Abstract
Background
The optimal therapy for submassive pulmonary embolism remains in question. The following meta-analysis compiles the current evidence comparing Catheter-Directed Thrombolysis (CDT) versus Systemic Anticoagulation (SA).
Methods
An electronic search through PubMed and Google scholar revealed studies comparing CDT versus SA in terms of mortality and major bleeding events. Thirty-day, 90-day, and one-year mortality results were analyzed.
Results
Six studies were included in the meta-analysis. Thirty-day and one-year mortality were less with CDT compared to SA (OR 0.27 [CI 0.11-0.67]; and OR 0.50 [CI 0.28-0.89]). Ninety-day mortality was similar between the two methods (OR 0.57 [CI 0.17-1.92]). Compilation of all studies reporting at least greater than 30-day mortality revealed less mortality with CDT (OR 0.51 [0.30-0.86]). Major bleeding was similar between the two treatments (OR 1.63 [CI 0.63-4.20]).
Conclusion
CDT has less 30-day and 1-year mortality with equivalent rates of major bleeding compared to SA for treatment of submassive pulmonary embolism.
Keywords: Submassive pulmonary embolism, catheter-directed thrombolysis, systematic anticoagulation, thrombolytic, heparin, hemodynamic instability
1. INTRODUCTION
Acute pulmonary embolism (PE) is associated with high morbidity and is one of the leading causes of cardiovascular mortality. Treatment options include systemic thrombolysis, catheter-directed interventions, and surgical thromboembolectomy. Systemic anticoagulation is the standard of care for most PEs. Massive (high-risk) PEs - defined as PEs causing hemodynamic instability - confer high hospital mortality and therefore are treated with thrombolytic therapy [1]. Systemic anticoagulation (SA) is the treatment of choice with the goal of stabilization and eventual dissolution of the clot. Surgical interventions are an option of last resort due to the associated morbidity and mortality in the treatment of acute PE [2-3].
Submassive (intermediate-risk) PEs, defined as PEs that cause no hemodynamic changes and less mortality risk than massive PEs but cause increased right heart strain, remain in question in regards to treatment. The PEITHO trial showed no difference in mortality with systemic thrombolytics versus SA alone but found less risk of shock with more risk of significant bleeding with systemic thrombolytics [4].
The recent development of catheter-directed therapies, including Catheter-Directed Thrombolysis (CDT), ultrasound-accelerated thrombolysis (USAT), and pharmacomechanical thrombectomy, introduced more methods to treat acute PE [6]. Several recent studies have suggested a clinical benefit of CDT in the treatment of submassive PEs [5], but data comparing CDT versus SA alone remain controversial. With a delivery system that focuses thrombolytic administration on the embolism itself at a lower dose, CDT may confer a safer approach to clot dissolution. This meta-analysis compiles the current evidence comparing CDT versus SA where thirty-day, 90-day, and one-year mortality results are analyzed between six different studies.
2. METHODS
2.1. Data Collection
An electronic search through PubMed and Google scholar revealed studies comparing CDT versus SA for submassive PE treatment in terms of mortality. While the SA group received only systemic anticoagulation (i.e., heparin), the CDT group received both the catheter intervention for focused delivery of thrombolytics and systemic anticoagulation. Keywords included the following: Submassive, Pulmonary Embolism, Catheter, Thrombolysis, Systemic, Heparin, Thrombolytics, Systemic Anticoagulation, Mortality, and Survival. Submassive PE was defined as evidence of PE with signs of concomitant heart strain (i.e., echocardiographic evidence of increased right ventricular pressure, septal flattening, or systolic dysfunction; elevated troponin levels) without hypotension or hemodynamic instability similar to the criteria used in the PEITHO trial [4]. Major bleeding, defined as requiring red blood cell transfusion or that according to the criteria of the International Society of Thrombosis and Hemostasis, [7] was a secondary endpoint. Abstracts were reviewed for relevancy and to check whether further reading of the manuscript was warranted. Inclusion criteria included presenting 30-day, 90-day, or one-year all-cause mortality results. Studies including other types of PE were excluded. Information was organized and compiled in its respective period.
2.2. Statistical Analysis
Endpoints for the meta-analysis reflected the mortality period as listed in the inclusion criteria. Statistical analyses used the Review Manager Version 5.3 (The Cochrane Collaboration, Copenhagen, Denmark) software program. Forest plots were created with the DerSimonian and Laird fixed-effects model. The odds ratio (OR) with a 95% confidence interval (CI) was reported. The value marking no significance via confidence interval was 1. An I2 greater than 50% suggested significant heterogeneity. If significant heterogeneity existed, a random-effects model was used instead.
3. RESULTS
3.1. Selection Process
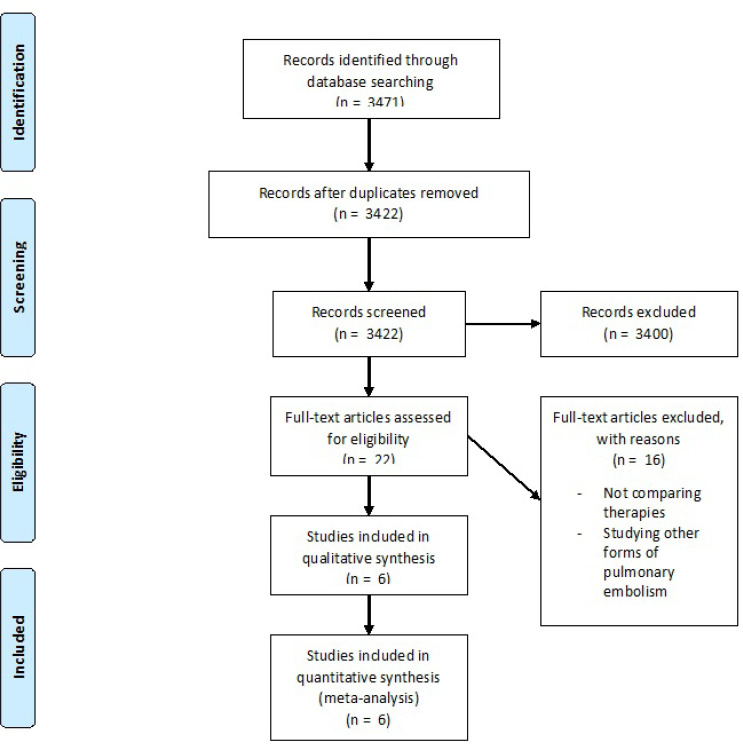
A total of 3471 studies were initially identified as potential candidates for the analysis (Fig. 1). After further screening and employing exclusion criteria, six studies met the criteria for the meta-analysis (Table 1). Baseline characteristics were similar between the two groups except for CDT having more patients with diabetes mellitus and SA having more coronary artery disease and stroke history (Table 2). A total of 391 CDT and 460 SA patients were included in the meta-analysis.
Fig. (1).
Flow Diagram of Study Selection
Table 1.
Details of selected studies.
| Study | Type | Number of CDT | Number of SA | Device (Dose Protocol) | Contribution |
|---|---|---|---|---|---|
| Kucher 2014 [8] | Randomized controlled trial | 30 | 29 | EkoSonic Endovascular System (rtPA 1 mg/hr for 5 hours, then 0.5 mg/hr for 10 hours) | 90-day mortality, Greater than 30-day mortality, Major bleeding |
| Avgerinos 2016 [9] | Retrospective, propensity score-matched | 64 | 64 | EkoSonic Endovascular System (tPA 2-4 mg bolus followed by 0.5-1 mg/hr) | 90-day mortality, Greater than 30-day mortality, Major bleeding |
| Lat 2018 [10] | Retrospective | 13 | 109 | unspecified | 90-day mortality, Greater than 30-day mortality, Major bleeding |
| Mawri 2018 [11] | Retrospective, propensity score-matched | 120 | 120 | unspecified | 30-day mortality, One-year mortality, Greater than 30-day mortality |
| Schissler 2018 [12] | Retrospective | 65 | 39 | EkoSonic Endovascular System (alteplase 0-5 mg bolus followed by 0.5-1 mg/hr infusion) | One-year mortality, Greater than 30-day mortality, Major bleeding |
| D’Auria 2020 [13] | Retrospective, propensity score-matched | 99 | 99 | Unspecified (tPA 1mg/hr for up to 12 hours) | 30-day mortality, One-year mortality, Greater than 30-day mortality, Major bleeding |
CDT - Catheter-Directed Thrombolysis; SA - Systemic Anticoagulation; tPA - tissue plasminogen activator; rtPA - recombinant tissue plasminogen activator
Table 2.
Baseline characteristics of studies selected.
| Characteristic | CDT (Range) | SA (Range) | Studies Included |
|---|---|---|---|
| Total Patients | 391 (13-120) | 460 (29-120) | Kucher 2014 [8], Avgerinos 2016 [9], Lat 2018 [10], Mawri 2018 [11], Schissler 2018 [12], D’Auria 2020 [13] |
| Age (years) | 57.8 (54.0-64.0) | 59.7 (58.0-62.0) | Kucher 2014 [8], Avgerinos 2016 [9], Schissler 2018 [12], D’Auria 2020 [13] |
| Female (%) | 54.2 (52.3-63.0) | 47.6 (41.0-64.1) | Kucher 2014 [8], Avgerinos 2016 [9], Schissler 2018 [12], D’Auria 2020 [13] |
| Hypertension (%) | 52.2 (46.2-67.0) | 52.3 (50.0-56.4) | Kucher 2014 [8], Avgerinos 2016 [9], Schissler 2018 [12] |
| Smoking History (%) | 22.6 (13.0-32.3) | 20.5 (13.0-28.2) | Kucher 2014 [8], Avgerinos 2016 [9], Schissler 2018 [12] |
| Diabetes Mellitus (%) | 20.0 (20.0-20.0) | 11.8 (10.3-14.0) | Kucher 2014 [8], Schissler 2018 [12] |
| Congestive Heart Failure (%) | 3.4 (1.5-7.0) | 4.8 (2.0-10.9) | Kucher 2014 [8], Avgerinos 2016 [9], Schissler 2018 [12], D’Auria 2020 [13] |
| Coronary Artery Disease (%) | 9.8 (7.0-10.9) | 12.5 (3.0-15.6) | Kucher 2014 [8], Avgerinos 2016 [9], D’Auria 2020 [13] |
| Chronic Lung Disease (%) | 18.4 (12.0-27.7) | 19.8 (10.9-28.2) | Avgerinos 2016 [9], Schissler 2018 [12], D’Auria 2020 [13] |
| Stroke (%) | 8.4 (0.0-12.3) | 14.7 (3.0-23.1) | Kucher 2014 [8], Schissler 2018 [12] |
| Hx of DVT/PE (%) | 18.9 (14.0-23.1) | 18.3 (4.7-28.2) | Avgerinos 2016 [9], Schissler 2018 [12], D’Auria 2020 [13] |
CDT - Catheter Directed Thrombolysis; SA - Systemic Anticoagulation; DVT - Deep Vein Thrombosis; PE - Pulmonary Embolism
3.2. Mortality Rates
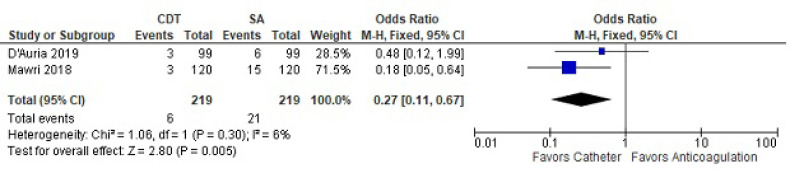
For 30-day mortality, two studies with a total of 219 treated patients and 219 controls were analyzed (Fig. 2). Thirty-day mortality was less with CDT compared to SA (OR 0.27 [CI 0.11-0.67]). There was no significant heterogeneity between the two studies (I2=6).
Fig. (2).
Thirty-day mortality between catheter-directed thrombolysis versus systemic anticoagulation for submassive pulmonary embolism.
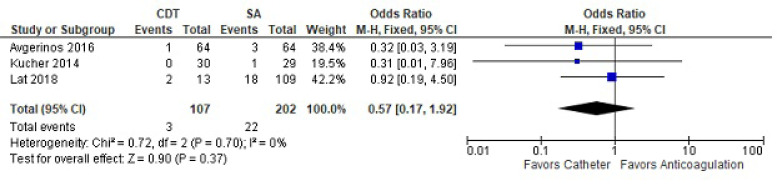
For 90-day mortality, three studies with a total of 107 treated and 202 controls were reviewed (Fig. 3). Ninety-day mortality was similar between the two methods (OR 0.57 [CI 0.17-1.92]). There was no heterogeneity in the analysis.
Fig. (3).
Ninety-day mortality between catheter-directed thrombolysis versus systemic anticoagulation for submassive pulmonary embolism.
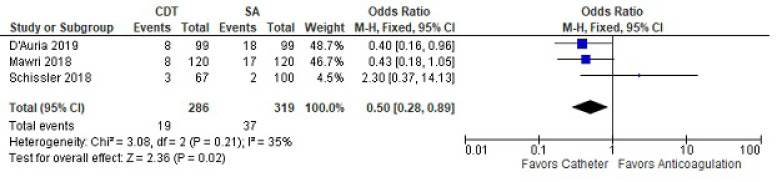
One-year mortality included three studies in the analysis (Fig. 4). One-year mortality was less with CDT compared to SA (OR 0.5 [CI 0.28-0.89]). There was no heterogeneity between the two studies.
Fig. (4).
One-year mortality between catheter-directed thrombolysis versus systemic anticoagulation for submassive pulmonary embolism.
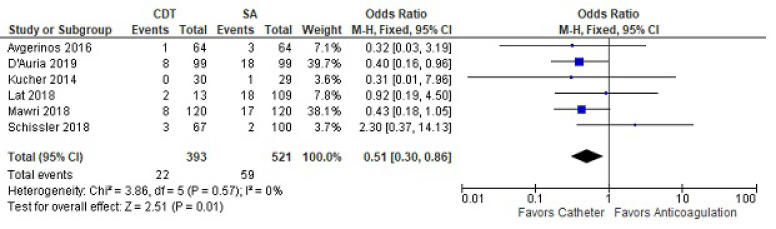
Compilation of all studies reporting at least greater than 30-day mortality revealed less mortality with CDT (OR 0.51 [0.30-0.86]) (Fig. 5). This included 393 treated and 521 control subjects. There was no heterogeneity among the studies.
Fig. (5).
Greater than 30-day mortality between catheter-directed thrombolysis versus systemic anticoagulation for submassive pulmonary embolism.
3.3. Major Bleeding
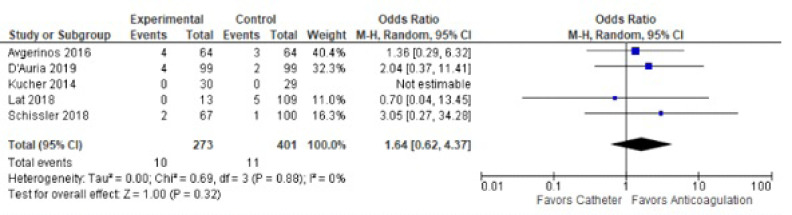
Major bleeding was similar between the two studies in treated groups (OR 1.63 [CI 0.63-4.20]) (Fig. 6). There was no heterogeneity between the studies.
Fig. (6).
Major bleeding rates between catheter-directed thrombolysis versus systemic anticoagulation for submassive pulmonary embolism.
4. DISCUSSION
Results of the meta-analysis show better all-cause mortality outcomes at 30 days and 1 year with CDT compared to SA alone for submassive PEs. The rate of major bleeding was similar between the two therapies.
While the results show a benefit with CDT at 30-day and one year, 90-day mortality remained similar between the two groups. This may be explained by the exclusion of the studies by Mawri and D’Auria in the 90-day mortality analysis [11-13]. Both studies presented a significant amount of mortality for systemic anticoagulation in 30-day (Mawri) and at one year (D’Auria). The causes of death in the studies were not clearly presented, but D’Auria was able to conclude that 12 of the 15 known causes of death were PE-associated [13]. Neither study could be added into the 90-day mortality analysis since they lacked the results at that time frame. Furthermore, it needs to be noted that the only randomized controlled trial was included in the 90-day mortality analysis and not 30-day or one year. The ULTIMA randomized controlled trial by Kucher et al. showed no difference in mortality, having only one mortality (from pancreatic cancer) in the SA arm [10]. However, compiling an analysis of greater than 30-day mortality, which included all the studies, showed a mortality benefit for CDT.
The role of systemic thrombolysis in submassive PE is controversial [14]. The PEITHO trial showed that systemic thrombolytic therapy may improve hemodynamics but with an increased risk of significant bleeding [4]. CDT was developed to achieve a hemodynamic benefit similar to thrombolysis while minimizing complications by utilizing localized delivery with lower thrombolytic agent dosages. It has a benefit for patients with massive, hemodynamically unstable PEs [6]. However, data on its clinical efficacy with submassive PE remain limited. Some studies show statistically significant improvement in 30-day mortality with CDT compared to SA [11,13].
The only randomized controlled trial comparing submassive PE patients treated with CDT and anticoagulation with heparin alone (ULTIMA trial) showed decreased right ventricular dilation and pulmonary artery pressure by 24 hours without increased risk for major bleeding. The right ventricular (RV) - to - left ventricular (LV) diameter ratio decreased more with CDT and anticoagulation than heparin alone after 24 hours. One death did occur in the SA group but was attributed to pancreatic cancer [8]. Our results do not show a difference between CDT and SA, with both modalities demonstrating similar rates of major bleeding as the risk of the treatment modalities.
These results support the feasibility of the findings presented in the SEATTLE II study. The SEATTLE II study presented the effectiveness of CDT in reducing the right ventricular diameter (via the RV-to-LV diameter ratio) via a prospective, single-arm, multicenter trial. This was with a 10% bleeding rate, but no intracranial hemorrhage was observed. While the study compiled both massive and submassive PEs, a comparison between the two showed similar drops in the RV-to-LV diameter ratio and pulmonary artery systolic pressure, with massive PEs causing more major bleeding events [5].
The question of whether thrombolysis with thrombotic agents is effective for submassive pulmonary embolism stems from the cumulative data regarding systemic thrombolytic versus SA. Two meta-analyses showed no difference in mortality between the use of systemic thrombolytics versus SA [15, 16]. Nakamura showed that thrombolytics did reduce clinical deterioration, defined as needing surgical embolectomy, catheter intervention, catecholamine, or vasopressor administration for shock, cardiopulmonary resuscitation, or experiencing significant hypotension. A difference was observed in supporting thrombolytics when combining mortality with clinical deterioration [15]. While Cao did show a trend in favor of thrombolytics for reducing mortality, it was statistically nonsignificant with a CI of 0.19-1.05 [16]. Both meta-analyses showed an increase in minor bleeding, but not major bleeding [15, 16], although Nakamura did show increased intracranial bleeding with thrombolytics [16]. However, CDT administers the thrombolytic therapy directly to the embolism and at lower doses, which could explain the difference between CDT and SA in our meta-analysis compared to systemic thrombolytics and SA in the other meta-analyses.
4.1. Limitations
Despite this analysis being promising for CDT as a better treatment for submassive PE, there are also limitations to the analyses. The selection of studies is mainly retrospective and observational. This is due to a lack of studies in the literature. This allows for biases that would be eliminated from randomization. More randomized controlled trials are needed involving increased sample sizes of patients, eliminating biases, thereby rendering the ability to solidify a conclusion. Nonetheless, this is the first meta-analysis of its kind and compiles all the current studies comparing the therapies for submassive PE. Heterogeneity was also minimal among the selected studies. Finally, the difference in clot burden in different patients despite the consistency in patient factors can skew some of these outcomes related to CDT.
CONCLUSION
Patients with submassive PE treated with CDT have lower mortality compared to those treated with standard anticoagulation. The rate of major bleeding is equivocal between the two therapies. However, most studies were observational and nonrandomized. While CDT is a promising option, more randomized controlled trials are required to establish a clear benefit.
CONSENT FOR PUBLICATION
Not applicable.
STANDARD OF REPORTING
PRISMA guidelines and methodologies are followed.
FUNDING
None.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
We would like to thanks Dr. Bujji Ainapurapu for helping out with the editing and references.
SUPPLEMENTARY MATERIAL
Supplementary material is available on the publisher’s website along with the published article.
REFERENCES
- 1.Avgerinos E.D., Chaer R.A. Catheter-directed interventions for acute pulmonary embolism. J. Vasc. Surg. 2015;61(2):559–565. doi: 10.1016/j.jvs.2014.10.036. [DOI] [PubMed] [Google Scholar]
- 2.Guyatt G.H., Eikelboom J.W., Gould M.K., et al. 9th. 2 Suppl. Vol. 141. American college of chest physicians evidence-based clinical practice guidelines; 2012. Approach to outcome measurement in the prevention of thrombosis in surgical and medical patients: Antithrombotic therapy and prevention of thrombosis. pp. e185S–e194S. Chest. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Konstantinides S.V., Torbicki A., Agnelli G., Danchin N., Fitzmaurice D., Galiè N., Gibbs J.S., Huisman M.V., Humbert M., Kucher N., Lang I., Lankeit M., Lekakis J., Maack C., Mayer E., Meneveau N., Perrier A., Pruszczyk P., Rasmussen L.H., Schindler T.H., Svitil P., Vonk Noordegraaf A., Zamorano J.L., Zompatori M. 2014 ESC guidelines on the diagnosis and management of acute pulmonary embolism. Eur. Heart J. 2014;35(43):3033–3069k. doi: 10.1093/eurheartj/ehu283. [published correction appears in Eur Heart J. 2015 Oct 14;36(39):2666]. [published correction appears in Eur Heart J. 2015 Oct 14;36(39):2642]. [DOI] [PubMed] [Google Scholar]
- 4.Meyer G., Vicaut E., Danays T., Agnelli G., Becattini C., Beyer-Westendorf J., Bluhmki E., Bouvaist H., Brenner B., Couturaud F., Dellas C., Empen K., Franca A., Galiè N., Geibel A., Goldhaber S.Z., Jimenez D., Kozak M., Kupatt C., Kucher N., Lang I.M., Lankeit M., Meneveau N., Pacouret G., Palazzini M., Petris A., Pruszczyk P., Rugolotto M., Salvi A., Schellong S., Sebbane M., Sobkowicz B., Stefanovic B.S., Thiele H., Torbicki A., Verschuren F., Konstantinides S.V., PEITHO Investigators Fibrinolysis for patients with intermediate-risk pulmonary embolism. N. Engl. J. Med. 2014;370(15):1402–1411. doi: 10.1056/NEJMoa1302097. [DOI] [PubMed] [Google Scholar]
- 5.Piazza G., Hohlfelder B., Jaff M.R., Ouriel K., Engelhardt T.C., Sterling K.M., Jones N.J., Gurley J.C., Bhatheja R., Kennedy R.J., Goswami N., Natarajan K., Rundback J., Sadiq I.R., Liu S.K., Bhalla N., Raja M.L., Weinstock B.S., Cynamon J., Elmasri F.F., Garcia M.J., Kumar M., Ayerdi J., Soukas P., Kuo W., Liu P.Y., Goldhaber S.Z., SEATTLE II Investigators A prospective, single-arm, multicenter trial of ultrasound-facilitated, catheter-directed, low-dose fibrinolysis for acute massive and submassive pulmonary embolism: the SEATTLE II study. JACC Cardiovasc. Interv. 2015;8(10):1382–1392. doi: 10.1016/j.jcin.2015.04.020. [DOI] [PubMed] [Google Scholar]
- 6.Liang N.L., Avgerinos E.D., Marone L.K., Singh M.J., Makaroun M.S., Chaer R.A. Comparative outcomes of ultrasound-assisted thrombolysis and standard catheter-directed thrombolysis in the treatment of acute pulmonary embolism. Vasc. Endovascular Surg. 2016;50(6):405–410. doi: 10.1177/1538574416666228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schulman S., Kearon C. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J. Thromb. Haemost. 2005;3(4):692–694. doi: 10.1111/j.1538-7836.2005.01204.x. [DOI] [PubMed] [Google Scholar]
- 8.Kucher N., Boekstegers P., Müller O.J., Kupatt C., Beyer-Westendorf J., Heitzer T., Tebbe U., Horstkotte J., Müller R., Blessing E., Greif M., Lange P., Hoffmann R.T., Werth S., Barmeyer A., Härtel D., Grünwald H., Empen K., Baumgartner I. Randomized, controlled trial of ultrasound-assisted catheter-directed thrombolysis for acute intermediate-risk pulmonary embolism. Circulation. 2014;129(4):479–486. doi: 10.1161/CIRCULATIONAHA.113.005544. [DOI] [PubMed] [Google Scholar]
- 9.Avgerinos E.D., Liang N.L., El-Shazly O.M., Toma C., Singh M.J., Makaroun M.S., Chaer R.A. Improved early right ventricular function recovery but increased complications with catheter-directed interventions compared with anticoagulation alone for submassive pulmonary embolism. J. Vasc. Surg. Venous Lymphat. Disord. 2016;4(3):268–275. doi: 10.1016/j.jvsv.2015.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lat T., Lipatov K., Chen W., et al. Comparison of outcomes between catheter-directed thrombolysis and systemic anticoagulation in patients with submassive pulmonary embolism. Am. J. Respir. Crit. Care Med. 2018;197:A6345. [Google Scholar]
- 11.Mawri S., Ali M., Gorgis S., Aurora L., Giordano M., Hegab S., Schwartz S., Koenig G. Comparative study of outcomes using catheter-directed thrombolysis versus anticoagulation alone for management of submassive pulmonary embolism. Catheter. Cardiovasc. Interv. 2018;91:S14. [Google Scholar]
- 12.Schissler A.J., Gylnn R.J., Sobieszczyk P.S., Waxman A.B. Ultrasound-assisted catheter-directed thrombolysis compared with anticoagulation alone for treatment of intermediate-risk pulmonary embolism. Pulm. Circ. 2018;8(4):2045894018800265. doi: 10.1177/2045894018800265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.D’Auria S., Sezer A., Thoma F., Sharbaugh M., McKibben J., Maholic R., Avgerinos E.D., Rivera-Lebron B.N., Toma C. Outcomes of catheter-directed thrombolysis vs. standard medical therapy in patients with acute submassive pulmonary embolism. Pulm. Circ. 2020;10(1):2045894019898368. doi: 10.1177/2045894019898368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kearon C., Akl E.A., Ornelas J., Blaivas A., Jimenez D., Bounameaux H., Huisman M., King C.S., Morris T.A., Sood N., Stevens S.M., Vintch J.R.E., Wells P., Woller S.C., Moores L. Antithrombotic therapy for VTE disease: CHEST guideline and expert panel report. Chest. 2016;149(2):315–352. doi: 10.1016/j.chest.2015.11.026. [DOI] [PubMed] [Google Scholar]
- 15.Nakamura S., Takano H., Kubota Y., Asai K., Shimizu W. Impact of the efficacy of thrombolytic therapy on the mortality of patients with acute submassive pulmonary embolism: A meta-analysis. J. Thromb. Haemost. 2014;12(7):1086–1095. doi: 10.1111/jth.12608. [DOI] [PubMed] [Google Scholar]
- 16.Cao Y., Zhao H., Gao W., Wang Y., Cao J. Systematic review and meta-analysis for thrombolysis treatment in patients with acute submassive pulmonary embolism. Patient Prefer. Adherence. 2014;8:275–282. doi: 10.2147/PPA.S56280. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material is available on the publisher’s website along with the published article.