Abstract
Vigorous exercise generates large amounts of reactive oxygen species (ROS) as a result of the consumption of large volumes of O2 in athletes, causing some athletes to consume antioxidants in the erroneous belief that this will counteract the damaging effects of ROS. There is currently no convincing evidence to support the benefits of antioxidant supplementation in acute physical exercise and exercise training. On the contrary, exogenous antioxidants prevent some physiological functions of free radicals that are needed for cell signaling, causing higher dosages of antioxidants to hamper or prevent performance-enhancing and health-promoting training adaptation such as mitochondrial biogenesis, skeletal and cardiac muscle hypertrophy, and improved insulin sensitivity. However, there remains the perception that antioxidants can counterbalance oxidative stress and benefit exercise adaptation and performance in athletes. It is likely that the negative effects of high doses of antioxidant supplementation exceed their potential benefits. We discuss some proposed pathways of potential side effects of exogenous antioxidant supplementation in athletes.
Keywords: Antioxidants, Athletes, Reactive oxygen and nitrogen species, Nuclear factor erythroid 2-related factor 2
1. Introduction
Antioxidants are commonly consumed by athletes as a nutritional strategy with purported benefits, as indicated by reports from the American College of Sports Medicine (ACSM) that over 50% of elite endurance and male collegiate athletes consumed doses of antioxidant supplements daily that were higher than the recommended daily allowance (RDA).1 Consumption of antioxidants is largely based on their presumed benefits against exercise-induced oxidative stress and so prevent or minimize free radical mediated harms on skeletal muscle damage and muscle fatigue.2 Frequent beliefs are that antioxidants are unlikely to be toxic since they are often “natural products”, and that they mitigate the harmful effects of reactive oxygen species (ROS) on cell structure and function. This misconception is based on the erroneous assumption that an accumulation of reactive oxygen and nitrogen species (RONS) levels represents a “biochemical accident” that can be prevented by antioxidants, and that RONS fulfill no beneficial physiological functions. Instead, there is much evidence that RONS have important roles in physiological signaling pathways that regulate response to acute and repeated bouts of exercise.3 For example, oxidative stress induced by physical exercise is essential to training adaptation, which can be negated by the use of antioxidants.4 Physical exercise augments ROS levels, which in turn increases gene expression of signaling pathways involved in cell proliferation and adaptation.5
Antioxidants are often used based on intuition and without a complete understanding of the multiple functions of ROS to counteract the damaging effects of free radicals, with most studies on the benefits of antioxidants in athletes undertaken in the 1970's and 1980's.6 However, the late 1990's heralded an increased understanding of ROS biology, where free radicals (e.g., nitric oxide, superoxide, hydrogen peroxide) were shown to be important components of the complex signaling network of cells.7 The appropriate balance between antioxidants and free radicals is necessary prerequisite for obtaining physiological adaptation.8, 9, 10 This raises a concern about the use of antioxidants in attenuating or even negating the beneficial roles of ROS in normal cell signaling, particularly in athletes.
2. Rethinking the role of RONS in exercise
The “free radicals” concept was first introduced by Gomberg in 190011 followed by Kharach who suggested that free radicals could participate in chemical reactions.12 The free radical theory was first proposed by Harman in 1956.13 Later advances in molecular biology and free radical research techniques led to great progress in our understanding of free radical biology. Dillard first introduced free radical theory to the field of sports science in 1978,14 and the important role of free radicals in exercise-induced fatigue subsequently attracted great attention.15
Free radicals are atoms or molecules containing one or more unpaired electrons in their outer rings or valency shells, and are produced in mitochondria, endoplasmic reticulum, nucleus, plasma membrane and vacuoles. The oxygen and nitrogen are converted into reactive oxygen and nitrogen species after obtaining unpaired electrons, including superoxide anion, hydroxyl radical, hydrogen peroxide, nitric oxide and nitrogen dioxide. RONS are very reactive and unstable, usually attack body tissues, glands, cell membranes and biological macromolecules, etc., and cause oxidative damage. In particular, there is a clear correlation between oxidative stress and physical activity.16
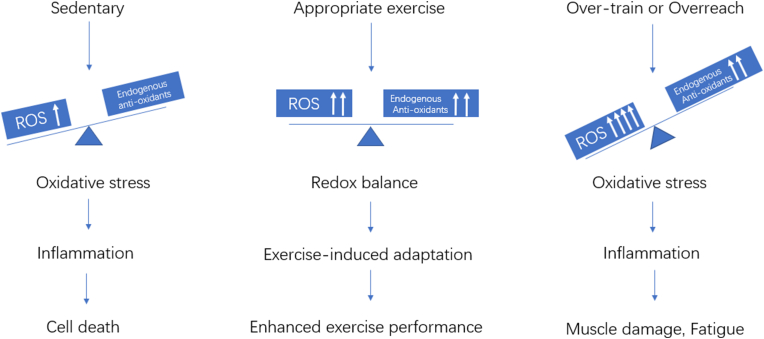
Sedentary lifestyles are characterized by increased oxidative stress resulting from RONS derived from a lower mitochondrial density due to a lack of movement. Lower levels of physical activity reduce ATP demand and leads to an accumulation of electrons in the mitochondrial respiratory chain. Increased ROS levels damage mitochondria, causing the nucleus to release transcription factors for the generation of new healthy mitochondria; this process creates an inflammatory condition, leading to programmed cell death.17
The association between exercise and free radical generation was discovered in the late 1970s.18 Strenuous exercise generates RONS as a consequence of metabolism within the mitochondria during muscle contraction.19 Early studies reported that ROS production led to oxidative damage in the muscle during and after exercise,20 with later studies confirming that ROS production plays a key role in regulating signals required for muscle adaptation in response to exercise.21 For example, the degree of physiological adaptation (i.e., maximal oxygen uptake (VO2 max), time trials, and Wingate tests) was greater in the “moderate” and “high” groups than in the “low” exercise-induced oxidative stress group.22 Redox balance is maintained within physiological limits in most exercise conditions to minimize potential oxidative damage and limits the need for supplementation with exogenous antioxidants; however, intense and prolonged exercise (usually over-train or overreach) increases production of RONS and elicits a deleterious redox environment leading to impaired exercise capacity and health.23
Optimized regular exercise upregulates endogenous antioxidant systems and reverses the deleterious effect of RONS.24 Chronic exercise augments oxidative stress in muscles and other cells due to a 10- to 15-fold increase in oxygen consumption associated with exercise.25 The constant oxidative stress produced by chronic exercise causes well-trained athletes to have a more highly developed endogenous antioxidant system capacity, such as increases in superoxide dismutase and glutathione peroxidase to cellular accumulation of free radicals.26
RONS generated during exercise stimulate two important redox-sensitive signaling pathways: nuclear factor κ B (NF-κB) and mitogen activated protein kinase (MAPK). Activation of these pathways leads to induction of antioxidant enzymes including mitochondrial superoxide dismutase (Mn SOD) and glutathione peroxidase (GPX) as well as inducible nitric oxide synthase.27 There will be minimal damage to skeletal muscle structure and sports performance if RONS levels do not exceed the capacity of endogenous antioxidant to maintain redox homeostasis.28 However, excess free radical levels produced during and after strenuous exercise (e.g. over-train or overreach) causes muscle damage, fatigue and decreased performance29 (Fig. 1). Several studies reported detrimental effects of antioxidant supplementation on exercise capacity (Fig. 1 and Table 1).
Fig. 1.
Sedentary, appropriate exercise, over-train and redox hemostasis.
Table 1.
Potential harms of high doses of antioxidants supplementation on exercise adaptation in athletes.
| Antioxidants | Protocol | Exercise | Design | Potential harms | References |
|---|---|---|---|---|---|
| Vitamin C | 1 g/d, 8 wk | Static bicycle,65–80% VO2max, 40 min/d, 3d/w, 8 wk | Randomized, double-blind | Reduced mitochondrial biogenesis; Decreased exercise-induced adaptation | Gomez-C, 200845 |
| 1 g/d, 2 wk | Motorised treadmill, −15% decline,60% VO2max, 30min | Randomized, double-blind | Delayed post-exercise recovery. | Close,200646 | |
| 1 g/d,3 wk | High-intensity hilly training, 2–3 times/w, 3 wk | Randomized, double-blind | Increased oxidative stress | Braakhuis, 201447 | |
| 1 g/d,3 wk | steady state ride (60min), performance ride (30min); 70%VO2max | Cross-over | Increased plasma monoaldehyde levels | Bryant,200348 | |
| Vitamin E | 400IU/d, 6wk | Swimming, 4 sessions/w, 6 wk | Randomized, double-blind | Decreased exercise-induced adaptation | Sharman, 197149 |
| 1200IU/d, 2wk | a one-repetition maximum (RM); a 10RM resistance exercise | Randomized | Increased lipid peroxidation. | McBride, 199850 | |
| 800IU/, 8wk | triathlon | Randomized, double-blind | Increased oxidative stress | Nieman, 200451 | |
| >or = 400IU/d | N/A | Meta-analysis | Increased all-cause mortality | Miller,200552 | |
| Vitamin C + E | (Vit C 1g + Vit E 400IU)/d, 4wk | biking or running (20 min), circuit training (45 min); 5d/w, 4 wk | Randomized, double-blind, controlled | Decreased exercise-induced adaptation | Ristow,200953 |
| Vit C 1g + Vit E 235 mg/d, 11wk | high-intensity interval sessions; steady state continuous sessions (30–60 min); 3–4 sessions/w, 11 wk | Randomized, double-blind, controlled | Reduced mitochondrial biogenesis | Paulsen, 201454 | |
| Vit C 0.5g + Vit E 400IU/d, 16wk | Cycling; 5 times/w, 12wk | Randomized, double-blind, controlled | Increased oxidative stress | Yfanti,201255 | |
| Vit C 1 g/d + Vit E 235 mg/d, 3 wk | 30s all-out cycling sprints, 4–6 repetitions, 3 sessions/wk,3wk | Randomized, double-blind, controlled | Blunted sprint interval training-induced exercise adaptation | Wyckelsma,202056 | |
| Resveratrol | 250 mg/d, 8 wk | cycle ergometer (twice a week), Crossfit (once a week); 8wk | Randomized, double-blind, controlled | Blunt exercise-induced improvements in cardiovascular health parameters | Gliemann, 201357 |
3. Antioxidants supplementation in exercise
Athletes often take antioxidants supplementation to prevent the harmful effects of exercise-induced oxidative stress and enhance exercise performance. Proper antioxidants supplementation may be beneficial under some circumstances, such as overtraining, overreach, muscle damage, and high-altitude or hypoxic training. Earlier studies suggested that supplementation with vitamin E improved exercise performance in climbers at high altitudes30 and also the athletic performance of sled dogs,31 while supplementation with vitamin C improved exercise performance in untrained college students32 and athletes.33 Moreover, a combination of vitamins E and C increased aerobic capacity after long-term exercise training.34
However, more recent studies do not support the idea that antioxidant supplementation is beneficial to human health35, 36, 37 and exercise adaptation.38, 39, 40, 41, 42 Furthermore, high doses of exogenous antioxidants disturb the balance between free radicals and endogenous antioxidant mechanisms and alters physiological adaptive responses.43 Supplementation with antioxidants negatively impacts exercise capacity, adaptive gene expression and protein synthesis to alter skeletal muscle and cardiovascular health44 (Table 1).
4. Potential negative effects of exogenous antioxidants on exercise adaptation
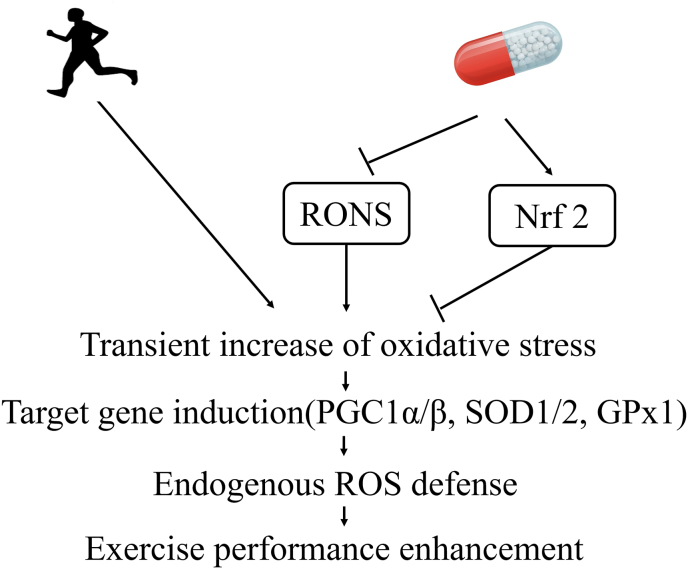
In general, antioxidants can be divided into either RONS sequestrants (such as vitamin C, vitamin E, and vitamins C plus E) or Nrf-2 activators (such as resveratrol and flavonoids). Consuming excessive amounts of antioxidants during exercise training paradoxically increases muscle fatigue and delays recovery.46 A general mechanism by which chronic ingestion of high doses of these antioxidants can scavenge free radicals and prevent the activation of Nrf2 is illustrated in Fig. 2.
Fig. 2.
Antioxidants negating the benefits of oxidative stress in exercise-induced adaptation.
4.1. RONS scavengers
Exogenous RONS scavengers can reduce the negative ROS/RNS effects, but can also impede physiological signaling acticated by ROS/RNS related to cellular adaptation to exercise, such as signal transduction cascades (e.g., Nrf2) and calcium signaling activated by lipid peroxidation.58,59 Vitamin C and other RONS scavengers prevent the transient elevation of RONS and therefore prevent the activation of Nrf2 and inositol triphosphate system triggered by lipid oeroxidation induced by increases in ROS.
4.1.1. Vitamin C
Vitamin C (ascorbic acid or ascorbate) is a water-soluble antioxidant that is able to react with numerous ROS derivatives. Ascorbic acid scavenges free radicals such as O2-, H2O2, OH−, and aqueous peroxyl radicals, and undergoes two-electron oxidation to dehydroascorbic acid, with intermediate formation of the relatively unreactive ascorbyl radical. The RDA for vitamin C is 75 mg/day for adult females and 90 mg/day for adult males, and the Tolerable Upper Intake Level (UL)is set at 2000 mg/day for adults. Excessive levels of vitamin C are excreted by the kidneys.60
The earliest study of the effects of vitamin C on athletic performance appeared promising.61 However, high concentrations of vitamin C can act as a pro-oxidant agent and stimulate lipid peroxidation, suggesting that vitamin C is not ergogenic.47,48 Potential negative effects of vitamin C supplementation in athletes include:
-
(1)
Decreased exercise-induced adaptation. Supplementation with vitamin C hampers training induced adaptations in endurance performance. A double-blind randomized controlled trial by Gomez-Cabrera et al. compared the effectiveness of eight weeks of endurance training in a group that was supplemented with an oral dose of 1 g of vitamin C as compared to a placebo treated group. Athletes not supplemented with vitamin C had an eleven percent greater improvement in VO2max, suggesting that vitamin C supplementation prevents crucial cellular adaptations to exercise.45
-
(2)
Delayed post-exercise recovery. The effects of vitamin C supplementation on ROS production and delayed-onset muscle soreness (DOMS) following downhill running were investigated by Close et al.30. Participants were assigned to one of two groups: an ascorbic acid group receiving 1 g of ascorbic acid 2 h before and for 14 days after downhill running, and a placebo treated group. While both groups experienced DOMS and impaired muscle function post-exercise, those receiving ascorbic acid also experienced delayed recovery, suggesting that vitamin C could hinder post-exercise recovery of muscle function.46
-
(3)
Increased lipid peroxidation. Supplementation with 1 g/d of vitamin C for 3 weeks increased plasma monoaldehyde levels (marker of oxidative stress resulting from lipid peroxidation) after cycling for 90 min.48
-
(4)
Diminished mitochondrial biogenesis. Long-term administration of high doses of vitamin C inhibits skeletal muscle adaptation to endurance training and attenuates improvements of endurance performance. The adverse effects of vitamin C on exercise adaptation were cause by reduced expression of transcription factors involved in mitochondrial biogenesis, such as peroxisome proliferator-activated receptor gamma coactivator-1 alpha (PGC-1), nuclear respiratory factor 1 (NRF-1), and mitochondrial transcription factor A (TFAM)45 which are all activated by exercise induced increases in RONS.
Intake of vitamin C from dietary sources are unlikely to share the deleterious effects that occur with the use of supplements because antioxidants in foods are biochemically balanced in that they are part of a combination of redox agents in oxidized and reduced forms; this balance may be lacking in exogenous supplements.62 Vitamin C levels in food products usually ranges from 24.12 mg/100g to 217.52 mg/100g and are unlikely to reach the concentrations found in exogenous supplements.
4.1.2. Vitamin E
Vitamin E (tocopherol and tocotrienol) is a lipid-soluble chain-breaking antioxidant present in cell membranes. Vitamin E is capable of scavenging lipid-derived peroxyl radicals, and like vitamin C, appears to react relatively poorly with H2O2. The RDA for vitamin E is 15 mg/day (∼22 international units (IU) from natural sources or 33 IU from synthetic sources), and the UL is set at 1000 mg/day (1500IU), with the amount of vitamin E related to levels of dietary polyunsaturated fats. Tocopherol (vitamin E) is the main chain-breaking antioxidant in lipids, plasma, and red cells.63
There are only a few studies on the effects of consuming high doses (or chronic consumption) of vitamin E on exercise performance. Potential negative effects of high doses of vitamin E supplementation in athletes include:
-
(1)
Decreased exercise-induced adaptation. Supplementation with vitamin E (400 IU/day for 6 wk) did not improve endurance performance in a study reported by Sharman et al. Moreover, the placebo-treated group demonstrated greater improvements of cardiorespiratory function with exercise training compared with the antioxidant group, which could be the first report of the unfavorable effects of supplementation with antioxidants.49
-
(2)
Increased lipid peroxidation. Supplementation with natural vitamin E for two weeks (992 mg/d) increased levels of thiobarbituric acid-reactive substances (by products of lipid peroxidation) following high intensity resistance training.50
-
(3)
Increased oxidative stress. A randomized, double blind involving thirty-eight ironman triathletes who received vitamin E (800 IU/day alpha-tocopherol) or placebo for 2 months reported that vitamin E increased oxidative stress by promoting lipid peroxidation and inflammation.51
-
(4)
Increased all-cause mortality. There is a positive association between increased high-sensitivity C-reactive protein (hs-CRP levels, marker of inflammation) and high-doses of ingested vitamin E (400 IU/day or more), which is probably responsible for an increase in all-cause mortality in women.52
4.1.3. Combination of vitamins C and E
Vitamin C assists in recycling of cellular vitamin E and acts synergistically to affect H2O2-based redox signaling through its competition with SODs, suggesting that consuming a combination of vitamin C and E can help protect against lipid peroxidation damage such as increasing the leakiness of cell membranes.64,65 Potential negative effects of high doses of vitamin C plus vitamin E supplementation in athletes include:
-
(1)
Decreased exercise-induced adaptation. A study of the effects of a combination of vitamins C and E on insulin sensitivity in previously untrained and trained healthy young men, consumed before and after a four-week exercise intervention period, reported that exercise-induced ROS production improved insulin resistance and augmented adaptive responses that promoted natural antioxidant defense capacity. Supplementation with a combination of vitamins C and E to reduce exercise-dependent ROS formation abolished the health-promoting effects of exercise.66 Other studies also suggest that 12 weeks of supplementation with high-doses of a combination of vitamin C and E (vitamin C 1000 mg/day and vitamin E 600 mg/day) blunted the increases in total lean and muscle thickness normally observed after resistance training.67
-
(2)
Reduced mitochondrial biogenesis. Treatment with a combination of vitamin C (1000 mg/day) and vitamin E (235 mg/day) for 11 weeks blunts increases in cytochrome c oxidase subunit IV (COX4, a mitochondrial marker) and cytosolic peroxisome proliferator-activated receptor-γ co-activator 1α (PGC-1α), which are important for improving muscular endurance and exercise performance.54
-
(3)
Increased oxidative stress. Supplementation with a combination of vitamin C and E increased levels of lipid peroxidation (marker of oxidative stress) in ironman triathletes, suggesting a pro-oxidative effect of the supplementation.68 Other studies also reported that supplementation with a combination of vitamin C and E increased plasma protein oxidation and lipid peroxidation in well-trained subjects,55 leading to greater oxidative damage in half- and full-ironman triathlons athletes. Athletes in the latter study consumed high doses of the antioxidants for prolonged periods (vitamin C: 1095 ± 447 mg/day for 4.9 ± 4.7 yr; vitamin E: 314 ± 128 mg/day for 5.6 ± 5.2 yr).68 Thus, These studies suggest that supplementation with high-doses of a combination of vitamin C and E can lead to a loss of adaptation of physiological and/or biological changes due to exercise.
In addition, it is important to note that minerals and trace elements, especially selenium, are widely consumed as micronutrient supplements by approximately 50% of athletes.69 The RDA for selenium is 55 mg/day and the UL is set as 400 mg/day for adults. Indeed, selenium is an essential component of selenoproteins and plays an important role in maintaining the normal function of the important antioxidant enzyme glutathione peroxidase (GPx).70 Of interest is that treating with sodium selenite (200 μg/day) for 3 weeks significantly increased GPx and further reduced plasma lipid hydroperoxide levels immediately postexercise in overweight healthy individuals.71 However, a clinical trial reported that selenium supplementation had no beneficial effects on exercise training-induced adaptation and athletic performance in youth.72 Of note are studies reporting that selenium supplementation dampens the rate of exercise-induced mitochondrial density,73, 74, 75 which represents mitochondrial adaptations to chronic and acute exercise and which is an important predictor of aerobic capacity of athletes. To summarize, there is a lack of studies showing clear beneficial effects of selenium supplementation on athletic performance. However, given the potential of adverse effects of selenium on exercise-induced mitochondrial adaptations and the possible safety issues at high doses, selenium is not a recommended candidate of antioxidant supplementation in athletes.
4.2. Nrf2 activators
Excessive antioxidant levels lead to reductive stress.76 Reductive stress is the opposite of oxidative stress, and partly arises from excessive activation of Nrf2 leading to disorders of the cardiovascular, neurological, neoplastic and metabolic systems. Chronic reductive stress can induce oxidative stress, which in turn stimulates greater reductive stress formation by positive feedback regulation.77
Persistent activation of Nrf2, as in the case with high and chronic doses of Nrf2 activators, can lead to reductive stress. A recent study shed some light on Nrf2 signaling in defective autophagy. Autophagy describes cell adaptation to stressful conditions caused by degrading defective or redundant proteins, and aggregates cellular organelles to be repurposed for energy supplies to promote cell survival.78,79 Autophagy and oxidative stress are reciprocally linked. In addition to regulation by Keap1, levels of active Nrf2 are also regulated by autophagy and p62, a ubiquitin binding protein acting as a scaffold for several protein aggregates and which triggers their degradation through proteasomes or lysosomes via autophagy.80
Autophagy degrades p62 under normal conditions. Oxidative stress upregulates p62 levels, with resultant sequestration of Keap1 and activation of Nrf2 and Nrf2-dependent antioxidant gene expression. In addition, oxidative conditions also activate NFκB consequent to p62 upregulation and TNF receptor-associated factor 6 (TRAF6) complex formation, to activate antioxidant-defense gene expression.81 There is an accumulation of p62 in autophagy-defective cells and tissues, causing P62 to bind and sequester Keap1 in aggregates, resulting in the constitutive activation of Nrf2 and antioxidant defenses. Elevated Nrf2 levels generates reductive stress leading to muscle atrophy and myopathy.27
4.2.1. Resveratrol
Resveratrol, an activator of Nrf2, is a naturally occurring antioxidant present in red wine that can offer cardiovascular protection by reducing oxidative stress and non-esterified fatty acid levels.82 At low concentrations (0.5 μM), resveratrol enhances endothelial NO production through a caveolae-dependent mechanism involving p42/44MAPK activation, while higher doses (25 μM) of resveratrol exert dose-related pro-oxidant effects, causing mitochondrial damage and endothelial cell death mediated by CYP2C983 and down-regulation of Akt phosphorylation.84 A randomized double-blinded study reported that treating 14 healthy aged men with resveratrol (250 mg/day) for 8 weeks blunted high-intensity exercise training gains in maximal oxygen uptake, mean arterial pressure, triglyceride concentrations, low-density lipoprotein, and several other cardiovascular health parameters.57
4.2.2. Herbal antioxidants
The use of herbal supplements by athletes has increased greatly during the past decade.85 Herbal products extracted from seeds, gums, roots, leaves, bark, berries, or flowers, contain phytochemicals such as carotenoids and polyphenols, including phenolic acids, alkaloids, flavonoids, glycosides, saponins, and lignans which are thought to provide health benefits. Epidemiological studies suggest that increased intake of dietary flavonoids is associated with a reduced risk of chronic diseases, including some cancers and cardiovascular diseases.86
Several studies highlight the role of herbal supplements in reducing exercise induced oxidative stress in athletes.60 However, herbal supplements also possess pro-oxidant activities when consumed in high doses or when metal ions are present; their pro-oxidant and/or antioxidant activity are dose dependent.87 Pro-oxidative activities of several polyphenols, such as quercetin, catechins, and gallic acid, occur in cell models.88 Cell survival and viability, thiol content, total antioxidant capacity, and SOD, CAT, and GST activities are reduced by quercetin at concentrations as low as 50 μM.89 Quercetin (50–250 μM) causes cytotoxicity, damage to DNA, apoptosis, and production of respiratory bursts, resulting in the generation of O2- and H2O2.90 High concentrations of phenolic antioxidants have pro-oxidant activities when transition metal ions such as iron and copper are present, forming chelators and reducing the antioxidant capacity.91
Phenolic antioxidants are converted to phenoxyl radicals, which in biological systems, can be the basis of a cascade of pro-oxidative events that are characterized first by autoxidation of a diphenol or polyphenol, concomitant with a univalent reduction of molecular oxygen, followed by dismutation of the O2- formed, and subsequent formation of hydroxyl radicals in a Fenton-type reaction.92 These diphenolic compounds are more cytotoxic than monophenolic substances because they produce larger quantities of reactive oxygen metabolites in the extracellular space.93 Quercetin can also interfere with mitochondrial biogenesis by decreasing mitochondrial copy number via decreased DNA polymerase subunit gamma (POLG) expression and excessive mitochondrial transcription factor A (TFAM) expression in irradiated murine bone marrow; these effects are not observed during total body irradiation without quercetin.94
Remarkably, the use of herbal antioxidants as the exogenous supplementation, do not have the recommended dietary allowance and the tolerable upper intake level in human. Moreover, the mechanisms of action of most herbal antioxidants are still not fully understood, including their potential beneficial effects on athletic performance, side effects, optimal dosage, and misuse. Clearly, more detailed research is need to understand the role of herbal supplements in antioxidant defenses and performance in athletes. Thus, herbal antioxidants are not currently recommended for athletes.
5. Summary
The consumption of antioxidants produces dose-related effects, with beneficial effects occurring at low doses and negative effects occurring when high doses are consumed.95 The negative effects of antioxidant supplementation occur when consuming doses that are 5–17 times greater than the RDA, while doses of antioxidants based on the RDA may be sufficient to maintain the body's antioxidant defenses, even for competitive endurance athletes.96
There are potential negative effects related to consuming high doses of antioxidants by athletes and individuals participating in regular exercise.97 Long-term administration of high doses of antioxidants inhibits redox-sensitive signaling pathways, and reduces exercise-induced physiological adaptations such as mitochondrial biogenesis, improved antioxidant capacity, increased insulin sensitivity, and muscle hypertrophy.98, 99, 100
In conclusion, ingestion of supposedly “healthy “compounds, such as antioxidants, vitamins and some herbal compounds can not only be ineffective but could also aggravate oxidative stress in athletes. Thus, athletes should be advised to avoid consuming high-doses of antioxidants for extended periods as this can potentially inhibit exercise induced physiological adaptations of skeletal muscle and cardiovascular health.
Author contributions
Drs. Shunchang Li and Babatunde Fasipe performed the literature search and data analysis, and Drs. Shunchang Li and Ismail Laher drafted and critically revised the manuscript.
Declaration of competing interest
The authors declare no conflict of interest.
Acknowledgements
This work was supported by National Natural Science Foundation of China [grant number 31971104].
Contributor Information
Shunchang Li, Email: lishunchang_1983@163.com.
Babatunde Fasipe, Email: fasipe.adegbola@gmail.com.
Ismail Laher, Email: ilaher@mail.ubc.ca.
References
- 1.Rodriguez N.R., Di Marco N.M., Langley S. American College of Sports Medicine position stand. Nutrition and athletic performance. Med Sci Sports Exerc. 2009;41(3):709–731. doi: 10.1249/MSS.0b013e31890eb86. [DOI] [PubMed] [Google Scholar]
- 2.Muñoz D., Barrientos Vicho G., Alves Vas J., et al. Oxidative stress, lipid peroxidation indexes and antioxidant vitamins in long and middle distance athletes during a sport season. J Sports Med Phys Fit. 2017;58(12):1713–1719. doi: 10.23736/S0022-4707.17.07887-2. [DOI] [PubMed] [Google Scholar]
- 3.Thirupathi A., Wang M., Lin J.K., et al. Effect of different exercise modalities on oxidative stress: a systematic review. BioMed Res Int. 2021 doi: 10.1155/2021/1947928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Morales-Alamo D., Calbet J.A.L. AMPK signaling in skeletal muscle during exercise: role of reactive oxygen and nitrogen species. Free Radic Biol Med. 2016;98:68–77. doi: 10.1016/j.freeradbiomed.2016.01.012. [DOI] [PubMed] [Google Scholar]
- 5.Pesta D., Roden M. The janus head of oxidative stress in metabolic diseases and during physical exercise. Curr Diabetes Rep. 2017;17(6):41. doi: 10.1007/s11892-017-0867-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Buzina R., Suboticanec K. Vitamin C and physical working capacity. Int J Vitam Nutr Res Suppl. 1985;27:157–166. [PubMed] [Google Scholar]
- 7.Ogrunc M. Reactive oxygen species: the good, the bad, and the enigma. Mol Cell Oncol. 2014;1(3) doi: 10.4161/23723548.2014.964033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gomez-Cabrera M.C., Domenech E., Viña J. Moderate exercise is an antioxidant: upregulation of antioxidant genes by training. Free Radic Biol Med. 2008;44(2):126–131. doi: 10.1016/j.freeradbiomed.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 9.Vargas-Mendoza N., Angeles-Valencia M., Morales-González Á., et al. Oxidative stress, mitochondrial function and adaptation to exercise: new perspectives in nutrition. Life. 2021;11(11) doi: 10.3390/life11111269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ji L.L., Yeo D., Kang C., et al. The role of mitochondria in redox signaling of muscle homeostasis. J Sport Health Sci. 2020;9(5):386–393. doi: 10.1016/j.jshs.2020.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gomberg M. An instance of trivalent carbon:triphenylmethyl. J Am Chem Soc. 1900;20:757–771. [Google Scholar]
- 12.Kharasch M.S., Margolis E., White P.C., et al. The peroxide effect in the halogenation of aromatic side chains. J Am Chem Soc. 1937;59(7):1405–1406. [Google Scholar]
- 13.Harman D. The free radical theory of aging: effect of age on serum copper levels. J Gerontol. 1965;20:151–153. doi: 10.1093/geronj/20.2.151. [DOI] [PubMed] [Google Scholar]
- 14.Dillard C.J., Litov R.E., Savin W.M., et al. Effects of exercise, vitamin E, and ozone on pulmonary function and lipid peroxidation. J Appl Physiol. 1978;45(6):927–932. doi: 10.1152/jappl.1978.45.6.927. [DOI] [PubMed] [Google Scholar]
- 15.Gomez-Cabrera M.C., Carretero A., Millan-Domingo F., et al. Redox-related biomarkers in physical exercise. Redox Biol. 2021;42 doi: 10.1016/j.redox.2021.101956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Simioni C., Zauli G., Martelli A.M., et al. Oxidative stress: role of physical exercise and antioxidant nutraceuticals in adulthood and aging. Oncotarget. 2018;9(24):17181–17198. doi: 10.18632/oncotarget.24729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lessiani G., Santilli F., Boccatonda A., et al. Arterial stiffness and sedentary lifestyle: role of oxidative stress. Vasc Pharmacol. 2016;79:1–5. doi: 10.1016/j.vph.2015.05.017. [DOI] [PubMed] [Google Scholar]
- 18.Droge W. Free radicals in the physiological control of cell function. Physiol Rev. 2002;82(1):47–95. doi: 10.1152/physrev.00018.2001. [DOI] [PubMed] [Google Scholar]
- 19.Leon-Lopez J., Calderon-Soto C., Perez-Sanchez M., et al. Oxidative stress in elite athletes training at moderate altitude and at sea level. Eur J Sport Sci. 2018;18(6):832–841. doi: 10.1080/17461391.2018.1453550. [DOI] [PubMed] [Google Scholar]
- 20.Dong J., Chen P., Wang R., et al. NADPH oxidase: a target for the modulation of the excessive oxidase damage induced by overtraining in rat neutrophils. Int J Biol Sci. 2011;7(6):881–891. doi: 10.7150/ijbs.7.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bjorklund G., Chirumbolo S. Role of oxidative stress and antioxidants in daily nutrition and human health. Nutrition. 2017;33:311–321. doi: 10.1016/j.nut.2016.07.018. [DOI] [PubMed] [Google Scholar]
- 22.Margaritelis N., Theodorou A., Paschalis V., et al. Adaptations to endurance training depend on exercise-induced oxidative stress: exploiting redox interindividual variability. Acta Physiol. 2017;222 doi: 10.1111/apha.12898. [DOI] [PubMed] [Google Scholar]
- 23.Parker L., Shaw C.S., Stepto N.K., et al. Exercise and glycemic control: focus on redox homeostasis and redox-sensitive protein signaling. Front Endocrinol. 2017;8:87. doi: 10.3389/fendo.2017.00087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Korge P., Calmettes G., Weiss J.N. Increased reactive oxygen species production during reductive stress: the roles of mitochondrial glutathione and thioredoxin reductases. Biochim Biophys Acta. 2015;1847(6-7):514–525. doi: 10.1016/j.bbabio.2015.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Powers S., Deruisseau K., Quindry J., et al. Dietary antioxidants and exercise. J Sports Sci. 2004;22:81–94. doi: 10.1080/0264041031000140563. [DOI] [PubMed] [Google Scholar]
- 26.Thomas D., Burke L., Erdman K. Nutrition and athletic performance. Med Sci Sports Exerc. 2016;48:543–568. doi: 10.1249/MSS.0000000000000852. [DOI] [PubMed] [Google Scholar]
- 27.Bellezza I., Giambanco I., Minelli A., et al. Nrf2-Keap1 signaling in oxidative and reductive stress. Biochim Biophys Acta Mol Cell Res. 2018;1865(5):721–733. doi: 10.1016/j.bbamcr.2018.02.010. [DOI] [PubMed] [Google Scholar]
- 28.Mello R., Mello R., Gomes D., et al. Oxidative stress and antioxidant biomarker responses after a moderate-intensity soccer training session. Res Sports Med. 2017;25(3):322–332. doi: 10.1080/15438627.2017.1345738. [DOI] [PubMed] [Google Scholar]
- 29.Wang F., Wang X. Effects of exercise-induced ROS on the pathophysiological functions of skeletal muscle. Oxid Med Cell Longev. 2021 doi: 10.1155/2021/3846122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Simon-Schnass I., Pabst H. Influence of vitamin E on physical performance. Int J Vitam Nutr Res. 1988;58(1):49–54. [PubMed] [Google Scholar]
- 31.Piercy R.J., Hinchcliff K.W., Morley P.S., et al. Association between vitamin E and enhanced athletic performance in sled dogs. Med Sci Sports Exerc. 2001;33(5):826–833. doi: 10.1097/00005768-200105000-00024. [DOI] [PubMed] [Google Scholar]
- 32.Hoogerwerf A., Hoitink A.W. The influence of vitamin c administration on the mechanical efficiency of the human organism. Int Z Angew Physiol. 1963;20:164–172. doi: 10.1007/BF00699449. [DOI] [PubMed] [Google Scholar]
- 33.Howald H., Segesser B., Körner W.F. Ascorbic acid and athletic performance. Ann N Y Acad Sci. 1975;258:458–464. doi: 10.1111/j.1749-6632.1975.tb29304.x. [DOI] [PubMed] [Google Scholar]
- 34.Aguiló A., Tauler P., Sureda A., et al. Antioxidant diet supplementation enhances aerobic performance in amateur sportsmen. J Sports Sci. 2007;25(11):1203–1210. doi: 10.1080/02640410600951597. [DOI] [PubMed] [Google Scholar]
- 35.Bjelakovic G., Nikolova D., Gluud L.L., et al. Mortality in randomized trials of antioxidant supplements for primary and secondary prevention: systematic review and meta-analysis. JAMA. 2007;297(8):842–857. doi: 10.1001/jama.297.8.842. [DOI] [PubMed] [Google Scholar]
- 36.Pădureanu V., Florescu D.N., Pădureanu R., et al. Role of antioxidants and oxidative stress in the evolution of acute pancreatitis (Review) Exp Ther Med. 2022;23(3):197. doi: 10.3892/etm.2022.11120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang V.X., Sze K.M., Chan L.K., et al. Antioxidant supplements promote tumor formation and growth and confer drug resistance in hepatocellular carcinoma by reducing intracellular ROS and induction of TMBIM1. Cell Biosci. 2021;11(1):217. doi: 10.1186/s13578-021-00731-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gomez-Cabrera M.C., Borrás C., Pallardó F.V., et al. Decreasing xanthine oxidase-mediated oxidative stress prevents useful cellular adaptations to exercise in rats. J Physiol. 2005;567(Pt 1):113–120. doi: 10.1113/jphysiol.2004.080564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Knez W.L., Peake J.M. The prevalence of vitamin supplementation in ultraendurance triathletes. Int J Sport Nutr Exerc Metabol. 2010;20(6):507–514. doi: 10.1123/ijsnem.20.6.507. [DOI] [PubMed] [Google Scholar]
- 40.Weight L.M., Myburgh K.H., Noakes T.D. Vitamin and mineral supplementation: effect on the running performance of trained athletes. Am J Clin Nutr. 1988;47(2):192–195. doi: 10.1093/ajcn/47.2.192. [DOI] [PubMed] [Google Scholar]
- 41.Close G.L., Ashton T., Cable T., et al. Ascorbic acid supplementation does not attenuate post-exercise muscle soreness following muscle-damaging exercise but may delay the recovery process. Br J Nutr. 2006;95(5):976–981. doi: 10.1079/bjn20061732. [DOI] [PubMed] [Google Scholar]
- 42.Teixeira V.H., Valente H.F., Casal S.I., et al. Antioxidants do not prevent postexercise peroxidation and may delay muscle recovery. Med Sci Sports Exerc. 2009;41(9):1752–1760. doi: 10.1249/MSS.0b013e31819fe8e3. [DOI] [PubMed] [Google Scholar]
- 43.Merry T.L., Ristow M. Do antioxidant supplements interfere with skeletal muscle adaptation to exercise training? J Physiol. 2016;594(18):5135–5147. doi: 10.1113/JP270654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Antonioni A., Fantini C., Dimauro I., et al. Redox homeostasis in sport: do athletes really need antioxidant support? Res Sports Med. 2019;27(2):147–165. doi: 10.1080/15438627.2018.1563899. [DOI] [PubMed] [Google Scholar]
- 45.Gomez-Cabrera M.-C., Domenech E., Romagnoli M., et al. Oral administration of vitamin C decreases muscle mitochondrial biogenesis and hampers training-induced adaptations in endurance performance. Am J Clin Nutr. 2008;87(1):142–149. doi: 10.1093/ajcn/87.1.142. [DOI] [PubMed] [Google Scholar]
- 46.Close G.L., Ashton T., Cable T., et al. Ascorbic acid supplementation does not attenuate post-exercise muscle soreness following muscle-damaging exercise but may delay the recovery process. Br J Nutr. 2006;95(5):976–981. doi: 10.1079/bjn20061732. [DOI] [PubMed] [Google Scholar]
- 47.Braakhuis A.J., Hopkins W.G., Lowe T.E. Effects of dietary antioxidants on training and performance in female runners. Eur J Sport Sci. 2014;14(2):160–168. doi: 10.1080/17461391.2013.785597. [DOI] [PubMed] [Google Scholar]
- 48.Bryant R.J., Ryder J., Martino P., et al. Effects of vitamin E and C supplementation either alone or in combination on exercise-induced lipid peroxidation in trained cyclists. J Strength Condit Res. 2003;17(4):792–800. doi: 10.1519/1533-4287(2003)017<0792:eoveac>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 49.Sharman I.M., Down M.G., Sen R.N. The effects of vitamin E and training on physiological function and athletic performance in adolescent swimmers. Br J Nutr. 1971;26(2):265–276. doi: 10.1079/bjn19710033. [DOI] [PubMed] [Google Scholar]
- 50.McBride J.M., Kraemer W.J., Triplett-McBride T., et al. Effect of resistance exercise on free radical production. Med Sci Sports Exerc. 1998;30(1):67–72. doi: 10.1097/00005768-199801000-00010. [DOI] [PubMed] [Google Scholar]
- 51.Nieman D.C., Henson D.A., McAnulty S.R., et al. Vitamin E and immunity after the kona triathlon world championship. Med Sci Sports Exerc. 2004;36(8):1328–1335. doi: 10.1249/01.mss.0000135778.57355.ca. [DOI] [PubMed] [Google Scholar]
- 52.Miller E., Pastor-Barriuso R., Dalal D., et al. Meta-analysis: high-dosage vitamin E supplementation may increase all-cause mortality. Ann Intern Med. 2005;142(1):37–46. doi: 10.7326/0003-4819-142-1-200501040-00110. [DOI] [PubMed] [Google Scholar]
- 53.Ristow M., Zarse K., Oberbach A., et al. Antioxidants prevent health-promoting effects of physical exercise in humans. Proc Natl Acad Sci U S A. 2009;106(21):8665–8670. doi: 10.1073/pnas.0903485106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Paulsen G., Cumming K.T., Holden G., et al. Vitamin C and E supplementation hampers cellular adaptation to endurance training in humans: a double-blind, randomised, controlled trial. J Physiol. 2014;592(8):1887–1901. doi: 10.1113/jphysiol.2013.267419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yfanti C., Fischer C.P., Nielsen S., et al. Role of vitamin C and E supplementation on IL-6 in response to training. J Appl Physiol. 2012;112(6):990–1000. doi: 10.1152/japplphysiol.01027.2010. [DOI] [PubMed] [Google Scholar]
- 56.Wyckelsma V.L., Venckunas T., Brazaitis M., et al. Vitamin C and E treatment blunts sprint interval training-induced changes in inflammatory mediator-, calcium-, and mitochondria-related signaling in recreationally active elderly humans. Antioxidants. 2020;9(9) doi: 10.3390/antiox9090879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gliemann L., Schmidt J.F., Olesen J., et al. Resveratrol blunts the positive effects of exercise training on cardiovascular health in aged men. J Physiol. 2013;591(20):5047–5059. doi: 10.1113/jphysiol.2013.258061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bouayed J., Bohn T. Exogenous antioxidants--Double-edged swords in cellular redox state: health beneficial effects at physiologic doses versus deleterious effects at high doses. Oxid Med Cell Longev. 2010;3(4):228–237. doi: 10.4161/oxim.3.4.12858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Roehrs M., Valentini J., Paniz C., et al. The relationships between exogenous and endogenous antioxidants with the lipid profile and oxidative damage in hemodialysis patients. BMC Nephrol. 2011;12:59. doi: 10.1186/1471-2369-12-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hamishekar H., Ranjdoost F., Asgharian P., et al. Vitamins, are they safe? Adv Pharmaceut Bull. 2016;6(4):467–477. doi: 10.15171/apb.2016.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Howald H., Segesser B., Korner W.F. Ascorbic acid and athletic performance. Ann N Y Acad Sci. 1975;258:458–464. doi: 10.1111/j.1749-6632.1975.tb29304.x. [DOI] [PubMed] [Google Scholar]
- 62.Crilly M., Tryon L., Erlich A., et al. The role of Nrf2 in skeletal muscle contractile and mitochondrial function. J Appl Physiol. 2016;121(3):730–740. doi: 10.1152/japplphysiol.00042.2016. 1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Burton G.W., Joyce A., Ingold K.U. Is vitamin E the only lipid-soluble, chain-breaking antioxidant in human blood plasma and erythrocyte membranes? Arch Biochem Biophys. 1983;221(1):281–290. doi: 10.1016/0003-9861(83)90145-5. [DOI] [PubMed] [Google Scholar]
- 64.Gross M., Baum O., Hoppeler H. Antioxidant supplementation and endurance training: win or loss? Eur J Sport Sci. 2011;11(1):27–32. [Google Scholar]
- 65.Higgins M.R., Izadi A., Kaviani M. Antioxidants and exercise performance: with a focus on vitamin E and C supplementation. Int J Environ Res Publ Health. 2020;17(22) doi: 10.3390/ijerph17228452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ristow M., Zarse K., Oberbach A., et al. Antioxidants prevent health-promoting effects of physical exercise in humans. Proc Natl Acad Sci U S A. 2009;106(21):8665–8670. doi: 10.1073/pnas.0903485106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bjørnsen T., Salvesen S., Berntsen S., et al. Vitamin C and E supplementation blunts increases in total lean body mass in elderly men after strength training. Scand J Med Sci Sports. 2016;26(7):755–763. doi: 10.1111/sms.12506. [DOI] [PubMed] [Google Scholar]
- 68.Knez W.L., Jenkins D.G., Coombes J.S. Oxidative stress in half and full Ironman triathletes. Med Sci Sports Exerc. 2007;39(2):283–288. doi: 10.1249/01.mss.0000246999.09718.0c. [DOI] [PubMed] [Google Scholar]
- 69.Heffernan S.M., Horner K., De Vito G., et al. The role of mineral and trace element supplementation in exercise and athletic performance: a systematic review. Nutrients. 2019;11(3) doi: 10.3390/nu11030696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kieliszek M., Lipinski B. Selenium supplementation in the prevention of coronavirus infections (COVID-19) Med Hypotheses. 2020;143 doi: 10.1016/j.mehy.2020.109878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Savory L.A., Kerr C.J., Whiting P., et al. Selenium supplementation and exercise: effect on oxidant stress in overweight adults. Obesity. 2012;20(4):794–801. doi: 10.1038/oby.2011.83. [DOI] [PubMed] [Google Scholar]
- 72.Margaritis I., Tessier F., Prou E., et al. Effects of endurance training on skeletal muscle oxidative capacities with and without selenium supplementation. J Trace Elem Med Biol. 1997;11(1):37–43. doi: 10.1016/S0946-672X(97)80008-9. [DOI] [PubMed] [Google Scholar]
- 73.Zamora A.J., Tessier F., Marconnet P., et al. Mitochondria changes in human muscle after prolonged exercise, endurance training and selenium supplementation. Eur J Appl Physiol Occup Physiol. 1995;71(6):505–511. doi: 10.1007/BF00238552. [DOI] [PubMed] [Google Scholar]
- 74.Sun H.J., Rathinasabapathi B., Wu B., et al. Arsenic and selenium toxicity and their interactive effects in humans. Environ Int. 2014;69:148–158. doi: 10.1016/j.envint.2014.04.019. [DOI] [PubMed] [Google Scholar]
- 75.Fernández-Lázaro D., Fernandez-Lazaro C.I., Mielgo-Ayuso J., et al. The role of selenium mineral trace element in exercise: antioxidant defense system, muscle performance, hormone response, and athletic performance. A systematic review. Nutrients. 2020;12(6) doi: 10.3390/nu12061790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Shanmugam G., Wang D., Gounder S.S., et al. Reductive stress causes pathological cardiac remodeling and diastolic dysfunction. Antioxidants Redox Signal. 2020;32(18):1293–1312. doi: 10.1089/ars.2019.7808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Xiao W., Loscalzo J. Metabolic responses to reductive stress. Antioxidants Redox Signal. 2020;32(18):1330–1347. doi: 10.1089/ars.2019.7803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Pal R., Palmieri M., Loehr J.A., et al. Src-dependent impairment of autophagy by oxidative stress in a mouse model of Duchenne muscular dystrophy. Nat Commun. 2014;5:4425. doi: 10.1038/ncomms5425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kimmelman A.C., White E. Autophagy and tumor metabolism. Cell Metabol. 2017;25(5):1037–1043. doi: 10.1016/j.cmet.2017.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Jiao J., Demontis F. Skeletal muscle autophagy and its role in sarcopenia and organismal aging. Curr Opin Pharmacol. 2017;34:1–6. doi: 10.1016/j.coph.2017.03.009. [DOI] [PubMed] [Google Scholar]
- 81.White E. Deconvoluting the context-dependent role for autophagy in cancer. Nat Rev Cancer. 2012;12(6):401–410. doi: 10.1038/nrc3262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Castrejón-Tellez V., Rodríguez-Pérez J.M., Pérez-Torres I., et al. The effect of resveratrol and quercetin treatment on PPAR mediated uncoupling protein (UCP-) 1, 2, and 3 expression in visceral white adipose tissue from metabolic syndrome rats. Int J Mol Sci. 2016;17(7):1069. doi: 10.3390/ijms17071069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Posadino A.M., Cossu A., Giordo R., et al. Resveratrol alters human endothelial cells redox state and causes mitochondrial-dependent cell death. Food Chem Toxicol. 2015;78:10–16. doi: 10.1016/j.fct.2015.01.017. [DOI] [PubMed] [Google Scholar]
- 84.Pasciu V., Maria P., Cossu A., et al. Akt downregulation by flavin oxidase-induced ROS generation mediates dose-dependent endothelial cell damage elicited by natural antioxidants. Toxicol Sci. 2009;114(1):101–112. doi: 10.1093/toxsci/kfp301. [DOI] [PubMed] [Google Scholar]
- 85.Galati G., O'Brien P.J. Potential toxicity of flavonoids and other dietary phenolics: significance for their chemopreventive and anticancer properties. Free Radic Biol Med. 2004;37(3):287–303. doi: 10.1016/j.freeradbiomed.2004.04.034. [DOI] [PubMed] [Google Scholar]
- 86.Hollman P.C.H., Geelen A., Kromhout D. Dietary flavonol intake may lower stroke risk in men and women. J Nutr. 2010;140(3):600–604. doi: 10.3945/jn.109.116632. [DOI] [PubMed] [Google Scholar]
- 87.Wätjen W., Michels G., Steffan Br, et al. Low concentrations of flavonoids are protective in rat H4IIE cells whereas high concentrations cause DNA damage and apoptosis. J Nutr. 2005;135(3):525–531. doi: 10.1093/jn/135.3.525. [DOI] [PubMed] [Google Scholar]
- 88.Sergedien≐ E., Jönsson K., Szymusiak H., et al. Prooxidant toxicity of polyphenolic antioxidants to HL-60 cells: description of quantitative structure-activity relationships. FEBS Lett. 1999;462(3):392–396. doi: 10.1016/s0014-5793(99)01561-6. [DOI] [PubMed] [Google Scholar]
- 89.Robaszkiewicz A., Balcerczyk A., Bartosz G. Antioxidative and prooxidative effects of quercetin on A549 cells. Cell Biol Int. 2007;31(10):1245–1250. doi: 10.1016/j.cellbi.2007.04.009. [DOI] [PubMed] [Google Scholar]
- 90.Hodnick W.F., Kung F.S., Roettger W.J., et al. Inhibition of mitochondrial respiration and production of toxic oxygen radicals by flavonoids: a structure-activity study. Biochem Pharmacol. 1986;35(14):2345–2357. doi: 10.1016/0006-2952(86)90461-2. [DOI] [PubMed] [Google Scholar]
- 91.Decker E.A. Phenolics: prooxidants or antioxidants? Nutr Rev. 1997;55(11 Pt 1):396–398. doi: 10.1111/j.1753-4887.1997.tb01580.x. [DOI] [PubMed] [Google Scholar]
- 92.Passi S., Picardo M., Nazzaro-Porro M. Comparative cytotoxicity of phenols in vitro. Biochem J. 1987;245(2):537–542. doi: 10.1042/bj2450537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Mostafavi-Pour Z., Ramezani F., Keshavarzi F., et al. The role of quercetin and vitamin C in Nrf2-dependent oxidative stress production in breast cancer cells. Oncol Lett. 2017;13(3):1965–1973. doi: 10.3892/ol.2017.5619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Chen R., Lin J., Hong J., et al. Potential toxicity of quercetin: the repression of mitochondrial copy number via decreased POLG expression and excessive TFAM expression in irradiated murine bone marrow. Toxicol Rep. 2014;30(1):450–458. doi: 10.1016/j.toxrep.2014.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Neubauer O., Yfanti C. In: Antioxidants in Sport Nutrition. Boca Raton (FL): CRC Press/Taylor & Francis (C) 2015. Lamprecht M., editor. Taylor & Francis Group, LLC.; 2015. Antioxidants in athlete's basic nutrition: considerations towards a guideline for the intake of vitamin C and vitamin E. [PubMed] [Google Scholar]
- 96.Mason S.A., Trewin A.J., Parker L., et al. Antioxidant supplements and endurance exercise: current evidence and mechanistic insights. Redox Biol. 2020;35 doi: 10.1016/j.redox.2020.101471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Gomez-Cabrera M.C., Ristow M., Vina J. Antioxidant supplements in exercise: worse than useless? Am J Physiol Endocrinol Metab. 2012;302(4):E476–E477. doi: 10.1152/ajpendo.00567.2011. author reply E8-9. [DOI] [PubMed] [Google Scholar]
- 98.Merry T.L., Ristow M. Do antioxidant supplements interfere with skeletal muscle adaptation to exercise training? J Physiol. 2016;594(18):5135–5147. doi: 10.1113/JP270654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Peternelj T.T., Coombes J.S. Antioxidant supplementation during exercise training: beneficial or detrimental? Sports Med. 2011;41(12):1043–1069. doi: 10.2165/11594400-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 100.Nikolaidis M.G., Kerksick C.M., Lamprecht M., et al. Does vitamin C and E supplementation impair the favorable adaptations of regular exercise? Oxid Med Cell Longev. 2012 doi: 10.1155/2012/707941. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]