Abstract
In this study, the effects of Bacillus species–fermented products (synbiotics [SYNs]) and essential oils (EOs) on the growth performance, gut morphology, cecal short-chain fatty acid (SCFA) levels, and microbiota of broilers were investigated. A total of 180 one-day-old unsexed broiler chicks (Ross 308) were randomly assigned to 5 dietary treatments as follows: basal diet (control group), basal diet plus enramycin (10 mg/kg; A group), basal diet plus SYNs (3 × 1011 CFU spore/kg of feed; SYN group), basal diet plus EOs (100 mg/kg; EO group), and basal diet plus SYNs and EOs (SYN + EO group), with 6 replicate cages per treatment group and 6 birds per cage. The SYN+EO treatment resulted in a higher (P = 0.003) average daily gain at 1 to 14 d of age than did the control and EO treatments. SYNs had a significant effect on the average daily gain at 1 to 14 d (P < 0.001) and 1 to 35 d (P = 0.045) of age. EOs had a significant effect on the villus height of the duodenum (P = 0.015) and jejunum (P = 0.027). Superoxide dismutase (SOD) and mucin 2 (MUC2) mRNA expression in the duodenum, jejunum, and ileum in the SYN + EO group was higher (P < 0.001) than that in any of the other groups. The SYN+EO treatment resulted in higher (P < 0.001) 2-methylbutyric acid and 3-methylbutyric acid levels in the cecal digesta of the broilers than did the control treatment. Cecal species evenness in the SYN + EO group was higher (P < 0.001) than that in the control group. The abundance of the phylum Firmicutes in the cecal digesta of the broilers was higher (P < 0.001) in the SYN+EO group than in the control group. SYNs had a significant effect (P < 0.001) on the abundance of the genus Lactobacillus in the cecal digesta of the broilers. The abundance of the genus Lactobacillus was positively associated with 2-methylbutyric acid and 3-methylbutyric acid levels. The 2-methylbutyric acid and 3-methylbutyric acid levels were positively correlated with the villus height of the duodenum and ileum. These results suggest that simultaneous supplementation with SYNs and EOs can increase the average daily gain, improve gut health–associated gene expression, increase SCFA levels, and modulate the gut microbiota composition of broilers.
Key words: Bacillus, broiler, essential oil, fermented product, microbiota
INTRODUCTION
Antibiotics at subtherapeutic doses and for prolonged periods have been extensively used as feed additives to improve animal performance through the inhibition of pathogenic bacterial growth in the gut. However, current global trends in poultry production involve a reduction in the use of antibiotics as growth promoters because of the risk of antibiotic resistance (Marshall and Levy, 2011). In response to the increasing global demand for alternatives to antibiotics in poultry production, numerous natural feed additives have been developed. Among the potential candidates, probiotics and essential oils (EOs) have been explored as alternatives because they exhibit various biological benefits, such as antimicrobial, antioxidant, and immunomodulatory effects (Simon et al., 2001; Stevanović et al., 2018).
Bacillus species–based probiotics are the most commonly used probiotics in animal production because of their sporulation ability, which enables their survival under harsh environmental conditions (low pH, bile salts, and heat), spore germination in the gastrointestinal tract, and production of antimicrobial substances (Ramlucken et al., 2020). Bacillus species–based probiotics can promote animal growth and prevent pathogenic infections in poultry (Abudabos et al., 2013; Abudabos et al., 2017; Grant et al., 2018; Mohamed et al., 2022). Our previous studies have demonstrated that antimicrobial substances derived from fermented products obtained from Bacillus species (B. subtilis or B. licheniformis) can inhibit the growth of pathogens, particularly gram-positive bacteria (Horng et al., 2019). Supplementation with Bacillus species–fermented products (synbiotics [SYNs]) can reduce the risk of enteric diseases, improve growth performance, modulate gut microbiota, and alleviate inflammation in broilers (Cheng et al., 2018, 2021a, 2021b; Chen and Yu, 2020, 2021; Yu et al., 2021).
EOs are natural plant extracts that exhibit high antibacterial activity against gram-negative bacteria, such as enterohemorrhagic Escherichia coli, Salmonella typhimurium, and Salmonella enterica (Helander et al., 1998; Bartkiene et al., 2020). EOs also exhibit antifungal activity by blocking cell communication mechanisms, fungal biofilm formation, and mycotoxin production (Nazzaro et al., 2017). EOs are effective in preventing lipid oxidation and promoting antioxidant activity in broilers (Adaszyńska-Skwirzyńska and Szczerbińska, 2017; Mohebodini et al., 2021). Supplementation with EOs can improve growth performance, reduce coccidiosis, and alleviate necrotic enteritis in broilers (Giannenas et al., 2003; Khattak et al., 2014; Zhang et al., 2021; Pham et al., 2022). A recent meta-analysis also shows that all type of EOs have positive effects on body weight, average daily gain, feed conversion ratio, digestibility, and cecal microbiota in broilers (Irawan et al., 2021).
To the best of our knowledge, the effects of combined supplementation with SYNs and EOs, which may exert synergistic effects, have not yet been investigated in broilers. Antimicrobial substances derived from SYNs exhibit higher antimicrobial activity against gram-positive bacteria than against gram-negative bacteria, whereas EOs exhibit high antibacterial activity against gram-negative bacteria (Helander et al., 1998; Cheng et al., 2018; Bartkiene et al., 2020). Spores of Bacillus species can prevent pathogen invasion in the gut through competitive exclusion, and EOs have antioxidant properties (Adaszyńska-Skwirzyńska and Szczerbińska, 2017; Chen and Yu, 2020; Zhang et al., 2021; Shi et al., 2022; Abd El-Hack et al., 2022). Therefore, we hypothesize that the combination of SYNs and EOs is more likely to modulate growth performance, intestinal morphology, and gut microbiota than are in-feed antibiotics. This study evaluated the individual and combined effects of SYNs and EOs (as replacements for antibiotic growth promoters) on growth performance, gut morphology, cecal short-chain fatty acid (SCFA) levels, and microbiota community in broilers.
MATERIALS AND METHODS
Bacillus Species–Fermented Products and EOs
Details of the preparation of SYNs obtained from Bacillus species (B. subtilis and B. licheniformis) are provided in our previous studies (Cheng et al., 2018, 2021a). B. subtilis–fermented products and B. licheniformis–fermented products were produced separately and mixed into the diet at the indicated concentrations after the determination of bacterial counts. In brief, solid-state fermentation substrates were mixed with water in a space bag to obtain the required initial moisture content, and the mixture was autoclaved at 121°C for 30 min. After cooling, the substrates were inoculated with 4% (v/w) inoculum of B. subtilis or B. licheniformis, mixed carefully under sterile conditions, and incubated at 30°C in a chamber with free oxygen and at relative humidity above 80%. After 6 d of solid-state fermentation, SYNs were dried at 50°C for 2 d and homogenized through mechanical agitation. The concentrations of B. subtilis and B. licheniformis spores in fermented products were 1.1 × 1014 CFU/g and 6.9 × 1013 CFU/g, respectively. The EO blend (basil, caraway, laurel, lemon, oregano, sage, tea, and thyme) used in this study is a commercially available feed additive (Tecnaroma, São Paulo, Brasil), and its chemical composition was analyzed using gas chromatography–mass spectrometry (GC–MS; Bruker GC-MS System, Billerica, MA). The main constituents of the EO blend were as follows: cymene (31.4%), thymol (18.3%), santalone (10.2%), cinnamaldehyde (3.2%), sylvestrene (1.88%), and thujene (1.6%).
Experimental Design
The animal protocol was approved by the Institutional Animal Care and Use Committee of National Ilan University (109-26). A total of 180 one-day-old unsexed broiler chicks (Ross 308) with an average body weight of 46.8 ± 0.39 g were obtained from a local commercial hatchery and were randomly assigned to one of 5 treatments, with 6 replicate cages per treatment group and 6 birds per cage. The broilers were reared in temperature-controlled stainless-steel cages. The treatments were as follows: 1) basal diet (control group), 2) basal diet plus enramycin (10 mg/kg; A group), 3) basal diet plus SYNs (1.5 × 1011 CFU B. subtilis spore/kg of feed and 1.5 × 1011 CFU B. licheniformis spore/kg of feed; SYN group), 4) basal diet plus EOs (100 mg/kg; EO group), and 5) basal diet plus SYNs and EOs (SYN+EO group). The experimental diets were formulated to meet all the minimum nutrient requirements for birds (Nutrient Requirements for Poultry, 1994, Table 1). Enramycin, SYNs, and EOs were added to the diets in powder form. The broilers were fed the test diets from 1 to 35 d of age; the broilers aged 1 to 14 d and 15 to 35 d were considered to be in the starter and growth phrases, respectively. The birds were given drinking water and feed ad libitum, and a 20-h/4-h light/darkness cycle was applied. The ambient temperature on d 1 to 3 was set to 33°C and was gradually reduced to 30°C on d 4 to 7, 27°C on d 8 to 14, and 24°C on d 15 to 35. Newcastle disease/infectious bronchitis vaccines were administered by nose drop on d 4 and 14. Growth performance (average body weight, average daily gain, average daily feed intake, and feed conversion ratio) was calculated from 1 to 35 d of age. The mortality of the broilers was monitored daily. At the end of the experiment, the broilers were humanely killed by carbon dioxide inhalation.
Table 1.
Composition of basal diets.
| Item | D 1 to 14 | D 15 to 35 |
|---|---|---|
| Ingredient, g kg−1 | ||
| Corn, yellow | 554.2 | 607.3 |
| Soybean meal | 355.2 | 315.3 |
| Fish meal | 39.9 | 36.3 |
| Vegetable oil | 35.2 | 30.2 |
| Limestone | 15.2 | 12.7 |
| Salt | 3.0 | 3.0 |
| Monocalcium phosphate | 9.2 | 7.8 |
| Mineral premix1 | 2.0 | 2.0 |
| Vitamin premix2 | 2.0 | 2.0 |
| DL-methionine | 2.0 | 2.0 |
| L-lysine | 1.0 | 0.6 |
| Choline chloride | 0.5 | 0.5 |
| Calculated value, g kg−1 | ||
| Dry matter | 88.9 | 88.7 |
| Crude protein | 221.6 | 206.3 |
| Analyzed calcium | 10.2 | 8.7 |
| Analyzed total phosphorus | 6.9 | 6.3 |
| Lysine | 11.2 | 9.5 |
| Methionine + Cystine | 8.5 | 7.6 |
| ME, kcal/kg | 3081.1 | 3057.2 |
Supplied per kg of diet: 32 mg of Mn (MnSO4·H2O), 16 mg of Fe (FeSO4·7H2O), 24 mg of Zn (ZnO), 2 mg of Cu (CuSO4·5H2O), 800 μg of I (KI), 200 μg of Co (CoSO4), and 60 μg of Se.
Supplied per kg of diet: 5,232 IU of vitamin A, 800 IU of vitamin D, 8.3 IU of vitamin E, 2.2 mg of menadione, 2 mg of pyridoxine HCl, 8 mg of cyanocobalamin, 10 mg of nicotine amid, 0.3 mg of folic acid, 20 mg of D-biotin, and 160 mg of choline chloride.
Intestinal Histomorphology Analysis
Two broilers per replicate were randomly chosen and 6 replicates (12 birds/group, n = 6) were used for small intestine morphology analysis. The intestinal morphology of the broilers was analyzed at 3 locations: 2 cm after the gizzard (duodenum), before the Meckel's diverticulum (jejunum), and before the ileocecal transition (ileum). The samples were fixed in 10% neutral-buffered formalin solution (Thermo Fisher Scientific, Waltham, MA). Subsequently, 5-μm thick-sections (3 cross-sections from each sample) were cut using a microtome (Thermo Fisher Scientific), mounted on microscope slides, and stained with hematoxylin and eosin. Each section was imaged using a digital camera coupled to an Olympus CKX41 microscope (Olympus, Tokyo, Japan).
Intestinal Gene Expression Analysis
Two broilers per replicate were randomly chosen and pooled and 4 replicates (8 birds/group, n = 4) were used for gene expression analysis. Total RNA was extracted from the small intestine (duodenum, jejunum, and ileum) using the TRIzol reagent extraction method (Thermo Fisher Scientific) and reverse-transcribed using an iScript cDNA Synthesis kit (Bio-Rad, Hercules, CA). The expression of genes (SOD, MUC2, and interleukin-6 [IL-6]) was measured on a CFX connect PCR detection system (Bio-Rad) using an iQ SYBR Green Supermix kit (Bio-Rad). 18S rRNA was used for normalization. The specific oligonucleotide primers were as follows: SOD, forward: 5′-AGGGGGTCATCCACTTCC-3′, reverse: 5′-CCCATTTGTGTTGTCTCCAA-3′; MUC2, forward: 5′-GCCTGCCCAGGAAATCAAG-3′, reverse: 5′-CGACAAGTTTGCTGGCACAT-3′; IL-6, forward: 5′-AGGACGAGATGTGCAAGAAGTTC-3′, reverse: 5′-TTGGGCAGGTTGAGGTTGTT-3′; and 18S rRNA, forward: 5′-ATAACGAACGAGACTCTGGCA-3′, reverse: 5′-CGGACATCTAAGGGCATCACA-3′. The threshold cycle (Ct) values were obtained, and the relative gene expression was calculated using the following formula: 2−ΔΔCt.
Chromatographic Analysis of Short-Chain Fatty Acids
Two broilers per replicate were randomly chosen and pooled and four replicates (8 birds/group, n = 4) were used for cecal SCFA extraction. Cecal digesta was homogenized in 10% isobutanol and centrifuged. The resulting supernatant was mixed with chloroform and NaOH. The aqueous phase of the mixture was removed and mixed with isobutanol, pyridine, and isobutyl chloroformate. After sonication, the mixture was extracted with hexane and centrifuged. The resulting supernatant was analyzed using GC–MS (Bruker GC-MS System). The SCFA measured were formic acid, acetic acid, propionic acid, butyric acid, isobutyric acid, pentanoic acid, 2-methylbutyric acid, 3-methylbutyric acid, hexanoic acid, and 4-methylpentanoic acid.
16S rRNA Gene Sequencing
Two broilers per replicate were randomly chosen and pooled and 4 replicates (8 birds/group, n = 4) were used for 16S rRNA gene sequencing. The same chicks were used for SCFA analysis and 16S rRNA gene sequencing. Microbial genomic DNA was extracted from cecal digesta using a ZymoBIOMICS DNA Miniprep Kit (Zymo Research, Irvine, CA) according to the manufacturer's instructions. The purity and integrity of total DNA were detected using a Quibt 2.0 Fluorometer (Thermo Scientific) and 1.5% agarose gel electrophoresis, respectively. The V3–V4 hypervariable regions of the 16S rRNA gene were amplified using 341F-805R primers and were sequenced on a HiSeq 2500 system (Illumina, San Diego, CA). QIIME software (version 1.9.0) was used for data quality control, which involved filtering out the primers, barcodes, and low-quality sequences. FLASH software (version 1.2.11) was used to merge high-quality paired-end reads into tags, and USEARCH software (version 10.0) was used to cluster the tags into operational taxonomic units at 97% sequence similarity. UniFrac distance metrics and standard multivariate statistics were used for principal component analysis (PCA) and principal coordinate analysis (PCoA) conducted using QIIME software. The R package corrplot (version 0.84) was used conducting for Pearson correlation analysis.
Statistical Analysis
Individual cages were considered as replicates that were defined as the experimental units. The differences among the dietary treatment groups were analyzed through one-way ANOVA followed by Tukey's honestly significant difference test conducted using SAS software (version 9.4, 2012; SAS Institute, Cary, NC). Two-way ANOVA was conducted to evaluate the main effects of SYNs and EOs and their interactions (SYNs × EOs). A P value of ≤0.05 indicated a significant difference. PCoA was conducted using UniFrac distance metrics coupled with standard multivariate statistics.
RESULTS
Effect of SYNs and EOs on the Growth Performance and Gut Morphology of Broilers
The effect of SYNs and EOs on the growth performance of the broilers is summarized in Table 2. No dead birds were observed during the experimental period. No significant intergroup differences in body weight or the feed conversion ratio were identified during the experimental period. The SYN + EO treatment resulted in a higher (P = 0.003) average daily gain at 1 to 14 d of age than did the control and EO treatments. SYNs had a significant effect on the average daily gain at 1 to 14 d (P < 0.001) and 1 to 35 d (P = 0.045) of age. The average daily feed intake at 1 to 14 d of age in the SYN+EO group was higher (P = 0.011) than that in the EO group. SYNs had a significant effect (P = 0.002) on the average daily feed intake at 1 to 14 d of age. The effect of SYNs and EOs on the gut morphology of the broilers is summarized in Table 3. The average crypt depths in the duodenum (P = 0.01) and ileum (P = 0.024) of the broilers in the A group were higher than those in the control group. The average ratio of the villus height to the crypt depth in the ileum in the A group was lower (P = 0.025) than that in the control group. EOs had a significant effect on the villus height (P = 0.015) and crypt depth (P = 0.025) in the duodenum and the villus height (P = 0.027) in the jejunum. SYNs had a significant effect on the crypt depth (P = 0.011) and the villus height to crypt depth ratio (P = 0.033) in the duodenum. A significant interaction effect (P = 0.023) between SYNs and EOs on the ileal villus height was observed.
Table 2.
Effect of Bacillus species–fermented products and essential oils on growth performance of broilers.
|
P value |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| C1 | A | SYN | EO | SYN+ EO | SEM | P value | SYN | EO | SYN × EO | |
| Body weight (g/bird) | ||||||||||
| 1 d | 46.1 | 46.2 | 47.3 | 47.3 | 47.2 | 0.39 | 0.753 | 0.570 | 0.581 | 0.560 |
| 35 d | 1,896.5 | 1,966.7 | 1,974.7 | 1,936.8 | 1,973.9 | 15.18 | 0.446 | 0.113 | 0.574 | 0.559 |
| Average daily gain (g/d/bird) | ||||||||||
| 1–14 d | 25.3b | 26.8ab | 27.1ab | 24.8b | 28.2a | 0.34 | 0.003 | < 0.001 | 0.614 | 0.198 |
| 15–35 d | 71.2 | 73.6 | 73.7 | 73.3 | 72.9 | 0.68 | 0.808 | 0.530 | 0.693 | 0.397 |
| 1–35 d | 48.3 | 50.2 | 50.4 | 49.1 | 50.6 | 0.38 | 0.262 | 0.045 | 0.570 | 0.707 |
| Average daily feed intake (g/d/bird) | ||||||||||
| 1–14 d | 31.1ab | 32.9ab | 32.5ab | 30.4b | 33.9a | 0.37 | 0.011 | 0.002 | 0.607 | 0.122 |
| 15–35 d | 102.6 | 98.9 | 101.4 | 105.5 | 102.3 | 1.78 | 0.869 | 0.462 | 0.540 | 0.738 |
| 1–35 d | 66.9 | 65.9 | 66.9 | 67.9 | 68.1 | 0.91 | 0.956 | 0.950 | 0.496 | 0.982 |
| Feed conversion ratio | ||||||||||
| 1–14 d | 1.2 | 1.2 | 1.2 | 1.2 | 1.2 | 0.01 | 0.430 | 0.316 | 0.365 | 0.985 |
| 15–35 d | 1.4 | 1.3 | 1.4 | 1.4 | 1.4 | 0.03 | 0.891 | 0.344 | 0.908 | 0.864 |
| 1–35 d | 1.3 | 1.3 | 1.3 | 1.3 | 1.3 | 0.02 | 0.897 | 0.227 | 0.811 | 0.873 |
Means in the row without common superscripts are significantly different (P ≤ 0.05).
C = Basal diet; A = Basal diet plus enramycin (10 mg/kg); SYN = Basal diet plus Bacillus species–fermented products; EO = Basal diet plus essential oils; SYN+EO = Basal diet plus Bacillus species–fermented products and essential oils.
Table 3.
Effect of Bacillus species–fermented products and essential oils on gut morphology of broilers.
|
P value |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| C1 | A | SYN | EO | SYN+ EO | SEM | P value | SYN | EO | SYN × EO | ||
| Villus height (μm) | 1,312.4 | 1,340.6 | 1,383.6 | 1,521.9 | 1,448.3 | 27.09 | 0.083 | 0.981 | 0.015 | 0.167 | |
| Duodenum | Crypt depth (μm) | 173.4b | 270.6a | 208.3ab | 202.5ab | 251.1ab | 10.42 | 0.010 | 0.011 | 0.025 | 0.644 |
| Villus length: crypt depth | 7.9 | 5.2 | 6.8 | 7.7 | 5.8 | 0.33 | 0.051 | 0.033 | 0.358 | 0.539 | |
| Villus height (μm) | 982.2 | 1,094.4 | 1055.0 | 1,107.2 | 1,145.6 | 21.51 | 0.147 | 0.229 | 0.027 | 0.703 | |
| Jejunum | Crypt depth (μm) | 124.2 | 171.1 | 165.0 | 138.3 | 159.4 | 8.39 | 0.373 | 0.117 | 0.821 | 0.606 |
| Villus length: crypt depth | 8.0 | 6.6 | 7.2 | 8.12 | 7.4 | 0.31 | 0.583 | 0.354 | 0.836 | 0.985 | |
| Villus height (μm) | 957.2 | 847.8 | 834.4 | 905.4 | 984.4 | 19.95 | 0.052 | 0.593 | 0.238 | 0.023 | |
| Ileum | Crypt depth (μm) | 147.8b | 194.4a | 146.1b | 162.4ab | 166.1ab | 5.51 | 0.024 | 0.928 | 0.149 | 0.815 |
| Villus length: crypt depth | 6.6a | 4.4b | 5.8ab | 5.8ab | 5.9ab | 0.23 | 0.025 | 0.538 | 0.475 | 0.326 | |
Means in the same row without common superscripts are significantly different (P ≤ 0.05).
C = Basal diet; A = Basal diet plus enramycin (10 mg/kg); SYN = Basal diet plus Bacillus species–fermented products; EO = Basal diet plus essential oils; SYN+EO = Basal diet plus Bacillus species–fermented products and essential oils.
Effect of SYNs and EOs on the Intestinal Gene Expression and Cecal SCFA Levels of Broilers
The effect of SYNs and EOs on the intestinal gene expression of the broilers is summarized in Table 4. The duodenum SOD mRNA levels in the SYN + EO group were higher (P < 0.001) than those in the other groups. SYNs alone or in combination with EOs resulted in higher (P < 0.001) MUC2 mRNA expression in the duodenum than did the control treatment. All the treatments downregulated (P < 0.001) IL-6 mRNA expression in the duodenum. SOD and MUC2 mRNA expression levels in the jejunum of the broilers in the SYN, EO, and SYN + EO groups were higher (P < 0.001) than those in the control group. IL-6 mRNA expression in the jejunum in the A group was lower (P = 0.001) than that in the control group. SOD and MUC2 mRNA expression levels in the ileum of the broilers in the SYN + OE group were higher (P < 0.001) than those in the other groups. SYNs and EOs had a significant effect (P < 0.05) on SOD and MUC2 mRNA expression in the duodenum, jejunum, and ileum. Significant interaction effects (P < 0.05) between SYNs and EOs on SOD and IL-6 mRNA expression in the duodenum and SOD and MUC2 mRNA expression in the jejunum and ileum were observed. The effect of SYNs and EOs on the cecal SCFA levels of the broilers is summarized in Table 5. No significant intergroup differences were found in formic acid, acetic acid, propionic acid, pentanoic acid, hexanoic acid, and 4-methylpentanoic acid levels. Butyric acid levels in cecal digesta in the enramycin group were higher (P = 0.05) than those in the control group. Cecal isobutyric acid levels in the A group were lower (P = 0.019) than those in the SYN + EO group. The levels of 2-methylbutyric acid and 3-methylbutyric acid in cecal digesta in the SYN + EO group were higher (P < 0.001) than those in the control, A, and SYN groups. EOs had a significant effect on isobutyric acid (P = 0.028), 2-methylbutyric acid (P < 0.001), and 3-methylbutyric acid (P < 0.001) levels in cecal digesta; SYNs had a significant effect (P = 0.028) on 3-methylbutyric acid levels in cecal digesta.
Table 4.
Effect of Bacillus species–fermented products and essential oils on antioxidant and tight-junction gene expression in small intestines of broilers.
|
P value |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| C1 | A | SYN | EO | SYN+ EO | SEM | P value | SYN | EO | SYN × EO | ||
| SOD | 1.0b | 2.3b | 2.6b | 2.4b | 10.1a | 0.77 | < 0.001 | < 0.001 | < 0.001 | < 0.001 | |
| Duodenum | MUC2 | 1.0c | 1.8bc | 2.4b | 1.6bc | 3.5a | 0.21 | < 0.001 | < 0.001 | 0.002 | 0.270 |
| IL-6 | 1.0a | 0.5bc | 0.6b | 0.5bc | 0.4c | 0.06 | < 0.001 | < 0.001 | < 0.001 | 0.002 | |
| SOD | 0.9d | 1.2cd | 1.6bc | 2.0b | 4.9a | 0.39 | < 0.001 | < 0.001 | < 0.001 | < 0.001 | |
| Jejunum | MUC2 | 1.0c | 1.3bc | 1.6b | 1.6b | 4.5a | 0.30 | < 0.001 | < 0.001 | < 0.001 | < 0.001 |
| IL-6 | 1.0a | 0.5b | 0.8ab | 0.9a | 0.8ab | 0.05 | 0.001 | 0.089 | 0.727 | 0.503 | |
| SOD | 1.0b | 1.6b | 1.4b | 1.5b | 4.0a | 0.30 | < 0.001 | 0.002 | 0.002 | 0.014 | |
| Ileum | MUC2 | 1.0b | 1.8b | 1.1b | 1.6b | 3.6a | 0.27 | < 0.001 | 0.015 | 0.002 | 0.023 |
| IL-6 | 1.0 | 1.2 | 1.1 | 1.0 | 0.3 | 0.11 | 0.053 | 0.224 | 0.077 | 0.081 | |
Means in the same row without common superscripts are significantly different (P ≤ 0.05).
C = Basal diet; A = Basal diet plus enramycin (10 mg/kg); SYN = Basal diet plus Bacillus species–fermented products; EO = Basal diet plus essential oils; SYN+EO = Basal diet plus Bacillus species–fermented products and essential oils.
Table 5.
Effect of Bacillus species–fermented products and essential oils on cecal short-chain fatty acid levels of broilers.
|
P value |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| C1 | A | SYN | EO | SYN+ EO | SEM | P value | SYN | EO | SYN × EO | |
| Formic acid (μM) | 0 | 0.8 | 5.2 | 1.7 | 23.4 | 3.19 | 0.091 | 0.078 | 0.180 | 0.262 |
| Acetic acid (μM) | 3,064.9 | 1,184.5 | 2,715.6 | 2,208.7 | 2,527.2 | 277.10 | 0.261 | 0.981 | 0.437 | 0.617 |
| Propionic acid (μM) | 573.4 | 212.3 | 508.3 | 461.7 | 422.3 | 61.52 | 0.444 | 0.738 | 0.529 | 0.934 |
| Butyric acid (μM) | 646.8a | 89.1b | 426.6ab | 284.2ab | 234.8ab | 64.87 | 0.050 | 0.334 | 0.061 | 0.535 |
| Isobutyric acid (μM) | 49.2ab | 20.4b | 38.3ab | 74.1ab | 85.7a | 7.52 | 0.019 | 0.980 | 0.028 | 0.450 |
| Pentanoic acid (μM) | 78.7 | 28.3 | 21.3 | 32.4 | 49.2 | 3.66 | 0.111 | 0.618 | 0.957 | 0.151 |
| 2-methylbutyric acid (μM) | 18.6bc | 8.3c | 21.3bc | 32.4ab | 49.2a | 3.66 | < 0.001 | 0.054 | < 0.001 | 0.150 |
| 3-methylbutyric acid (μM) | 14.9bc | 11.0c | 18.7bc | 27.9ab | 41.4a | 2.81 | < 0.001 | 0.028 | < 0.001 | 0.181 |
| Hexanoic acid (μM) | 4.7 | 2.9 | 5.7 | 4.4 | 4.5 | 0.39 | 0.300 | 0.550 | 0.391 | 0.651 |
| 4-methylpentanoic acid (μM) | 0.8 | 0.3 | 0.7 | 0.9 | 0.8 | 0.09 | 0.201 | 0.662 | 0.675 | 0.903 |
Means in the same row without common superscripts are significantly different (P ≤ 0.05).
C = Basal diet; A = Basal diet plus enramycin (10 mg/kg); SYN = Basal diet plus Bacillus species–fermented products; EO = Basal diet plus essential oils; SYN+EO = Basal diet plus Bacillus species–fermented products and essential oils.
Effect of SYNs and EOs on Cecal Microbiota
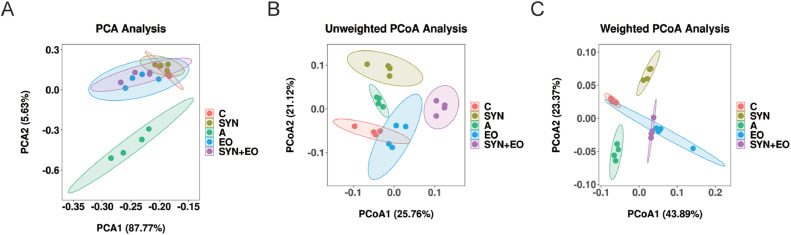
The effects of SYNs and EOs on the cecal microbial diversity of the broilers are summarized in Table 6. Cecal species richness (Fisher alpha) in the SYN group was higher (P = 0.016) than that in the A and EO groups. Cecal species evenness (Shannon and Enspie alpha) in the SYN + EO group was higher (P < 0.001) than that in the control group. SYNs had a significant effect on cecal species richness (Chao1 and Fisher alpha; P = 0.005 and P = 0.01, respectively) and evenness (Shannon and Enspie alpha; P < 0.001 and P = 0.004, respectively) in cecal digesta. EOs had a significant effect on cecal species richness (Fisher alpha; P = 0.031) and evenness (Shannon and Enspie alpha; P < 0.001 and P < 0.001, respectively) in cecal digesta. A significant interaction effect (Enspie alpha; P < 0.001) between SYNs and EOs on species evenness in cecal digesta was observed. PCA revealed the formation of a bacterial cluster in the enramycin group that was distinct from those observed in the other treatment groups (Figure 1A). Unweighted PCoA (qualitative traits) and weighted PCoA (quantitative traits) revealed significant intergroup differences in the bacterial community composition in cecal digesta (Figures 1B and 1C).
Table 6.
Effect of Bacillus species–fermented products and essential oils on cecal microbial diversity of broilers.
|
P value |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| C1 | A | SYN | EO | SYN+ EO | SEM | P value | SYN | EO | SYN × EO | |
| Chao1 | 111.3 | 111.5 | 119.0 | 109.0 | 117.0 | 1.29 | 0.051 | 0.005 | 0.373 | 0.958 |
| Fisher alpha | 13.2ab | 12.7b | 14.3a | 12.5b | 13.4ab | 0.20 | 0.016 | 0.010 | 0.031 | 0.918 |
| Shannon | 4.4c | 4.5b | 4.6ab | 4.6ab | 4.6a | 0.02 | < 0.001 | < 0.001 | 0.001 | < 0.001 |
| Enspie | 11.4b | 13.2a | 13.6a | 14.1a | 13.6a | 0.23 | < 0.001 | 0.004 | < 0.001 | < 0.001 |
Means in the same row without common superscripts are significantly different (P ≤ 0.05).
C = Basal diet; A = Basal diet plus enramycin (10 mg/kg); SYN = Basal diet plus Bacillus species–fermented products; EO = Basal diet plus essential oils; SYN+EO = Basal diet plus Bacillus species–fermented products and essential oils.
Figure 1.
Advanced analysis of bacterial communities in cecal digesta. (A) Principal component analysis of cecal digesta of broilers receiving basal diet without treatment (C), basal diet plus enramycin (A), basal diet plus Bacillus species–fermented products (SYN), basal diet plus essential oils (EO), and basal diet plus Bacillus species–fermented products and essential oils (SYN+EO; n = 4). Principal coordinate analysis of quantitative traits (unweighted UniFrac distances) (B), and qualitative traits (weighted UniFrac distances) (C) of cecal bacterial communities of the control, A, SYN, EO, and SYN+EO groups (n = 4).
Effects of SYNs and EOs on Cecal Bacterial Taxonomic Composition
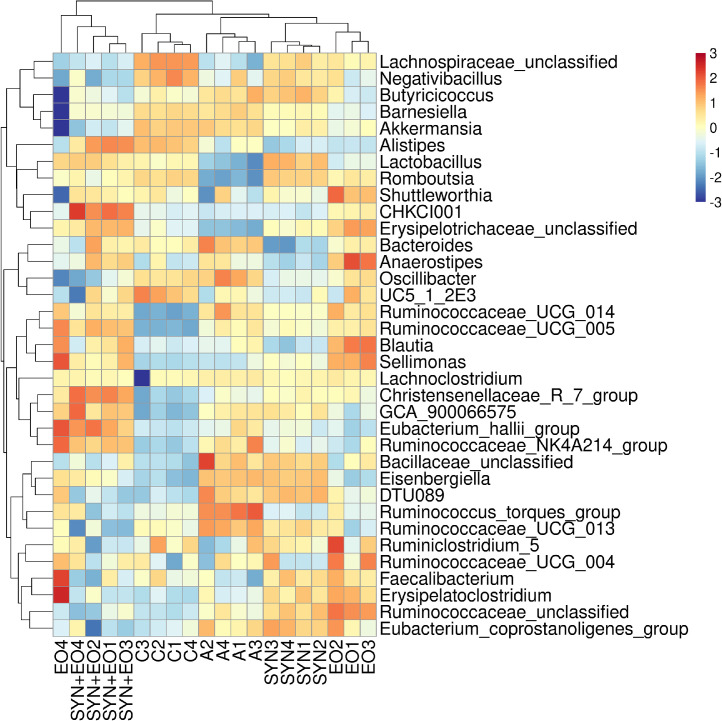
The effect of SYNs and EOs on the bacterial taxonomy in the cecal digesta of the broilers is summarized in Table 7. At the phylum level, the abundance of the phylum Firmicutes in the EO group was higher (P < 0.001) than that in the control and A groups. The abundance of the phylum Bacteroidetes in the EO group was lower (P < 0.001) than that in the control, A, and SYN + EO groups. The ratio of the phyla Firmicutes to Bacteroidetes in the EO group was higher (P = 0.001) than that in the control, A, and SYN + EO groups. EOs had a significant effect on the abundance of the phyla Firmicutes (P < 0.001) and Bacteroidetes (P = 0.01) and the ratio of the phyla Firmicutes to Bacteroidetes (P = 0.05). Significant interaction effects between SYNs and EOs on the abundance of the phyla Firmicutes (P < 0.001) and Bacteroidetes (P < 0.001) and the ratio of the phyla Firmicutes to Bacteroidetes (P = 0.002) were observed. At the genus level, the abundance of the unclassified Lachnospiraceae, Lactobacillus, and Romboutsia genera in the enramycin group was lower (P < 0.001) than that in the control, SYN, and EO groups. The abundance of the Barnesiella genus in the EO group was lower (P < 0.001) than that in the control, enramycin, and SYN-treated groups. The abundance of the Alistipes genus in the control and SYN+EO groups was higher (P < 0.001) than that in the other groups. The abundance of the Blautia, Faecalibacterium, and Erysipelatoclostridium genera in the EO group was higher than that in the A (P < 0.001) and SYN + EO groups (P = 0.007). The abundance of the Ruminococcus torques genus in the enramycin group was higher (P < 0.001) than that in the other groups. The abundance of the Bacteroides genus in the A group was higher (P < 0.001) than that in the SYN and EO groups. The abundance of the unclassified Ruminococcaceae genus in the SYN and EO groups was higher (P = 0.002) than that in the control and SYN + EO groups. The abundance of the Ruminococcaceae UCG 014 genus in the A and EO groups was higher (P < 0.001) than that in the control and SYN groups. The abundance of the Eubacterium hallii group genus in the SYN + EO group was higher (P = 0.02) than that in the control and SYN groups. The abundance of the Akkermansia genus in the control group was higher (P < 0.001) than that in the other groups. The abundance of the Anaerostipes genus in the EO group was higher (P = 0.008) than that in the control and SYN groups. SYNs had a significant effect on the abundance of unclassified Lachnospiraceae (P = 0.002), Lactobacillus (P < 0.001), Blautia (P = 0.004), and Akkermansia (P < 0.001). EOs had a significant effect on the abundance of unclassified Lachnospiraceae (P < 0.001), Barnesiella (P < 0.001), Blautia (P < 0.001), Bacteroides (P = 0.039), Ruminococcaceae UCG 014 (P < 0.001), Romboutsia (P < 0.001), Eubacterium hallii group (P = 0.011), Akkermansia (P < 0.001), and Anaerostipes (P = 0.002). Significant interaction effects between SYNs and EOs on the abundance of Barnesiella (P = 0.002), Alistipes (P < 0.001), Blautia (P = 0.025), Bacteroides (P = 0.012), unclassified Ruminococcaceae (P < 0.001), Faecalibacterium (P = 0.007), Erysipelatoclostridium (P = 0.007), Ruminococcaceae UCG 014 (P < 0.001), Romboutsia (P = 0.039), and Akkermansia (P < 0.001) were observed. Figure 2 presents an overview of the genus-level taxonomy and a heat map of the 35 most abundant genera in cecal digesta. Specific microbial community clusters, such as the Ruminococcus torques group and Ruminococcaceae UCG 013 genera, were identified in the A group. Microbial community clusters, such as unclassified Bacillaceae, Eisenbergiella, and the Clostridiales bacterium DTU089, partially overlapped between the A and SYN groups. Similar microbial community clusters, such as Faecalibacterium, Erysipelatoclostridium, and unclassified Ruminococcaceae, were identified in the SYN and EO groups. Specific microbial community clusters, including the Clostridiales bacterium CHKCI001, Christensenellaceae R-7 group, GCA 900066575, and Eubacterium hallii group, were identified only in the SYN + EO group.
Table 7.
Effect of Bacillus species–fermented products and essential oils on taxonomic assignment and ranking of cecal bacteria in broilers.
|
P value |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| C1 | A | SYN | EO | SYN+ EO | SEM | P value | SYN | EO | SYN × EO | |
| Phylum | ||||||||||
| Firmicutes | 67.9c | 68.9c | 78.2ab | 81.4a | 75.3ab | 1.27 | < 0.001 | 0.104 | < 0.001 | < 0.001 |
| Bacteroidetes | 27.9a | 27.6a | 20.2bc | 17.6c | 23.9ab | 1.00 | < 0.001 | 0.498 | 0.010 | < 0.001 |
| Firmicutes: Bacteroidetes | 2.4b | 2.5b | 3.9ab | 4.8a | 3.2b | 0.25 | 0.001 | 0.787 | 0.050 | 0.002 |
| Genus | ||||||||||
| Lachnospiraceae_unclassified | 21.4a | 15.0d | 19.1ab | 17.3bc | 15.5cd | 0.58 | < 0.001 | 0.002 | < 0.001 | 0.634 |
| Lactobacillus | 11.9bc | 5.6d | 17.7a | 10.7c | 14.8ab | 1.00 | < 0.001 | < 0.001 | 0.064 | 0.404 |
| Barnesiella | 13.6a | 13.5a | 10.4ab | 6.2c | 9.0bc | 0.70 | < 0.001 | 0.821 | < 0.001 | 0.002 |
| Alistipes | 10.5a | 8.8b | 8.0b | 8.0b | 10.9a | 0.30 | < 0.001 | 0.475 | 0.360 | < 0.001 |
| Blautia | 4.4bc | 5.5bc | 3.6c | 11.9a | 7.2b | 0.73 | < 0.001 | 0.004 | < 0.001 | 0.025 |
| Ruminococcus_torques_group | 4.3b | 13.2a | 3.4b | 5.3b | 3.7b | 0.88 | < 0.001 | 0.068 | 0.312 | 0.629 |
| Bacteroides | 3.6ab | 5.2a | 1.7c | 3.4bc | 3.9ab | 0.30 | < 0.001 | 0.094 | 0.039 | 0.012 |
| Ruminococcaceae_unclassified | 2.7b | 3.3ab | 3.9a | 4.1a | 2.8b | 0.16 | 0.002 | 0.761 | 0.574 | < 0.001 |
| Faecalibacterium | 3.2ab | 2.4b | 3.9ab | 4.7a | 2.5b | 0.26 | 0.007 | 0.114 | 0.972 | 0.007 |
| Erysipelatoclostridium | 2.7ab | 3.0b | 3.6ab | 4.2a | 2.8b | 0.15 | 0.007 | 0.114 | 0.972 | 0.007 |
| Ruminococcaceae_UCG_014 | 0.8c | 4.1a | 2.4bc | 4.1a | 3.0ab | 0.32 | < 0.001 | 0.378 | < 0.001 | < 0.001 |
| Romboutsia | 3.6a | 0.1c | 4.3a | 1.8b | 1.3b | 0.36 | < 0.001 | 0.551 | < 0.001 | 0.039 |
| Eubacterium_hallii_group | 1.3b | 1.6ab | 1.4b | 1.6ab | 2.3a | 0.11 | 0.020 | 0.074 | 0.011 | 0.247 |
| Akkermansia | 3.7a | 2.9b | 1.3b | 0.6b | 0.3b | 0.32 | < 0.001 | < 0.001 | < 0.001 | < 0.001 |
| Anaerostipes | 1.0b | 1.3ab | 0.9b | 2.0a | 1.6ab | 0.13 | 0.008 | 0.204 | 0.002 | 0.597 |
C = Basal diet; A = Basal diet plus enramycin (10 mg/kg); SYN = Basal diet plus Bacillus species–fermented products; EO = Basal diet plus essential oils; SYN+EO = Basal diet plus Bacillus species–fermented products and essential oils.
Means in the same row without common superscripts are significantly different (P ≤ 0.05).
Figure 2.
Taxonomic composition analysis of cecal digesta. Heatmap indicating the dominant 35 genera (y-axis) across different treatment groups (x-axis): basal diet without treatment (C), basal diet plus enramycin, (A), basal diet plus Bacillus species–fermented products (SYN), basal diet plus essential oils (EO), and basal diet plus Bacillus species–fermented products and essential oils (SYN+EO); n = 4.
Correlations Among Dominant Microbial Genera, SCFA Levels, and Gut Morphology
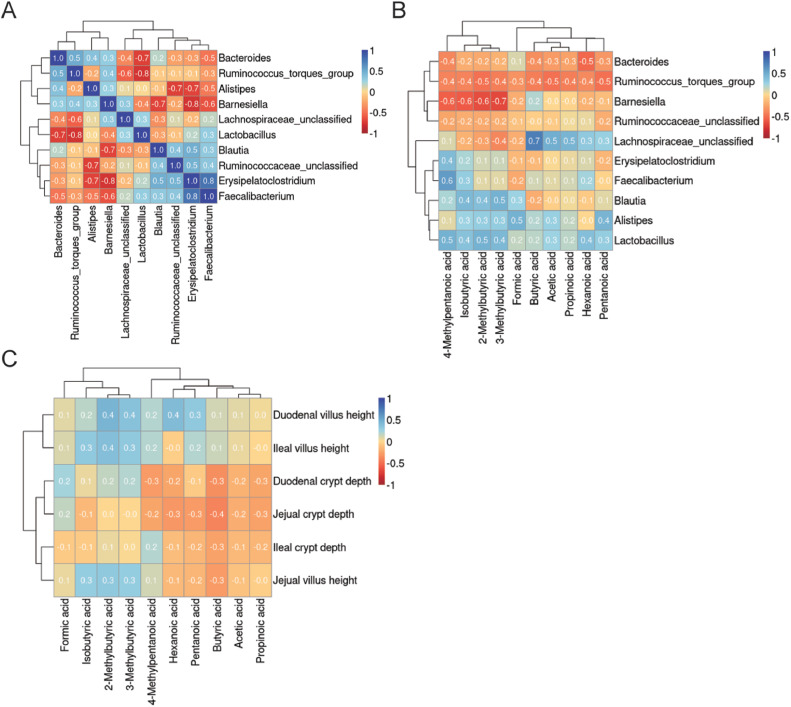
The abundance of the Bacteroides genus was positively associated with the abundance of the Barnesiella, Alistipes, and Ruminococcus torques group genera (Figure 3A). The abundance of the Lactobacillus genus was negatively correlated with the abundance of the Barnesiella, Ruminococcus torques group, and Bacteroides genera (Figure 3A). The abundance of the Blautia, unclassified Ruminococcaceae, Erysipelatoclostridium, and Faecalibacterium genera was positively associated (Figure 3A). The abundance of the Ruminococcus torques group genus was negatively correlated with all SCFA levels (Figure 3B). By contrast, the abundance of the Lactobacillus genus was positively associated with all SCFA levels (Figure 3B). The abundance of the unclassified Lachnospiraceae genus was positively correlated with butyric acid, acetic acid, propionic acid, hexanoic acid, and pentanoic acid but negatively associated with isobutyric acid, 2-methylbutyric acid, 3-methylbutyric acid, and formic acid (Figure 3B). Isobutyric acid, 2-methylbutyric acid, and 3-methylbutyric acid levels were positively correlated with the intestinal villus height (duodenum, jejunum, and ileum; Figure 3C). Hexanoic acid, pentanoic acid, butyric acid, acetic acid, and propionic acid levels were negatively associated with the intestinal crypt depth (duodenum, jejunum, and ileum; Figure 3C).
Figure 3.
Correlation analysis of cecal microbiota. (A) Correlations among dominant microbial genera. (B) Correlations among dominant microbial genera and short-chain fatty acids. (C) Correlations among short-chain fatty acids and gut morphology. Positive correlations are displayed in blue; negative correlations are displayed in red. The values from +1 to −1 indicate the strength of the association.
DISCUSSION
Bacillus species are the most commonly used probiotic microorganisms in animal production. Dietary supplementation with B. subtilis or B. licheniformis can enhance growth performance and mitigate C. perfringens-induced necrotic enteritis in broilers (Knap et al., 2010; Abudabos et al., 2013; Gong et al., 2018; Hussein et al., 2020; Mohamed et al., 2022). Both B. subtilis and B. licheniformis show some similarities with antibiotic growth promoters in terms of improving growth performance in broilers (Musa et al., 2019). Dietary supplementation with B. licheniformis is more effective than dietary supplementation with the same amount of B. subtilis (Xu et al., 2021). Our previous studies have demonstrated that B. subtilis– or B. licheniformis–fermented products that contain Bacillus spores can modulate immunity and gut microbiota, leading to improved health and growth performance in broilers (Cheng et al., 2018; Chen and Yu, 2020; Cheng et al., 2021a,b). Combined supplementation with B. subtilis and B. licheniformis does not affect the growth performance of broilers (Mutuş et al., 2006) but can improve the feed conversion ratio of broilers (Midilli et al., 2008). In the present study, dietary supplementation with SYNs containing the same amounts of B. subtilis and B. licheniformis spores increased the average daily gain and feed intake in the broilers. These findings are in partial agreement with the results of Midilli et al. (2008), who observed that simultaneous supplementation with B. subtilis and B. licheniformis improved the feed conversion ratio of broilers. SYNs contain not only B. subtilis and B. licheniformis spores but also Bacillus species–derived antimicrobial peptides. Therefore, the mechanisms underlying the growth performance improvement associated with B. subtilis– and B. licheniformis–fermented products and their spores separately may differ. Recent studies have reported that EOs can improve growth performance, immunity, and intestinal morphology (Hesabi Nameghi et al., 2019; Mohebodini et al., 2021; Abd El-Hack et al., 2022). In the present study, EOs alone or in combination with SYNs improved the intestinal villus height of the broilers but did not affect their growth performance. These findings are in partial agreement with the results of Irawan et al. (2021), who also observed that a positive effect of EOs inclusion is detected on increasing intestinal villus height of the broilers. Bioactive compounds in EOs play a key role in biological functions, and the chemical composition of EOs depends on the plants from which they are extracted. The major bioactive compounds of the EO blend used in the present study were cymene and thymol. A meta-analysis indicates that thymol-based EOs have a positive effect on feed conversion ratio, but negative effects on body weight and average weight gain in broilers (Irawan et al., 2021). Little is known about the effect of cymene- and thymol-based EOs on the growth performance of broilers, and the effect of dietary supplementation with cymene- and thymol-based EOs on broilers warrants further investigation. Taken together, our findings demonstrated that supplementation with SYNs can improve the average daily gain and feed intake of broilers.
Our previous studies have demonstrated that supplementation with B. licheniformis–fermented products reduces bacterial diversity (richness and evenness) in the feces of broilers (Chen and Yu, 2020). Broilers fed B. subtilis–fermented products exhibited reduced bacterial richness in their cecal digesta (Chen and Yu, 2021). By contrast, in the present study, combined supplementation with B. subtilis– and B. licheniformis–fermented products increased bacterial evenness in cecal digesta. These findings indicate that the individual or combined effects of B. subtilis– and B. licheniformis–fermented products may differentially regulate bacterial diversity in the gut. Different species of Bacillus produce antimicrobial peptides with different modes of antibacterial action (Sumi et al., 2015). Therefore, antimicrobial peptides derived from B. subtilis– and B. licheniformis–fermented products may interact in the gut, thereby regulating bacterial evenness in the cecal digesta of broilers. Numerous studies have reported that EOs have antibacterial and antifungal activity against poultry pathogens (Helander et al., 1998; Nazzaro et al., 2017; Abd El-Hack et al., 2022). Similar to SYNs, EOs increased bacterial evenness in cecal digesta in the present study. However, the SYN+EO treatment did not further increase bacterial evenness in the cecal digesta of the broilers. Our in vitro study demonstrated that EOs failed to inhibit the growth of B. subtilis and B. licheniformis (data not shown). These results suggest no interaction effect of SYNs and EOs on the bacterial diversity of broilers.
It has been reported that EOs can promote the abundance of the Lactobacillus genus in the cecal digesta of broilers (Irawan et al., 2021). SYNs (B. licheniformis spores, mannan-oligosaccharides, and β-glucans) in combination with capsaicin and curcumin-based EOs also increase the abundance of the Lactobacillus genus in the ileal mucosa of broilers during subclinical necrotic enteritis (Emami et al., 2020). In this study, some bacteria, such as those from the Lactobacillus genus, in the cecal digesta of the broilers were specifically regulated by SYNs. SYNs increased the abundance of the Lactobacillus genus, and this effect was also observed in the SYN + EO group. Dietary supplementation with Lactobacillus species is potentially beneficial for the growth performance and intestinal health of broilers (Wang et al., 2021). Our previous studies have demonstrated that SYNs can increase the abundance of the Lactobacillus genus in the feces or cecal digesta of broilers, and that the abundance of the Lactobacillus genus is positively correlated with growth performance (Chen and Yu, 2020; Cheng et al., 2021b). In the present study, the abundance of the Lactobacillus genus in the cecal digesta was positively correlated with SCFA levels. The abundance of the Blautia genus in the EO group was higher than that in the SYN + EO group. Blautia is a member of the family Lachnospiraceae and can produce SCFA through the degradation of complex polysaccharides for energy utilization by the host (Biddle et al., 2013). In the present study, the abundance of the Blautia genus in cecal digesta was also positively correlated with SCFA levels, particularly those of isobutyric acid, 2-methylbutyric acid, and 3-methylbutyric acid. SCFA levels in cecal digesta were positively correlated with gut morphology. These findings are in agreement with the findings of Liao et al. (2020), who observed that SCFA improve the intestinal morphology of broilers. In the present study, the SYN + EO treatment specifically increased 2-methylbutyric acid and 3-methylbutyric acid levels in the cecal digesta of the broilers. Branched-chain fatty acids, such as 2-methylbutyric acid and 3-methylbutyric acid, are formed upon the fermentation of branched amino acids (Gilbert et al., 2018). Branched-chain fatty acids exert anti-inflammatory effects on lipopolysaccharide-stimulated human enterocytes (Yan et al., 2018). In the present study, inflammatory gene expression levels in the duodenum of the broilers in the SYN, EO, and SYN+EO groups were lower than those in the control group. Taken together, these findings demonstrated that SYNs or EOs can increase the abundance of beneficial bacteria in the guts of broilers, and the abundance of these beneficial bacteria in the gut is not reduced after combined supplementation with SYN and EO.
It has been demonstrated that SYNs in combination with EOs can improve growth performance in broilers during subclinical necrotic enteritis (Emami et al., 2020). However, combined supplementation with B. subtilis and EOs cannot improve the growth performance of broilers of different ages (Fernandez-Alarcon et al., 2017). In the present study, the SYN + EO treatment resulted in a higher average daily gain for starter-phase broilers than did EOs alone. This discrepancy may be attributed to the distinct formulas (probiotics only vs. probiotics and probiotics-derived antimicrobial peptides) and concentrations (5.0 × 108 CFU B. subtilis/kg of feed vs. 3 × 1011 CFU B. subtilis and B. licheniformis/kg of feed) of probiotics and bioactive compounds of EOs (carvacrol-, cinnamaldehyde-, cineol-, and pepper extract–based vs. cymene- and thymol-based). Furthermore, small intestinal antioxidant gene and mucin gene expression in the SYN+EO group was higher than that in the SYN and EO groups. Branched-chain fatty acid levels in the SYN + EO group were higher than those in the control group. Branched-chain fatty acid levels were positively correlated with the villus height in the duodenum and ileum of the broilers. SYN + EO treatment also modulated cecal microbiota differently than did SYNs or EOs alone. Furthermore, the SYN + EO treatment exerted an effect similar to that of antibiotic growth promoters on the growth performance of the broilers. The effects of the SYN+EO treatment on intestinal gene expression, cecal SCFA levels, and cecal microbiota were superior to those of antibiotic growth promoters. Therefore, the combination of SYNs and EOs is more likely to regulate intestinal gene expression, increase gut SCFA levels, and modulate gut microbiota than are in-feed antibiotics.
In conclusion, combined supplementation with SYNs and EOs can increase the average daily gain, improve gut health–associated gene expression, increase cecal SCFA levels, and modulate the gut microbiota composition of broilers. This study provides valuable insights into how SYNs, EOs, and their combination differentially improve the health of broilers in terms of the regulation of gene expression, SCFA levels, and gut microbiota.
ACKNOWLEDGMENTS
We thank Dr. Tzu-Cheng Chang (National Ilan University, Yilan, Taiwan) for the gas chromatography-mass spectrometry support. This work was supported by the Ministry of Science and Technology [MOST 108-2313-B-197-003 and MOST 110-2313-B-197-005-MY3] in Taiwan.
DISCLOSURES
The authors have no conflicts of interest to report.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.psj.2022.101970.
Appendix. Supplementary materials
REFERENCES
- Abd El-Hack M.E., El-Saadony M.T., Saad A.M., Salem H.M., Ashry N.M., Abo Ghanima M.M., Shukry M., Swelum A.A., Taha A.E., El-Tahan A.M., AbuQamar S.F., El-Tarabily K.A. Essential oils and their nanoemulsions as green alternatives to antibiotics in poultry nutrition: a comprehensive review. Poult. Sci. 2022;101 doi: 10.1016/j.psj.2021.101584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abudabos A.M., Alyemni A.H., Al Marshad B.A. Bacillus subtilis PB6 based-probiotic (CloSTATTM) improves intestinal morphological and microbiological status of broiler chickens under Clostridium perfringens challenge. Int. J. Agric. Biol. 2013;15:978–982. [Google Scholar]
- Abudabos A.M., Alyemni A.H., Dafalla Y.M., Khan R.U. Effect of organic acid blend and Bacillus subtilis alone or in combination on growth traits, blood biochemical and antioxidant status in broilers exposed to Salmonella typhimurium challenge during the starter phase. J. Appl. Anim. Res. 2017;45:538–542. [Google Scholar]
- Adaszyńska-Skwirzyńska M., Szczerbińska D. Use of essential oils in broiler chicken production – a review. Ann. Anim. Sci. 2017;17:317–335. [Google Scholar]
- Bartkiene E., Ruzauskas M., Bartkevics V., Pugajeva I., Zavistanaviciute P., Starkute V., Zokaityte E., Lele V., Dauksiene A., Grashorn M., Hoelzle L.E., Mendybayeva A., Ryshyanova R., Gruzauskas R. Study of the antibiotic residues in poultry meat in some of the EU countries and selection of the best compositions of lactic acid bacteria and essential oils against Salmonella enterica. Poult. Sci. 2020;99:4065–4076. doi: 10.1016/j.psj.2020.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biddle A., Stewart L., Blanchard J., Leschine S. Untangling the genetic basis of fibrolytic specialization by Lachnospiraceae and Ruminococcaceae in diverse gut communities. Diversity. 2013;5:627–640. [Google Scholar]
- Chen J.Y., Yu Y.H. Bacillus subtilis-fermented products ameliorate the growth performance and alter cecal microbiota community in broilers under lipopolysaccharide challenge. Poult. Sci. 2021;100:875–886. doi: 10.1016/j.psj.2020.10.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y.C., Yu Y.H. Bacillus licheniformis-fermented products improve growth performance and the fecal microbiota community in broilers. Poult. Sci. 2020;99:1432–1443. doi: 10.1016/j.psj.2019.10.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y.H., Zhang N., Han J.C., Chang C.W., Hsiao F.S.H., Yu Y.H. Optimization of surfactin production from Bacillus subtilis in fermentation and its effects on Clostridium perfringens-induced necrotic enteritis and growth performance in broilers. J. Anim. Physiol. Anim. Nutr. 2018;102:1232–1244. doi: 10.1111/jpn.12937. [DOI] [PubMed] [Google Scholar]
- Cheng Y.H., Horng Y.B., Dybus A., Yu Y.H. Bacillus licheniformis-fermented products improve growth performance and intestinal gut morphology in broilers under Clostridium perfringens challenge. J. Poult. Sci. 2021;58:30–39. doi: 10.2141/jpsa.0200010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y.H., Horng Y.B., Chen W.J., Hua K.F., Dybus A., Yu Y.H. Effect of fermented products produced by Bacillus licheniformis on the growth performance and cecal microbial community of broilers under coccidial challenge. Animals. 2021;11:1245. doi: 10.3390/ani11051245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emami N.K., Calik A., White M.B., Kimminau E.A., Dalloul R.A. Effect of probiotics and multi-component feed additives on microbiota, gut barrier and immune responses in broiler chickens during subclinical necrotic Enteritis. Front. Vet. Sci. 2020;7 doi: 10.3389/fvets.2020.572142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Alarcon M.F., Trottier N., Steibel J.P., Lunedo R., Campos D.M.B., Santana A.M., Jr Pizauro J.M., Furlan R.L., Furlan L.R. Interference of age and supplementation of direct-fed microbial and essential oil in the activity of digestive enzymes and expression of genes related to transport and digestion of carbohydrates and proteins in the small intestine of broilers. Poult. Sci. 2017;96:2920–2930. doi: 10.3382/ps/pex039. [DOI] [PubMed] [Google Scholar]
- Giannenas I., Florou-Paneri P., Papazahariadou M., Christaki E., Botsolglou N.A., Spais A.B. Effect of dietary supplementation with oregano essential oil on performance of broilers after experimental infection with Eimeria tenella. Arch. Anim. Nutr. 2003;57:99–106. doi: 10.1080/0003942031000107299. [DOI] [PubMed] [Google Scholar]
- Gilbert M.S., Ijssennagger N., Kies A.K., van Mil S.W.C. Protein fermentation in the gut; implications for intestinal dysfunction in humans, pigs, and poultry. Am. J. Physiol. Gastrointest. Liver Physiol. 2018;315:G159–G170. doi: 10.1152/ajpgi.00319.2017. [DOI] [PubMed] [Google Scholar]
- Gong L., Wang B., Mei X., Xu H., Qin Y., Li W., Zhou Y. Effects of three probiotic Bacillus on growth performance, digestive enzyme activities, antioxidative capacity, serum immunity, and biochemical parameters in broilers. Anim. Sci. J. 2018;89:1561–1571. doi: 10.1111/asj.13089. [DOI] [PubMed] [Google Scholar]
- Grant A., Gay C.G., Lillehoj H.S. Bacillus spp. as direct-fed microbial antibiotic alternatives to enhance growth, immunity, and gut health in poultry. Avian Pathol. 2018;47:339–351. doi: 10.1080/03079457.2018.1464117. [DOI] [PubMed] [Google Scholar]
- Helander I.M., Alakomi H.L., Latva-Kala K., Mattila-Sandholm T., Pol I., Smid E.J., Gorris L.G.M., von Wright A. Characterization of the action of selected essential oil components on gram-negative bacteria. J. Agric. Food Chem. 1998;46:3590–3595. [Google Scholar]
- Hesabi Nameghi A., Edalatian O., Bakhshalinejad R. Effects of a blend of thyme, peppermint and eucalyptus essential oils on growth performance, serum lipid and hepatic enzyme indices, immune response and ileal morphology and microflora in broilers. J. Anim. Physiol. Anim. Nutr. 2019;103:1388–1398. doi: 10.1111/jpn.13122. [DOI] [PubMed] [Google Scholar]
- Horng Y.B., Yu Y.H., Dybus A., Hsiao F.S.H., Cheng Y.H. Antibacterial activity of Bacillus species-derived surfactin on Brachyspira hyodysenteriae and Clostridium perfringens. AMB Express. 2019;9:188. doi: 10.1186/s13568-019-0914-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussein E.O.S., Ahmed S.H., Abudabos A.M., Suliman G.M., Abd El-Hack M.E., Swelum A.A., Alowaimer A.N. Ameliorative effects of antibiotic-, probiotic- and phytobiotic-supplemented diets on the performance, intestinal health, carcass traits, and meat quality of Clostridium perfringens-infected broilers. Animals. 2020;10 doi: 10.3390/ani10040669. pii:E669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irawan A., Hidayat C., Jayanegara A., Ratriyanto A. Essential oils as growth-promoting additives on performance, nutrient digestibility, cecal microbes, and serum metabolites of broiler chickens: a meta-analysis. Anim. Biosci. 2021;34:1499–1513. doi: 10.5713/ab.20.0668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khattak F., Ronchi A., Castelli P., Sparks N. Effects of natural blend of essential oil on growth performance, blood biochemistry, cecal morphology, and carcass quality of broiler chickens. Poult. Sci. 2014;93:132–137. doi: 10.3382/ps.2013-03387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knap I., Lund B., Kehlet A.B., Hofacre C., Mathis G. Bacillus licheniformis prevents necrotic enteritis in broiler chickens. Avian Dis. 2010;54:931–935. doi: 10.1637/9106-101509-ResNote.1. [DOI] [PubMed] [Google Scholar]
- Liao X., Shao Y., Sun G., Yang Y., Zhang L., Guo Y., Luo X., Lu L. The relationship among gut microbiota, short-chain fatty acids, and intestinal morphology of growing and healthy broilers. Poult. Sci. 2020;99:5883–5895. doi: 10.1016/j.psj.2020.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall B.M., Levy S.B. Food animals and antimicrobials: impacts on human health. Clin. Microbiol. Rev. 2011;24:718–733. doi: 10.1128/CMR.00002-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Midilli M., Alp M., Kocabağli N., Muğlali Ö.H., Turan N., Yılmaz H., Çakir S. Effects of dietary probiotic and prebiotic supplementation on growth performance and serum IgG concentration of broilers. S. Afr. J. Anim. Sci. 2008;38:21–27. [Google Scholar]
- Mohamed T.M., Sun W., Bumbie G.Z., Elokil A.A., Mohammed K.A.F., Zebin R., Hu P., Wu L., Tang Z. Feeding Bacillus subtilis ATCC19659 to broiler chickens enhances growth performance and immune function by modulating intestinal morphology and cecum microbiota. Front. Microbiol. 2022;12 doi: 10.3389/fmicb.2021.798350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohebodini H., Jazi V., Ashayerizadeh A., Toghyani M., Tellez-Isaias G. Productive parameters, cecal microflora, nutrient digestibility, antioxidant status, and thigh muscle fatty acid profile in broiler chickens fed with Eucalyptus globulus essential oil. Poult. Sci. 2021;100 doi: 10.1016/j.psj.2020.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musa B.B., Duan Y., Khawar H., Sun Q., Ren Z., Elsiddig Mohamed M.A., Abbasi I.H.R., Yang X. Bacillus subtilis B21 and Bacillus licheniformis B26 improve intestinal health and performance of broiler chickens with Clostridium perfringens-induced necrotic enteritis. J. Anim. Physiol. Anim. Nutr. 2019;103:1039–1049. doi: 10.1111/jpn.13082. [DOI] [PubMed] [Google Scholar]
- Mutuş R., Kocabagli N., Alp M., Acar N., Eren M., Gezen S.S. The effect of dietary probiotic supplementation on tibial bone characteristics and strength in broilers. Poult. Sci. 2006;85:1621–1625. doi: 10.1093/ps/85.9.1621. [DOI] [PubMed] [Google Scholar]
- Nazzaro F., Fratianni F., Coppola R., Feo V. Essential oils and antifungal activity. Pharmaceuticals. 2017;10:86. doi: 10.3390/ph10040086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pham V.H., Abbas W., Huang J., He Q., Zhen W., Guo Y., Wang Z. Effect of blending encapsulated essential oils and organic acids as an antibiotic growth promoter alternative on growth performance and intestinal health in broilers with necrotic enteritis. Poult. Sci. 2022;101 doi: 10.1016/j.psj.2021.101563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramlucken U., Lalloo R., Roets Y., Moonsamy G., Jansenvan van Rensburg C., Thantsha M.S. Advantages of Bacillus-based probiotics in poultry production. Livest. Sci. 2020;241 [Google Scholar]
- Shi S., Liu J., Dong J., Hu J., Liu Y., Feng J., Zhou D. Research progress on the regulation mechanism of probiotics on the microecological flora of infected intestines in livestock and poultry. Lett. Appl. Microbiol. 2022;74:647–655. doi: 10.1111/lam.13629. [DOI] [PubMed] [Google Scholar]
- Simon O., Jadamus A., Vahjen W. Probiotic feed additives - effectiveness and expected modes of action. J. Anim. Feed Sci. 2001;10:51–67. [Google Scholar]
- Stevanović Z.D., Bošnjak-Neumüller J., Pajić-Lijaković I., Raj J., Vasiljević M. Essential oils as feed additives-future perspectives. Molecules. 2018;23:1717. doi: 10.3390/molecules23071717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumi C.D., Yang B.W., Yeo I.C., Hahm Y.T. Antimicrobial peptides of the genus Bacillus: a new era for antibiotics. Can. J. Microbiol. 2015;61:93–103. doi: 10.1139/cjm-2014-0613. [DOI] [PubMed] [Google Scholar]
- Wang B., Gong L., Zhou Y., Tang L., Zeng Z., Wang Q., Zou P., Yu D., Li W. Probiotic Paenibacillus polymyxa 10 and Lactobacillus plantarum 16 enhance growth performance of broilers by improving the intestinal health. Anim. Nutr. 2021;7:829–840. doi: 10.1016/j.aninu.2021.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y., Yu Y., Shen Y., Li Q., Lan J., Wu Y., Zhang R., Cao G., Yang C. Effects of Bacillus subtilis and Bacillus licheniformis on growth performance, immunity, short chain fatty acid production, antioxidant capacity, and cecal microflora in broilers. Poult. Sci. 2021;100 doi: 10.1016/j.psj.2021.101358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Y., Wang Z., Wang D., Lawrence P., Wang X., Kothapalli K.S.D., Greenwald J., Liu R., Park H.G., Brenna J.T. BCFA-enriched vernix-monoacylglycerol reduces LPS-induced inflammatory markers in human enterocytes in vitro. Pediatr. Res. 2018;83:874–879. doi: 10.1038/pr.2017.297. [DOI] [PubMed] [Google Scholar]
- Yu Y.H., Wu C.M., Chen W.J., Hua K.F., Liu J.R., Cheng Y.H. Effectiveness of Bacillus licheniformis-fermented products and their derived antimicrobial lipopeptides in controlling coccidiosis in broilers. Animals. 2021;11:3576. doi: 10.3390/ani11123576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L.Y., Peng Q.Y., Liu Y.R., Ma Q.G., Zhang J.Y., Guo Y.P., Xue Z., Zhao L.H. Effects of oregano essential oil as an antibiotic growth promoter alternative on growth performance, antioxidant status, and intestinal health of broilers. Poult. Sci. 2021;100 doi: 10.1016/j.psj.2021.101163. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.