Abstract

Photoredox catalysis typically relies on the use of single chromophores, whereas strategies, in which two different light absorbers are combined, are rare. In photosystems I and II of green plants, the two separate chromophores P680 and P700 both absorb light independently of one another, and then their excitation energy is combined in the so-called Z-scheme, to drive an overall reaction that is thermodynamically very demanding. Here, we adapt this concept to perform photoredox reactions on organic substrates with the combined energy input of two red photons instead of blue or UV light. Specifically, a CuI bis(α-diimine) complex in combination with in situ formed 9,10-dicyanoanthracenyl radical anion in the presence of excess diisopropylethylamine catalyzes ca. 50 dehalogenation and detosylation reactions. This dual photoredox approach seems useful because red light is less damaging and has a greater penetration depth than blue or UV radiation. UV–vis transient absorption spectroscopy reveals that the subtle change in solvent from acetonitrile to acetone induces a changeover in the reaction mechanism, involving either a dominant photoinduced electron transfer or a dominant triplet–triplet energy transfer pathway. Our study illustrates the mechanistic complexity in systems operating under multiphotonic excitation conditions, and it provides insights into how the competition between desirable and unwanted reaction steps can become more controllable.
Keywords: photocatalysis, spectroscopy, mechanistic analysis, electron transfer, energy transfer
Introduction
Merging photoredox catalysis with other fields of chemistry has become increasingly popular over the past decade, including combinations with transition-metal catalysis,1−7 organocatalysis,8−10 biocatalysis,11−14 electrochemistry,15−19 or asymmetric catalysis.20,21 While an increasing number of photoredox strategies rely on biphotonic excitation involving the consecutive absorption of two (visible) photons, combinations of two independent photoactive catalysts are underexplored.22,23 In natural photosynthesis, two separate chlorophyll molecules called P680 and P700 absorb light with maxima in the red spectral range at 680 and 700 nm (Figure 1a), and their combined excitation energy is used to drive an overall reaction that would be unattainable based on the absorption of a single visible light quantum.24 The electron transfer chain and the photoexcitation events in photosystems I and II as drawn in Figure 1a resemble the letter Z, and consequently have been termed Z-scheme. In the context of artificial photosynthesis and photochemical water splitting, many researchers have made use of the Z-scheme strategy,25,26 but in synthetic organic photoredox catalysis, this approach seems underexplored yet. In the work presented herein, we have sought to apply the Z-scheme concept to an artificial photoredox system, operating based on low-energy input radiation (red light) to catalyze chemical reactions of organic molecules that would normally require blue or UV illumination. Our principal motivation was to explore to what extent the mimicry of a natural photoredox strategy can be applied in a useful manner to modern photocatalysis. From a more practical viewpoint, the use of a single photocatalyst absorbing blue or UV photons looks most straightforward at first glance, yet the consecutive absorption of two lower-energy photons can be advantageous because red or near-infrared light causes substantially less photodamage and has typically much greater penetration depth into colored solutions in reaction vessels.27−29 Red light furthermore provides an opportunity to excite photocatalysts more selectively, to prevent undesirable side reactions.27−30 On the other hand, red photons provide a significantly smaller amount of energy per photon than blue or UV photons: for instance, a blue photon with a wavelength (λ) of 410 nm carries an energy of 3.0 eV, while a red photon with a wavelength (λ) of 620 nm bears only 2.0 eV. Consequently, the synthetic opportunities for monophotonic applications with red light are considerably more limited than with blue light, due to the lower photonic energy.
Figure 1.

(a) Z-scheme of bacterial photosynthesis with the two key chromophores P680 of photosystem II (PS II) and P700 of photosystem I (PS I). Redox cofactors between P680 and P700 are not shown for simplicity. P700 is excited in its charge-neutral form and only accepts an electron from PS II once the photoexcited P700 (*P700) has been quenched oxidatively by the primary electron acceptor of PS I. (b) Combination of chromophores used herein for red light-driven photocatalysis. In a key mechanistic pathway, 9,10-dicyanoanthracene (DCA) is reduced by the photoexcited CuI complex (dap = 2,9-dianisyl-1,10-phenanthroline) and is then itself photoexcited.
Dual organic photoredox catalysis with two independent photocatalysts is very rare yet.31,32 Somewhat more common is the use of systems in which two closely related photocatalysts collaborate,33,34 including several examples where one of the two catalysts converts to the other under light irradiation.35−39 In those approaches, however, the photophysical and photochemical properties of the two catalysts cannot be optimized independently of each other, and a rational method development is only possible with limited degrees of freedom. When using biphotonic excitation strategies, typically a multitude of (productive and unproductive) mechanistic steps are viable,40,41 making the deliberate tuning of electron transfer, energy transfer, or triplet–triplet annihilation steps all the more desirable,22 and this is best possible in a system with two mutually independent photocatalysts. The exploration of this strategy seems relevant not only to accomplish new reactions that were so far unattainable with monophotonic excitation, but furthermore to perform known reactions that are thermodynamically demanding with lower-energy input light than previously possible.22,31
This latter aspect attracted our interest for the present study, in particular against the background of the recent surge of interest in red light-driven photocatalysis. As noted above, red and near-infrared photons have comparatively low energy content, and therefore monophotonic excitation strategies as used in the majority of studies performed with red light until now typically only permit the turnover of activated substrates.27−30,42−50
For instance, this includes α-brominated ketones (Figure 2a),27,42 the trifluoromethylation of alkenes based on CF3I (Figure 2b),43,51 the transition-metal co-catalyzed cross-coupling based on aryl diazonium salts (Figure 2c),28 an atom transfer radical addition (ATRA) reaction with benzyl bromide (Figure 2d),52 and the fluoroalkylation of aniline on the basis of C4F9I (Figure 2e).45 The examples in Figure 2 collectively illustrate the point that until now, red light has been mostly employed for activated substrates including α-functionalized ketones, polyfluorinated alkyl iodides, diazonium salts, and benzyl halides. Thus, the development of a biphotonic excitation strategy to push the limits of what is thermodynamically possible with red light seemed a worthy goal to us, in addition to demonstrating a new concept in photoredox catalysis.
Figure 2.
(a–e) Previously reported examples of red light-driven transformations.28,42,43,45,52 (f) Photocatalytic system used herein.
Blue or green light-absorbing photocatalysts are widespread,53−55 whereas alternatives that feature sizeable extinction coefficients in the red spectral range are less common.28,42−45,47−49,52 Osmium polypyridyls are a well-known option,28,56−62 but in the spirit of our research program geared at the development of new photocatalysts based on Earth-abundant transition metals,63−65 CuI complexes attracted our attention. Many tetrahedral complexes of this type have long been known but received much attention for photoredox catalysis only recently.10,66−74 Most of them absorb predominantly in the blue or green,72,75−81 whereas the [Cu(dap)2]+ compound (dap = 2,9-dianisyl-1,10-phenanthroline, Figure 2f) stands out in its capacity to absorb up to ca. 650 nm.70,82−84 With its photoactive excited state storing 2.05 eV and an excited-state oxidation potential of −1.4 V vs SCE,70,83,84 [Cu(dap)2]+ looked like an attractive alternative to precious OsII polypyridyls and was therefore chosen as the primary photocatalyst (Figure 1b).
The choice of the secondary photocatalyst was inspired by recent photoredox studies, in which radical anions (or their derivatives) were invoked as catalytically active species,35,36,85−89 including biphotonic as well as monophotonic (photoelectrochemical) excitation strategies.16,17,19,90−95 The radical anion of 9,10-dicyanoanthracene (DCA•–, Figure 1b) absorbs not only in the blue and green spectral ranges as exploited previously,35 but furthermore features prominent bands at 642 and 706 nm.96 Thus, we anticipated that a steady concentration of DCA•– could be formed upon continuous red irradiation of [Cu(dap)2]+ in the presence of excess diisopropylethylamine, and that furthermore DCA•– could be excited with the same light to reach a highly reactive excited state (Figure 1b). The respective doublet excited-state lifetimes of such anion radicals are usually in the subnanosecond time range and do not permit diffusion-controlled reactions.97−102 We speculated that photoreaction could nevertheless occur upon long-term irradiation, for example on the basis of preaggregated DCA•– and substrates.93,103−105
Our study shows that this expectation is fulfilled for ca. 50 examples of dehalogenation and detosylation reactions, which typically require blue light as energy input. Transient absorption studies reveal an unanticipated complexity of the overall reaction mechanism, yet support the view of the Z-scheme-like process in Figure 1b as a key contributor.
Results and Discussion
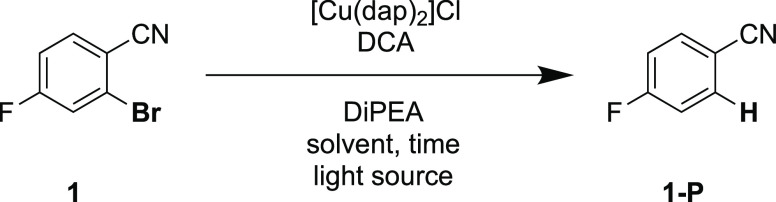
We started our investigation with fluorinated bromobenzonitrile (1) as substrate and initially employed a continuous-wave (cw) laser with an output wavelength of 635 nm and a power of 500 mW for the photoexcitation. Using 1 mol % of [Cu(dap)2]Cl along with 10 mol % of DCA (Table 1, entry 1), we determined a yield of 91% for product 1-P in MeCN-d3 after illumination over 16 h. After 6 h of irradiation under these conditions, 79% of the starting material 1 was already consumed (entry 2). Since the cw laser irradiates a comparatively small area with high excitation density (due to its collimated beam), a high-power light-emitting diode (LED, 3.8 W) with output wavelengths centered at 623 nm was used to achieve a more homogeneous irradiation of the reaction vessel. This resulted in a yield of 86% for 1-P after 6 h (entry 3), and therefore we continued our investigations with this light source. Analysis of the reaction progress over time (Supporting Information, Figure S1) revealed that the reaction dramatically slows once a conversion near 80–85% is reached. Screening of different additives (see the Supporting Information, Section 2.3.2) indicated that the addition of 0.5 equiv of cesium carbonate enables essentially complete conversion of the starting material 1 within 5 h (entry 4). This added salt can have diverse possible effects (see further discussion in Section 2.3.3 in the Supporting Information), but this was not investigated in detail. Control experiments in the absence of copper catalyst, DCA, sacrificial electron donor, or light resulted in no conversion of substrate 1 (entries 5–8).
Table 1. Optimization of a Photocatalytic Debromination Reaction with Red Light at 20 °Ca.
| entry | [Cu(dap)2]Cl/mol % | DCA/mol % | solvent | additive | time/h | light source | yield (conv.)/%b |
|---|---|---|---|---|---|---|---|
| 1 | 1 | 10 | MeCN-d3 | none | 16 | 635 nm cw laser | 91 (91) |
| 2 | 1 | 10 | MeCN-d3 | none | 6 | 635 nm cw laser | 79 (79) |
| 3 | 1 | 10 | MeCN-d3 | none | 6 | 623 nm LED | 86 (87) |
| 4 | 1 | 10 | MeCN-d3 | 0.5 equiv Cs2CO3 | 5 | 623 nm LED | 95 (99)c |
| 5 | 0 | 10 | MeCN-d3 | 0.5 equiv Cs2CO3 | 5 | 623 nm LED | 0 (0) |
| 6 | 1 | 0 | MeCN-d3 | 0.5 equiv Cs2CO3 | 6 | 623 nm LED | 2 (2) |
| 7 | 1 | 10 | MeCN-d3 | 0.5 equiv Cs2CO3 | 6 | 623 nm LED | 0 (0)d |
| 8 | 1 | 10 | MeCN-d3 | 0.5 equiv Cs2CO3 | 16 | no light | 0 (0) |
Reaction conditions: 25 mM substrate 1 and 20 equiv DiPEA (diisopropylethylamine) in 2 mL of deaerated MeCN-d3. Sample irradiated in a quartz cuvette under an argon atmosphere at 20 °C.
Yields and conversions (in parentheses) were determined by quantitative 19F{1H}-NMR analysis using 4-fluorotoluene as the internal standard.
Analysis of solutions under identical conditions in nondeuterated solvent on an analytical high-performance liquid chromatography (HPLC) setup as a complementary method gave a yield (conversion) of 95% (95%).
Reaction performed in the absence of DiPEA.
With these optimized conditions, we then investigated different types of light-driven reactions. Dehalogenations of (activated) aryl halides (as a class of frequently used substrates for reductive transformations)35,36,106,107 served as first benchmark reactions. Reductive debrominations of aromatic substrates (Figure 3) such as benzonitriles (1 and 2), acetophenone (3), trifluormethylbenzene (4), benzoic ester (5), and benzothiazole (6) are readily possible in excellent yields. Furthermore, benzylic debromination (7) as well as deiodination of aryl substrates without (8) and with electron-donating substituents (9) are achievable with good to excellent yields. Unsurprisingly, for the more challenging dechlorination108 of activated aryl chloride (10) and the debromination of unactivated naphthyl bromide (11) only comparatively low conversions and yields were observed. Investigation of a substrate with an aliphatic iodide (12) furthermore demonstrated the limitation of our system with respect to reductive dehalogenation reactions. The lower yield despite longer irradiation times for aryl chlorides is in line with the reactivity observed in a previous study that used DCA and white light for carbon–carbon bond formation reactions between aryl radicals and suitable radical interceptors.35 For the light-induced reductive dehalogenation, that previous study reported similar performance as we observe for our [Cu(dap)2]Cl/DCA system. For example, the previous study reported a yield of 85% for 4-bromobenzonitrile in 5 h with white light,35 whereas we observe a yield of 95% with red light (Figure 3, substrate 1). Based on this comparison, we expect a comparable substrate scope of aryl halides for carbon–carbon bond formation reactions with our catalytic system as in the previous study.
Figure 3.
Hydrodehalogenation of selected aryl halides by red light-driven photoredox catalysis. Reaction conditions: 25 mM substrate, 1 mol % [Cu(dap)2]Cl, 10 mol % DCA, 0.5 equiv Cs2CO3, and 20 equiv DiPEA in 2 mL of MeCN-d3 irradiated with a 623 nm high-power LED (Thorlabs Solis-623C, 3.8 W) under argon at 20 °C. Yields and conversions (in parentheses) were determined by quantitative 19F{1H}-NMR or 1H-NMR analysis using 4-fluorotoluene or mesitylene as internal standards. aSubstrate concentration lowered to 20 mM.
As a next class of reactions, we investigated detosylations of phenol substrates. Detosylations have recently been investigated with other photocatalytic systems, but typically blue light is needed for these reactions.38,109−112 All detosylation reactions of phenols with different electron-donating as well as electron-withdrawing substituents (13–17) resulted in very high conversions, and NMR yields above 95% within 5 h (Figure 4a). The double detosylation of naphthalene-diol 18 was possible in 84% yield, although a longer irradiation time of 16 h was needed. For the detosylation reactions explored here, full conversion was achievable without the addition of Cs2CO3, contrasting our findings above in the hydrodehalogenation reactions. This observation suggests that halide anions as leaving groups interfere with our catalytic system, whereas byproducts related to tosylates as leaving groups seem to be less problematic. Indeed, titration of bromide ions to [Cu(dap)2]Cl without irradiation suggested decreasing stability of this complex with increasing bromide concentration (see the Supporting Information, Section 4.3.5). Furthermore, anion-induced quenching of the excited state can have a significant impact on (unproductive) static excited-state deactivation of [Cu(dap)2]+ and could serve as a reasonable explanation for the observable reactivity depending on the leaving group and additive of the reaction.113−116 Due to the complexity of the overall system, further investigations regarding this aspect were not performed. A previous study of reductive dehalogenation with copper(I) photocatalysts did not observe a reactivity dependence on halide anions resulting from the dehalogenation reaction.117
Figure 4.

Red light-driven photoredox detosylation of protected phenolic (a) and nitrogen-containing substrates (b). Reaction conditions: 25 mM substrate, 1 mol % [Cu(dap)2]Cl, 10 mol % DCA and 20 equiv DiPEA in 2 mL of MeCN-d3 irradiated with a 623 nm high-power LED (Thorlabs Solis-623C, 3.8 W) under argon at 20 °C. Yields and conversions (in parentheses) were determined by quantitative 19F{1H}-NMR or 1H-NMR analyses using 4-fluorotoluene or mesitylene as internal standards. The experiment with substrate 20 and product isolation was performed on a 220 μmol scale. Further details are in the Supporting Information. aConditions changed to 10 mM substrate, 2 mol % [Cu(dap)2]Cl and 15 mol % DCA. bSubstrate concentration lowered to 20 mM. cOnly mono-detosylation observed. d5 equiv of DiPEA used.
In addition to protected phenols, tosylated nitrogen-containing groups furthermore attracted our attention118 as an additional class of compounds that might be suitable as substrates for energy-demanding red light-driven reduction reactions (Figure 4b). Experiments with unsubstituted (19) and tert-butyl-substituted carbazoles (20) resulted in excellent yields, and a carbazole with electron-withdrawing acetyl substituents (21) also worked very well. Furthermore, diarylamines with cyano (22), trifluormethyl (23), and bromo substituents (24) were successfully detosylated with excellent conversions and yields within 5 h, while a chlorinated analogue (25) needed an extended irradiation time of 12 h to achieve similar conversion and yield. Phenoxazine (26) and di(p-anisyl)amine (27) required significantly longer reaction times and only comparatively modest product yields were obtained even after 16 h of irradiation. Aromatic heterocycles such as imidazoles (28), benzimidazoles (29), and indoles (30 and 31) gave excellent yields, whereas tosylated pyrrole (32) was deprotected with a considerably lower yield of 67% within 12 h. The red light-driven detosylation reaction was furthermore extendable to substrates with nonaromatic substituents attached to the protected nitrogen atom. The direct comparison of benzyl anilines with a trifluoromethyl (33) and a methyl group (34) reveals that the electron-withdrawing trifluoromethyl-substituent is beneficial. A similar effect is seen for a tosylated acetamide (35), which reacts much better than more electron-rich methylated (36), butylated (37) analogues, or the primary aniline 38. A twofold tosyl-protected aniline (39) reacted selectively to the mono-detosylated product with very good yields (in line with the observed poor reactivity of the mono-tosylated substrate 38, which is the product of this reaction). Moving onward to nonaromatic ring structures further underscored that electron-withdrawing ketone functional groups, as in protected lactames (40) or oxazolidone (41), are beneficial for substrate activation. With purely aliphatic substrates such as pyrrolidone (42), no reaction occurred. Protected melatonin (43) was chosen as a representative example for a substrate bearing several functional groups, and excellent conversion and yield were achieved within 5 h in this case.
A preparative scale reaction was performed with substrate 20 on a 220 μmol scale (details in the Supporting Information, Section 2.2) and resulted in 79% of isolated product. Whilst the focus of our dehalogenations and detosylations was on the replacement of a functional or protective group by a hydrogen atom, the involved radical intermediates can in principle also be trapped by suitable radical interceptors.35,119,120 For example, N-methylpyrrole 44 and 1,3,5-trimethoxybenzene 45 were used successfully as aryl radical trapping reagents for substrate 1 with 84% yield of product 44-P and 69% yield of product 45-P (Figure 5a). Both of these reactions were furthermore performed on a 250 μmol scale, resulting in isolated product yields of 77 and 43%, respectively.
Figure 5.
Investigations of carbon–carbon bond formation reactions (a); carbon–sulfur (b) and carbon–oxygen (c) bond cleavage reactions as well as an attempted demesylation reaction (d) using red light-driven photoredox catalysis. Reaction conditions: 25 mM substrate, 1 mol % [Cu(dap)2]Cl, 10 mol % DCA and 20 equiv DiPEA in 2 mL of MeCN-d3 irradiated with a 623 nm high-power LED (Thorlabs Solis-623C, 3.8 W) under argon at 20 °C. Yields and conversions (in parentheses) were determined by quantitative 19F{1H}-NMR or 1H-NMR analyses using 4-fluorotoluene or mesitylene as internal standards. The reactions, in which 44-P and 45-P were isolated, were performed on a 250 μmol scale. Further details are in the Supporting Information, Section 2.2. aSubstrate concentration lowered to 15 mM.
Finally, we concentrated on substrates with carbon–sulfur or carbon–oxygen bonds and explored the possibility of reductive C–S and C–O bond cleavage reactions (Figure 5b/c). While substrate 46 reacted smoothly within 5 h, the cleavage of an anhydride (47) needed prolonged irradiation times of 14 h. For protected benzoins, tosylates (48) as well as acetates (49) are both suitable leaving groups in our catalytic system (Figure 5c).121 It is worth mentioning that for substrates 48 and 49 a cleavage of the C–O bond is observed, unlike in the detosylation reactions of Figure 4, in which the O–S bonds are cleaved. In contrast to successful detosylations with carbazoles, a demesylation reaction (50) is not possible (Figure 5d).
Mechanistic Investigations
In catalytic systems relying on biphotonic excitation, the elucidation of the reaction mechanism is often very challenging because a multitude of light-induced elementary steps are usually conceivable. This is the case for example in catalytic systems operating on the basis of triplet–triplet annihilation upconversion,122,123 consecutive photoinduced electron transfer mechanisms,123 or two-photon absorption pathways.124 Complete mechanistic study of catalytic systems with two photoactive species or with two (competing) reaction pathways is even more challenging.125,126 In exceptional cases tailor-made systems can give valuable insights into the biphotonic mechanisms,32,127,128 but this is not (readily) possible, or not investigated in studies with more synthetically oriented focus.27,129 In our catalytic system, [Cu(dap)2]+ absorbs up to 650 nm hence transient UV–vis absorption studies are only viable at longer wavelengths. The copper catalyst and DCA•– absorb in the same range of the visible spectrum (see the Supporting Information, Section 4.1), and therefore selective excitation of either one of these two species in the presence of the other is not possible, thereby further complicating mechanistic studies by time-resolved laser spectroscopy. This imposes clear limits regarding the level of detail at which mechanistic investigations can be performed with our catalytic system.
The mechanistic proposal in Figure 1b involves photoinduced electron transfer (PET) from [Cu(dap)2]+ to DCA, and the DCA radical anion as photoexcitable species leading to substrate activation.35,90,130 However, when considering the photophysical and photochemical characteristics of the overall catalytic system, this is not a priori the only possible mechanistic interpretation of its observable photoreactivity. In the following, we discuss two different plausible mechanisms, one based on the above-mentioned initial photoinduced electron transfer step (PET mechanism in Figure 6a), and the other based on an initial triplet–triplet energy transfer (TTET) step between 3MLCT-excited [Cu(dap)2]+ and DCA (TTET mechanism in Figure 6b).
Figure 6.
(a) Reaction mechanism based on an initial photoinduced electron transfer (PET) step between 3MLCT-excited [Cu(dap)2]Cl and 9,10-dicyanoanthracene (DCA). Gray circles mark the elementary reaction steps of (1) oxidative quenching of 3*[Cu(dap)2]+ (abbreviated as 3*[CuI]) by DCA, (2) spontaneous reduction of the oxidized copper photocatalyst ([CuII]) by DiPEA, and (3) substrate activation after excitation of the DCA radical anion. (b) Reaction mechanism based on an initial triplet–triplet energy transfer (TTET) step between 3MLCT-excited [Cu(dap)2]Cl and DCA. Gray circles mark the elementary reaction steps of (I) TTET, (II) reductive quenching of 3*DCA by DiPEA, and (III) substrate activation after excitation of DCA•–. The doublet excited state of that radical anion is extremely short-lived,101 and therefore, 2*DCA•– is set in quotation marks, to emphasize the possibility that the photoreaction could in fact predominantly occur from preaggregated DCA•–/substrate encounter complexes, or could even involve some DCA photodegradation products.
In both mechanisms, initially only [Cu(dap)2]Cl is excited because this is initially the only species absorbing the red cw laser or LED light (Figure S5). PET from 3*[Cu(dap)2]Cl to DCA (step 1 in Figure 6a) is exergonic by 0.5 eV (see the Supporting Information, Section 4.2.1); hence, DCA•– should indeed be accessed directly. DiPEA could then reduce the oxidized copper complex ([CuII]) back to its initial CuI form (step 2 in Figure 6a), to close the catalytic copper cycle.
An alternative pathway that could lead to the formation DCA•– is a so-called sensitization-initiated electron transfer as presented in Figure 6b. This pathway must be considered because the initial TTET step from 3*[Cu(dap)2]Cl to DCA to yield 3*DCA (step I in Figure 6b) is exergonic by 0.25 eV.83,84,131 Depending on exact conditions (solvent, ionic strength), the initial (exergonic) PET and TTET elementary steps in Figure 6a/b will therefore compete directly with one another. Based on the triplet energy of DCA (1.8 eV) and its ground state reduction potential (−0.93 V vs SCE), an excited-state reduction potential of roughly 0.87 V vs SCE can be estimated,132,133 which should be sufficient to oxidize DiPEA (step II in Figure 6b).134 Consequently, if 3*DCA is formed, then onward reaction to DCA•– via spontaneous electron transfer from DiPEA seems plausible.
Regardless of whether DCA•– is formed directly via PET (Figure 6a) or via a sequence of TTET and electron donation from DiPEA (Figure 6b), the next productive step of the overall catalytic mechanism must excite DCA•–, ultimately leading to substrate activation (step 3 in Figure 6a and step III in Figure 6b) and completion of one catalytic turnover.
Pulsed excitation of an acetonitrile solution of [Cu(dap)2]Cl (100 μM) at 532 nm in the presence of 500 μM of DCA (corresponding to the solubility limit) results initially in a dominant transient absorption band around 590 nm, corresponding to 3*[Cu(dap)2]+ (green trace in Figure 7a). At the same time, the characteristic spectroscopic features of the DCA radical anion with absorption maxima at 642 and 705 nm (the latter wavelength is marked by a vertical green line in Figure 7a) are already detectable. The same two absorption bands are observable in the UV–vis spectrum of electrochemically generated DCA•– (top trace in Figure 7e). After longer time delays following the laser pulse (500 ns delay shown as a brown trace in Figure 7a), a (weak) new absorption band around 440 nm appears. Based on comparison to the literature and a reference experiment with sensitized TTET from [Ru(bpy)3]2+ (bottom trace in Figure S7e), the transient absorption band at 440 nm in Figure 7a/e is unambiguously attributable to the lowest triplet excited state of DCA (3*DCA).135 Evidently, both the PET (Figure 6a) and TTET (Figure 6b) mechanisms operate in acetonitrile, as suspected from the outset (see above).
Figure 7.
[Cu(dap)2]Cl (100 μM) in deaerated acetonitrile (a) and in deaerated acetone (b) was excited at 532 nm (30 mJ) in the presence of DCA (500 μM), and transient UV–vis absorption spectra were recorded with different time delays after the laser pulse (see insets), time-integrated over 200 ns. Kinetic traces over the first 9 μs after the laser pulse monitoring the transient absorption signals at 440 nm (main contribution from 3*DCA, violet traces) and 705 nm (main contribution from DCA•–, green traces) for the same solutions as in (a) and (b) are presented in (c) for acetonitrile and in (d) for acetone. A comparison of the spectral traces recorded with a delay of 500 ns from (a) and (b) in both solvents (red traces, middle) to the electrochemically generated DCA•– reference in acetonitrile (top) and the transient signals of 3*DCA reference generated by energy transfer from [Ru(bpy)3]2+ in acetonitrile are presented in (e). Further details are provided in the text and in Section 4.1 in the Supporting Information.
To gain some semiquantitative insight into the relative importance of these PET and TTET mechanisms, it is useful to consider the kinetic traces in Figure 7c. Following excitation of [Cu(dap)2]+ at 532 nm and a time delay of 500 ns, 3*[Cu(dap)2]+ has largely disappeared and the remaining signals are predominantly due to DCA•– (mΔOD = 23.5 at 705 nm) and 3*DCA (mΔOD = 10.1 at 440 nm). Assuming that the molar extinction coefficient of DCA•– at 705 nm (ε705) is 8400 M–1 cm–1 (as reported previously),96 and further assuming that the molar extinction coefficient of 3*DCA at 440 nm (ε440) is 9000 M–1 cm–1,135 one estimates maximum concentrations of 2.80 μM for DCA•– and 1.12 μM for 3*DCA. The simple comparison of these two concentrations suggests that the PET mechanism of Figure 6a contributes to roughly 70%, whereas the TTET mechanism of Figure 6b contributes to approximately 30% under these conditions in acetonitrile. This crude estimation is associated with considerable uncertainty, given the experimental limitations of the catalytic system and considering certain simplifications implicit to the above analysis (see the Supporting Information, Section 4.3 for details). However, the key point is that the PET mechanism (Figure 6a) is dominant, whereas the TTET is less relevant in acetonitrile.
Analogous experiments performed under the same conditions in acetone yielded the opposite result (Figure 7b/d). In this solvent, the maximum transient absorbance and consequently the concentration of DCA•– (green trace detected at 705 nm) is clearly lower than that of 3*DCA (violet trace detected at 440 nm). A direct comparison to reference spectra is provided in Figure 7e, indicating a dominant energy transfer pathway and less contribution from direct electron transfer. Using the same analysis as described above, we estimate that the TTET mechanism now dominates with about 70% over the PET mechanism, which contributes with roughly 30% to the photoreaction of the excited copper complex. Thus, the data in Figure 7 illustrate that even subtle changes in the reaction conditions (here a change in solvent from acetonitrile to acetone) can lead to a change in the dominant mechanistic pathway.136 This does not lead to a drastic change in the observed overall chemical reactivity in our system (for dehalogenation reactions in acetone, see the Supporting Information, Table S1), presumably because both mechanisms of Figure 6 are similarly productive, as they both ultimately lead to DCA•– as key species.
Based on steady-state and time-resolved luminescence quenching experiments (Figures S18–S20), the rate constant for the initial reaction step leading to deactivation of the 3MLCT-excited state of [Cu(dap)2]+ is on the order of ∼(6–7) × 109 M–1 s–1 in both acetone and acetonitrile (details in the Supporting Information, Section 4). This rate constant is roughly a factor of 2 below the diffusion limit for bimolecular quenching in acetonitrile (2 × 1010 M–1 s–1) at 20 °C.131 Our luminescence quenching experiments with [Cu(dap)2]+ and DCA revealed a static component in addition to the dynamic quenching, suggesting that the PET and TTET elementary steps can also occur in preaggregated [Cu(dap)2]+/DCA adducts (see the Supporting Information, Section 4.3.2).137 A more detailed analysis of the rate for formation of DCA•– and 3*DCA tentatively points toward a static quenching mechanism for the initial PET step, while for the TTET step a dynamic quenching step is detectable (Supporting Information, Section 4). Control experiments with substrate 1 or DiPEA as a quencher for excited [Cu(dap)2]+ result in rate constants of 7 × 106 M–1 s–1 or even below 106 M–1 s–1, respectively. For the synthetically relevant DiPEA (0.5 M) and substrate concentrations (25 mM), this results in pseudo-first-order rate constants of 5 × 105 and ∼2 × 105 s–1, both being substantially below the pseudo-first-order rate constant for the reaction of 3*[Cu(dap)2]+ with 0.5 mM DCA (∼(6–7) × 109 M–1 s–1 × 0.0005 M = ∼(3–4) × 106 s–1). This short analysis confirms that direct oxidative quenching by the substrate and reductive quenching by the sacrificial donor are not kinetically competitive with the PET and TTET steps in Figure 6.
In the PET mechanism, the copper catalyst is recovered with the sacrificial electron donor (step 2 in Figure 6a) while in the sensitized TTET mechanism [Cu(dap)2]+ is not redox-active and 3*DCA is quenched by DiPEA (step II in Figure 6b). In the following, we focus on these reactions with the sacrificial electron donor. By monitoring the kinetics of the UV–vis transient absorption signal associated with [Cu(dap)2]2+ at 380 nm (Supporting Information, Figure S26 and Section 4.3.3) as a function of DiPEA concentration in acetonitrile, a rate constant of ∼1 × 107 M–1 s–1 is determined for electron transfer from DiPEA to [Cu(dap)2]2+. An analogous experiment monitoring the reduction of 3*DCA by DiPEA in acetone provided a rate constant of 2.5 × 106 M–1 s–1 (Figure S25). Evidently, both of these rate constants are substantially below the diffusion limit, which likely reflects the fact they both occur with only small driving forces.
The last step of our proposed catalytic cycle is the substrate activation by 2*DCA•– (step 3 in Figure 6a, and step III in Figure 6b). While earlier studies reported a lifetime in the range of several nanoseconds for 2*DCA•–,138,139 this was questioned later, and in particular the luminescence of 2*DCA•– was doubted. Lifetimes on the picosecond timescale seem more realistic for 2*DCA•–,101,140−142 in line with the excited-state lifetimes reported for other radical anions.97,102,143−145 Furthermore, concerning the reactivity and stability of DCA•–, a variety of very different observations were reported in the literature, including claims of reasonably good stability of DCA•–,146 as well as observations of comparatively rapid degradation reactions with the solvent, reaction intermediates or oxygen.140,147−149 Overall, the stability and reactivity of DCA•– seem to be highly dependent on the actual conditions in solution, and it seems that these aspects are sometimes overlooked. In our catalytic system, it seems plausible that DCA•– accumulates over time as a result of [Cu(dap)2]Cl irradiation in the presence of excess DiPEA. Consequently, after some time, the two proposed catalytic cycles in Figure 2f are effectively decoupled. Given the very short lifetime of 2*DCA•– it seems furthermore plausible that preassociation between DCA•– and substrate might play an important role in successful product formation,103,150,151 and we cannot rigorously exclude the possibility that some of its photodegradation products interfere in the overall mechanism.99,102,141,149,152 Under cw laser irradiation of [Cu(dap)2]+ at 635 nm with a power of 500 mW, our photocatalytic system exhibits reasonably good stability (Supporting Information, Section 4.3.5). Indirect analysis of the substrate activation step in our photocatalytic system revealed conversions of over 80% for substrates with reduction potentials below −2.3 V vs SCE and a notable decrease for substrates with more negative reduction potentials. This observation seems in good agreement with the estimated excited-state reduction potential of −2.6 V vs SCE for 2*DCA•– (see the Supporting Information, Section 4.3.4 for details).101
In summary, the two mechanisms in Figure 6 contribute to different extents in different solvents, and furthermore, other photoactive species related to the copper complex or DCA could contribute to the overall reaction.147−149 One specific possibility not discussed here is for example triplet–triplet annihilation upconversion of 3*DCA•– to yield 1*DCA•–, followed by reduction of the latter with DiPEA. The PET versus TTET competition illustrated in Figure 6 captures however the main essence of the [Cu(dap)2]Cl/DPA dual photoredox system.
Summary and Conclusions
The concept of dual photocatalysis (Figure 1b), in which two different photoredox catalysts are combined, allows the use of red light for thermodynamically demanding reduction reactions. Roughly 50 examples of chemical transformations including dehalogenations of aryl halides, detosylations, as well as carbon–carbon bond formations illustrate the good catalytic performance of the [Cu(dap)2]Cl/DCA couple. Our approach of mimicking the Z-scheme of natural photosynthesis (Figure 1) pools the energy of two red photons, and consequently the scope of chemical transformations that can be driven by red light is considerably broadened beyond the current state of the art (Figure 2). Multiphoton excitation-based mechanisms that rely on red light are yet very rare (lower part of Figure 8),27 and most studies performed in this context until now relied on blue or green light (upper part of Figure 8).22
Figure 8.
On a timeline, different multiphotonic mechanistic strategies for photoredox catalysis with visible light are assigned to the year within the last decade, in which they became popular (top) and when these were adapted to systems with red or near-IR light excitation (bottom).22 In the years 2014,36 2015,122 2016,31 2017,153 2018,32 and 2019,33,126 different mechanisms were reported with blue and green excitation light (background color classifies the excitation light color), while in 2019, an example with red to near-IR irradiation was reported.27
Mechanistic studies of the different reaction types in Figure 6 are particularly tricky because several different (competing) reaction pathways are usually opened up by multiphoton excitation.111,120,154−156 Our study illustrates this aspect quite clearly. In the initial photoinduced elementary step, electron transfer and triplet–triplet energy transfer compete with one another as seen unambiguously in transient absorption spectroscopy (Figure 7), and while the electron transfer process dominates in acetonitrile, triplet–triplet energy transfer becomes dominant in acetone. Since both of these elementary reaction steps ultimately lead to the formation of the key catalytic species (DCA•–), this does not affect the overall catalytic performance. For other photocatalytic systems, it is however conceivable that a subtle change of conditions activates unproductive or counterproductive reaction steps, and this could then drastically affect the reaction outcome and yield. Our study furthermore illustrates the point that a photoredox reaction does not necessarily follow a single mechanism, but that in fact multiple mechanisms can run in parallel and all contribute to product formation. The more complex the photocatalytic systems become, the more likely this probably gets.136,157,158
The combination of [Cu(dap)2]+ and DCA complements and expands the known photochemical applications of these two individual components when used separately.68,77,83,89,117,159−167 Red light-driven applications play important roles in other important contexts, for example, hydrogen production,47,48,168,169 medical applications,158,169−173 and polymerizations.174−178 Now, red light as well as multiphoton excitation-based mechanisms seem to become of increasing interest for synthetic organic photoredox chemistry,50,126,179−181 and we hope the insights gained from our work will be useful in that greater context.
Glossary
Abbreviations
- cw
continuous wave
- Cu
copper
- dap
2,9-bis(4-methoxyphenyl)-1,10-phenanthroline
- DCA
9,10-dicyanoanthracene
- DiPEA
diisopropylethylamine
- PET
photoinduced electron transfer
- LED
light-emitting diode
- MeCN
acetonitrile
- TTET
triplet–triplet energy transfer
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/jacsau.2c00265.
Synthetic protocols and characterization data, description of methods and equipment, and additional spectroscopic and electrochemical data (PDF)
Author Contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.
This work was funded by the Swiss National Science Foundation through grant number 200021_178760.
The authors declare no competing financial interest.
Supplementary Material
References
- Twilton J.; Le C.; Zhang P.; Shaw M. H.; Evans R. W.; MacMillan D. W. C. The Merger of Transition Metal and Photocatalysis. Nat. Rev. Chem. 2017, 1, 0052 10.1038/s41570-017-0052. [DOI] [Google Scholar]
- Skubi K. L.; Blum T. R.; Yoon T. P. Dual Catalysis Strategies in Photochemical Synthesis. Chem. Rev. 2016, 116, 10035–10074. 10.1021/acs.chemrev.6b00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu C.; Yue H.; Chu L.; Rueping M. Recent Advances in Photoredox and Nickel Dual-Catalyzed Cascade Reactions: Pushing the Boundaries of Complexity. Chem. Sci. 2020, 11, 4051–4064. 10.1039/D0SC00712A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarver P. J.; Bacauanu V.; Schultz D. M.; DiRocco D. A.; Lam Y.; Sherer E. C.; MacMillan D. W. C. The Merger of Decatungstate and Copper Catalysis to Enable Aliphatic C(sp3)–H Trifluoromethylation. Nat. Chem. 2020, 12, 459–467. 10.1038/s41557-020-0436-1. [DOI] [PubMed] [Google Scholar]
- Le C.; Chen T. Q.; Liang T.; Zhang P.; MacMillan D. W. C. A Radical Approach to the Copper Oxidative Addition Problem: Trifluoromethylation of Bromoarenes. Science 2018, 360, 1010–1014. 10.1126/science.aat4133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye Y.; Sanford M. S. Merging Visible-Light Photocatalysis and Transition-Metal Catalysis in the Copper-Catalyzed Trifluoromethylation of Boronic Acids with CF3I. J. Am. Chem. Soc. 2012, 134, 9034–9037. 10.1021/ja301553c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin M. D.; Kim S.; Toste F. D. Photoredox Catalysis Unlocks Single-Electron Elementary Steps in Transition Metal Catalyzed Cross-Coupling. ACS Cent. Sci. 2016, 2, 293–301. 10.1021/acscentsci.6b00090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicewicz D. A.; MacMillan D. W. C. Merging Photoredox Catalysis with Organocatalysis: The Direct Asymmetric Alkylation of Aldehydes. Science 2008, 322, 77–80. 10.1126/science.1161976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw M. H.; Shurtleff V. W.; Terrett J. A.; Cuthbertson J. D.; MacMillan D. W. C. Native Functionality in Triple Catalytic Cross-Coupling: sp3 C-H Bonds as Latent Nucleophiles. Science 2016, 352, 1304–1308. 10.1126/science.aaf6635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong M.; Pannecoucke X.; Jubault P.; Poisson T. Recent Advances in Photocatalyzed Reactions Using Well-Defined Copper(I) Complexes. Beilstein J. Org. Chem. 2020, 16, 451–481. 10.3762/bjoc.16.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao X.; Turek-Herman J. R.; Choi Y. J.; Cohen R. D.; Hyster T. K. Photoenzymatic Synthesis of α-Tertiary Amines by Engineered Flavin-Dependent “Ene”-Reductases. J. Am. Chem. Soc. 2021, 143, 19643–19647. 10.1021/jacs.1c09828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner N. J. A New Twist on Catalytic Teamwork. Nature 2018, 560, 310–311. 10.1038/d41586-018-05933-0. [DOI] [PubMed] [Google Scholar]
- Litman Z. C.; Wang Y.; Zhao H.; Hartwig J. F. Cooperative Asymmetric Reactions Combining Photocatalysis and Enzymatic Catalysis. Nature 2018, 560, 355–359. 10.1038/s41586-018-0413-7. [DOI] [PubMed] [Google Scholar]
- Guo X.; Okamoto Y.; Schreier M. R.; Ward T. R.; Wenger O. S. Enantioselective Synthesis of Amines by Combining Photoredox and Enzymatic Catalysis in a Cyclic Reaction Network. Chem. Sci. 2018, 9, 5052–5056. 10.1039/C8SC01561A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verschueren R. H.; De Borggraeve W. M. Electrochemistry and Photoredox Catalysis: A Comparative Evaluation in Organic Synthesis. Molecules 2019, 24, 2122. 10.3390/molecules24112122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barham J. P.; König B. Synthetic Photoelectrochemistry. Angew. Chem., Int. Ed. 2020, 59, 11732–11747. 10.1002/anie.201913767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowper N. G. W.; Chernowsky C. P.; Williams O. P.; Wickens Z. K. Potent Reductants via Electron-Primed Photoredox Catalysis: Unlocking Aryl Chlorides for Radical Coupling. J. Am. Chem. Soc. 2020, 142, 2093–2099. 10.1021/jacs.9b12328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J.; Cao J.; Wu X.; Wang H.; Yang X.; Tang X.; Toh R. W.; Zhou R.; Yeow E. K. L.; Wu J. Unveiling Extreme Photoreduction Potentials of Donor-Acceptor Cyanoarenes to Access Aryl Radicals from Aryl Chlorides. J. Am. Chem. Soc. 2021, 143, 13266–13273. 10.1021/jacs.1c05994. [DOI] [PubMed] [Google Scholar]
- Chernowsky C. P.; Chmiel A. F.; Wickens Z. K. Electrochemical Activation of Diverse Conventional Photoredox Catalysts Induces Potent Photoreductant Activity. Angew. Chem., Int. Ed. 2021, 60, 21418–21425. 10.1002/anie.202107169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gualandi A.; Cozzi P. G.; Rodeghiero G.; Jansen T. P.; Perciaccante R. Stereoselective Synergystic Organo Photoredox Catalysis with Enamines and Iminiums. Phys. Sci. Rev. 2020, 5, 20180098 10.1515/psr-2018-0098. [DOI] [Google Scholar]
- Xiang S.-H.; Tan B. Advances in Asymmetric Organocatalysis over the Last 10 Years. Nat. Commun. 2020, 11, 3786 10.1038/s41467-020-17580-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaser F.; Kerzig C.; Wenger O. S. Multi-Photon Excitation in Photoredox Catalysis: Concepts, Applications, Methods. Angew. Chem., Int. Ed. 2020, 59, 10266–10284. 10.1002/anie.201915762. [DOI] [PubMed] [Google Scholar]
- Cesana P. T.; Li B. X.; Shepard S. G.; Ting S. I.; Hart S. M.; Olson C. M.; Martinez Alvarado J. I.; Son M.; Steiman T. J.; Castellano F. N.; Doyle A. G.; MacMillan D. W. C.; Schlau-Cohen G. S. A Biohybrid Strategy for Enabling Photoredox Catalysis with Low-Energy Light. Chem 2022, 8, 174–185. 10.1016/j.chempr.2021.10.010. [DOI] [Google Scholar]
- Blankenship R. E.Molecular Mechanisms of Photosynthesis; Blackwell Science Ltd.: Oxford, U.K., 2008. [Google Scholar]
- Favereau L.; Makhal A.; Pellegrin Y.; Blart E.; Petersson J.; Göransson E.; Hammarström L.; Odobel F. A Molecular Tetrad That Generates a High-Energy Charge-Separated State by Mimicking the Photosynthetic Z-Scheme. J. Am. Chem. Soc. 2016, 138, 3752–3760. 10.1021/jacs.5b12650. [DOI] [PubMed] [Google Scholar]
- Wang Y.; Suzuki H.; Xie J.; Tomita O.; Martin D. J.; Higashi M.; Kong D.; Abe R.; Tang J. Mimicking Natural Photosynthesis: Solar to Renewable H2 Fuel Synthesis by Z-Scheme Water Splitting Systems. Chem. Rev. 2018, 118, 5201–5241. 10.1021/acs.chemrev.7b00286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravetz B. D.; Pun A. B.; Churchill E. M.; Congreve D. N.; Rovis T.; Campos L. M. Photoredox Catalysis Using Infrared Light via Triplet Fusion Upconversion. Nature 2019, 565, 343–346. 10.1038/s41586-018-0835-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravetz B. D.; Tay N. E. S.; Joe C. L.; Sezen-Edmonds M.; Schmidt M. A.; Tan Y.; Janey J. M.; Eastgate M. D.; Rovis T. Development of a Platform for Near-Infrared Photoredox Catalysis. ACS Cent. Sci. 2020, 6, 2053–2059. 10.1021/acscentsci.0c00948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sellet N.; Cormier M.; Goddard J.-P. The Dark Side of Photocatalysis: Near-Infrared Photoredox Catalysis for Organic Synthesis. Org. Chem. Front. 2021, 8, 6783–6790. 10.1039/D1QO01476E. [DOI] [Google Scholar]
- Gisbertz S.; Reischauer S.; Pieber B. Overcoming Limitations in Dual Photoredox/Nickel-Catalysed C–N Cross-Couplings Due to Catalyst Deactivation. Nat. Catal. 2020, 3, 611–620. 10.1038/s41929-020-0473-6. [DOI] [Google Scholar]
- Kerzig C.; Goez M. Combining Energy and Electron Transfer in a Supramolecular Environment for the “Green” Generation and Utilization of Hydrated Electrons through Photoredox Catalysis. Chem. Sci. 2016, 7, 3862–3868. 10.1039/C5SC04800A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu A.; Chen Y.; Guo J.-J.; Yu N.; An Q.; Zuo Z. Cerium-Catalyzed Formal Cycloaddition of Cycloalkanols with Alkenes through Dual Photoexcitation. J. Am. Chem. Soc. 2018, 140, 13580–13585. 10.1021/jacs.8b08781. [DOI] [PubMed] [Google Scholar]
- Connell T. U.; Fraser C. L.; Czyz M. L.; Smith Z. M.; Hayne D. J.; Doeven E. H.; Agugiaro J.; Wilson D. J. D.; Adcock J. L.; Scully A. D.; Gómez D. E.; Barnett N. W.; Polyzos A.; Francis P. S. The Tandem Photoredox Catalysis Mechanism of [Ir(ppy)2(dtb-bpy)]+ Enabling Access to Energy Demanding Organic Substrates. J. Am. Chem. Soc. 2019, 141, 17646–17658. 10.1021/jacs.9b07370. [DOI] [PubMed] [Google Scholar]
- Li H.; Tang X.; Pang J. H.; Wu X.; Yeow E. K. L.; Wu J.; Chiba S. Polysulfide Anions as Visible Light Photoredox Catalysts for Aryl Cross-Couplings. J. Am. Chem. Soc. 2021, 143, 481–487. 10.1021/jacs.0c11968. [DOI] [PubMed] [Google Scholar]
- Neumeier M.; Sampedro D.; Májek M.; de la Peña O’Shea V. A.; Jacobi von Wangelin A.; Pérez-Ruiz R. Dichromatic Photocatalytic Substitutions of Aryl Halides with a Small Organic Dye. Chem. – Eur. J. 2018, 24, 105–108. 10.1002/chem.201705326. [DOI] [PubMed] [Google Scholar]
- Ghosh I.; Ghosh T.; Bardagi J. I.; König B. Reduction of Aryl Halides by Consecutive Visible Light-Induced Electron Transfer Processes. Science 2014, 346, 725–728. 10.1126/science.1258232. [DOI] [PubMed] [Google Scholar]
- Brandl F.; Bergwinkl S.; Allacher C.; Dick B. Consecutive Photoinduced Electron Transfer (ConPET): The Mechanism of the Photocatalyst Rhodamine 6G. Chem. – Eur. J. 2020, 26, 7946–7954. 10.1002/chem.201905167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKenzie I. A.; Wang L.; Onuska N. P. R.; Williams O. F.; Begam K.; Moran A. M.; Dunietz B. D.; Nicewicz D. A. Discovery and Characterization of an Acridine Radical Photoreductant. Nature 2020, 580, 76–80. 10.1038/s41586-020-2131-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naumann R.; Lehmann F.; Goez M. Generating Hydrated Electrons for Chemical Syntheses by Using a Green Light-Emitting Diode (LED). Angew. Chem., Int. Ed. 2018, 57, 1078–1081. 10.1002/anie.201711692. [DOI] [PubMed] [Google Scholar]
- Neumann S.; Kerzig C.; Wenger O. S. Quantitative Insights into Charge-Separated States from One- and Two-Pulse Laser Experiments Relevant for Artificial Photosynthesis. Chem. Sci. 2019, 10, 5624–5633. 10.1039/C9SC01381D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orazietti M.; Kuss-Petermann M.; Hamm P.; Wenger O. S. Light-Driven Electron Accumulation in a Molecular Pentad. Angew. Chem., Int. Ed. 2016, 55, 9407–9410. 10.1002/anie.201604030. [DOI] [PubMed] [Google Scholar]
- Lee J.; Papatzimas J. W.; Bromby A. D.; Gorobets E.; Derksen D. J. Thiaporphyrin-Mediated Photocatalysis Using Red Light. RSC Adv. 2016, 6, 59269–59272. 10.1039/C6RA11374E. [DOI] [Google Scholar]
- Matsuzaki K.; Hiromura T.; Tokunaga E.; Shibata N. Trifluoroethoxy-Coated Subphthalocyanine Affects Trifluoromethylation of Alkenes and Alkynes Even under Low-Energy Red-Light Irradiation. ChemistryOpen 2017, 6, 226–230. 10.1002/open.201600172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goulet-Hanssens A.; Rietze C.; Titov E.; Abdullahu L.; Grubert L.; Saalfrank P.; Hecht S. Hole Catalysis as a General Mechanism for Efficient and Wavelength-Independent Z → E Azobenzene Isomerization. Chem 2018, 4, 1740–1755. 10.1016/j.chempr.2018.06.002. [DOI] [Google Scholar]
- Yerien D. E.; Cooke M. V.; García Vior M. C.; Barata-Vallejo S.; Postigo A. Radical Fluoroalkylation Reactions of (Hetero)Arenes and Sulfides under Red Light Photocatalysis. Org. Biomol. Chem. 2019, 17, 3741–3746. 10.1039/C9OB00486F. [DOI] [PubMed] [Google Scholar]
- Watanabe K.; Terao N.; Kii I.; Nakagawa R.; Niwa T.; Hosoya T. Indolizines Enabling Rapid Uncaging of Alcohols and Carboxylic Acids by Red Light-Induced Photooxidation. Org. Lett. 2020, 22, 5434–5438. 10.1021/acs.orglett.0c01799. [DOI] [PubMed] [Google Scholar]
- Rupp M. T.; Auvray T.; Shevchenko N.; Swoboda L.; Hanan G. S.; Kurth D. G. Substituted 2,4-Di(Pyridin-2-yl)pyrimidine-Based Ruthenium Photosensitizers for Hydrogen Photoevolution under Red Light. Inorg. Chem. 2021, 60, 292–302. 10.1021/acs.inorgchem.0c02955. [DOI] [PubMed] [Google Scholar]
- Huang J.; Sun J.; Wu Y.; Turro C. Dirhodium(II,II)/NiO Photocathode for Photoelectrocatalytic Hydrogen Evolution with Red Light. J. Am. Chem. Soc. 2021, 143, 1610–1617. 10.1021/jacs.0c12171. [DOI] [PubMed] [Google Scholar]
- Ishikawa Y.; Kameyama T.; Torimoto T.; Maeda H.; Segi M.; Furuyama T. Red-Light-Activatable Ruthenium Phthalocyanine Catalysts. Chem. Commun. 2021, 57, 13594–13597. 10.1039/D1CC06307C. [DOI] [PubMed] [Google Scholar]
- Obah Kosso A. R.; Sellet N.; Baralle A.; Cormier M.; Goddard J.-P. Cyanine-Based near Infra-Red Organic Photoredox Catalysis. Chem. Sci. 2021, 12, 6964–6968. 10.1039/D1SC00998B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mei L.; Moutet J.; Stull S. M.; Gianetti T. L. Synthesis of CF3-Containing Spirocyclic Indolines via a Red-Light-Mediated Trifluoromethylation/Dearomatization Cascade. J. Org. Chem. 2021, 86, 10640–10653. 10.1021/acs.joc.1c01313. [DOI] [PubMed] [Google Scholar]
- Mei L.; Veleta J. M.; Gianetti T. L. Helical Carbenium Ion: A Versatile Organic Photoredox Catalyst for Red-Light-Mediated Reactions. J. Am. Chem. Soc. 2020, 142, 12056–12061. 10.1021/jacs.0c05507. [DOI] [PubMed] [Google Scholar]
- Shon J. H.; Teets T. S. Photocatalysis with Transition Metal Based Photosensitizers. Comments Inorg. Chem. 2020, 40, 53–85. 10.1080/02603594.2019.1694517. [DOI] [Google Scholar]
- Prier C. K.; Rankic D. A.; MacMillan D. W. C. Visible Light Photoredox Catalysis with Transition Metal Complexes: Applications in Organic Synthesis. Chem. Rev. 2013, 113, 5322–5363. 10.1021/cr300503r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero N. A.; Nicewicz D. A. Organic Photoredox Catalysis. Chem. Rev. 2016, 116, 10075–10166. 10.1021/acs.chemrev.6b00057. [DOI] [PubMed] [Google Scholar]
- Scattergood P. A.; Sinopoli A.; Elliott P. I. P. Photophysics and Photochemistry of 1,2,3-Triazole-Based Complexes. Coord. Chem. Rev. 2017, 350, 136–154. 10.1016/j.ccr.2017.06.017. [DOI] [Google Scholar]
- Haruki R.; Sasaki Y.; Masutani K.; Yanai N.; Kimizuka N. Leaping across the Visible Range: Near-Infrared-to-Violet Photon Upconversion Employing a Silyl-Substituted Anthracene. Chem. Commun. 2020, 56, 7017–7020. 10.1039/D0CC02240C. [DOI] [PubMed] [Google Scholar]
- Sasaki Y.; Oshikawa M.; Bharmoria P.; Kouno H.; Hayashi-Takagi A.; Sato M.; Ajioka I.; Yanai N.; Kimizuka N. Near-Infrared Optogenetic Genome Engineering Based on Photon-Upconversion Hydrogels. Angew. Chem., Int. Ed. 2019, 58, 17827–17833. 10.1002/anie.201911025. [DOI] [PubMed] [Google Scholar]
- Noble B.; Peacock R. D. Absorption, Circular Dichroism, and Luminescence Spectroscopy of Electrogenerated Δ-[Ru(bipy)3]+/0/- and Δ-[Os(bipy)3]+/0/- (bipy = 2,2′-bipyridine). Inorg. Chem. 1996, 35, 1616–1620. 10.1021/ic951096e. [DOI] [PubMed] [Google Scholar]
- Shaw G. B.; Styers-Barnett D. J.; Gannon E. Z.; Granger J. C.; Papanikolas J. M. Interligand Electron Transfer Dynamics in [Os(bpy)3]2+: Exploring the Excited State Potential Surfaces with Femtosecond Spectroscopy. J. Phys. Chem. A 2004, 108, 4998–5006. 10.1021/jp0363850. [DOI] [Google Scholar]
- Hamann T. W.; Gstrein F.; Brunschwig B. S.; Lewis N. S. Measurement of the Free-Energy Dependence of Interfacial Charge-Transfer Rate Constants Using ZnO/H2O Semiconductor/Liquid Contacts. J. Am. Chem. Soc. 2005, 127, 7815–7824. 10.1021/ja0436188. [DOI] [PubMed] [Google Scholar]
- Bergkamp M. A.; Gütlich P.; Netzel T. L.; Sutin N. Lifetimes of the Ligand-to-Metal Charge-Transfer Excited States of Iron(III) and Osmium(III) Polypyridine Complexes. Effects of Isotopic Substitution and Temperature. J. Phys. Chem. A 1983, 87, 3877–3883. 10.1021/j100243a018. [DOI] [Google Scholar]
- Wenger O. S. Photoactive Complexes with Earth-Abundant Metals. J. Am. Chem. Soc. 2018, 140, 13522–13533. 10.1021/jacs.8b08822. [DOI] [PubMed] [Google Scholar]
- Larsen C. B.; Wenger O. S. Photoredox Catalysis with Metal Complexes Made from Earth-Abundant Elements. Chem. – Eur. J. 2018, 24, 2039–2058. 10.1002/chem.201703602. [DOI] [PubMed] [Google Scholar]
- Wegeberg C.; Wenger O. S. Luminescent First-Row Transition Metal Complexes. JACS Au 2021, 1, 1860–1876. 10.1021/jacsau.1c00353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minozzi C.; Caron A.; Grenier-Petel J. C.; Santandrea J.; Collins S. K. Heteroleptic Copper(I)-Based Complexes for Photocatalysis: Combinatorial Assembly, Discovery, and Optimization. Angew. Chem., Int. Ed. 2018, 57, 5477–5481. 10.1002/anie.201800144. [DOI] [PubMed] [Google Scholar]
- Nicholls T. P.; Constable G. E.; Robertson J. C.; Gardiner M. G.; Bissember A. C. Brønsted Acid Cocatalysis in Copper(I)-Photocatalyzed α-Amino C-H Bond Functionalization. ACS Catal. 2016, 6, 451–457. 10.1021/acscatal.5b02014. [DOI] [Google Scholar]
- Hossain A.; Bhattacharyya A.; Reiser O. Copper’s Rapid Ascent in Visible-Light Photoredox Catalysis. Science 2019, 364, eaav9713 10.1126/science.aav9713. [DOI] [PubMed] [Google Scholar]
- Reiser O. Shining Light on Copper: Unique Opportunities for Visible-Light-Catalyzed Atom Transfer Radical Addition Reactions and Related Processes. Acc. Chem. Res. 2016, 49, 1990–1996. 10.1021/acs.accounts.6b00296. [DOI] [PubMed] [Google Scholar]
- Knorn M.; Rawner T.; Czerwieniec R.; Reiser O. [Copper(phenanthroline)(bisisonitrile)]+-Complexes for the Visible-Light-Mediated Atom Transfer Radical Addition and Allylation Reactions. ACS Catal. 2015, 5, 5186–5193. 10.1021/acscatal.5b01071. [DOI] [Google Scholar]
- Engl S.; Reiser O. Making Copper Photocatalysis Even More Robust and Economic: Photoredox Catalysis with [CuII(dmp)2Cl]Cl. Eur. J. Org. Chem. 2020, 2020, 1523–1533. 10.1002/ejoc.201900839. [DOI] [Google Scholar]
- Hernandez-Perez A. C.; Collins S. K. Heteroleptic Cu-Based Sensitizers in Photoredox Catalysis. Acc. Chem. Res. 2016, 49, 1557–1565. 10.1021/acs.accounts.6b00250. [DOI] [PubMed] [Google Scholar]
- Nicholls T. P.; Bissember A. C. Developments in Visible-Light-Mediated Copper Photocatalysis. Tetrahedron Lett. 2019, 60, 150883 10.1016/j.tetlet.2019.06.042. [DOI] [Google Scholar]
- Jacob C.; Baguia H.; Dubart A.; Oger S.; Thilmany P.; Beaudelot J.; Deldaele C.; Peruško S.; Landrain Y.; Michelet B.; Neale S.; Romero E.; Moucheron C.; Van Speybroeck V.; Theunissen C.; Evano G. A General Synthesis of Azetidines by Copper-Catalysed Photoinduced Anti-Baldwin Radical Cyclization of Ynamides. Nat. Commun. 2022, 13, 560 10.1038/s41467-022-28098-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fayad R.; Bui A. T.; Shepard S. G.; Castellano F. N. Photochemical Upconversion in Water Using Cu(I) MLCT Excited States: Role of Energy Shuttling at the Micellar/Water Interface. ACS Appl. Energy Mater. 2020, 3, 12557–12564. 10.1021/acsaem.0c02492. [DOI] [Google Scholar]
- Gimeno L.; Blart E.; Rebilly J. N.; Coupeau M.; Allain M.; Roisnel T.; Quarré de Verneuil A.; Gourlaouen C.; Daniel C.; Pellegrin Y. Non-Symmetrical Sterically Challenged Phenanthroline Ligands and Their Homoleptic Copper(I) Complexes with Improved Excited-State Properties. Chem. – Eur. J. 2020, 26, 11887–11899. 10.1002/chem.202001209. [DOI] [PubMed] [Google Scholar]
- Sandoval-Pauker C.; Molina-Aguirre G.; Pinter B. Status Report on Copper (I) Complexes in Photoredox Catalysis; Photophysical and Electrochemical Properties and Future Prospects. Polyhedron 2021, 199, 115105 10.1016/j.poly.2021.115105. [DOI] [Google Scholar]
- Baguia H.; Deldaele C.; Romero E.; Michelet B.; Evano G. Copper-Catalyzed Photoinduced Radical Domino Cyclization of Ynamides and Cyanamides: A Unified Entry to Rosettacin, Luotonin A, and Deoxyvasicinone. Synthesis 2018, 50, 3022–3030. 10.1055/s-0037-1610134. [DOI] [Google Scholar]
- McCusker C. E.; Castellano F. N. Design of a Long-Lifetime, Earth-Abundant, Aqueous Compatible Cu(I) Photosensitizer Using Cooperative Steric Effects. Inorg. Chem. 2013, 52, 8114–8120. 10.1021/ic401213p. [DOI] [PubMed] [Google Scholar]
- Yang P.; Yang X.; Wu B. Synthesis, Structure, and Spectroscopic and Electrochemical Properties of Copper(II/I) Complexes with Symmetrical and Unsymmetrical 2,9-Diaryl-1,10-phenanthroline Ligands. Eur. J. Inorg. Chem. 2009, 2951–2958. 10.1002/ejic.200900024. [DOI] [Google Scholar]
- Cuttell D. G.; Kuang S.-M.; Fanwick P. E.; McMillin D. R.; Walton R. A. Simple Cu(I) Complexes with Unprecedented Excited-State Lifetimes. J. Am. Chem. Soc. 2002, 124, 6–7. 10.1021/ja012247h. [DOI] [PubMed] [Google Scholar]
- Cetin M. M.; Hodson R. T.; Hart C. R.; Cordes D. B.; Findlater M.; Casadonte D. J. Jr.; Cozzolino A. F.; Mayer M. F. Characterization and Photocatalytic Behavior of 2,9-Di(aryl)-1,10-phenanthroline Copper(I) Complexes. Dalton Trans. 2017, 46, 6553–6569. 10.1039/C7DT00400A. [DOI] [PubMed] [Google Scholar]
- Pirtsch M.; Paria S.; Matsuno T.; Isobe H.; Reiser O. [Cu(dap)2Cl] As an Efficient Visible-Light-Driven Photoredox Catalyst in Carbon-Carbon Bond-Forming Reactions. Chem. – Eur. J. 2012, 18, 7336–7340. 10.1002/chem.201200967. [DOI] [PubMed] [Google Scholar]
- Kern J.-M.; Sauvage J.-P. Photoassisted C – C Coupling via Electron Transfer to Benzylic Halides by a Bis(di-imine) Copper(I) Complex. J. Chem. Soc., Chem. Commun. 1987, 8, 546–548. 10.1039/C39870000546. [DOI] [Google Scholar]
- Ghosh I.; Marzo L.; Das A.; Shaikh R.; König B. Visible Light Mediated Photoredox Catalytic Arylation Reactions. Acc. Chem. Res. 2016, 49, 1566–1577. 10.1021/acs.accounts.6b00229. [DOI] [PubMed] [Google Scholar]
- Naumann R.; Kerzig C.; Goez M. Laboratory-Scale Photoredox Catalysis Using Hydrated Electrons Sustainably Generated with a Single Green Laser. Chem. Sci. 2017, 8, 7510–7520. 10.1039/C7SC03514D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naumann R.; Lehmann F.; Goez M. Micellized Tris(bipyridine)ruthenium Catalysts Affording Preparative Amounts of Hydrated Electrons with a Green Light-Emitting Diode. Chem. – Eur. J. 2018, 24, 13259–13269. 10.1002/chem.201801955. [DOI] [PubMed] [Google Scholar]
- Giedyk M.; Narobe R.; Weiß S.; Touraud D.; Kunz W.; König B. Photocatalytic Activation of Alkyl Chlorides by Assembly-Promoted Single Electron Transfer in Microheterogeneous Solutions. Nat. Catal. 2020, 3, 40–47. 10.1038/s41929-019-0369-5. [DOI] [Google Scholar]
- Mandigma M. J. P.; Žurauskas J.; MacGregor C. I.; Edwards L. J.; Shahin A.; D’Heureuse L.; Yip P.; Birch D. J. S.; Gruber T.; Heilmann J.; John M. P.; Barham J. P. An Organophotocatalytic Late-Stage N–CH3 Oxidation of Trialkylamines to N-Formamides with O2 in Continuous Flow. Chem. Sci. 2022, 13, 1912–1924. 10.1039/D1SC05840A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H.; Kim H.; Lambert T. H.; Lin S. Reductive Electrophotocatalysis: Merging Electricity and Light to Achieve Extreme Reduction Potentials. J. Am. Chem. Soc. 2020, 142, 2087–2092. 10.1021/jacs.9b10678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W.; Carpenter K. L.; Lin S. Electrochemistry Broadens the Scope of Flavin Photocatalysis: Photoelectrocatalytic Oxidation of Unactivated Alcohols. Angew. Chem., Int. Ed. 2020, 59, 409–417. 10.1002/anie.201910300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandigma M. J. P.; Domański M.; Barham J. P. C-Alkylation of Alkali Metal Carbanions with Olefins. Org. Biomol. Chem. 2020, 18, 7697–7723. 10.1039/D0OB01180K. [DOI] [PubMed] [Google Scholar]
- Wu S.; Žurauskas J.; Domański M.; Hitzfeld P. S.; Butera V.; Scott D. J.; Rehbein J.; Kumar A.; Thyrhaug E.; Hauer J.; Barham J. P. Hole-Mediated Photoredox Catalysis: Tris(p-substituted)biarylaminium Radical Cations as Tunable, Precomplexing and Potent Photooxidants. Org. Chem. Front. 2021, 8, 1132–1142. 10.1039/D0QO01609H. [DOI] [Google Scholar]
- Huang H.; Lambert T. H. Electrophotocatalytic SNAr Reactions of Unactivated Aryl Fluorides at Ambient Temperature and Without Base. Angew. Chem., Int. Ed. 2020, 59, 658–662. 10.1002/anie.201909983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H.; Strater Z. M.; Lambert T. H. Electrophotocatalytic C-H Functionalization of Ethers with High Regioselectivity. J. Am. Chem. Soc. 2020, 142, 1698–1703. 10.1021/jacs.9b11472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen S. U.; Christensen T. B.; Thomasen T.; Daasbjerg K. New Methods for the Accurate Determination of Extinction and Diffusion Coefficients of Aromatic and Heteroaromatic Radical Anions in N,N-Dimethylformamide. J. Electroanal. Chem. 1998, 454, 123–143. 10.1016/S0022-0728(98)00195-8. [DOI] [Google Scholar]
- Gosztola D.; Niemczyk M. P.; Svec W.; Lukas A. S.; Wasielewski M. R. Excited Doublet States of Electrochemically Generated Aromatic Imide and Diimide Radical Anions. J. Phys. Chem. A 2000, 104, 6545–6551. 10.1021/jp000706f. [DOI] [Google Scholar]
- La Porte N. T.; Martinez J. F.; Chaudhuri S.; Hedström S.; Batista V. S.; Wasielewski M. R. Photoexcited Radical Anion Super-Reductants for Solar Fuels Catalysis. Coord. Chem. Rev. 2018, 361, 98–119. 10.1016/j.ccr.2018.01.018. [DOI] [Google Scholar]
- Marchini M.; Gualandi A.; Mengozzi L.; Franchi P.; Lucarini M.; Cozzi P. G.; Balzani V.; Ceroni P. Mechanistic Insights into Two-Photon-Driven Photocatalysis in Organic Synthesis. Phys. Chem. Chem. Phys. 2018, 20, 8071–8076. 10.1039/C7CP08011E. [DOI] [PubMed] [Google Scholar]
- Reischauer S.; Pieber B. Emerging Concepts in Photocatalytic Organic Synthesis. iScience 2021, 24, 102209 10.1016/j.isci.2021.102209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckwith J. S.; Aster A.; Vauthey E. The Excited-State Dynamics of the Radical Anions of Cyanoanthracenes. Phys. Chem. Chem. Phys. 2021, 24, 568–577. 10.1039/D1CP04014F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieth A. J.; Gonzalez M. I.; Kudisch B.; Nava M.; Nocera D. G. How Radical Are “Radical” Photocatalysts? A Closed-Shell Meisenheimer Complex Is Identified as a Super-Reducing Photoreagent. J. Am. Chem. Soc. 2021, 143, 14352–14359. 10.1021/jacs.1c06844. [DOI] [PubMed] [Google Scholar]
- Haimerl J.; Ghosh I.; König B.; Vogelsang J.; Lupton J. M. Single-Molecule Photoredox Catalysis. Chem. Sci. 2019, 10, 681–687. 10.1039/C8SC03860K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephenson C. R. J.; McClain E. J.; Monos T. M.; Mori M.; Beatty J. W. Design and Implementation of a Catalytic Electron Donor-Acceptor Complex Platform for Radical Trifluoromethylation and Alkylation. ACS Catal. 2020, 10, 12636–12641. 10.1021/acscatal.0c03837. [DOI] [Google Scholar]
- Brimioulle R.; Lenhart D.; Maturi M. M.; Bach T. Enantioselective Catalysis of Photochemical Reactions. Angew. Chem., Int. Ed. 2015, 54, 3872–3890. 10.1002/anie.201411409. [DOI] [PubMed] [Google Scholar]
- Li K.; Wan Q.; Yang C.; Chang X.-Y. Y.; Low K.-H. H.; Che C.-M. Air-Stable Blue Phosphorescent Tetradentate Platinum(II) Complexes as Strong Photo-Reductant. Angew. Chem., Int. Ed. 2018, 57, 14129–14133. 10.1002/anie.201808642. [DOI] [PubMed] [Google Scholar]
- López-Calixto C. G.; Liras M.; de la Peña O’Shea V. A.; Pérez-Ruiz R. Synchronized Biphotonic Process Triggering C–C Coupling Catalytic Reactions. Appl. Catal., B 2018, 237, 18–23. 10.1016/j.apcatb.2018.05.062. [DOI] [Google Scholar]
- Costentin C.; Robert M.; Savéant J. M. Fragmentation of Aryl Halide π Anion Radicals. Bending of the Cleaving Bond and Activation vs Driving Force Relationships. J. Am. Chem. Soc. 2004, 126, 16051–16057. 10.1021/ja045989u. [DOI] [PubMed] [Google Scholar]
- Qiang-Liu; Liu Y. X.; Song H. J.; Wang Q. M. Electron Transfer Photoredox Catalysis: Development of a Photoactivated Reductive Desulfonylation of an Aza-Heteroaromatic Ring. Adv. Synth. Catal. 2020, 362, 3110–3115. 10.1002/adsc.202000578. [DOI] [Google Scholar]
- Hasegawa E.; Nakamura S.; Oomori K.; Tanaka T.; Iwamoto H.; Wakamatsu K. Competitive Desulfonylative Reduction and Oxidation of α-Sulfonylketones Promoted by Photoinduced Electron Transfer with 2-Hydroxyaryl-1,3-Dimethylbenzimidazolines under Air. J. Org. Chem. 2021, 86, 2556–2569. 10.1021/acs.joc.0c02666. [DOI] [PubMed] [Google Scholar]
- Hendy C. M.; Smith G. C.; Xu Z.; Lian T.; Jui N. T. Radical Chain Reduction via Carbon Dioxide Radical Anion (CO2•-). J. Am. Chem. Soc. 2021, 143, 8987–8992. 10.1021/jacs.1c04427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasegawa E.; Izumiya N.; Miura T.; Ikoma T.; Iwamoto H.; Takizawa S. Y.; Murata S. Benzimidazolium Naphthoxide Betaine Is a Visible Light Promoted Organic Photoredox Catalyst. J. Org. Chem. 2018, 83, 3921–3927. 10.1021/acs.joc.8b00282. [DOI] [PubMed] [Google Scholar]
- McMillin D. R.; Kirchhoff J. R.; Goodwin K. V. Exciplex Quenching of Photo-Excited Copper Complexes. Coord. Chem. Rev. 1985, 64, 83–92. 10.1016/0010-8545(85)80043-6. [DOI] [Google Scholar]
- Everly R. M.; McMillin D. R. Concentration-Dependent Lifetime of Cu(NN)2+ Systems: Exciplex Quenching from the Ion Pair State. Photochem. Photobiol. 1989, 50, 711–716. 10.1111/j.1751-1097.1989.tb02900.x. [DOI] [Google Scholar]
- Kutal C. Spectroscopic and Photochemical Properties of d10 Metal Complexes. Coord. Chem. Rev. 1990, 99, 213–252. 10.1016/0010-8545(90)80064-Z. [DOI] [Google Scholar]
- Vining W. J.; Caspar J. V.; Meyer T. J. The Influence of Environmental Effects on Excited-State Lifetimes. The Effect of Ion Pairing on Metal-to-Ligand Charge Transfer Excited States. J. Phys. Chem. B 1985, 89, 1095–1099. 10.1021/j100253a010. [DOI] [Google Scholar]
- Michelet B.; Deldaele C.; Kajouj S.; Moucheron C.; Evano G. A General Copper Catalyst for Photoredox Transformations of Organic Halides. Org. Lett. 2017, 19, 3576–3579. 10.1021/acs.orglett.7b01518. [DOI] [PubMed] [Google Scholar]
- Schoenebeck F.; Murphy J. A.; Zhou S. Z.; Uenoyama Y.; Miclo Y.; Tuttle T. Reductive Cleavage of Sulfones and Sulfonamides by a Neutral Organic Super-Electron-Donor (S.E.D.) Reagent. J. Am. Chem. Soc. 2007, 129, 13368–13369. 10.1021/ja074417h. [DOI] [PubMed] [Google Scholar]
- Jin S.; Dang H. T.; Haug G. C.; He R.; Nguyen V. D.; Nguyen V. T.; Arman H. D.; Schanze K. S.; Larionov O. V. Visible Light-Induced Borylation of C–O, C–N, and C–X Bonds. J. Am. Chem. Soc. 2020, 142, 1603–1613. 10.1021/jacs.9b12519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chmiel A. F.; Williams O. P.; Chernowsky C. P.; Yeung C. S.; Wickens Z. K. Non-Innocent Radical Ion Intermediates in Photoredox Catalysis: Parallel Reduction Modes Enable Coupling of Diverse Aryl Chlorides. J. Am. Chem. Soc. 2021, 143, 10882–10889. 10.1021/jacs.1c05988. [DOI] [PubMed] [Google Scholar]
- Cutulic S. P. Y.; Findlay N. J.; Zhou S. Z.; Chrystal E. J. T.; Murphy J. A. Metal-Free Reductive Cleavage of C-O σ-Bonds in Acyloin Derivatives by an Organic Neutral Super-Electron-Donor. J. Org. Chem. 2009, 74, 8713–8718. 10.1021/jo901815t. [DOI] [PubMed] [Google Scholar]
- Majek M.; Faltermeier U.; Dick B.; Pérez-Ruiz R.; JacobivonWangelin A. Application of Visible-to-UV Photon Upconversion to Photoredox Catalysis: The Activation of Aryl Bromides. Chem. – Eur. J. 2015, 21, 15496–15501. 10.1002/chem.201502698. [DOI] [PubMed] [Google Scholar]
- Glaser F.; Kerzig C.; Wenger O. S. Sensitization-Initiated Electron Transfer via Upconversion: Mechanism and Photocatalytic Applications. Chem. Sci. 2021, 12, 9922–9933. 10.1039/D1SC02085D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fajardo J.; Barth A. T.; Morales M.; Takase M. K.; Winkler J. R.; Gray H. B. Photoredox Catalysis Mediated by Tungsten(0) Arylisocyanides. J. Am. Chem. Soc. 2021, 143, 19389–19398. 10.1021/jacs.1c07617. [DOI] [PubMed] [Google Scholar]
- Coles M. S.; Quach G.; Beves J. E.; Moore E. G. A Photophysical Study of Sensitization-Initiated Electron Transfer: Insights into the Mechanism of Photoredox Activity. Angew. Chem., Int. Ed. 2020, 59, 9522–9526. 10.1002/anie.201916359. [DOI] [PubMed] [Google Scholar]
- Chatterjee A.; König B. Birch-Type Photoreduction of Arenes and Heteroarenes by Sensitized Electron Transfer. Angew. Chem., Int. Ed. 2019, 58, 14289–14294. 10.1002/anie.201905485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fayad R.; Engl S.; Danilov E. O.; Hauke C. E.; Reiser O.; Castellano F. N. Direct Evidence of Visible Light-Induced Homolysis in Chlorobis(2,9-dimethyl-1,10-phenanthroline)copper(II). J. Phys. Chem. Lett. 2020, 11, 5345–5349. 10.1021/acs.jpclett.0c01601. [DOI] [PubMed] [Google Scholar]
- Kerzig C.; Guo X.; Wenger O. S. Unexpected Hydrated Electron Source for Preparative Visible-Light Driven Photoredox Catalysis. J. Am. Chem. Soc. 2019, 141, 2122–2127. 10.1021/jacs.8b12223. [DOI] [PubMed] [Google Scholar]
- Ghosh I.; Shaikh R. S.; König B. Sensitization-Initiated Electron Transfer for Photoredox Catalysis. Angew. Chem., Int. Ed. 2017, 56, 8544–8549. 10.1002/anie.201703004. [DOI] [PubMed] [Google Scholar]
- Herrera-Luna J. C.; Díaz D. D.; Jiménez M. C.; Pérez-Ruiz R. Highly Efficient Production of Heteroarene Phosphonates by Dichromatic Photoredox Catalysis. ACS Appl. Mater. Interfaces 2021, 13, 48784–48794. 10.1021/acsami.1c14497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montalti M.; Credi A.; Prodi L.; Gandolfi M. T.. Handbook of Photochemistry, Third ed.; CRC Press, 2006. [Google Scholar]
- Leonhardt H.; Weller A. Elektronenübertragungsreaktionen des angeregten Perylens. Ber. Bunsen-Ges. Phys. Chem. 1963, 67, 791–795. 10.1002/bbpc.19630670810. [DOI] [Google Scholar]
- Rehm D.; Weller A. Kinetics of Fluorescence Quenching by Electron and H-Atom Transfer. Isr. J. Chem. 1970, 8, 259–271. 10.1002/ijch.197000029. [DOI] [Google Scholar]
- Zhu Y.; He Y. Q.; Tian W. F.; Wang M.; Zhou Z. Z.; Song X. R.; Ding H. X.; Xiao Q. Dual Cobalt and Photoredox Catalysis Enabled Redox-Neutral Annulation of 2-Propynolphenols. Adv. Synth. Catal. 2021, 363, 3372–3377. 10.1002/adsc.202100221. [DOI] [Google Scholar]
- Darmanyan A. P. Experimental Study of Singlet-Triplet Energy Transfer in Liquid Solutions. Chem. Phys. Lett. 1984, 110, 89–94. 10.1016/0009-2614(84)80152-9. [DOI] [Google Scholar]
- Glaser F.; Larsen C. B.; Kerzig C.; Wenger O. S. Aryl Dechlorination and Defluorination with an Organic Super-Photoreductant. Photochem. Photobiol. Sci. 2020, 19, 1035–1041. 10.1039/D0PP00127A. [DOI] [PubMed] [Google Scholar]
- Garakyaraghi S.; Danilov E. O.; McCusker C. E.; Castellano F. N. Transient Absorption Dynamics of Sterically Congested Cu(I) MLCT Excited States. J. Phys. Chem. A 2015, 119, 3181–3193. 10.1021/acs.jpca.5b00901. [DOI] [PubMed] [Google Scholar]
- Eriksen J.; Lund H.; Nyvad A. I.; Yamato T.; Mitchell R. H.; Dingle T. W.; Williams R. V.; Mahedevan R. Electron-Transfer Fluorescence Quenching of Radical Ions. Acta Chem. Scand. 1983, 37b, 459–466. 10.3891/acta.chem.scand.37b-0459. [DOI] [Google Scholar]
- Fujita M.; Ishida A.; Majima T.; Takamuku S. Lifetimes of Radical Anions of Dicyanoanthracene, Phenazine, and Anthraquinone in the Excited State from the Selective Electron-Transfer Quenching. J. Phys. Chem. C 1996, 100, 5382–5387. 10.1021/jp953203w. [DOI] [Google Scholar]
- Breslin D. T.; Fox M. A. A Strongly Chemiluminescent Dioxetanimine Dianion Fragmentation: Reaction of the Dicyanoanthracene Radical Anion with Superoxide Ion. J. Am. Chem. Soc. 1993, 115, 11716–11721. 10.1021/ja00078a009. [DOI] [Google Scholar]
- Breslin D. T.; Fox M. A. Excited-State Behavior of Thermally Stable Radical Ions. J. Phys. Chem. C 1994, 98, 408–411. 10.1021/j100053a011. [DOI] [Google Scholar]
- Janzen E. G.; Rudy B. C.; Lopp I. G.; Happ J. W. Chemiluminescent Oxygenation of 9,10-Dicyanoanthracene Radical Anion. J. Chem. Soc. D: Chem. Commun. 1970, 491–492. 10.1039/c29700000491. [DOI] [Google Scholar]
- Zeman C. J.; Kim S.; Zhang F.; Schanze K. S. Direct Observation of the Reduction of Aryl Halides by a Photoexcited Perylene Diimide Radical Anion. J. Am. Chem. Soc. 2020, 142, 2204–2207. 10.1021/jacs.9b13027. [DOI] [PubMed] [Google Scholar]
- Wu S.; Kaur J.; Karl T. A.; Tian X.; Barham J. P. Synthetic Molecular Photoelectrochemistry: New Frontiers in Synthetic Applications, Mechanistic Insights and Scalability. Angew. Chem., Int. Ed. 2022, 61, e202107811 10.1002/anie.202107811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujitsuka M.; Majima T. Reaction Dynamics of Excited Radical Ions Revealed by Femtosecond Laser Flash Photolysis. J. Photochem. Photobiol., C 2018, 35, 25–37. 10.1016/j.jphotochemrev.2017.12.003. [DOI] [Google Scholar]
- Kellett M. A.; Whitten D. G.; Gould I. R.; Bergmark W. R. Surprising Differences in the Reactivity of Cyanoaromatic Radical Anions Generated by Photoinduced Electron Transfer. J. Am. Chem. Soc. 1991, 113, 358–359. 10.1021/ja00001a052. [DOI] [Google Scholar]
- Ohashi M.; Kudo H.; Yamada S. Photochemical Reaction of Dicyanoanthracene with Acetonitrile in the Presence of an Aliphatic Amine. A Novel Photochemical Amination. J. Am. Chem. Soc. 1979, 101, 2201–2202. 10.1021/ja00502a045. [DOI] [Google Scholar]
- Hasegawa E.; Brumfield M. A.; Mariano P. S.; Yoon U. C. Photoadditions of Ethers, Thioethers, and Amines to 9,10-Dicyanoanthracene by Electron Transfer Pathways. J. Org. Chem. 1988, 53, 5435–5442. 10.1021/jo00258a007. [DOI] [Google Scholar]
- Freccero M.; Mella M.; Albini A. The Photochemical Reactions of 9,10-Anthracenedicarbonitrile and 1,4-Naphthalenedicarbonitrile in Acetonitrile in the Presence of Bases. Tetrahedron 1994, 50, 2115–2130. 10.1016/S0040-4020(01)85074-2. [DOI] [Google Scholar]
- Rosso C.; Filippini G.; Cozzi P. G.; Gualandi A.; Prato M. Highly Performing Iodoperfluoroalkylation of Alkenes Triggered by the Photochemical Activity of Perylene Diimides. ChemPhotoChem 2019, 3, 193–197. 10.1002/cptc.201900018. [DOI] [Google Scholar]
- Tian X.; Karl T. A.; Reiter S.; Yakubov S.; de Vivie-Riedle R.; König B.; Barham J. P. Electro-Mediated PhotoRedox Catalysis for Selective C(sp3)–O Cleavages of Phosphinated Alcohols to Carbanions. Angew. Chem., Int. Ed. 2021, 60, 20817–20825. 10.1002/anie.202105895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawner T.; Lutsker E.; Kaiser C. A.; Reiser O. The Different Faces of Photoredox Catalysts: Visible-Light-Mediated Atom Transfer Radical Addition (ATRA) Reactions of Perfluoroalkyl Iodides with Styrenes and Phenylacetylenes. ACS Catal. 2018, 8, 3950–3956. 10.1021/acscatal.8b00847. [DOI] [Google Scholar]
- Kohlmann T.; Naumann R.; Kerzig C.; Goez M. 3-Aminoperylene and Ascorbate in Aqueous SDS, One Green Laser Flash ... and Action! Sustainably Detoxifying a Recalcitrant Chloro-Organic. Photochem. Photobiol. Sci. 2017, 16, 1613–1622. 10.1039/C7PP00311K. [DOI] [PubMed] [Google Scholar]
- Engl P. S.; Häring A. P.; Berger F.; Berger G.; Pérez-Bitrián A.; Ritter T. C-N Cross-Couplings for Site-Selective Late-Stage Diversification via Aryl Sulfonium Salts. J. Am. Chem. Soc. 2019, 141, 13346–13351. 10.1021/jacs.9b07323. [DOI] [PubMed] [Google Scholar]
- Capaldo L.; Ravelli D. Hydrogen Atom Transfer (HAT): A Versatile Strategy for Substrate Activation in Photocatalyzed Organic Synthesis. Eur. J. Org. Chem. 2017, 2017, 2056–2071. 10.1002/ejoc.201601485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alektiar S. N.; Wickens Z. K. Photoinduced Hydrocarboxylation via Thiol-Catalyzed Delivery of Formate across Activated Alkenes. J. Am. Chem. Soc. 2021, 143, 13022–13028. 10.1021/jacs.1c07562. [DOI] [PubMed] [Google Scholar]
- Stevenson B. G.; Spielvogel E. H.; Loiaconi E. A.; Wambua V. M.; Nakhamiyayev R. V.; Swierk J. R. Mechanistic Investigations of an α-Aminoarylation Photoredox Reaction. J. Am. Chem. Soc. 2021, 143, 8878–8885. 10.1021/jacs.1c03693. [DOI] [PubMed] [Google Scholar]
- Aydogan A.; Bangle R. E.; Cadranel A.; Turlington M. D.; Conroy D. T.; Cauët E.; Singleton M. L.; Meyer G. J.; Sampaio R. N.; Elias B.; Troian-Gautier L. Accessing Photoredox Transformations with an Iron(III) Photosensitizer and Green Light. J. Am. Chem. Soc. 2021, 143, 15661–15673. 10.1021/jacs.1c06081. [DOI] [PubMed] [Google Scholar]
- Creutz S. E.; Lotito K. J.; Fu G. C.; Peters J. C. Photoinduced Ullmann C-N Coupling: Demonstrating the Viability of a Radical Pathway. Science 2012, 338, 647–651. 10.1126/science.1226458. [DOI] [PubMed] [Google Scholar]
- Johnson M. W.; Hannoun K. I.; Tan Y.; Fu G. C.; Peters J. C. A Mechanistic Investigation of the Photoinduced, Copper-Mediated Cross-Coupling of an Aryl Thiol with an Aryl Halide. Chem. Sci. 2016, 7, 4091–4100. 10.1039/C5SC04709A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean E. B.; Lee A. L. Dual Copper- and Photoredox-Catalysed Reactions. Tetrahedron 2018, 74, 4881–4902. 10.1016/j.tet.2018.07.032. [DOI] [Google Scholar]
- Liang Y.; Zhang X.; MacMillan D. W. C. Decarboxylative sp3 C-N Coupling via Dual Copper and Photoredox Catalysis. Nature 2018, 559, 83–88. 10.1038/s41586-018-0234-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q. Y.; Gockel S. N.; Lutovsky G. A.; DeGlopper K. S.; Baldwin N. J.; Bundesmann M. W.; Tucker J. W.; Bagley S. W.; Yoon T. P. Decarboxylative Cross-Nucleophile Coupling via Ligand-to-Metal Charge Transfer Photoexcitation of Cu(II) Carboxylates. Nat. Chem. 2022, 14, 94–99. 10.1038/s41557-021-00834-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amos S. G. E.; Garreau M.; Buzzetti L.; Waser J. Photocatalysis with Organic Dyes: Facile Access to Reactive Intermediates for Synthesis. Beilstein J. Org. Chem. 2020, 16, 1163–1187. 10.3762/bjoc.16.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C.; Yang J.-D.; Li Y.-H.; Li X.; Cheng J.-P. 9,10-Dicyanoanthracene Catalyzed Decarboxylative Alkynylation of Carboxylic Acids under Visible-Light Irradiation. J. Org. Chem. 2016, 81, 12357–12363. 10.1021/acs.joc.6b02385. [DOI] [PubMed] [Google Scholar]
- Pandey G.; Laha R. Visible-Light-Catalyzed Direct Benzylic C(sp3)-H Amination Reaction by Cross-Dehydrogenative Coupling. Angew. Chem., Int. Ed. 2015, 54, 14875–14879. 10.1002/anie.201506990. [DOI] [PubMed] [Google Scholar]
- Nakamura M.; Miki M.; Majima T. Substituent Effect on the Photoinduced Electron-Transfer Reaction of Para-Substituted Triphenylphosphines Sensitised by 9,10-Dicyanoanthracene. J. Chem. Soc., Perkin Trans. 2 2000, 2, 1447–1452. 10.1039/b001617i. [DOI] [Google Scholar]
- Whittemore T. J.; Xue C.; Huang J.; Gallucci J. C.; Turro C. Single-Chromophore Single-Molecule Photocatalyst for the Production of Dihydrogen Using Low-Energy Light. Nat. Chem. 2020, 12, 180–185. 10.1038/s41557-019-0397-4. [DOI] [PubMed] [Google Scholar]
- Shen Z.; Zheng S.; Xiao S.; Shen R.; Liu S.; Hu J. Red-Light-Mediated Photoredox Catalysis Enables Self-Reporting Nitric Oxide Release for Efficient Antibacterial Treatment. Angew. Chem., Int. Ed. 2021, 60, 20452–20460. 10.1002/anie.202107155. [DOI] [PubMed] [Google Scholar]
- Zhang C.; Guo X.; Da X.; Wang Z.; Wang X.; Zhou Q. A Ru-Anthraquinone Dyad with Triple Functions of PACT, Photoredox Catalysis and PDT upon Red Light Irradiation. Dalton Trans. 2021, 50, 10845–10852. 10.1039/D1DT01088C. [DOI] [PubMed] [Google Scholar]
- Qi S.; Jin Z.; Jian Y.; Hou Y.; Li C.; Zhao Y.; Wang X.; Zhou Q. Photo-Induced Mitochondrial DNA Damage and NADH Depletion by -NO2 Modified Ru(II) Complexes. Chem. Commun. 2021, 57, 4162–4165. 10.1039/D1CC00258A. [DOI] [PubMed] [Google Scholar]
- Sun W.; Li S.; Häupler B.; Liu J.; Jin S.; Steffen W.; Schubert U. S.; Butt H.-J.; Liang X.-J.; Wu S. An Amphiphilic Ruthenium Polymetallodrug for Combined Photodynamic Therapy and Photochemotherapy In Vivo. Adv. Mater. 2017, 29, 1603702 10.1002/adma.201603702. [DOI] [PubMed] [Google Scholar]
- Zhou Z.; Song J.; Nie L.; Chen X. Reactive Oxygen Species Generating Systems Meeting Challenges of Photodynamic Cancer Therapy. Chem. Soc. Rev. 2016, 45, 6597–6626. 10.1039/C6CS00271D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truong V. X.; Barner-Kowollik C. Red-Light Driven Photocatalytic Oxime Ligation for Bioorthogonal Hydrogel Design. ACS Macro Lett. 2021, 10, 78–83. 10.1021/acsmacrolett.0c00767. [DOI] [PubMed] [Google Scholar]
- Stafford A.; Ahn D.; Raulerson E. K.; Chung K. Y.; Sun K.; Cadena D. M.; Forrister E. M.; Yost S. R.; Roberts S. T.; Page Z. A. Catalyst Halogenation Enables Rapid and Efficient Polymerizations with Visible to Far-Red Light. J. Am. Chem. Soc. 2020, 142, 14733–14742. 10.1021/jacs.0c07136. [DOI] [PubMed] [Google Scholar]
- Cao H.; Wang G.; Xue Y.; Yang G.; Tian J.; Liu F.; Zhang W. Far-Red Light-Induced Reversible Addition–Fragmentation Chain Transfer Polymerization Using a Man-Made Bacteriochlorin. ACS Macro Lett. 2019, 8, 616–622. 10.1021/acsmacrolett.9b00320. [DOI] [PubMed] [Google Scholar]
- Shanmugam S.; Xu J.; Boyer C. Light-Regulated Polymerization under Near-Infrared/Far-Red Irradiation Catalyzed by Bacteriochlorophyll A. Angew. Chem., Int. Ed. 2016, 55, 1036–1040. 10.1002/anie.201510037. [DOI] [PubMed] [Google Scholar]
- Wang Q.; Popov S.; Feilen A.; Strehmel V.; Strehmel B. Rational Selection of Cyanines to Generate Conjugate Acid and Free Radicals for Photopolymerization upon Exposure at 860 nm. Angew. Chem., Int. Ed. 2021, 60, 26855–26865. 10.1002/anie.202108713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole J. P.; Chen D.-F.; Kudisch M.; Pearson R. M.; Lim C.-H.; Miyake G. M. Organocatalyzed Birch Reduction Driven by Visible Light. J. Am. Chem. Soc. 2020, 142, 13573–13581. 10.1021/jacs.0c05899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freitag M.; Möller N.; Rühling A.; Strassert C. A.; Ravoo B. J.; Glorius F. Photocatalysis in the Dark: Near-Infrared Light Driven Photoredox Catalysis by an Upconversion Nanoparticle/Photocatalyst System. ChemPhotoChem 2019, 3, 24–27. 10.1002/cptc.201800212. [DOI] [Google Scholar]
- Katsurayama Y.; Ikabata Y.; Maeda H.; Segi M.; Nakai H.; Furuyama T. Direct Near Infrared Light–Activatable Phthalocyanine Catalysts. Chem. – Eur. J. 2022, 28, e202103223 10.1002/chem.202103223. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.