1. Introduction
Atrial fibrillation (AF) is the most common arrhythmia in clinical practice, and is associated with the occurrence of thromboembolisms, heart failure, morality, and the quality of life (QoL) [1], [2]. Catheter ablation (CA) of AF has been demonstrated to be superior to antiarrhythmic drugs (AADs) in maintaining sinus rhythm and is increasingly performed worldwide [3], [4]. Since it has been reported that muscular pulmonary venous sleeves are responsible for the initiation and maintenance of AF, electrical pulmonary vein isolation (PVI) has been established as an essential strategy of AF ablation [4], [5], [6].
Although it is known that the PVI is less successful in persistent AF (PeAF) patients with more advanced atrial remodeling, randomized studies including STAR AF II trial have not demonstrated that an adjunctive ablation lesion set targeting sites outside of the pulmonary veins (PVs) at the time of the index procedure improves the clinical efficacy [7], [8], [9], [10], [11]. Therefore, PVI is still the cornerstone of CA not only of paroxysmal AF (PAF) but of PeAF.
The cryoballoon (CB) ablation is an anatomically based simplified technology, and both the CB and radiofrequency (RF) ablation are in routine clinical use for AF ablation worldwide [3], [12], [13], [14], [15]. The FIRE and ICE trial was a prospective, multicenter, randomized, non-inferiority trial comparing the CB and RF ablation in patients with PAF [12]. CB ablation has a comparable efficacy and safety to RF ablation with a shorter procedure time, left atrial (LA) dwell time, and total fluoroscopy time than RF ablation. Since the FIRE AND ICE trial was reported, some non-randomized observational studies have demonstrated a similar efficacy of CA between the CB and RF ablation not only of PAF but also of PeAF [16], [17], [18], [19]. However, there have been no prospective randomized studies comparing the clinical outcome between CB and RF ablation for the treatment of PeAF.
2. Methods
2.1. Aim
The aim of the CRRF-PeAF study is to compare the efficacy and safety of CA of AF between CB and RF ablation in patients with PeAF.
2.2. Primary hypothesis and study design
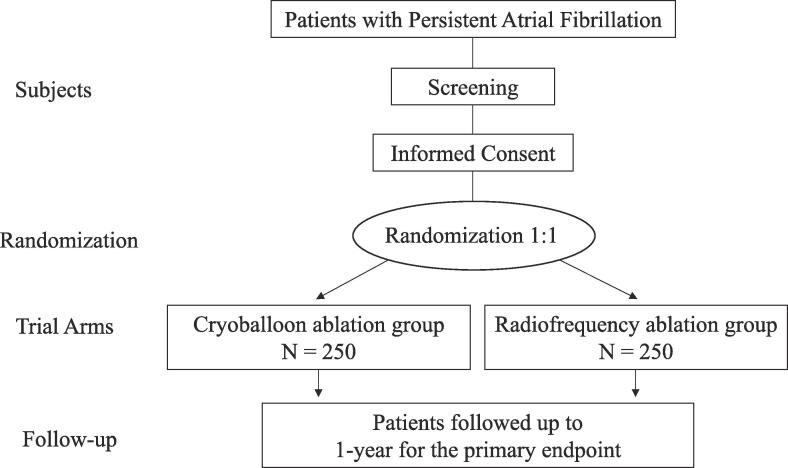
The primary hypothesis of the CRRF-PeAF study is that CB ablation is not inferior to RF ablation with respect to the clinical efficacy in patients with PeAF. To assess the hypothesis, the CRRF-PeAF study is designed as a prospective, multicenter, randomized, controlled study comparing the efficacy and safety of CA between CB and RF ablation at 1-year in patients with PeAF from April 8, 2021 to March 31, 2029 (Fig. 1). The enrollment period is from April 8, 2021 to March 31, 2025. The study cohort will include 500 patients with PeAF from 12 hospitals in Japan. The enrollment and study periods will be extended if the number of enrolled patients is not achieved during the period, and these will be shortened if the number of enrolled patients is achieved earlier.
Fig. 1.
Flow Chart of the Study.
2.3. Primary endpoint
The primary endpoint of the CRRF-PeAF study will be the freedom from any atrial tachyarrhythmias (ATs) lasting ≥ 30 s occurring outside the blanking period (BP) of 90 days at 1-year following the index procedure. Repeat ablation procedures for any ATs during the BP will also be considered as a recurrence.
2.4. Secondary endpoints
The key secondary endpoints are as follows:
-
1.
Freedom from any ATs at 3-years following the index procedure.
-
2.
Change and percentage change in the left ventricular ejection fraction at 1-year and 3-years.
-
3.
Change and percentage change in the creatinine clearance and brain natriuretic peptide level at 1-year and 3-years.
-
4.
Cardiovascular events (hospitalizations due to heart failure and/or angina pectoris, myocardial infarctions, strokes, transient ischemic attacks) at 1-year and 3-years.
-
5.
All-cause mortality and cardiovascular mortality at 1-year and 3-years.
-
6.
Change and percentage change in the QoL at 1-year and 3-years.
-
7.
The success rate of the PVI, total procedural time, LA dwell time, total fluoroscopy time, radiation exposure, and quantity of contrast media.
-
8.
Efficacy of an adjunctive ablation lesion set.
-
9.
Complication rate.
2.5. Inclusion and Exclusion criteria
2.5.1. Inclusion criteria
Subjects must meet all of the following criteria:
-
1.
Diagnosed with PeAF with a continuous AF episode of > 7 days but ≤ 1 year in accordance with the 2014 AHA/ACC/HRS guidelines3.
-
2.
Age 20–85 years.
-
3.
Planned CA using CB or RF ablation.
-
4.
Capable of complying with the protocol and providing written informed consent.
2.5.2. Exclusion Criteria
Those who meet any of the following criteria are ineligible for the study:
-
1.
Previous CA and/or surgical procedure of AF.
-
2.
AF lasting over 1 year (long-standing PeAF).
-
3.
LA dimension (LAD) > 50 mm (parasternal long-axis view).
-
4.
Woman currently or possibly pregnant.
-
5.
Enrollment in another investigational drug and/or device study.
2.6. Study Procedures
Patients will undergo trans-esophageal echocardiography and/or contrast-enhanced CT to exclude the presence of thrombi in the LA appendage and LA before the CA. AADs will be discontinued for at least 5 half-lives before the CA, except for amiodarone. Oral anticoagulation therapy will be prescribed at a minimum of 4 weeks before the CA.
During the procedure of CA, systemic anticoagulation will be conducted with intravenous heparin to maintain an activated clotting time of 300 to 400 s during the procedure. The esophageal temperature will be monitored with a temperature probe (SensiTherm Multi, Abbott, Chicago, USA; Esophastar, Japan Lifeline, Tokyo, Japan; Circa, Boston Scientific, Marlborough, USA). A PVI will be mandatorily performed in both groups. A cavotricuspid isthmus ablation will be performed in patients who have clinical or inducible cavotricuspid isthmus–dependent atrial flutter. Other adjunctive ablation lesion sets targeting sites outside of the PVs including non-PV AF triggers will be conducted according to the hospital’s standard strategy.
2.7. The CB ablation
The detailed procedure has been described elsewhere [15]. The 4th generation CB will be used in the CB ablation group. In brief, an 8.5-F sheath (SL0, Abbott) will be exchanged for a 15-F steerable sheath (FlexCath, Medtronic, Minneapolis, USA) after the transseptal puncture. The 28-mm CB will be introduced into the LA over a spiral mapping catheter (Achieve, Medtronic) through the sheath and will be inflated proximal to each PV and pushed gently, aiming for complete sealing at the antral aspect of the PV. Contrast medium will be injected from the distal lumen of the CB to verify a complete PV antrum occlusion. A 180-second or time to isolation plus 120-second freeze cycle will be performed at each PV [15], [20]. An electrical PVI with bidirectional block will be confirmed with the Achieve or other multielectrode catheter. When the initial freezing fails to isolate the PV, the CB will be repositioned, and the second freeze cycle will be applied. When the CB ablation cannot achieve the PVI, additional focal ablation will be performed with a Freezor Max (Medtronic) or RF catheter.
A 3-D electroanatomic mapping system will be used to delineate the PV anatomy and aid in navigation (EnSite system, Abbott; CARTO system, Biosense Webster, Diamond Bat, USA; Rhythmia system, Boston Scientific). The right phrenic nerve (PN) will be stimulated with a mapping catheter positioned in the superior vena cava to prevent PN palsy during the CB ablation of the right-sided PVs. PN capture will be assessed by monitoring the diaphragmatic contraction and diaphragmatic compound motor action potentials recorded from a surface electrocardiogram lead positioned over the right diaphragm. The CB ablation will be stopped immediately when attenuation or loss of the diaphragmatic contractions is detected.
2.8. RF ablation
After the transseptal puncture, a 3D geometry of the LA and PVs will be depicted by a circular mapping (Lasso, Biosense Webster; Advisor, AFocus, Optima, Abbott; EP star Libero, Japan Lifeline), PentaRay (Biosense Webster), HD grid (Abbott), or Orion (Boston Scientific) catheter. The ablation of the ipsilateral superior and inferior PVs will be jointly performed under navigation using the 3-D mapping system. RF ablation will be performed with an open-irrigated catheter (Thermocool SmartTouch SF, Thermocool SmartTouch; Biosense Webster; TactiCath SE, Flexibility, Abbott; Intellanav StablePoint, Intella Tip MiFi, Boston Scientific). The RF ablation settings will be according to the hospital’s standard strategy, generally with a power of 30–50 W, targeting an ablation index of 450–550 for CARTO and lesion index of 4.0–5.0 for NavX. The power and duration will usually be reduced to 20–25 W for 20 s on the LA posterior wall near the esophagus. Contact force (CF) data will be continuously monitored throughout the procedure to achieve at least 10 g (mean) with a vector perpendicular to the tissue and with an upper limit of 50 g. An electrical PVI and bidirectional block will be confirmed with a multielectrode catheter.
2.9. Follow-up
Personal study visits will be scheduled at 1 month and then every 1 to 3 months after the index procedure. Study visits will include a medical history, physical examination, and 12-lead ECG. The patients will be provided with an ambulatory one-channel electrogram recorder (HCG-801, OMRON Healthcare, Kyoto, Japan), and electrograms will be recorded twice daily for 1-year regardless of any symptoms after the index procedure except for during the BP [21], [22]. In addition, ambulatory electrograms will be recorded when the patient has any symptoms during the study period. In patients with cardiac implantable electronic devices, home monitoring will be adapted to facilitate the continuous monitoring of AF episodes. Twenty-four hour Holter recordings will be performed at 3, 12, 24, and 36 months after the index procedure. Echocardiography and QoL questionnaires will also be obtained at baseline and 12, 24, and 36 months after the procedure. The QoL will be assessed by AFEQT questionnaires [23]. AADs will be recommended to be discontinued after the BP unless any recurrent ATs are observed. Anticoagulation will be continued for at least 3 months, and the continuation of anticoagulation will be decided based on the CHADS2 /CHA2DS2-VASc score according to the guidelines [3]. In case of recurrences outside the BP, a 2nd catheter ablation will be recommended.
Adverse events (AEs) will be collected during the study period. An AE is defined as any untoward medical occurrence in a subject during this study, regardless of whether or not the AE is related to the ablation procedure.
2.10. Sample size and power
The target sample size is 500 (250 in each group). The sample size was calculated based on the primary hypothesis. The freedom rate of ATs after CA of PeAF has been reported to be comparable between the CB and RF ablation, ranging from 50 − 70 % at 1-year in the observational studies [16], [17], [18], [24], [25], [26], [27], [28]. Based on those data, a freedom rate at 1-year of 60% in both groups is assumed. The non-inferiority hypothesis will be evaluated using a log-rank test with a non-inferiority margin for a hazard ratio of 1.5. The sample size is calculated as 239 patients per group with a power of 80% based on a significance level of 2.5%. To cope with a potential loss-to-follow-up, a minimum of 500 patients (250 patients per group) will be enrolled in this study. Any withdrawn patients will not be replaced.
When the primary endpoint is observed in one-third of the total sample size (160 patients), early stopping of the study for futility will be considered based on the conditional power at an interim analysis, which will be reviewed by the Data and Safety Monitoring Board (DSMB). A simulation suggested a very limited power loss with a potential early stopping for futility, and the targeted power is expected to be achieved with this sample size.
2.11. Randomization and allocation factor
The patients will be registered and randomly assigned to either the CB or RF ablation group in a 1:1 ratio with a UMIN INDICE cloud system. The treatment allocation will be based on a covariate-adaptive randomization (minimization) scheme including the age (<75 vs. ≥ 75 years), sex, and LAD (<45 vs. ≥ 45 mm) as covariates, which may influence the evaluation of the efficacy and safety of the CA [26], [29], [30], [31], [32].
2.12. Data quality control and management
The principal investigator will authorize access to the electronic Case Report Form (CRF) system for investigators. The principal investigator will take full responsibility for the accuracy and reliability of all the data entered in the CRFs. The principal investigator and other investigators must not disclose the information contained in the CRFs to third parties. Only investigators can access the data.
2.13. Statistical analysis
Efficacy analyses will be performed on the basis of the intention-to-treat principle. A per-protocol basis analysis will also be performed as a supplemental analysis. When non-inferiority is demonstrated on the primary endpoint, the superiority hypothesis will also be evaluated. The Kaplan-Meier method and log-rank test will be used to estimate or compare the AT recurrence free survival for the time-to-event variables.
Categorical variables will be presented as absolute and relative frequencies and compared using a chi-square test or Fisher’s exact test. Continuous variables will be presented as the mean ± standard deviation for normally distributed data or median with the interquartile range (25th−75th percentiles) for skewed data and analyzed with the Student’s t-test or Wilcoxon rank-sum test, as appropriate. A P value < 0.05 will be considered statistically significant. A multivariable Cox regression analysis will be performed to investigate the predictors associated with clinical outcomes. A statistical analysis will be performed using up-to-date versions of Stata software (StataCorp, College Station, USA). The patient demographic data and outcomes of CA in each group will be collected descriptively as presented in Table 1, Table 2. A detailed plan for the interim analysis and final analysis will be prespecified in the statistical analysis plan, which will be prepared and finalized prior to the database lock.
Table 1.
Patient Characteristics at Baseline.
| Age, years, n (%) |
| Female sex, n (%) |
| Height, cm |
| Weight, kg |
| Body mass index, kg/m2 |
| Months since paroxysmal AF onset |
| Months since persistent AF onset |
| Duration of the longest AF episode, months |
| Failed antiarrhythmic drugs, n |
| Cardioversion before enrollment |
| NYHA functional class |
| mEHRA |
| AFEQT |
| History of a heart failure hospitalization, n (%) |
| Number of heart failure hospitalizations, n (%) |
| Systolic blood pressure, mmHg |
| Diastolic blood pressure, mmHg |
| Heart rate, /min |
| Congestive heart failure, n (%) |
| Hypertension, n (%) |
| Diabetes mellitus, n (%) |
| Stroke and/or transient ischemic attack, n (%) |
| Vascular disease, n (%) |
| Abnormal renal/liver function, n (%) |
| Structural heart disease, n (%) |
| Coronary artery disease |
| Valvular heat disease |
| Dilated cardiomyopathy |
| Hypertrophic cardiomyopathy |
| Others |
| Post-open heart surgery |
| CHADS2 score |
| CHA2DS2-VASc score |
| HAS-BLED score |
| Echocardiographic data |
| Left ventricular ejection fraction (modified Simpson’s method), % |
| Left ventricular diastolic dimension, mm |
| Left ventricular systolic dimension, mm |
| Left atrial dimension, mm |
| Left atrial volume, ml |
| Left atrial volume index |
| Left ventricular inflow velocity pattern |
| Valvular heart disease |
| Cardiac Implantable Electronic Device |
| Pacemaker |
| ICD |
| CRT-P |
| CRT-D |
| History of anti-arrhythmic drug use, n (%) |
| Disopyramide |
| Cibenzoline |
| Pirmenol |
| Procainamide |
| Quinidine |
| Aprindine |
| Pilsicainide |
| Flecainide |
| Propafenone |
| Amiodarone |
| Sotalol |
| Bepridil |
| Others |
| Laboratory data |
Table 2.
The Ablation Procedure and Complications.
| Total procedure time (groin puncture to catheter extraction), min |
| Left atrial dwell time, min |
| Total fluoroscopy time, min |
| Total absorbed dose, mGy |
| PVI success, n (%) |
| For cryoablation |
| Total freeze cycles, n |
| Freeze cycles per vein, n |
| Time to isolation per vein, s |
| Additional freeze cycles, n |
| Total freeze time, s |
| Freeze time per vein, s |
| Minimum balloon temperature, ℃ |
| The need for touch up ablation, n (%) |
| For radiofrequency ablation |
| Type of ablation catheter |
| Output and duration of radiofrequency application |
| Total radiofrequency application time, s |
| Other adjunctive ablation, n (%) |
| Use and type of 3D mapping system, n (%) |
| Use of general anesthesia, n (%) |
| Use of esophageal temperature probe, n (%) |
| Quantity of contrast media, ml |
| Number of case experiences per operator, n |
| Complications, n (%) |
| Pericardial effusion requiring drainage |
| Pericardial effusion not requiring drainage |
| Transient ischemic attack |
| Cerebral infarction |
| Other thromboembolisms |
| Transient phrenic nerve paralysis |
| Prolonged phrenic nerve paralysis |
| Severe pulmonary vein stenosis (>70% reduction in pulmonary vein diameter) |
| Hematoma at the puncture site |
| Pseudoaneurysm at the puncture site |
| Gastric hypomotility |
| Death |
| Others |
| Discharge prescription, n (%) |
| Oral anticoagulant |
| Antiarrhythmic drugs |
| Disopyramide |
| Cibenzoline |
| Pirmenol |
| Procainamide |
| Quinidine |
| Aprindine |
| Pilsicainide |
| Flecainide |
| Propafenone |
| Amiodarone |
| Sotalol |
| Bepridil |
| Others |
| Antiplatelet drug |
| Beta-blocker |
| Angiotensin converting enzyme inhibitor/Angiotensin II receptor blocker |
| Angiotensin receptor-neprilysin inhibitor |
| Ivabradine |
| Digitalis |
| Calcium channel blocker |
| Loop diuretic |
| Statin |
2.14. Study organization
The research group consists of investigators at 12 hospitals in Japan and an independent data monitoring committee. The Certified Review Board (CRB) and DSMB regularly monitors the recruitment and conduct of the study, data quality, timeliness, serious AEs, and further AEs selected at their discretion during the course of the study. All study hospitals will be required to have performed at least 30 procedures of CA of AF annually, and to have at least 2 operators who have completed at least 50 procedures of CA of AF and a system that enables emergency cardiovascular surgery, in order for them to be able to participate in this study. As operator experience and the hospital CA volume will influence the efficacy and safety of the CA, we will also assess the relation between the CA outcome and operator experience/hospital CA volume.
2.15. Ethics
The study is registered at the Japan Registry of Clinical Trials (jRCT) identifier (jRCT1052210003). The study is being conducted in accordance with the Declaration of Helsinki and the Ethical Guidelines for Clinical Studies issued by the Ministry of Health, Labour and Welfare, Japan. This study received approval from the CRB of the Osaka City University Hospital, Japan (OCU009E, March 1, 2021). CRB approved informed consent form (written in accordance with the applicable laws for clinical research) will be obtained from every patient prior to study enrollment.
3. Discussion
Although the PVI is an essential strategy of AF ablation, the role of CB ablation in PeAF has not been well evaluated. The STOP Persistent AF trial was a prospective, multicenter, single arm study, which evaluated the safety and efficacy of the PVI with CB ablation in patients with PeAF (continuous episodes < 6 months) [24]. A total of 165 patients with PeAF (70% male, age 65 ± 9 years, LAD 42 ± 6 mm) were treated with CB ablation at 25 sites in the United States, Canada, and Japan. The total procedural, LA dwell, and fluoroscopy times were 121 ± 46 min, 102 ± 41 min, and 19 ± 16 min, respectively. The freedom from any ATs after a 90-day BP was 54.8% (95% confidence interval 46.7% − 62.1%).
Ciconte et al. retrospectively compared the efficacy of the PVI at 1-year as an index procedure for PeAF between the CB and RF ablation with the CF technology [28]. Among 100 consecutive patients (74% male, age 62 ± 10 years), 50 underwent RF ablation (LAD 47 ± 6 mm) and 50 CB ablation (LAD 46 ± 7 mm). The procedure and fluoroscopy times were significantly shorter in the CB ablation than RF ablation (91 ± 42 vs. 140 ± 47 min and 15 ± 7 vs. 20 ± 7 min; P < 0.01). The freedom from ATs at 1-year was comparable between the CB and RF ablation (60% vs. 56%, P = 0.71).
Isolation of the posterior LA (box isolation) has been proposed as an adjunctive ablation lesion set targeting sites outside of the PVs because the arrhythmogenicity of the posterior LA wall has a similar embryological background as the PVs [33]. Yokokawa et al. compared the efficacy of CA in patients with PeAF between the CB and an RF box ablation (PVI plus LA roof line + LA bottom line) with CF technology in patients with PeAF [25]. A total of 167 consecutive patients with PeAF (mean age 64 ± 9 years, LAD 46 ± 6 mm) underwent CB ablation (n = 90, LAD 46 ± 5 mm, an additional LA roof line was created in 33 patients) or RF box ablation (n = 77, LAD 46 ± 6 mm) in the study, and the freedom from ATs was comparable between the CB and RF box ablation (41% vs. 51%, P = 0.22) during a follow-up period of 21 ± 10 months.
It has been reported that the LA size is associated with the ablation outcome in both CB and RF ablation [30], [31]. Ikenouchi et al. analyzed the impact of the LA size on the efficacy of CB and RF ablation [32]. A total of 2,224 AF patients (64 ± 11 years, 65.5% male) who underwent a PVI were analyzed. The patients undergoing the CB and RF ablation were propensity score matched, and 376 matched pairs were evaluated. As a result, the efficacy was comparable between RF and CB in patients without LA enlargement, however, CB was inferior to RF in patients with LA enlargement. According to those studies, the patients with enlarged LA (LAD > 50 mm) will be excluded from the CRRF-PeAF study.
Although both the CB and RF ablation are increasingly used worldwide not only for PAF but PeAF, there are no prospective randomized data comparing the 2 ablation technologies in patients with PeAF. The CRRF-PeAF study will compare the efficacy and safety of CA of AF between these 2 CA technologies in patients with PeAF. It will provide critical evidence to guide selection of CA technologies in the treatment of PeAF.
4. Conclusions
The CRRF-PeAF study will be the first to compare the efficacy and safety of CA of PeAF between CB and RF ablation in a prospective, multicenter, randomized, controlled fashion.
Declaration of Competing Interest
Kazuhiro Satomi report lecture/consultant/advisory honoraria from Abbott and Medtronic.
Wataru Shimizu, Junichi Nitta, Atsushi Kobori, Kengo Kusano report lecture/consultant/advisory honoraria from Medtronic.
Satoshi Nagase belongs to a donation course of Medtronic.
Takeshi Aiba belonged to a donation course of Medtronic.
Acknowledgments
Acknowledgement
We thank Mr. John Martin for his help in the preparation of the article.
Role of the funding source
The CRRF-PeAF study is partly supported by the Intramural Research Fund 21-3-1 (Kusano) for Cardiovascular Diseases of National Cerebral and Cardiovascular Center.
References
- 1.Kopecky S.L., Gersh B.J., McGoon M.D., Whisnant J.P., Holmes D.R., Jr., Ilstrup D.M., Frye R.L. The natural history of lone atrial fibrillation. A population-based study over three decades. New Engl. J. Med. 1987;317:669–674. doi: 10.1056/NEJM198709103171104. [DOI] [PubMed] [Google Scholar]
- 2.Prystowsky E.N., Benson D.W., Jr., Fuster V., Hart R.G., Kay G.N., Myerburg R.J., Naccarelli G.V., Wyse D.G. Management of patients with atrial fibrillation. A statement for healthcare professionals. From the subcommittee on electrocardiography and electrophysiology, american heart association. Circulation. 1996;93:1262–1277. doi: 10.1161/01.cir.93.6.1262. [DOI] [PubMed] [Google Scholar]
- 3.Calkins H., Hindricks G., Cappato R., Kim Y.H., Saad E.B., Aguinaga L., Akar J.G., Badhwar V., Brugada J., Camm J., Chen P.S., Chen S.A., Chung M.K., Cosedis Nielsen J., Curtis A.B., Davies D.W., Day J.D., d'Avila A., Natasja de Groot N.M.S., Di Biase L., Duytschaever M., Edgerton J.R., Ellenbogen K.A., Ellinor P.T., Ernst S., Fenelon G., Gerstenfeld E.P., Haines D.E., Haissaguerre M., Helm R.H., Hylek E., Jackman W.M., Jalife J., Kalman J.M., Kautzner J., Kottkamp H., Kuck K.H., Kumagai K., Lee R., Lewalter T., Lindsay B.D., Macle L., Mansour M., Marchlinski F.E., Michaud G.F., Nakagawa H., Natale A., Nattel S., Okumura K., Packer D., Pokushalov E., Reynolds M.R., Sanders P., Scanavacca M., Schilling R., Tondo C., Tsao H.M., Verma A., Wilber D.J., Yamane T., Document R. hrs/ehra/ecas/aphrs/solaece expert consensus statement on catheter and surgical ablation of atrial fibrillation. Europace : European pacing, arrhythmias, and cardiac electrophysiology : journal of the working groups on cardiac pacing, arrhythmias, and cardiac cellular electrophysiology of the European Society of Cardiology. 2017;2018(20):e1–e160. doi: 10.1093/europace/eux274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wilber D.J., Pappone C., Neuzil P., De Paola A., Marchlinski F., Natale A., Macle L., Daoud E.G., Calkins H., Hall B., Reddy V., Augello G., Reynolds M.R., Vinekar C., Liu C.Y., Berry S.M., Berry D.A., ThermoCool A.F.T.I. Comparison of antiarrhythmic drug therapy and radiofrequency catheter ablation in patients with paroxysmal atrial fibrillation: a randomized controlled trial. JAMA. 2010;303:333–340. doi: 10.1001/jama.2009.2029. [DOI] [PubMed] [Google Scholar]
- 5.Haissaguerre M., Jais P., Shah D.C., Takahashi A., Hocini M., Quiniou G., Garrigue S., Le Mouroux A., Le Metayer P., Clementy J. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. New Engl. J. Med. 1998;339:659–666. doi: 10.1056/NEJM199809033391003. [DOI] [PubMed] [Google Scholar]
- 6.Jais P., Haissaguerre M., Shah D.C., Chouairi S., Gencel L., Hocini M., Clementy J. A focal source of atrial fibrillation treated by discrete radiofrequency ablation. Circulation. 1997;95:572–576. doi: 10.1161/01.cir.95.3.572. [DOI] [PubMed] [Google Scholar]
- 7.Verma A., Jiang C.Y., Betts T.R., Chen J., Deisenhofer I., Mantovan R., Macle L., Morillo C.A., Haverkamp W., Weerasooriya R., Albenque J.P., Nardi S., Menardi E., Novak P., Sanders P., Investigators S.A.I. Approaches to catheter ablation for persistent atrial fibrillation. New Engl. J. Med. 2015;372:1812–1822. doi: 10.1056/NEJMoa1408288. [DOI] [PubMed] [Google Scholar]
- 8.Oral H., Knight B.P., Tada H., Ozaydin M., Chugh A., Hassan S., Scharf C., Lai S.W., Greenstein R., Pelosi F., Jr., Strickberger S.A., Morady F. Pulmonary vein isolation for paroxysmal and persistent atrial fibrillation. Circulation. 2002;105:1077–1081. doi: 10.1161/hc0902.104712. [DOI] [PubMed] [Google Scholar]
- 9.Pak H.N., Park J., Park J.W., Yang S.Y., Yu H.T., Kim T.H., Uhm J.S., Choi J.I., Joung B., Lee M.H., Kim Y.H., Shim J. Electrical posterior box isolation in persistent atrial fibrillation changed to paroxysmal atrial fibrillation: a multicenter, prospective, randomized study. Circulation. Arrhythmia Electrophysiol. 2020;13 doi: 10.1161/CIRCEP.120.008531. [DOI] [PubMed] [Google Scholar]
- 10.Yu H.T., Shim J., Park J., Kim I.S., Kim T.H., Uhm J.S., Joung B., Lee M.H., Kim Y.H., Pak H.N. Pulmonary vein isolation alone versus additional linear ablation in patients with persistent atrial fibrillation converted to paroxysmal type with antiarrhythmic drug therapy: a multicenter, prospective, randomized study. Circulation. Arrhythmia Electrophysiol. 2017;10 doi: 10.1161/CIRCEP.116.004915. [DOI] [PubMed] [Google Scholar]
- 11.Dixit S., Marchlinski F.E., Lin D., Callans D.J., Bala R., Riley M.P., Garcia F.C., Hutchinson M.D., Ratcliffe S.J., Cooper J.M., Verdino R.J., Patel V.V., Zado E.S., Cash N.R., Killian T., Tomson T.T., Gerstenfeld E.P. Randomized ablation strategies for the treatment of persistent atrial fibrillation: Rasta study. Circulation. Arrhythmia Electrophysiol. 2012;5:287–294. doi: 10.1161/CIRCEP.111.966226. [DOI] [PubMed] [Google Scholar]
- 12.Kuck K.H., Brugada J., Furnkranz A., Metzner A., Ouyang F., Chun K.R., Elvan A., Arentz T., Bestehorn K., Pocock S.J., Albenque J.P., Tondo C., Fire I.I. Cryoballoon or radiofrequency ablation for paroxysmal atrial fibrillation. New Engl. J. Med. 2016;374:2235–2245. doi: 10.1056/NEJMoa1602014. [DOI] [PubMed] [Google Scholar]
- 13.Wazni O.M., Dandamudi G., Sood N., Hoyt R., Tyler J., Durrani S., Niebauer M., Makati K., Halperin B., Gauri A., Morales G., Shao M., Cerkvenik J., Kaplon R.E., Nissen S.E., Investigators S.A.F.T. Cryoballoon ablation as initial therapy for atrial fibrillation. New Engl. J. Med. 2021;384:316–324. doi: 10.1056/NEJMoa2029554. [DOI] [PubMed] [Google Scholar]
- 14.Andrade J.G., Wells G.A., Deyell M.W., Bennett M., Essebag V., Champagne J., Roux J.F., Yung D., Skanes A., Khaykin Y., Morillo C., Jolly U., Novak P., Lockwood E., Amit G., Angaran P., Sapp J., Wardell S., Lauck S., Macle L., Verma A., Investigators E.-A. Cryoablation or drug therapy for initial treatment of atrial fibrillation. New Engl. J. Med. 2021;384:305–315. doi: 10.1056/NEJMoa2029980. [DOI] [PubMed] [Google Scholar]
- 15.Miyamoto K., Doi A., Hasegawa K., Morita Y., Mishima T., Suzuki I., Kaseno K., Nakajima K., Kataoka N., Kamakura T., Wada M., Yamagata K., Ishibashi K., Inoue Y.Y., Nagase S., Noda T., Aiba T., Asakura M., Izumi C., Noguchi T., Tada H., Takagi M., Yasuda S., Kusano K.F. Multicenter study of the validity of additional freeze cycles for cryoballoon ablation in patients with paroxysmal atrial fibrillation: The ad-balloon study. Circulation. Arrhythmia Electrophysiol. 2019;12 doi: 10.1161/CIRCEP.118.006989. [DOI] [PubMed] [Google Scholar]
- 16.Koektuerk B., Yorgun H., Hengeoez O., Turan C.H., Dahmen A., Yang A., Bansmann P.M., Gorr E., Hoppe C., Turan R.G., Horlitz M. Cryoballoon ablation for pulmonary vein isolation in patients with persistent atrial fibrillation: one-year outcome using second generation cryoballoon. Circulation. Arrhythmia Electrophysiol. 2015;8:1073–1079. doi: 10.1161/CIRCEP.115.002776. [DOI] [PubMed] [Google Scholar]
- 17.Tondo C., Iacopino S., Pieragnoli P., Molon G., Verlato R., Curnis A., Landolina M., Allocca G., Arena G., Fassini G., Sciarra L., Luzi M., Manfrin M., Padeletti L. ClinicalService SPI Pulmonary vein isolation cryoablation for patients with persistent and long-standing persistent atrial fibrillation: clinical outcomes from the real-world multicenter observational project. Heart Rhythm. 2018;15:363–368. doi: 10.1016/j.hrthm.2017.10.038. [DOI] [PubMed] [Google Scholar]
- 18.Akkaya E., Berkowitsch A., Zaltsberg S., Greiss H., Hamm C.W., Sperzel J., Neumann T., Kuniss M. Ice or fire? Comparison of second-generation cryoballoon ablation and radiofrequency ablation in patients with symptomatic persistent atrial fibrillation and an enlarged left atrium. J. Cardiovasc. Electrophysiol. 2018;29:375–384. doi: 10.1111/jce.13402. [DOI] [PubMed] [Google Scholar]
- 19.Kobori A., Sasaki Y., Pak M., Ishikura M., Murai R., Okada T., Toyota T., Taniguchi T., Kim K., Ehara N., Kinoshita M., Kihara Y., Furukawa Y. Comparison of cryoballoon and contact force-sensing radiofrequency ablation for persistent atrial fibrillation in clinical practice. Circulation J.: Official J. Japanese Circulation Soc. 2022;86:290–298. doi: 10.1253/circj.CJ-21-0608. [DOI] [PubMed] [Google Scholar]
- 20.Reissmann B., Wissner E., Deiss S., Heeger C., Schlueter M., Wohlmuth P., Lemes C., Mathew S., Maurer T., Sohns C., Saguner A., Santoro F., Hayashi K., Riedl J., Ouyang F., Kuck K.H., Metzner A. First insights into cryoballoon-based pulmonary vein isolation taking the individual time-to-isolation into account. Europace : European pacing, arrhythmias, and cardiac electrophysiology : journal of the working groups on cardiac pacing, arrhythmias, and cardiac cellular electrophysiology of the European Society of Cardiology. 2017;19:1676–1680. doi: 10.1093/europace/euw233. [DOI] [PubMed] [Google Scholar]
- 21.Kobori A., Shizuta S., Inoue K., Kaitani K., Morimoto T., Nakazawa Y., Ozawa T., Kurotobi T., Morishima I., Miura F., Watanabe T., Masuda M., Naito M., Fujimoto H., Nishida T., Furukawa Y., Shirayama T., Tanaka M., Okajima K., Yao T., Egami Y., Satomi K., Noda T., Miyamoto K., Haruna T., Kawaji T., Yoshizawa T., Toyota T., Yahata M., Nakai K., Sugiyama H., Higashi Y., Ito M., Horie M., Kusano K.F., Shimizu W., Kamakura S., Kimura T., Investigators U.-A.-T. Adenosine triphosphate-guided pulmonary vein isolation for atrial fibrillation: The unmasking dormant electrical reconduction by adenosine triphosphate (under-atp) trial. Eur. Heart J. 2015;36:3276–3287. doi: 10.1093/eurheartj/ehv457. [DOI] [PubMed] [Google Scholar]
- 22.Kaitani K., Inoue K., Kobori A., Nakazawa Y., Ozawa T., Kurotobi T., Morishima I., Miura F., Watanabe T., Masuda M., Naito M., Fujimoto H., Nishida T., Furukawa Y., Shirayama T., Tanaka M., Okajima K., Yao T., Egami Y., Satomi K., Noda T., Miyamoto K., Haruna T., Kawaji T., Yoshizawa T., Toyota T., Yahata M., Nakai K., Sugiyama H., Higashi Y., Ito M., Horie M., Kusano K.F., Shimizu W., Kamakura S., Morimoto T., Kimura T., Shizuta S., Investigators E.-A.-T. Efficacy of antiarrhythmic drugs short-term use after catheter ablation for atrial fibrillation (east-af) trial. Eur. Heart J. 2016;37:610–618. doi: 10.1093/eurheartj/ehv501. [DOI] [PubMed] [Google Scholar]
- 23.Spertus J., Dorian P., Bubien R., Lewis S., Godejohn D., Reynolds M.R., Lakkireddy D.R., Wimmer A.P., Bhandari A., Burk C. Development and validation of the atrial fibrillation effect on quality-of-life (afeqt) questionnaire in patients with atrial fibrillation. Circulation. Arrhythmia Electrophysiol. 2011;4:15–25. doi: 10.1161/CIRCEP.110.958033. [DOI] [PubMed] [Google Scholar]
- 24.Su W.W., Reddy V.Y., Bhasin K., Champagne J., Sangrigoli R.M., Braegelmann K.M., Kueffer F.J., Novak P., Gupta S.K., Yamane T., Calkins H., Investigators S.P.A. Cryoballoon ablation of pulmonary veins for persistent atrial fibrillation: Results from the multicenter stop persistent af trial. Heart rhythm. 2020;17:1841–1847. doi: 10.1016/j.hrthm.2020.06.020. [DOI] [PubMed] [Google Scholar]
- 25.Yokokawa M., Chugh A., Latchamsetty R., Ghanbari H., Crawford T., Jongnarangsin K., Cunnane R., Saeed M., Sunkara B., Tezcan M., Bogun F., Pelosi F., Jr., Morady F., Oral H. Cryoballoon antral pulmonary vein isolation vs contact force-sensing radiofrequency catheter ablation for pulmonary vein and posterior left atrial isolation in patients with persistent atrial fibrillation. Heart rhythm. 2018;15:1835–1841. doi: 10.1016/j.hrthm.2018.06.047. [DOI] [PubMed] [Google Scholar]
- 26.Nishimura T., Yamauchi Y., Aoyagi H., Tsuchiya Y., Shigeta T., Nakamura R., Yamashita M., Asano M., Nakamura T., Suzuki H., Shimura T., Kurabayashi M., Keida T., Sasano T., Hirao K., Okishige K. The clinical impact of the left atrial posterior wall lesion formation by the cryoballoon application for persistent atrial fibrillation: Feasibility and clinical implications. J. Cardiovasc. Electrophysiol. 2019;30:805–814. doi: 10.1111/jce.13879. [DOI] [PubMed] [Google Scholar]
- 27.Sawhney V., Schilling R.J., Providencia R., Cadd M., Perera D., Chatha S., Mercer B., Finlay M., Halimi F., Pavin D., Anselme F., Cebron J.P., Chun J., Schmidt B., Defaye P., Dhillon G., Boveda S., Albenque J.P., Tayebjee M., de Asmundis C., Chierchia G., Hunter R.J. Cryoablation for persistent and longstanding persistent atrial fibrillation: Results from a multicentre european registry. Europace : European pacing, arrhythmias, and cardiac electrophysiology : journal of the working groups on cardiac pacing, arrhythmias, and cardiac cellular electrophysiology of the European Society of Cardiology. 2020;22:375–381. doi: 10.1093/europace/euz313. [DOI] [PubMed] [Google Scholar]
- 28.Ciconte G., Baltogiannis G., de Asmundis C., Sieira J., Conte G., Di Giovanni G., Saitoh Y., Irfan G., Mugnai G., Hunuk B., Chierchia G.B., Brugada P. Circumferential pulmonary vein isolation as index procedure for persistent atrial fibrillation: A comparison between radiofrequency catheter ablation and second-generation cryoballoon ablation. Europace : European pacing, arrhythmias, and cardiac electrophysiology : journal of the working groups on cardiac pacing, arrhythmias, and cardiac cellular electrophysiology of the European Society of Cardiology. 2015;17:559–565. doi: 10.1093/europace/euu350. [DOI] [PubMed] [Google Scholar]
- 29.Berruezo A., Tamborero D., Mont L., Benito B., Tolosana J.M., Sitges M., Vidal B., Arriagada G., Mendez F., Matiello M., Molina I., Brugada J. Pre-procedural predictors of atrial fibrillation recurrence after circumferential pulmonary vein ablation. Eur. Heart J. 2007;28:836–841. doi: 10.1093/eurheartj/ehm027. [DOI] [PubMed] [Google Scholar]
- 30.Olshansky B., Heller E.N., Mitchell L.B., Chandler M., Slater W., Green M., Brodsky M., Barrell P., Greene H.L. Are transthoracic echocardiographic parameters associated with atrial fibrillation recurrence or stroke? Results from the atrial fibrillation follow-up investigation of rhythm management (affirm) study. J. Am. Coll. Cardiol. 2005;45:2026–2033. doi: 10.1016/j.jacc.2005.03.020. [DOI] [PubMed] [Google Scholar]
- 31.Njoku A., Kannabhiran M., Arora R., Reddy P., Gopinathannair R., Lakkireddy D., Dominic P. Left atrial volume predicts atrial fibrillation recurrence after radiofrequency ablation: a meta-analysis. Europace : European pacing, arrhythmias, and cardiac electrophysiology : journal of the working groups on cardiac pacing, arrhythmias, and cardiac cellular electrophysiology of the European Society of Cardiology. 2018;20:33–42. doi: 10.1093/europace/eux013. [DOI] [PubMed] [Google Scholar]
- 32.Ikenouchi T., Inaba O., Takamiya T., Inamura Y., Sato A., Matsumura Y., Sato H., Hirakawa A., Takahashi Y., Goya M., Sasano T., Nitta J. The impact of left atrium size on selection of the pulmonary vein isolation method for atrial fibrillation: cryoballoon or radiofrequency catheter ablation. Am. Heart J. 2021;231:82–92. doi: 10.1016/j.ahj.2020.10.061. [DOI] [PubMed] [Google Scholar]
- 33.Ho S.Y., Cabrera J.A., Sanchez-Quintana D. Left atrial anatomy revisited. Circulation. Arrhythmia Electrophysiol. 2012;5:220–228. doi: 10.1161/CIRCEP.111.962720. [DOI] [PubMed] [Google Scholar]