Abstract
The present report describes a case of Echinococcus multilocularis infection in nutria (Myocastor coypus) culled in the central area of Slovenia. Post-mortem exam showed multiple cystic lesions in the liver. Gross examination, as well as parasitological and histopathological examinations, revealed numerous cysts of various sizes, filled with yellow clear fluid and displacing most of the liver parenchyma. The cyst lumina contained numerous protoscolices approximately 100 μm in diameter and calcareous corpuscles. The protoscolices had two visible suckers and a rostellum with birefringent hooks. The lesions were consistent with an E. multilocularis cyst. Molecular analysis confirmed that the nutria was infected with E. multilocularis. To our knowledge, this is the first report of echinococcosis in nutria in Slovenia that presents gross, parasitological, and histological lesions and the result of molecular analysis. Nutrias in Slovenia are dangerous invaders but can also be a relevant bioindicator of the presence of the parasite in the environment.
Keywords: Nutria, Myocastor coypus, Echinococcus multilocularis, Pathology, PCR, Bioindicator
Graphical abstract
1. Introduction
Human alveolar echinococcosis (AE) is a zoonotic infection caused by the metacestode stage of the tapeworm Echinococcus multilocularis and it is usually fatal if left untreated (Eckert and Deplazes, 2004). In temperate and arctic regions of Europe, it is considered as one of the most serious zoonotic diseases. The life cycle of E. multilocularis is mainly sylvatic, involving the red foxes (Vulpes vulpes), wolves (Canis lupus), raccoon dogs (Nyctereutes procyonoides), golden jackals (Canis aureus), and wild cats (Felis sylvestris) as the main definitive host (Romig et al., 2006). Domestic dogs and cats can also be definitive hosts and therefore may be the most important source of infection for humans (Seimenis, 2003). The life cycle also includes intermediate hosts such as muskrats, arvicolas, nutrias, murids, etc. (Kern et al., 2003). Humans can act as an aberrant intermediate host and are infected by ingestion of eggs that are excreted in the faeces of the definitive hosts. In immunocompetent humans, the asymptomatic incubation period of AE is usually about 5–15 years (Gottstein et al., 2015b). The metacestode stage proliferates and slowly invades the liver and surrounding tissues to produce tumour-like lesions, resembling neoplasia (Moro and Schantz, 2009).
In Europe, the risk of human infection with E. multilocularis was considered in the past to be restricted to certain geographic regions. Recently, however, the spread of the parasite to several new areas such as Denmark, the Netherlands, Poland, Romania, Slovakia, and Slovenia, as well as the increase in human AE incidence in Austria, France, and Switzerland, suggest that the disease is spreading, most likely due to the increase in red fox prevalence (Schweiger et al., 2007; Vergles Rataj et al., 2010; Miterpáková and Dubinský, 2011; Schneider et al., 2013; Karamon et al., 2014; Gottstein et al., 2015a; Marcinkutė et al., 2015; Oksanen et al., 2016). In Slovenia, 428 red fox carcasses were collected as road casualties between 2002 and 2005. A prevalence of 2.6% was found for the presence of E. multilocularis in the intestinal contents of the foxes (Vergles Rataj et al., 2010).
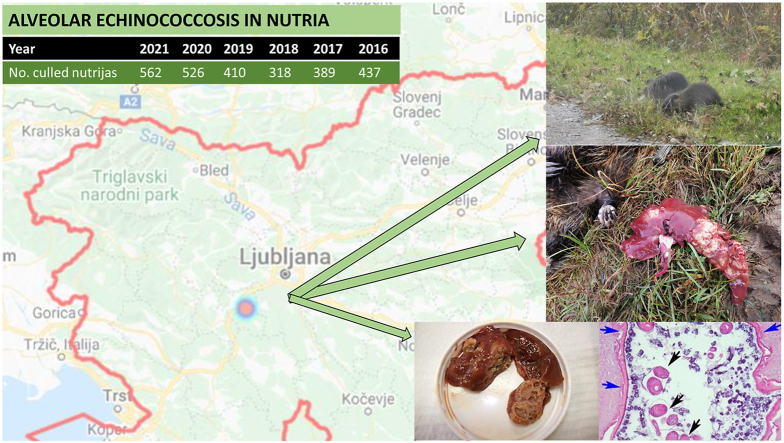
Nutria (Myocastor coypus) is a large semi-aquatic rodent which originates from South America. However, due to escapes and releases from fur farms, there are now large feral populations in North America, Europe, and Asia. Their burrows penetrate and damage riverbanks, dikes, and irrigation facilities (ISSG 2011). This species has been nominated by the International Union for Conservation of Nature as one of the “100 worst invaders in the world”. In Slovenia, as evident in the Central Slovenian Hunting Information System (OSLIS) database (http://oslis.gozdis.si/), the annual cull by hunters has increased from one in 2005 to 526 in 2020, mainly in the central region near Ljubljana, the capital of Slovenia with 300,000 inhabitants. From this hunting region, rivers flow into the Ljubljanica river, which flows directly into the city centre, surrounded with parks for recreation and dog walking. According to recent research (Alagić et al., 2019), there is an increasing problem with nutrias on the riverbanks of the Ljubljanica river in the city centre.
The aim of the presented work was to describe a case of echinococcosis in nutria in Slovenia, and to identify nutria not only as a dangerous invader, but also as a bioindicator of the presence of E. multilocularis in environment.
2. Material and methods
In March 2020 during the regular annual harvest nutria was culled on the hunting ground Borovnica (45°56ʼ33.5ʼʼy 14°21ʼ33.5ʼʼx) in the central area of Slovenia. Post-mortem examination revealed severe liver lesions, therefore liver sample was sent to Veterinary Faculty, University of Ljubljana, for pathomorphological and parasitological examination.
Liver was examined macroscopically and microscopically. Liver samples with cysts for histopathology were fixed in 10% buffered formalin and routinely embedded in paraffin. Four-μm thick tissue sections were first deparaffinized and then stained with haematoxylin and eosin (HE).
A part of the liver tissue was referred to the Institute of Microbiology and Immunology, Faculty of Medicine, University of Ljubljana, for molecular analyses. Genomic DNA was extracted from the affected tissue using a commercial QIAamp DNA Mini Kit (Qiagen, Germany), following the manufacturer's protocol for DNA purification from tissues. Fragments of cytochrome c oxidase subunit 1, cox1 (Marinova et al., 2017), NADH dehydrogenase subunit 1, nad1 (Huttner et al., 2008; Marinova et al., 2017), NADH dehydrogenase subunit 5, nad5 (Roelfsema et al., 2016), and small ribosomal RNA, rrnS (Beato et al., 2010), mitochondrial genes were amplified by conventional PCRs as described previously with modifications according to Šoba et al. (2020). The PCR products were sequenced from both strands. The sequences obtained were edited using CLC Main Workbench 7.9.1 (CLC Bio, Denmark) and compared to available sequences from GenBank database using BLAST.
3. Results
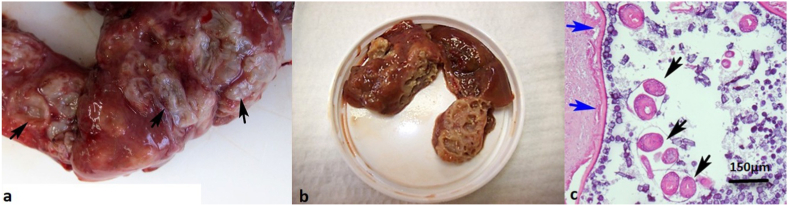
Gross examination of liver revealed numerous cysts of varying size, measuring from 3 to 5 mm, filled with yellow clear fluid (Fig. 1a and b). Some of the contents of the cysts were transferred to a slide and examined under a light microscope. We observed protoscolices of Echinococcus tapeworm.
Fig. 1.
Pathomorphological finding in the liver of a nutria (Myocastor coypus) with echinococcosis. Numerous cysts of varying sizes on the surface of the liver (arrows). b Numerous fluid-filled cysts in the cut section of the liver. c Haematoxylin and eosin (HE) stain of single cyst with several protoscolices (black arrows). The cyst is lined by an eosinophilic, hyaline outer membrane (blue arrows) and an inner germinal epithelial layer. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Histopathological examination revealed multilocular alveolar hydatid cysts that almost completely replaced the hepatic parenchyma. The cysts consisted of numerous round to oval cysts measuring up to 5 mm in diameter. The cysts were lined by an eosinophilic, hyaline, laminated outer membrane and an inner germinal epithelial layer containing basophilic nuclei, eosinophilic, flocculent to granular material, and numerous basophilic calcareous corpuscles. The cyst lumina contained numerous protoscolices and calcareous corpuscles. Protoscolices were about 100 μm in diameter and had two visible suckers and a rostellum with birefringent hooks (Fig. 1c). Most cysts were surrounded by a thick rim of granulation tissue moderately infiltrated with lymphocytes, histiocytes and heterophils, and in the adjacent parenchyma there were multifocal areas of granulation tissue and hepatocellular necrosis with multifocal areas of mineralization. There were severe degenerative lesions in the surrounding liver parenchyma. The morphology of the lesions was consistent with an E. multilocularis cysts.
DNA extracted from the affected tissue was successfully amplified yielding the products of 444, 530, 297 and 777 bp for the mitochondrial genes cox1, nad1, nad5 and rrnS, respectively. Sequence data were generated from all four loci. Species identification was obtained by comparing the sequences with available sequences from the GenBank database using BLAST. The analyses confirmed that the nutria was infected with E. multilocularis. The nucleotide sequences identified in this study have been deposited in the GenBank database under the accession numbers MW558108, MW558226, MW560731, MW560732.
4. Discussion
Nutria is a new game species in Slovenia, and its numbers are increasing very fast. As it is an invasive alien species, it is allowed to be hunted all year round and there are no hunting bag limits. A meta-analysis study (Oksanen et al., 2016) showed that nutrias seem to play little or no role in the life cycle of E. multilocularis within the EU. Nutrias (pooled prevalence (PP) 1.0%) could play a role in the life cycle of E. multilocularis in areas with medium to high PP in red foxes. Reports of echinococcosis in free-living nutrias are rare and show a low prevalence. Hepatic lesions were found in only two out of 531 nutrias examined in France, corresponding to a prevalence of 0.4% (Umhang et al., 2013), and in only seven of 119 nutrias examined in Germany, of which only one contained fertile protoscolices (Hartel et al., 2004). In the studies by Umhang et al. (2013) and Hartel et al. (2004), nutrias were trapped, but not described grossly or histopathologically. In the first study, lesions were examined for the presence of protoscolices and then PCR analysis was performed, whereas in the second study, examination of native tissue and, in cases of doubt, histological examination or nested PCR was performed. The cited studies were the only studies to report echinococcosis in nutria in a large systematic review of the geographic distribution of E. multilocularis in definitive and intermediate hosts in the European Union (Oksanen et al., 2016).
Slovenian law requires hunters to hunt with a trained hunting dog when hunting nutria. After killing the nutria, the dog jumps into the water and brings the culled nutria to the hunter. If the dog penetrates the skin and the wall of the abdominal cavity with its teeth, it can become infected with E. multilocularis. After the kill, the nutria is eviscerated on the spot. Usually, the hunter throws the organs into the bushes, grass, etc. or gives them to the dog as a reward. In Borovnica, where our nutria was culled, there are many small vegetable and fruit gardens located near water and forest. The organs left behind are food for the foxes, but also for another carnivore animals (dogs, cats). They can excrete the eggs of the tapeworm in the gardens, which poses a threat for human infection.
The red fox is known to be the most important definitive host of E. multilocularis. In Slovenia and other European countries, fox population is in expansion, therefore E. multilocularis prevalence has increased in foxes within areas to which it is known to be endemic (Combes et al., 2012; Schweiger et al., 2007). In the study from Slovenia (Vergles Rataj et al., 2010), the highest prevalence of E. multilocularis was 7% in red foxes in the southern region. The lowest prevalence was 1.3% in the central Slovenia, where our nutria was culled. However, this region is rich in rivers and most nutrias are culled in the central Slovenia region. Moreover, the number of golden jackals, another definitive host, is increasing very rapidly in this region, from 1 in 2015 to 23 in 2020 (OSLIS), and the first golden jackal was found in Slovenia in 2005 (Krofel and Potočnik, 2008).
According to the records of the Slovenian Hunting Association, there are almost 21,000 hunters and only 91 of them are veterinarians by profession. So, there is a lack of knowledge about diseases, especially zoonoses. Even though in the Eu regulation (EC) No. 853/2004, annex III, section IV, chapter III: handling of small wild game it is written that a trained person must examine the killed animal, this is usually the case in Slovenia for big game.
In Slovenia, echinococcosis in humans is included in the list of notifiable diseases and the mean annual incidence rate was 0.27 per 100,000 inhabitants in the period from 2014 to 2018 (NIJZ, 2019). Nevertheless, reporting rarely includes information on the causative species, which means that AE and cystic echinococcosis caused by Echinococcus granulosus sensu lato are both reported as echinococcosis and the incidence specifically for AE is therefore not known. However, the raising number of foxes and nutrias might present a potential threat for AE in humans. In neighbouring Croatia, the first human case of AE was confirmed in 2017 (Dušek et al., 2020) and the disease appears to be emerging in Hungary (Dezsényi et al., 2021). Nevertheless, the results of our study and those of others (Umhang et al., 2013) lead us to consider nutrias can be a relevant bioindicators of the presence of E. multilocularis in environment. The results also suggest that if nutrias are listed as hunting prey and pests, they could be used to detect the presence of E. multilocularis in areas considered free of this parasite.
Funding
The authors acknowledge the financial support from the Slovenian Research Agency (research core funding Nos. P4-0096 and P3-0083).
Availability of data and material
Not applicable.
Code availability
Not applicable.
Author's contributions
MK culled nutria, transported samples of liver to the Veterinary Faculty University of Ljubljana and prepared first draft manuscript. TŠ performed gross examination and histopathology and was involved in the interpretation of results and manuscript drafting. BŠ performed the molecular analysis, obtained accession numbers in GenBank, was involved in the interpretation of the results and manuscript drafting. AVR performed parasitological examination and was involved in the interpretation of results and manuscript drafting. All authors read and approved the final version of the manuscript.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
The authors give their consent for the publication of this manuscript in the International Journal for Parasitology: Parasites and Wildlife.
Declaration of competing interest
The authors declare no competing interests.
Acknowledgements
We thank the Hunting association of Slovenia to publish the results.
This research was funded by the Slovenian Research Agency, grant number P4-0092 and P3-0083.
References
- Alagić A., Flajšman K., Adamič M., Bužan E., Pokorny B. 2019. Conflicts between Humans and Game Species in Non-hunting Areas: a Case Study from Slovenia; pp. 127–128.https://www.iugb2019.mi.lt/wp-content/uploads/2019/09/34th-IUGB-Congress-Proceedings-LT.pdf Abstract book. Kaunas. [Google Scholar]
- Beato S., Parreira R., Calado M., Gracio M.A. Apparent dominance of the G1-G3 genetic cluster of Echinococcus granulosus strains in the central inland region of Portugal. Parasitol. Int. 2010;59:638–642. doi: 10.1016/j.parint.2010.08.004. https://doi:10.1016/j.parint.2010.08.004 [DOI] [PubMed] [Google Scholar]
- Combes B., Comte S., Raton V., Raoul F., Boue F., Umhang G., Favier S., Dunoyer C., Woronoff N., Giraudouxet P. Westward spread of Echinococcus multilocularis in foxes, France, 2005-2010. Emerg. Infect. Dis. 2012;18:2059–2062. doi: 10.3201/eid1812.120219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dezsényi B., Dubóczki Z., Strausz T., Csulak E., Czoma V., Káposztás Z., Fehérvári M., Somorácz A., Csilek A., Oláh A., Almási K., Patonai A., Görög D., Széll Z., Tolnai Z., Sréter T., Danka J., Auer H., Grüner B., Barth T.F.E., Casulli A. Emerging human alveolar echinococcosis in Hungary (2003-2018): a retrospective case series analysis from a multi-centre study. BMC Infect. Dis. 2021;21:168. doi: 10.1186/s12879-021-05859-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dušek D., Vince A., Kurelac I., Papić N., Višković K., Deplazes P., Beck R. Human alveolar echinococcosis, Croatia. Emerg. Infect. Dis. 2020;26:364–366. doi: 10.3201/eid2602.181826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckert J., Deplazes P. Biological, epidemiological, and clinical aspects of echinococcosis, a zoonosis of increasing concern. Clin. Microbiol. Rev. 2004;17:107–135. doi: 10.1128/cmr.17.1.107-135.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottstein B., Stojkovic M., Vuitton D.A., Millon L., Marcinkute A., Deplazes P. Threat of alveolar echinococcosis to public health – a challenge for Europe. Trends Parasitol. 2015;31:207–412. doi: 10.1016/j.pt.2015.06.001. [DOI] [PubMed] [Google Scholar]
- Gottstein B., Wang J., Boubaker G., Marinova I., Spiliotis M., Muller N., Hemphill A. Susceptibility versus resistance in alveolar echinococcosis (larval infection with Echinococcus multilocularis) Vet. Parasitol. 2015;213:103–109. doi: 10.1016/j.vetpar.2015.07.029. [DOI] [PubMed] [Google Scholar]
- Hartel K.S., Spittler H., Doering H., Winkelmann J., Hoerauf A., Reiter-Owona I. The function of wild nutria (Myocastor coypus) as intermediate hosts for Echinococcus multilocularis in comparison to wild muskrats (Ondatra zibethicus) Int. J. Med. Microbiol. 2004;293:62–63. [Google Scholar]
- Huttner M., Nakao M., Wassermann T., Siefert L., Boomker J.D., Dinkel A., Sako Y., Mackenstedt U., Romig T., Ito A. Genetic characterization and phylogenetic position of Echinococcus felidis (Cestoda: taeniidae) from the African lion. Int. J. Parasitol. 2008;38:861–868. doi: 10.1016/j.ijpara.2007.10.013. https://doi:10.1016/j.ijpara.2007.10.013 [DOI] [PubMed] [Google Scholar]
- ISSG . 2011. Global Invasive Species Database (GISD). Invasive Species Specialist Group of the IUCN Species Survival Commission.http://www.issg.org/database [Google Scholar]
- Karamon J., Kochanowski M., Sroka J., Cencek T., Różycki M., Chmurzyńska E., Bilska-Zając E. The prevalence of Echinococcus multilocularis in red foxes in Poland-current results (2009-2013) Parasitol. Res. 2014;113:317–322. doi: 10.1007/s00436-013-3657-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kern P., Bardonnet K., Renner E., Auer H., Pawlowski Z., Ammann R.W., Vuitton D.A., Kern P. European echinococcosis registry: human alveolar echinococcosis, Europe, 1982-2000. Emerg. Infect. Dis. 2003;9:343–349. doi: 10.3201/eid0903.020341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krofel M., Potočnik H. First record of a golden jackal (Canis aureus) in the Savinja Valley (Northern Slovenia) Nat. Slov. 2008;10:57–62. http://www.dlib.si/?URN=URN:NBN:SI:DOC-D8E3DACL [Google Scholar]
- Marcinkutė A., Šarkūnas M., Moks E., Saarma U., Jokelainen P., Bagrade G., Laivacuma S., Strupash K., Sokolovash V., Deplazesi P. Echinococcus infections in the Baltic region. Vet. Parasitol. 2015;213:121–131. doi: 10.1016/j.vetpar.2015.07.032. [DOI] [PubMed] [Google Scholar]
- Marinova I., Spiliotis M., Wang J., Muhtarov M., Chaligiannis I., Sotiraki S., Rainova I., Gottstein B., Boubaker G. Molecular characterization of Echinococcus granulosus isolates from Bulgarian human cystic echinococcosis patients. Parasitol. Res. 2017;116:1043–1054. doi: 10.1007/s00436-017-5386-1. https://doi:10.1007/s00436-017-5386-1 [DOI] [PubMed] [Google Scholar]
- Miterpáková M., Dubinský P. Fox tapeworm (Echinococcus multilocularis) in Slovakia – summarizing the long-term monitoring. Helminthol. 2011;48:155–161. doi: 10.2478/s11687-011-0023-5. [DOI] [Google Scholar]
- Moro P., Schantz P.M. Echinococcosis: a review. Int. J. Infect. Dis. 2009;13:125–133. doi: 10.1016/j.ijid.2008.03.037. [DOI] [PubMed] [Google Scholar]
- National Institute of Public Health of the Republic of Slovenia . National Institute of Public Health; Ljubljana, Slovenia: 2019. Epidemiološko Spremljanje Nalezljivih Bolezni V Sloveniji V Letu 2018; Annual Epidemiological Report on Communicable Diseases in Slovenia, 2018.https://www.nijz.si/sites/www.nijz.si/files/uploaded/epidemiolosko_spremljanje_nalezljivih_bolezni_v_sloveniji_v_letu_2018.pdf Available online: [Google Scholar]
- Oksanen A., Siles-Lucas M., Karamon J., Possenti A., Conraths F.J., Romig T., Wysocki P., Mannocci A., Mipatrini D., La Torre G., Boufana B., Casulli A. The geographical distribution and prevalence of Echinococcus multilocularis in animals in the European Union and adjacent countries: a systematic review and meta-analysis. Parasit. Vectors. 2016;9:519. doi: 10.1186/s13071-016-1746-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roelfsema J.H., Nozari N., Pinelli E., Kortbeek L.M. PCRs for differential diagnosis of cestodes. Exp. Parasitol. 2016. Novel;161:20–26. doi: 10.1016/j.exppara.2015.12.010. https://doi:10.1016/j.exppara.2015.12.010 [DOI] [PubMed] [Google Scholar]
- Romig T., Dinkel A., Mackenstedt U. The present situation of echinococcosis in Europe. Parasitol. Int. 2006;55:187–191. doi: 10.1016/j.parint.2005.11.028. [DOI] [PubMed] [Google Scholar]
- Schneider R., Aspöck H., Auer H. Unexpected increase of alveolar echincoccosis, Austria, 2011. Emerg. Infect. Dis. 2013;19:475–477. doi: 10.3201/eid1903.120595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweiger A., Ammann R.W., Candinas D., Clavien P.A., Eckert J., Gottstein B., Halkic N. Human alveolar echinococcosis after fox population increase, Switzerland. Emerg. Infect. Dis. 2007;13:878–882. doi: 10.3201/eid1306.061074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seimenis A. Overview of the epidemiological situation on echinococcosis in Mediterranean region. Acta Trop. 2003;85:191–195. doi: 10.1016/s0001-706x(02)00272-3. [DOI] [PubMed] [Google Scholar]
- Šoba B., Gašperšič Š., Keše D., Kotar T. Molecular characterization of Echinococcus granulosus sensu lato from humans in Slovenia. Pathogens. 2020;9:562. doi: 10.3390/pathogens9070562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umhang G., Richomme C., Boucher J.M., Guedon G., Boué F. Nutrias and muskrats as bioindicators for the presence of Echinococcus multilocularis in new endemic areas. Vet. Parasitol. 2013;197:283–287. doi: 10.1016/j.vetpar.2013.05.003. [DOI] [PubMed] [Google Scholar]
- Vergles Rataj A., Bidovec A., Žele D., Vengušt G. Echinococcus multilocularis in the red fox (Vulpes vulpes) in Slovenia. Eur. J. Wildl. Res. 2010;56:819–822. doi: 10.1007/s10344-010-0417-6. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.