Highlights
-
•
WML load is associated with lower cognition at baseline and follow-up.
-
•
Amyloid is associated with baseline but not follow-up executive functioning.
-
•
Tau is not associated with cognitive scores at baseline or follow-up.
-
•
WMLs may be an early biomarker of cognitive changes in healthy older adults.
Keywords: Cognitively normal older adults, Magnetic resonance imaging, White matter lesions, Cognitive decline
Abbreviations: AD, Alzhemier's disease; WML, White matter lesion; SCD, subjective cognitive decline
Abstract
Background
Research suggests that cerebral small vessel disease (CSVD), amyloid, and pTau contribute to age-related cognitive decline. It remains unknown how these factors relate to one another and how they jointly contribute to cognitive decline in normal aging. This project examines the association between these factors and their relationship to cognitive decline in cognitively unimpaired older adults without subjective cognitive decline.
Methods
A total of 230 subjects with cerebrospinal fluid (CSF) Aß42, CSF pTau181, white matter lesions (WMLs) used as a proxy of CSVD, and cognitive scores from the Alzheimer’s Disease Neuroimaging Initiative were included. Associations between each factor and cognitive score were investigated using regression models. Furthermore, relationships between the three pathologies were also examined using regression models.
Results
At baseline, there was an inverse association between WML load and Aß42 (t = −4.20, p <.001). There was no association between WML load and pTau (t = 0.32, p = 0.75), nor with Aß42 and pTau (t = 0.51, p =.61). Correcting for age, sex and education, baseline WML load was associated with baseline ADAS-13 scores (t = 2.59, p =.01) and lower follow-up executive functioning (t = −2.84, p =.005). Baseline Aß42 was associated with executive function at baseline (t = 3.58, p<.004) but not at follow-up (t = 1.05, p = 0.30), nor with ADAS-13 at baseline (t = −0.24, p = 0.81) or follow-up (t = 0.09, p = 0.93). Finally, baseline pTau was not associated with any cognitive measure at baseline or follow-up.
Conclusion
Both baseline Aß42 and WML load are associated with some baseline cognition scores, but only baseline WML load is associated with follow-up executive functioning. This finding suggests that WMLs may be one of the earliest clinical manifestations that contributes to future cognitive decline in cognitively healthy older adults. Given that healthy older adults with WMLs exhibit declines in cognitive functioning, they may be less resilient to future pathology increasing their risk for cognitive impairment due to dementia than those without WMLs.
1. Introduction
Alzheimer’s disease (AD) is characterized by several neuropathological brain changes such as senile plaques (ß-amyloid, Aß) and neurofibrillary tangles (Tau) deposition (Perrin et al., 2009, Sperling et al., 2011). These accumulations have been associated with the hallmarked progressive cognitive decline observed in people with AD. Both cerebrospinal (CSF) Aß and tau pathologies (Donohue et al., 2017, Stomrud et al., 2010, Verberk et al., 2020) and position emission tomography (PET) Aß and tau (Aschenbrenner et al., 2018, Schöll et al., 2016, Sperling et al., 2019) have been associated with cognitive decline in healthy older adults. Tau pathology markers have been observed to be better predictors of cognitive decline than amyloid (Aschenbrenner et al., 2018, Malpas et al., 2021).
Another pathological change, cerebral small vessel disease (CSVD), results in structural damage to the white matter of the brain that is often observed as white matter hyperintensities (WMHs) in T2w or FLAIR MRI in both non-clinical healthy aging populations and individuals with cognitive decline (Rhodius-Meester et al., 2017) or as white matter lesions (WMLs) in T1w MRIs. An association between high WMH load (i.e., WMLs appearing as hyperintensities in T2 or FLAIR MRI) and decreased cognitive functioning in healthy older adults is often reported. A meta-analysis observed that CSVD impacts all cognitive domains in healthy older adults, with the strongest association seen between WMHs and both attention and executive functioning (Kloppenborg and Geerlings, 2014). WMH load increases a healthy older adults’ risk for future development of mild cognitive impairment (MCI) (Boyle et al., 2016) and dementia (Prins et al., 2004). CSVD also continues to contribute to cognitive decline observed in AD (Kaskikallio et al., 2020) because many cases of AD have a mixed etiology (Prins and Scheltens, 2015). CSVD (as measured by WMH) has been observed to precede neurodegeneration and cognitive decline in MCI, AD, and Parkinson’s disease (Dadar et al., 2020a, Dadar et al., 2018c), suggesting that CSVD may be involved with the etiology of cognitive decline in normal aging and cognitive impairment due to MCI and Alzheimer’s disease.
While many studies have examined the effects of CSF tau and amyloid on cognitive functioning (Donohue et al., 2017, Stomrud et al., 2010, Verberk et al., 2020) and the relationship between WMH and amyloid (Dadar et al., 2020a, Hedden et al., 2012, Vemuri et al., 2015) there is limited research examining the interaction between WMH, amyloid, and tau and their joint effects on cognitive decline in healthy older adults. One study, observed no association between WMH burden and amyloid-PET but found that increased WMH burden was associated with lower executive functioning whereas amyloid measured with PET had no effect on cognitive function (Marchant et al., 2012). Another study observed the association between WMHs and tau- and amyloid-PET but did not compare them to cognitive decline (Graff-Radford et al., 2019). They observed that increases in WMHs were associated with increases in amyloid-PET in cognitively healthy older adults, but no relationship between tau-PET and WMH burden was observed (Graff-Radford et al., 2019).
One difficulty when studying AD-related pathologies is that they occur years prior to the onset of clinical symptoms (Craig-Schapiro et al., 2009, Sperling et al., 2011), emphasizing the need to evaluate these changes in the healthy aging population. The goal of this study was thus to expand on current research by examining the relationship between cognitive changes and all three factors (CSVD, amyloid, and tau) in cognitively unimpaired older adults. The novelty of this paper was to focus on cognitively healthy older adults by excluding people with subjective cognitive decline (SCD). Excluding people with SCD was necessary to avoid possible confounds caused by cognitively healthy older adults that may be close to clinical disease onset (Rabin et al., 2017). Another aim was to explore whether WMLs, amyloid, and tau are present in healthy older adults who do not yet have SCD. We wanted to examine how these three AD-related changes at baseline are associated with current cognitive function and cognitive decline in healthy older adults. Furthermore, we also examined how WMLs, amyloid, and tau are related to one another.
2. Methods
2.1. Alzheimer’s Disease Neuroimaging Initiative
Data used in the preparation of this article were obtained from the Alzheimer’s Disease Neuroimaging Initiative (ADNI) database (adni.loni.usc.edu). The ADNI was launched in 2003 as a public–private partnership, led by Principal Investigator Michael W. Weiner, MD. The primary goal of ADNI has been to test whether serial magnetic resonance imaging (MRI), positron emission tomography (PET), other biological markers, and clinical and neuropsychological assessment can be combined to measure the progression of MCI and early AD. Participants were between 55 and 90 years old at the time of recruitment. The study received ethical approval from the review boards of all participating institutions. Written informed consent was obtained from participants or their study partner.
2.2. Participants
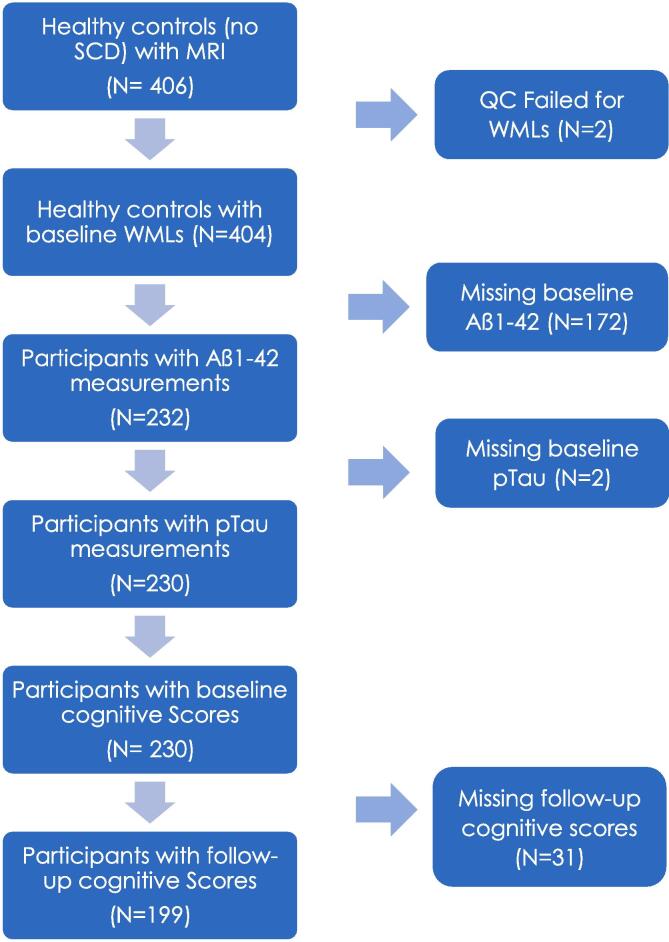
ADNI-1 and ADNI-GO did not measure subjective cognitive decline; therefore, we applied the following study inclusion and exclusion criteria to the 406 healthy older adults classified as cognitively normal controls at baseline with no SCD from the ADNI-2 and ADNI-3 cohorts with MRI scans. Full participant inclusion/exclusion criteria can be downloaded from https://www.adni-info.org. Briefly, Participants were between 55 and 90 years old at the time of recruitment and had no evidence of cognitive decline on either the Mini Mental Status Examination or Clinical Dementia Rating. Cognitively normal older healthy adults were considered if they were labelled as ‘Normal control’ and if they scored <16 on the Cognitive Change Index, signifying no indication of SCD. The following inclusion criteria were applied to the 406 healthy controls: having an MRI scan from which WML load could be estimated and having both pTau and amyloid measures. Inclusion criteria also included baseline executive functioning, memory, and ADAS-13 scores. These criteria resulted in 230 participants for our study at baseline. At follow-up, 199 of these participants had cognitive scores. Fig. 1 summarizes the methodology used to select participants. This project is a cohort study design that followed healthy older adults over a period of time.
Fig. 1.
Flowchart summarizing the participant inclusion and exclusion criteria based on WMLs, Aß42, pTau, and cognitive score measurements. Abbreviations: SCD, subjective cognitive decline; QC, quality control; WMLs, white matter lesions.
We used executive functioning (ADNI-EF; Gibbons et al., 2012) and memory (ADNI-MEM; Crane et al., 2012) composite scores that have been previously developed and validated in ADNI. Alzheimer’s Disease Assessment Scale- Cog13 (ADAS-13;Mohs et al., 1997) scores were also included for all participants. The ADAS-13 measures severity of cognitive decline, for a total of 85 points, with higher scores indicating greater severity. These scores were downloaded from the ADNI public website.
To obtain CSF samples, lumbar punctions were performed as described in the ADNI procedures manual. CSF Aß42 and phosphorylated p-Tau181 (pTau) were measured using the multiplex xMAP Luminex platform (Luminex Corp, Austin, TX, USA) with the INNO-BIA AlzBio3 kit (Innogenetics) (Olsson et al., 2005, Shaw et al., 2009). The Elecsys Aß42 assay has not been established for measuring concentrations above 1700 pg/ml. Any values above this concentration were, therefore, truncated at 1700 pg/ml (Sutphen et al., 2018).
2.3. Structural MRI acquisition and processing
All participants were imaged using a 3 T scanner with T1-weighted imaging parameters (see https://adni.loni.usc.edu/methods/mri-tool/mri-analysis/ for the detailed MRI acquisition protocol). Baseline scans were downloaded from ADNI.
T1w scans for each participant were pre-processed through our standard pipeline including noise reduction (Coupe et al., 2008), intensity inhomogeneity correction (Sled et al., 1998), and intensity normalization into a range [0–100]. The pre-processed images were then both linearly (9 parameters: 3 translation, 3 rotation, and 3 scaling) (Dadar et al., 2018a) and nonlinearly (1 mm3 grid) (Avants et al., 2008) registered to the MNI-ICBM152-2009c average template (Fonov et al., 2011). The quality of the linear and nonlinear registrations was visually verified by an experienced rater (co-author MD).
2.4. WML measurements
WML measurements were obtained through a previously validated WML segmentation technique and a library of manual segmentations based on 50 ADNI participants (independent of the 230 studied here). WMLs were automatically segmented at baseline using the T1w contrasts, along with a set of location and intensity features obtained from a library of manually segmented scans in combination with a random forest classifier to detect the WMLs in new images (Dadar et al., 2017a, Dadar et al., 2017b). WML load often reflects cerebrovascular disease, thus WML load was used as a proxy for cerebrovascular pathology and was defined as the volume of all voxels identified as WMLs in the standard space (in mm3) and are thus normalized for head size. WML volumes were log-transformed to achieve normal distribution. This WML technique has been developed and extensively tested specifically for assessment of WMLs/WMHs in multi-center studies including validation in the ADNI cohort. For example, these techniques have been used in examining WMHs in HIV infection studies(Sanford et al., 2019) and multi-center studies including, The Parkinson’s Markers Initiative (Dadar et al., 2020b) and UK Brain bank (Dadar et al., 2020c) to assess WMHs in Parkinson’s, and the National Alzheimer’s Coordinating Center (Anor et al., 2021) and ADNI (Dadar et al., 2019) to assess WMLs/WMHs in MCI and AD.
2.5. Statistical analysis
Analyses were performed using MATLAB R2019b. Participant demographic information is presented in Table 1. Linear regression models were conducted to examine whether baseline WMLs, amyloid, and pTau would influence cognitive scores. Correction of multiple comparisons was completed using false discovery rate (FDR), p-values are reported as raw values with significance determined by FDR correction. CognitiveScore_bl represents executive function (or memory composite) score at baseline. For baseline measurements, 230 participants were included for both the executive functioning and memory composite models. Both CSF Aß42 and CSF pTau levels were downloaded from ADNI.
| (1) |
Table 1.
Descriptive statistics for the participants included in this study. Data are number (N) or mean ± standard deviation.
| Full Sample Healthy Older Adults | Follow-up Sample | Baseline only Sample | |
|---|---|---|---|
| Participants (Ntotal) | 230 | 199 | 31 |
| Female (N) | 129 (56%) | 108 (54%) | 21 (67%) |
| Age at baseline (years) | 73.08 ± 6.49 | 73.26 ± 6.42 | 71.98 ± 6.94 |
| Education (years) | 16.68 ± 2.45 | 16.65 ± 2.53 | 16.87 ± 1.91 |
| ADNI-EF at baseline | 1.00 ± 0.85 | 0.99 ± 0.85 | 1.08 ± 0.90 |
| ADNI-EF at follow-up | ---- | 0.96 ± 0.89 | ---- |
| ADNI-MEM at baseline | 1.06 ± 0.58 | 1.09 ± 0.60 | 0.88 ± 0.47 |
| ADNI-MEM at follow-up | ---- | 1.22 ± 0.621 | ---- |
| ADAS-13 at baseline | 10.09 ± 4.72 | 9.65 ± 4.57 | 12.93 ± 4.802 |
| ADAS-13 at follow-up | ---- | 9.09 ± 4.76 | ---- |
| CSF Aß42 at baseline (pg/mL) | 1228 ± 445 | 1235 ± 448 | 1187 ± 430 |
| CSF pTau181 at baseline (pg/mL) | 21.55 ± 9.06 | 21.52 ± 9.12 | 21.73 ± 8.81 |
| WML load at baseline | 8.67 ± 0.54 | 8.68 ± 0.56 | 8.61 ± 0.42 |
| Systolic blood pressure | 133.40 ± 19.09 | 133.10 ± 18.90 | 135.29 ± 20.48 |
| Diastolic blood pressure | 74.17 ± 11.12 | 74.07 ± 11.50 | 74.84 ± 8.37 |
| BMI | 27.02 ± 4.70 | 27.00 ± 4.65 | 27.11 ± 5.06 |
Notes: ADNI = Alzheimer’s Disease Neuroimaging Initiative. EF = Executive functioning. MEM = Memory. CSF = Cerebrospinal fluid. BMI = Body mass index. ADAS-13, Alzheimer’s Disease Assessment Scale-13; WMLs, White matter lesions ADNI-EF and ADNI-MEM are composite scores that are z-scored values; ADAS-13 is a raw score; WMLs are log-transformed. 1Follow-up participants had higher memory composites (ADNI-MEM, p <.001) and lower ADAS-13 (p =.045) scores at follow-up than baseline. 2The sample with only baseline measures had marginally significantly higher ADAS-13 scores than the sample with follow-up measures (p <.001, uncorrected).
For follow-up measurements, 199 participants had follow-up scores over periods 1–2 years (n = 45), and 2–3 years (n = 154) that were included for both the executive functioning and memory composite models. Baseline cognitive scores were also included in the follow-up model to ensure that the follow-up results account for more variance than what can be explained by the baseline scores.
| (2) |
A linear regression was completed to examine whether baseline amyloid and pTau influence white matter lesions. All 230 participants were included in this analysis:
| (3) |
A linear regression was also completed to examine whether baseline pTau and amyloid are associated. All 230 participants were included in this analysis:
| (4) |
Additional t-tests were completed to compare the baseline characteristics of the 31 participants who did not have follow-up data to that of the 199 participants that had follow-up data to observe if there was evidence of selective attrition. The participants’ age, sex, education, CSF Aß42, CSF p-Tau181, ADNI-EF, ADNI-MEM, ADAS-13, and WML load were compared between the two groups. At baseline, those that did not have follow-up scores had significantly increased ADAS-13 scores (p <.001, uncorrected); that is those who did not have follow-ups had lower performance at baseline in general cognitive functioning. No other group differences were significant (See Table 1).
A secondary exploratory analysis was also completed using the same participant numbers to examine anatomic specificity of the WMLs and their association with cognition. Linear regressions were completed to examine whether baseline regional (i.e., frontal, temporal, parietal, and occipital) WMLs, amyloid, and pTau was associated with cognitive scores. Correction of multiple comparisons was not completed because of the exploratory nature of this analysis, p-values are thus reported as raw values without correction. CognitiveScore_bl was represents executive function (or memory composite) score at baseline.
| (5) |
| (6) |
3. Results
3.1. Cognitive score analysis
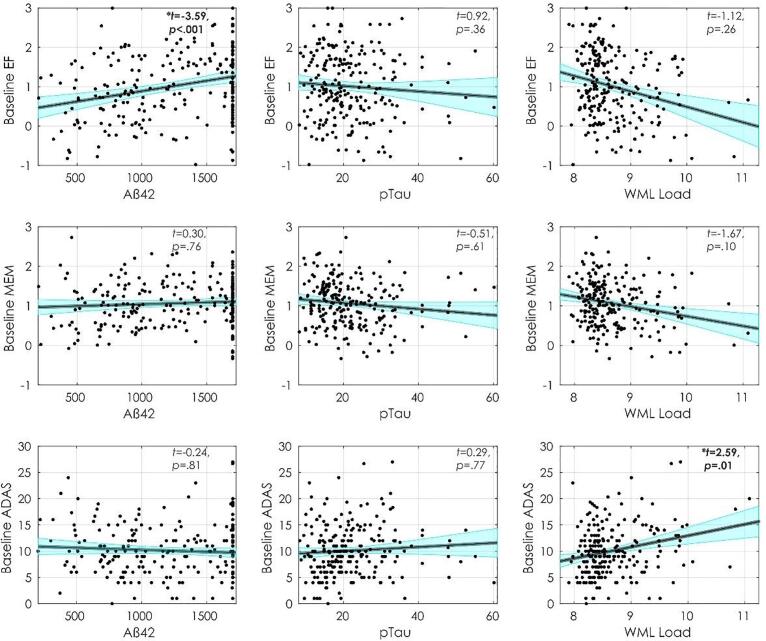
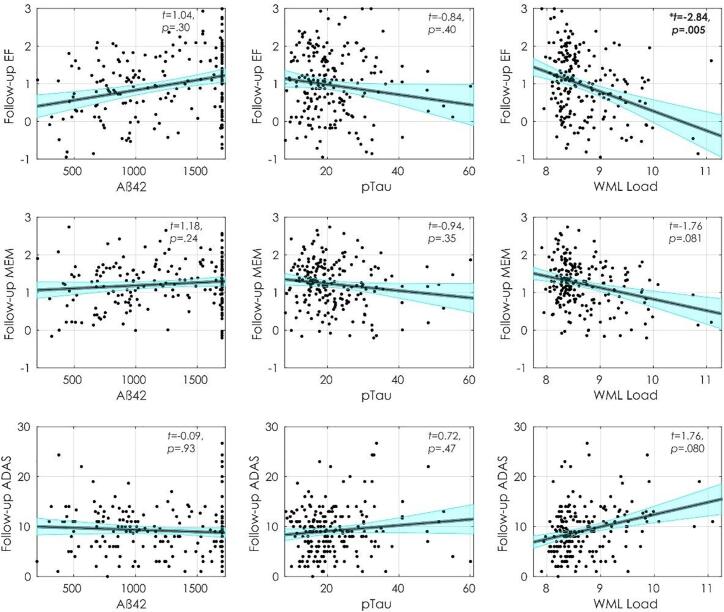
Fig. 2, Fig. 3 display executive functioning composite, memory composite, and ADAS-13 scores with Aß42, pTau, and WML at baseline and follow-up. Table 2 summarizes the regression model results. At baseline, increased executive functioning scores were associated with higher CSF Aß42 (t = 3.58, p<.001) and higher education (t = 2.95, p =.003), and inversely associated with age (t = −5.63, p<.001). At follow-up, lower executive functioning scores were associated with increased WMLs (t = −2.84, p =.005), male sex (t = −2.22, p =.027), and increased age (t = −1.99, p =.049), however, age and sex were no longer significant after FDR correction. At follow-up, increased executive functioning scores were associated with increased education (t = 4.39, p <.001) and increased baseline executive functioning (t = 12.38, p <.001).
Fig. 2.
Baseline cognitive scores and their relationship with Aß42, pTau, and WML load. Notes: Figures show baseline executive functioning (first row), memory (second row) and ADAS-13 (third row) scores and their association with Aß42 (first column), pTau (Second column), and WML load (third column). Abbreviations: EF, executive functioning; MEM, memory; ADAS, Alzheimer’s Disease Assessment Scale; WML, white matter lesion.
Fig. 3.
Follow-up cognitive scores and their relationship with Aß42, pTau, and WML load. Notes: Figures show follow-up executive functioning (first row), memory (second row) and ADAS-13 (third row) scores and their association with Aß42 (first column), pTau (Second column), and WML load (third column). Abbreviations: EF, executive functioning; MEM, memory; ADAS, Alzheimer’s Disease Assessment Scale; WML, white matter lesion.
Table 2.
Regression model outputs.
| Model | ADNI-EF Baseline |
ADNI-EF Follow-up |
ADNI-MEM Baseline |
ADNI-MEM Follow-up |
ADAS-13 Baseline |
ADAS-13 Follow-up |
WML load | Aß42 |
|---|---|---|---|---|---|---|---|---|
| Age |
ß= −0.05, t = −5.63, p <.001* |
ß= −0.01, t = −1.99, p =.049 |
ß= −0.03, t = −4.26, p <.001* |
ß= −0.001, t = −0.19, p =.85 |
ß=0.11, t = 2.04, p =.042 |
ß= −0.001, t = −0.01, p = 0.99 |
ß=0.03, t = 5.03, p <.001* | ß= −6.25, t = −1.29, p =.20 |
| Sex | ß=0.05, t = 0.53, p =.60 |
ß= −0.18, t = −2.22, p =.027 |
ß= −0.26, t = −3.56 p <.001* |
ß= −0.13, t = −2.21, p =.028 |
ß=1.50, t = 2.39, p =.018 | ß= −0.17, t = −0.32, p =.75 |
ß= −0.02, t = −0.23, p =.82 |
ß=54.17, t = 0.89, p =.37 |
| Education | ß=0.06, t = 2.95, p =.003* |
ß=0.07, t = 4.39, p <.001* |
ß=0.04, t = 2.81, p =.005* |
ß= 0.02, t = 1.97, p =.050 |
ß= −0.12, t = −0.99, p =.32 |
ß=0.03, t = 0.29, p =.77 |
---- | ---- |
| WML load | ß= −0.11, t = −1.12, p =.26 |
ß= −0.22, t = −2.84, p =.005* |
ß= −0.12, t = −1.67, p =.096 |
ß= −0.10, t = −1.76, p =.081 |
ß=1.59, t = 2.59, p =.01* |
ß=0.89, t = 1.76, p =.080 | ---- | ---- |
| Aß42 |
ß=0.001, t = 3.58, p <.001* |
ß=0.001, t = 1.05, p =.30 |
ß=0.001, t = 0.30, p =.76 |
ß= 0.001, t = 1.18 p =.24 |
ß= −0.001, t = −0.24, p =.81 |
ß=-0.001, t = 0.09, p =.93 |
ß= −0.001, t = −4.20, p <.001* |
---- |
| pTau | ß=0.005, t = 0.92, p =.35 |
ß= −0.003, t = −0.84, p =.40 | ß= −0.002, t = −0.51, p =.61 |
ß= −0.003, t = −0.94, p =.35 |
ß=0.01, t = 0.29, p =.76 |
ß=0.02, t = 0.72, p =.47 |
ß=0.001, t = 0.32, p =.75 |
ß=1.76, t = 0.51, p =.61 |
| Cognitive score at baseline | ---- |
ß= 0.65, t = 12.38, p <.001* |
---- | ß=0.75, t = 14.28, p <.001* | ---- | ß=0.68 t = 11.66, p <.001* | ---- | ---- |
Notes: Significant results are shown in bold. * Represents results that were significant after false discovery rate correction for multiple comparisons. Abbreviations: ADAS-13, Alzheimer’s Disease Assessment Scale-13; WML, White matter lesion.
At baseline, increased memory was associated with higher education (t = 2.81, p =.005). Lower memory scores were associated with increased age (t = −4.26, p <.001) and male sex (t = −3.56, p <.001). At follow-up, increased memory was associated higher baseline memory (t = 14.28, p <.001). At follow-up increased memory was also associated with increased education (t = 1.97, p =.05) and lower memory scores were associated with male sex (t = −2.21, p =.028) and marginally associated with increased WMLs (t = 1.76, p =.081). However, the association between education, sex, and WML with follow-up did not remain significant after FDR correction.
At baseline, higher ADAS-13 scores (i.e., worse performance) were associated with increased WMLs (t = 2.59, p =.01), age (t = 2.04, p =.042), and male sex (t = 2.39, p =.018); age and sex did were no longer significant after FDR correction. At follow-up, higher ADAS-13 scores were associated with higher baseline ADAS-13 scores (t = 11.66, p <.001) and marginally associated with increased WMLs (t = 1.76, p =.08).
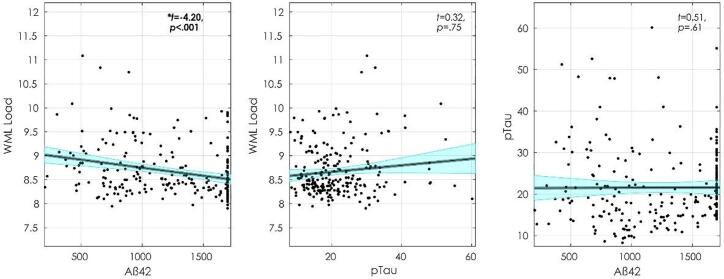
3.2. WML, Tau, and amyloid analysis
Fig. 4 displays the baseline associations between WMLs, CSF Aß42, and pTau. At baseline, WMLs were associated with increased CSF Aß42 (t = −4.20, p <.001) and age (t = 5.03, p <.001) but not pTau (t = 0.32, p = 0.75). CSF Aß42 was not observed to be associated with pTau (t = 0.51, p =.61), age (t = −1.29, p =.020) or male sex (t = 0.89, p = 0.37).
Fig. 4.
Baseline measurements of WML load, Aß42, and pTau. Figures show, from left to right, WML association with Aß42, WML association with pTau, and pTau association with Aß42. Abbreviations: WML, white matter lesion.
Analyses were repeated using the CSF Aß42/40 ratio. However, only a limited sample of participants (102 at baseline and 79 at follow-up) had CSF Aß42/40 data. Tables for both demographic data and linear regressions as well as figures showing baseline and follow-up data are provided in supplementary material. The results were similar but the association between amyloid and WML was lost which could be due to the smaller sample size.
Visual inspection of Fig. 2, Fig. 3 show that the Aß42 and pTau values have skewed distributions which may impact the results. As noted in the methods, Aß42 values were truncated at 1700 pg/ml, therefore, a log transformation of these values did not improve the distribution. To examine the influence of this skew on results, amyloid values were binarized based using the ADNI recommended threshold of 980 pg/ml for amyloid positivity/negativity. The analyses were then repeated with amyloid as a categorical variable (i.e., amyloid positive or amyloid negative) and the results remained the same. The pTau values were log-transformed, which slightly improved the distribution. However, completing the analysis with the log-transformed values of pTau did not change the results of any results.
3.3. Secondary exploratory analysis
Table 3 summarizes the regression model results, with bolded values representing results that differed from the original analysis. Almost all results were similar to that reported in the total WML analysis, results reported here are ones that differed from that of the total WML approach. WMLs were not associated with baseline ADAS-13 scores in the frontal or occipital region. This finding indicates that the baseline ADAS-13 association with whole brain WMLs was driven by the association between increased ADAS-13 scores and increased temporal (t = 1.59, p =.016) and parietal WMLs (t = 3.15, p =.001). An association in the parietal region was observed between increased WMLs and lower memory scores (t = −2.05, p =.042), this association was not observed in any other region. For the occipital region, follow-up executive functions was not associated with WMLs, whereas lower memory scores were associated with increased WMLs (t = −2.35, p =.019).
Table 3.
Secondary exploratory analysis regression model outputs.
| ADNI-EF Baseline |
ADNI-EF Follow-up |
ADNI-MEM Baseline |
ADNI-MEM Follow-up |
ADAS-13 Baseline |
ADAS-13 Follow-up |
|
|---|---|---|---|---|---|---|
| Frontal | ||||||
| WML load | ß= −0.10, t = −0.88, p =.38 |
ß= −0.19, t = −2.32, p =.02 |
ß= −0.10, t = −1.26, p =.21 |
ß= −0.09, t = −1.50, p =.13 |
ß= 1.05, t = −1.58, p =.12 |
ß=0.92, t = 1.72, p =.09 |
| Aß42 | ß=0.001, t = 3.76, p <.001 |
ß=0.001, t = 1.35, p =.18 |
ß= 0.001, t = 0.50, p =.61 |
ß= 0.001, t = 1.38, p =.17 |
ß= −0.001, t = −0.62, p =.54 |
ß=-0.001, t = −0.04, p =.96 |
| pTau | ß= −0.005, t = −0.93, p =.35 |
ß= −0.003, t = −0.83, p =.40 | ß= −0.002, t = −0.49, p =.62 |
ß= −0.003, t = −0.93, p =.35 |
ß=0.01, t = 0.29, p =.77 |
ß=0.02, t = 0.69, p =.49 |
| Temporal | ||||||
| WML load | ß= −0.009, t = −0.08, p =.93 |
ß= −0.18, t = −2.17, p =.03 |
ß= −0.11, t = −1.47, p =.14 |
ß= −0.08, t = −1.29, p =.20 |
ß= 1.59, t = 2.42, p =.016 |
ß=0.70, t = 1.27, p =.20 |
| Aß42 | ß=0.001, t = 3.92, p <.001 |
ß=0.001, t = 1.18, p = 0.24 |
ß= 0.001, t = 0.39, p =.69 |
ß= 0.001, t = 1.33, p =.19 |
ß= −0.001, t = −0.34, p =.73 |
ß=-0.001, t = −0.06, p =.95 |
| pTau | ß= 0.005, t = 0.90, p =.37 |
ß= −0.004, t = −0.98, p =.33 |
ß= −0.002, t = −0.58, p =.56 |
ß= −0.003, t = −1.02, p =.31 |
ß=0.01, t = 0.41, p =.69 |
ß=0.02, t = 0.81, p =.42 |
| Parietal | ||||||
| WML load | ß= −0.07, t = −1.26, p =.21 |
ß= −0.15, t = −3.30, p =.001 |
ß= −0.09, t = −2.05, p =.042 |
ß= −0.05, t = −1.50, p =.14 |
ß= 1.13, t = 3.15, p =.001 |
ß=0.42, t = 1.36, p =.18 |
| Aß42 | ß=0.001, t = 3.49, p <.001 |
ß=0.001, t = 0.89, p = 0.37 |
ß= 0.001, t = 0.15, p =.88 |
ß= 0.001, t = 1.22, p =.22 |
ß= −0.001, t = −0.01, p =.99 |
ß=-0.001, t = −0.01, p =.99 |
| pTau | ß= 0.005, t = 0.84, p =.40 |
ß= −0.004, t = −1.03, p =.30 |
ß= −0.002, t = −0.63, p =.53 |
ß= −0.003, t = −1.04, p =.30 |
ß=0.01, t = 0.49, p =.63 |
ß=0.02, t = 0.82, p =.41 |
| Occipital | ||||||
| WML load | ß= −0.04, t = −0.46, p =.65 |
ß= −0.05, t = −0.75, p =.46 |
ß= −0.02, t = −0.38, p =.70 |
ß= −0.11, t = −2.35, p =.019 |
ß= 1.02, t = 1.83, p =.07 |
ß=0.75, t = 1.59, p =.11 |
| Aß42 | ß=0.001, t = 3.85, p <.001 |
ß=0.001, t = 1.63, p = 0.10 |
ß= 0.001, t = 0.69, p =.49 |
ß= 0.001, t = 1.16, p =.25 |
ß= −0.001, t = −0.58, p =.57 |
ß=-0.001, t = −0.03, p =.98 |
| pTau | ß= 0.005, t = 0.89, p =.37 |
ß= −0.004, t = −0.90, p =.36 |
ß= −0.002, t = −0.54, p =.59 |
ß= −0.003, t = −0.98, p =.33 |
ß=0.01, t = 0.36, p =.72 |
ß=0.02, t = 0.79, p =.43 |
Notes: Results that differ from Table 2 (total WML) results are shown in bold. Abbreviations: ADAS-13, Alzheimer’s Disease Assessment Scale-13; WML, White matter lesion.
4. Discussion
The current study investigated the effects of WMLs, CSF Aß42, and CSF pTau on current and future cognitive decline in cognitively unimpared older adults without SCD. We observed that CSF pTau was not associated with current or future decline for either memory or executive function in this cohort. CSF Aß42 burden was however associated with decreased baseline executive functioning. Increased WML load was associated with reduced performance on baseline global cognition (as measured by ADAS-13) and follow-up executive functioning. WML load was also related to baseline CSF Aß42 but not pTau. CSF Aß42 was not associated with CSF pTau. These results suggest WMLs may contribute to the initial stages of cognitive decline in healthy older adults. While additional data and analysis are necessary, healthy older adults with WML pathology may be less resilient to future pathology and at greater risk of cognitive impairment due to dementia than those without WMLs.
In this sample of cognitively unimpaired participants with no subjective cognitive decline, WML loads appeared to have the largest association with cognitive changes. CSVD, as measured by WMLs, may be one of the first changes observed in the healthy older adult population that affects cognitive functioning. Other changes that occur later in the aging process (i.e., tau and amyloid accumulation) may require less deposition to result in deterioration in cognitive functioning in those with high WML loads. Reducing WML may thus reduce the risk of future cognitive decline and the impact of other pathologies. The observation that future cognitive decline is impacted in healthy aging by WMLs supports previous findings suggesting that WMHs increase the risk of MCI and dementia (Prins and Scheltens, 2015) by lowering the threshold for cognitive decline due to Alzheimer’s dementia. Risk factors that contribute to WMHs (e.g., hypertension and cardiovascular disease) are also associated with cognitive decline in healthy older adults (Marchant et al., 2012, Walker et al., 2017) and a higher conversion rate from MCI to AD (Ettorre et al., 2012). Taken together, these findings provide support an aggressive treatment strategy of vascular factors to help reduce future cognitive decline. For example, previous research has observed that people who undergo antihypertensive treatment exhibit less cognitive decline (Streit et al., 2019) and a reduced risk of dementia (Ding et al., 2020) than those with untreated high blood pressure. However, a recent meta-analysis suggests there is limited evidence to support the use of aggressive antihypertensive treatment to reduce cognitive decline (Dallaire-Théroux et al., 2021).
In the secondary analysis, we observed some regional results that expanded beyond the results observed in the global WML approach. We observed that the parietal region is the main region driving the baseline ADAS-13 association with whole brain WMLs. Furthermore, parietal WMLs were also observed to be associated with lower baseline memory scores. Memory is one of the principal cognitive domains impacted in AD (Knopman et al., 2021). Furthermore, previous research has indicated that increased WMH volume in the parietal region is elevated among older adults at risk for AD and predicts future AD diagnosis (Brickman, 2013). Thus, this association between increased parietal region WMLs with lower memory and global cognition scores may be reflective of early AD-related pathology.
In MCI and AD, both PET and CSF tau and amyloid deposition is reported to be a strong predictor of cognitive decline (Bejanin et al., 2017, Lim et al., 2014, Rolstad et al., 2011). It is well-known that this accumulation of pathological amyloid and tau begins years before deficits in cognitive functioning can be detected (Leal et al., 2018), and thus may occur during healthy aging. The relationship between tau and amyloid in healthy older adults is, however, less prominent. Nevertheless, some associations between CSF tau and amyloid (Donohue et al., 2017, Stomrud et al., 2010, Verberk et al., 2020) with reduced cognitive performance in healthy older adults have been observed. CSF pTau was not associated with cognitive decline in our sample of healthy older adults without subjective cognitive decline. The sample of participants in this study comprises healthy older adults who exhibit low overall pTau burdens making it difficult to detect associations between tau and cognition at this stage (Jack et al., 2017). Amyloid deposition may occur on average 13.3 years earlier than tau accumulation (Therneau et al., 2021). Thus, it is not surprising that Aß42 was associated with baseline executive functioning in the absence of associations between pTau and cognition. The fastest rates of cognitive decline are observed in those who have both elevated amyloid and tau deposition (Sperling et al., 2019). Therefore, the low pTau levels in our sample may have influenced why we did not observe an association between pTau and cognition at baseline or follow-up or between Aß42 and cognition at follow-up in our healthy older adult sample.
The participants who did not have follow-up data had higher ADAS-13 scores (i.e., more cognitive impairment) at baseline than those who had follow-up data. This finding suggests that those participants may be closer to the dementia/AD trajectory. In the current study, we only have 2–3 years of follow-up data so we cannot examine differences in people who develop more advanced accumulation or convert to dementia/AD. Future research should examine longer follow-ups to determine whether cognitively normal healthy older adults with WMHs have a faster rate of cognitive decline, if they are more likely to convert to dementia/AD, and if elevated amyloid and tau contribute to this decline. In this study, while we did observe a relationship between WMLs and amyloid at baseline we did not observe a relationship between WMLs and tau. This finding is similar to a previous study that reported no associations between tau-PET and WMH burden, but reported that amyloid-PET load correlated with WMH (Graff-Radford et al., 2019).
The image processing methods employed in this study have been developed and extensively validated for use in multi-center and multi-scanner studies. These processing methods have previously observed WMHs effects in PD (Dadar et al., 2021, 2020a), healthy aging and AD (Dadar et al., 2020a). Therefore, the lack of association between WML with amyloid, tau, and some cognitive scores is not the result of poor sensitivity of the image processing methods employed. It should also be noted that this WML/WMH segmentation technique on T1w images has shown strong correlations to WMH measures obtained from that of T2w/PD and FLAIR images (Dadar et al., 2018b).
The limitations of the current study include the relatively short follow-up period, limited sample size, and high participant education. Our follow-up times were limited to 3 years because we included participants from ADNI-3. Longer follow-ups may demonstrate stronger associations between tau and amyloid pathologies and cognitive decline. The relatively limited sample in this study did not allow for the testing of the interaction between amyloid, tau, and WMLs on cognition with enough statistical power. The high education of this sample may limit the generalizability of these results to other populations. Some research has shown that WMHs may also be associated with other pathologies than cerebrovascular disease (McAleese et al., 2021). However, the methodologies needed to differentiate these pathologies are unavailable in this dataset, limiting our ability to identify the cause of the WMLs. Future research is needed to examine the underlying pathologies associated with WMLs.
5. Conclusion
The current study sought to examine the relationship between CSVD as measured by WML load, and amyloid and tau measured by CSF, and their effects on cognitive functioning in healthy older adults without subjective cognitive decline. Our findings suggest that in healthy older adults who are not likely to be in the preclinical AD stage, as reflected by low tau levels, WMLs may be one of the first pathologies to contribute to age-related cognitive decline. It is possible that WML burden may preceded tau and amyloid deposition, however, without being able to determine who converts to AD, this hypothesis needs to be further examined. Despite being unable to determine which participants will eventually develop dementia in this study, our findings that WMLs contribute to cognitive decline aligns with previous work suggesting that vascular biomarkers may improve our current understanding of AD pathophysiology and dementia (Sweeney et al., 2019).
CRediT authorship contribution statement
Cassandra Morrison: Conceptualization, Methodology, Formal analysis, Writing – original draft, Writing – review & editing. Mahsa Dadar: Conceptualization, Methodology, Formal analysis, Writing – review & editing. Sylvia Villeneuve: Conceptualization, Supervision, Writing – review & editing. D. Louis Collins: Conceptualization, Methodology, Funding acquisition, Writing – review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgments
Data collection and sharing for this project was funded by the Alzheimer's Disease Neuroimaging Initiative (ADNI) (National Institutes of Health Grant U01 AG024904) and DOD ADNI (Department of Defense award number W81XWH-12-2-0012). ADNI is funded by the National Institute on Aging, the National Institute of Biomedical Imaging and Bioengineering, and through generous contributions from the following: AbbVie, Alzheimer’s Association; Alzheimer’s Drug Discovery Foundation; Araclon Biotech; BioClinica, Inc.; Biogen; Bristol-Myers Squibb Company; CereSpir, Inc.; Cogstate; Eisai Inc.; Elan Pharmaceuticals, Inc.; Eli Lilly and Company; EuroImmun; F. Hoffmann-La Roche Ltd and its affiliated company Genentech, Inc.; Fujirebio; GE Healthcare; IXICO Ltd.; Janssen Alzheimer Immunotherapy Research & Development, LLC.; Johnson & Johnson Pharmaceutical Research & Development LLC.; Lumosity; Lundbeck; Merck & Co., Inc.; Meso Scale Diagnostics, LLC.; NeuroRx Research; Neurotrack Technologies; Novartis Pharmaceuticals Corporation; Pfizer Inc.; Piramal Imaging; Servier; Takeda Pharmaceutical Company; and Transition Therapeutics. The Canadian Institutes of Health Research is providing funds to support ADNI clinical sites in Canada. Private sector contributions are facilitated by the Foundation for the National Institutes of Health (www.fnih.org). The grantee organization is the Northern California Institute for Research and Education, and the study is coordinated by the Alzheimer’s Therapeutic Research Institute at the University of Southern California. ADNI data are disseminated by the Laboratory for Neuro Imaging at the University of Southern California.
Funding information
Alzheimer’s Disease Neuroimaging Initiative; This research was supported by a grant from the Canadian Institutes of Health Research and The Louise & André Charron Family.
Financial disclosures
Dr. Morrison is supported by a postdoctoral fellowship from Canadian Institutes of Health Research, Funding Reference Number: MFE-176608.
Dr. Dadar Dr. Dadar reports receiving research funding from the Healthy Brains for Healthy Lives (HBHL), Alzheimer Society Research Program (ASRP), and Douglas Research Centre (DRC).
Dr. Villeneuve reports work supported by a Canada Research Chair, a Canadian Institutes of Health Research Foundation Grant, a Canada Fund for Innovation Grant, an Alzheimer’s Association Grant, and an Alzheimer’s society of Canada and Fonds de recherche Sante Quebec fellowship.
Dr. Collins reports receiving research funding from Canadian Institutes of Health research, the Canadian National Science and Engineering Research Council, Brain Canada, the Weston Foundation, and the Famille Louise & André Charron.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.nicl.2022.103096.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Anor C.J., Dadar M., Collins D.L., Tartaglia M.C. The longitudinal assessment of neuropsychiatric symptoms in mild cognitive impairment and Alzheimer’s Disease and Their Association With White Matter Hyperintensities in the National Alzheimer’s Coordinating Center’s Uniform Data Set. Biol. Psychiatry Cogn. Neurosci. Neuroimaging. 2021;6(1):70–78. doi: 10.1016/j.bpsc.2020.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aschenbrenner, A.J., Gordon, B.A., Benzinger, T.L.S., Morris, J.C., Hassenstab, J.J., 2018. Influence of tau PET, amyloid PET, and hippocampal volume on cognition in Alzheimer disease. Neurology 91(9), e859-e866. doi:10.1212/WNL.0000000000006075. [DOI] [PMC free article] [PubMed]
- Avants B.B., Epstein C.L., Grossman M., Gee J.C. Symmetric diffeomorphic image registration with cross-correlation: Evaluating automated labeling of elderly and neurodegenerative brain. Med. Image Anal. 2008;12(1):26–41. doi: 10.1016/j.media.2007.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bejanin A., Schonhaut D.R., La Joie R., Kramer J.H., Baker S.L., Sosa N., Ayakta N., Cantwell A., Janabi M., Lauriola M., O’Neil J.P., Gorno-Tempini M.L., Miller Z.A., Rosen H.J., Miller B.L., Jagust W.J., Rabinovici G.D. Tau pathology and neurodegeneration contribute to cognitive impairment in Alzheimer’s disease. Brain. 2017;140(12):3286–3300. doi: 10.1093/brain/awx243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle P.A., Yu L., Fleischman D.A., Leurgans S., Yang J., Wilson R.S., Schneider J.A., Arvanitakis Z., Arfanakis K., Bennett D.A. White matter hyperintensities, incident mild cognitive impairment, and cognitive decline in old age. Ann. Clin. Transl. Neurol. 2016;3(10):791–800. doi: 10.1002/acn3.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brickman A.M. Contemplating Alzheimer’s disease and the contribution of white matter hyperintensities. Curr. Neurol. Neurosci. Rep. 2013;13(12):1–9. doi: 10.1007/s11910-013-0415-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coupe P., Yger P., Prima S., Hellier P., Kervrann C., Barillot C. An optimized blockwise nonlocal means denoising filter for 3-D magnetic resonance images. IEEE Trans. Med. Imaging. 2008;27(4):425–441. doi: 10.1109/TMI.2007.906087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig-Schapiro R., Fagan A.M., Holtzman D.M. Biomarkers of Alzheimer’s disease. Neurobiol. Dis. 2009;35(2):128–140. doi: 10.1016/j.nbd.2008.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crane P.K., Carle A., Gibbons L.E., Insel P., Mackin R.S., Gross A., Jones R.N., Mukherjee S., Curtis S.M., Harvey D., Weiner M., Mungas D. Development and assessment of a composite score for memory in the Alzheimer’s Disease Neuroimaging Initiative (ADNI) Brain Imaging Behav. 2012;6(4):502–516. doi: 10.1007/s11682-012-9186-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dadar M., Camicioli R., Duchesne S., Collins D.L. The temporal relationships between white matter hyperintensities, neurodegeneration, amyloid beta, and cognition. Alzheimer’s Dement. Diagnosis, Assess. Dis. Monit. 2020;1:e12091. doi: 10.1002/dad2.12091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dadar M., Fereshtehnejad S.M., Zeighami Y., Dagher A., Postuma R.B., Collins D.L. White Matter Hyperintensities Mediate Impact of Dysautonomia on Cognition in Parkinson’s Disease. Mov. Disord. Clin. Pract. 2020;7(6):639–647. doi: 10.1002/mdc3.13003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dadar M., Fonov V.S., Collins D.L. A comparison of publicly available linear MRI stereotaxic registration techniques. Neuroimage. 2018;174:191–200. doi: 10.1016/j.neuroimage.2018.03.025. [DOI] [PubMed] [Google Scholar]
- Dadar M., Maranzano J., Ducharme S., Carmichael O.T., Decarli C., Collins D.L., Initiative A.D.N. Validation of T1w-based segmentations of white matter hyperintensity volumes in large-scale datasets of aging. Hum. Brain Mapp. 2018;39(3):1093–1107. doi: 10.1002/hbm.23894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dadar M., Gee M., Shuaib A., Duchesne S., Camicioli R. Cognitive and motor correlates of grey and white matter pathology in Parkinson’s disease. NeuroImage Clin. 2020;27 doi: 10.1016/j.nicl.2020.102353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dadar M., Maranzano J., Ducharme S., Collins D.L. White matter in different regions evolves differently during progression to dementia. Neurobiol. Aging. 2019;76:71–79. doi: 10.1016/j.neurobiolaging.2018.12.004. [DOI] [PubMed] [Google Scholar]
- Dadar M., Maranzano J., Misquitta K., Anor C.J., Fonov V.S., Tartaglia M.C., Carmichael O.T., Decarli C., Collins D.L. NeuroImage Performance comparison of 10 different classification techniques in segmenting white matter hyperintensities in aging. Neuroimage. 2017;157:233–249. doi: 10.1016/j.neuroimage.2017.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dadar M., Miyasaki J., Duchesne S., Camicioli R. White matter hyperintensities mediate the impact of amyloid ß on future freezing of gait in Parkinson’s disease. Park. Relat. Disord. 2021;85:95–101. doi: 10.1016/j.parkreldis.2021.02.031. [DOI] [PubMed] [Google Scholar]
- Dadar, M., Pascoal, T.A., Manitsirikul, S., Misquitta, K., Fonov, V.S., Carmela, M., Breitner, J., Rosa-neto, P., Carmichael, O.T., Decarli, C., Collins, D.L., 2017b. Validation of a Regression Technique for Segmentation of White Matter Hyperintensities in Alzheimer’s Disease 36(8), 1758–1768. [DOI] [PubMed]
- Dadar M., Zeighami Y., Yau Y., Fereshtehnejad S.M., Maranzano J., Postuma R.B., Dagher A., Collins D.L. White matter hyperintensities are linked to future cognitive decline in de novo Parkinson’s disease patients. NeuroImage Clin. 2018;20:892–900. doi: 10.1016/j.nicl.2018.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallaire-Théroux C., Quesnel-Olivo M.-H., Brochu K., Bergeron F., O’Connor S., Turgeon A.F., Laforce R.J., Verreault S., Camden M.-C., Duchesne S. Evaluation of intensive vs standard blood pressure reduction and association with cognitive decline and dementia: a systematic review and meta-analysis. JAMA Network Open. 2021;4(11):e2134553. doi: 10.1001/jamanetworkopen.2021.34553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding J., Davis-Plourde K.L., Sedaghat S., Tully P.J., Wang W., Phillips C., Pase M.P., Himali J.J., Gwen Windham B., Griswold M., Gottesman R., Mosley T.H., White L., Guðnason V., Debette S., Beiser A.S., Seshadri S., Ikram M.A., Meirelles O., Tzourio C., Launer L.J. Antihypertensive medications and risk for incident dementia and Alzheimer’s disease: a meta-analysis of individual participant data from prospective cohort studies. Lancet Neurol. 2020;19:61–70. doi: 10.1016/S1474-4422(19)30393-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donohue M.C., Sperling R.A., Petersen R., Sun C.K., Weiner M., Aisen P.S. Association between elevated brain amyloid and subsequent cognitive decline among cognitively normal persons. JAMA - J. Am. Med. Assoc. 2017;317:2305–2316. doi: 10.1001/jama.2017.6669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ettorre E., Cerra E., Marigliano B., Vigliotta M., Vulcano A., Fossati C., De Benedetto G., Servello A., Andreozzi P., Marigliano G., Servello A., Andreozzi P. Role of cardiovascular risk factors (CRF) in the patients with mild cognitive impairment (MCI) Arch. Gerontol. Geriatr. 2012;54:330–332. doi: 10.1016/j.archger.2011.04.025. [DOI] [PubMed] [Google Scholar]
- Gibbons L.E., Carle A.C., Mackin R.S., Harvey D., Mukherjee S., Insel P., Curtis S.M., Mungas D., Crane P.K. A composite score for executive functioning, validated in Alzheimer’s Disease Neuroimaging Initiative (ADNI) participants with baseline mild cognitive impairment. Brain Imaging Behav. 2012;6(4):517–527. doi: 10.1007/s11682-012-9176-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graff-Radford J., Arenaza-Urquijo E.M., Knopman D.S., Schwarz C.G., Brown R.D., Rabinstein A.A., Gunter J.L., Senjem M.L., Przybelski S.A., Lesnick T., Ward C., Mielke M.M., Lowe V.J., Petersen R.C., Kremers W.K., Kantarci K., Jack C.R., Vemuri P. White matter hyperintensities: Relationship to amyloid and tau burden. Brain. 2019;142(8):2483–2491. doi: 10.1093/brain/awz162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedden T., Mormino E.C., Amariglio R.E., Younger A.P., Schultz A.P., Becker J.A., Buckner R.L., Johnson K.A., Sperling R.A., Rentz D.M. Cognitive profile of amyloid burden and white matter hyperintensities in cognitively normal older adults. J. Neurosci. 2012;32(46):16233–16242. doi: 10.1523/JNEUROSCI.2462-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack C.R., Wiste H.J., Weigand S.D., Therneau T.M., Lowe V.J., Knopman D.S., Gunter J.L., Senjem M.L., Jones D.T., Kantarci K., Machulda M.M., Mielke M.M., Roberts R.O., Vemuri P., Reyes D.A., Petersen R.C. Defining imaging biomarker cut points for brain aging and Alzheimer’s disease. Alzheimer’s Dement. 2017;13(3):205–216. doi: 10.1016/j.jalz.2016.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaskikallio A., Karrasch M., Koikkalainen J., Lötjönen J., Rinne J.O., Tuokkola T., Parkkola R., Grönholm-Nyman P. White matter hyperintensities and cognitive impairment in healthy and pathological aging: a quantified Brain MRI study. Dement. Geriatr. Cogn. Disord. 2020;48(5–6):297–307. doi: 10.1159/000506124. [DOI] [PubMed] [Google Scholar]
- Kloppenborg R.P., Geerlings M.I. Presence and progression of white matter hyperintensities and cognition. Neurology. 2014;82(23):2127–2138. doi: 10.1212/WNL.0000000000000505. [DOI] [PubMed] [Google Scholar]
- Knopman D.S., Amieva H., Petersen R.C., Chételat G., Holtzman D.M., Hyman B.T., Nixon R.A., Jones D.T. Alzheimer disease. Nat. Rev. Dis. Primers. 2021;7(1) doi: 10.1038/s41572-021-00269-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leal S.L., Lockhart S.N., Maass A., Bell R.K., Jagust W.J. Subthreshold amyloid predicts tau deposition in aging. J. Neurosci. 2018;38(19):4482–4489. doi: 10.1523/JNEUROSCI.0485-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim Y.Y., Maruff P., Pietrzak R.H., Ames D., Ellis K.A., Harrington K., Lautenschlager N.T., Szoeke C., Martins R.N., Masters C.L., Villemagne V.L., Rowe C.C. Effect of amyloid on memory and non-memory decline from preclinical to clinical Alzheimer’s disease. Brain. 2014;137(1):221–231. doi: 10.1093/brain/awt286. [DOI] [PubMed] [Google Scholar]
- Malpas C.B., Sharmin S., Kalincik T. The histopathological staging of tau, but not amyloid, corresponds to antemortem cognitive status, dementia stage, functional abilities and neuropsychiatric symptoms. Int. J. Neurosci. 2021;131(8):800–809. doi: 10.1080/00207454.2020.1758087. [DOI] [PubMed] [Google Scholar]
- Marchant N.L., Reed B.R., DeCarli C.S., Madison C.M., Weiner M.W., Chui H.C., Jagust W.J. Cerebrovascular disease, beta-amyloid, and cognition in aging. Neurobiol. Aging. 2012;33(5):1006.e25–1006.e36. doi: 10.1016/j.neurobiolaging.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAleese K.E., Miah M., Graham S., Hadfield G.M., Walker L., Johnson M., Colloby S.J., Thomas A.J., DeCarli C., Koss D., Attems J. Frontal white matter lesions in Alzheimer’s disease are associated with both small vessel disease and AD-associated cortical pathology. Acta Neuropathol. 2021;142(6):937–950. doi: 10.1007/s00401-021-02376-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohs R.C., Knopman D.S., Petersen R.C., Ferris S.H., Ernesto C., Grundman M., Sano M., Bieliauskas L., Geldmacher D.S., Clark C., Thal L. Development of cognitive instruments for use in clinical trials of antidementia drugs: Additions to the Alzheimer’s Disease Assessment Scale that broaden its scope. Alzheimer Dis. Assoc. Disord. 1997;11(Suppl2):S13–S21. [PubMed] [Google Scholar]
- Olsson A., Vanderstichele H., Andreasen N., De Meyer G., Wallin A., Holmberg B., Rosengren L., Vanmechelen E., Blennow K. Simultaneous measurement of β-amyloid(1–42), total Tau, and phosphorylated Tau (Thr181) in cerebrospinal fluid by the xMAP technology. Clin. Chem. 2005;51(2):336–345. doi: 10.1373/clinchem.2004.039347. [DOI] [PubMed] [Google Scholar]
- Perrin R., Fagan A.M., Holtzman D.M. Multi-modal techniques for diagnosis and prognosis of Alzheimer’s disease. Nature. 2009;461(7266):916–922. doi: 10.1038/nature08538.Multi-modal. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prins N.D., Scheltens P. White matter hyperintensities, cognitive impairment and dementia: An update. Nat. Rev. Neurol. 2015;11(3):157–165. doi: 10.1038/nrneurol.2015.10. [DOI] [PubMed] [Google Scholar]
- Prins N.D., Van Dijk E.J., Den Heijer T., Vermeer S.E., Koudstaal P.J., Oudkerk M., Hofman A., Breteler M.M.B. Cerebral white matter lesions and the risk of dementia. Arch. Neurol. 2004;61(10):1531–1534. doi: 10.1001/archneur.61.10.1531. [DOI] [PubMed] [Google Scholar]
- Rabin L.A., Smart C.M., Amariglio R.E. Subjective cognitive decline in preclinical Alzheimer’s Disease. Annu. Rev. Clin. Psychol. 2017;13:369–396. doi: 10.1146/annurev-clinpsy-032816-045136. [DOI] [PubMed] [Google Scholar]
- Rhodius-Meester H.F.M., Benedictus M.R., Wattjes M.P., Barkhof F., Scheltens P., Muller M., van der Flier W.M. MRI visual ratings of brain atrophy and white matter hyperintensities across the spectrum of cognitive decline are differently affected by age and diagnosis. Front. Aging Neurosci. 2017;9:1–12. doi: 10.3389/fnagi.2017.00117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolstad S., Berg A.I., Bjerke M., Blennow K., Johansson B., Zetterberg H., Wallin A. Amyloid-beta42 is associated with cognitive impairment in healthy elderly and subjective cognitive impairment. J. Alzheimer’s Dis. 2011;26(1):135–142. doi: 10.3233/JAD-2011-110038. [DOI] [PubMed] [Google Scholar]
- Sanford, R., Strain, J., Dadar, M., Maranzano, J., Bonnet, A., Mayo, N.E., Scott, S.C., Fellows, L.K., Ances, B.M., Collins, D.L., Neurologie, D., Cedex, R., 2019. HIV infection and cerebral small vessel disease are independently associated with brain atrophy and cognitive impairment 33(7), 1197–1205. https://doi.org/10.1097/QAD.0000000000002193.HIV. [DOI] [PMC free article] [PubMed]
- Schöll M., Lockhart S.N., Schonhaut D.R., O’Neil J.P., Janabi M., Ossenkoppele R., Baker S.L., Vogel J.W., Faria J., Schwimmer H.D., Rabinovici G.D., Jagust W.J. PET imaging of tau deposition in the aging human brain. Neuron. 2016;89(5):971–982. doi: 10.1016/j.neuron.2016.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw L.M., Vanderstichele H., Knapik-Czajka M., Clark C.M., Aisen P.S., Petersen R.C., Blennow K., Soares H., Simon A., Lewczuk P., Dean R., Siemers E., Potter W., Lee V.M.Y., Trojanowski J.Q. Cerebrospinal fluid biomarker signature in alzheimer’s disease neuroimaging initiative subjects. Ann. Neurol. 2009;65(4):403–413. doi: 10.1002/ana.21610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sled J.G., Zijdenbos A.P., Evans A.C. A nonparametric method for automatic correction of intensity nonuniformity in mri data. IEEE Trans. Med. Imaging. 1998;17(1):87–97. doi: 10.1109/42.668698. [DOI] [PubMed] [Google Scholar]
- Sperling R.A., Aisen P.S., Beckett L.A., Bennett D.A., Craft S., Fagan A.M., Iwatsubo T., Jack C.R., Kaye J., Montine T.J., Park D.C., Reiman E.M., Rowe C.C., Siemers E., Stern Y., Yaffe K., Carrillo M.C., Thies B., Morrison-Bogorad M., Wagster M.V., Phelps C.H. Toward defining the preclinical stages of Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s Dement. 2011;7(3):280–292. doi: 10.1016/j.jalz.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperling R.A., Mormino E.C., Schultz A.P., Betensky R.A., Papp K.V., Amariglio R.E., Hanseeuw B.J., Buckley R., Chhatwal J., Hedden T., Marshall G.A., Quiroz Y.T., Donovan N.J., Jackson J., Gatchel J.R., Rabin J.S., Jacobs H., Yang H.S., Properzi M., Kirn D.R., Rentz D.M., Johnson K.A. The impact of amyloid-beta and tau on prospective cognitive decline in older individuals. Ann. Neurol. 2019;85(2):181–193. doi: 10.1002/ana.25395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stomrud E., Hansson O., Zetterberg H., Blennow K., Minthon L., Londos E. Correlation of longitudinal cerebrospinal fluid biomarkers with cognitive decline in healthy older adults. Arch. Neurol. 2010;67(2):217–223. doi: 10.1001/archneurol.2009.316. [DOI] [PubMed] [Google Scholar]
- Streit S., Poortvliet R.K.E., Den Elzen W.P.J., Blom J.W., Gussekloo J. Systolic blood pressure and cognitive decline in older adults with hypertension. Ann. Fam. Med. 2019;17(2):100–107. doi: 10.1370/afm.2367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutphen C.L., McCue L., Herries E.M., Xiong C., Ladenson J.H., Holtzman D.M., Fagan A.M. Longitudinal decreases in multiple cerebrospinal fluid biomarkers of neuronal injury in symptomatic late onset Alzheimer's disease. Alzheimer's & Dementia. 2018;14(7):869–879. doi: 10.1016/j.jalz.2018.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweeney M.D., Montagne A., Sagare A.P., Nation D.A., Schneider L.S., Chui H.C., Harrington M.G., Pa J., Law M., Wang D.J., Jacobs R.E. Vascular dysfunction—the disregarded partner of Alzheimer's disease. Alzheimer's & Dementia. 2019;15(1):158–167. doi: 10.1016/j.jalz.2018.07.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Therneau, T.M., Knopman, D.S., Lowe, V.J., Botha, H., Graff-Radford, J., Jones, D.T., Vemuri, P., Mielke, M.M., Schwarz, C.G., Senjem, M.L. and Gunter, J.L., 2021. Relationships between β-amyloid and tau in an elderly population: An accelerated failure time model. Neuroimage, 242, p.118440. doi:10.1016/j.neuroimage.2021.118440. [DOI] [PMC free article] [PubMed]
- Vemuri P., Lesnick T.G., Przybelski S.A., Knopman D.S., Preboske G.M., Kantarci K., Raman M.R., Machulda M.M., Mielke M.M., Lowe V.J., Senjem M.L., Gunter J.L., Rocca W.A., Roberts R.O., Petersen R.C., Jack C.R. Vascular and amyloid pathologies are independent predictors of cognitive decline in normal elderly. Brain. 2015;138(3):761–771. doi: 10.1093/brain/awu393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verberk I.M.W., Hendriksen H.M.A., van Harten A.C., Wesselman L.M.P., Verfaillie S.C.J., van den Bosch K.A., Slot R.E.R., Prins N.D., Scheltens P., Teunissen C.E., Van der Flier W.M. Plasma amyloid is associated with the rate of cognitive decline in cognitively normal elderly: the SCIENCe project. Neurobiol. Aging. 2020;89:99–107. doi: 10.1016/j.neurobiolaging.2020.01.007. [DOI] [PubMed] [Google Scholar]
- Walker K.A., Power M.C., Gottesman R.F. Defining the relationship between hypertension, cognitive decline, and dementia: a review. Curr. Hypertens. Rep. 2017;19(3):24. doi: 10.1007/s11906-017-0724-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.