Highlights
-
•
Evaluation of adult Ehlers Danlos Syndrome (EDS) patients undergoing Posterior Lumbar Fusion
-
•
Matched study controlling for potential confounding variables
-
•
Adult EDS patients found not to be at increased risk of 90-day adverse events
-
•
Within five years, EDS patients also did not have increased risk of reoperation
Keywords: Posterior Lumbar Fusion, 90-Day Complications, Ehlers Danlos, Reoperations, Readmissions, Pearldiver
Abbreviations: EDS, Ehlers-Danlos syndrome; PLF, Posterior lumbar fusion; ECI, Elixhauser Comorbidity Index; CPT, Current Procedural Terminology; ICD, International Classification of Diseases; AAE, Any Adverse Events; SAE, Severe Adverse Events; MAE, Minor Adverse Events; OR, Odds Ratio
Abstract
Background
Ehlers Danlos Syndrome (EDS) is a rare connective tissue disorder that results from mutations in collagen genes. Potentially related to laxity and resultant degenerative changes, adult EDS patients may require posterior lumbar fusion (PLF). However, with low numbers, adequately powered outcome studies have been limited. The purpose of this study was to investigate risk of complications, readmissions and reoperations in adult patients with EDS following single-level PLF,
Methods
A retrospective study using the 2010 to 2020 MSpine Pearldiver dataset was performed. Adult patients undergoing single-level PLF (excluding any with anterior procedures) with and without EDS for which at least 90-day follow up was available were identified. Any cases performed for trauma, tumor, or infection were excluded.
Single-level PLF EDS patients were then matched 1:4 with PLF non-EDS patients based on age, sex, and Elixhauser Comorbidity Index (ECI). Rates of ninety-day any, severe, and minor adverse events as well as readmissions were tabulated and compared with chi-square tests. Multivariate logistical regression was then performed (controlling for age, sex, and ECI).
Reoperation surgeries over five years were assessed, Kaplan-Meier survival curves generated, and curves of those with and without EDS were compared with log rank test.
Results
In total, there were 170,100 single-level PLF case identified, of which 242 (0.14%) had EDS. After matching, there were 957 without EDS and 239 with EDS. On multivariate regression, there were no significant differences in 90-day any, severe, or minor adverse events, or readmissions (p>0.05 for each). Over five years, there were also not significant differences in rates of reoperation (p> 0.05).
Conclusions
For EDS patients undergoing PLF, the current study identified similar 90-day adverse events and 5-year reoperation rates compared to those without EDS. These findings may be useful for patient counseling and surgical planning for those with this rare condition.
Introduction
Ehlers-Danlos syndrome (EDS) comprises a heterogenous group of rare, heritable disorders that affect connective tissue principally in the skin, bones and ligaments, and blood vessels, causing joint hypermobility, skin hyperextensibility, and tissue fragility [1]. These traits result directly from underlying collagen dysfunction, with most forms of EDS resulting from mutations in one of the genes encoding fibrillar collagens (types I, II, III, V, or XI) or enzymes involved in the synthesis of these collagens [2,3].
Owing to the collagen-related connective tissues defects causing hypermobility, patients with EDS may develop spinal issues [4]. This has received attention for those undergoing surgery for deformity early in life [5], [6], [7], [8], however limited attention has been given to adults EDS patients undergoing spinal procedures for degenerative etiologies.
Limited case reports of patients undergoing varying surgeries have suggested that patients with EDS are at increased risk for bleeding and poor wound healing due to vascular abnormalities and tissue fragility [3,9,10]. It has been suggested that this risk may be contributed to by platelet function abnormalities, and the severity of the EDS has been suggested to correlated with bleeding risk [11].
In general, adult PLF procedures are associated high rates of success [12], but only limited case reports are available about spinal surgery in adult EDS patients [13,14]. Consequently, the current study was performed to examine adult EDS patients who underwent single-level PLF for demographic details, 90-day perioperative complications, and five-year surgery need for revision procedures relative to a matched non-EDS population.
Methods
Patient Sample
The present study made use of data from the 2010-2020 MSpine Pearldiver database. This large national insurance claims database contains both inpatient and outpatient information for over 1.15 million United States patients. The MSpine dataset contains data for commercially insured, Medicare, and Medicaid patients. It contains all information on any patient who underwent a spinal procedure in the Pearldiver database. Our Investigative Review Board found studies using this database to be exempt from review based on the de-identified nature of the data.
Patients over eighteen years of age undergoing PLF with or without laminectomy were identified using the Current Procedural Terminology (CPT) codes 22612, 22630, and 22633. Patients who had a primary diagnosis code of fractures, infection, neoplasm, or were not in the database for at least 90 days following the surgery were excluded. Further, patients who had multi-level fusions or had concurrent anterior fusion were excluded.
Patients with a diagnosis of EDS (any type) were then identified using the following International Classification of Diseases (ICD) codes: ICD-9-D-75683 and ICD10 codes ICD-10-D-Q796, ICD-10-D-Q7960, ICD-10-D-Q7961, ICD-10-D-Q7962, ICD-10-D-Q7963, and ICD-10-D-Q7969. Additional patient characteristics abstracted from the dataset included: age, sex, and ECI (Elixhauser Comorbidity Index, which was calculated by an algorithm included in the PearlDiver analysis package). Those without and with Ehlers Danlos Diagnosis were then matched 4:1 based on age, sex, and ECI to eliminate any significant differences in the variables between the two groups.
Postoperative outcomes
Perioperative outcomes were abstracted from the dataset based on ICD codes. Individual adverse events were aggregated into severe adverse events (SAE), minor adverse events (MAE), and any adverse event (AAE).
SAEs included: surgical site infection, sepsis, dep vein thrombosis, pulmonary embolism, myocardial infection, cardiac arrest, and pancreatitis. MAEs included: pneumonia, urinary tract infection, acute kidney injury, wound dehiscence, transfusion, and hematoma. AAEs were noted if there was the occurrence of a serious or minor adverse event. Readmissions were separately noted.
The rate of reoperation up to five-year survival after index surgery was then investigated. Reoperations were identified based on any subsequent surgery, including fusion, decompression, and/or revision or removal of instrumentation.
Statistical Analyses
Univariate comparison of non-matched patient characteristics (age, sex, and ECI) was performed with chi-square tests. Based on identified differences between the groups, 1:4 matching using age, sex, and ECI was performed, and chi-square tests were repeated.
The rate of aggregated complications between the matched non-EDS and EDS groups were compared with univariate chi-square tests and multivariate logistical regression (controlling for age, sex, and ECI) to define odds ratio (OR) and 95% confidence interval (CI).
Reoperation rates were determined using Kaplan-Meier analyses to determine timing and incidence. A log-rank (Mantel-Cox) test was performed to compare survival rate between the matched groups.
Statistical analyses were performed using Pearldiver statistical software (Pearldiver, Colorado Springs, CO) and graphics and survival analyses were generated with Graphpad Prism 9 (GraphPad Software, San Diego, CA). Significance was set at p < 0.05.
Results
Study cohorts
Based on the inclusion criteria, 170,342 PLF patients were identified, of which 242 (0.14%) had EDS (Table 1). There were statistically significant differences between those without and with EDS for age (59.61 vs 44.33, p < 0.001) and sex (58.5% female vs 86.8% female, p < 0.01) but not ECI.
Table 1.
Descriptive Characteristics of single-level PLF patients with and without Ehlers Danlos Syndrome
| Non-Matched PLF Groups | Matched PLF Groups | |||||
|---|---|---|---|---|---|---|
| Non-EDS | EDS | P -Value | Non-EDS | EDS | P - Value | |
| Sample Size | N = 170100 | N = 242 | N = 957 | N = 239 | ||
| Age (in years): Mean (SD) | 59.61 (12.49) |
44.33 (14.26) |
p < 0.001 | 44.83 (13.97) |
44.64 (14.09) |
p = 0.87 |
| Sex Female Male |
99519 (58.5%) 70581 (41.5%) |
210 (86.8%) 32 (13.2%) |
p < 0.001 |
829 (86.6%) 128 (13.7%) |
207 (86.6%) 32 (13.4%) |
p = 1.00 |
| ECI: Mean (SD) |
4.01 (3.25) |
4.76 (3.37) |
p = 0.27 | 4.72 (3.35) |
4.72 (3.36) |
p = 1.00 |
Significance set to p <0.05.
Matched based on Age, Sex, ECI 4:1 Non-EDS (Ehlers Danlos Syndrome) to EDS
PLF = Posterior Lumbar Fusion
To address the inherent differences in the study cohorts, matching was done based on age, sex, and ECI. This resulted in 957 non-EDS patients and 239 patients in the EDS patients. Once this was done, there were no residual differences for age, sex, or ECI (Table 1).
Ninety-day adverse outcomes
The incidence of 90-day adverse events and readmissions are shown in Table 2. Even with the large national population, the incidence of several the individual adverse events were low. Notably, to adhere to privacy considerations, numbers of ten or less cannot be displayed and are indicated in the table with an asterisk.
Table 2.
Univariate analyses of 90-day complications of Matched Groups
| No Ehlers Danlos Diagnosis | Ehlers Danlos Diagnosis | P - Value | |
|---|---|---|---|
| Sample Size | N = 957 | N = 239 | |
| Any Adverse Events | 138 (14.4%) | 33 (13.8%) | p = 0.92 |
| Severe Adverse Events | 65 (6.8%) | 12 (5.0%) | p = 0.38 |
| Surgical Site Infection | 37 (3.9%) | * | |
| Sepsis | 11 (1.1%) | * | |
| Deep Vein Thrombosis | 11 (1.1%) | * | |
| Pulmonary Embolism | * | * | |
| Myocardial Infarction | * | 0 | |
| Cardiac Arrest | * | 0 | |
| Pancreatitis | * | 0 | |
| Minor Adverse Events | 102 (10.7%) | 29 (12.1%) | p = 0.49 |
| Pneumonia | 16 (1.7%) | * | |
| Urinary Tract Infection | 49 (5.1%) | 18 (7.5%) | |
| Acute Kidney Injury | 11 (1.1%) | * | |
| Wound Dehiscence | 21 (2.2%) | * | |
| Transfusion | * | 0 | |
| Hematoma | * | * | |
| Readmissions | 91 (9.5%) | 28 (11.7%) | p = 0.33 |
Significance set to p < 0.05
Matched for ECI, Age, Sex
An * indicates 10 or less patients
Based on the above considerations, the univariate and multivariate statistical comparisons were done for the aggregated adverse events only (AAE, SAE, MAE, and readmission). Univariate analyses of these outcomes revealed no statistical differences (Table 2). Similarly, multivariate analyses controlling for age, sex, and ECI to address any residual differences after matching revealed no statistical differences (Table 3).
Table 3.
Multivariate analyses of 90-day complications and readmissions of Ehlers Danlos patients
| Odds Ratio | 95% Confidence Interval | P - Value | |
|---|---|---|---|
| Any Adverse Event | 0.95 | 0.62 – 1.44 | p = 0.81 |
| Severe Adverse Event | 0.99 | 0.38 – 1.37 | p = 0.32 |
| Minor Adverse Event | 1.17 | 0.74 – 1.82 | p = 0.50 |
| Readmissions | 1.26 | 0.79 – 1.97 | p = 0.31 |
Significance set to p < 0.05
Controlled for ECI, Age, Sex, EDS diagnosis
Five- year reoperations
Patient were then tracked out to five years. By that time, the non-EDS groups had 49.6% still in the dataset and the EDS group had 40.6% still in the dataset.
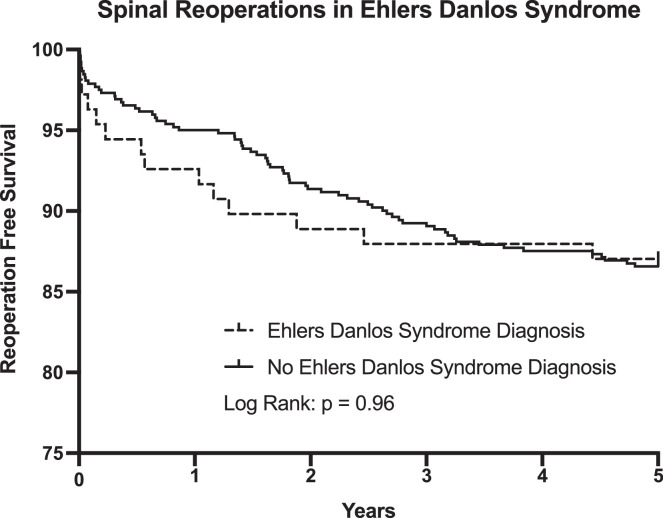
The rate of any subsequent surgeries within five years of both groups was compiled using a Kaplan Meier curve. There were 70 observed reoperations in the control group, for a survival rate of 86.6%, and 14 observed reoperations in the Ehlers Danlos group, for a survival rate of 87.0%. Based on these numbers, a Kaplan Meier curve was generated and there was no significant difference between the rate of survival between the two groups (p = 0.96, Fig. 1).
Fig. 1.
The Kaplan-Meier curve showing five-year instrumentation survival following PLF comparing the two matched groups. Significance was determined with p < 0.05.
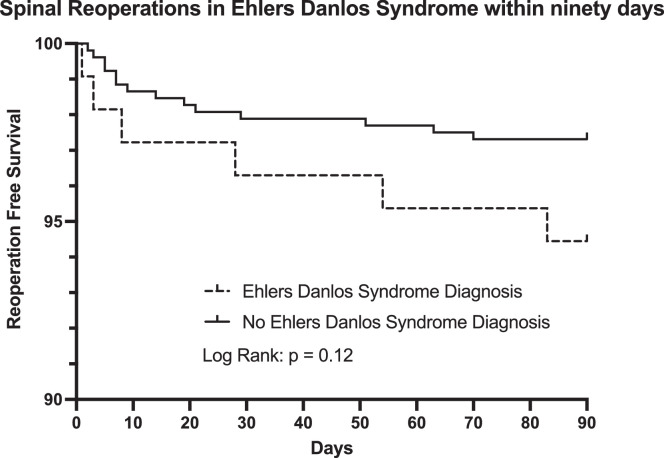
During the first ninety days following surgery, there was fourteen reoperations in control group, compared to six reoperations in the EDS group (Fig. 2). There was no significant difference in reoperation (p = 0.12). The most common reasons for reoperations in both the first ninety days and over five years for the EDS group was infection and complications with the implant.
Fig. 2.
Discussion
Ehlers-Danlos Syndrome (EDS) is comprised of a set of rare genetic connective tissue conditions [15,16]. Based on resultant laxity and potential lumbar degenerative changes, patients with EDS may undergo PLF in their adult years, but the perioperative and long-term outcomes of PLF for EDS patients are not well understood.
EDS patients were not found to have greater 90-day perioperative adverse events for any of the metrics assessed, including AAE, SAE, MAE, and readmission. This is consistent to the findings of Matur et al. who in a study of fifty-six pediatric deformity EDS patients, found that they do not have a greater risk of perioperative adverse events [17]. Nonetheless, this different from Rabenhorst et al. single-institution study of six pediatric EDS patients who found elevated adverse events in this patient population [18].
These findings are consistent with the few case reports available showing that adult EDS patients don't appear to have an increased risk of complications [13,14]. However, Uehara et al. reports concern for vascular fragility [14]. The large sample size of the current study makes it stand out from these prior reports [19]. Of additional note, one could question if the laxity inherent to the EDS population might predispose them to longer term adverse outcomes and/or revisions. However, five years revision rates were not found to be significantly different.
As with any national database study, there are limitations inherent to the coding and conditions specific factors. However, as noted above, it is only with such tools that larger number can be assessed and studied. In fact, it is possible that some of the comparisons were not sufficiently powered for all analyses, but based on the numbers available, no significant differences could be detected. Additionally, EDS is a heterogenous disease with many subtypes, however due to a limit in ICD coding, it was not possible to perform adequate analysis on EDS subtypes. The study was limited in determining how patients obtained their EDS diagnosis. PLF is a heterogenous surgery, and that difference in how the surgery might have been performed cannot fully be accounted for. An additional limitation is that not there was attrition of patients from the dataset for the five-year follow up comparison, and this should be considered with interpretation of those results.
Overall, based on the largest reported cohort of EDS patients undergoing single-level PLS, the current study found that adult EDS patients did not have any significant difference in 90-day adverse events, or 5-year reoperations compared to non-EDS patients following PLF. The literature on orthopaedic surgery in EDS patients is sparse. These findings may be useful for patient counseling and surgical planning for those with this rare condition.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Funding
The current study received funding from the Yale School of Medicine Medical Student Fellowship. The manuscript submitted does not contain information about medical devices/drugs.
Footnotes
Given his role as Editor in Chief, Jonathan Grauer, MD had no involvement in the peer-review of this article and has no access to information regarding its peer-review. Full responsibility for the editorial process for this article was delegated to Tobias Mattei, MD.
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.xnsj.2022.100136.
Appendix. Supplementary materials
References
- 1.Lawrence EJ. The clinical presentation of Ehlers-Danlos syndrome. Adv Neonatal Care. 2005;5:301–314. doi: 10.1016/j.adnc.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 2.Malfait F, Francomano C, Byers P, et al. The 2017 international classification of the Ehlers–Danlos syndromes. Am J Med Genet Part C Semin Med Genet. 2017;175:8–26. doi: 10.1002/ajmg.c.31552. [DOI] [PubMed] [Google Scholar]
- 3.De Paepe A, Malfait F. The Ehlers-Danlos syndrome, a disorder with many faces. Clin Genet. 2012;82:1–11. doi: 10.1111/j.1399-0004.2012.01858.x. [DOI] [PubMed] [Google Scholar]
- 4.van Dijk FS, Mancini GMS, Maugeri A, et al. Ehlers Danlos syndrome, kyphoscoliotic type due to Lysyl Hydroxylase 1 deficiency in two children without congenital or early onset kyphoscoliosis. Eur J Med Genet. 2017;60:536–540. doi: 10.1016/j.ejmg.2017.07.011. [DOI] [PubMed] [Google Scholar]
- 5.Henderson FC, Austin C, Benzel E, et al. Neurological and spinal manifestations of the Ehlers–Danlos syndromes. Am J Med Genet Part C Semin Med Genet. 2017;175:195–211. doi: 10.1002/ajmg.c.31549. [DOI] [PubMed] [Google Scholar]
- 6.Stern CM, Pepin MJ, Stoler JM, et al. Musculoskeletal Conditions in a Pediatric Population with Ehlers-Danlos Syndrome. J Pediatr. 2017;181:261–266. doi: 10.1016/j.jpeds.2016.10.078. [DOI] [PubMed] [Google Scholar]
- 7.Jasiewicz B, Potaczek T, Tesiorowski M, et al. Spine deformities in patients with Ehlers-Danlos syndrome, type IV - late results of surgical treatment. Scoliosis. 2010;5:1–7. doi: 10.1186/1748-7161-5-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Natarajan D, Samartzis D, Wong YW, et al. Natural history of spinal deformity in a patient with Ehlers-Danlos syndrome: Case report with 20-year follow-up. Spine J. 2011;11:e1. doi: 10.1016/j.spinee.2011.02.029. [DOI] [PubMed] [Google Scholar]
- 9.Henneton P, Legrand A, Giunta C, et al. Arterial fragility in kyphoscoliotic Ehlers-Danlos syndrome. BMJ Case Rep. 2018;2018:10–13. doi: 10.1136/bcr-2018-224423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang JS, Sponseller PD, Yazici M, et al. Vascular complications from anterior spine surgery in three patients with ehlers-danlos syndrome. Spine (Phila Pa 1976) 2009;34:153–157. doi: 10.1097/BRS.0b013e31818d58da. [DOI] [PubMed] [Google Scholar]
- 11.Artoni A, Bassotti A, Abbattista M, et al. Hemostatic abnormalities in patients with Ehlers–Danlos syndrome. J Thromb Haemost. 2018;16:2425–2431. doi: 10.1111/jth.14310. [DOI] [PubMed] [Google Scholar]
- 12.Galimberti F, Lubelski D, Healy AT, et al. A Systematic Review of Lumbar Fusion Rates With and Without the Use of rhBMP-2. Spine (Phila Pa 1976) 2015;40:1132–1139. doi: 10.1097/BRS.0000000000000971. [DOI] [PubMed] [Google Scholar]
- 13.Lindley EM, Patti BN, Taylor M, et al. Lumbar artificial disc replacement in Ehlers-Danlos syndrome: A case report and discussion of clinical management. Int J Spine Surg. 2012 Dec 1;6:124–129. doi: 10.1016/j.ijsp.2012.02.006. PMID: 25694881; PMCID: PMC4300890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Uehara M, Oba H, Hatakenaka T, et al. Posterior Spinal Fusion for Severe Spinal Deformities in Musculocontractural Ehlers-Danlos Syndrome: Detailed Observation of a Novel Case and Review of 2 Reported Cases. World Neurosurg. Nov 2020;143:454–461. doi: 10.1016/j.wneu.2020.08.085. Epub 2020 Aug 19. PMID: 32822956. [DOI] [PubMed] [Google Scholar]
- 15.Miller E, Grosel JM. A review of Ehlers-Danlos syndrome. Journal of the American Academy of Physician Assistants. Apr 2020;33(4):23–28. doi: 10.1097/01.JAA.0000657160.48246.91. [DOI] [PubMed] [Google Scholar]
- 16.Taj FT, Sajjan VV, Singh D. Ehlers-Danlos syndrome. Indian Dermatol Online J. Nov 2014;5(Suppl 1):S68–S70. doi: 10.4103/2229-5178.144554. PMID: 25506578; PMCID: PMC4252965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Matur AV, Nouri A, Huang S, et al. Complications in Children with Ehlers-Danlos Syndrome Following Spine Surgery: Analysis of the Pediatric National Surgery Quality Improvement Program Database. World Neurosurg. Jan 2020;133:e473–e478. doi: 10.1016/j.wneu.2019.09.046. Epub 2019 Sep 14. PMID: 31526884. [DOI] [PubMed] [Google Scholar]
- 18.Rabenhorst BM, Garg S, Herring JA. Posterior spinal fusion in patients with Ehlers-Danlos syndrome: a report of six cases. J Child Orthop. Jun 2012;6(2):131–136. doi: 10.1007/s11832-012-0393-3. Epub 2012 Mar 9. PMID: 23730344; PMCID: PMC3364348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tinkle B, Castori M, Berglund B, et al. Hypermobile Ehlers-Danlos syndrome (a.k.a. Ehlers-Danlos syndrome Type III and Ehlers-Danlos syndrome hypermobility type): Clinical description and natural history. Am J Med Genet C Semin Med Genet. Mar 2017;175(1):48–69. doi: 10.1002/ajmg.c.31538. Epub 2017 Feb 1. PMID: 28145611. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.