Abstract
Introduction
Apolipoprotein E (APOE) ε4 is the strongest genetic risk factor for Alzheimer's disease and related dementias (ADRDs), affecting many different pathways that lead to cognitive decline. Exercise is one of the most widely proposed prevention and intervention strategies to mitigate risk and symptomology of ADRDs. Importantly, exercise and APOE ε4 affect similar processes in the body and brain. While both APOE ε4 and exercise have been studied extensively, their interactive effects are not well understood.
Methods
To address this, male and female APOE ε3/ε3, APOE ε3/ε4, and APOE ε4/ε4 mice ran voluntarily from wean (1 month) to midlife (12 months). Longitudinal and cross‐sectional phenotyping were performed on the periphery and the brain, assessing markers of risk for dementia such as weight, body composition, circulating cholesterol composition, murine daily activities, energy expenditure, and cortical and hippocampal transcriptional profiling.
Results
Data revealed chronic running decreased age‐dependent weight gain, lean and fat mass, and serum low‐density lipoprotein concentration dependent on APOE genotype. Additionally, murine daily activities and energy expenditure were significantly influenced by an interaction between APOE genotype and running in both sexes. Transcriptional profiling of the cortex and hippocampus predicted that APOE genotype and running interact to affect numerous biological processes including vascular integrity, synaptic/neuronal health, cell motility, and mitochondrial metabolism, in a sex‐specific manner.
Discussion
These data in humanized mouse models provide compelling evidence that APOE genotype should be considered for population‐based strategies that incorporate exercise to prevent ADRDs and other APOE‐relevant diseases.
Keywords: Alzheimer's disease, apolipoprotein E, dementia, exercise, running
1. BACKGROUND
Aging and apolipoprotein E (APOE) ε4 are the strongest risk factors for Alzheimer's disease and related dementias (ADRDs). 1 With APOE ε4 implicated in unfavorable systemic changes such as high body mass index, dysregulated cholesterol concentrations, and aberrant metabolism, as well as deficits in cerebral health such as changes in cerebral metabolism, cerebrovasculature, and neuronal health, the APOE ε4 allele has been targeted to help reverse these risks. 2 , 3 , 4 , 5 , 6 , 7 The cerebral changes caused by APOE ε4 emerge in humans at early ages and can worsen with advancing age. 8 , 9 , 10 , 11 , 12 Further, the impact of APOE ε4 dosage (such as in the APOE ε3/ε4 vs. APOE ε4/ε4 genotype) on peripheral and brain health during aging is understudied. Targeting APOE ε4 through pharmacological interventions has resulted in both beneficial and damaging outcomes meaning therapies targeting APOE‐dependent pathways will likely need to be tailored to specific mechanisms. 13 , 14 , 15 , 16
While pharmacological interventions are still being investigated, others have turned to non‐pharmacological interventions to reduce risk for ADRDs, such as exercise. 13 , 17 Studies in mice show benefits of exercise to peripheral health, as well as improvements to cognitive function. 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 Though the cognitive changes due to exercise have been controversial, with human studies showing either no change or improvements with exercise, it is widely accepted that exercise affects the body in a generally positive manner (i.e., decreasing weight/fat mass, improving metabolism and circulation, and elevating mood). 19 , 28 , 29 , 30 , 31 , 32 , 33 While understanding the effect of exercise on neuronal health is critical, other compartments of the brain are largely neglected. It is essential to understand how exercise affects all mechanisms that pertain to ADRD risk, such as metabolism and vascular health.
It is unknown if the detrimental effects associated with APOE ε4 can be mitigated by exercise, or conversely, whether the effects of exercise are impacted by APOE ε4 genotype. Studies in previous models of APOE mice are usually completed in one sex, with some, but not all, studies showing age‐related cognitive deficits. Other mouse studies show worsened ADRD pathology in APOE ε4 mice, begging the question whether APOE ε4's effects on ADRD can be influenced by exercise. Studies on exercise in humans are performed later in life after symptom onset, typically measuring improvements to activities of daily living and quality of life. While important, it is necessary to understand whether running can influence risk factors for dementia before symptomology. We evaluated the systemic and cerebral effects of running across APOE ε3/ε3, APOE ε3/ε4, and APOE ε4/ε4 litter‐matched mice during early aging. We show that chronic running affects multiple ADRD‐relevant phenotypes in both the periphery and the brain, but these effects are both APOE genotype‐ and sex‐specific.
RESEARCH IN CONTEXT
Systematic Review: The authors have surveyed literature through traditional methods, conference presentations, and other online platforms (Alzforum.org). While exercise is often studied after dementia onset in humans, studies of exercise in dementia mouse models have had significant limitations.
Interpretation: Our findings support that apolipoprotein E (APOE) ε4 dosage affects the body and brain and interacts with running in a unique pattern that differs between males and females. These results should be considered during the development of strategies to prevent or reduce risk of human dementia through exercise.
Future Directions: This work highlights the interactions between APOE ε4 and running in male and female mice; however, interrogation of specific mechanisms is still necessary. While here we studied early aging (birth to midlife), future studies can determine the effects of advanced aging, APOE ε4 dosage, and running. Finally, human trials would be useful to validate these APOE ε4 genotype effects on the body and brain.
2. METHODS
2.1. Mouse husbandry
Novel APOE mouse strains were created on C57BL/6J (B6) and maintained at The Jackson Laboratory as previously described. 34 Mice were kept in a 12/12‐hour light/dark cycle (06:00–18:00 light) and fed ad libitum 6% kcal fat standard mouse chow. Experimental cohorts were generated by intercrossing male and female APOE ε3/ε4 mice to create APOE ε3/ε3, APOE ε3/ε4, and APOE ε4/ε4 male and female littermate controls. As with previous studies, APOE ε3/ε3 mice served as the control genotype in these studies to standardize any human APOE insertion differences. 3 , 35 , 36 , 37 Animals were divided as evenly as possible per litter into running and sedentary cohorts. All experiments were approved by the Institutional Animal Care and Use Committee (IACUC) at The Jackson Laboratory.
2.2. Exercise by voluntary running
Mice were group‐housed into two or three per cage and given 24‐hour access to an unlocked (running) or locked (sedentary) running wheel (Innovive). At 5 months, mice were singly housed for the remaining duration of the experiment to enable data to be collected on individual mice. Mice were not returned to group housing to prevent fighting. At 6 and 11 months, running mice were tracked for number of rotations per minute during the dark cycle when they are most active using trackable running wheels (Med Associates, Inc.). Nights during which fewer than 700 minutes of data were tracked were considered incomplete and excluded from analysis. For each mouse, sum of rotations per night (n = 7 + nights tracked) was calculated and then averaged across all nights.
2.3. Harvesting, tissue preparation, plasma collection
All mice were euthanized by intraperitoneal injection of a lethal dose of ketamine (100 mg/ml)/xylazine (20 mg/ml). Mice were perfused intracardially with 1X phosphate buffered saline. Brains were carefully dissected, hemisected sagittally, and one half was then snap frozen on solid CO2 for later dissection and RNA sequencing. At timepoints throughout the experiment, blood plasma was collected via cheek bleed. Blood was carefully collected in K2 EDTA (1.0 mg) microtainer tubes (BD Biosciences), allowed to sit at room temperature for at least 30 minutes, and then centrifuged at 21°C for 10 minutes at 5031 g. Plasma was carefully collected and stored at –20°C. At the harvest timepoint (12 months), blood was collected in K2 EDTA (1.0 mg) microtainer tubes (BD Biosciences) through cardiac puncture. Plasma total cholesterol (mg/dL), direct low‐density lipoprotein (LDL; mg/dL), and high‐density lipoprotein (HDL; mg/dL) concentrations were characterized on the Beckman Coulter AU680 chemistry analyzer. All samples were profiled at the same time at the end of the experiment to avoid batch effects.
2.4. Nuclear magnetic resonance imaging
Each cohort was subjected to nuclear magnetic resonance (NMR) imaging at 6 and 11 months (female: n = 9–15), male: n = 11–15). NMR was performed as previously described. 38 Weight was measured, and mice were briefly placed into a Plexiglas tube 2.5 in. by 8 in., which was then subjected to NMR (EchoMRI). Magnetic field was measured by a 5‐gauss magnet. Measurements included weight, lean muscle mass, and fat mass, as well as fat percentage ([fat/body weight] × 100).
2.5. Murine daily activities and indirect calorimetry
After NMR measurements (female: n = 9–11 per genotype/activity, male: n = 9–12 per genotype/activity), mice were measured for energy balance through indirect calorimetry measurement cages (Sable Promethion). Briefly, these specialized cages continuously measure food and water intake, general activity (pedometers), wheel running behavior, energy expenditure (kcal/hr), and respiratory quotient (RQ). Measurements are collected for 5 days in 5‐minute interval bins. The RQ is a ratio of the volume of carbon dioxide (CO2) released over the volume of oxygen (O2) absorbed. RQ has been widely used in humans and mice as a tool to determine the starting substrate for energy metabolism (carbohydrate RQ ≈1, protein RQ ≈0.8, fat RQ ≈0.7, anaerobic respiration RQ ≈0, and multiple energy sources RQ ≈0.8). 39 , 40 , 41 , 42 , 43 , 44
2.6. RNA sequencing, linear modeling, and GSEA
We performed RNA sequencing on six brains per group (sex/genotype/activity) at 12 months. RNA extraction, library construction, RNA sequencing, and seq quality control were performed as described previously. 34 , 38 Genes were then filtered by (1) removing all genes that did not vary in expression (gene count change across all samples was 0) and (2) removing all genes that did not have at least five reads in 50% of the samples. Remaining genes (20,641) were normalized using DEseq2. 45 Principal component analysis (PCA) on the variance stabilized data (vst) identified outliers. To allow for the evaluation of APOE ε4 allele dosage, each linear model included two genotype comparisons: (1) APOE ε3/ε4 to APOE ε3/ε3 and (2) APOE ε4/ε4 to APOE ε3/ε3. Linear models were run separately for (1) cortex – female, (2) cortex – male, (3) hippocampus – female, and (4) hippocampus – male. β‐estimates were obtained for all four linear models that evaluated the main effects of APOE genotype (APOE ε3/ε4, APOE ε4/ε4, ref: APOE ε3/ε3) and running (run, ref: sed), as well as the interaction between APOE genotype and running (APOE ε3/ε4:Run, APOE ε4/ε4:Run). For each linear model, gseGO from the clusterProfiler package was run on genes significant for each factor. Gene set enrichment analysis (GSEA) was used to determine Gene Ontology (GO) terms for the genes significant for the main (running, APOE ε3/ε4, APOE ε4/ε4) and interacting factors (APOE ε3/ε4:Run and APOE ε4/ε4:Run). Normalized enrichment scores (NES) from GSEA were used to identify terms that were positively or negatively associated with each factor. GO terms were ordered based on the NES. Terms with a positive NES had more genes higher on the ranked list (i.e., more positive β values) and the terms with a negative NES containing more genes lower on the ranked list (i.e., more negative β values). Enriched GO terms had overlapping biological functions that we termed “vascular integrity,” “cellular motility,” “immune system response,” “mitochondrial metabolism,” and “synaptic/neuronal health.” The top 20 most positive and negative GO terms were visualized for the cortex and hippocampus for both females and males (Figures S10–S24 in supporting information).
2.7. Statistical analysis
For running comparisons within sex and age, a one‐way analysis of variance (ANOVA) with Tukey's multiple comparison was performed. To determine age‐related decline a two‐sided paired t‐test was performed.
For all weights, body composition analysis, murine daily activities, and energy expenditure, a two‐way ANOVA for APOE genotype, activity, and the interaction between APOE genotype and activity was calculated. Bonferroni post hoc corrections were calculated and significance within genotype (the effect of running per genotype) was visualized.
3. RESULTS
3.1. APOE genotype did not affect voluntary running from young to midlife
To determine the effects of one APOE ε4 allele to two APOE ε4 alleles, we compared the APOE ε3/ε4 or APOE ε4/ε4 genotypes to the control, APOE ε3/ε3 genotype (Figure 1A). Previous studies have shown that females run more than males; therefore, we assessed the sexes separately. 38 There was no difference in voluntary running during the dark cycle across APOE genotypes; however, there was expected variation between individual mice within the APOE genotypes (Figure 1B,C; Figures S1–S2 in supporting information). There was an age‐dependent decrease in voluntary running from 6 to 11 months; however, there was no difference between APOE genotypes (Figure 1D–G). These findings show that running is not a variable between the APOE genotypes, and therefore not a confound in subsequent analyses.
FIGURE 1.
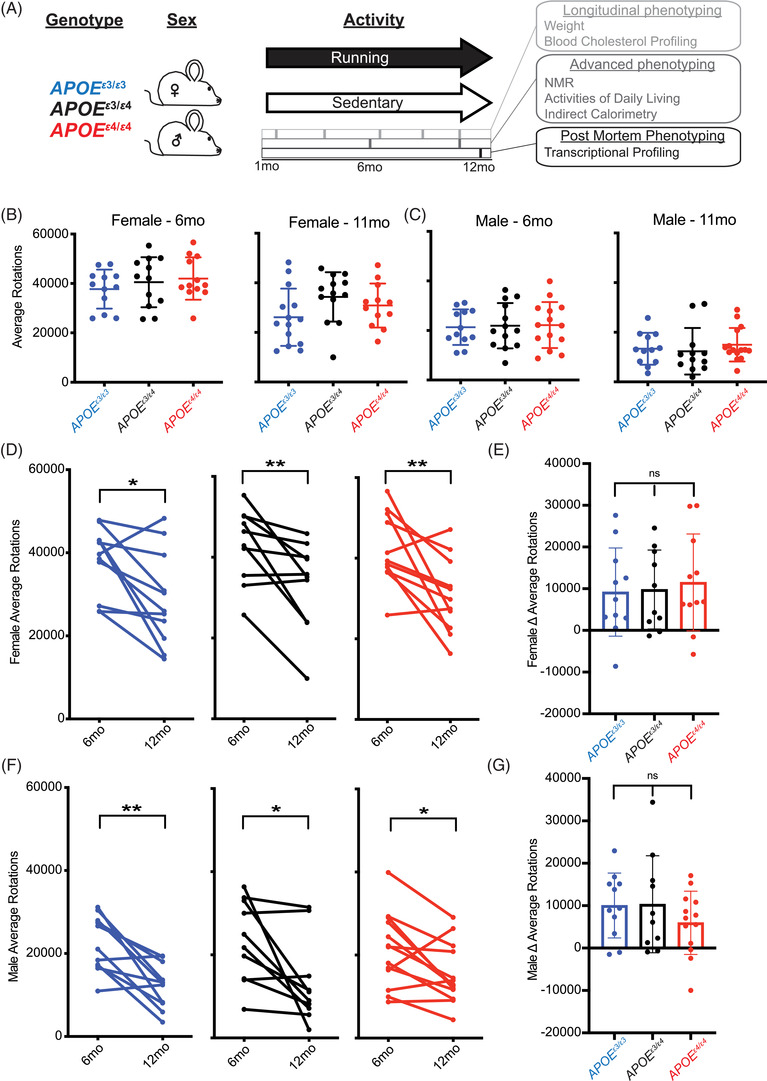
Voluntary chronic running to midlife is not different between apolipoprotein E (APOE) genotypes. A, Schematic of the voluntary running paradigm in which APOE ε3/ε3, APOE ε3/ε4, and APOE ε4/ε4 male and female mice were introduced to a locked (control – sedentary) or unlocked (treatment – running) running wheel at 1 month until 12 months (midlife). Longitudinal, advanced, and post mortem phenotyping is indicated. B‐C, No difference in running (average rotations across multiple consecutive nights) between APOE genotypes at both 6 and 11 months in female (B) or male (C) mice (n = 10–15). D‐G, Average rotations per mouse at 6 and 12 months showed an age‐dependent decrease for both females (D) and males (F); however, the change over time was not significantly different between genotypes (E,G) (n = 10–15). Data presented as mean ± standard deviation, one‐way analysis of variance with Tukey's multiple comparison performed for B,C,E,G. Two‐sided paired t‐test performed for D,F. *P < .05, **P < .01
3.2. APOE genotype and running interact in a sex‐specific manner to modulate general markers of healthy aging
Weight, body composition (e.g., lean mass, fat mass, and fat percentage) and cholesterol levels are commonly used as a general proxy for health in humans. 46 , 47 , 48 These biometrics are typically measured at routine physicals and are considered indicative of general health status, and markers for obesity, cardiovascular disruption, and lipid dysregulation. 49 , 50 , 51 , 52 We examined whether running affected weight, body composition, and cholesterol across APOE genotypes. Monthly weights (from 1 to 12 months) revealed an expected age‐dependent weight gain in sedentary mice that was significantly attenuated by running (Figure 2A–F; Figure S3A–D in supporting information). In females, but not males, the APOE ε4/ε4 genotype caused a greater running‐based attenuation in weight gain compared to APOE ε3/ε3 and APOE ε3/ε4. These results suggest that the beneficial effects of running on weight loss are APOE genotype‐dependent in females only.
FIGURE 2.
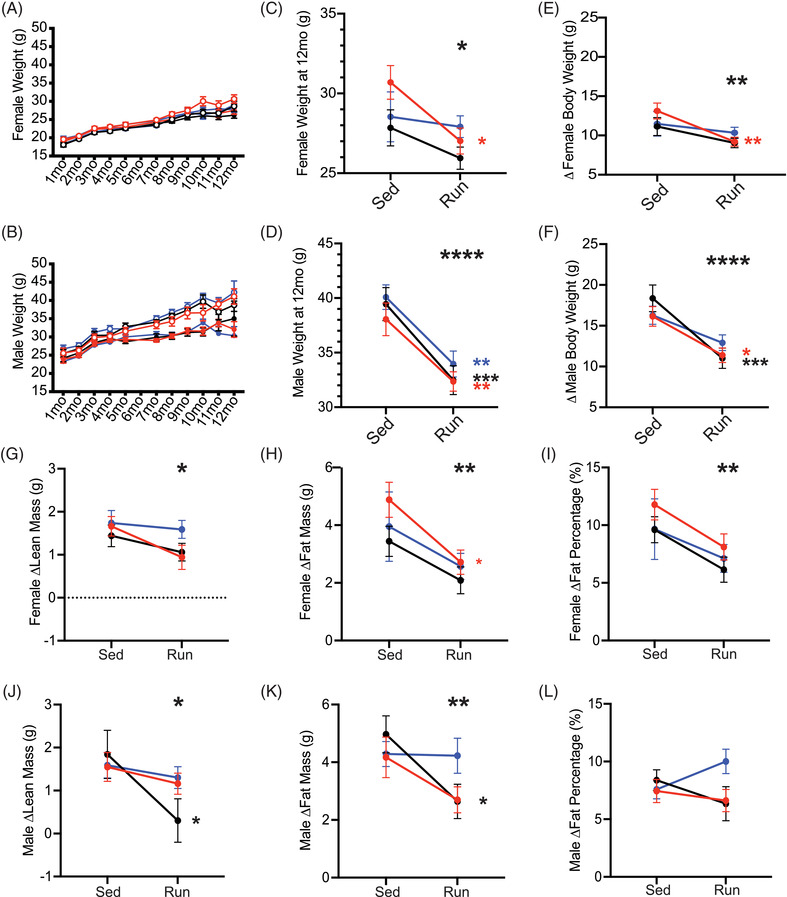
Running attenuated age‐dependent weight gain and fat accumulation across apolipoprotein E (APOE) genotypes. A‐B, Expected age‐dependent weight gain from 1 to 12 months in females (A, n = 9–15) and males (B, n = 11–15). C‐D, Running mice weighed significantly less at 12 months in both females (C) and males (D). E‐F, Running significantly attenuated age‐dependent weight gain (the difference in body weight from 1 to 12 months) in both females (E) and males (F). G‐I, Significant effect of running on the change in lean mass (G), fat mass (H), and fat percentage (I) between 6 and 11 months, with an overall reduction in running mice compared to sedentary mice across all APOE genotypes in females. J‐L, Running had a significant reduction on the change in lean mass (J) and fat mass (K) between 6 and 11 months, but no change in fat percentage (L) in male mice. Data presented as mean ± standard error of the mean, two‐way analysis of variance performed for APOE genotype (significant marked above “Sed” column, indicating an effect of APOE genotype), Running (significance marked above “Run” column, indicating an effect of running), and the interaction between APOE genotype:Running (significance marked to the right of the graph). Bonferroni's multiple comparisons performed for within‐genotype running effects (significance marked in smaller stars directly to the right of the run column, within graph limits, in the color of the genotype). *P < .05, **P < .01, ***P < .001, ****P < .0001
Overall, running mice had a lower fat composition compared to sedentary mice for both sexes at 6 and 11 months (Figures S4,5 in supporting information). In females, only APOE ε4/ε4 mice showed a significant attenuation of fat mass and fat percentage in running compared to sedentary mice (Figure 2G–I; Figure S3E–G). There were no APOE genotype differences in male mice; however, there was an effect of running on lean and fat mass. This effect was most pronounced in APOE ε3/ε4 male mice, with running attenuating lean and fat mass (Figure 2J–L; Figure S3H–J). Running attenuated the age‐related increase in lean and fat mass across all genotypes and sexes. However, there was a pronounced reduction of age‐related fat mass accumulation in female APOE ε4/ε4 running mice. Also, male APOE ε3/ε4 running mice showed the greatest reduction in age‐related lean and fat mass accumulation.
No effect of running or APOE genotype was determined for total cholesterol or HDL concentration at 12 months (Figure S6 in supporting information). There was a significant sex‐specific effect of APOE genotype on LDL concentration in the plasma. In running females, LDL concentrations decreased in an APOE ε4 dose‐dependent manner (Figure S6H). Conversely, in running males, LDL concentrations were significantly lower than sedentary mice (Figure S6K). Cholesterol composition in running mice did not correlate with running distance for both sexes (Figure S6C,F,I,L,O,R).
Collectively, these data demonstrate that weight, body composition, and cholesterol levels, commonly used markers of healthy aging, are significantly affected by voluntary running, but the effects are dependent upon both sex and APOE genotype.
3.3. Running affects murine daily activities in an APOE genotype‐ and sex‐specific manner
In humans, prior to more severe cognitive decline in ADRDs, activities of daily living (i.e., sleep, general movement, feeding) are often disrupted. 53 , 54 , 55 To evaluate murine daily activities in mice, we measured feeding and walking behavior (pedometers) across four dark cycles (active/awake period), and three light cycles (inactive/sleep period) at 11 months. Feeding behavior revealed significant changes in the dark cycle, but not in the light cycle for both sexes. In females, running mice consumed more food than sedentary mice during the dark cycle across all APOE genotypes (Figure 3A–C; Figure S7 in supporting information). However, in males, there was an interaction between APOE genotype and running during the dark cycle (Figure 3D–F). In sedentary mice, male APOE ε3/ε4 ate more than APOE ε3/ε3 and APOE ε4/ε4. This pattern was not apparent in running mice, suggesting running mitigates the APOE genotype differences observed in sedentary mice (Figure 3D; Figure S7).
FIGURE 3.
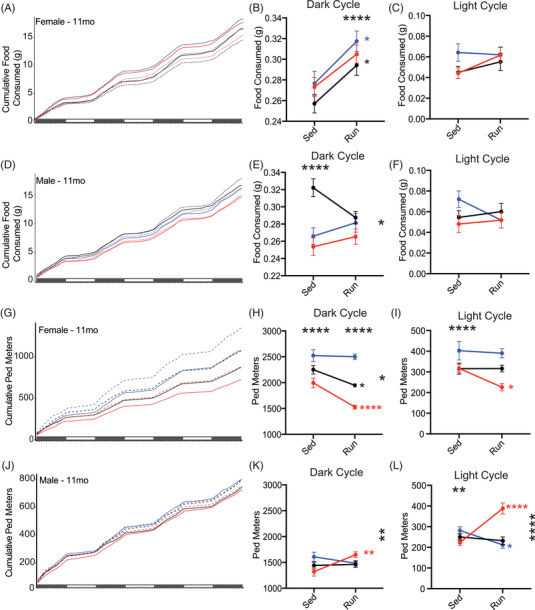
Murine daily activities are influenced by apolipoprotein E (APOE) genotype and running. A,D, Cumulative food consumed per gram for female (A, n = 9–11) and male (D, n = 9–12) mice across four dark cycles and three light cycles. B‐C, Running significantly increased food consumed during the dark cycle (B), but not the light cycle (C) in female mice. E‐F, APOE genotype and APOE genotype:running interaction affected food consumption in males during the dark cycle (E), but no effect was seen during the light cycle (F). G,J, General movement (cumulative ped meters) for female (G) and male (J) mice across four dark cycles and three light cycles. H‐I, APOE genotype, running, and APOE genotype:running interaction all significantly affected ped meters during the dark cycle (H) with running decreasing ped meters differently across the genotypes. Only APOE genotype was significant during light cycle (I) in female mice. K‐L, APOE genotye:Running interaction significantly affected ped meters in males, with APOE ε4/ε4 showing increased ped meters during the dark cycle (K), as well as the light cycle (L). There was also an APOE genotype effect (L). Solid lines indicate Run mice, dashed lines indicate Sed mice (A,D,G,J). Data presented as mean ± standard error of the mean, two‐way analysisof variance performed for APOE genotype (significant marked above “Sed” column, indicating an effect of APOE genotype), running (significance marked above “Run” column, indicating an effect of running), and APOE genotype:running interaction (significance marked in to the right of the graph). Bonferroni's multiple comparisons performed for within‐genotype running effects (significance marked in smaller stars directly to the right of the run column, within graph limits, in the color of the genotype). *P < .05, **P < .01, ***P < .001, ****P < .0001
We next determined whether movement in the home cage was affected by APOE genotype and/or running by measuring walking (Ped meters; see Methods). Female sedentary mice showed an APOE ε4 dose‐dependent increase in Ped meters that was attenuated by running (Figure 3G,H; Figure S7). During the light cycle only APOE ε4/ε4 females showed a significant reduction in Ped meters compared to their sedentary counterparts (Figure 3I; Figure S7). In male mice, both APOE genotype and running interacted to alter Ped meters during both the dark and light cycle; however, running more strikingly increased cumulative Ped meters of APOE ε4/ε4 mice compared to the other APOE genotypes (Figure 3K,L; Figure S7).
These results show that APOE genotype modulates the effects of running on natural home cage behaviors such as feeding and general movement, considered equivalent to activities of daily living in humans.
3.4. APOE genotype affects running‐dependent increase in energy expenditure during the dark cycle
Previous studies in humans demonstrated APOE genotype affects metabolism on a cellular, regional, and organismal level. 37 , 56 To determine whether running and APOE genotype affect metabolic processes, energy expenditure (kcal/hr) was measured at 11 months. In female sedentary mice, energy expenditure showed an APOE ε4 dose‐dependent increase during the dark cycle. In general, running resulted in significantly higher energy expenditure in the dark cycle in male and female mice. However, this effect was not observed in male APOE ε3/ε4 mice (Figure 4A–C; Figure S8 in supporting information). This suggests the APOE ε3/ε4 genotype attenuates the effects of running in a sexually dimorphic manner. During the light cycle, all genotypes showed decreased energy expenditure with running; except for female APOE ε3/ε3 mice that showed an increase (Figure 4D–F; Figure S8). Subtle but significant changes in substrate usage (based on RQ; see Methods) were also determined across groups in both the light and dark cycle (Figure 4G–L; Figure S8). Significant changes were small; however, they may worsen with more advancing age (Figure 4H–L; Figure S8). These results highlight that APOE genotype and running affect energy expenditure; however, changes in starting energy substrate usage were minute (Figure 4A–F).
FIGURE 4.
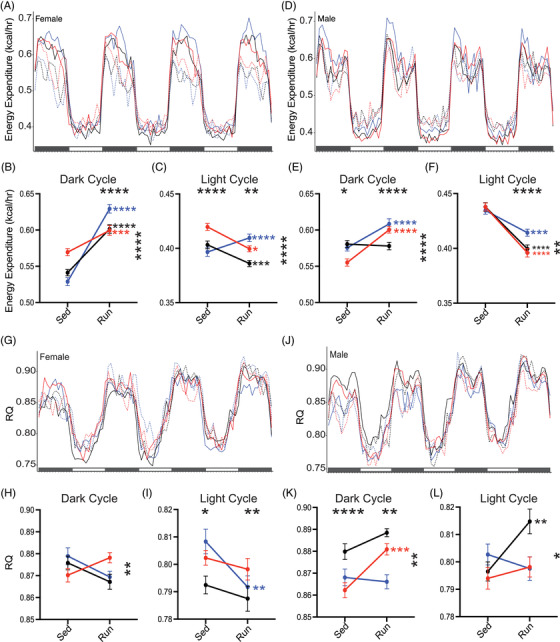
Running influences energy expenditure differently between females and male apolipoprotein E (APOE) mice. A‐C, Energy expenditure (kcal/hr) across four dark cycles and three light cycles for female mice (n = 9–11). Energy expenditure (kcal/hr) significantly affected by APOE gentoype:running, and running during the dark cycle (B), with an increase in energy expenditure in running mice. APOE genotype:running, APOE genotype, and running all influenced light cycle energy expenditure (C), with APOE ε3/ε3 increasing with running while APOE ε3/ε4 and APOE ε4/ε4 decreased energy expenditure with running. D‐F, Energy expenditure (kcal/hr) across four dark cycles and three light cycles for male mice (n = 9–12). E, APOE genotype:running, APOE genotype, and running all affected dark cycle energy expenditure in male mice. F, APOE genotype:running and running showed an overall decrease in energy expenditure in running male mice. G‐I, Respiratory quotient (RQ) across four dark cycles and three light cycles for female mice (G). APOE genotype:running significantly affected RQ during the dark cycle for female mice (H). APOE genotype and running significantly affected RQ during the light cycle for female mice (I). J‐L, RQ across four dark cycles and three light cycles for male mice (J). APOE genotype:running, APOE genotype, and running all significantly affected RQ during the dark cycle in male mice (K). APOE genotype:running significantly affected RQ during the light cycle (L). Solid lines indicate Run mice, dashed lines indicate Sed mice (A,D,G,J). Data presented as mean ± standard error of the mean, two‐way analysis of variance performed for APOE genotype (significant marked above “Sed” column, indicating an effect of APOE genotype), Running (significance marked above “Run” column, indicating an effect of running), and the interaction between APOE genotype:running (significance marked to the right of the graph). Bonferroni's multiple comparisons performed for within genotype running effects (significance marked in smaller stars directly to the right of the run column, within graph limits, in the color of the genotype). *P < .05, **P < .01, ***P < .001, ****P < .0001
3.5. APOE genotype causes subtle sex‐specific changes to the effects of running on the aging brain
Unbiased transcriptional profiling was used to capture molecular effects across APOE genotype and activities (12 groups per brain region, Figure 5A; see Methods). PCA revealed brain region (PC1, 65%) and sex (PC2, 20%) were the primary drivers of variance, suggesting APOE genotype and running are not exerting strong effects (Figure 5B). Therefore, to determine subtle effects of APOE genotype and running, linear modeling was used for male or female cortex or hippocampus samples separately (four linear models in total; Figure 5C). Supporting the PCA data, linear modeling revealed fewer than 200 significant genes in females for the cortex and hippocampus, and fewer than 800 genes in males (Figure 5D–G). These numbers align with published data from human studies but are somewhat fewer than previous mouse studies (Figure S12). Several significant genes including Ephx1 (main effect: APOE ε3/ε4), Ctsf (main effect: APOE ε4/ε4), C3 (interaction APOE ε3/ε4:Run), and Cav3 (interaction APOE ε4/ε4:Run) are known to function in lipid homeostasis, neuroinflammation, and membrane integrity, key processes implicated in ADRD risk (Figure 5H).
FIGURE 5.
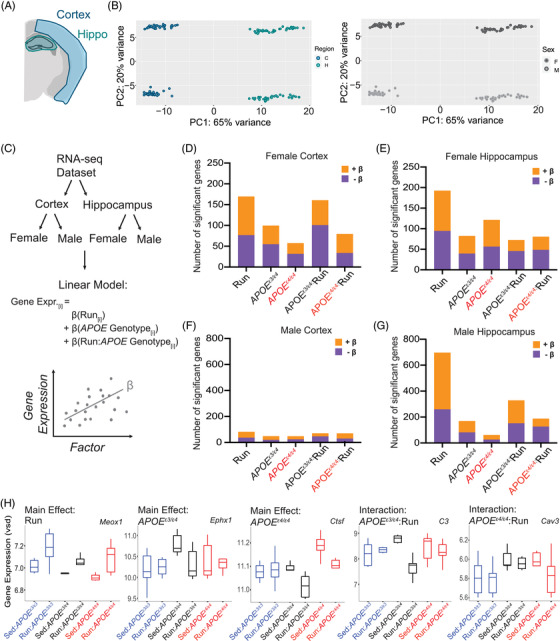
Transcriptional profiling reveals subtle changes due to apolipoprotein E (APOE) genotype and running in the cortex and hippocampus. A, Diagram of the cortical and hippocampal regions of the brain taken for transcriptional profiling (n = 6 per sex/genotype/activity). B, Principal components analysis revealed clear separations between brain regions (cortex and hippocampus, 65% variance explained), as well as by sex (female and male, 20% of variance explained). C, Schematic of the computational analysis approach; first RNA‐seq was separated by brain region, next separated again by sex. Four linear models were run to examine gene expression as it varies with running, APOE genotype, and the interaction between APOE genotype:running. β‐value is the association of the gene with the factor tested—positive β‐value indicates a positive correlation, negative β‐value indicates a negative correlation. D‐G, Number of significant genes (false discovery rate corrected) for female cortex (D), female hippocampus (E), male cortex (F), and male hippocampus (G). H, Example of a significant gene for each of the main effects and interactive effects: Meox1 (Hippocampus, Male), Ephx1 (Hippocampus, Male), Ctsf (Hippocampus, Female), C3 (Hippocampus, Male), Cav3 (Cortex, Male)
Through Gene Set Enrichment Analysis (GSEA), for the female cortex, GO terms showed positive normalized enrichment scores (NES) for the main effects (running, APOE ε3/ε4, and APOE ε4/ε4), but negative NES for the interactive terms (APOE ε3/ε4:Run, APOE ε4/ε4:Run) for vascular and synaptic/neuronal functions (Figure 6A–C)). Also, interestingly, in males, there were few significantly enriched GO terms for APOE ε3/ε4, suggesting in males, but not females, the APOE ε3/ε4 genotype exerts little to no effect compared to the APOE ε3/ε3 genotype on genes associated with vascular integrity–related processes (Figure 6D). Transcriptional profiling supplemented our peripheral findings, determining that running and APOE genotype interact in sex‐specific ways to influence mechanisms involved in dementia‐relevant biological processes.
FIGURE 6.
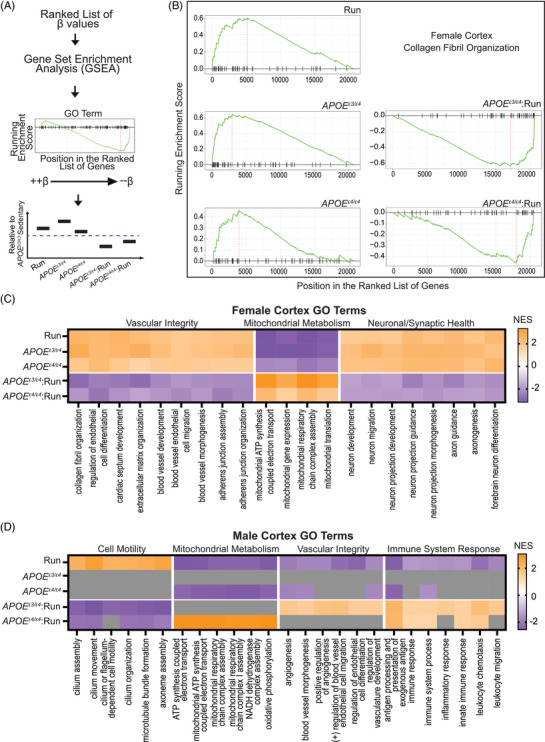
Gene set enrichment analysis (GSEA) predicts apolipoprotein E (APOE) genotype and running interact to mitigate main effects. A, Schematic of computational approach; β‐values from linear models were passed through GSEA for gene ranking, GSEA plots were used to visualize results and main effects of running, APOE genotype, and APOE genotype:running were interpreted per Gene Ontology (GO) term. B, GSEA plots for “Collagen Fibril Organization” in the female cortex. Main effects of running and APOE ε3/ε4, and APOE ε4/ε4 all show positive enrichment scores, while the interactions, APOE ε3/ε4:run and APOE ε4/ε4:run reveal negative enrichment scores. C, In the female cortex data GO terms that fit the pattern shown in (B), colored by normalized enrichment score (NES), are represented specifically vascular integrity, mitochondrial metabolism, and synaptic/neuronal health. D, The pattern observed in male cortex was different than that seen in female cortex (B,C) with APOE ε3/ε4 appearing more similar to APOE ε3/ε3 (indicated by gray boxes) for enrichment terms grouped as cell motility, mitochondrial metabolism, vascular integrity, and immune system response
4. DISCUSSION
Exercise is generally considered to have beneficial effects, but our results show APOE genotype impacts the effects of running. Significant interactions between APOE genotype and running were observed across body weight, body composition, murine daily activities, systemic metabolism, and cortical and hippocampal gene expression. As with previous studies, to enable genotype comparisons between littermates, APOE ε3/ε3 were considered controls in all comparisons. 3 , 35 , 36 , 37 This was because APOE ε3 is considered the neutral allele in human studies, although this design meant comparisons between human APOE and mouse Apoe could not be made. Male and female mice were evaluated separately as ADRD risk varies between the sexes, with higher risk in women compared to men. 57 , 58 Sex is typically used as a covariate in human studies, but our data show that APOE genotype and sex interact across multiple domains. Additionally, there is a lack of consideration that odds ratios are sex‐specific when assessing clinical trials, obfuscating the effects of sex. Our data suggest APOE genotype for each sex should be considered for studies assessing exercise interventions to reduce risk for dementia and more broadly any diseases for which APOE genotype has been associated.
While the brain has been shown to be plastic throughout adulthood, environmental influences can exert a greater effect on a younger brain compared to an older brain, 19 , 23 , 38 , 59 , 60 , 61 , 62 prompting us to study the effects of APOE genotype and running from early age to midlife. We assessed 12‐month‐old mice to understand the effect of APOE and running up until midlife, likening our findings to prodromal studies in humans. 63 , 64 APOE genotype‐specific effects may also be apparent at older ages so studying later timepoints in the mouse, even beginning running at midlife, would be informative. This would relate closely to some human clinical trials that conduct studies on older, affected human populations (i.e., nursing home/hospice patients). 65 , 66 , 67 Additionally, it is unknown if exercise affects APOE production in the brain and periphery. While APOE production is assumed to be stable, it is possible that expression is directly or indirectly affected by exercise‐related changes, such as BDNF, FNDC5/Irisin, and other systemic factors that promote neuroprotection. However, one human study suggested that exercise paradigms helped preserve cognition in APOE ε4 carriers more effectively over APOE ε3 controls suggesting APOE ε4 carriers to be more responsive to exercise‐induced myokines; however, the APOE levels were not tested. 68 It is still necessary to carry out further clinical analyses, and while many ADRD studies include exercise as an intervention, the mechanisms by which APOE ε4 and exercise interact to affect amyloid beta deposition, tau tangle accumulation, neuroinflammation, vascular disruption, and other important ADRD pathologies are still to be performed. 69 , 70 , 71
Transcriptomic approaches have revolutionized our understanding of ADRDs and have therefore become a hypothesis‐generating tool for identifying the molecular pathways impacted by genetic and environmental risk factors. Therefore, we used transcriptional profiling to identify interactions between APOE genotype and running. Our data revealed a reversal of NES direction from the main effects and the interaction of APOE genotype and running. This was unexpected, as we saw similar patterns of positive (or negative) enrichment for (1) running compared to sedentary, (2) APOE ε3/ε4 compared to APOE ε3/ε3, and (3) APOE ε4/ε4 compared to APOE ε3/ε3. These results contradict the assumption that running would have the opposite effect on the brain as APOE ε4, particularly when considering each of these terms collectively (vascular, immune, mitochondrial, neuronal/synaptic). We propose that there is a possibility for overcompensation for the APOE ε4 allele. While evidence shows APOE ε4 causes gains and losses of APOE function across many processes, it is unknown whether there is a preemptive response that has not been considered. Further, the APOE ε3/ε4 genotype may be responding to early aging phenotypes different than APOE ε4/ε4 genotype. Precise experimentation on this phenomenon is needed in both mice and humans to better understand which APOE ε4‐specific pathways are mitigated by running. Last, while these models are key for interpretation of APOE biology, other important pathological interactions (e.g., amyloid or tau) are not present in this study. Future studies are necessary to interrogate the interactions among APOE, exercise, and hallmark ADRD pathologies to provide further translatable outcomes.
Advancements in RNA sequencing have made it cheaper and faster to sequence the brains of ADRD patients (Religious Orders Study/Memory and Aging Project [ROSMAP], Mayo Clinic [MAYO], Alzheimer's Disease Sequencing Project [ADSP]). Recently, research programs have explored whether APOE influences the human cerebral transcriptome. In three largescale Accelerating Medicines Partnership–Alzheimer's Disease studies, reports revealed few to no gene expression changes in multiple brain regions in APOE ε4+ cases compared to noncarriers (ROSMAP: syn8456629, MAYO: syn8466812, Mount Sinai Brain Bank [MSBB]: syn8484987) 72 , 73 , 74 (Figure S12). The ROSMAP dataset analysis showed no differences due to APOE ε4 status across the dorsolateral prefrontal cortex region. 72 The MAYO dataset showed a significant differential expression (DE) of only 173 genes between APOE ε3/ε4 and APOE ε3/ε3, and a significant DE of only 88 genes between APOE ε4/ε4 and APOE ε3/ε3 in the temporal cortex. 73 The MSBB reported fewer than five genes DE between all APOE genotype comparisons in the frontal pole region, parahippocampal gyrus region, frontal superior temporal gyrus region, and inferior frontal gyrus region. 74 Our mouse data align more closely with these human studies, possibly due to litter‐matched mice, and further analyses using GSEA showed subtle changes that escaped detection through traditional DE analysis. Moving forward, our data show the importance of including heterozygous genotypes (e.g., APOE ε3/ε4) and varying degrees of chronic voluntary exercise (e.g., low, medium, high) in mouse studies to improve the alignment to ADRD in human studies.
The APOE ε4 allele emerged as our early hominin ancestors adapted to changes in habitat and food availability to include more aerobic exercise such as running. 75 The APOE ε4 allele was beneficial for storage of fats, increasing cholesterol. While the APOE ε4 conferred longer lifespan 200,000 years ago, the diet and exercise of an individual was drastically different. 75 Currently, Western culture sees some of the highest rates of ADRD, due to the interaction between APOE ε4 and our environment, and as we show, running. This work supports that APOE ε4 interacts with running in a genotype‐ and sex‐specific manner to influence peripheral and central risk factors for diseases such as ADRDs.
CONFLICTS OF INTEREST
The authors declare they have no competing interests or disclosures.
AUTHOR CONTRIBUTIONS
Kate E. Foley and Gareth R. Howell conceived and designed this project. Kate E. Foley, Cory A. Diemler, Amanda A. Hewes performed mouse experiments. Kate E. Foley performed IF, experimental analysis, and bioinformatic analysis. Kate E. Foley and Gareth R. Howell consulted for statistical approach and analysis. Kate E. Foley and Gareth R. Howell wrote and prepared this manuscript. All authors read and approved the final manuscript.
Supporting information
Supporting Information
ACKNOWLEDGMENTS
Research reported in this publication was partially supported by The Jackson Laboratory's Genetic Engineering Technologies Scientific Service. The authors wish to thank Todd Hoffert from Clinical Assessment Services for blood chemistry, Heidi Munger and the Genome Technologies group for RNA sequencing. We would also like to thank Dr. Gregory Carter and Dr. Christoph Pruess for their continued advice on computational analysis and support for these projects. The authors are supported by funding from the National Institutes of Health, National Institute on Aging U54 AG054345. This work was supported by T32HD007065 to Kate Foley. Also, the authors are especially grateful to Tucker Taft and his wife Phyllis R. Yale, and the estate of Bennett Bradford and his daughter, Deborah Landry for their generous and thoughtful support of Alzheimer's research at The Jackson Laboratory. These funding sources supported study design, data collection and interpretation, and writing of the manuscript. The mice used in this study were created by the IU/JAX/PITT Model Organism Development and Evaluation for Late‐onset Alzheimer's Disease (MODEL‐AD) Center funded by the National Institute on Aging (U54 AG054345). This work was also supported by the National Institutes of Health grant to The Jackson Laboratory Nathan Shock Center of Excellence in the Basic Biology of Aging (AG038070). The funding organizations played no role in the design and conduct of the study; in the management, analysis, and interpretation of the data; or in the preparation, review, or approval of the article.
Foley KE, Diemler CA, Hewes AA, Garceau DT, Sasner M, Howell GR. APOE ε4 and exercise interact in a sex‐specific manner to modulate dementia risk factors. Alzheimer's Dement. 2022;8:e12308. 10.1002/trc2.12308
REFERENCES
- 1. Sando SB, Melquist S, Cannon A, et al. APOE epsilon 4 lowers age at onset and is a high risk factor for Alzheimer's disease; a case control study from central Norway. BMC Neurol. 2008;8:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kresovich JK, Garval EL, Martinez Lopez AM, et al. Associations of body composition and physical activity level with multiple measures of epigenetic age acceleration. Am J Epidemiol. 2021;190:984‐993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Area‐Gomez E, Larrea D, Pera M, et al. APOE4 is associated with differential regional vulnerability to bioenergetic deficits in aged APOE mice. Sci Rep. 2020;10:4277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Montagne A, Nation DA, Sagare AP, et al. APOE4 leads to blood‐brain barrier dysfunction predicting cognitive decline. Nature. 2020;581:71‐76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Montagne A, Nation DA, Zlokovic BV. APOE4 accelerates development of dementia after stroke: is there a role for cerebrovascular dysfunction? Stroke. 2020;51:699‐700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Montagne A, Nikolakopoulou AM, Huuskonen MT, et al. APOE4 accelerates advanced‐stage vascular and neurodegenerative disorder in old Alzheimer's mice via cyclophilin A independently of amyloid‐β. Nature Aging. 2021;1:506‐520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Swain RA, Harris AB, Wiener EC, et al. Prolonged exercise induces angiogenesis and increases cerebral blood volume in primary motor cortex of the rat. Neuroscience. 2003;117:1037‐1046. [DOI] [PubMed] [Google Scholar]
- 8. Growdon JH, Hyman BT. APOE genotype and brain development. JAMA Neurol. 2014;71:7‐8. [DOI] [PubMed] [Google Scholar]
- 9. Reynolds CA, Smolen A, Corley RP, et al. APOE effects on cognition from childhood to adolescence. Neurobiol Aging. 2019;84:239.e1‐239.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mc Donald J, Krainc D. Alzheimer gene APOE epsilon4 linked to brain development in infants. JAMA. 2014;311:298‐299. [DOI] [PubMed] [Google Scholar]
- 11. Dean DC 3rd, Jerskey BA, Chen K, et al. Brain differences in infants at differential genetic risk for late‐onset Alzheimer disease: a cross‐sectional imaging study. JAMA Neurol. 2014;71:11‐22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chang L, Douet V, Bloss C, et al. Gray matter maturation and cognition in children with different APOE epsilon genotypes. Neurology. 2016;87:585‐594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Williams T, Borchelt DR, Chakrabarty P. Therapeutic approaches targeting Apolipoprotein E function in Alzheimer's disease. Mol Neurodegener. 2020;15:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Xiong M, Jiang H, Serrano JR, et al. APOE immunotherapy reduces cerebral amyloid angiopathy and amyloid plaques while improving cerebrovascular function. Sci Transl Med. 2021;13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Liu CC, Liu CC, Kanekiyo T, Xu H, Bu G. Apolipoprotein E and Alzheimer disease: risk, mechanisms and therapy. Nat Rev Neurol. 2013;9:106‐118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yamazaki Y, Zhao N, Caulfield TR, Liu CC, Bu G. Apolipoprotein E and Alzheimer disease: pathobiology and targeting strategies. Nat Rev Neurol. 2019;15:501‐518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gupta R, Khan R, Cortes CJ. Forgot to exercise? Exercise derived circulating myokines in alzheimer's disease: a perspective. Front Neurol. 2021;12:649452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zhou W, Barkow JC, Freed CR. Running wheel exercise reduces alpha‐synuclein aggregation and improves motor and cognitive function in a transgenic mouse model of Parkinson's disease. PLoS One. 2017;12:e0190160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zheng J, Sun X, Ma C, Li BM, Luo F. Voluntary wheel running promotes myelination in the motor cortex through Wnt signaling in mice. Mol Brain. 2019;12:85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zhang J, Guo Y, Wang Y, Song L, Zhang R, Du Y. Long‐term treadmill exercise attenuates Abeta burdens and astrocyte activation in APP/PS1 mouse model of Alzheimer's disease. Neurosci Lett. 2018;666:70‐77. [DOI] [PubMed] [Google Scholar]
- 21. Yuede CM, Zimmerman SD, Dong H, et al. Effects of voluntary and forced exercise on plaque deposition, hippocampal volume, and behavior in the Tg2576 mouse model of Alzheimer's disease. Neurobiol Dis. 2009;35:426‐432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. van Praag H, Shubert T, Zhao C, Gage FH. Exercise enhances learning and hippocampal neurogenesis in aged mice. J Neurosci. 2005;25:8680‐8685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tapia‐Rojas C, Aranguiz F, Varela‐Nallar L, Inestrosa NC. Voluntary running attenuates memory loss, decreases neuropathological changes and induces neurogenesis in a mouse model of Alzheimer's disease. Brain Pathol. 2016;26:62‐74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Soto I, Graham LC, Richter HJ, et al. APOE stabilization by exercise prevents aging neurovascular dysfunction and complement induction. PLoS Biol. 2015;13:e1002279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Nigam SM, Xu S, Kritikou JS, Marosi K, Brodin L, Mattson MP. Exercise and BDNF reduce Abeta production by enhancing alpha‐secretase processing of APP. J Neurochem. 2017;142:286‐296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Nichol K, Deeny SP, Seif J, Camaclang K, Cotman CW. Exercise improves cognition and hippocampal plasticity in APOE epsilon4 mice. Alzheimers Dement. 2009;5:287‐294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mifflin KA, Frieser E, Benson C, Baker G, Kerr BJ. Voluntary wheel running differentially affects disease outcomes in male and female mice with experimental autoimmune encephalomyelitis. J Neuroimmunol. 2017;305:135‐144. [DOI] [PubMed] [Google Scholar]
- 28. Zheng F, Cai Y. Concurrent exercise improves insulin resistance and nonalcoholic fatty liver disease by upregulating PPAR‐gamma and genes involved in the beta‐oxidation of fatty acids in ApoE‐KO mice fed a high‐fat diet. Lipids Health Dis. 2019;18:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Stimpson NJ, Davison G, Javadi AH. Joggin' the Noggin: towards a physiological understanding of exercise‐induced cognitive benefits. Neurosci Biobehav Rev. 2018;88:177‐186. [DOI] [PubMed] [Google Scholar]
- 30. Khodadadi D, Gharakhanlou R, Naghdi N, et al. Treadmill exercise ameliorates spatial learning and memory deficits through improving the clearance of peripheral and central amyloid‐beta levels. Neurochem Res. 2018;43:1561‐1574. [DOI] [PubMed] [Google Scholar]
- 31. Ruegsegger GN, Booth FW. Running from disease: molecular mechanisms associating dopamine and leptin signaling in the brain with physical inactivity, obesity, and type 2 diabetes. Front Endocrinol (Lausanne). 2017;8:109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Chieffi S, Messina G, Villano I, et al. Neuroprotective effects of physical activity: evidence from human and animal studies. Front Neurol. 2017;8:188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hassan M, Aguib Y, Yacoub M. Molecular mechanisms of cardiovascular benefits of exercise: running for cover from heart disease. Glob Cardiol Sci Pract. 2016;2016:e201603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Foley KE, Garceau DT, Kotredes KP, Carter GW, Sasner M, Howell GR. APOEε3/ε4 and APOEε4/ε4 genotypes drive unique gene signatures in the cortex of young mice. Front Aging Neurosci. 2022:14:838436. 10.3389/fnagi.2022.838436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zhao N, Ren Y, Yamazaki Y, et al. Alzheimer's risk factors age, APOE genotype, and sex drive distinct molecular pathways. Neuron. 2020;106:727‐742.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Johnson LA, Torres ER, Impey S, Stevens JF, Raber J. Apolipoprotein E4 and insulin resistance interact to impair cognition and alter the epigenome and metabolome. Sci Rep. 2017;7:43701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Johnson LA. APOE and metabolic dysfunction in Alzheimer's disease. Int Rev Neurobiol. 2020;154:131‐151. [DOI] [PubMed] [Google Scholar]
- 38. Foley KE, Yang HS, Graham LC, Howell GR. Transcriptional profiling predicts running promotes cerebrovascular remodeling in young but not midlife mice. BMC Genomics. 2019;20:860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Bond ND, Guo J, Hall KD, McPherron AC. Modeling energy dynamics in mice with skeletal muscle hypertrophy fed high calorie diets. Int J Biol Sci. 2016;12:617‐630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Longo KA, Charoenthongtrakul S, Giuliana DJ, et al. The 24‐hour respiratory quotient predicts energy intake and changes in body mass. Am J Physiol Regul Integr Comp Physiol. 2010;298:R747‐54. [DOI] [PubMed] [Google Scholar]
- 41. Brown JD, Naples SP, Booth FW. Effects of voluntary running on oxygen consumption, RQ, and energy expenditure during primary prevention of diet‐induced obesity in C57BL/6N mice. J Appl Physiol. 2012;113:473‐478. [DOI] [PubMed] [Google Scholar]
- 42. Patel H, Kerndt CC, Bhardwaj A. Physiology, Respiratory Quotient. StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022. Available from: https://www.ncbi.nlm.nih.gov/books/NBK531494/ [PubMed] [Google Scholar]
- 43. McClave SA, Lowen CC, Kleber MJ, McConnell JW, Jung LY, Goldsmith LJ. Clinical use of the respiratory quotient obtained from indirect calorimetry. JPEN J Parenter Enteral Nutr. 2003;27:21‐26. [DOI] [PubMed] [Google Scholar]
- 44. Wooley JA. Indirect calorimetry: applications in practice. Respir Care Clin N Am. 2006;12:619‐633. [DOI] [PubMed] [Google Scholar]
- 45. Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA‐seq data with DESeq2. Genome Biol. 2014;15:550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Woolley C, Thompson C, Hakendorf P, Horwood C. The effect of age upon the interrelationship of bmi and inpatient health outcomes. J Nutr Health Aging. 2019;23:558‐563. [DOI] [PubMed] [Google Scholar]
- 47. Yan LL, Daviglus ML, Liu K, et al. BMI and health‐related quality of life in adults 65 years and older. Obes Res. 2004;12:69‐76. [DOI] [PubMed] [Google Scholar]
- 48. Gandhi PK, Revicki DA, Huang IC. Adolescent body weight and health‐related quality of life rated by adolescents and parents: the issue of measurement bias. BMC Public Health. 2015;15:1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Klop B, Elte JW, Cabezas MC. Dyslipidemia in obesity: mechanisms and potential targets. Nutrients. 2013;5:1218‐1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Knight JA. Diseases and disorders associated with excess body weight. Ann Clin Lab Sci. 2011;41:107‐121. [PubMed] [Google Scholar]
- 51. Flock MR, Green MH. Kris‐Etherton PM. Effects of adiposity on plasma lipid response to reductions in dietary saturated fatty acids and cholesterol. Adv Nutr. 2011;2:261‐274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Natarajan P, Ray KK, Cannon CP. High‐density lipoprotein and coronary heart disease: current and future therapies. J Am Coll Cardiol. 2010;55:1283‐1299. [DOI] [PubMed] [Google Scholar]
- 53. Katz S. Assessing self‐maintenance: activities of daily living, mobility, and instrumental activities of daily living. J Am Geriatr Soc. 1983;31:721‐727. [DOI] [PubMed] [Google Scholar]
- 54. Prizer LP, Zimmerman S. Progressive support for activities of daily living for persons living with dementia. Gerontologist. 2018;58:S74‐S87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Giebel CM, Sutcliffe C, Stolt M, et al. Deterioration of basic activities of daily living and their impact on quality of life across different cognitive stages of dementia: a European study. Int Psychogeriatr. 2014;26:1283‐1293. [DOI] [PubMed] [Google Scholar]
- 56. Williams HC, Farmer BC, Piron MA, et al. APOE alters glucose flux through central carbon pathways in astrocytes. Neurobiol Dis. 2020;136:104742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Bretsky PM, Buckwalter JG, Seeman TE, et al. Evidence for an interaction between apolipoprotein E genotype, gender, and Alzheimer disease. Alzheimer Dis Assoc Disord. 1999;13:216‐221. [DOI] [PubMed] [Google Scholar]
- 58. Farrer LA, Cupples LA, Haines JL, et al. Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease. A meta‐analysis. APOE and Alzheimer Disease Meta Analysis Consortium. JAMA. 1997;278:1349‐1356. [PubMed] [Google Scholar]
- 59. Erickson KI, Gildengers AG, Butters MA. Physical activity and brain plasticity in late adulthood. Dialogues Clin Neurosci. 2013;15:99‐108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Zajac MS, Pang TY, Wong N, et al. Wheel running and environmental enrichment differentially modify exon‐specific BDNF expression in the hippocampus of wild‐type and pre‐motor symptomatic male and female Huntington's disease mice. Hippocampus. 2010;20:621‐636. [DOI] [PubMed] [Google Scholar]
- 61. Rodriguez JJ, Noristani HN, Olabarria M, et al. Voluntary running and environmental enrichment restores impaired hippocampal neurogenesis in a triple transgenic mouse model of Alzheimer's disease. Curr Alzheimer Res. 2011;8:707‐717. [DOI] [PubMed] [Google Scholar]
- 62. Vecchio LM, Meng Y, Xhima K, Lipsman N, Hamani C, Aubert I. The neuroprotective effects of exercise: maintaining a healthy brain throughout aging. Brain Plast. 2018;4:17‐52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Wang S, Lai X, Deng Y, Song Y. Correlation between mouse age and human age in anti‐tumor research: significance and method establishment. Life Sci. 2020;242:117242. [DOI] [PubMed] [Google Scholar]
- 64. Flurkey K, Currer JM, Harrison DE. The Mouse in Biomedical Research. Elsevier; 2007. [Google Scholar]
- 65. Lamb SE, Sheehan B, Atherton N, et al. Dementia and Physical Activity (DAPA) trial of moderate to high intensity exercise training for people with dementia: randomised controlled trial. BMJ. 2018;361:k1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Karssemeijer EGA, Aaronson JA, Bossers WJ, Smits T, Olde Rikkert MGM, Kessels RPC. Positive effects of combined cognitive and physical exercise training on cognitive function in older adults with mild cognitive impairment or dementia: a meta‐analysis. Ageing Res Rev. 2017;40:75‐83. [DOI] [PubMed] [Google Scholar]
- 67. Toots A, Littbrand H, Bostrom G, et al. Effects of exercise on cognitive function in older people with dementia: a randomized controlled trial. J Alzheimers Dis. 2017;60:323‐332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Jensen CS, Simonsen AH, Siersma V, et al. Patients with Alzheimer's disease who carry the APOE epsilon4 allele benefit more from physical exercise. Alzheimers Dement (N Y). 2019;5:99‐106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Head D, Bugg JM, Goate AM, et al. Exercise engagement as a moderator of the effects of apoe genotype on amyloid deposition. Arch Neurol. 2012;69:636‐643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Ngandu T, Lehtisalo J, Solomon A, et al. A 2 year multidomain intervention of diet, exercise, cognitive training, and vascular risk monitoring versus control to prevent cognitive decline in at‐risk elderly people (FINGER): a randomised controlled trial. Lancet. 2015;385:2255‐2263. [DOI] [PubMed] [Google Scholar]
- 71. Tokgoz S, Claassen J. Exercise as potential therapeutic target to modulate alzheimer's disease pathology in APOE epsilon4 carriers: a systematic review. Cardiol Ther. 2021;10:67‐88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Allen M, Carrasquillo MM, Funk C, et al. Human whole genome genotype and transcriptome data for Alzheimer's and other neurodegenerative diseases. Sci Data. 2016;3:160089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. De Jager PL, Ma Y, McCabe C, et al. A multi‐omic atlas of the human frontal cortex for aging and Alzheimer's disease research. Sci Data. 2018;5:180142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Wang M, Beckmann ND, Roussos P, et al. The Mount Sinai cohort of large‐scale genomic, transcriptomic and proteomic data in Alzheimer's disease. Sci Data. 2018;5:180185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Raichlen DA, Alexander GE. Exercise, APOE genotype, and the evolution of the human lifespan. Trends Neurosci. 2014;37:247‐255. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information


