Photobiol. B: Biol. 2020, 204, 111787. 10.1016/j.jphotobiol.2020.111787. [DOI] [PubMed] [Google Scholar]
- Kiani-Esfahani A.; Tavalaee M.; Deemeh M. R.; Hamiditabar M.; Nasr-Esfahani M. H. DHR123: an alternative probe for assessment of ROS in human spermatozoa. Syst. Biol. Reprod. Med. 2012, 58, 168–74. 10.3109/19396368.2012.681420. [DOI] [PubMed] [Google Scholar]
- Abreu I. A.; Cabelli D. E. Superoxide dismutases -a review of the metal-associated mechanistic variations. Biochim. Biophys. Acta 2010, 1804, 263–274. 10.1016/j.bbapap.2009.11.005. [DOI] [PubMed] [Google Scholar]
- Crack J. C.; Green J.; Cheesman M. R.; Le Brun N. E.; Thomson A. J. Superoxide-mediated amplification of the oxygen-induced switch from [4Fe-4S] to [2Fe-2S] clusters in the transcriptional regulator FNR. Proc. Natl. Acad. Sci. U.S.A. 2007, 104, 2092–2097. 10.1073/pnas.0609514104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lion Y.; Gandin E.; Vorst A. On the production of nitroxide radicals by singlet oxygen reaction: An EPR study. Photochem. Photobiol. 1980, 31, 305–309. 10.1111/j.1751-1097.1980.tb02545.x. [DOI] [Google Scholar]
- Dikalov S.; Skatchkov M.; Bassenge E. Spin trapping of superoxide radicals and peroxynitrite by 1-hydroxy-3-carboxy-pyrrolidine and 1-hydroxy-2,2,6,6-tetramethyl-4-oxo-piperidine and the stability of corresponding nitroxyl radicals towards biological reductants. Biochem. Biophys. Res. Commun. 1997, 231, 701–704. 10.1006/bbrc.1997.6174. [DOI] [PubMed] [Google Scholar]
- Nakagawa T.; Okamoto K.; Hanada H.; Katoh R. Probing with randomly interleaved pulse train bridges the gap between ultrafast pump-probe and nanosecond flash photolysis. Opt. Lett. 2016, 41, 1498–1501. 10.1364/ol.41.001498. [DOI] [PubMed] [Google Scholar]
- Song L.; Varma C. A.; Verhoeven J. W.; Tanke H. J. Influence of the triplet excited state on the photobleaching kinetics of fluorescein in microscopy. Biophys. J. 1996, 70, 2959–2968. 10.1016/s0006-3495(96)79866-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasche V.; Lindqvist L. Reactions between the Triplet State of Fluorescein and Oxygen. J. Phys. Chem. 1964, 68, 817–823. 10.1021/j100786a019. [DOI] [Google Scholar]
- Shigeya N.; Yoshiharu S.; Shiro K.; Hiroshi K. ESR study on fluorescein semiquinone radical. Bull. Chem. Soc. Jpn. 1974, 47, 2121–2125. 10.1246/bcsj.47.2121. [DOI] [Google Scholar]
- Yasui H.; Nishinaga Y.; Taki S.; Takahashi K.; Isobe Y.; Shimizu M.; Koike C.; Taki T.; Sakamoto A.; Katsumi K.; Ishii K.; Sato K. Near-infrared photoimmunotherapy targeting GPR87: Development of a humanised anti-GPR87 mAb and therapeutic efficacy on a lung cancer mouse model. EBioMedicine 2021, 67, 103372. 10.1016/j.ebiom.2021.103372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsubone T. M.; Martins W. K.; Pavani C.; Junqueira H. C.; Itri R.; Baptista M. S. Enhanced efficiency of cell death by lysosome-specific photodamage. Sci. Rep. 2017, 7, 6734. 10.1038/s41598-017-06788-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Z.; Liu J.; Huang J.; Rees T. W.; Wang Y.; Wang H.; Li X.; Chao H.; Stang P. J. A self-assembled Ru–Pt metallacage as a lysosome-targeting photosensitizer for 2-photon photodynamic therapy. Proc. Natl. Acad. Sci. U.S.A. 2019, 116, 20296–20302. 10.1073/pnas.1912549116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahalingam S. M.; Ordaz J. D.; Low P. S. Targeting of a photosensitizer to the mitochondrion enhances the potency of photodynamic therapy. ACS Omega 2018, 3, 6066–6074. 10.1021/acsomega.8b00692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X.; Zhao Y.; Zhang T.; Xing D. Mitochondria-specific agents for photodynamic cancer therapy: A key determinant to boost the efficacy. Adv. Healthc. Mater. 2021, 10, 2001240. 10.1002/adhm.202001240. [DOI] [PubMed] [Google Scholar]
- Chen J.; Rogers S. C.; Kavdia M. Analysis of kinetics of dihydroethidium fluorescence with superoxide using xanthine oxidase and hypoxanthine assay. Ann. Biomed. Eng. 2013, 41, 327–337. 10.1007/s10439-012-0653-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.