Summary
Background
Kidney benefits have been demonstrated for both sodium-glucose cotransporter 2 inhibitors (SGLT2i) and glucagon-like peptide-1 receptor agonists (GLP1RA) compared with placebo in patients with type 2 diabetes. This study aimed to compare the impacts of SGLT2i and GLP1RA on the trend of estimated glomerular filtration rate (eGFR) and other kidney outcomes.
Methods
Using a real-world population-based database, the Hong Kong Hospital Authority (HA) database, of patients with type 2 diabetes between January 2008 and December 2020, patients started on SGLT2i were compared with those started on GLP1RA, with one-to-one propensity-score matching. Primary outcome was a composite of sustained ≥50% eGFR decline, end-stage kidney disease (ESKD), incident macroalbuminuria and kidney-related mortality. Secondary outcome was the rate of eGFR decline.
Findings
A total of 2551 SGLT2i and 2551 GLP1RA new users were analyzed. At baseline, mean age was 56·2 years, with mean eGFR 78·0 mL/min/1·73m2 and 11·9% having macroalbuminuria. Upon median follow-up of 13 months (IQR: 5-27), SGLT2i users had a lower risk of composite kidney outcomes (HR=0·77, 95%CI 0·62–0·96, p = 0·02), mainly driven by a reduction in ESKD (HR=0·53, p = 0·01). SGLT2i users also tended to have a lower risk of incident macroalbuminuria (HR=0·74, p = 0·05). Subgroup analyses of the benefits of SGLT2i use on composite kidney outcomes did not reveal interaction by age, sex, baseline eGFR/albuminuria status, hemoglobin A1c (HbA1c) and renin-angiotensin-system inhibitor use. Furthermore, SGLT2i users had a slower eGFR decline than GLP1RA users (SGLT2i: -1·19 mL/min/1·73m2/year, GLP1RA: -1·95 mL/min/1·73m2/year, p < 0·01).
Interpretation
Our results suggest that SGLT2i might be superior to GLP1RA in reducing kidney outcomes among patients with type 2 diabetes. Future trials are needed to corroborate our findings.
Funding
None.
Keywords: Diabetes mellitus, type 2; GLP-1 analogue; Incretins; Kidney outcomes; Sodium-glucose transporter 2 inhibitors
Research in context.
Evidence before this study
Both sodium-glucose cotransporter 2 inhibitors (SGLT2i) and glucagon-like peptide-1 receptor agonists (GLP1RA) have renal benefits compared with placebo in patients with type 2 diabetes. We searched PubMed using the MeSH terms “type 2 diabetes mellitus”, “GLP-1 analogue”, “sodium-glucose transporter 2 inhibitors”, and “kidney outcomes” for articles without language restriction published up to May 6, 2022. We could not find any head-to-head clinical trials directly comparing the renal effects of these two classes of agents, and only limited data are available from real world cohort studies and network meta-analyses.
Added value of this study
Using a propensity-score matched population-based cohort, upon median follow-up of 13 months, SGLT2i users had a significantly lower risk of composite kidney outcomes than GLP1RA. This was mainly driven by a reduction in incident end-stage kidney disease. SGLT2i users also tended to have a lower risk of incident macroalbuminuria. In addition, SGLT2i users had a significantly slower eGFR decline than GLP1RA users.
Implications of all the available evidence
Based on real-world evidence, our results suggest that SGLT2i might be superior to GLP1RA in reducing kidney outcomes among patients with type 2 diabetes.
Alt-text: Unlabelled box
Introduction
Chronic kidney disease (CKD) is a major burden in patients with type 2 diabetes. The regulatory requirement of cardiovascular outcome trials (CVOTs) for all novel anti-diabetic agents has led to the unexpected discovery of the cardiovascular and kidney benefits associated with sodium-glucose cotransporter 2 inhibitors (SGLT2i) and glucagon-like peptide-1 receptor agonist (GLP1RA).1 Four CVOTs have explored composite kidney outcomes as secondary analyses: EMPA-REG for empagliflozin,2 DECLARE-TIMI 58 for dapagliflozin3 and CANVAS for canagliflozin4 showing >40% reduction in composite kidney outcomes; and VERTIS for ertugliflozin5 showing 20% reduction in composite kidney outcomes. To date, there are two large randomized controlled trial (RCT) of SGLT2i dedicated to examining kidney outcomes in type 2 diabetes patients with CKD (CREDENCE for canagliflozin6 and DAPA-CKD trial for dapagliflozin7), which confirmed the kidney benefits demonstrated in the four CVOTs as mentioned above. For GLP1RA, there is yet a dedicated RCT to examine the primary kidney outcomes. All the available reno-protective effects of GLP1RA were derived from secondary analyses in some of the CVOTs including LEADER for liraglutide,8 SUSTAIN-6 for semaglutide,9 EXSCEL for exenatide10 and REWIND for dulaglutide.11 The kidney benefits were mainly driven by benefits on new-onset macroalbuminuria. In the latest meta-analysis, including the recent AMPLITUDE-O for efpeglenatide, GLP1RA showed kidney benefits, including a predominantly eGFR-based kidney outcome.12 The dedicated RCT for GLP1RA on kidney outcomes in type 2 diabetes, the FLOW trial for semaglutide, is ongoing with results eagerly awaited.
To date, there is yet a head-to-head RCT comparing SGLT2i and GLP1RA for their kidney benefits. Although there was a network meta-analysis of nine RCTs of SGLT2i/GLP1RA vs placebo suggesting potential superiority of SGLT2i over GLP1RA in kidney outcomes in type 2 diabetes, there were certain limitations acknowledged by the authors, including varying definitions of kidney events in different RCTs included.13 A recent Scandinavian propensity-score weighted cohort compared cardiac and kidney benefits between SGLT2i and GLP1RA users, but significant missing values of eGFR and albuminuria status limited the detailed analyses of kidney outcomes.14 Results of such direct comparison are essential to inform diabetes care providers in offering eligible patients with type 2 diabetes the optimal anti-diabetic agents in reducing kidney outcomes.
Hence, we carried out this population-based analysis of patients with type 2 diabetes who were started on SGLT2i or GLP1RA to compare their impacts on the trend of eGFR and other kidney outcomes.
Methods
Data source
Electronic medical records of patients were retrieved from the Hong Kong Hospital Authority (HA) database. The HA is a statutory body that manages all public hospitals and ambulatory clinics in Hong Kong. The service is available to all Hong Kong residents (>7·3 million), covering approximately 80% of all routine hospital admissions in Hong Kong. The database has been used in previous studies involving long-term follow-up of patients treated with various anti-diabetic medications.15, 16, 17 Patients with type 2 diabetes managed in the HA public health clinics receive regular diabetic complication screening. During each diabetic complication screening session, patients are assessed clinically and have laboratory investigations to determine their control of diabetes, its related cardiovascular risk factors and the presence of diabetic complications.18 These include assessment of eGFR and albuminuria status for diabetic kidney disease.
Study design and patient population
We conducted a propensity-score matched cohort study using a territory-wide cohort of adult patients (age ≥18 years) with type 2 diabetes managed in the HA between 1st January 2008 and 31st December 2020 in Hong Kong SAR, China. The ‘active comparator, new user’ study design19 was adopted to identify patients with type 2 diabetes who had been started on SGLT2i or GLP1RA, respectively. Index dates were defined as the date of the first SGLT2i or GLP1RA prescription. Those who had end-stage kidney disease (ESKD; defined by the need for dialysis, kidney transplantation, or eGFR <15 mL/min/1·73m2) before the index date were excluded from the analysis. eGFR measurements were determined using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation.20 To capture the eGFR slope before the index date, only those who had at least two eGFR measurements prior to the index date were included, with at least one eGFR measurement within 180 days of the index date. It was additionally required for ≥180 days between the first and the last eGFR measurements before the index date to reliably estimate the eGFR changes before the index date.21 Length of follow up was calculated from the index date until the occurrence of outcomes of interest, date of death, treatment crossover (i.e. patients crossing over from SGLT2i to GLP1RA, or vice versa), treatment discontinuation, or 31st December 2020, whichever occurred earlier.
Definition of covariates
Demographics (age and sex), anthropometric parameters (body mass index [BMI]) and blood pressure readings were obtained. A range of laboratory parameters was obtained: eGFR, HbA1c, fasting glucose, lipid profile, duration of diabetes and albuminuria status. Albuminuria status was defined as below: macroalbuminuria if urine albumin/creatinine ratio [UACR] > 34 mg/mmol Cr, microalbuminuria if UACR > 3·4 to ≤ 34 mg/mmol Cr, and normoalbuminuria if UACR ≤ 3·4 mg/mmol Cr. Furthermore, Charlson comorbidity index (CCI) and use of medications in the 6 months before the index date (insulin, metformin, sulfonylurea, thiazolidinedione, dipeptidyl peptidase-4 inhibitors, alpha-glucosidase inhibitors, angiotensin-converting enzyme inhibitors [ACEI], angiotensin receptor blockers [ARB], beta-blockers, calcium channel blockers, diuretics, other anti-hypertensive drugs, and lipid-lowering drugs) were recorded.
Definition of outcomes
The primary outcome was the composite kidney outcomes, which consisted of a sustained decline in eGFR of ≥50% (confirmed by subsequent eGFR measurement at least 30 days apart), ESKD (defined by eGFR <15 mL/min/1·73m2 [confirmed by subsequent eGFR measurement at least 30 days apart], requirement of dialysis or kidney transplantation), incident macroalbuminuria (confirmed by subsequent UACR measurement) and kidney-related mortality. The secondary outcome was the slopes of the eGFR decline after the index date.
The complete list of disease diagnosis codes, procedure codes, drug codes, and laboratory criteria for each clinical diagnosis is shown in Supplementary Table 1.
Statistical analyses
Multiple imputation by chained equations was performed to deal with missing data of laboratory parameters using other observed demographic, clinical characteristics, and drug treatments. Laboratory parameters were imputed 20 times and then used to generate multiple-imputation linear predictions by applying Rubin's combination rules.22 The data were assumed to be missing completely at random (MCAR) as the Little's MCAR test (Chi-square distance of 4434 with degrees of freedom 4408 and p-value 0.388) was not significant. A logistic regression model was constructed to estimate the propensity-score for each patient in SGLT2i and GLP1RA group through the covariates described above, including index date, age, sex, pre-existing morbidities, CCI, and drug history in the past 6 months. Patients in SGLT2i and GLP1RA groups were matched using the 1-to-1 propensity-score with a caliper width of 0·01. Standardized mean difference (SMD) was used to assess the balance of baseline covariates between SGLT2i and GLP1RA groups, with SMDs ≤0·1 indicating sufficient balance after matching.23
The crude incidence rate (per 10,000 person-years) of each outcome event in SGLT2i and GLP1RA groups was estimated. The hazard ratio (HR) and 95% confidence interval (CI) of each outcome were estimated using Cox proportional hazard regressions. Cumulative incidence curves of event outcomes were plotted. Sensitivity analysis was conducted to adjust imbalanced characteristics in Cox regression to minimize residual confounding effect.
Differences in eGFR slope at 18 months post-index date between SGLT2i and GLP1RA groups by a linear mixed regression model, while differences in eGFR slope at 12 months pre-index date were also assessed with a linear mixed regression model. Subgroup analyses by sex (male vs female), age (<65 years vs ≥65 years), albuminuria status (normo- to micro-albuminuria vs macroalbuminuria), ACEI or ARB use prior to the index date (yes vs no), and baseline HbA1c (<8% vs ≥8%) were conducted.
Several sensitivity analyses were performed: (i) we applied intention-to-treat analysis in the evaluation of various kidney outcomes among SGLT2i and GLP1RA users; and (ii) we excluded the 1st month or 1st–2nd months short-term change from eGFR slope estimation to avoid false negatives associated with the initial short-term eGFR decline,24 since an acute dip in eGFR is commonly seen following SGLT2i initiation.25,26
All statistical analyses were performed using STATA version 16·0 (StataCorp LP, College Station, Texas). All significance tests were two‐tailed. P values <0·05 were taken to indicate statistical significance. The study builds on anonymised publicly available data from official authorities and no patient-identifying data was used. Ethical approval for this study was granted by the Institutional Review Board of the University of HK/HA HK West Cluster (Reference No. UW21-320).
This study was reported according to the Reporting of studies Conducted using Observational Routinely-collected Data (RECORD), extended from the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guideline.
Role of the funding source
The funders had no roles in study design, data collection, data analysis, interpretation of the data, as well as in the writing of the report and in the decision to submit the paper for publication. DTW Lui, KCB Tan and CKH Wong had full access to the data in the study and had final responsibility for the decision to submit for publication.
Results
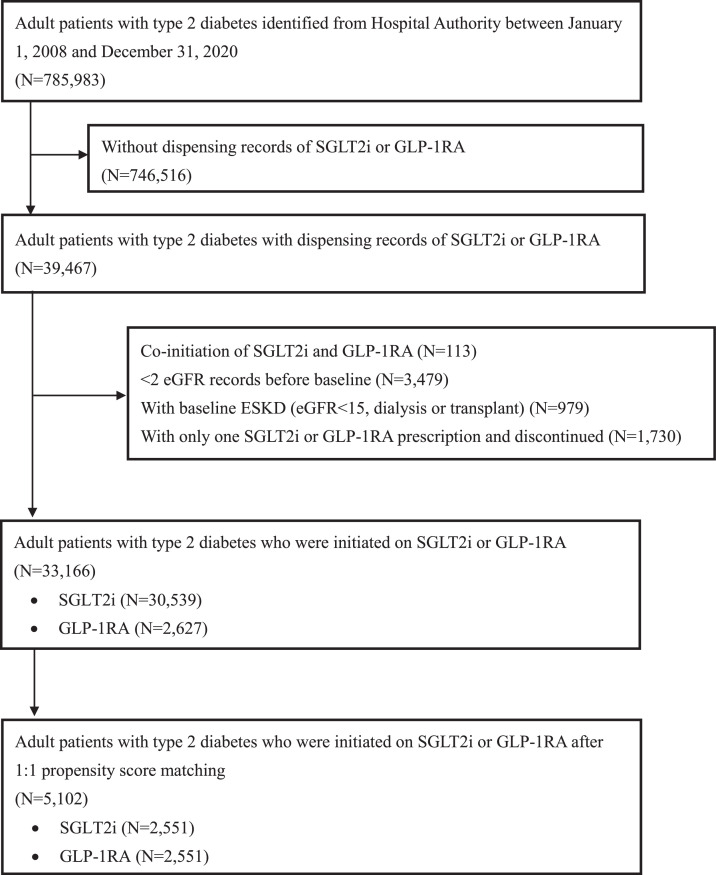
Before matching, a total of 33,166 patients with type 2 diabetes met the eligibility criteria. (Table 1) There was slightly more male predominance in SGLT2i users, with older age than GLP1RA users. On the other hand, the baseline HbA1c and kidney function reflected by eGFR were better among SGLT2i users. The mean BMI was also lower among SGLT2i users. Regarding the SGLT2i use, the majority were empagliflozin (66·2%) and dapagliflozin (33·6%). Regarding GLP1RA use, liraglutide was most prescribed (46·4%), followed by dulaglutide (30·6%) and exenatide (18·7%). After 1:1 propensity-score matching with highly overlapping propensity-score distribution (Supplementary Figure 1), 5102 patients were included in this analysis: 2551 SGLT2i users and 2551 GLP1RA users (Table 1). The study flow diagram is shown in Figure 1. All baseline characteristics were well-matched after propensity-score matching, indicated by all SMDs ≤0·1, except BMI (SMD 0·133). The cohort's mean age was 56·2±12·8 years, with slight male predominance (56·0% men). The mean BMI was 30·0±7·4 kg/m2. This cohort consisted of patients with suboptimal glycemic control (mean baseline HbA1c 8·9±1·6% [73·5±18·0 mmol/mol]) and longstanding diabetes (mean duration was 13·9 years). Most were on metformin (82·9%) and insulin (69·7%). Regarding baseline kidney function, most patients (71·2%) had eGFR ≥ 60 mL/min/1·73m2, and most (75·1%) were already on ACEI/ARB. During follow-up, among the SGLT2i group (median: 14 months; IQR: 6-30), 612 (24·0%) discontinued SGLT2i and 246 (9·6%) were co-prescribed with GLP1RA; among the GLP1RA group (median: 12 months; IQR: 4-12), 761 (29·8%) discontinued GLP1RA and 669 (26·2%) were co-prescribed with SGLT2i.
Table 1.
Baseline characteristics after multiple imputation and one-to-one propensity score matching.
| Before one-to-one propensity score matching |
After one-to-one propensity score matching |
|||||||
|---|---|---|---|---|---|---|---|---|
| Factors, mean±standard deviation or % (n) | All patients (N = 33166) | SGLT2i (N = 30,539) | GLP1RA (N = 2627) | SMD | All patients (N = 5102) | SGLT2i (N = 2551) | GLP1RA (N = 2551) | SMD† |
| Socio-demographics | ||||||||
| Sex | 0·086 | 0·000 | ||||||
| Male | 59·9% | 60·3% | 56·0% | 56·0% | 56·0% | 56·0% | ||
| Female | 40·1% | 39·7% | 44·0% | 44·0% | 44·0% | 44·0% | ||
| Age, year | 61·4±11·3 | 61·8±11·1 | 56·0±12·2 | 0·499 | 56·2±12·8 | 56·3±13·3 | 56·0±12·3 | 0·020 |
| Clinical factors | ||||||||
| HbA1c, % | 8·6±1·5 | 8·6±1·5 | 8·9±1·7 | 0·212 | 8·9±1·6 | 8·8±1·6 | 8·9±1·7 | 0·042 |
| HbA1c, mmol/mol | 70·7±16·9 | 70·4±16·7 | 74·1±18·4 | 73·5±18·0 | 73·1±17·7 | 73·9±18·3 | ||
| Fasting glucose, mmol/L | 9·2±3·2 | 9·2±3·1 | 9·6±3·6 | 0·119 | 9·5±3·5 | 9·5±3·4 | 9·5±3·6 | 0·024 |
| Systolic blood pressure, mmHg | 137·3±25·4 | 137·2±24·0 | 138·0±26·0 | 0·042 | 137·9±25·6 | 137·8±23·3 | 137·9±24·6 | 0·002 |
| Diastolic blood pressure, mmHg | 77·8±15·3 | 77·7±14·6 | 79·0±15·0 | 0·113 | 79·0±15·0 | 79·1±13·7 | 79·0±15·1 | 0·005 |
| Low-density lipoprotein cholesterol, mmol/L | 2·1±0·8 | 2·1±0·8 | 2·2±0·8 | 0·155 | 2·2±0·8 | 2·2±0·8 | 2·2±0·8 | 0·030 |
| Total cholesterol to high-density lipoprotein cholesterol ratio | 3·7±1·2 | 3·7±1·2 | 4·0±1·2 | 0·230 | 4·0±1·3 | 4·0±1·4 | 4·0±1·2 | 0·012 |
| Triglyceride, mmol/L | 1·8±1·4 | 1·8±1·4 | 2·1±1·4 | 0·198 | 2·0±1·5 | 2·0±1·7 | 2·1±1·4 | 0·013 |
| Body mass index, kg/m2 | 27·8±5·6 | 27·6±5·9 | 30·4±6·7 | 0·604 | 30·0±7·4 | 29·7±6·8 | 30·4±6·7 | 0·133 |
| eGFR, mL/min/1·73m2 | 79·4±21·5 | 79·7±20·6 | 76·6±29·4 | 0·123 | 78·0±28·3 | 78·2±28·1 | 77·9±28·6 | 0·012 |
| ≥60 | 80·4% | 81·4% | 69·0% | 0·472 | 71·2% | 71·5% | 70·9% | 0·026 |
| 45-59 | 12·5% | 12·7% | 9·8% | 10·2% | 10·3% | 10·1% | ||
| 30-44 | 5·6% | 4·9% | 14·2% | 13·6% | 13·4% | 13·8% | ||
| 15-29 | 1·4% | 0·9% | 7·0% | 5·0% | 4·7% | 5·2% | ||
| eGFR slope in pre-index period, mL/min/1·73m2/year (mean±standard error) | -2·3±0·4 | -2·4±0·4 | -1·9±0·3 | -2·4±0·3 | -2·5±0·4 | -2·3±0·2 | ||
| Rapid decline in eGFR by 3 mL/min/1·73m2/year | 34·9% | 34·6% | 38·4% | 0·079 | 37·8% | 37·7% | 37·8% | 0·001 |
| Rapid decline in eGFR by 5 mL/min/1·73m2/year | 30·4% | 30·1% | 33·7% | 0·077 | 33·4% | 33·4% | 33·3% | 0·002 |
| Duration of diabetes, year | 13·3±8·6 | 13·3±8·6 | 14·0±9·0 | 0·088 | 13·9±9·0 | 13·8±9·0 | 14·0±9·0 | 0·018 |
| Charlson Comorbidity Index | 4·5±1·8 | 4·6±1·8 | 3·9±1·8 | 0·407 | 3·9±1·9 | 3·9±2·0 | 3·9±1·8 | 0·039 |
| Albuminuria | 0·364 | 0·017 | ||||||
| Normal | 54·1% | 55·1% | 42·5% | 44·1% | 44·9% | 43·4% | ||
| Microalbuminuria | 32·5% | 32·3% | 34·3% | 34·4% | 34·0% | 34·8% | ||
| Macroalbuminuria | 13·4% | 12·6% | 23·2% | 21·5% | 21·1% | 21·9% | ||
| Use of anti-diabetic medications (6 months prior to baseline) | ||||||||
| Insulin | 41·6% | 38·9% | 72·2% | 0·711 | 69·7% | 68·1% | 71·4% | 0·073 |
| Metformin | 88·5% | 89·1% | 81·4% | 0·216 | 82·9% | 82·9% | 82·9% | 0·001 |
| Sulfonylurea | 62·4% | 63·9% | 45·1% | 0·384 | 46·2% | 46·5% | 45·8% | 0·014 |
| Thiazolidinedione | 19·5% | 19·2% | 23·1% | 0·096 | 22·5% | 22·0% | 22·9% | 0·021 |
| Dipeptidyl peptidase-4 inhibitors | 48·6% | 49·4% | 40·4% | 0·181 | 40·7% | 41·1% | 40·4% | 0·014 |
| Alpha-glucosidase inhibitors | 1·9% | 1·9% | 2·1% | 0·017 | 2·0% | 2·0% | 2·0% | 0·000 |
| Use of anti-hypertensive medications (6 months prior to baseline) | ||||||||
| Angiotensin-converting enzyme inhibitors / angiotensin receptor blockers | 71·4% | 71·0% | 76·1% | 0·115 | 75·1% | 74·4% | 75·9% | 0·034 |
| Beta blocker | 43·4% | 43·6% | 40·9% | 0·055 | 40·2% | 40·2% | 40·3% | 0·001 |
| Calcium channel blockers | 51·4% | 50·7% | 58·4% | 0·153 | 56·6% | 55·7% | 57·5% | 0·036 |
| Diuretics | 19·6% | 19·3% | 22·8% | 0·084 | 22·3% | 22·7% | 22·0% | 0·017 |
| Others anti-hypertensive drugs | 7·6% | 7·3% | 10·9% | 0·126 | 10·6% | 10·7% | 10·5% | 0·009 |
| Use of lipid-lowering agents (6 months prior to baseline) | 81·7% | 82·0% | 78·2% | 0·096 | 78·0% | 78·2% | 77·9% | 0·009 |
| Type of SGLT2i used in the first prescription | ||||||||
| Canagliflozin | NA | 0·3% (84) | NA | NA | NA | 0·4% | NA | NA |
| Dapagliflozin | 33·4% (10,193) | 36·5% | ||||||
| Empagliflozin | 66·2% (20,216) | 63·2% | ||||||
| Ertugliflozin | 0·0% (6) | 0·0% | ||||||
| Type of GLP1RA used in the first prescription | ||||||||
| Exenatide | NA | NA | 18·7% (492) | NA | NA | NA | 18·9% | NA |
| Liraglutide | 46·4% (1220) | 47·1% | ||||||
| Lixisenatide | 4·2% (111) | 4·4% | ||||||
| Dulaglutide | 30·6% (804) | 29·7% | ||||||
Abbreviations: eGFR = Estimated glomerular filtration rate; HbA1c = Hemoglobin A1c; NA = Not applicable; SMD = Standardized mean difference.
Standardized mean difference ≤0·1 indicates sufficient balance after matching.
Figure 1.
Study flow diagram.
The study flow diagram shows the inclusion andexclusion criteria for patients in SGLT2i group and GLP1RA group.
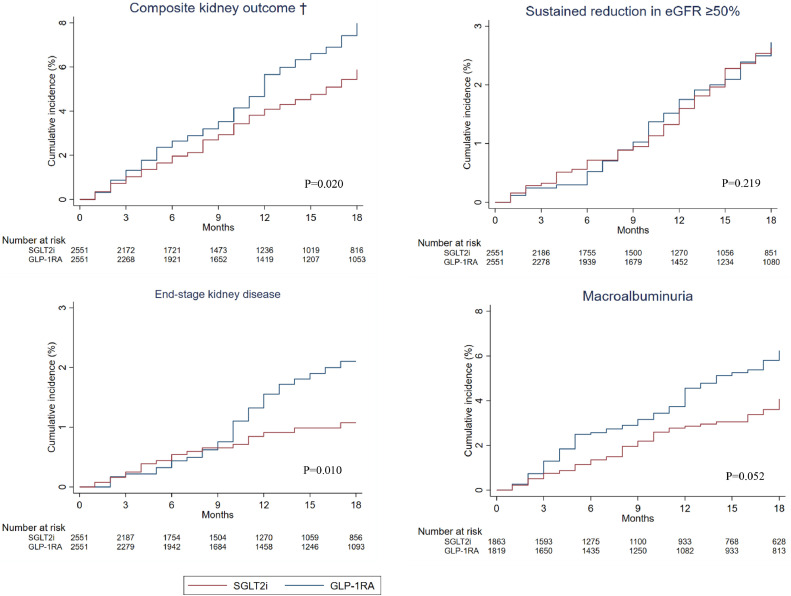
Upon a median follow-up of 13 months (IQR: 5-27), 153 (6·0%) SGLT2i users and 187 (7·3%) GLP1RA users reached the composite kidney outcomes, with a crude incidence rate of 385 and 518 per 10,000 person-years, respectively. (Table 2) As depicted in cumulative incidence plots in Figure 2, SGLT2i use was associated with a lower risk of composite kidney outcomes than GLP1RA use (HR=0·77, 95% CI 0·62–0·96, p = 0·02). When looking into the individual components of the composite kidney outcomes, SGLT2i users had a lower incidence of ESKD than GLP1RA users (HR=0·53, 95% CI 0·33–0·86, p = 0·01), although the difference in the risk of sustained reduction in eGFR ≥50% did not reach statistical significance. Furthermore, we observed a trend towards a lower risk of incident macroalbuminuria among SGLT2i users than GLP1RA users (HR=0·74, 95% CI 0·55–1·00, p = 0·05). Subgroup analyses for the composite kidney outcomes did not show significant interaction according to age, sex, baseline eGFR/albuminuria status, ACEI/ARB use, and baseline HbA1c. (Table 3) In view of the potential imbalance in BMI between SGLT2i and GLP1RA groups (SMD 0·133), sensitivity analysis was performed with further adjustment for baseline BMI for various kidney outcomes, which showed no significant difference from the main analysis. (Supplementary Table 4)
Table 2.
Risks of various kidney outcomes between SGLT2i and GLP1RA users.
| Outcomes | SGLT2i (N = 2551) |
GLP1RA (N = 2551) |
SGLT2i vs GLP1RA |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cumulative incidence |
Crude incidence rate (Events / 10,000 person-years) |
Cumulative incidence |
Crude incidence rate (Events / 10,000 person-years) |
||||||||||
| New events | Rate | Estimate | 95% CI | Follow-up person-years | New events | Rate | Estimate | 95% CI | Follow-up person-years | HR† | 95% CI | P-value | |
| Composite kidney outcome* | 153 | 6·00% | 384·5 | (326·0, 450·5) | 3979 | 187 | 7·33% | 518·1 | (446·5, 597·9) | 3610 | 0·771 | (0·620, 0·959) | 0·020 |
| Sustained reduction in eGFR ≥50% | 73 | 2·86% | 179·2 | (140·4, 225·3) | 4074 | 85 | 3·33% | 226·8 | (181·1, 280·4) | 3748 | 0·817 | (0·592, 1·127) | 0·219 |
| End-stage kidney disease | 27 | 1·06% | 65·6 | (43·2, 95·5) | 4115 | 46 | 1·80% | 120·2 | (88·0, 160·4) | 3826 | 0·532 | (0·328, 0·862) | 0·010 |
| Incident macroalbuminuria | 79 | 4·34% | 260·2 | (206·0, 324·3) | 3036 | 101 | 5·42% | 362·6 | (295·4, 440·6) | 2785 | 0·743 | (0·551, 1·003) | 0·052 |
Abbreviations: HR = Hazard ratio; CI = Confidence interval; eGFR = Estimated glomerular filtration rate.
Composite kidney outcome included sustained reduction in eGFR ≥50%, end-stage kidney disease (defined by eGFR <15mL/min/1·73m2, dialysis or kidney transplant), incident macroalbuminuria, or kidney-related mortality.
HR <1 indicates SGLT2i users had lower risks of kidney outcomes compared to GLP1RA users.
There was no death from kidney causes identified in this study during the follow-up period.
Figure 2.
Cumulative incidence plots of various kidney outcomes for SGLT2i and GLP1RA users.
† Cumulative incidence plots of composite kidney outcome, sustained reduction in eGFR ≥50%, end-stage kidney disease (defined by eGFR <15mL/min/1.73m2, dialysis or kidney transplant), and incident macroalbuminuria over 18-month follow-up. Red line represents SGLT2i group and blue line represents GLP1RA group. The table below cumulative incidence plots shows respective number of patients at risk in each group at 3-month intervals. P value refers to test of hazard ratio by Cox proportional hazard regression, with P value <0·05 indicates significant difference in risk of event outcome between SGLT2i group and GLP1RA group.
Table 3.
Subgroup analyses of the risk of composite kidney outcomes according to baseline characteristics.
| SGLT2i (N = 2551) |
GLP1RA (N = 2551) |
SGLT2i vs GLP1RA |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cumulative incidence |
Cumulative incidence |
|||||||||
| N | New events | Rate | N | New events | Rate | HR† | 95% CI | P-value | P-value for interaction | |
| Male | 1429 | 85 | 5·95% | 1429 | 114 | 7·98% | 0·720 | (0·540, 0·960) | 0·025 | 0·403 |
| Female | 1122 | 68 | 6·06% | 1122 | 73 | 6·51% | 0·854 | (0·611, 1·194) | 0·356 | |
| Age <65 | 1856 | 106 | 5·71% | 1898 | 127 | 6·69% | 0·821 | (0·630, 1·071) | 0·146 | 0·310 |
| Age ≥65 | 695 | 47 | 6·76% | 653 | 60 | 9·19% | 0·650 | (0·443, 0·955) | 0·028 | |
| Normo- to micro-albuminuria | 1986 | 117 | 5·89% | 1981 | 136 | 6·87% | 0·806 | (0·627, 1·037) | 0·094 | 0·547 |
| Macroalbuminuria | 565 | 36 | 6·37% | 570 | 51 | 8·95% | 0·674 | (0·434, 1·045) | 0·078 | |
| eGFR <60 mL/min/1·73 m2 | 727 | 61 | 8·39% | 743 | 89 | 11·98% | 0·733 | (0·526, 1·021) | 0·066 | 0·376 |
| eGFR ≥60 mL/min/1·73 m2 | 1824 | 92 | 5·04% | 1808 | 98 | 5·42% | 0·867 | (0·648, 1·161) | 0·338 | |
| With use of ACEI/ARB | 1898 | 134 | 7·06% | 1935 | 168 | 8·68% | 0·760 | (0·602, 0·959) | 0·021 | 0·498 |
| Without use of ACEI/ARB | 653 | 19 | 2·91% | 616 | 19 | 3·08% | 0·849 | (0·453, 1·592) | 0·610 | |
| HbA1c <8% | 809 | 38 | 4·70% | 742 | 44 | 5·93% | 0·841 | (0·542, 1·305) | 0·440 | 0·764 |
| HbA1c ≥8% | 1742 | 115 | 6·60% | 1809 | 143 | 7·90% | 0·750 | (0·583, 0·964) | 0·025 | |
Abbreviations: HR = Hazard ratio; CI = Confidence interval; eGFR = Estimated glomerular filtration rate; ACEI/ARB = Angiotensin-converting enzyme inhibitors/angiotensin receptor blockers.
HR <1 indicates SGLT2i users had lower risk of kidney outcomes compared to GLP1RA users.
In the intention-to-treat analysis, the hazard ratio (HR) (SGLT2i users, vs GLP1RA users as reference) for composite kidney outcome was 0·89 (95% CI 0·75 – 1·05; p = 0·157), HR for sustained reduction in eGFR ≥50% was 1·04 (95% CI 0·84 – 1·29, p = 0·726), end-stage kidney disease was 1·19 (95% CI 0·87 – 1·63, p = 0·266), and incident macroalbuminuria was 0·65 (95% CI 0·06 – 7·09, p = 0·721).
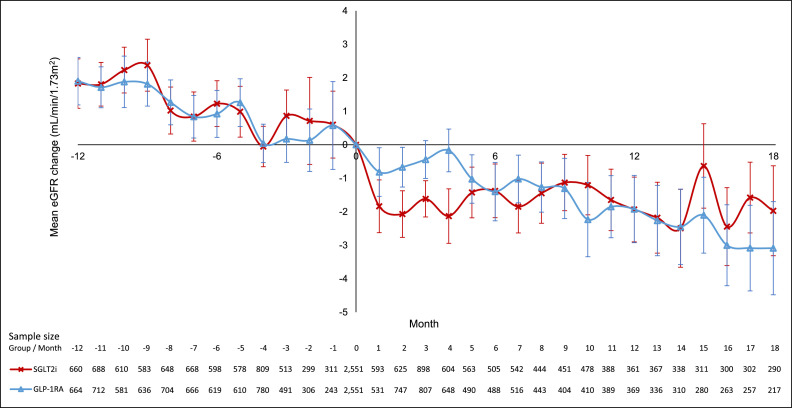
Figure 3 summarizes the eGFR changes over time for SGLT2i and GLP1RA users, respectively. After propensity-score matching, the pre-index date eGFR slope was comparable between the SGLT2i and GLP1RA groups (SGLT2i: -2·50 mL/min/1·73m2, 95% CI -3·30 – -1·70; GLP1RA: -2·31 mL/min/1·73m2, 95% CI -2·77 – -1·85; p = 0·57). After the index date, we noted an acute dip in eGFR at 1-month after initiation of SGLT2i, which is well-recognized25,26 and was not seen in the GLP1RA group. Despite the acute dip in eGFR at 1-month after initiation of SGLT2i, SGLT2i users had a slower eGFR decline than GLP1RA users (SGLT2i: -1·19 mL/min/1·73m2/year vs GLP1RA: -1·95 mL/min/1·73m2/year, p < 0·01). Sensitivity analyses showed consistently slower eGFR decline among SGLT2i users even after excluding the initial 1st-month and 1st-2nd month eGFR dips. Subgroup analyses revealed no significant interaction based on baseline GFR (p for interaction=0·28). (Supplementary Table 5)
Figure 3.
Changes in eGFR before and after initiation of SGLT2i or GLP1RA.
Line plot of changes in eGFR (mL/min/1.73m2) before and after initiation of SGLT2i or GLP1RA, with baseline eGFR as reference value. Red line, markers (cross), and error bars represent SGLT2i group, and blue line, markers (triangle), and error bars represent GLP1RA group. Error bars show the standard error of changes in eGFR at each month. The table below line plot shows the sample size of changes in eGFR of each group at each month.
Discussion
To our knowledge, this is the largest real-world population-based propensity-score matched cohort allowing head-to-head comparison between SGLT2i and GLP1RA for kidney outcomes. Importantly, our study revealed that SGLT2i was superior to GLP1RA in terms of composite kidney outcomes, driven by the reduction in incident ESKD. We also observed a trend towards less incident albuminuria among SGLT2i users. Furthermore, consistent with the composite kidney outcomes, the rate of eGFR decline was also lower among SGLT2i than GLP1RA users. Our results provided important novel information which may weigh SGLT2i over GLP1RA in clinical practice when considering kidney protection for patients with type 2 diabetes.
Two recent studies compared the kidney outcomes between SGLT2i and GLP1RA using network meta-analyses. The earlier network meta-analysis27 focused on ESKD (defined by eGFR <15 mL/min/1·73m2 or requirement of kidney replacement therapy) as the only kidney outcome for comparison, which showed that both agents have beneficial effects on ESKD. The authors concluded that SGLT2i and GLP1RA probably did not have different effects on ESKD (odds ratio 0·91, 95% CI 0·69 – 1·20), though the level of certainty was low to moderate. Another network meta-analysis has looked into the kidney outcomes in more detail – defined as a composite of incident macroalbuminuria, ESKD, kidney function decline, and kidney-related death.13 The study showed that both SGLT2i and GLP1RA could reduce kidney outcomes regardless of albuminuria status, with SGLT2i superior over GLP1RA in kidney outcomes. Nonetheless, the superiority of SGLT2i should be interpreted with caution as kidney outcome definitions varied across RCTs. Moreover, patient-level data were not analyzed in these network meta-analyses. With a unified definition of the kidney outcome, we showed that SGLT2i was indeed superior in terms of composite kidney outcomes, driven by hard outcomes of ESKD, together with a trend towards a lower risk of incident macroalbuminuria.
Recently, there was a propensity-score matched retrospective cohort analysis of detailed kidney outcomes among patients with type 2 diabetes in Japan treated with SGLT2i (n = 541) and GLP1RA (n = 265) respectively,28 which was the first in the literature to compare both agents head-to-head for kidney outcomes directly. The definitions of kidney outcomes were different from CVOTs – defined as annual eGFR decline by >15% and/or worsening in UACR category. The Japanese group demonstrated superior kidney benefits of SGLT2i over GLP1RA in composite kidney outcomes, >15% annual eGFR decline and the rate of eGFR decline. Nonetheless, a few issues limited the generalizability of their results. Firstly, the inclusion criteria of the patients in the Japanese cohort were more heterogeneous: while SGLT2i users were limited to those with CKD, there was no such restriction among GLP1RA users in the analysis. These might create potential bias despite the subsequent propensity-score matching. Furthermore, the potential difference in the pre-index date rates of eGFR decline between the two groups was not considered in the propensity-score matching. Last but not least, the sample size was relatively small, with the study potentially underpowered. Although another Scandinavian cohort study included a large number of patients with type 2 diabetes (87,525 new users of SGLT2i and 63,921 new users of GLP1RA) suggested that serious kidney events (kidney replacement therapy, hospitalization for kidney-related events, and death from kidney-related causes) were lower among SGLT2i users,14 the significant missing values of eGFR and albuminuria status precluded detailed analyses of kidney outcomes as in the Japanese study. These limitations were addressed in our study. Moreover, we showed that the superiority of SGLT2i in composite kidney outcomes, in line with those adopted in various CVOTs. The benefit remained consistent across various subgroups, including age, sex, baseline eGFR/albuminuria status, concomitant ACEI/ARB use, and baseline HbA1c. Of note, the kidney benefits of SGLT2i over GLP1RA in this study were mainly driven by reduction in end-stage kidney disease, whereas a trend was observed in sustained eGFR decline ≥50%. The fact that there was no statistically significant difference in sustained eGFR decline ≥50% but a significant difference in ESKD might be because in the subgroup with eGFR <30 mL/min/1·73m2, there was a higher proportion of patients reaching ESKD without/before sustained eGFR decline ≥50% among GLP1RA (13 out of 133) than SGLT2i users (5 out of 121).
Evaluation of the eGFR slopes revealed a few interesting observations. First of all, in the subgroup analysis (Supplementary Table 5), patients with preserved eGFR on the whole had faster eGFR decline than those with eGFR <60 mL/min/1·73m2. This might be due to the differences in baseline use of ACEI/ARB (70·6% among those with baseline eGFR ≥60 mL/min/1·73m2 vs 86·3% among those with baseline eGFR <60 mL/min/1·73m2, p < 0.001). Secondly, some analyses from GLP1RA studies such as LEADER suggested that the potential kidney benefits with GLP1RA became stronger with declining eGFR.29 This phenomenon was also seen in our study, and the eGFR decline was slower in subjects with eGFR <60 mL/min/1·73m2 than those with eGFR ≥60 mL/min/1·73m2 in the GLPRA subgroup. Furthermore, in the subgroup with baseline eGFR <60 mL/min/1·73m2, while the GLP1RA users achieved a decline of 1 mL/min/1·73m2 which was the normal rate of decline, there was hardly any decline in eGFR in users of SGLT2i. In CVOTs involving SGLT2i such as the VERTIS-CV trial using ertugliflozin,30 the chronic eGFR slope reported with ertugliflozin users varied from -0.45 to +0.67 mL/min/1·73m2 per year during the 5-year follow-up. Hence, our result was consistent with those reported in CVOTs. After the initial eGFR dip with SGLT2i commonly observed, the eGFR gradually returned towards baseline. The mechanisms responsible for the subsequent eGFR increase over time have not been fully elucidated. It might represent an adaptation in the downstream sodium reabsorption pathways, including tubular sodium-glucose cotransporter 1 or sodium-hydrogen exchanger bioactivity, leading to a new state of tubuloglomerular feedback equilibrium and afferent redilatation.30
Mechanistically, SGLT2i and GLP1RA had different effects on the kidney, explaining their differences in both eGFR-based outcomes and albuminuria.31 SGLT2i inhibits glucose and sodium transport via SGLT2 and GLUT2 transporters, responsible for 90% of glucose reabsorption, thereby inducing glucosuria, diuresis, natriuresis, and uric acid excretion. On the other hand, although GLP1RA may possess natriuretic effects via sodium hydrogen exchanger-3, mechanistic studies and clinical trials failed to exhibit kidney-related hemodynamic vasoconstriction in response to GLP1RA, resulting in an overall neutral eGFR effect.32 This might partly explain the difference in the eGFR slope and eGFR-based outcomes between the two classes of anti-diabetic agents. Regarding albuminuria, both SGLT2i and GLP1RA exert anti-inflammatory effects via inducing suppression of inflammatory markers such as TGF-beta, IL-6 and TNF-alpha. The observed trend towards a lower risk of incident macroalbuminuria among SGLT2i users warrants further studies to confirm our findings.
In the intention-to-treat analysis, the HR for the composite kidney outcome was consistent with the main analysis, though not reaching statistical significance. This may be attributed to the fact that among the SGLT2i group, 24·0% discontinued SGLT2i and 9·6% were co-prescribed with GLP1RA; and among the GLP1RA group, 29·8% discontinued GLP1RA and 26·2% were co-prescribed with SGLT2i. Hence, the significant proportion of GLP1RA co-prescribed with SGLT2i might have negated the margin of benefit between SGLT2i and GLP1RA users in the intention-to-treat analysis.
Our results should be interpreted bearing certain limitations. Firstly, the duration of follow-up was relatively short, and the number of events was relatively small. Secondly, because of the sample size, some subgroup analyses were not powered to detect the differences between SGLT2i and GLP1RA. Thirdly, all our subjects were East Asians and the propensity-score matched cohort had long duration of diabetes. Not all agents within SGLT2i and GLP1RA classes were covered in our study. Predominantly empagliflozin and dapagliflozin were prescribed in the SGLT2i group, and liraglutide and dulaglutide in the GLP1RA group. Hence, these factors will limit the generalizability of our results. Fourthly, similar to all large-scale pharmacovigilance studies using electronic medical record databases, drug adherence could not be ascertained. Fifthly, despite our attempts in balancing a range of patient characteristics, in common with all epidemiological studies, retrospective database analysis cannot exclude residual confounders and infer causation. Sixthly, details on the cause of kidney failure or kidney biopsy results were not available in this database. The cause of ESKD in this cohort was presumably mostly due to diabetes. Last but not least, data on treatment-related adverse events such as gastrointestinal intolerance to GLP1RA were not available in the current dataset. Whether these contribute to the results remains to be elucidated. In conclusion, our real-world population-based analysis suggested that SGLT2i was superior to GLP1RA in reducing kidney outcomes among patients with type 2 diabetes.
Funding
None.
Contributors
Conceptualization - DTW Lui, KCB Tan and CKH Wong. Data curation - ICH Au, EHM Tang and CKH Wong. Formal analysis - ICH Au, EHM Tang and CKH Wong. Funding acquisition - None. Investigation - DTW Lui, KCB Tan and CKH Wong. Methodology - DTW Lui, ICHA, EHMT and CKH Wong. Project administration - DTW Lui, KCB Tan and CKH Wong. Resources - None. Software - None. Supervision - DTW Lui, KCB Tan and CKH Wong. Validation - DTW Lui, ICHA, EHMT and CKH Wong. Visualization - DTW Lui, ICHA, EHMT and CKH Wong. Writing - original draft - DTW Lui, ICH Au and CKH Wong. Writing - review & editing - CL Cheung, CH Lee, YC Woo, TW, and KCB Tan.
Data sharing statement
The data that support the findings of this study were provided by the Hong Kong Hospital Authority. Restrictions apply to the availability of these data, which were used under license for this study.
Declaration of interests
None to report.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.eclinm.2022.101510.
Contributor Information
David Tak Wai Lui, Email: dtwlui@hku.hk.
Ivan Chi Ho Au, Email: auchiho@hku.hk.
Kathryn Choon Beng Tan, Email: kcbtan@hku.hk.
Carlos King Ho Wong, Email: carlosho@hku.hk.
Appendix. Supplementary materials
References
- 1.Brown E, Heerspink HJL, Cuthbertson DJ, Wilding JPH. SGLT2 inhibitors and GLP-1 receptor agonists: established and emerging indications. Lancet. 2021;398(10296):262–276. doi: 10.1016/S0140-6736(21)00536-5. [DOI] [PubMed] [Google Scholar]
- 2.Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373(22):2117–2128. doi: 10.1056/NEJMoa1504720. [DOI] [PubMed] [Google Scholar]
- 3.Wiviott SD, Raz I, Bonaca MP, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019;380(4):347–357. doi: 10.1056/NEJMoa1812389. [DOI] [PubMed] [Google Scholar]
- 4.Neal B, Perkovic V, Mahaffey KW, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377(7):644–657. doi: 10.1056/NEJMoa1611925. [DOI] [PubMed] [Google Scholar]
- 5.Cannon CP, Pratley R, Dagogo-Jack S, et al. Cardiovascular outcomes with ertugliflozin in type 2 diabetes. N Engl J Med. 2020;383(15):1425–1435. doi: 10.1056/NEJMoa2004967. [DOI] [PubMed] [Google Scholar]
- 6.Perkovic V, Jardine MJ, Neal B, et al. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med. 2019;380(24):2295–2306. doi: 10.1056/NEJMoa1811744. [DOI] [PubMed] [Google Scholar]
- 7.Heerspink HJL SB, Correa-Rotter R. Dapagliflozin in patients with chronic kidney disease. N Engl J Med. 2020;383:1436–1446. doi: 10.1056/NEJMoa2024816. [DOI] [PubMed] [Google Scholar]
- 8.Marso SP, Daniels GH, Brown-Frandsen K, et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375(4):311–322. doi: 10.1056/NEJMoa1603827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marso SP, Bain SC, Consoli A, et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375(19):1834–1844. doi: 10.1056/NEJMoa1607141. [DOI] [PubMed] [Google Scholar]
- 10.Holman RR, Bethel MA, Mentz RJ, et al. Effects of once-weekly exenatide on cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2017;377(13):1228–1239. doi: 10.1056/NEJMoa1612917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gerstein HC, Colhoun HM, Dagenais GR, et al. Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): a double-blind, randomised placebo-controlled trial. Lancet. 2019;394(10193):121–130. doi: 10.1016/S0140-6736(19)31149-3. [DOI] [PubMed] [Google Scholar]
- 12.Sattar N, Lee MMY, Kristensen SL, et al. Cardiovascular, mortality, and kidney outcomes with GLP-1 receptor agonists in patients with type 2 diabetes: a systematic review and meta-analysis of randomised trials. Lancet Diabetes Endocrinol. 2021;9(10):653–662. doi: 10.1016/S2213-8587(21)00203-5. [DOI] [PubMed] [Google Scholar]
- 13.Kawai Y, Uneda K, Yamada T, et al. Comparison of effects of SGLT-2 inhibitors and GLP-1 receptor agonists on cardiovascular and renal outcomes in type 2 diabetes mellitus patients with/without albuminuria: a systematic review and network meta-analysis. Diabetes Res Clin Pract. 2022;183 doi: 10.1016/j.diabres.2021.109146. [DOI] [PubMed] [Google Scholar]
- 14.Ueda P, Wintzell V, Dahlqwist E, et al. The comparative cardiovascular and renal effectiveness of sodium-glucose co-transporter-2 inhibitors and glucagon-like peptide-1 receptor agonists: a Scandinavian cohort study. Diabetes Obes Metab. 2021;24(3):473–485. doi: 10.1111/dom.14598. [DOI] [PubMed] [Google Scholar]
- 15.Yang A, Wu H, Lau ESH, et al. Trends in glucose-lowering drug use, glycemic control, and severe hypoglycemia in adults with diabetes in Hong Kong, 2002-2016. Diabetes Care. 2020;43(12):2967–2974. doi: 10.2337/dc20-0260. [DOI] [PubMed] [Google Scholar]
- 16.Wong CKH, Man KKC, Shi M, et al. Intensification with dipeptidyl peptidase-4 inhibitor, insulin, or thiazolidinediones and risks of all-cause mortality, cardiovascular diseases, and severe hypoglycemia in patients on metformin-sulfonylurea dual therapy: a retrospective cohort study. PLoS Med. 2019;16(12) doi: 10.1371/journal.pmed.1002999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ke C, Stukel TA, Shah BR, et al. Age at diagnosis, glycemic trajectories, and responses to oral glucose-lowering drugs in type 2 diabetes in Hong Kong: a population-based observational study. PLoS Med. 2020;17(9) doi: 10.1371/journal.pmed.1003316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lui DTW, Lee CH, Chan YH, et al. HbA1c variability, in addition to mean HbA1c, predicts incident hip fractures in Chinese people with type 2 diabetes. Osteoporos Int. 2020;31(10):1955–1964. doi: 10.1007/s00198-020-05395-z. [DOI] [PubMed] [Google Scholar]
- 19.Lund JL, Richardson DB, Stürmer T. The active comparator, new user study design in pharmacoepidemiology: historical foundations and contemporary application. Curr Epidemiol Rep. 2015;2(4):221–228. doi: 10.1007/s40471-015-0053-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Inker LA, Eneanya ND, Coresh J, et al. New creatinine- and cystatin C-based equations to estimate GFR without race. N Engl J Med. 2021;385(19):1737–1749. doi: 10.1056/NEJMoa2102953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Heerspink HJL, Karasik A, Thuresson M, et al. Kidney outcomes associated with use of SGLT2 inhibitors in real-world clinical practice (CVD-REAL 3): a multinational observational cohort study. Lancet Diabetes Endocrinol. 2020;8(1):27–35. doi: 10.1016/S2213-8587(19)30384-5. [DOI] [PubMed] [Google Scholar]
- 22.Leyrat C, Seaman SR, White IR, et al. Propensity score analysis with partially observed covariates: how should multiple imputation be used? Stat Methods Med Res. 2019;28(1):3–19. doi: 10.1177/0962280217713032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang Z, Kim HJ, Lonjon G, Zhu Y, written on behalf of AMEB-DCTCG Balance diagnostics after propensity score matching. Ann Transl Med. 2019;7(1):16. doi: 10.21037/atm.2018.12.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Khan MS, Bakris GL, Shahid I, Weir MR, Butler J. Potential role and limitations of estimated glomerular filtration rate slope assessment in cardiovascular trials: a review. JAMA Cardiol. 2022;7(5):549–555. doi: 10.1001/jamacardio.2021.5151. [DOI] [PubMed] [Google Scholar]
- 25.Heerspink HJL, Cherney DZI. Clinical implications of an acute dip in eGFR after SGLT2 inhibitor initiation. Clin J Am Soc Nephrol. 2021;16(8):1278–1280. doi: 10.2215/CJN.02480221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kraus BJ, Weir MR, Bakris GL, et al. Characterization and implications of the initial estimated glomerular filtration rate 'dip' upon sodium-glucose cotransporter-2 inhibition with empagliflozin in the EMPA-REG OUTCOME trial. Kidney Int. 2021;99(3):750–762. doi: 10.1016/j.kint.2020.10.031. [DOI] [PubMed] [Google Scholar]
- 27.Palmer SC, Tendal B, Mustafa RA, et al. Sodium-glucose cotransporter protein-2 (SGLT-2) inhibitors and glucagon-like peptide-1 (GLP-1) receptor agonists for type 2 diabetes: systematic review and network meta-analysis of randomised controlled trials. BMJ. 2021;372:m4573. doi: 10.1136/bmj.m4573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kobayashi K, Toyoda M, Hatori N, et al. Comparison of renal outcomes between sodium glucose co-transporter 2 inhibitors and glucagon-like peptide 1 receptor agonists. Diabetes Res Clin Pract. 2022 doi: 10.1016/j.diabres.2022.109231. [DOI] [PubMed] [Google Scholar]
- 29.Górriz JL SM, Navarro-González JF. Receptor agonists and diabetic kidney disease: a call of attention to nephrologists. J Clin Med. 2020;30(9) doi: 10.3390/jcm9040947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cherney DZI, Cosentino F, Dagogo-Jack S, et al. Ertugliflozin and slope of chronic eGFR: prespecified analyses from the randomized VERTIS CV trial. Clin J Am Soc Nephrol. 2021;16(9):1345–1354. doi: 10.2215/CJN.01130121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ninčević V, Omanović Kolarić T, Roguljić H, Kizivat T, Smolić M, Bilić Ćurčić I. Renal benefits of SGLT 2 inhibitors and GLP-1 receptor agonists: evidence supporting a paradigm shift in the medical management of Type 2 diabetes. Int J Mol Sci. 2019;20(23) doi: 10.3390/ijms20235831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cherney DZI, Bakris GL. Novel therapies for diabetic kidney disease. Kidney Int Suppl. 2018;8(1):18–25. doi: 10.1016/j.kisu.2017.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.